Abstract
16S rRNA sequence data have been used to provide a molecular basis for an accurate system for identification of members of the genus Mycobacterium. Previous studies have shown that Mycobacterium species demonstrate high levels (>94%) of 16S rRNA sequence similarity and that this method cannot differentiate between all species, i.e., M. gastri and M. kansasii. In the present study, we have used the recA gene as an alternative sequencing target in order to complement 16S rRNA sequence-based genetic identification. The recA genes of 30 Mycobacterium species were amplified by PCR, sequenced, and compared with the published recA sequences of M. tuberculosis, M. smegmatis, and M. leprae available from GenBank. By recA sequencing the species showed a lower degree of interspecies similarity than they did by 16S rRNA gene sequence analysis, ranging from 96.2% between M. gastri and M. kansasii to 75.7% between M. aurum and M. leprae. Exceptions to this were members of the M. tuberculosis complex, which were identical. Two strains of each of 27 species were tested, and the intraspecies similarity ranged from 98.7 to 100%. In addition, we identified new Mycobacterium species that contain a protein intron in their recA genes, similar to M. tuberculosis and M. leprae. We propose that recA gene sequencing offers a complementary method to 16S rRNA gene sequencing for the accurate identification of the Mycobacterium species.
Mycobacteria are aerobic or microaerophilic rods that are characterized by acid-fast properties and high G+C contents (8). The conventional identification of Mycobacterium species is still based on biochemical analysis, which is both time-consuming and labor-intensive, since cultivation of many of the slowly growing Mycobacterium species may take weeks. In addition, the biochemical tests are further hindered by the increasing number of rare and newly discovered disease-causing Mycobacterium species. Alternative methods, such as cell wall lipid chromatography, require pure culture and significant amounts of bacteria that normally need at least 3 weeks of growth (9). The DNA-based diagnostic methods that are available such as methods that use the DNA probe technology developed by Gen-Probe are rapid and relatively sensitive, but their chief drawback is that they have limited numbers of species-specific probes (12).
One of the most accurate molecular identification methods is based on 16S rRNA gene sequences, with identification commonly being achieved by comparison of the variable regions in the 16S rRNA gene. Within the Mycobacterium genus, the interspecies percent similarity of the 16S rRNA gene sequences is relatively high, from 94.3% between M. chelonae and M. xenopi to 100% between M. kansasii and M. gastri (17). In this study, we chose to investigate the genetic relatedness of Mycobacterium species using the recA gene, which exists in all bacteria due to its important function in homologous DNA recombination, DNA damage repair, and induction of the SOS response (13). As part of the SOS response, recA coordinates the induction of over 20 genes involved in DNA repair, DNA synthesis, DNA recombination, and cell division (11, 13). Bacterial classification and identification derived from the results of recA gene sequence analysis have previously been studied, with a focus on gram-negative bacteria and a few gram-positive bacteria (4–6). Comparative phylogenetic analyses based on the recA and 16S rRNA gene sequences of various bacterial species have demonstrated highly similar branching patterns (6), indicating that the recA gene is a good choice for use in molecular systematic studies and species identification. However, the recA genes of Mycobacterium species have been far less studied. To date, only three sequences from Mycobacterium species are published or available from GenBank. The lengths of the recA sequences of M. tuberculosis and M. leprae are 2,373 and 2,136 bp, respectively. Both contain a protein intron, and these protein introns in the two species vary in size and location within the recA gene (2). The recA gene of M. smegmatis does not contain the protein intron and is 1,050 bp in length (15).
The primary purpose of this study was to determine the potential of recA gene sequencing for the identification of Mycobacterium species, of which more than 80 have been described (12), by using characterized reference strains as a foundation. The secondary purpose of the study was to determine the utility of recA gene sequencing in comparison with that of 16S rRNA gene sequencing, particularly among species for which the similarity is above 99%.
MATERIALS AND METHODS
Bacterial species and media.
The reference strains used in this study are listed in Table 1. All strains, stocked at −20°C in skim milk, were inoculated into BACTEC 12B liquid medium (Becton-Dickinson, Oakville, Ontario, Canada) and were subcultured onto either Middlebrook 7H10 agar or Lowenstein-Jensen slant and grown under optimum conditions, depending on the species. A loopful of bacteria from a solid-medium culture was resuspended in 1 ml of sterile distilled H2O containing 4- to 6-mm-diameter glass beads. The mixture was vortexed for 2 min for mechanical breakage, transferred to a 1.5-ml microcentrifuge tube, and boiled for 10 min. The resulting crude lysate was stored at −20°C until PCR.
TABLE 1.
Mycobacterium species and strains used in the studya
| Organism | Strains tested | % Similarity | % Divergence |
|---|---|---|---|
| Slow-growing species | |||
| M. africanum | ATCCb 25420T | NAc | |
| M. asiaticum | ATCC 25276T, ATCC 25274 | 99.4 | 0.6 |
| M. avium | ATCC 25291T, ATCC 35717 | 99.3 | 0.7 |
| M. bovis | ATCC 35720, ATCC 35726 | 100 | 0.0 |
| M. gastri | ATCC 15754T, EB 1609 | 100 | 0.0 |
| M. gordonae | ATCC 14470T, ATCC 35756 | 99.6 | 0.4 |
| M. intracellulare | ATCC 13950T, ATCC 25122 | 100 | 0.0 |
| M. kansasii | ATCC 12478T, ATCC 35755 | 100 | 0.0 |
| M. leprae | (X73822)d | NA | |
| M. marinum | ATCC 927T, ATCC 11564 | 99.6 | 0.4 |
| M. microti | ATCC 19422T, ATCC 11152 | 100 | 0.0 |
| M. nonchromogenicum | ATCC 19531, ATCC 35783 | 99.6 | 0.4 |
| M. scrofulaceum | ATCC 19981T, ATCC 35788 | 98.7 | 1.3 |
| M. shimodei | ATCC 27962T | NA | |
| M. simiae | ATCC 25275T, 8988/68 | 99.9 | 0.1 |
| M. szulgai | ATCC 35799T, NCTCe 10829 | 99.9 | 0.1 |
| M. terrae | ATCC 15755T, EB 1614 | 100 | 0.0 |
| M. triviale | ATCC 23292T, TMC 1543 | 100 | 0.0 |
| M. tuberculosis | H37Rv, ATCC 27294T (X58485), Canetti (AJ000012) | 100 | 0.0 |
| M. xenopi | ATCC 19250T, EB 1362 | 99.7 | 0.3 |
| Fast-growing species | |||
| M. abscessus | ATCC 19977T, ATCC 23003 | 100 | 0.0 |
| M. album | ATCC 29676, ATCC 29677 | 99.2 | 0.5 |
| M. aurum | ATCC 23366T | NA | |
| M. chelonae | ATCC 19237, ATCC 35749 | 99.9 | 0.1 |
| M. fortuitum | ATCC 6841T, ATCC 6842 | 100 | 0.0 |
| M. gadium | ATCC 27726T, CASAL 1080 | 100 | 0.0 |
| M. mucogenicum | ATCC 49650T, ATCC 49651 | 98.7 | 0.9 |
| M. peregrinum | ATCC 14467T, ATCC 23015 | 96.2 | 3.9 |
| M. phlei | ATCC 11758T, ATCC 27206 | 99.8 | 0.2 |
| M. porcinum | ATCC 33776T, ATCC 33775 | 100 | 0.0 |
| M. smegmatis | ATCC 19420T, mc2 155 (X99208) | 99.6 | 0.4 |
The percent similarity and percent divergence are indicated for all species of which two strains each were tested.
ATCC, American Type Culture Collection, Manassas, Va.
NA, not applicable.
Sequences obtained from GenBank; accession numbers are given in parentheses.
NCTC, National Collection of Type Cultures, London, England.
Primer design and PCR.
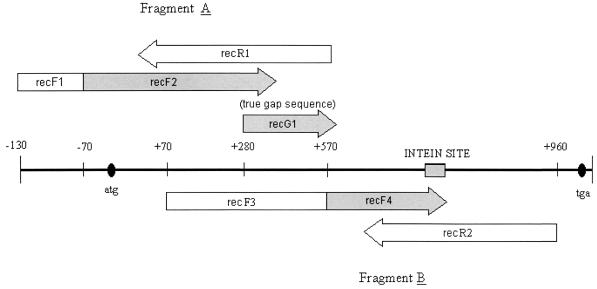
Four relatively conserved regions were identified by aligning the available Mycobacterium recA sequences found in GenBank, and these four regions were used to design four pairs of degenerate primers. All oligonucleotides were synthesized by the DNA Core Facility, Bureau of Microbiology, Health Canada. PCR was performed with the GeneAmp PCR system 9600 (PE Biosystems, Foster City, Calif.). The reaction mix consisted of 5 μl of crude DNA lysate, each deoxynucleoside triphosphate at a concentration of 200 μM, each primer at a concentration of 1 μM, 1× PCR buffer with 1.5 mM MgCl2 (Qiagen Inc., Valencia, Calif.), and 1.25 U of Taq (Qiagen Inc.) with 1× Q solution (Qiagen Inc.) for a total volume of 50 μl. For the amplification of the first PCR product, fragment A (Fig. 1), a forward degenerate primer (recF1; 5′-GGT GTT CGN CTA NTG TGG TG-3′) was paired with a reverse degenerate primer (recR1; 5′-AGC TGG TTG ATG AAG ATY GC-3′). For those strains from which a product was not amplified, seminested PCR was performed with a 1/100 dilution of the recF1-recR1 PCR product by using forward degenerate primer recF2 (5′-GYG TCA CSG CCA ACC GAY C-3′) and recR1. The cycles used were 5 min at 94°C, followed by 30 cycles of 94°C for 1 min, 48°C for 1 min, and 72°C for 1 min, with a final extension at 72°C for 10 min. For the amplification of the second product, fragment B, forward degenerate primer recF3 (5′-GGC AAR GGY TCG GTS ATG C-3′) and reverse primer recR2 (5′-TTG ATC TTC TTC TCG ATC TC-3′) were used in a touchdown PCR protocol. Conditions were 94°C for 5 min; 5 cycles of 94°C for 1 min, 50°C for 1 min (with a decrease of 1°C each cycle), and 72°C for 1 min; 25 cycles of 94°C for 1 min, 45°C for 1 min, and 72°C for 1 min; and a final extension of 72°C for 10 min. For amplification of recA gene fragment B that contained an intein (a posttranslationally self-splicing protein intron), as well as for difficult templates with significant nonspecific amplification or weak or no amplification, a different forward primer, recF4, was used. The sequence of primer recF4 is complementary to that of primer recR1 and is used in a seminested PCR with a 1/100 dilution of the recF3-recR2 product. Conditions for this PCR are 94°C for 5 min; 3 cycles of 94°C for 1 min, 40°C for 1 min, 2 min of ramping to 72°C, and 72°C for 1 min; 27 cycles of 94°C for 1 min, 55°C for 1 min, 1 min of ramping to 72°C, and 72°C for 1 min; and a final extension of 72°C for 10 min. A schematic representation of the primer location along the gene can be found in Fig. 1.
FIG. 1.
Schematic illustration of the primer pairs and sites used in the amplification of the recA gene. The shaded segments correspond to primers applied for seminested PCR. Primer recG1 was used to determine the true sequence of the gap left between primers recR1 and recF4 when seminested PCR was performed.
PCR product detection, purification, and quantitation.
PCR products were visualized by UV detection of an ethidium bromide-stained 1.5% agarose gel following electrophoresis. The purification of the remaining PCR product was achieved with Microcon-100 microconcentrators (Millipore Corp., Bedford, Mass.) by following the manufacturer's instruction. On occasion, the PCR product was shown to contain a second nonspecific band on the gel, in which case the desired product was cut out of the gel and was purified with the QIAquick Gel Extraction Kit (Qiagen Inc.) by following the manufacturer's instructions. The concentration of the purified PCR product was determined spectrophotometrically by measuring the A260.
DNA sequencing and sequencing analysis.
The ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Biosystems) was used for the sequencing of the PCR product. The sequencing reaction required 4 μl of premix, 3.2 pmol of sequencing primer, and 150 ng of PCR product template in a total volume of 10 μl. PCR primers were used for sequencing, as was primer recG1 (5′-CTS GAR ATC GCC GAC ATG CTG-3′) (Fig. 1). The sequencing reaction and template preparation were performed in accordance with the instructions of the manufacturer (PE Biosystems). The sequencing product was purified with the Centricep columns (Princeton Separations, Adelphia, N.J.) recommended by PE Biosystems.
The sequencing output was analyzed by using the DNA Sequence Analyzer computer software (PE Biosystems). The Lasergene program, version 4.01 (DNASTAR, Inc., Madison, Wis.), was used for sequence assembly, sequence alignment, and phylogenetic analysis. Multiple sequence alignments were determined by using the Clustal method algorithm.
RESULTS
PCR with the designed primers yielded two products which have overlapping sequences corresponding to the first ∼970 bp of the ∼1-kb recA gene (Fig. 1). By using forward primers recF1 or recF2 in either a separate or a seminested reaction, fragment A was obtained from all species tested. Fragment A is homologous to the 5′ end of the recA gene and also contains a short stretch of DNA homologous to the sequence upstream of the recA gene. As a result, the exact size of this PCR product varies slightly among species (data not shown). When compared with the recA sequences of M. tuberculosis and M. leprae (2, 3), the fragment A sequence was determined to contain the region from 1 to 587 bp of the recA gene. Fragment B was obtained from most of the organisms tested by using primer set recF3 and recR2, and the sequencing results indicated that the size of this product was 907 bp, from 67 to 974 bp of the recA gene.
The sequences of PCR fragments A and B were combined to yield a usable nucleotide sequence of a minimum of 915 bp up to a maximum of ∼970 bp of the recA gene (excluding the protein intron region found in certain species). Thus, the first 915 bp of all species was used for sequence alignment and analysis of the recA gene, which revealed the presence of a large number of nucleotide substitutions among the species tested, with interspecies similarities ranging from 75.7% between M. leprae and M. aurum or M. mucogenicum to 96.2% between M. gastri and M. kansasii (Table 2). Six of the species tested, M. fortuitum, M. peregrinum, M. album, M. porcinum, M. aurum, and M. mucogenicum, contain an extra glutamine near the N terminus at amino acid position 4, while the rest of the species studied do not have the insertion.
TABLE 2.
Interspecies similarity of partial recA gene sequences (∼915 bp)a
| Species | % Similarity
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. africanum | M. bovis | M. microti | M. abscessus | M. chelonae | M. xenopi | M. szulgai | M. intracellulare | M. avium | M. gastri | M. kansasii | M. gordonae | M. asiaticum | M. simiae | M. scrofulaceum | M. gadium | M. phlei | M. shimoidei | M. terrae | M. nonchromogenicum | M. triviale | M. marinum | M. leprae | M. smegmatis | M. mucogenicum | M. fortuitum | M. peregrinum ATCC 14467 | M. peregrinum ATCC 23015 | M. porcinum | M. album | M. aurum | |
| M. tuberculosis | 100 | 100 | 100 | 81.5 | 81.7 | 86.3 | 89.4 | 89.7 | 90.0 | 91.4 | 90.7 | 88.9 | 89.8 | 89.8 | 89.7 | 85.9 | 86.4 | 86.7 | 86.7 | 86.1 | 84.9 | 89.5 | 83.3 | 85.5 | 84.3 | 85.1 | 84.5 | 84.9 | 86.1 | 84.6 | 81.5 |
| M. africanum | 100 | 100 | 81.5 | 81.7 | 86.3 | 89.4 | 89.7 | 90.0 | 91.4 | 90.7 | 88.9 | 89.8 | 89.8 | 89.7 | 85.9 | 86.4 | 86.7 | 86.7 | 86.1 | 84.9 | 89.5 | 83.3 | 85.5 | 84.3 | 85.1 | 84.5 | 84.9 | 86.1 | 84.6 | 81.5 | |
| M. bovis | 100 | 81.5 | 81.7 | 86.3 | 89.4 | 89.7 | 90.0 | 91.4 | 90.7 | 88.9 | 89.8 | 89.9 | 89.7 | 85.9 | 86.4 | 86.7 | 86.7 | 86.1 | 84.9 | 89.5 | 83.3 | 85.5 | 84.3 | 85.1 | 84.5 | 84.9 | 86.1 | 84.6 | 81.5 | ||
| M. microti | 81.5 | 81.7 | 86.3 | 89.4 | 89.7 | 90.0 | 91.4 | 90.7 | 88.9 | 89.8 | 89.9 | 89.7 | 85.9 | 86.4 | 86.7 | 86.7 | 86.1 | 84.9 | 89.5 | 83.3 | 85.5 | 84.3 | 85.1 | 84.5 | 84.9 | 86.1 | 84.6 | 81.5 | |||
| M. abscessus | 90.4 | 81.1 | 83.0 | 85.5 | 83.6 | 83.0 | 82.9 | 83.6 | 82.7 | 84.5 | 84.2 | 85.0 | 84.9 | 82.9 | 84.4 | 85.0 | 84.2 | 82.0 | 78.9 | 87.4 | 88.4 | 85.5 | 85.2 | 84.5 | 85.9 | 85.5 | 82.3 | ||||
| M. chelonae | 80.0 | 82.9 | 85.1 | 84.2 | 83.2 | 82.9 | 81.7 | 82.2 | 85.1 | 84.5 | 85.2 | 84.2 | 82.6 | 84.4 | 85.0 | 84.0 | 81.1 | 78.4 | 85.5 | 87.9 | 85.0 | 86.0 | 85.7 | 86.2 | 85.0 | 82.3 | |||||
| M. xenopi | 85.3 | 86.6 | 85.6 | 85.7 | 85.4 | 84.5 | 84.8 | 87.5 | 86.3 | 83.0 | 82.9 | 86.6 | 84.9 | 84.6 | 83.8 | 84.2 | 80.8 | 82.9 | 81.5 | 82.7 | 82.3 | 82.2 | 83.4 | 83.2 | 78.5 | ||||||
| M. szulgai | 88.7 | 90.0 | 90.8 | 90.6 | 89.1 | 90.1 | 89.7 | 89.4 | 85.2 | 86.8 | 86.5 | 86.5 | 85.7 | 85.4 | 90.5 | 83.0 | 85.0 | 84.4 | 84.8 | 84.2 | 84.1 | 85.2 | 85.0 | 80.9 | |||||||
| M. intracellulare | 95.9 | 91.1 | 90.2 | 90.2 | 90.0 | 95.0 | 95.4 | 87.7 | 89.7 | 88.5 | 90.8 | 90.7 | 88.8 | 88.9 | 84.6 | 87.6 | 87.6 | 87.6 | 87.5 | 87.8 | 89.4 | 88.0 | 84.3 | ||||||||
| M. avium | 91.1 | 90.4 | 89.1 | 90.0 | 94.3 | 95.1 | 87.5 | 89.9 | 88.4 | 91.3 | 89.9 | 89.1 | 88.8 | 84.1 | 87.6 | 87.4 | 87.2 | 87.8 | 87.6 | 88.8 | 86.8 | 84.4 | |||||||||
| M. gastri | 96.2 | 89.9 | 90.8 | 90.2 | 90.4 | 86.3 | 87.7 | 87.2 | 87.4 | 87.2 | 85.5 | 90.9 | 86.0 | 85.2 | 84.3 | 85.5 | 85.2 | 85.4 | 86.4 | 85.5 | 82.0 | ||||||||||
| M. kansasii | 89.4 | 89.9 | 90.1 | 90.0 | 86.5 | 87.6 | 86.4 | 87.2 | 86.7 | 85.0 | 91.0 | 86.1 | 85.1 | 84.3 | 85.3 | 85.1 | 84.9 | 86.5 | 84.4 | 81.2 | |||||||||||
| M. gordonae | 90.1 | 89.1 | 89.1 | 87.1 | 87.8 | 86.6 | 87.8 | 87.6 | 86.0 | 88.9 | 82.1 | 85.5 | 83.4 | 85.0 | 84.5 | 84.3 | 86.0 | 85.2 | 80.9 | ||||||||||||
| M. asiaticum | 90.1 | 89.6 | 86.8 | 87.8 | 86.3 | 88.5 | 87.8 | 86.6 | 89.4 | 84.6 | 86.1 | 85.5 | 86.3 | 85.5 | 85.3 | 86.4 | 85.7 | 82.4 | |||||||||||||
| M. simiae | 94.5 | 86.1 | 87.9 | 87.8 | 90.4 | 89.8 | 88.6 | 88.9 | 84.5 | 87.3 | 87.2 | 86.6 | 87.1 | 87.5 | 88.8 | 87.0 | 83.9 | ||||||||||||||
| M. scrofulaceum | 87.0 | 88.8 | 87.8 | 89.9 | 89.5 | 89.4 | 88.7 | 84.6 | 87.3 | 86.7 | 87.2 | 86.8 | 87.3 | 88.7 | 85.7 | 83.2 | |||||||||||||||
| M. gadium | 94.8 | 87.3 | 86.7 | 87.6 | 87.3 | 85.1 | 79.6 | 87.5 | 86.6 | 87.5 | 87.3 | 87.5 | 88.0 | 87.2 | 84.9 | ||||||||||||||||
| M. phlei | 87.1 | 88.5 | 88.7 | 88.5 | 86.6 | 79.9 | 89.3 | 88.2 | 88.2 | 88.2 | 88.4 | 88.9 | 88.8 | 85.9 | |||||||||||||||||
| M. shimoidei | 88.5 | 88.3 | 86.3 | 85.6 | 81.8 | 84.6 | 83.8 | 84.9 | 85.7 | 85.7 | 85.9 | 85.9 | 83.8 | ||||||||||||||||||
| M. terrae | 93.2 | 89.0 | 86.2 | 81.9 | 88.9 | 87.1 | 87.8 | 88.7 | 88.8 | 89.1 | 87.7 | 84.9 | |||||||||||||||||||
| M. nonchromogenicum | 88.9 | 86.0 | 82.5 | 88.4 | 87.5 | 88.9 | 89.4 | 89.5 | 89.5 | 87.8 | 84.5 | ||||||||||||||||||||
| M. triviale | 85.2 | 79.7 | 87.2 | 87.0 | 86.4 | 86.4 | 86.4 | 86.7 | 86.0 | 84.7 | |||||||||||||||||||||
| M. marinum | 83.2 | 84.8 | 84.3 | 84.6 | 83.7 | 83.7 | 85.4 | 83.7 | 80.6 | ||||||||||||||||||||||
| M. leprae | 78.5 | 78.6 | 79.7 | 79.4 | 79.3 | 80.3 | 79.6 | 75.7 | |||||||||||||||||||||||
| M. smegmatis | 89.8 | 92.2 | 92.2 | 92.0 | 93.2 | 90.9 | 86.2 | ||||||||||||||||||||||||
| M. mucogenicum | 89.0 | 89.6 | 89.6 | 90.1 | 89.3 | 85.4 | |||||||||||||||||||||||||
| M. fortuitum | 94.3 | 94.0 | 94.4 | 90.9 | 84.7 | ||||||||||||||||||||||||||
| M. peregrinum ATCC 14467 | 96.2 | 95.4 | 90.7 | 85.0 | |||||||||||||||||||||||||||
| M. peregrinum ATCC 23015 | 95.7 | 90.6 | 85.4 | ||||||||||||||||||||||||||||
| M. porcinum | 91.8 | 85.8 | |||||||||||||||||||||||||||||
| M. album | 85.1 | ||||||||||||||||||||||||||||||
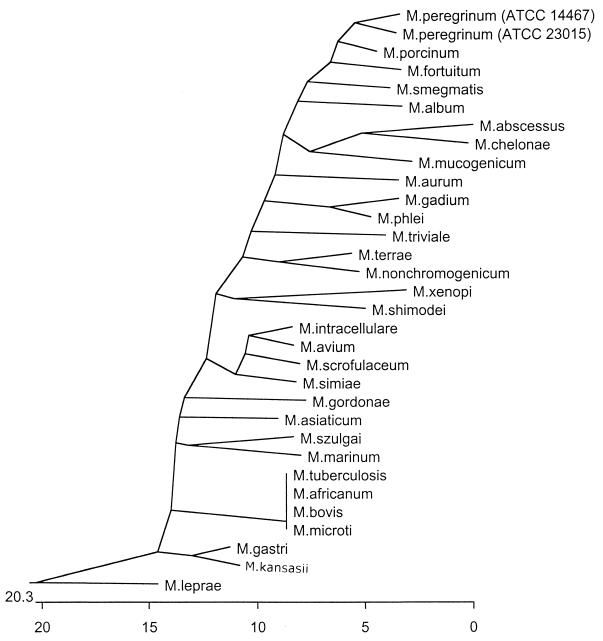
The intraspecies variability has been determined with 2 reference strains of each of 27 of the Mycobacterium species tested in this study (Table 1). No intraspecies variability was present in 13 of the species, whereas 13 species demonstrated an intraspecies variability that ranged from 98.7 to 99.9%. Comparison of two strains of M. peregrinum, however, resulted in a 96.2% similarity. The 16S rRNA gene sequences of these two strains were also determined in our laboratory (data not shown) and indicated a 100% similarity. A phylogenetic tree of the 31 species, including those species tested as well as M. leprae and M. tuberculosis, was generated with the type strain of each species set when possible as well as with both M. peregrinum strains (Fig. 2).
FIG. 2.
Phylogenetic relationships of 31 Mycobacterium species derived from the sequence of the recA gene (excluding the intein regions). The tree was generated from the alignment obtained with the Megalign tool of Lasergene (DNAstar Inc.) with the Clustal method algorithm.
Amplification of fragment B from M. xenopi, M. asiaticum, M. shimoidei, and members of the M. tuberculosis clade resulted in a PCR product ∼1 kb larger than expected (data not shown), suggesting the presence of a DNA insertion. Sequencing results for M. xenopi indicated that the PCR product was indeed the desired fragment of the recA gene in which 1,092 bp of extraneous DNA was inserted. In these cases, another forward primer, recF4, approximately 500 bp downstream from the recA gene start codon, was used instead of recF3 in a seminested reaction. In addition, the complete protein intron of M. xenopi ATCC 19250 was sequenced by using a pair of primers specifically designed from the sequenced recA region that flanked the intein and was compared to the intein of M. leprae (3). The size of the insertion fragment in M. xenopi is 1 amino acid residue short of the size of the protein intron in M. leprae. The missing amino acid residue was identified as L140. More importantly, the sites of insertion within the recA genes of these two species are identical. Despite the similarity in size and location, the two protein introns show only 86% similarity at the protein level and 77.8% similarity at the DNA level (data not shown). Likewise, M. shimoidei and M. asiaticum were also found to contain insertions that resemble the intein of M. leprae (partial sequences were determined).
DISCUSSION
Despite its role in DNA recombination and repair, the recA genes of Mycobacterium species have not been studied extensively. The only known recA sequences available prior to this study were those of M. tuberculosis, M. leprae, and M. smegmatis.
Sequence alignment and analysis of the recA genes that belong to 31 species of mycobacteria revealed the presence of a large number of nucleotide substitutions among the species tested. Unlike the 16S rRNA gene, in which variability is confined to certain areas of the gene, the sequence similarities of the recA genes of Mycobacterium species are significantly lower among species (≤96.2%) and variability occurs throughout the recA gene. The majority of substitutions were found to be confined to the third position of a codon, also known as the wobble position, allowing a conserved amino acid sequence across the genus, thereby preserving the important functions of the RecA protein. This pattern of sequence divergence is analogous to the more extensively studied recA sequences of Escherichia coli (13).
Previous studies have found that the recA gene of M. smegmatis (15) contains an extra glutamine residue near the N terminus, at amino acid position 4 (nucleotide positions 10 to 12), whereas M. tuberculosis and M. leprae do not have this extra glutamine (2, 3). In this study, we have also found that M. fortuitum, M. peregrinum, M. album, M. porcinum, M. aurum, and M. mucogenicum also contain the extra glutamine at the exact same location. These are all nonchromogenic rapid growers (8). The sequence alignment of all species tested revealed that these species share a very high degree of recA sequence similarity and are clustered together on the phylogenetic tree, apart from all slow growers.
Comparison of the results of recA gene-based sequence analysis and the results of 16S rRNA gene-based sequence analysis revealed a general likeness in the relative position of each species within the tree, with a division between the rapid growers and slow growers (Fig. 2) (17). Excluding members of the M. tuberculosis complex, there is greater variability between the species by recA gene sequence analysis (from 75.7 to 96.2%) than by 16S rRNA gene sequence analysis (94.3 to 100%) (17). 16S rRNA sequence analysis is unable to distinguish M. kansasii from M. gastri due to their identical sequences, whereas our preliminary findings demonstrate that these species can be differentiated by the recA gene sequence analysis, which indicated 96.2% similarity. While the two species can be differentiated on the basis of their photochromogenicities, recA gene sequence analysis can prove to be useful in cases in which identification may rely exclusively on molecular methods when growth is unsuccessful. Since M. kansasii is considered clinically significant, whereas M. gastri is not, the distinction between the two is essential (18). Furthermore, M. bovis and M. marinum, which are 99.4% similar by 16S rRNA gene sequence analysis (17), are 89.5% similar by recA gene sequence analysis (Table 2). Alternatively, upon sequencing of the species in the M. tuberculosis complex, it was found that the sequences of M. tuberculosis, M. bovis, M. microti, and M. africanum were identical and that recA gene sequencing would therefore not serve as a method for differentiation between the members of this complex.
The other focus of this study was evaluation of the degree of intraspecies variation of the recA gene. The recA gene sequence was available for two reference strains of each of 27 of the 31 species tested (Table 1). The sequencing results revealed that overall the intraspecies sequence variation is insignificant: the greatest variation was observed for M. scrofulaceum (ATCC 19981 and ATCC 35788) and M. mucogenicum (ATCC 49650 and ATCC 49651), with 1.3% variations for each species. All other species have 0.9 to 0% divergence. The slight divergence seen within the same species could demonstrate the presence of recA alleles. Several species of Mycobacterium have been found to exhibit a number of 16S rRNA and/or rpoB alleles (7, 10, 14, 16). As suggested previously for these two genes, the use of only one reference strain for each species may not provide a large enough wealth of information for the accurate identification of species and subspecies (1, 7). Although these findings are generated from a small data set, the recA sequences derived from American Type Culture Collection reference strains, including, when possible, the type strain of each species, allude to the viability of this method. These sequences can be used as a foundation on which to base a database system, analogous to the 16S rRNA gene system, with reference type strains serving as standards for identification.
We have found in this study that, in general, the interspecies deviation of the recA gene sequence was more than 3.8%, while the intraspecies variation was less than 1.3%, with one exception: M. peregrinum. Strains ATCC 14467T and ATCC 23015 of M. peregrinum show 3.9% divergence, the same divergence that exists between M. gastri and M. kansasii, which cannot be differentiated by 16S rRNA gene sequence analysis. Comparison of the 16S rRNA gene sequences of M. peregrinum ATCC 14467T (GenBank accession number AF130308) and ATCC 23015 performed in our laboratory showed that they had 100% similarity. In addition, the biochemical profile determined in our laboratory indicated that the profiles of these two strains follow that of M. peregrinum: 3-day arylsulfatase positivity, positivity for iron uptake and nitrate, tolerance of 5% NaCl, no growth at 42°C, and fermentation of mannitol (9). However, ATCC 23015 has a positive 10-day Tween test result and does not develop a dark pink color, unlike the type strain, when growing on MacConkey agar without crystal violet. The percent similarity of the recA genes between these two strains would suggest that they may perhaps, like M. kansasii and M. gastri, be two closely related species or subspecies, as they do occupy similar positions in the M. fortuitum cluster of the phylogenetic tree shown in Fig. 2. This does not, however, eliminate the possibility that the discrepancy may be due to the presence of different copies of recA in the genome.
Previous studies have indicated the presence of an extraneous DNA sequence known as a posttranslationally self-splicing protein intron (or intein) in the recA genes of M. tuberculosis and M. leprae (2, 3). As of yet, the function of this element is unknown, but it has been hypothesized that the intein contributes to a novel regulatory mechanism needed for recA expression rather than being just a selfish element (2). After first reporting the existence of an insertion element in the M. tuberculosis recA gene in 1991 (3), Davis and coworkers (2) discovered the presence of a protein intron in M. leprae, another highly pathogenic Mycobacterium species. Their study indicated that the insertion elements in the recA genes of these two species differ in size, sequence, as well as location with respect to the gene, which led them to propose that the acquisition of the inteins in these two species occurred through independent pathways. Furthermore, they concluded that the intein is confined to M. tuberculosis and M. leprae only on the basis of the fact that none of the other Mycobacterium species that they studied contains the insertion. However, we report on the presence of an intein in other Mycobacterium species, including M. xenopi, M. shimoidei, and M. asiaticum, none of which were included in the study of Davis et al. (2). Predictably, all members of the M. tuberculosis complex tested in this study were found to contain the intein. The insertion site of the intein of M. xenopi was identical to that of M. leprae, and the sizes differed by one amino acid residue. Despite their similarities in size and location, the sequences of the protein introns of these two species were quite different (86% protein sequence homology). By determination of partial sequences, the sequences of the inteins of M. shimodei and M. asiaticum also appeared to resemble that of the intein of M. leprae. On the basis of these results, we conclude, first, that M. leprae, M. xenopi, M. asiaticum, and M. shimoidei acquired the protein intron through a common pathway that differs from the mechanism used by M. tuberculosis. Second, the sequence variation provides little information on whether these species acquired the intein simultaneously from a common source or from other intron-containing species at various stages of their evolution. In any case, the high degree of sequence variation among inteins of the same origin suggests that the acquisition of the intein may have occurred early in the evolution of these species (2) and that significant sequence variation is the accumulated product of spontaneous mutations over the generations.
The discovery of the intein in the recA genes of the two most pathogenic species of mycobacteria had led Davis and coworkers (2) to propose a selection hypothesis. They hypothesized that the presence of the intein in two major human pathogens raises the possibility that inteins play a role in intracellular survival or pathogenesis (2). Both M. tuberculosis and M. leprae are pathogenic slow growers. This is also true of M. xenopi, M. shimoidei, and M. asiaticum, which seems to support the notion of selection among pathogenic species. We predict that a more extensive study of recA may reveal the existence of more intron-containing Mycobacterium species.
A target gene must be sufficiently conserved among strains of species for use for genotypic identification. We conclude that the recA gene of Mycobacterium species is less conserved at the nucleic acid level than the 16S rRNA gene and is thus potentially useful for identification. Phylogenetic analysis based on recA gene sequence analysis grouped the Mycobacterium species generally in the same way as that based on phylogenetic studies by 16S rRNA gene sequence analysis. Therefore, we believe that recA sequencing can be used in conjunction with 16S rRNA-based species identification, particularly when 16S rRNA sequences share high similarity values.
REFERENCES
- 1.Clayton R A, Sutton G, Hinkle P S, Bult C, Fields C. Intraspecific variation in small-subunit rRNA sequences in GenBank: why single sequences may not adequately represent prokaryotic taxa. Int J Syst Bacteriol. 1995;45:595–599. doi: 10.1099/00207713-45-3-595. [DOI] [PubMed] [Google Scholar]
- 2.Davis E O, Thangaraj H S, Brooks P C, Colston M J. Evidence of selection for protein introns in the RecAs of pathogenic mycobacteria. EMBO J. 1994;13:699–703. doi: 10.1002/j.1460-2075.1994.tb06309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis E O, Sedgwick S G, Colston M J. Novel structure of the recA locus of Mycobacterium tuberculosis implies processing of the gene product. J Bacteriol. 1991;173:5653–5662. doi: 10.1128/jb.173.18.5653-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duwat P, Ehrlich S D, Gruss A. A general method for cloning recA genes of gram-positive bacteria by polymerase chain reaction. J Bacteriol. 1992;174:5171–5175. doi: 10.1128/jb.174.15.5171-5175.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dybvig K, Hollingshead S K, Heath D G, Clewell D B, Sun F, Woodard A. Degenerate oligonucleotide primers for enzymatic amplification of recA sequences from gram-positive bacteria and mycoplasmas. J Bacteriol. 1992;174:2729–2732. doi: 10.1128/jb.174.8.2729-2732.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisen J A. The RecA protein as a model molecule for molecular systematic studies of bacteria: comparison of trees of RecAs and 16S rRNAs from the same species. J Mol Evol. 1995;41:1105–1123. doi: 10.1007/BF00173192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gingeras T R, Ghandour G, Wang E, Berno A, Small P E, Drobniewski F, Alland D, Desmond E, Holodniy M, Drenkow J. Simultaneous genotyping and species identification using hybridization pattern recognition analysis of generic Mycobacterium DNA arrays. Gene Res. 1998;8:435–448. doi: 10.1101/gr.8.5.435. [DOI] [PubMed] [Google Scholar]
- 8.Goodfellow M, Magee J G. Taxonomy of mycobacteria. In: Gangadharam P R J, Jenkins P A, editors. Mycobacteria I. Basic aspects. New York, N.Y: Chapman & Hall; 1998. pp. 1–49. [Google Scholar]
- 9.Heifets L B, Jenkins P A. Speciation of mycobacteria in clinical laboratories. In: Gangadharam P R J, Jenkins P A, editors. Mycobacteria I. Basic aspects. New York, N.Y: Chapman & Hall; 1998. pp. 308–350. [Google Scholar]
- 10.Kim B-J, Lee S-H, Lyu M-A, Kim S-J, Bai G-H, Kim S-J, Chae G-T, Kim E-C, Cha C-Y, Kook Y-H. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB) J Clin Microbiol. 1999;37:1714–1720. doi: 10.1128/jcm.37.6.1714-1720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S S, Rehrauer W M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metchock B G, Nolte F S, Wallace R J., Jr . Mycobacterium. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 399–437. [Google Scholar]
- 13.Miller R V, Kokjohn T A. General microbiology of recA: environmental and evolutionary significance. Annu Rev Microbiol. 1990;44:365–394. doi: 10.1146/annurev.mi.44.100190.002053. [DOI] [PubMed] [Google Scholar]
- 14.Ninet B, Monod M, Embler S, Pawlowski J, Metral C, Rohner P, Auckenthaler R, Hirschel B. Two different 16S rRNA genes in a mycobacterial strain. J Clin Microbiol. 1996;34:2531–2536. doi: 10.1128/jcm.34.10.2531-2536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papavinasasundaram K G, Movahedzadeh F, Keer J T, Stoker N G, Colston M J, Davis E O. Mycobacterial recA is cotranscribed with a potential regulatory gene called recX. Mol Microbiol. 1997;24:141–153. doi: 10.1046/j.1365-2958.1997.3441697.x. [DOI] [PubMed] [Google Scholar]
- 16.Reischl U, Feldmann K, Naumann L, Gaugler B J M, Ninet B, Hirschel B, Emler S. 16S rRNA sequence diversity in Mycobacterium celatum strains caused by presence of two different copies of 16S rRNA gene. J Clin Microbiol. 1998;36:1761–1764. doi: 10.1128/jcm.36.6.1761-1764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogall T, Wolters J, Flohr T, Bottger E C. Towards a phylogeny and definition of species at the molecular level within the genus Mycobacterium. Int J Syst Bacteriol. 1990;40:323–330. doi: 10.1099/00207713-40-4-323. [DOI] [PubMed] [Google Scholar]
- 18.Wayne L G, Sramek H A. Agents of newly recognized or encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]