Abstract
In this report, we present 18F-FDG PET/CT findings of reactive left axillary and supraclavicular hypermetabolic lymphadenopathy, as well as ipsilateral deltoid muscle injection site radiotracer uptake, related to recent coronavirus disease 2019 (COVID-19) vaccination in a patient with osteosarcoma. With the growing number of patients receiving COVID-19 vaccine, recognition of benign characteristic 18F-FDG PET/CT image findings will ensure staging and restaging accuracy and avoid unnecessary biopsy.
Keywords: COVID-19, vaccination, lymphadenopathy
Reactive lymphadenopathy is a recognized side effect related to vaccination. With messenger RNA–based coronavirus disease 2019 (COVID-19) vaccines, efficacy depends on the activation of dendritic cells after administration. These activated antigen-presenting cells must then migrate to the draining lymph nodes and present the translated protein to the node-based B and T cells in order to create robust humoral and cell-mediated adaptive immunity (1). As more oncologic patients start to receive COVID-19 vaccines, it is important to recognize the benign 18F-FDG PET/CT imaging features immediately after vaccination to ensure staging accuracy and prevent unnecessary biopsy.
CASE REPORT
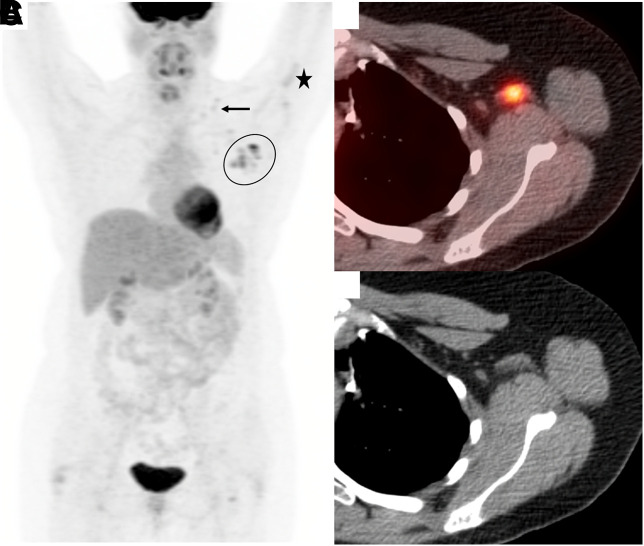
A 40-y-old woman with a history of metastatic left proximal tibia osteosarcoma underwent surveillant 18F-FDG PET/CT. There was no radiotracer uptake suggestive of tumor recurrence. However, multiple morphologically benign-appearing hypermetabolic lymph nodes were visualized at the left axillary and supraclavicular regions, as well as focal uptake in the left deltoid muscle (Fig. 1). On further interview, the patient revealed receiving her second dose of the BNT162b2 vaccine (Pfizer/BioNTech) against SARS-CoV-2 the day before the 18F-FDG PET/CT scan. She also stated that she had experienced pain at the left-upper-arm injection site, intense body aches, headaches, and a slight fever. Combined with inoculation history and characteristic imaging features, as well as exclusion of tracer injection at ipsilateral arm, a diagnosis of reactive lymphadenopathy secondary to COVID-19 vaccination was achieved.
FIGURE 1.
(A) Maximum-intensity-projection image demonstrating cluster of hypermetabolic left axillary lymph nodes (encircled), cluster of hypermetabolic supraclavicular lymph nodes (arrow), and faint intramuscular radiotracer uptake (star). (B and C) Representative left axillary lymph node that appears benign on axial CT image (C) but demonstrates increased uptake (SUVmax, 7.1) on axial 18F-FDG PET/CT image (B).
Reactive lymphadenopathy is one of the well-documented reactions after intramuscular injection of COVID-19 vaccine and is likely secondary to a robust vaccine-elicited immune response (2,3). The efficacy of messenger RNA COVID-19 vaccine depends on encoding dendritic cell migration to draining lymph nodes in order to kickstart the complex humoral and cell-mediated response and, ultimately, to establish immunity (2). There are rich draining lymph nodes at the axillary region that may show an immediate response after vaccination (4,5). 18F-FDG is a glucose analog and is nonspecifically trapped in metabolically active tumor cells and benign conditions such as infection and inflammation, potentially leading to false-positive interpretations on oncologic 18F-FDG PET/CT scans (6). Among the most common etiologies of hypermetabolic axillary lymph nodes on 18F-FDG PET/CT scans are malignancies, reactive changes, and lymphatic drainage of radiotracer extravasation.
CONCLUSION
With more oncologic patients receiving messenger RNA COVID-19 vaccines, it is important for nuclear radiologists to recognize the characteristic benign 18F-FDG uptake after vaccination. A detailed COVID-19 vaccination history, including the inoculation time, which arm was injected, and any side effects, should be acquired before the 18F-FDG PET/CT scan to ensure the accuracy of staging or restaging and to avoid an unnecessary biopsy.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1. Lindsay KE, Bhosle SM, Zurla C, et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET-CT and near-infrared imaging. Nat Biomed Eng. 2019;3:371–380. [DOI] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 MRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coates EE, Costner PJ, Nason MC, et al. Lymph node activation by PET/CT following vaccination with licensed vaccines for human papillomaviruses. Clin Nucl Med. 2017;42:329–334. [DOI] [PubMed] [Google Scholar]
- 5. Thomassen A, Lerberg Nielsen A, Gerke O, Johansen A, Petersen H. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38:894–898. [DOI] [PubMed] [Google Scholar]
- 6. Stumpe KD, Dazzi H, Schaffner A, von Schulthess GK. Infection imaging using whole-body FDG-PET. Eur J Nucl Med. 2000;27:822–832. [DOI] [PubMed] [Google Scholar]