Abstract
Kampo medicine has been practiced as traditional medicine (TM) in Japan. Kampo medicine uses Kampo formulae that are composed of multiple crude drugs to make Kampo formulae. In Japan, Kampo formulae are commonly used instead of or combined with Western medicines. If drug therapy that follows the guidelines for neuropathic pain does not work or cannot be taken due to side effects, various Kampo formulae are considered as the next line of treatment. Since Kampo formulae are composed of two or more kinds of natural crude drugs, and their extracts contain many ingredients with pharmacological effects, one Kampo formula usually has multiple effects. Therefore, when selecting a formula, we consider symptoms other than pain. This review outlines the Kampo formulae that are frequently used for pain treatment and their crude drugs and the basic usage of each component. In recent years, Yokukansan (YKS) has become one of the most used Kampo formulae for pain treatment with an increasing body of baseline research available. We outline the known and possible mechanisms by which YKS exerts its pharmacologic benefits as an example of Kampo formulae’s potency and holistic healing properties.
Keywords: neuropathic pain, analgesic effect, Kampo formula, Kampo medicine, Yokukansan
Introduction
Tricyclic antidepressants, calcium channel α2δ ligands such as gabapentin and pregabalin, and serotonin noradrenaline reuptake inhibitors (SNRIs) are the first choices for treating neuropathic pain according to the Japan Pain Clinic Society’s guidelines for drug therapy (Sumitani et al., 2018). An extract from inflammatory rabbit skin inoculated with vaccinia virus, Neurotropin, and tramadol are second-line treatments. The third-line treatment is potent opioids, such as morphine, fentanyl, and oxycodone. Carbamazepine is the first choice for trigeminal neuralgia, while lamotrigine and baclofen are the second choices. However, the actual efficacy rates of these drugs are low (Finnerup et al., 2015). In many cases, patients have been unable to take them due to side effects. In such cases, Kampo formulae are used as a treatment option in Japan. The Japanese Ministry of Health, Labor, and Welfare has officially approved the clinical use of Kampo formulae as an ethical pharmaceutical. Kampo medicine has been practiced as traditional medicine (TM) in Japan. Kampo medicine uses Kampo formulae that are composed of multiple crude drugs to make Kampo formulae. In recent years, the need for Kampo medicine has increased, and more than 80% of doctors prescribe Kampo formulae clinically (Ito et al., 2012; Moschik et al., 2012). We herein review examples of the clinical uses of Kampo formulae for neuropathic pain and their action mechanisms based on findings in the literature.
Kampo Formulae for Neuropathic Pain
Kampo formulae are composed of two or more kinds of natural crude drugs, and the decoctions of their mixtures are generally administered. One of the characteristics of Kampo formulae is that they are multi-component formulations, unlike most Western medicines. A single medicine has an analgesic effect along with various other effects such as improving blood flow and coldness while reducing swelling and stress. Therefore, when selecting a Kampo formula, we check for symptoms other than pain.
Table 1 shows examples. For example, patients whose pain is exacerbated by cold stimulation require medicine that relieves pain and simultaneously warms the body. Patients with an impaired blood flow receive medicine that improves the blood flow while reducing pain. Since the factors that make the pain worse can also be improved simultaneously, the therapeutic effect is higher than the administration of analgesics alone. The second feature is that almost no side effects are developed. Kampo medicines have a long history (Kuchta, 2019), and in the process, ineffective and poisonous components have been naturally eliminated. At present, only potent and safe Kampo formulae are cataloged.
TABLE 1.
Treatment strategies and examples of drug selection in Kampo medicine.
| Characteristic symptoms other than pain | Treatment strategies | Representative example of Kampo formulae | References |
| Cold | Warm | Goshajinkigan (GJG) | Takayama et al., 2018; Matsubara et al., 2021 |
| Heat/inflammation | Cool/anti-inflammatory | Eppikajutsuto (EJT) | Kogure et al., 2013; Shinkai et al., 2017 |
| Microangiopathy | Improving blood flow | Keishibukuryogan (KBG) | Endo et al., 2008; Fujita et al., 2010 |
| Dropsy/abnormal water metabolism | Improving water metabolism | Goreisan (GRS) | Yano et al., 2017; Murakami et al., 2021 |
| Stress/anxiety | Antistress/antianxiety | Yokukansan (YKS) | Katahira et al., 2017; Wada et al., 2017 |
| Decreased physical strength/immune deficiency | Improving physical fitness/improving immunity | Juzentaihoto (JTT) | Ishikawa et al., 2017; Takaku et al., 2020 |
Table 2 lists the Kampo formulae frequently used to treat chronic pain, their crude drugs, and each crude drug’s main actions and components, classified according to their effects based on Kampo medicine. (TM) has been added to certain terms to indicate that their meaning in this content is in relation to traditional Kampo medicine. Crude drugs with mainly analgesic and anti-inflammatory effects are assigned to Class 1, those with anti-stress effects are assigned to Class 2, and those that improve blood (TM) disturbances are assigned to Class 3. Blood (TM) is a red fluid that supports the nutrition and metabolism of the body, and its disturbance patterns include static blood (TM) and blood (TM) deficiency (The Editing Committee for the Dictionary of Kampo Medicine, the Japan Society for Oriental Medicine, 2020). In addition, crude drugs that enhance the digestive function and improve physical strength are assigned to Class 4, whereas those that improve water metabolism, suppress swelling, and confer a diuretic effect are assigned to Class 5. Class 4 includes crude drugs that have a qi (TM)-tonifying effect. Qi is a fundamental energy required for life activities. Class 5 includes drugs that have a fluid (TM)-regulating effect. Fluid is a colorless fluid that supports nutrition and metabolism including interstitial fluid and lymph. Weights (g) indicate the amount of each crude drug used to produce each Kampo formula, and the drugs marked with (*) are those that play the most central role in each Kampo formula (Takayama, 2019). Aside from Goshajinkigan (GJG) and Yokukansan (YKS), the contents of crude drugs differ depending on the manufacturers, so some patterns were shown. These doses of crude drugs are mixed, and the decoction is administered.
TABLE 2.
Kampo formulae for chronic pain and crude constituent drugs.
| Classification | Crude drugs |
Kampo formulae |
References | |||||||||||||||||
| Latin name | English name | Main effects | Major component | GJG | EJT |
KBG |
GRS |
YKS | JTT |
KJT | SKT |
|||||||||
| (1) | (2) | (1) | (2) | (1) | (2) | (3) | (1) | (2) | (1) | (2) | (3) | |||||||||
| 1 | Aconiti radix processa | Processed Aconiti root | Analgesia, cardiotonic, warm | Aconitine | 1.0 | 0.5 or 1.0 | Yu et al., 2012; Deng et al., 2021; Qiu et al., 2021. | |||||||||||||
| Glycyrrhizae radix | Glycyrrhiza | Analgesia, antiinflammation, antitussive | Glycyrrhizin | 2.0 | 2.0 | 1.5 | 1.0 | 1.5 | 2.0 | 1.5 | 1.5 | 2.0 | Kamei et al., 2005; Wang et al., 2015 | |||||||
| Paeoniae radix | Peony root | Analgesia, antiinflammation, improving static blood (TM), sedation | Paeoniflorin | 3.0 | 4.0 | 3.0 | 3.0 | 4.0 | 2.0 | 2.5 | 2.0 | Li et al., 2014; Yin et al., 2016; Xin et al., 2019. | ||||||||
| Cinnamomi cortex | Cinnamon bark | Analgesia, antiinflammation, perspiration, warm | Cinnamaldehyde | 1.0 | 3.0 | 4.0 | 3.0 | 2.0 | 1.5 | 3.0 | 3.0 | 4.0* | 2.5 | 2.5 | 2.0 | Iwasaki et al., 2008; Churihar et al., 2016; Lee et al., 2018. | ||||
| Gypsum fibrosum | Gypsum | Antiinflammation, sedation | Calcium sulfate | 8.0 | 8.0 | Liu et al., 2021. | ||||||||||||||
| Ephedrae Herba | Ephedrae Herb | Antiinflammation, perspiration, antitussive | Ephedrine | 6.0* | 6.0* | Miyagoshi et al., 1986; Wu et al., 2014; Cheng et al., 2017. | ||||||||||||||
| Scutellariae radix | Scutellaria root | Antiinflammation | Baicalin | 2.0 | 2.0 | 2.0 | Shimizu et al., 2018. | |||||||||||||
| 2 | Pinelliae tuber | Pinellia tuber | Antistress, sedation, antitussive | Homogentisic acid | 4.0 | 4.0 | 4.0 | Goto et al., 2013; Lin et al., 2019. | ||||||||||||
| Bupleuri radix | Bupleurum root | Antistress, antiinflammation, analgesia | Saikosaponin | 2.0 | 5.0* | 5.0* | 5.0* | Shin et al., 2019; Guo et al., 2020; Xu et al., 2021. | ||||||||||||
| Uncariae uncis cum ramulus | Uncaria hook | Antistress, vasodilation, analgesia | Rhynchophylline | 3.0* | Pengsuparp et al., 2001; Loh et al., 2017; Qiao et al., 2021. | |||||||||||||||
| 3 | Persicae semen | Peach kernel | Improving static blood (TM), antiinflammation | Amygdalin | 3.0* | 4.0* | Hao et al., 2019; He et al., 2020. | |||||||||||||
| Moutan cortex | Moutan bark | Improving static blood (TM) | Paeonol | 3.0 | 3.0* | 4.0* | Hirai et al., 1983. | |||||||||||||
| Rehmanniae radix | Rehmannia root | Tonifying blood (TM), analeptic | Catalpol | 5.0* | 3.5 | 3.0 | Leong et al., 2018; Wu et al., 2019. | |||||||||||||
| Angelicae acutilobae radix | Japanese angelica root | Tonifying blood (TM), analeptic | Ligustilide | 3.0 | 3.5 | 3.0 | Shimizu et al., 1991; Hatano et al., 2004. | |||||||||||||
| Achyranthis radix | Achyranthes root | Improving static blood (TM), improving of fluid (TM), analgesia | Ecdysterone | 3.0 | Luo et al., 2009; Jung et al., 2015; He et al., 2017. | |||||||||||||||
| Cnidii rhizoma | Cnidium rhizome | Tonifying blood (TM), analgesia, analeptic | Cnidilide | 3.0 | 3.0 | 3.0 | Choi et al., 2016; Lee et al., 2016; Ningsih et al., 2020. | |||||||||||||
| 4 | Ginseng radix | Ginseng | Tonifying qi (TM), analeptic, stomachic | Ginsenoside | 2.5* | 3.0* | 2.0 | 2.0 | 2.0 | Zhang et al., 2019; Fan et al., 2021; Qu et al., 2021. | ||||||||||
| Corni fructus | Cornus fruit | Tonifying qi (TM), analeptic | Loganin | 3.0 | Dong et al., 2018. | |||||||||||||||
| Dioscoreae rhizoma | Dioscorea rhizome | Tonifying qi (TM), analeptic, antitussive | Diosgenin | 3.0 | Kim et al., 2012. | |||||||||||||||
| Zizyphi fructus | Jujube | Tonifying qi (TM), analeptic, antistress | Zizyphus saponin | 3.0 | 3.0 | 4.0 | 2.0 | 2.0 | 2.0 | Peng et al., 2000; Irshad et al., 2020; Wang et al., 2020. | ||||||||||
| Zingiberis rhizoma | Ginger | Stomachic, warm | Gingerol | 1.0 | 0.8 | 1.0 | 0.5 or 1.0 | 1.0 | 2.0 | Yoshikawa et al., 1994; Chang et al., 1995; Chrubasik et al., 2005. | ||||||||||
| Astragali radix | Astragalus root | Tonifying qi (TM), analeptic, cardiotonic | Formononetin | 2.5 | 3.0 | Zhang et al., 2011; Wei et al., 2021. | ||||||||||||||
| 5 | Atractylodis rhizoma | Atractylodes rhizome | Improving static blood (TM), anti-edema, stomachic | Atractylon | 4.5# | 3.0 | 4.0# | 3.5 | 3.0# | Hwang et al., 1996; Shi et al., 2019; Zhang et al., 2021. | ||||||||||
| Atractylodis lanceae rhizoma | Atractylodes lancea rhizome | Improving of fluid (TM), anti-edema, stomachic, perspiration | Atractylodin | 4.0 | 4.0 | 4.5# | 3.0 | 4.0# | 3.0# | 4.0 | Yamahara et al., 1990; Koonrungsesomboon et al., 2014; Yu et al., 2017. | |||||||||
| Alismatis tuber | Alisma tuber | Improving of fluid (TM), anti-edema | Alisol | 3.0 | 6.0 | 5.0 | 4.0 | Makino et al., 2002; Han et al., 2013. | ||||||||||||
| Poria | Poria sclerotium | Improving of fluid (TM), anti-edema, stomachic, antistress | Eburicoic acid | 3.0 | 3.0 | 4.0 | 4.5* | 3.0* | 3.0* | 4.0 | 3.5 | 3.0 | Nukaya et al., 1996; Lee et al., 2012; Lu et al., 2021. | |||||||
| Polyporus | Polyporus sclerotium | Improving of fluid (TM), anti-edema, antiinflammation | Ergosterol | 4.5 | 3.0 | 3.0 | Sun and Yasukawa, 2008; Zhang et al., 2010. | |||||||||||||
| Plantaginis semen | Plantago seed | Improving of fluid (TM), anti-edema, antiinflammation, antitussive | Aucubin | 3.0 | Park and Chang, 2004; Tzeng et al., 2016; Li et al., 2020. | |||||||||||||||
All crude drugs are listed in the 17th edition of the Japanese Pharmacopeia (Pharmaceutical and Medical Device Regulatory Science Society of Japan, 2017). Class 1, crude drugs with analgesic and anti-inflammatory effects; Class 2, drugs with anti-stress effects; Class 3, drugs with blood flow-improving effects; Class 4, drugs that enhance the digestive function and improve physical strength; and Class 5, drugs that improve water metabolism, suppress swelling, and confer a diuretic effect. Traditional medicine (TM) is added to the terms used in the content of traditional Kampo medicine. Weights (g) indicate the amount of each crude drug to produce each Kampo formula, and the crude drugs marked with (*) and bold are the most active components of each medicine (Takayama, 2019). Except for GJG and YKS, the contents of crude drugs differ depending on the manufacturers, so some patterns were shown. One of the crude drugs marked with (#) (Atractylodis rhizoma or Atractylodis lanceae rhizoa) is used. GJG, Goshajinkigan; EJT, Eppikajutsuto; KBG, Keishibukuryogan; GRS, Goreisan; YKS, Yokukansan; JTT, Juzentaihoto; KJT, Keishikajutsubuto; SKT, Saikokeishito.
Goshajinkigan (Chinese Name: Niu Che Sen Qi Wan)
Goshajinkigan was first described in Ji Sheng Fang published in 1253 in China (Yan and Liu, 2012). It is a well-balanced combination of Class 3–5 crude drugs plus Aconiti radix processa and Cinnamomi cortex, which have strong analgesic and warming effects. This combination is suitable for patients who have a decreased physical function, are extremely tired, and complain of coldness, especially in the lower limbs, a dry mouth, and dysuria. GJG is often prescribed for inferior limb pain and lower back pain (Hamaguchi et al., 2017). Recent reports have suggested that GJG may prevent chemotherapy-induced peripheral neuropathy (Nishioka et al., 2011; Cascella and Muzio, 2017).
Eppikajutsuto (Yue Bi Jia Zhu Tang)
Eppikajutsuto (EJT) was first described in Jin Gui Yao Lue published around 200 AD in China (Zhang, 2020). EJT mainly includes Class 1 crude drugs, such as Ephedrae Herba, Gypsum fibrosum, and Glycyrrhizae radix, which have anti-inflammatory activities, and Atractylodis lanceae rhizoma, which improves an uneven distribution of water, such as in case of edema. EJT is useful for relieving edema and knee effusion caused by allergies and inflammation, especially rheumatoid arthritis (Kogure et al., 2013). Since EJT has a very strong anti-inflammatory effect, it is an alternative to non-steroidal anti-inflammatory drugs in patients with gastrointestinal disorders.
Keishibukuryogan (Gui Zhi Fu Ling Wan)
Keishibukuryogan (KBG), which was first described in Jin Gui Yao Lue (Zhang, 2020), is a Kampo formula that improves various symptoms caused by a decreased blood flow and stagnation (Nozaki et al., 2007; Tomita et al., 2017). It is composed of Persicae semen and Moutan cortex, which belong to Class 3, and Paeoniae radix, which improves the blood flow. In addition, the analgesic and anti-inflammatory effects of Paeoniae radix and Cinnamomi cortex, which belong to Class 1, are also present. The administration of KBG is reported to warm diseased limbs and improve post-stroke cold sensations and numbness in the affected body parts by increasing the peripheral blood flow (Fujita et al., 2010).
Goreisan (Wu Ling San)
Goreisan (GRS) was first described in Jin Gui Yao Lue (Zhang, 2020) and Shang Hang Lun published around 200 AD in China (Zhang, 1999). GRS consists of Atractylodis lanceae rhizoma (or Atractylodis rhizoma), Alismatis tuber, Poria, and Polyporus (Class 5), which relieve water retention in such conditions as edema, oliguria, and diarrhea and Cinnamomi cortex, which has analgesic, anti-inflammatory, and warming effects. GRS is administered to patients with exacerbated pain due to swelling. Changes in barometric pressure that accompany weather changes can exacerbate pain, and GRS is effective in such cases (Kurihara et al., 2018).
Yokukansan (Yi Gan San)
YKS was first described in a Chinese medical book Bao Ying Cuo Yao published in 1556 (Kai and Ji, 2016). One characteristic of YKS is that it is mainly composed of Bupleuri radix and Uncariae uncis cum ramulus (Class 2), which have anti-stress effects. In addition, it contains crude drugs from Classes 3 to 5. It is useful for patients with a weak constitution, especially those with frustration and anger due to increased sensitivity to stress. Originally, YKS was administered to patients with symptoms of emotional irritability, neurosis, and insomnia and to infants suffering from night crying and convulsions (de Caires and Steenkamp, 2010). The crude drug components of YKS, including Glycyrrhizae radix, Bupleuri radix, Uncariae uncis cum ramulus, and Cnidii rhizome, have analgesic effects. Thus, YKS is also used to treat various pain disorders, including fibromyalgia, post-herpetic neuralgia, phantom-limb pain, headache, and trigeminal neuralgia (Nakamura et al., 2009; Yamaguchi, 2015; Sugasawa, 2016; Akiyama and Hasegawa, 2019). Many studies have been published concerning the mechanism of analgesic action of YKS. Chronic pain causes stress, and stress further promotes and exacerbates pain (Hannibal and Bishop, 2014). YKS is effective in such cases.
Juzentaihoto (Shi Quan Da Bu Tang)
Juzentaihoto (JTT) was first described in Taiping Huimin Heji Ju Fang published (1151) in China (Ping et al., 2017). Long-lasting pain deprives patients of physical strength and reduces their willingness to fight illness. Chronic pain may alter immune response, which can affect recovery from chronic pain (Herzberg et al., 1994; Sunagawa et al., 2000; Bethea and Fischer, 2021). The main components of JTT, Ginseng radix, and Astragali radix (Class 4) improve fatigue, malaise, loss of appetite, and weakened immunity. JTT should improve physical strength to fight illness (Yamakawa et al., 2016). In addition, JTT contains crude drugs from Classes 3 to 5. Glycyrrhizae radix, Paeoniae radix, and Cinnamomi cortex, which have analgesic effects, contribute to a well-balanced formula. JTT is frequently used for cancer patients because it enhances immune function (Saiki et al., 2017; Ogawa-Ochiai et al., 2021).
Keishikajutsubuto (Gui Zhi Jia Zhu Fu Tang)
Keishikajutsubuto (KJT) was produced by Japanese doctor Todo Yoshimasu (1702–1773) and described in Hoki (Yoshimasu, 1181). KJT is mainly composed of Class 1 drugs with anti-inflammatory and analgesic effects. In addition to its strong analgesic effect, Aconiti radix processa, Atractylodis lanceae rhizoma (Class 5), Cinnamomi cortex (Class 1), and Zingiberis rhizoma (Class 4) variously offer warming and diuretic effects. KJT is effective for joint pain and neuralgia associated with coldness and swelling (Nakanishi et al., 2012). Although the crude constituent drugs are similar to EJT, EJT contains Gypsum fibrosum and Ephedrae Herba, which have strong anti-inflammatory effects, and treats cases without coldness.
Kampo Formulae for Trigeminal and Glossopharyngeal Neuralgias
In Western medicine, treatment strategies differ between trigeminal neuralgia and neuralgia in other parts of the body. Similarly, the drugs used in Kampo medicine are slightly different. Trigeminal neuralgia is divided into idiopathic trigeminal neuralgia caused by the compression of blood vessels around the nerve and symptomatic trigeminal neuralgia caused by organic diseases, such as tumors, other than vascular compression. Drug treatment is less invasive than surgery and is often the first treatment choice, including to achieve pain relief before surgery. In addition, drug administration is performed when surgical therapy, radiation therapy, and nerve block cannot be performed, or when symptoms recur. As mentioned above, the first-line drug is carbamazepine, an antiepileptic drug, but its number needed to harm is 3.4 (Cruccu et al., 2008). Its side effects, including gastrointestinal symptoms, light-headedness, drowsiness, drug eruption, and myelosuppression, also cause dose reduction or discontinuation of administration. In such cases, the carbamazepine dosage may be reduced by concomitant use of Kampo formulae. Kampo formula treatment is a useful countermeasure against side effects caused by long-term carbamazepine use. Frequent treatments for trigeminal neuralgia include GRS (Kido et al., 2017), Saikokeshito (SKT) and KJT.
Glossopharyngeal neuralgia is paroxysmal pain induced by coughing, swallowing, mastication, conversation, and yawning. It occurs mainly in the back of the ear, behind the tongue, tonsils, and pharynx and just below the lower jaw angle. The incidence is reportedly 0.2/100,000, making it a very rare disease. GRS is a common glossopharyngeal neuralgia treatment that it seems to confer anti-inflammatory effects and helps reduce edema around the nerve.
The reason GRS works for trigeminal and glossopharyngeal neuralgias is unclear. However, according to oriental medical theory, neuralgia is caused by the swelling of nerves. Therefore, GRS, which has a diuretic effect, is effective against these neuralgias. KJT would be better for cases with strong symptoms of coldness.
Saikokeshito (Chai Hu Gui Zhi Tang)
Saikokeshito was first described in Jin Gui Yao Lue (Zhang, 2020) and Shang Hang Lun (Zhang, 1999). SKT is usually given to patients with cold accompanied by gastrointestinal symptoms. Still, most crude drugs such as Glycyrrhizae radix, Paeoniae radix, Cinnamomi cortex, Scutellariae radix, and Bupleuri radix have analgesic and anti-inflammatory effects. SKT has been reported to exert analgesic activity in a rat trigeminal neuralgia model (Sunagawa et al., 2001). Some reports indicate the efficacy of SKT for epilepsy (Aimi et al., 1976). Therefore, SKT may have an anticonvulsant effect and may be effective for trigeminal neuralgia.
Action Mechanisms of Yokukansan for Neuropathic Pain
Kampo medicines have a long history, and although their effectiveness has been empirically recognized, their mechanisms of action have not been completely clarified. However, in recent years, basic research on the Kampo formula has been actively conducted, and evidence based Kampo medicine treatments are also being carried out. For physicians who are trained under Western medicine, evidence-based drug selection is more familiar and easier to understand than narrative-based ones. In this section we will consider the mechanism through which Kampo formulae exert their analgesic effects, using YKS as an example.
YKS has been found clinically effective for diseases with chronic pain, including post-herpetic neuralgia, central post-stroke pain, post-traumatic spinal cord injury pain, thalamic syndrome, complex regional pain syndrome (CRPS; Nakamura et al., 2009), trigeminal neuralgia (Yamaguchi, 2015; Takinami et al., 2017), phantom pain (Sugasawa, 2016), migraine (Akiyama and Hasegawa, 2019), and headache (Kimura et al., 2008). Mitsuhata et al. (2010) administered YKS to 121 patients with chronic pain who did not respond to conventional drug therapy or nerve block treatment and found it effective in 73 patients (60%). They also found YKS to be effective in 25 of 47 chronic lumbar and inferior limb pain cases (53%), 3 of 6 cervical or lumbar post-surgery syndrome cases (50%), 13 of 20 post-herpetic neuralgia cases (65%), 6 of 8 herpes zoster neuralgia cases (75%), 7 of 15 cervical spondylosis/cervical spondylotic radiculopathy cases (47%), 2 of 4 perineal pain cases (50%), and 6 of 6 CRPS cases (100%). Considering that all of these entities are intractable painful diseases, the efficacy rate seems to be relatively high.
The analgesic effect of YKS has been proven in some animal models, including a chronic constriction injury (CCI) model (Suzuki et al., 2012; Suga et al., 2015), partial sciatic nerve ligation (PSL) model (Ebisawa et al., 2015), bone metastasis model (Nakao et al., 2019), and adjuvant arthritis model (Honda et al., 2013). Several factors are involved in the complex development and promotion of neuropathic pain. Increased reactivity of the dorsal horn of the spinal cord, i.e., central sensitization, is considered one cause of hyperalgesia and allodynia. Central sensitization includes the following: (1) enhancement of excitatory synaptic transmission, (2) attenuation of inhibitory synaptic transmission, (3) activation of glial cells, and (4) dysfunction of the descending pain modulatory system.
Enhancement of Excitatory Synaptic Transmission and Yokukansan
From the terminal of the primary afferent nerve C-fiber, neurotransmitters like glutamate and substance P act on each receptor in the dorsal horn of the spinal cord. Continuous or repetitive stimulation from the primary nerve promotes excitatory synaptic transmission by activating and phosphorylating the glutamate receptor, N-methyl-D-aspartate (NMDA) receptor.
YKS was observed to attenuate excessive glutamate release from presynaptic sites (Takeda et al., 2008). The removal of glutamate in the synaptic cleft is mainly carried out by the two glutamate transporters in astrocytes: glutamate transporter 1 (GLT- 1) and glutamate/aspartate transporter. YKS has been reported to promote the GLT-1-mediated uptake of glutamate using cultured astrocytes (Ueki et al., 2018). This action appears to be due to Glycyrrhizin and its metabolite, 18β-glycyrrhetinic acid, as well as a compound found in Glycyrrhizae radix (Kawakami et al., 2010). Furthermore, YKS has an antagonistic effect on the NMDA receptor. Isoliquiritigenin, a component of Glycyrrhizae radix, acts as the antagonist (Kawakami et al., 2011). Thus, YKS may suppress excessive neurotransmission mediated by glutamate. Suzuki et al. (2012) reported that YKS inhibited mechanical and cold allodynia in the rat CCI model and reduced the cerebrospinal fluid dialyzate level of glutamate increased by stimulation with a brush or acetone.
Attenuation of Inhibitory Synaptic Transmission and Yokukansan
The hypofunctions of GABAergic neurons, which are inhibitory interneurons in the spinal dorsal horn, occurred in rodents with chronic pain (Fu et al., 2017). YKS has been reported to reverse the reduction in pentobarbital-induced sleep durations in socially isolated mice (Egashira et al., 2011). It also exhibited anxiolytic effects (Kamei et al., 2009), which are thought to be mediated by GABAA receptors. Liao et al. (1995) reported that the water extract of Angelicae acutilobae radix binds to GABAA receptors in vitro. These findings suggest that YKS can be expected to exert an inhibitory effect on synaptic transmissions via the GABAergic neuron.
Activation of Glial Cells and Yokukansan
In animal models of schizophrenia (Furuya et al., 2013), multiple sclerosis (Nomura et al., 2017), and behavioral and psychological symptoms of dementia (Ikarashi et al., 2009), YKS suppresses glial cell (microglia and astrocytes) activity. The activation of these glial cells is associated with the development and persistence of neuropathic pain (Tsuda, 2018), so glial cells and their associated molecules became the targets of YKS treatment. Suga et al. (2015) reported that the administration of YKS inhibited the expression of activated astrocytes and astrogliosis in the CCI rat model. Ebisawa et al. (2015) reported that YKS inhibited the increased expression of interleukin-6 mRNA in the dorsal horn of the spinal cord in the PSL mouse model, and the expression was confirmed in astrocytes and/or microglia, not in neurons. Furthermore, only the administration of Atratylodis Lanceae rhizoma exhibited the same effect. These studies suggest that YKS is effective against neuropathic pain, as evidenced by the regulation of microglial and astrocytic functions, which indicate the formula’s potential mechanisms.
Dysfunction of the Descending Pain Modulatory System and Yokukansan
Descending neurons from the rostral ventromedial medulla mainly secrete serotonin. In contrast, neurons from the locus ceruleus secrete noradrenaline. Serotonin acts on 5-HT1A and 5-HT1B receptors, which are suppressive serotonin receptors in spinal dorsal horn neurons. Noradrenaline acts on α2 receptors, which suppress synaptic transmission. SNRI treats chronic pain with the expectation that this effect will be enhanced. Dysfunction of the descending pain modulatory system reportedly involves the development of chronic pain (Ossipov et al., 2014). YKS acts as an agonist of the 5-HT1A receptor; geissoschizine methyl ether, an alkaloid synthesized by the YKS component Uncariae uncis cum ramulus, is believed to play this role (Nishi et al., 2012; Yamaguchi et al., 2012). However, whether or not YKS improves the dysfunction of the descending pain modulatory system is unclear, so further studies are needed.
Other Actions of Yokukansan
The pre-administration of YKS attenuated the development of morphine antinociceptive tolerance, and suppression of glial cell activation may be one mechanism underlying this phenomenon (Takemoto et al., 2016; Katayama et al., 2018). A study that investigated orexin secretion found that orexin secretion was significantly increased in rats with morphine tolerance; however, YKS administration significantly suppressed it (Katayama et al., 2018). Orexin is a neuropeptide secreted from the hypothalamus. It has an analgesic effect (Yamamoto et al., 2003), but under pathological conditions of chronic pain, the excessive secretion of orexin may disrupt the pain modulatory system. The administration of an orexin receptor antagonist to rats with morphine tolerance, therefore suppressed the decrease in the pain threshold (Erami et al., 2012) and also exerted analgesic effects against acute and chronic pain (McDonald et al., 2016). We also found that YKS suppressed orexin secretion in a dose-dependent manner in healthy rats (Katahira et al., 2017). These findings suggest that the analgesic effect of YKS is partly involved in the inhibition of orexin secretion.
Oxytocin is also a neuropeptide secreted from the hypothalamus and has been reported to have a central-acting analgesic effect (Sun et al., 2018; González-Hernández et al., 2019). YKS administration also increased oxytocin secretion in rats (Kanada et al., 2018). The analgesic effect of YKS may be related to the secretagogue effect of oxytocin. Future studies should be conducted using pain model animals. Figure 1 summarizes the main actions of YKS.
FIGURE 1.
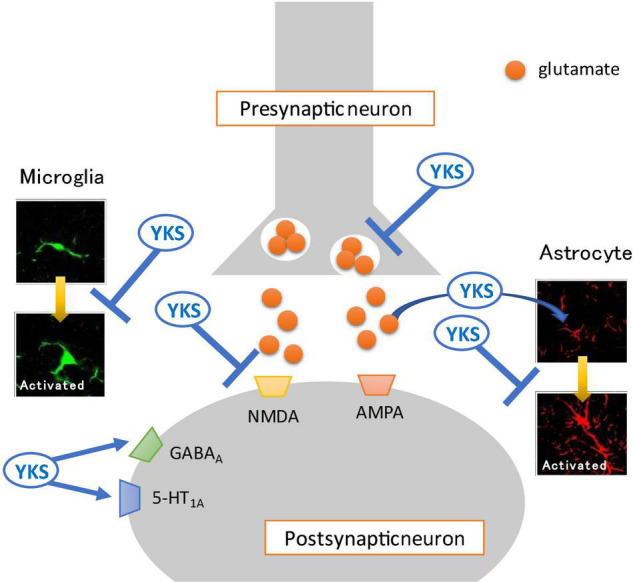
Mechanisms of action of Yokukansan for neuropathic pain. Several different mechanisms of action may act on neurotransmission in the spinal dorsal horn. (1) Attenuation of excessive glutamate release from presynaptic neurons. (2) Promotion of the uptake of glutamate into astrocytes. (3) Antagonistic effect on the glutamate receptor, N-methyl-D-aspartate (NMDA) receptor. (4) Agonistic effect on the GABAA receptor. (5) Inhibition of the activation of glia cells (microglia and astrocyte). (6) Agonistic effect on the serotonin 5-HT1A receptor. YKS, Yokukansan; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor.
Conclusion
The multiple ingredients that comprise Kampo formulae exert various beneficial effects. Although the individual pharmacological action of the components might be weak, the combination of these actions confers a holistic effect on intractable pain. This is an important point to consider in future pain treatment strategies. Multiple central sensitizations cause chronic pain; therefore, multi-component drugs, such as Kampo formulae, are more beneficial than seeking a strong analgesic effect with a single agent. In addition, identifying the active ingredients in the drugs used in traditional medicine can lead to the development of new drugs.
Author Contributions
YT and MS participated in the conception and design. KY and MS wrote the draft. All authors retrieved and reviewed the literature and accepted responsibility for the entire content of this manuscript and approved its submission.
Conflict of Interest
MS received a research grant from Tsumura & Co. (Tokyo, Japan). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
- SNRI
serotonin noradrenaline reuptake inhibitor
- GJG
Goshajinkigan
- EJT
Eppikajutsuto
- KBG
Keishibukuryogan
- GRS
Goreisan
- YKS
Yokukansan
- JTT
Juzentaihoto
- KJT
Keishikajutsubuto
- SKT
Saikokeishito
- CRPS
complex regional pain syndrome
- CCI
chronic constriction injury
- PSL
partial sciatic nerve ligation
- NMDA
N-methyl-D-aspartate.
References
- Aimi S., Saito T., Matsuda T. (1976). The effect of Saiko-Keishi-to on the treatment of epilepsy, referring to the improvement of the electroencephalogram of them [in Japanese, English abstract]. J. Jpn. Soc. Orient. Med. 27 99–116. [Google Scholar]
- Akiyama H., Hasegawa Y. (2019). Effectiveness of the traditional Japanese Kampo medicine Yokukansan for chronic migraine: A case report. Med 98:e17000. 10.1097/MD.0000000000017000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea J. R., Fischer R. (2021). Role of Peripheral Immune Cells for Development and Recovery of Chronic Pain. Front. Immunol. 12:641588. 10.3389/fimmu.2021.641588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Muzio M. R. (2017). Potential application of the Kampo medicine goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy. J. Integr. Med. 15 77–87. 10.1016/S2095-4964(17)60313-3 [DOI] [PubMed] [Google Scholar]
- Chang C. P., Chang J. Y., Wang F. Y., Chang J. G. (1995). The effect of Chinese medicinal herb Zingiberis rhizoma extract on cytokine secretion by human peripheral blood mononuclear cells. J. Ethnopharmacol. 48 13–19. 10.1016/0378-8741(95)01275-i [DOI] [PubMed] [Google Scholar]
- Cheng Y., Zhang Y., Xing H., Qian K., Zhao L., Chen X. (2017). Comparative Pharmacokinetics and Bioavailability of Three Ephedrines in Rat after Oral Administration of Unprocessed and Honey-Fried Ephedra Extract by Response Surface Experimental Design. Evid. Based Complement. Alternat. Med. 2017:2802193. 10.1155/2017/2802193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T. Y., Jun J. H., Park B., Lee J. A., You S., Jung J., et al. (2016). Concept of blood stasis in Chinese medical textbooks: a systematic review. Eur. J. Integr. Med. 8 158–164. 10.1016/j.eujim.2015.09.137 [DOI] [Google Scholar]
- Chrubasik S., Pittler M. H., Roufogalis B. D. (2005). Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 12 684–701. 10.1016/j.phymed.2004.07.009 [DOI] [PubMed] [Google Scholar]
- Churihar R., Solanki P., Vyas S., Hemant Tanwani H., Shubham Atal S. (2016). Analgesic activity of cinnamaldehyde per se and it’s interaction with diclofenac sodium and pentazocine in swiss albino mice. Int. J. Phamacog. 3 97–102. 10.13040/IJPSR.0975-8232.IJP.3(2).97-102 [DOI] [Google Scholar]
- Cruccu G., Gronseth G., Alksne J., Argoff C., Brainin M., Burchiel K., et al. (2008). AAN-EFNS guidelines on trigeminal neuralgia management. Eur. J. Neurol. 15 1013–1028. 10.1111/j.1468-1331.2008.02185.x [DOI] [PubMed] [Google Scholar]
- de Caires S., Steenkamp V. (2010). Use of Yokukansan (TJ-54) in the treatment of neurological disorders: a review. Phytother. Res. 24 1265–1270. 10.1002/ptr.3146 [DOI] [PubMed] [Google Scholar]
- Deng J., Han J., Chen J., Zhang Y., Huang Q., Wang Y., et al. (2021). Comparison of analgesic activities of aconitine in different mice pain models. PLoS One 16:e0249276. 10.1371/journal.pone.0249276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Feng Z. L., Chen H. B., Wang F. S., Lu J. H. (2018). Corni Fructus: a review of chemical constituents and pharmacological activities. Chin. Med. 13 1–20. 10.1186/s13020-018-0191-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa S., Andoh T., Shimada Y., Kuraishi Y. (2015). Yokukansan improves mechanical allodynia through the regulation of interleukin-6 expression in the spinal cord in mice with neuropathic pain. Evid. Based Complement. Alternat. Med. 2015:870687. 10.1155/2015/870687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira N., Nogami A., Iwasaki K., Ishibashi A., Uchida N., Takasaki K., et al. (2011). Yokukansan enhances pentobarbital-induced sleep in socially isolated mice: possible involvement of GABA(A)-benzodiazepine receptor complex. J. Pharmacol. Sci. 116 316–320. 10.1254/jphs.11079SC [DOI] [PubMed] [Google Scholar]
- Endo F., Oguchi T., Ikeda M., Sugimura T., Shiga Y., Yashi M., et al. (2008). Prospective clinical study of keishibukuryogan on pain caused by varicocele. J. Trad. Med. 25 52–54. 10.11339/jtm.25.52 [DOI] [Google Scholar]
- Erami E., Azhdari-Zarmehri H., Rahmani A., Ghasemi-Dashkhasan E., Semnanian S., Haghparast A. (2012). Blockade of orexin receptor 1 attenuates the development of morphine tolerance and physical dependence in rats. Pharmacol. Biochem. Behav. 103 212–219. 10.1016/j.pbb.2012.08.010 [DOI] [PubMed] [Google Scholar]
- Fan J., Liu S., Ai Z., Chen Y., Wang Y., Li Y., et al. (2021). Fermented ginseng attenuates lipopolysaccharide-induced inflammatory responses by activating the TLR4/MAPK signaling pathway and remediating gut barrier. Food Funct. 12 852–861. 10.1039/d0fo02404j [DOI] [PubMed] [Google Scholar]
- Finnerup N. B., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R. H., et al. (2015). Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 14 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H., Li F., Thomas S., Yang Z. (2017). Hyperbaric oxygenation alleviates chronic constriction injury (CCI)-induced neuropathic pain and inhibits GABAergic neuron apoptosis in the spinal cord. Scand. J. Pain 17 330–338. 10.1016/j.sjpain.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Fujita K., Yamamoto T., Kamezaki T., Matsumura A. (2010). Efficacy of keishibukuryogan, a traditional Japanese herbal medicine, in treating cold sensation and numbness after stroke: clinical improvement and skin temperature normalization in 22 stroke patients. Neurol. Med. Chir. 50 1–6. 10.2176/nmc.50.1 [DOI] [PubMed] [Google Scholar]
- Furuya M., Miyaoka T., Tsumori T., Liaury K., Hashioka S., Wake R., et al. (2013). Yokukansan promotes hippocampal neurogenesis associated with the suppression of activated microglia in Gunn rat. J. Neuroinflamm. 10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Hernández A., Espinosa, De Los Monteros-Zuñiga A. E., Martínez-Lorenzana G., Condés-Lara M. (2019). Recurrent antinociception induced by intrathecal or peripheral oxytocin in a neuropathic pain rat model. Exp. Brain Res. 237 2995–3010. 10.1007/s00221-019-05651-7 [DOI] [PubMed] [Google Scholar]
- Goto F., Morimoto N., Taiji H., Tsutumi T., Ogawa K. (2013). Treating pediatric psychogenic dizziness with a Japanese herbal medicine. Explore 9 41–43. 10.1016/j.explore.2012.10.005 [DOI] [PubMed] [Google Scholar]
- Guo J., Zhang F., Gao J., Guan X., Liu B., Wang X., et al. (2020). Proteomics-based screening of the target proteins associated with antidepressant-like effect and mechanism of Saikosaponin A. J. Cell Mol. Med. 24 174–188. 10.1111/jcmm.14695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi T., Yoshino T., Horiba Y., Watanabe K. (2017). Goshajinkigan for low back pain: an observational study. J. Altern. Complement. Med. 23 208–213. 10.1089/acm.2016.0276 [DOI] [PubMed] [Google Scholar]
- Han C. W., Kwun M. J., Kim K. H., Choi J. Y., Oh S. R., Ahn K. S., et al. (2013). Ethanol extract of Alismatis Rhizoma reduces acute lung inflammation by suppressing NF-κB and activating Nrf2. J. Ethnopharmacol. 146 402–410. 10.1016/j.jep.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Hannibal K. E., Bishop M. D. (2014). Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 94 1816–1825. 10.2522/ptj.20130597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao E., Pang G., Du Z., Lai Y.-H., Chen J.-R., Xie J., et al. (2019). Peach Kernel Oil Downregulates Expression of Tissue Factor and Reduces Atherosclerosis in ApoE knockout Mice. Int. J. Mol. Sci. 20:405. 10.3390/ijms20020405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano R., Takano F., Fushiya S., Michimata M., Tanaka T., Kazama I., et al. (2004). Water-soluble extracts from Angelica acutiloba Kitagawa enhance hematopoiesis by activating immature erythroid cells in mice with 5-fluorouracil-induced anemia. Exp. Hematol. 32 918–924. 10.1016/j.exphem.2004.07.003 [DOI] [PubMed] [Google Scholar]
- He X. Y., Wu L. J., Wang W. X., Xie P. J., Chen Y. H., Wang F. (2020). Amygdalin-A pharmacological and toxicological review. J. Ethnopharmacol. 254:112717. 10.1016/j.jep.2020.112717 [DOI] [PubMed] [Google Scholar]
- He X., Wang X., Fang J., Chang Y., Ning N., Guo H., et al. (2017). The genus Achyranthes: A review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 203 260–278. 10.1016/j.jep.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Herzberg U., Murtaugh M., Beitz A. J. (1994). Chronic pain and immunity: mononeuropathy alters immune responses in rats. Pain 59 219–225. 10.1016/0304-3959(94)90074-4 [DOI] [PubMed] [Google Scholar]
- Hirai A., Terano T., Hamazaki T., Sajiki J., Saito H., Tahara K., et al. (1983). Studies on the mechanism of antiaggregatory effect of Moutan Cortex. Thromb. Res. 3 29–40. 10.1016/0049-3848(83)90005-1 [DOI] [PubMed] [Google Scholar]
- Honda Y., Sunagawa M., Yoneyama S., Ikemoto H., Nakanishi T., Iwanami H., et al. (2013). Analgesic and anti-stress effects of Yokukansan in rats with adjuvant arthritis. Kampo Med. 64 78–85. 10.3937/kampomed.64.78 [DOI] [Google Scholar]
- Hwang J. M., Tseng T. H., Hsieh Y. S., Chou F. P., Wang C. J., Chu C. Y. (1996). Inhibitory effect of atractylon on tert-butyl hydroperoxide induced DNA damage and hepatic toxicity in rat hepatocytes. Arch. Toxicol. 70 640–644. 10.1007/s002040050323 [DOI] [PubMed] [Google Scholar]
- Ikarashi Y., Iizuka S., Imamura S., Yamaguchi T., Sekiguchi K., Kanno H., et al. (2009). Effects of yokukansan, a traditional Japanese medicine, on memory disturbance and behavioral and psychological symptoms of dementia in thiamine-deficient rats. Biol. Pharm. Bull. 32 1701–1709. 10.1248/bpb.32.1701 [DOI] [PubMed] [Google Scholar]
- Irshad Z., Hanif M. A., Ayub M. A., Hanif A., Afridi H. I. (2020). “Jujube,” in Medicinal Plants of South Asia, eds Hanif M. A., Nawaz H., Khan M. M., Byrne H. J. (Amsterdam: Elsevier; ), 451–463. [Google Scholar]
- Ishikawa S., Ishikawa T., Tezuka C., Asano K., Sunagawa M., Hisamitsu T. (2017). Efficacy of Juzentaihoto for Tumor Immunotherapy in B16 Melanoma Metastasis Model. Evid. Based Complement. Alternat. Med. 2017:6054706. 10.1155/2017/6054706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A., Munakata K., Imazu Y., Watanabe K. (2012). First nationwide attitude survey of Japanese physicians on the use of traditional Japanese medicine (kampo) in cancer treatment. Evid. Based Complement. Alternat. Med. 2012:957082. 10.1155/2012/957082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y., Tanabe M., Kobata K., Watanabe T. (2008). TRPA1 agonists—allyl isothiocyanate and cinnamaldehyde—induce adrenaline secretion. Biosci. Biotechnol. Biochem. 72 2608–2614. 10.1271/bbb.80289 [DOI] [PubMed] [Google Scholar]
- Jung S. K., Choi D. W., Kwon D. A., Kim M. J., Seong K. S., Shon D. H. (2015). Oral administration of achyranthis radix extract prevents TMA-induced allergic contact dermatitis by regulating th2 cytokine and chemokine production in vivo. Molecules 20 21584–21596. 10.3390/molecules201219788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai X., Ji X. (2016). Bao ying cuo yao. Beijing: China Press of Traditional Chinese Medicine. [Google Scholar]
- Kamei J., Miyata S., Ohsawa M. (2009). Involvement of the benzodiazepine system in the anxiolytic-like effect of Yokukansan (Yi-gan san). Prog. Neuropsychopharmacol. Biol. Psychiatry 33 1431–1437. 10.1016/j.pnpbp.2009.07.023 [DOI] [PubMed] [Google Scholar]
- Kamei J., Saitoh A., Asano T., Nakamura R., Ichiki H., Iiduka A., et al. (2005). Pharmacokinetic and pharmacodynamic profiles of the antitussive principles of Glycyrrhizae radix (licorice), a main component of the Kampo preparation Bakumondo-to (Mai-men-dong-tang). Eur. J. Pharmacol. 507 163–168. 10.1016/j.ejphar.2004.11.042 [DOI] [PubMed] [Google Scholar]
- Kanada Y., Katayama A., Ikemoto H., Takahashi K., Tsukada M., Nakamura A., et al. (2018). Inhibitory effect of the Kampo medicinal formula Yokukansan on acute stress-induced defecation in rats. Neuropsychiatr. Dis. Treat. 14 937–944. 10.2147/NDT.S156795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira H., Sunagawa M., Watanabe D., Kanada Y., Katayama A., Yamauchi R., et al. (2017). Antistress effects of Kampo medicine “Yokukansan” via regulation of orexin secretion. Neuropsychiatr. Dis. Treat. 13 863–872. 10.2147/NDT.S129418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama A., Kanada Y., Tsukada M., Akanuma Y., Takemura H., Ono T., et al. (2018). Yokukansan (Kampo medicinal formula) prevents the development of morphine tolerance by inhibiting the secretion of orexin A. Integr. Med. Res. 7 141–148. 10.1016/j.imr.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Z., Ikarashi Y., Kase Y. (2010). Glycyrrhizin and its metabolite 18β-glycyrrhetinic acid in Glycyrrhiza, a constituent herb of yokukansan, ameliorate thiamine deficiency-induced dysfunction of glutamate transport in cultured rat cortical astrocytes. Eur. J. Pharmacol. 626 154–158. 10.1016/j.ejphar.2009.09.046 [DOI] [PubMed] [Google Scholar]
- Kawakami Z., Ikarashi Y., Kase Y. (2011). Isoliquiritigenin is a novel NMDA receptor antagonist in Kampo medicine yokukansan. Cell. Mol. Neurobiol. 31 1203–1212. 10.1007/s10571-011-9722-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido H., Komasawa N., Fujiwara S., Minami T. (2017). Efficacy of go-rei-san for pain management in four patients with intractable trigeminal neuralgia [In Japanese, English Abstract]. Masui 66 184–186. [PubMed] [Google Scholar]
- Kim S., Shin S., Hyun B., Kong H., Han S., Lee A., et al. (2012). Immunomodulatory Effects of Dioscoreae Rhizome Against Inflammation through Suppressed Production of Cytokines Via Inhibition of the NF-κB Pathway. Immune Netw. 12 181–188. 10.4110/in.2012.12.5.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Shimizu S., Tanaka A., Suzuki M., Kinebuchi A., Inaki K., et al. (2008). Efficacy of yokukansan-based prescriptions for the treatment of patients with headache [In Japanese, English Abstract]. Kampo Med. 59 265–271. 10.3937/kampomed.59.265 [DOI] [Google Scholar]
- Kogure T., Harada N., Tatsumi T., Fujinaga H. (2013). Persistent undifferentiated arthritis successfully treated with the Japanese herbal medicine “Eppikajutsuto”. Eur. J. Integr. Med. 5 184–188. 10.1016/j.eujim.2012.11.001 [DOI] [Google Scholar]
- Koonrungsesomboon N., Na-Bangchang K., Karbwang J. (2014). Therapeutic potential and pharmacological activities of Atractylodes lancea (Thunb.) DC. Asian Pac. J. Trop. Med. 7 421–428. 10.1016/S1995-7645(14)60069-9 [DOI] [PubMed] [Google Scholar]
- Kuchta K. (2019). Traditional Japanese kampo medicine–history of ideas and practice; Part 1: from ancient shamanic practice to the medical academies of Edo. Trad. Kampo Med. 6 49–56. 10.1002/tkm2.1209 [DOI] [Google Scholar]
- Kurihara Y., Han C., Harada Y., Kobayashi H. (2018). General introduction to Kampo medicine - the nuts and bolts of Kampo -. Juntendo Med. J. 64 258–263. 10.14789/jmj.2018.64.JMJ18-R09 [DOI] [Google Scholar]
- Lee S. C., Wang S. Y., Li C. C., Liu C. T. (2018). Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J. Food Drug Anal. 26 211–220. 10.1016/j.jfda.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Lee Y. J., Yoon J. J., Kang D. G., Lee H. S. (2012). Effect of Poria cocos on hypertonic stress-induced water channel expression and apoptosis in renal collecting duct cells. J. Ethnopharmacol. 141 368–376. 10.1016/j.jep.2012.02.048 [DOI] [PubMed] [Google Scholar]
- Lee W. S., Shin J. S., Jang D. S., Lee K. T. (2016). Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 40 146–155. 10.1016/j.intimp.2016.08.021 [DOI] [PubMed] [Google Scholar]
- Leong P. K., Chen J., Ko K. M. (2018). “Development of Chinese Herbal Health Products for the Prevention of Aging-Associated Diseases,” in Natural Products and Drug Discovery, eds Mandal S. C., Mandal V., Konishi T. (Amsterdam: Elsevier; ), 73–104. 10.1016/B978-0-08-102081-4.00004-6 [DOI] [Google Scholar]
- Li C., Wen R., Liu W., Liu Q., Yan L. P., Wu J. X., et al. (2020). Diuretic Effect and Metabolomics Analysis of Crude and Salt-Processed Plantaginis Semen. Front. Pharmacol. 11:563157. 10.3389/fphar.2020.563157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wu P., Ning Y., Yan X., Zhu T., Ma C., et al. (2014). Sedative and hypnotic effect of freeze-dried paeoniflorin and Sini San freeze-dried powder in pentobarbital sodium-induced mice. J. Tradit. Chin. Med. 34 184–187. 10.1016/s0254-6272(14)60076-5 [DOI] [PubMed] [Google Scholar]
- Liao J. F., Jan Y. M., Huang S. Y., Wang H. H., Yu L. L., Chen C. F. (1995). Evaluation with receptor binding assay on the water extracts of ten CNS-active Chinese herbal drugs. Proc. Natl. Sci. Counc. Repub. China B 19 151–158. [PubMed] [Google Scholar]
- Lin S., Nie B., Yao G., Yang H., Ye R., Yuan Z. (2019). Pinellia ternata (Thunb.) Makino Preparation promotes sleep by increasing REM sleep. Nat. Prod. Res. 33 3326–3329. 10.1080/14786419.2018.1474466 [DOI] [PubMed] [Google Scholar]
- Liu K., Han S., Gao W., Tang Y., Han X., Liu Z., et al. (2021). Changes of Mineralogical Properties and Biological Activities of Gypsum and Its Calcined Products with Different Phase Structures. Evid. Based Complement. Alternat. Med. 2021:6676797. 10.1155/2021/6676797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y. C., Ch’ng Y. S., Tan C. S., Ahmad M., Asmawi M. Z., Yam M. F. (2017). Mechanisms of action of Uncaria rhynchophylla ethanolic extract for its vasodilatory effects. J. Med. Food 20 895–911. 10.1089/jmf.2016.3804 [DOI] [PubMed] [Google Scholar]
- Lu J., Tian J., Zhou L., Meng L., Chen S., Ma C., et al. (2021). Phytochemistry and Biological Activities of Poria. J. Chem. 2021:6659775. 10.1155/2021/6659775 [DOI] [Google Scholar]
- Luo C., Zhang Y., Chi L., Li L., Chen K. (2009). Protective effect and mechanism of ecdysterone on injury of focal cerebral infarct in rats. Medical J. Natl. Defend. Forces Southwest China 19 176–178. [Google Scholar]
- Makino B., Kobayashi M., Kimura K., Ishimatsu M., Sakakibara I., Higuchi M., et al. (2002). Local variation in the content of angiotensin II and arginine vasopressin receptor antagonistic terpenoids in the rhizomes of Alisma orientale. Planta Med. 68 226–231. 10.1055/s-2002-23129 [DOI] [PubMed] [Google Scholar]
- Matsubara Y., Okuda H., Harada K. H., Youssefian S., Koizumi A. (2021). Mechanical allodynia triggered by cold exposure in mice with the Scn11a p.R222S mutation: a novel model of drug therapy for neuropathic pain related to NaV1.9. Naunyn Schmiedeberg’s Arch. Pharmacol. 394 299–306. 10.1007/s00210-020-01978-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T., Liang H. A., Sanoja R., Gotter A. L., Kuduk S. D., Coleman P. J., et al. (2016). Pharmacological evaluation of orexin receptor antagonists in preclinical animal models of pain. J. Neurogenet. 30 32–41. 10.3109/01677063.2016.1171862 [DOI] [PubMed] [Google Scholar]
- Mitsuhata H., Nakamura Y., Kawagoe I., Tajima K., Kanai M., Konishi R., et al. (2010). Efficacy of yokukansan against neuropathic pain: clinical reports and the animal study [In Japanese, English Abstract]. Pain Kampo Med. 20 13–19. [Google Scholar]
- Miyagoshi M., Amagaya S., Ogihara Y. (1986). Antitussive effects of L-ephedrine, amygdalin, and makyokansekito (Chinese traditional medicine) using a cough model induced by sulfur dioxide gas in mice. Planta Med. 52 275–278. 10.1055/s-2007-969151 [DOI] [PubMed] [Google Scholar]
- Moschik E. C., Mercado C., Yoshino T., Matsuura K., Watanabe K. (2012). Usage and attitudes of physicians in Japan concerning traditional Japanese medicine (kampo medicine): a descriptive evaluation of a representative questionnaire-based survey. Evid. Based Complement. Alternat. Med. 2012:139818. 10.1155/2012/139818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Horie I., Hara-Chikuma M., Shimizu T., Matsumoto C., Isohama Y. (2021). Goreisan regulates AQP3 expression and improves diarrhea. Tradit. Kampo Med. 8 91–99. 10.1002/tkm2.1276 [DOI] [Google Scholar]
- Nakamura Y., Tajima K., Kawagoe I., Kanai M., Mitsuhata H. (2009). Efficacy of traditional herbal medicine, Yokukansan on patients with neuropathic pain [In Japanese, English Abstract]. Masui 58 1248–1255. [PubMed] [Google Scholar]
- Nakanishi M., Arimitsu J., Kageyama M., Otsuka S., Inoue T., Nishida S., et al. (2012). Efficacy of traditional Japanese herbal medicines—Keishikajutsubuto (TJ-18) and Bushi-matsu (TJ-3022)—against post-herpetic neuralgia aggravated by self-reported cold stimulation: a case series. J. Altern. Complement. Med. 18 686–692. 10.1089/acm.2010.0745 [DOI] [PubMed] [Google Scholar]
- Nakao K., Fujiwara A., Komasawa N., Jin D., Kitano M., Matsunami S., et al. (2019). Yokukansan alleviates cancer pain by suppressing matrix metalloproteinase-9 in a mouse bone metastasis model. Evid. Based Complement. Alternat. Med. 2019:3513064. 10.1155/2019/2956920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ningsih F. N., Okuyama T., To S., Nishidono Y., Okumura T., Tanaka K., et al. (2020). Comparative Analysis of Anti-inflammatory Activity of the Constituents of the Rhizome of Cnidium officinale Using Rat Hepatocytes. Biol. Pharm. Bull. 43 1867–1875. 10.1248/bpb.b20-00416 [DOI] [PubMed] [Google Scholar]
- Nishi A., Yamaguchi T., Sekiguchi K., Imamura S., Tabuchi M., Kanno H., et al. (2012). Geissoschizine methyl ether, an alkaloid in Uncaria hook, is a potent serotonin1A receptor agonist and candidate for amelioration of aggressiveness and sociality by yokukansan. Neuroscience 207 124–136. 10.1016/j.neuroscience.2012.01.037 [DOI] [PubMed] [Google Scholar]
- Nishioka M., Shimada M., Kurita N., Iwata T., Morimoto S., Yoshikawa K., et al. (2011). The Kampo medicine, Goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int. J. Clin. Oncol. 16 322–327. 10.1007/s10147-010-0183-1 [DOI] [PubMed] [Google Scholar]
- Nomura T., Bando Y., You H., Tanaka T., Yoshida S. (2017). Yokukansan reduces cuprizone-induced demyelination in the corpus callosum through anti-inflammatory effects on microglia. Neurochem. Res. 42 3525–3536. 10.1007/s11064-017-2400-z [DOI] [PubMed] [Google Scholar]
- Nozaki K., Goto H., Nakagawa T., Hikiami H., Koizumi K., Shibahara N., et al. (2007). Effects of keishibukuryogan on vascular function in adjuvant-induced arthritis rats. Biol. Pharm. Bull. 30 1042–1047. 10.1248/bpb.30.1042 [DOI] [PubMed] [Google Scholar]
- Nukaya H., Yamashiro H., Fukazawa H., Ishida H., Tsuji K. (1996). Isolation of inhibitors of TPA-induced mouse ear edema from Hoelen, Poria cocos. Chem. Pharm. Bull. 44 847–849. 10.1248/cpb.44.847 [DOI] [PubMed] [Google Scholar]
- Ogawa-Ochiai K., Katagiri T., Sato Y., Shirai A., Ishiyama K., Takami A., et al. (2021). Natural killer cell function changes by the Japanese Kampo Medicine juzentaihoto in General fatigue patients. Adv. Integr. Med. 8 33–43. 10.1016/j.aimed.2019.12.003 [DOI] [Google Scholar]
- Ossipov M. H., Morimura K., Porreca F. (2014). Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 8 143–151. 10.1097/SPC.0000000000000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. S., Chang I. M. (2004). Anti-inflammatory activity of aucubin by inhibition of tumor necrosis factor-α production in RAW 264.7 cells. Planta Med. 70 778–779. 10.1055/s-2004-827211 [DOI] [PubMed] [Google Scholar]
- Peng W. H., Hsieh M. T., Lee Y. S., Lin Y. C., Liao J. (2000). Anxiolytic effect of seed of Ziziphus jujuba in mouse models of anxiety. J. Ethnopharmacol. 72 435–441. 10.1016/s0378-8741(00)00255-5 [DOI] [PubMed] [Google Scholar]
- Pengsuparp T., Indra B., Nakagawasai O., Tadano T., Mimaki Y., Sashida Y., et al. (2001). Pharmacological studies of geissoschizine methyl ether, isolated from Uncaria sinensis Oliv., in the central nervous system. Eur. J. Pharmacol. 425 211–218. 10.1016/s0014-2999(01)01195-5 [DOI] [PubMed] [Google Scholar]
- Pharmaceutical and Medical Device Regulatory Science Society of Japan (2017). Japanese pharmacopoeia seventeenth edition (JP XVII) English version. Tokyo: Yakuji Nippo. [Google Scholar]
- Ping T., Min H., Ji H., Bian J. (2017). Taiping Huimin Heji Ju Fang. Beijin: People’s Health Publisher. [Google Scholar]
- Qiao Y. L., Zhou J. J., Liang J. H., Deng X. P., Zhang Z. J., Huang H. L., et al. (2021). Uncaria rhynchophylla ameliorates unpredictable chronic mild stress-induced depression in mice via activating 5-HT1A receptor: Insights from transcriptomics. Phytomedicine 81:153436. 10.1016/j.phymed.2020.153436 [DOI] [PubMed] [Google Scholar]
- Qiu L. Z., Zhou W., Yue L. X., Wang Y. H., Hao F. R., Li P. Y., et al. (2021). Repeated Aconitine Treatment Induced the Remodeling of Mitochondrial Function via AMPK–OPA1–ATP5A1 Pathway. Front. Pharmacol. 12:646121. 10.3389/fphar.2021.646121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q., Yang F., Zhao C., Liu X., Yang P., Li Z., et al. (2021). Effects of fermented ginseng on the gut microbiota and immunity of rats with antibiotic-associated diarrhea. J. Ethnopharmacol. 267:113594. 10.1016/j.jep.2020.113594 [DOI] [PubMed] [Google Scholar]
- Saiki I., Yokoyama S., Hayakawa Y. (2017). Effect of juzentaihoto/Shi-Quan-Da-Bu-Tang on malignant progression and metastasis of tumor cells. World J. Trad. Chin. Med. 3 26–45. 10.1248/bpb.23.677 [DOI] [PubMed] [Google Scholar]
- Shi K., Qu L., Lin X., Xie Y., Tu J., Liu X., et al. (2019). Deep-Fried Atractylodis Rhizoma Protects against Spleen Deficiency-Induced Diarrhea through Regulating Intestinal Inflammatory Response and Gut Microbiota. Int. J. Mol. Sci. 21:124. 10.3390/ijms21010124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Matsuzawa T., Suzuki S., Yoshizaki M., Morita N. (1991). Evaluation of Angelicae Radix (Touki) by the inhibitory effect on platelet aggregation. Chem. Pharm. Bull. 39 2046–2048. 10.1248/cpb.39.2046 [DOI] [PubMed] [Google Scholar]
- Shimizu T., Shibuya N., Narukawa Y., Oshima N., Hada N., Kiuchi F. (2018). Synergistic effect of baicalein, wogonin and oroxylin A mixture: multistep inhibition of the NF-κB signalling pathway contributes to an anti-inflammatory effect of Scutellaria root flavonoids. J. Nat. Med. 72 181–191. 10.1007/s11418-017-1129-y [DOI] [PubMed] [Google Scholar]
- Shin J. S., Im H. T., Lee K. T. (2019). Saikosaponin B2 Suppresses Inflammatory Responses Through IKK/IκBα/NF-κB Signaling Inactivation in LPS-Induced RAW 264.7 Macrophages. Inflammation 42 342–353. 10.1007/s10753-018-0898-0 [DOI] [PubMed] [Google Scholar]
- Shinkai T., Masumoto K., Chiba F., Tanaka N. (2017). A large retroperitoneal lymphatic malformation successfully treated with traditional Japanese Kampo medicine in combination with surgery. Surg. Case Rep. 3 1–4. 10.1186/s40792-017-0358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H., Sunagawa M., Ikemoto H., Nakanishi T., Fujiwara A., Okada M. (2015). The analgesic and anti-stress effects of a Kampo medicine (Yokukansan) in rats with chronic constriction injury–a comparative study with kamishoyosan. J. Integr. Ther. 2:5. 10.13188/2378-1343.1000009 [DOI] [Google Scholar]
- Sugasawa Y. (2016). Effect of yokukansan, Japanese herbal medicine, on phantom-limb pain. Middle East J. Anaesthesiol. 23 499–500. [PubMed] [Google Scholar]
- Sumitani M., Sakai T., Matsuda Y., Abe H., Yamaguchi S., Hosokawa T., et al. (2018). Executive summary of the clinical guidelines of pharmacotherapy for neuropathic pain: by the Japanese Society of Pain Clinicians. J. Anesth. 32 463–478. 10.1007/s00540-018-2501-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Zhou Q., Ba X., Feng X., Hu X., Cheng X., et al. (2018). Oxytocin relieves neuropathic pain through GABA release and presynaptic TRPV1 inhibition in spinal cord. Front. Mol. Neurosci. 11:248. 10.3389/fnmol.2018.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Yasukawa K. (2008). New anti-inflammatory ergostane-type ecdysteroids from the sclerotium of Polyporus umbellatus. Bioorg. Med. Chem. Lett. 18 3417–3420. 10.1016/j.bmcl.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Sunagawa M., Okada M., Guo S. Y., Hisamitsu T. (2000). Splenic natural killer cell activity is suppressed by ligation of unilateral mental nerve in rats [In Japanese, English abstract]. Masui Jap. J. Anesthesiol. 49 250–254. [PubMed] [Google Scholar]
- Sunagawa M., Okada M., Guo S. Y., Hisamitsu T. (2001). Effectiveness of Saiko-Keishi-To (TJ-10, a Kampo herbal medicine) for trigeminal neuralgia in rats [In Japanese, English abstract]. Masui Jap. J. Anesthesiol. 50 486–490. [PubMed] [Google Scholar]
- Suzuki Y., Mitsuhata H., Yuzurihara M., Kase Y. (2012). Antiallodynic effect of herbal medicine yokukansan on peripheral neuropathy in rats with chronic constriction injury. Evid. Based Complement. Alternat. Med. 2012:953459. 10.1155/2012/953459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaku S., Shimizu M., Takahashi H. (2020). Japanese Kampo Medicine Juzentaihoto Enhances Antitumor Immunity in CD1d-/- Mice Lacking NKT Cells. Integr. Cancer Ther. 19:1534735419900798. 10.1177/1534735419900798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K. (2019). Kampo Joyoshohou Kaisetsu, edition 2nd Edn. Chiba: Toyo Gakujutsu Shuppansha. [Google Scholar]
- Takayama S., Arita R., Kikuchi A., Ohsawa M., Kaneko S., Ishii T. (2018). Clinical practice guidelines and evidence for the efficacy of traditional Japanese herbal medicine (Kampo) in treating geriatric patients. Front. Nutr. 5:66. 10.3389/fnut.2018.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Itoh H., Tamano H., Yuzurihara M., Oku N. (2008). Suppressive effect of Yokukansan on excessive release of glutamate and aspartate in the hippocampus of zinc-deficient rats. Nutr. Neurosci. 11 41–46. 10.1179/147683008X301414 [DOI] [PubMed] [Google Scholar]
- Takemoto M., Sunagawa M., Okada M., Ikemoto H., Suga H., Katayama A., et al. (2016). Yokukansan, a Kampo medicine, prevents the development of morphine tolerance through the inhibition of spinal glial cell activation in rats. Integr. Med. Res. 5 41–47. 10.1016/j.imr.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takinami Y., Mita K., Nagai A., Yamakawa J., Ohara H. (2017). A case of trigeminal neuralgia successfully treated with yokukansan [in Japanese, English abstract]. Kampo Med. 68 358–361. 10.3937/kampomed.68.358 [DOI] [Google Scholar]
- The Editing Committee for the Dictionary of Kampo Medicine, the Japan Society for Oriental Medicine (2020). The Dictionary of Kampo Medicine -Basic terms-. Kyoto: Medical Yukon. [Google Scholar]
- Tomita T., Hirayama A., Matsui H., Aoyagi K. (2017). Effect of keishibukuryogan, a Japanese traditional Kampo prescription, on improvement of microcirculation and Oketsu and induction of endothelial nitric oxide: A live imaging study. Evid. Based Complement. Alternat. Med. 2017:3620130. 10.1155/2017/3620130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M. (2018). Modulation of pain and itch by spinal glia. Neurosci. Bull. 34 178–185. 10.1007/s12264-017-0129-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng T. F., Liu W. Y., Liou S. S., Hong T. Y., Liu I. M. (2016). Antioxidant-rich extract from plantaginis semen ameliorates diabetic retinal injury in a streptozotocin-induced diabetic rat model. Nutrients 8:572. 10.3390/nu8090572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki T., Kawakami Z., Kanno H., Omiya Y., Mizoguchi K., Yamamoto M. (2018). Yokukansan, a traditional Japanese medicine, enhances the glutamate transporter GLT-1 function in cultured rat cortical astrocytes. Evid. Based Complement. Alternat. Med. 2018:6804017. 10.1155/2018/6804017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada S., Inoguchi H., Hirayama T., Matsuoka Y. J., Uchitomi Y., Ochiai H., et al. (2017). Yokukansan for the treatment of preoperative anxiety and postoperative delirium in colorectal cancer patients: a retrospective study. Jpn. J. Clin. Oncol. 47 844–848. 10.1093/jjco/hyx080 [DOI] [PubMed] [Google Scholar]
- Wang H. L., Li Y. X., Niu Y. T., Zheng J., Wu J., Shi G. J., et al. (2015). Observing Anti-inflammatory and Anti-nociceptive Activities of Glycyrrhizin Through Regulating COX-2 and Pro-inflammatory Cytokines Expressions in Mice. Inflammation 38 2269–2278. 10.1007/s10753-015-0212-3 [DOI] [PubMed] [Google Scholar]
- Wang L., Jing N., Liu X., Jiang G., Liu Z. (2020). Nurturing and modulating gut microbiota with jujube powder to enhance anti-PD-L1 efficiency against murine colon cancer. J. Funct. Foods 64:103647. 10.1016/j.jff.2019.103647 [DOI] [Google Scholar]
- Wei H., Ding L., Wang X., Yan Q., Jiang C., Hu C., et al. (2021). Astragalus root extract improved average daily gain, immunity, antioxidant status and ruminal microbiota of early weaned yak calves. J. Sci. Food Agric. 101 82–90. 10.1002/jsfa.10617 [DOI] [PubMed] [Google Scholar]
- Wu C., Shan J., Feng J., Wang J., Qin C., Nie G., et al. (2019). Effects of dietary Radix Rehmanniae Preparata polysaccharides on the growth performance, immune response and disease resistance of Luciobarbus capito. Fish Shellfish Immunol. 89 641–646. 10.1016/j.fsi.2019.04.027 [DOI] [PubMed] [Google Scholar]
- Wu Z., Kong X., Zhang T., Ye J., Fang Z., Yang X. (2014). Pseudoephedrine/ephedrine shows potent anti-inflammatory activity against TNF-α-mediated acute liver failure induced by lipopolysaccharide/D-galactosamine. Eur. J. Pharmacol. 724 112–121. 10.1016/j.ejphar.2013.11.032 [DOI] [PubMed] [Google Scholar]
- Xin Q., Yuan R., Shi W., Zhu Z., Wang Y., Cong W. (2019). A review for the anti-inflammatory effects of paeoniflorin in inflammatory disorders. Life Sci. 237:116925. 10.1016/j.lfs.2019.116925 [DOI] [PubMed] [Google Scholar]
- Xu Y., Yu Y., Wang Q., Li W., Zhang S., Liao X., et al. (2021). Active components of Bupleurum chinense and Angelica biserrata showed analgesic effects in formalin induced pain by acting on Nav1.7. J. Ethnopharmacol. 269:113736. 10.1016/j.jep.2020.113736 [DOI] [PubMed] [Google Scholar]
- Yamaguchi K. (2015). Traditional Japanese herbal medicines for treatment of odontopathy. Front. Pharmacol. 6:176. 10.3389/fphar.2015.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T., Tsujimatsu A., Kumamoto H., Izumi T., Ohmura Y., Yoshida T., et al. (2012). Anxiolytic effects of yokukansan, a traditional Japanese medicine, via serotonin 5-HT1A receptors on anxiety-related behaviors in rats experienced aversive stress. J. Ethnopharmacol. 143 533–539. 10.1016/j.jep.2012.07.007 [DOI] [PubMed] [Google Scholar]
- Yamahara J., Matsuda H., Huang Q., Li Y. U. H. A. O., Fujimura H. (1990). Intestinal motility enhancing effect of Atractylodes lancea rhizome. J. Ethnopharmacol. 29 341–344. 10.1016/0378-8741(90)90044-t [DOI] [PubMed] [Google Scholar]
- Yamakawa J., Moriya J., Kobayashi J. (2016). Fatigue and Kampo (Japanese herbal) medicines: Hochuekkito and juzentaihoto. Methods Pharmacol. Toxicol. 2016 97–111. 10.3389/fphar.2020.00917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Saito O., Shono K., Aoe T., Chiba T. (2003). Anti-mechanical allodynic effect of intrathecal and intracerebroventricular injection of orexin-A in the rat neuropathic pain model. Neurosci. Lett. 347 183–186. 10.1016/s0304-3940(03)00716-x [DOI] [PubMed] [Google Scholar]
- Yan Y. H., Liu Y. J. Z. (2012). Yan Shi Ji Sheng Fang (TCM clinical Intangible Cultural Heritage Classic Reader), 1st Edn. Tianjin: Chinese Medical Science and Technology Press. [Google Scholar]
- Yano Y., Yano H., Takahashi H., Yoshimoto K., Tsuda S., Fujiyama K., et al. (2017). Goreisan Inhibits Upregulation of Aquaporin 4 and Formation of Cerebral Edema in the Rat Model of Juvenile Hypoxic-Ischemic Encephalopathy. Evid. Based. Complement. Alternat. Med. 2017:3209219. 10.1155/2017/3209219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Liu Y. Y., Wang T. X., Hu Z. Z., Qu W. M., Chen J. F., et al. (2016). Paeoniflorin exerts analgesic and hypnotic effects via adenosine A1 receptors in a mouse neuropathic pain model. Psychopharmacology 233 281–293. 10.1007/s00213-015-4108-6 [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Yamaguchi S., Kunimi K., Matsuda H., Okuno Y., Yamahara J., et al. (1994). Stomachic principles in ginger. III. An anti-ulcer principle, 6-gingesulfonic acid, and three monoacyldigalactosylglycerols, gingerglycolipids A, B, and C, from Zingiberis Rhizoma originating in Taiwan. Chem. Pharm. Bull. 42 1226–1230. 10.1248/cpb.42.1226 [DOI] [PubMed] [Google Scholar]
- Yoshimasu T. (1181). Hoki. Available online at: https://rmda.kulib.kyoto-u.ac.jp/item/rb00000733 (accessed August 16, 2021). [Google Scholar]
- Yu C., Xiong Y., Chen D., Li Y., Xu B., Lin Y., et al. (2017). Ameliorative effects of atractylodin on intestinal inflammation and co-occurring dysmotility in both constipation and diarrhea prominent rats. Kor. J. Physiol. Pharmacol. 21 1–9. 10.4196/kjpp.2017.21.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. Y., Wang S. J., Teng J. L., Ji X. M., Wu Z. C., Ma Q. C., et al. (2012). Effects of Radix aconiti lateralis preparata and Rhizoma zingiberis on energy metabolism and expression of the genes related to metabolism in rats. Chin. J. Integr. Med. 18 23–29. 10.1007/s11655-012-0964-7 [DOI] [PubMed] [Google Scholar]
- Zhang G., Zeng X., Han L., Wei J. A., Huang H. (2010). Diuretic activity and kidney medulla AQP1, AQP2, AQP3, V2R expression of the aqueous extract of sclerotia of Polyporus umbellatus FRIES in normal rats. J. Ethnopharmacol. 128 433–437. 10.1016/j.jep.2010.01.032 [DOI] [PubMed] [Google Scholar]
- Zhang S., Tang X., Tian J., Li C., Zhang G., Jiang W., et al. (2011). Cardioprotective effect of sulphonated formononetin on acute myocardial infarction in rats. Basic Clin. Pharmacol. Toxicol. 108 390–395. 10.1111/j.1742-7843.2011.00676.x [DOI] [PubMed] [Google Scholar]
- Zhang W. J., Zhao Z. Y., Chang L. K., Cao Y., Wang S., Kang C. Z., et al. (2021). Atractylodis Rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J. Ethnopharmacol. 266:113415. 10.1016/j.jep.2020.113415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Qiu Z., Qiu Y., Su T., Qu P., Jia A. (2019). Functional Regulation of Ginsenosides on Myeloid Immunosuppressive Cells in the Tumor Microenvironment. Integr. Cancer. Ther. 18:1534735419886655. 10.1177/1534735419886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. (1999). Shang Han Lun: On Cold Damage, Translation & Commentaries. Taos, NM: Paradigm Publications. [Google Scholar]
- Zhang Z. (2020). Essentials from the Golden Cabinet: Translation and Annotation of Jin Gui Yao Lue. Singapore: World Scientific. [Google Scholar]


