Abstract
Objectives
The aim of this study was to inform public health policy decisions through the assessment of IgG antibody seroprevalence in the population and the risk factors for SARS-CoV-2 infection.
Methods
The seroprevalence of IgG antibodies among different subpopulations at the end of the first and second waves of the pandemic was estimated. Various risk factors associated with seropositivity, including sociodemography, IgG antibodies against endemic human coronavirus, and vaccination status, were also assessed.
Results
For all 2433 consenting participants, the overall estimated seroprevalences at the end of first and second waves were 28.5% (95% CI 22.3–33.7%) and 71.5% (95% CI 62.8–80.5%), respectively. The accrual of IgG positivity was heterogeneous, with the highest seroprevalences found in urban slum populations (75.1%). Vaccine uptake varied among the subpopulations, with low rates (< 10%) among rural and urban slum residents. The majority of seropositive individuals (75%) were asymptomatic. Residence in urban slums (OR 2.02, 95% CI 1.57–2.6; p < 0.001), middle socioeconomic status (OR 1.77, 95% CI 1.17–2.67; p = 0.007), presence of diabetes (OR 1.721, 95% CI 1.148–2.581; p = 0.009), and hypertension (OR 1.75, 95% CI 1.16–2.64; p = 0.008) were associated with seropositivity in multivariable analyses.
Conclusion
Although considerable population immunity has been reached, with more than two-thirds seropositive, improved vaccination strategies among unreached subpopulations and high-risk individuals are suggested for better preparedness in future.
Key words: Seroprevalence, SARS-CoV-2, COVID, India
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused an unprecedented public health challenge in India, with over 32 million confirmed cases and over 400 000 deaths as of the end of August 2021 (JHU CSSE, 2021). India imposed a universal, strict lockdown, which began in March 2020 as the pandemic started, and lasted for several months.This provided a window of opportunity to prepare the healthcare system and helped in flattening the epidemic curve and reducing mortality. After the first wave — by the end of January 2021 — a low case fatality rate (CFR) of 1.4% was reported, with 154, 428 deaths among the 10.76 million cases (Purkayastha et al., 2021). However, the second wave, which was largely caused by the delta variant, caught the nation uninformed with its rapidity and magnitude. A much higher number of cases was reported during the second wave when compared with the first. India's most recent serosurvey, which is yet to be published, shows that two-thirds of the Indian population carries antibodies against COVID-19 either because of prior exposure to the virus or vaccination (Anand et al., 2021).
A serosurvey from 70 districts across India conducted between December 2020 and January 2021 reported a population-weighted seroprevalence of 24.1% (Murhekar et al., 2021). However, a cross-sectional study conducted among people living in urban slums around the same time reported a much higher seroprevalence of 57.9% (George et al., 2021). The extent of the spread of infection into the general population has been underestimated, because the majority of the infections are presymptomatic or asymptomatic (Wuet al., 2020). Indeed, there is heterogeneity in the transmission dynamics, with subsequent seroprevalence among the population. A vast majority (90–99%) of transmission in the community is reported to be asymptomatic (George et al., 2021; Kshatriet al.,2021). Such asymptomatic cases complicate and prevent the reliable estimation of transmission and planning of tracking strategies for containing the spread of infection. Nevertheless, asymptomatic infections contribute substantially to community transmission and population immunity (Oran et al., 2020). Based on the seroprevalence and proportion of asymptomatic transmission in the community, the estimated infection fatality rate (IFR) in India is reported to be 0.04%, which is low when compared with the reported IFR of 0.31% from high-income countries like the USA over the same period (Laxminarayan et al., 2021, Pei et al., 2020).This could be due to the much younger median age of the Indian population. Whether factors such as pre-existing seasonal coronavirus antibodies offer any partial cross-protection, leading to milder or asymptomatic disease, is unclear (Anderson et al., 2021; Song et al., 2021). With the number of cases dropping significantly, and the subsequent return to near normalcy occurring in most states, studying the current seroprevalence and vaccination rates in the community could help inform future response and prevention strategies.
This cross-sectional study assessed the seroprevalence of IgG antibodies to SARS-CoV-2 infection in Vellore district of Tamil Nadu, and compared the same among participants across four different subpopulations: urban slum, urban affluent, rural areas, and healthcare workers (HCW). IgG seropositivity was investigated in the four subgroups over two time periods — the end of wave 1 (January 2021) and the end of wave 2 (July 2021). Various risk factors associated with SARS CoV-2 infection were also evaluated — IgG antibodies against two other endemic strains (NL63 and OC43) of human coronavirus (HCoV) to evaluate possible cross-protection against SARS-CoV-2, and vaccination status at the end of wave 2. The seropositivity rates at the end of wave 1 and wave 2, combined with the vaccination status at the end of wave 2 for the four subgroups will provide valuable insight into the level of population immunity developed against SARS-CoV-2 infection. Based on this information, subgroups that warrant attention can be targeted for future prevention strategies.
Methods
Study design and participants
This cross-sectional serosurvey was performed among the permanent residents of Vellore district, Tamil Nadu in south India over two time periods: January 2021, at the end of wave 1; and July 2021, at the end of wave 2 (Figure 1 ). Vellore district, with six taluks (Vellore, Katpadi, Gudiyattam, Anaicut, Pernambet, and KV Kuppam), had 57% of the population living in rural areas and 43% in urban areas, according to the 2011 census. Villages in each of the taluks were randomly selected for the survey. Additionally, two different subpopulations in the urban area of Vellore — namely urban slums and urban affluent areas — were surveyed separately. The survey team randomly chose willing participants of all age groups from each sampling area. At least 30 individuals were enrolled from each random location, with a total of 250 individuals enrolled from each subpopulation. Healthcare workers who were willing to take part in the study were recruited from among doctors, nurses, paramedical staff, and support staff of Christian Medical College (CMC), Vellore.
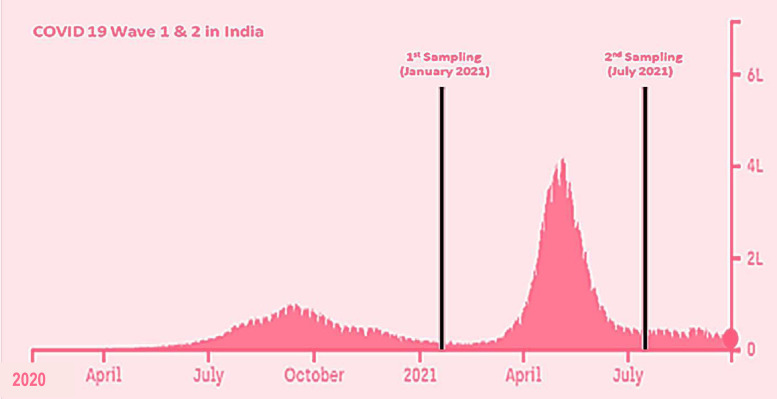
Figure 1.
COVID-19 peaks in India during wave 1 and wave 2, with two sampling periods (modified from Covid19India.org, 2021). The x-axis represents the timeline in months and the y-axis gives the number of infected cases in lakhs. The study samples at the end of wave 1 were collected in January 2021, and those at the end of wave 2 were collected in July 2021.
Procedure
Individuals who agreed to participate answered an interview-based semi-structured questionnaire after providing written informed consent or assent, as applicable. The questionnaire comprised questions relating to sociodemographic variables, including age, gender, and socioeconomic status, comorbidities, respiratory symptoms or fever in the 6 months prior to enrolment in the study, hospitalization for COVID-19 since March 2020, and mask usage in the workplace and public places. Vaccination status by the second wave was also included in the questionnaire at the end of wave 2. After administration of the questionnaire, 5 ml blood samples were drawn from the participants and transported in a sample carrier box with gel packs to the laboratory, where they were centrifuged. Plasma samples were collected and stored at −70°C until IgG ELISA testing was carried out.
SARS-CoV-2 serological testing was performed using the SCoV-2 Detect™ IgG ELISA kit (InBios International, Seattle, USA), an indirect ELISA that detects the presence of IgG antibodies against SARS-CoV-2 S proteins in human serum. Incurred sample reanalysis values above 1.1 were considered positive. The specificity of the SCoV-2 Detect™ IgG ELISA kit is reported to be 97.6% (Deshpande et al., 2021). Additionally, RecombivirusTM Human Anti-Human Coronavirus NL63/OC43 Spike 1 IgG ELISA Kit (Alpha International Diagnostics, USA) was used on human serum or plasma for quantifying IgG antibody specific for the S1 subunit of the spike protein of two other HCoV viruses (NL63 [alpha coronavirus] and OC43 [beta coronavirus]), which are etiologic agents for human endemic respiratory disease. Incurred sample optical density values above 3.5 were considered positive.
The primary outcome assessed was seropositivity (seroprevalence) to SARS-CoV-2 antibodies overall and in the different subgroups. The secondary outcomes included the various factors associated with seropositivity, symptomatic and asymptomatic infection, seropositivity to HCoV, and vaccination status.
The study was approved by the Institutional Review Board and Ethics Committee of CMC Vellore (number: 13165).
Statistical analysis
A target sample size of 1000 participants was estimated for each time point to detect differences by subpopulation (approximately 2000 participants for both waves), assuming a seroprevalence in the general population of 20% at the end of wave 1, with an α error of 0.05 and power of 80%, to detect at least a 12% difference in seroprevalence; this gave a sample size of 225 per subpopulation. In order to account for a 10% loss to follow-up from different subpopulations, 250 samples were targeted in each sampling period. The eventual sample was 2433 for both waves (1228 for wave 1 and 1205 for wave 2), which was slightly higher than the target sample size and, hence contributed to an increase in power.
The summary measures of participants, seroprevalences, and odds ratios (ORs) with 95% confidence intervals (95% CI) were calculated by subpopulation, time period, and participant characteristics. The seroprevalence estimates were calculated by subpopulation and previously known test performances. The overall seropositivity for each wave was weighted using the Vellore population. Post-stratification weighting was used to align the composition of the respondents’ sampling fraction with the known distribution of the whole population's subgroup proportions, thereby reducing sampling error. Weights were computed based on the expected proportion of the population for each location.
The risk factors for seropositivity were assessed by univariate mixed-effects logistic regression analysis clustered by time period. A p-value less than 0.05 was considered statistically significant. Multivariate mixed-effects logistic regression analysis was performed to adjust for potential confounding factors and clustering effects. Statistically significant risk factors identified in univariate analyses were selected as variables for the multivariate analyses. Statistical analyses were performed using SPSS version 21 and Stata 16 (College Station, Texas, USA).
Results
In total, 2433 willing participants from four subpopulations — rural, urban slums, urban affluent, and healthcare workers — were included in the study. Samples were collected from these four subpopulations at the end of wave 1 in January 2021 and wave 2 in July 2021, to give a total of 756 samples from the rural subpopulation, 645 from the urban slum group, 534 from urban affluent residents, and 498 from healthcare workers. The sample population comprised 56.6% females with a mean age of 37 years (SD ± 17.5). Of the participants, 18.7% were less than 20 years old, 41.8% were aged 21–40 years, 28.7% 41–60 years, and 10.9% over 60 years. The most commonly reported comorbidities were diabetes (11.1%), hypertension (10.7%), chronic obstructive pulmonary disease (COPD)/asthma (2.1%), and heart diseases (1.4%). The subpopulation-specific baseline demographic characteristics, including education, occupation, and socioeconomic class, are summarised in Table 1 .
Table 1.
Demographic characteristics
| Demographics | Categories | Total N = 2433; n (%) or n/N (%) | Rural N = 756; n (%) or n/N (%) | Urban slum N = 645; n (%) or n/N (%) | Urban affluent N = 534, n (%) or n/N (%) | Healthcare workers N = 498, n (%) or n/N (%) |
|---|---|---|---|---|---|---|
| Age | < 20 years | 454 (18.7) | 159 (21) | 198 (30.7) | 92 (17.2) | 5 (1) |
| 21–40 years | 1017 (41.8) | 220 (29.1) | 198 (30.7) | 181 (33.9) | 418 (83.9) | |
| 41–60 years | 698 (28.7) | 253 (33.5) | 185 (28.7) | 188 (35.2) | 72 (14.5) | |
| > 60 years | 264 (10.9) | 124 (16.4) | 64 (9.9) | 73 (13.7) | 3 (0.6) | |
| Gender | Male | 1,055(43.4) | 273 (36.1) | 255 (39.5) | 298 (55.8) | 229 (46) |
| Female | 1,378 (56.6) | 483 (63.9) | 390 (60.5) | 236 (44.2) | 269 (54) | |
| Education | Illiterate | 194 (8) | 107 (14.2) | 87 (13.5) | 0 (0) | 0 (0) |
| Primary to high school* | 1361 (55.9) | 573 (75.8) | 517 (80.2) | 213 (39.9) | 58 (11.6) | |
| Graduate | 878 (36.1) | 76 (10.1) | 41 (6.4) | 321 (60.1) | 440 (88.4) | |
| Occupation | Professional | 514 (21.1) | 8 (1.1) | 2 (0.3) | 126 (23.6) | 378 (75.9) |
| Semi-professional | 231 (9.5) | 28 (3.7) | 14 (2.2) | 107 (20) | 82 (16.5) | |
| Skilled worker | 528 (21.7) | 272 (36) | 116 (18) | 102 (19.1) | 38 (7.6) | |
| Unskilled/daily wage laborer | 174 (7.2) | 89 (11.8) | 85 (13.2) | 0 (0) | 0 (0) | |
| Housewife/unemployed/students | 986 (40.5) | 359 (47.5) | 428 (66.4) | 199 (37.3) | 0 (0) | |
| Socioeconomic class | Upper | 313 (12.9) | 0 (0) | 0 (0) | 116 (21.7) | 197 (39.6) |
| Middle | 923 (37.9) | 144 (19) | 75 (11.6) | 418 (78.3) | 286 (57.4) | |
| Lower | 1197 (49.2) | 612 (81) | 570 (88.4) | 0 (0) | 15 (3.0) | |
| Comorbidities | Diabetes | 271 (11.1) | 93 (12.3) | 82 (12.7) | 83 (15.5) | 13 (2.6) |
| Hypertension | 261 (10.7) | 99 (13.1) | 66 (10.2) | 73 (13.7) | 23 (4.6) | |
| COPD***/asthma | 51 (2.1) | 11 (1.5) | 5 (0.8) | 10 (1.9) | 25 (5) | |
| CAD/heart disease | 34 (1.4) | 13 (1.7) | 2 (0.3) | 16 (3) | 3 (0.6) | |
| Presence of symptoms** | 588 (24.2) | 103 (13.4) | 104 (16.1) | 151 (28.3) | 230 (46.2) | |
| Symptomatic infection requiring hospitalization | 99 (4.1) | 0 (0) | 6 (0.9) | 18 (3.4) | 75 (15.1) | |
| Mask usage in public places | Cloth | 1,414 (58.1) | 606 (80.2) | 584 (90.5) | 164 (30.7) | 60 (12) |
| Surgical | 855 (35.1) | 150 (19.8) | 57 (8.8) | 300 (56.2) | 348 (69.9) | |
| N95 | 164 (6.8) | 0(0) | 4 (0.6) | 70 (13.1) | 90 (18.1) | |
| HCoV antibody positivity among SARS-CoV-2 +ve | 391/391 (100) | 81/81 (100) | 136/136 (100) | 73/73 (100) | 101/101 (100) | |
| HCoV antibody positivity among SARS-CoV-2 –ve | 834/837 (99.6) | 221/221 (100) | 172/175 (98.2) | 222/222 (100) | 219/219 (100) | |
*< 12 years of formal education
**Suggestive of recent respiratory infection in the last 6 months
***COPD — chronic obstructive pulmonary disease
In total, 588 participants (24.2%) reported symptoms suggestive of acute respiratory infection or fever during the 6 months preceding sample collection. Symptoms were reported by 46.2% of healthcare workers, 28.3% of the urban affluent group, 16.1% of the urban slum residents, and 13.4% of the rural group. Overall reported symptom prevalences among participants were similar during wave 1 (27.6%) and wave 2 (20.7%). Among the SARS-CoV-2-positive participants, 30.7% and 22.5% were symptomatic during wave 1 and wave 2, respectively. Notably, only 4.1% of the study population had symptomatic infection requiring hospitalisation, most of which were for infection control purposes. In public places, cloth masks remained the most common type of mask used (58.1%), followed by surgical masks (35.1%), and N95 (6.8%), respectively. Other human coronavirus antibodies (NL63, OC43) were detected in almost all the participants: 99.6% of participants who were seronegative for SARS-CoV-2 and 100% of the participants who were seropositive for SARS-CoV-2 antibodies.
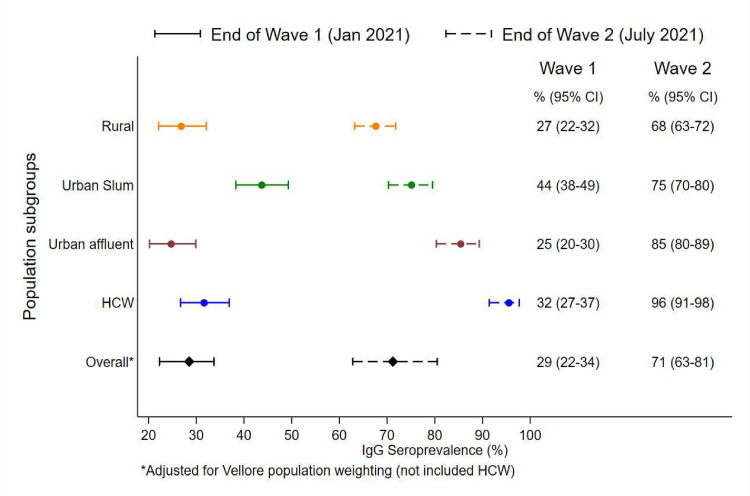
The overall weighted prevalence of SARS-CoV-2 antibodies adjusted for the population of Vellore was 28.5% (95% CI 22.3–33.7%) at the end of wave 1 and 71.6% (95% CI 62.8–80.5%) at the end of wave 2. At the end of wave 1, seroprevalence was found to be highest among individuals from urban slums (43.7%; 95% CI 38.1–49.4%), followed by healthcare workers (31.6%; 95% CI 26.5–37.0%), rural (26.8%; 95% CI 22.9–32.2%), and urban affluent (24.7%; 95% CI 20.0–30.0%). At the end of wave 2, seropositivity was highest among healthcare workers (95.5%; 95% CI 91.3–98.0%), with a high vaccination rate of 91.6% (95% CI 86.5–95.2%). The urban affluent had similarly high seropositivity of 85.4% (95% CI 80.2–89.6%), with 65.7% (95% CI 59.3–71.7%) of the participants being vaccinated. The urban slums and rural areas also had high seroprevalences of 75.1% (95% CI 70.2–79.7%) and 67.8% (95% CI 63.1–71.9%), respectively, with vaccination rates of only 6.6% (95% CI 4.2–9.8%) and 10.4% (95% CI 7.7–13.5%), respecitvely. The community seropositivities for IgG at the end of waves 1 and 2 for the four different subpopulations are shown in Table 2 and Figure 2 . The seroprevalences and vaccination rates for the different subpopulations at the end of wave 2 are shown in Figure 3 . Seropositivities according to different characteristics and time periods are shown in Table 3 . There was a slight shift in seropositivity noted among older age groups (over 60 and 40–60) in wave 1 to younger age groups (20–40 years and 1–20 years) in wave 2. This may be because of the larger proportion of the population who became infected during the second wave, thus reflecting the country's younger population.
Table 2.
Community seroprevalence and vaccination status in different subpopulations
| Subpopulation | End of wave 1 (January 2021) (N = 1228) |
End of wave 2 (July 2021) (N = 1205) |
||||
|---|---|---|---|---|---|---|
| Serology IgG, n/N (%) or % | 95% CI | Serology IgG, n/N (%) or % | 95% CI | Vaccination status#, n/N (%) | 95% CI | |
| Rural | 81/302 (26.8) | 22.9–32.2 | 307/454 (67.6) | 63.1–71.9 | 47/454 (10.4) | 7.7–13.5 |
| Urban slum | 136/311 (43.7) | 38.1–49.4 | 251/334 (75.1) | 70.2–79.7 | 22/334 (6.6) | 4.2–9.8 |
| Urban affluent | 73/295 (24.7) | 20.0–30.0 | 204/239 (85.4) | 80.2–89.6 | 157/239 (65.7) | 59.3–71.7 |
| Healthcare workers | 101/320 (31.6) | 26.5–37.0 | 170/178 (95.5) | 91.3–98.0 | 163/178 (91.6) | 86.5–95.2 |
| Overall weighted prevalence adjusted for Vellore population | 28.5 | 22.3–33.7 | 71.6 | 62.8–80.5 | 20.3 | 0–46.7 |
n — seropositives, N — total number of participants sampled, CI — confidence interval
Received at least one dose of vaccine
Figure 2.
Visual representation of community seroprevalence, with 95% confidence intervals, in various subpopulations at the end of wave 1 and wave 2. The x-axis represents IgG seropositivity as a percentage, and the y-axis shows the various subpopulations.
Figure 3.
Seropositivity and vaccination status (participants who received one dose and those fully vaccinated) in the total study population and the various subpopulations. The rural areas and urban slums had the lowest vaccination levels — of 10.4% and 6.6%, respectively. Healthcare workers had the highest vaccination rate of 91.6%, followed by the urban affluent subpopulation, with a rate of 65.7%.
Table 3.
Seropositivity by characteristics and time period
| Demographics | Categories | Seropositivity, n/N (%) | ||
|---|---|---|---|---|
| End of wave 1 | End of wave 2 | Overall | ||
| Subpopulation | Rural | 81/302 (26.8) | 307/454 (67.6) | 388/756 (51.3) |
| Urban slum | 136/311 (43.7) | 251/334 (75.1) | 387/645 (60.0) | |
| Urban affluent | 73/295 (24.7) | 204/239 (85.4) | 277/534 (51.9) | |
| Healthcare workers | 101/320 (31.6) | 170/178 (95.5) | 271/498 (54.4) | |
| Age | < 20 years | 75/237 (31.6) | 162/217 (74.7) | 237/454 (52.2) |
| 21–40 years | 166/540 (30.7) | 385/477 (80.7) | 551/1017 (54.2) | |
| 41–60 years | 105/317 (33.1) | 294/381 (77.2) | 399/698 (57.2) | |
| > 60 years | 45/134 (33.6) | 91/130 (70.0) | 136/264 (51.5) | |
| Gender | Male | 168/558 (30.1) | 379/497 (76.3) | 547/1,055 (51.8) |
| Female | 223/670 (33.3) | 553/708 (78.1) | 776/1378 (56.3) | |
| Education | Graduate | 161/534 (30.1) | 307/344 (89.2) | 468/878 (53.3) |
| Primary to high school* | 199/616 (32.3) | 552/745 (74.1) | 751/1361 (55.2) | |
| Illiterate | 31/78 (39.7) | 73/116 (62.9) | 104/194 (53.6) | |
| Occupation | Professional | 94/332 (28.3) | 170/182 (93.4) | 264/514 (51.4) |
| Semi-professional | 31/109 (28.4) | 111/122 (91.0) | 142/231 (61.5) | |
| Skilled worker | 71/214 (33.2) | 222/314 (70.7) | 293/528 (55.5) | |
| Unskilled/daily wage laborers | 29/63 (46.0) | 71/111 (64.0) | 100/174 (57.5) | |
| Housewife/unemployed/student | 166/510 (32.5) | 358/476 (75.2) | 524/985 (53.1) | |
| Socioeconomic class | Upper | 41/197 (20.8) | 106/116 (91.4) | 147/313 (47.0) |
| Middle | 188/554 (33.9) | 319/369 (86.4) | 507/923 (54.9) | |
| Lower | 162/477 (34.0) | 507/720 (70.4) | 669/1197 (55.9) | |
| Smoking habit | Smoker | 14/38 (36.8) | 0/0 (NA) | 14/38 (36.8) |
| Non-smoker | 377/1190 (31.7) | 932/1205 (77.3) | 1309/2395 (54.7) | |
| Alcohol Consumption | Alcohol consumer | 14/41 (34.1) | 2/2 (100.0) | 16/43 (37.2) |
| Non-consumer | 377/1187 (31.8) | 930/1203 (77.3) | 1307/2390 (54.7) | |
| Comorbidities | No comorbidities | 301/989 (30.4) | 749/976 (76.7) | 1050/1965 (53.4) |
| Diabetes | 33/70 (47.1) | 56/72 (77.8) | 89/142 (62.7) | |
| Hypertension | 28/70 (40.0) | 54/65 (83.1) | 82/135 (60.7) | |
| Asthma/chronic obstructive pulmonary disease | 3/30 (10.0) | 12/14 (85.7) | 15/44 (34.1) | |
| CAD/heart disease | 3/4 (75.0) | 6/8 (75.0) | 9/12 (75) | |
| Any two or more comorbidities | 23/65 (35.4) | 55/70 (78.6) | 78/135 (57.8) | |
| Presence of symptoms** | Yes | 120/339 (35.4) | 210/249 (84.3) | 330/588 (56.1) |
| No | 271/889 (30.5) | 722/956 (75.5) | 993/1845 (53.8) | |
| Close contact with confirmed cases | Yes | 102/320 (31.9) | 171/182 (94.0) | 273/502 (54.4) |
| No | 289/908 (31.8) | 761/1023 (74.4) | 1050/1931 (54.4) | |
| Mask usage in public places | Cloth | 218/645 (33.8) | 552/769 (71.8) | 770/1414 (54.5) |
| Surgical | 148/473 (31.3) | 333/382 (87.2) | 481/855 (56.3) | |
| N95 | 25/110 (22.7) | 47/54 (87.0) | 72/164 (43.9) | |
*< 12 years of formal education
**Suggestive of recent respiratory infection in the last 6 months
Mixed-effects logistic regression analysis of factors associated with seropositivity was adjusted for the clustering of time periods (Table 4 ). Healthcare workers and individuals among the urban affluent showed 2.1 and 1.5 times higher odds for seropositivity, respectively, when compared with the rural population in the univariate analysis. However, these were not found to be significant in the multivariate analysis. People living in urban slums showed about two times higher odds for seropositivity compared with those living in rural areas in both univariate and multivariate analysis, with an OR of 2.02 (95% CI 1.57–2.60; p < 0.001). Middle socioeconomic class based on the modified Kuppuswamy scale (Kattula et al., 2016) showed higher odds for seropositivity when compared with upper class both in univariate analysis, with an OR of 1.40 (95% CI 1.05–1.86; p = 0.024), and in multivariate analysis, with an OR of 1.77 (95% CI 1.17–2.67; p = 0.007). Although skilled workers showed a 30% lower risk of seropositivity in univariate analysis when compared with professional workers, the difference was not found to be significant on multivariate analysis. Among the comorbidities, diabetes and hypertension showed 1.5 and 1.3times higher odds for seropositivity in univariate analysis, respectively, as well as in multivariate analysis, with ORs of 1.70 (95% CI 1.15–2.58; p = 0.009) and 1.75 (95% CI 1.16–2.64; p = 0.008), respectively. There was no significant difference in protection noted between the use of N95 masks and the use of cloth masks in public places. However, use of surgical masks showed slightly higher odds for seropositivity of 1.38 (95% CI 1.13–1.28; p = 0.001) compared with cloth masks in univariate analysis. This difference was not found to be significant on multivariate analysis. Smoking habits and alcohol consumption were not found to be potential risk factors for seropositivity. The presence of symptoms suggestive of recent respiratory infection in the past 6 months and close contact with confirmed cases were not found to be independent risk factors for COVID-19 infection. Close contact with confirmed SARS-CoV-2-positive cases was also insignificant.
Table 4.
Univariate and multivariate analysis of factors associated with seropositivity, adjusted for clustering effects of time periods
| Demographics | Categories | Univariate odds ratio (OR) (95 % confidence interval) | p-value | Multivariate (adjusted) OR (95% confidence interval) | p-value |
|---|---|---|---|---|---|
| Subpopulation | Rural | (ref) | (ref) | ||
| Urban slum | 1.95 (1.52–2.49) | < 0.001 | 2.01 (1.57–2.59) | < 0.001 | |
| Urban affluent | 1.51 (1.17–1.95) | 0.002 | 0.95 (0.65–1.39) | 0.786 | |
| Healthcare workers | 2.13 (1.64–2.78) | < 0.001 | 1.472 (0.93–2.33) | 0.099 | |
| Age a | < 20 years | (ref) | |||
| 21–40 years | 1.13 (0.88–1.45) | 0.342 | |||
| 41–60 years | 1.10 (0.84–1.44) | 0.482 | |||
| > 60 years | 0.93 (0.66–1.31) | 0.695 | |||
| Gender a | Male | (ref) | |||
| Female | 1.14 (0.95–1.36) | 0.163 | |||
| Education | Graduate | (ref) | |||
| Primary to high school* | 0.76 (0.63–0.93) | 0.006 | 0.92 (0.66–1.29) | 0.641 | |
| Illiterate | 0.62 (0.44–0.89) | 0.009 | 0.77 (0.48–1.25) | 0.295 | |
| Occupation | Professional | (ref) | (ref) | ||
| Semi-professional | 1.13 (0.79–1.60) | 0.516 | 0.85 (0.53–1.36) | 0.496 | |
| Skilled worker | 0.70 (0.53–0.93) | 0.012 | 0.72 (0.42–1.23) | 0.223 | |
| Unskilled/daily wage laborer | 0.69 (0.47–1.04) | 0.074 | 0.81 (0.43–1.53) | 0.510 | |
| Housewife/unemployed/student | 1.81 (0.64–1.03) | 0.089 | 0.86 (0.51–1.47) | 0.584 | |
| Socioeconomic class | Upper | (ref) | (ref) | ||
| Middle | 1.39 (1.05–1.86) | 0.024 | 1.77 (1.17–2.67) | 0.007 | |
| Lower | 0.90 (0.68–1.19) | 0.471 | 1.04 (0.59–1.83) | 0.900 | |
| Smoking habita | Smoker | 1.25 (0.64–2.45) | 0.509 | ||
| Non-smoker | (ref) | ||||
| Alcohol consumptiona | Alcohol consumer | 1.16 (0.61–2.21) | 0.65 | ||
| Non-consumer | (ref) | ||||
| Comorbidities | No comorbidities | (ref) | (ref) | ||
| Diabetes | 1.46 (1.03–2.08) | 0.034 | 1.72 (1.15–2.58) | 0.009 | |
| Hypertension | 1.35 (0.94–1.93) | 0.101 | 1.75 (1.16–2.64) | 0.008 | |
| Asthma/chronic obstructive pulmonary disease | 0.56 (0.28–1.13) | 0.104 | 0.49 (0.24–1.02) | 0.056 | |
| CAD/heart disease | 2.21 (0.52–9.33) | 0.281 | 2.46 (0.59–10.23) | 0.216 | |
| Any two or more comorbidities | 1.19 (0.79–1.76) | 0.395 | 1.46 (0.97–2.21) | 0.073 | |
| Presence of symptoms**,a | Yes | 1.09 (0.91–1.32) | 0.329 | ||
| No | (ref) | ||||
| Close contact with confirmed casesa | Yes | 1.00 (0.82–1.22) | 0.998 | ||
| No | (ref) | ||||
| Mask usage (public places) | Cloth | (ref) | (ref) | ||
| Surgical | 1.38 (1.13–1.28) | 0.001 | 1.15 (0.89–1.47) | 0.271 | |
| N95 | 0.97 (0.67–1.39) | 0.868 | 0.80 (0.52–1.23) | 0.316 |
*< 12 years of formal education
**Suggestive of recent respiratory infection in the last 6 months
This factor was not included in the multivariate analysis because it was not significant in the univariate analysis
Discussion
Our results revealed a high community seroprevalence of SARS-CoV-2 in Vellore, India and demonstrated variability in transmission dynamics and vaccine utilization across different subpopulations. The overall weighted seroprevalence for Vellore district increased almost three-fold from 28.5% at the end of wave 1 in January 2021 to 71.6% at the end of wave 2 in July 2021, suggesting that population immunity had been achieved. Transmission dynamics varied across different subpopulations. Seroprevalence was highest among the urban slum population, both at the end of wave 1 (43.7%) and wave 2 (75.1%). As transmission progressed, the lower seroprevalences at the end of wave 1 among the rural and urban affluent subpopulations increased rapidly at the end of wave 2 to 67.6% and 85.4%, respectively, and probably reached considerable population immunity. The high seroprevalences achieved among the healthcare workers and the urban affluent was perhaps largely due to the high vaccination rates of 91.6% and 65.7%, respectively. In contrast, it is likely that natural infections had caused the high seroprevalences among the rural and urban slum populations, since vaccination rates in these populations were low (10.4% and 6.6%, respectively). The majority of individuals (75%) positive for SARS-CoV-2 antibodies did not report any symptoms in the 6 months prior to sample collection, suggesting significant asymptomatic transmission in the community.
Achieving population immunity, either by natural infection or vaccination, is a major goal for future management of the pandemic (Papachristodoulou et al., 2020). Emerging data from different parts of India suggest that this goal has been reached or is not far off (George et al., 2021; Laxminarayan et al., 2021, Dyer et al., 2021). The heterogeneous nature of transmission dynamics in the community was evident by the end of wave 1. High seroprevalence was reported among the urban slum population, characterized by overcrowding, where social distancing and diligent personal hygiene, such as frequent hand washing, were virtually impossible (George et al., 2021; Malaniet al., 2021). The subsequent spread of infection among other subpopulations followed, resulting in a large second wave in India between April and June 2021 (Gupta et al., 2021). The majority of transmissions in India were due to the prevalent delta variant (Sarkar et al., 2021; Adamoskiet al., 2021). However, a similar large second wave in Brazil was predominantly due to the gamma variant, with the delta variant only contributing a small proportion (De Souza et al., 2021; Singh et al., 2021). This evidence suggests that, although SARS-CoV-2 variants play a role in the transmission dynamics in the community, other factors such as overcrowding and personal hygiene play bigger roles.
For those living in the urban slum population, overcrowding was strongly associated with SARS-CoV-2 infection, with twice the risk for seropositivity on multivariate analysis when compared with the other subpopulations. Our study findings were similar to those of a multi-state serosurvey conducted by the Indian Council of Medical Research reporting the highest seroprevalence in urban slum areas, followed by urban non-slum and rural areas (Murhekar et al., 2021). Our observations that low and middle socioeconomic status and comorbidities such as diabetes mellitus and hypertension showed a higher risk for seropositivity in both univariate and multivariate analysis have been noted in other studies (Roy and Soumya, 2020; Royo et al., 2021). Since diabetes is an important risk factor for viral, bacterial, and fungal infections, presumably these emerging data suggest that there is an increased risk for SARS CoV-2 infection (Feehan et al., 2021, Mora et al., 2021).
The disease transmission among different age groups varied based on their exposure to infection at different time points during the pandemic. Our study found a shift in seroprevalence among the age groups between wave 1 and wave 2, which was similar to other observations (Laxminarayan et al., 2021; Miragliaet al., 2021). Among urban slum, rural, and urban affluent subpopulations, maximum seropositivity was seen in the 40–60 years and over 60 years age groups after wave 1, but at the end of wave 2 slightly higher seropositivity rates were seen in the 1–20 years and 20–40 years age groups. An age-stratified regional modelling study from France predicted that wave 2 would affect the middle-age and younger population more, which is in agreement with our observations (Roederer et al., 2021).
The vast majority of infections were asymptomatic. Among the individuals with SARS-CoV-2 antibodies, only 24.9% reported any symptoms during the recall period of 6 months. Several other studies from India and other parts of the world have reported similar high proportions of asymptomatic infection (Purkayastha et al., 2021; George et al., 2021; Feaster and Goh, 2020; Varghese and John, 2020). Although there is a possibility of recall bias, the high proportion of asymptomatic seroprevalence reported worldwide underscores the limitations of widely used syndromic surveillance for COVID-19. Asymptomatic transmission in the community coupled with the high exposure ratio undermines the utility of contact tracing and surveillance measures. Therefore, mitigation strategies targeting individuals with risk factors for severe disease, such as those with medical comorbidities and the elderly, might be more pragmatic and effective (Khan et al., 2021).
The reported infection fatality rate for COVID-19 in India of 0.04% is lower than that reported from several other countries, which could be attributed to the young median age of the population (Laxminarayan et al., 2021, Pei et al., 2020; Wagner et al., 2021, Yang et al., 2021). Cross-protective immunity due to other human coronaviruses (HCoV) are postulated to be contributing to this lower-than-expected IFR (Huang et al., 2020). To answer this question, our study evaluated IgG antibodies against two endemic strains of HCoV (NL63 and OC43) to analyze possible cross-protection against the current SARS-CoV-2 infection. Almost 100% of participants were found to be positive for HCoV antibodies. Similar estimates of high antibody titres due to the acquisition of infection in early childhood are common (Hovi et al., 1979; Ukkonenet al., 1984). However, infection by endemic HCoV strains provides little cross-protection against SARS-CoV. After an acute infection with endemic HCoV, no neutralizing antibodies against SARS-CoV are produced (Che et al., 2005; Liang et al., 2013). Whether endemic HCoV infections will impact COVID-19 severity, and if there is variation in antibody responses between HCoV strains, remains to be studied.
The healthcare worker group had seropositivity of 31.6% at the end of wave 1, which was comparable to the community seroprevalence in Vellore, indicating that the nosocomial acquisition of infection was the same as acquisition of infection in the community if appropriate personal protective measures were taken. The seropositivity in this group rose to 95.5% at the end of wave 2, which can be attributed to the mandatory vaccination drive among healthcare workers. In our study, 91.6% of health workers were vaccinated. Good vaccination take-up was also found among the urban affluent, with a vaccination rate of 65.7% by the second sampling in July 2021.
Many factors have played a role in driving the massive second wave of COVID-19 in a diverse country like India. Firstly, a large proportion of asymptomatic transmission occurred in a huge, susceptible population of the country. This corresponded with the period after travel restrictions were relaxed following the first wave. It was further fuelled by mass gatherings for various religious festivals and election campaigns (Patel et al., 2021). Secondly, the highly prevalent delta variant may have contributed to the larger second wave. India failed to vaccinate the majority and thus take advantage of the flattened epidemiological curve achieved during the initial period through enforced social distancing and strict lockdown. There have been issues with vaccine supply and acceptance (Wouters et al., 2021). As of October 13, 2021, only 19.9% of India's 1.4 billion population were fully vaccinated (Hannah et al., 2020).
The major limitation of our study was that these data may not be representative of the rest of the country. However, observations from the nationwide serosurvey showed a similar seroprevalence, and hence this study adds value and provides deeper insight. The simple random sampling used in our study for convenience may have caused minimal estimation errors. This method was chosen as practical and feasible while apprehension among the general public relating to the COVID-19 pandemic posed a major hindrance to the willingness of individuals to participate in the study.
Our results have important implications for the planning of future health policies. The considerable population immunity achieved predominantly by natural infection and vaccination in certain subpopulations makes future large-scale epidemics less likely. Patients who have had natural infections have been reported to possess comparable immune protection to those who have been vaccinated (Lumley et al., 2021; Khoury et al., 2021). While extensive vaccine drives have their place, focused vaccination of susceptible populations among vulnerable groups in order to decrease morbidity and mortality should be a priority.
High seroprevalences also encourage most activities, including businesses, tourism, and education, to remain open. However, the benefits of a high seroprevalence can be thwarted by the emergence of SARS-CoV-2 variants. The virus has unusually long genomic RNA, with error-prone proofreading mechanisms, and continues to mutate during infections. Therefore, well-coordinated genomic surveillance for emerging SARS-CoV-2 variants remains important.
Conclusion
Our results confirm the high seroprevalence of SARS-CoV-2 in Vellore, and provide insights into the heterogeneity and various factors associated with transmission of infection. Our study also found that urban slum and rural subpopulations need to be targeted for preventive strategies because they have the lowest vaccination rates. Going forward, improved vaccination strategies among less accessible subpopulations, focusing particularly on high-risk individuals, are suggested for better preparedness. Additionally, a rational approach involving a single dose of vaccine for the majority who are already positive for antibodies and thus have natural immunity could be considered. The meaningful inferences drawn from our results can be utilized to target high-risk groups that warrant attention, and to be better prepared to address future challenges.
Funding
A Wellcome Trust/DBT India Alliance Fellowship grant (IA/CPHS/16/1/502679) was awarded to GMV, while COVID grants from Capgemini Technology Services India Limited and Fluor Daniel India Private Limited were allocated to Indian Institute of Technology Madras. These funding sources had no role in the design or conduct of the study, in the collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript.
Ethical approval
The study was approved by the Institutional Review Board of Christian Medical College, Vellore (IRB minute 13165, dated July 22, 2020).
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors gratefully acknowledge Ms. Rohini Karthikeyan, Mr. S. Suresh Kumar, Ms. Merylin Sebastian, Ms. Emily Devasagayam, Mr. Vivegh V, Dr. Priyanka Gautam, and Dr. Mithun Mohan George for their technical help and support.
References
- Adamoski D, de Oliveira JC, Bonatto AC, Wassem R, Nogueira MB, Raboni SM, et al. SCB-UFPR COVID-19 team, 2. Large-scale screening of asymptomatic persons for SARS-CoV-2 variants of concern and gamma takeover. Brazil. Emerg Infect Dis. 2021;27(12) doi: 10.3201/eid2712.211326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Sandefur J, Subramanian A. Three new estimates of deaths in India during the pandemic. Center for Global Development. Jul 20, 2021. https://www.cgdev.org/blog/three-new-estimates-deaths-during-pandemic.
- Anderson EM, Goodwin EC, Verma A, Arevalo CP, Bolton MJ, Weirick ME, UPenn COVID Processing Unit. Betts MR, Wherry EJ, Meyer NJ, Cherry S, Bates P, Rader DJ, Hensley SE. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184(7) doi: 10.1016/j.cell.2021.02.010. et al. 1858–64.e10Epub 2021 Feb 9. PMID: 33631096; PMCID: PMC7871851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che XY, Qiu LW, Liao ZY, Wang YD, Wen K, Pan YX, et al. Antigenic cross-reactivity between severe acute respiratory syndrome-associated coronavirus and human coronaviruses 229E and OC43. J Infect Dis. 2005;191(12):2033–2037. doi: 10.1086/430355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covid19India.org (accessed on September 30, 2021 ).
- Deshpande K, Pt U, Kaduskar O, Vijay N, Rakhe A, Vidhate S, et al. Performance assessment of seven SARS-CoV-2 IgG enzyme-linked immunosorbent assays. J Med Virol. 2021 doi: 10.1002/jmv.27251. Jul 31:10.1002/jmv.27251. doi: 10.1002/jmv.27251. Epub ahead of print. PMID: 34331713PMCID: PMC8426713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza UJB, RN Dos Santos, Campos FS, Lourenço KL, da Fonseca FG, Spilki FR, Corona-Ômica Br/McTi Network High rate of mutational events in SARS-CoV-2 genomes across Brazilian geographical regions, February 2020 to June 2021. Viruses. 2021;13(9):1806. doi: 10.3390/v13091806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer O. Covid-19: Two thirds in India carry antibodies, while research suggests country's death toll is 10 times official figure. BMJ. 2021;374:n1856. doi: 10.1136/bmj.n1856. Jul 21. [DOI] [PubMed] [Google Scholar]
- George CE, Inbaraj LR, Chandrasingh S, de Witte LP. High seroprevalence of COVID-19 infection in a large slum in South India; what does it tell us about managing a pandemic and beyond? Epidemiol Infect. 2021;149:e39. doi: 10.1017/S0950268821000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaster M, Goh Y-Y. High proportion of asymptomatic SARS-CoV-2 infections in 9 long-term care facilities, Pasadena, California, USA, April 2020. Emerg Infect Dis. 2020;26:2416–2419. doi: 10.3201/eid2610.202694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehan AK, Denstel KD, Katzmarzyk PT, Velasco C, Burton JH, Price-Haywood EG, Seoane L. Community versus individual risk of SARS-CoV-2 infection in two municipalities of Louisiana, USA: an assessment of Area Deprivation Index (ADI) paired with seroprevalence data over time. PLoS One. 2021;16(11) doi: 10.1371/journal.pone.0260164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Kaur H, Yadav PD, Mukhopadhyay L, Sahay RR, Kumar A, et al. Clinical characterization and genomic analysis of samples from COVID-19 breakthrough infections during the second wave among the various states of India. Viruses. 2021;13(9):1782. doi: 10.3390/v13091782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah Ritchie, Mathieu Edouard, Rodés-Guirao Lucas, Appel Cameron, Giattino Charlie, Ortiz-Ospina Esteban. OurWorldInData.org; 2020. Coronavirus Pandemic (COVID-19)https://ourworldindata.org/coronavirus —. Published online atRetrieved from: '. ' [online resource] [Google Scholar]
- Hovi T, Kainulainen H, Ziola B, Salmi A. OC43 strain-related coronavirus antibodies in different age groups. J Med Virol. 1979;3(4):313–320. doi: 10.1002/jmv.1890030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11(1):4704. doi: 10.1038/s41467-020-18450-4. PMID: 32943637PMCID: PMC7499300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JHU CSSE Coronavirus COVID-19 Global Cases: https://arcg.is/0fHmTX (accessed on August 31, 2021).
- Kattula D, Venugopal S, Velusamy V, Sarkar R, Jiang V, Mahasampath Gowri S, et al. Measuring poverty in southern India: a comparison of socio-economic scales evaluated against childhood stunting. PLoS ONE. 2016;11(8) doi: 10.1371/journal.pone.0160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SMS, Qurieshi MA, Haq I, Majid S, Ahmad J, Ayub T, et al. Seroprevalence of SARS-CoV-2-specific IgG antibodies in Kashmir, India, 7 months after the first reported local COVID-19 case: results of a population-based seroprevalence survey from October to November 2020. BMJ Open. 2021;11(9) doi: 10.1136/bmjopen-2021-053791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Kshatri JS, Bhattacharya D, Kanungo S, Giri S, Palo SK, Parai D, et al. ICMR-RMRC [OdiSHA-COVID-19] Serosurvey Team. Serological surveys to inform SARS-CoV-2 epidemic curve: a cross-sectional study from Odisha, India. Sci Rep. 2021;11(1):10551. doi: 10.1038/s41598-021-89877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R, B CM, G VT, Arjun Kumar KV, Wahl B, Lewnard JA. SARS-CoV-2 infection and mortality during the first epidemic wave in Madurai, south India: a prospective, active surveillance study. Lancet Infect Dis. 2021;S1473-3099(21):00393–00395. doi: 10.1016/S1473-3099(21)00393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FY, Lin LC, Ying TH, Yao CW, Tang TK, Chen YW, et al. Immunoreactivity characterisation of the three structural regions of the human coronavirus OC43 nucleocapsid protein by Western blot: implications for the diagnosis of coronavirus infection. J Virol Methods. 2013;187(2):413–420. doi: 10.1016/j.jviromet.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley SF, Rodger G, Constantinides B, Sanderson N, Chau KK, Street TL, O'Donnell D, Howarth A, Hatch SB, Marsden BD, Cox S, James T, Warren F, Peck LJ, Ritter TG, de Toledo Z, Warren L, Axten D, Cornall RJ, Jones EY, Stuart DI, Screaton G, Ebner D, Hoosdally S, Chand M, Crook DW, O'Donnell AM, Conlon CP, Pouwels KB, Walker AS, Peto TEA, Hopkins S, Walker TM, Stoesser NE, Matthews PC, Jeffery K, Eyre DW. An observational cohort study on the incidence of SARS-CoV-2 infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status. Clin Infect Dis. 2021:ciab608. doi: 10.1093/cid/ciab608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malani A, Shah D, Kang G, Lobo GN, Shastri J, Mohanan M, et al. Seroprevalence of SARS-CoV-2 in slums versus non-slums in Mumbai, India. Lancet Glob Health. 2021;9(2):e110–e1e1. doi: 10.1016/S2214-109X(20)30467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia JL, NascimentoMonteiro C, Giannecchini Romagnolo A, Xavier Gomes R, Pitangueiras Mangueira C, Aparecida Rosseto-Welter E, et al. A seroprevalence survey of anti-SARS-CoV-2 antibodies among individuals 18 years of age or older living in a vulnerable region of the city of São Paulo, Brazil. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0255412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Lewnard JA, Kogut K, Rauch SA, Hernandez S, Wong MP, Huen K, Chang C, Jewell NP, Holland N, Harris E, Cuevas M, Eskenazi B. CHAMACOS-Project-19 Study Team. Risk factors associated with SARS-CoV-2 infection among farmworkers in Monterey County, California. JAMA Netw Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar MV, Bhatnagar T, Thangaraj JWV, Saravanakumar V, Kumar MS, Selvaraju S, et al. SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020–January 2021. Int J Infect Dis. 2021;108:145–155. doi: 10.1016/j.ijid.2021.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362–367. doi: 10.7326/M20-3012. Epub 2020 Jun 3. PMID: 32491919PMCID: PMC7281624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristodoulou E, Kakoullis L, Parperis K, Panos G. Long-term and herd immunity against SARS-CoV-2: implications from current and past knowledge. Pathog Dis. 2020;78(3) doi: 10.1093/femspd/ftaa025. ftaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Mukherjee S, Leifels M, Gautam R, Kaushik H, Sharma S, et al. Mega festivals like MahaKumbh, a largest mass congregation, facilitated the transmission of SARS-CoV-2 to humans and endangered animals via contaminated water. Int J Hyg Environ Health. 2021;237 doi: 10.1016/j.ijheh.2021.113836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei S, Yamana TK, Kandula S, Galanti M, Shaman J. Burden and characteristics of COVID-19 in the United States during 2020. Nature. 2020 doi: 10.1038/s41586-021-03914-4. Aug 26. [DOI] [PubMed] [Google Scholar]
- Purkayastha S, Kundu R, Bhaduri R, Barker D, Kleinsasser M, Ray D, et al. Estimating the wave 1 and wave 2 infection fatality rates from SARS-CoV-2 in India. BMC Res Notes. 2021;14(1):262. doi: 10.1186/s13104-021-05652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer T, Mollo B, Vincent C, Nikolay B, Llosa AE, Nesbitt R, et al. Seroprevalence and risk factors of exposure to COVID-19 in homeless people in Paris, France: a cross-sectional study. Lancet Public Health. 2021;6(4):e202–e2e9. doi: 10.1016/S2468-2667(21)00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. Low-income countries are more immune to COVID-19: a misconception. Indian Journal of Medical Sciences. 2020;72(1):5–7. doi: 10.25259/IJMS_26_2020. [DOI] [Google Scholar]
- Royo-Cebrecos, C, Vilanova, D., López, J, Arroyo, V., et al. Mass SARS-CoV-2 serological screening, a population-based study in the Principality of Andorra. The Lancet Regional Health — Europe 2021 May 20, 100119. doi: 10.1016/j.ejor.2015.01.016. [DOI] [PMC free article] [PubMed]
- Sarkar A, Chakrabarti AK, Dutta S. COVID-19 infection in India: a comparative analysis of the second wave with the first wave. Pathogens. 2021;10(9):1222. doi: 10.3390/pathogens10091222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BB, Ward MP, Lowerison M, Lewinson RT, Vallerand IA, Deardon R, et al. Meta-analysis and adjusted estimation of COVID-19 case fatality risk in India and its association with the underlying comorbidities. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, He WT, Callaghan S, Anzanello F, Huang D, Ricketts J, et al. Cross-reactive serum and memory B-cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. Nat Commun. 2021;12(1):2938. doi: 10.1038/s41467-021-23074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukkonen P, Hovi T, von Bonsdorff CH, Saikku P, Penttinen K. Age-specific prevalence of complement-fixing antibodies to sixteen viral antigens: a computer analysis of 58,500 patients covering a period of eight years. J Med Virol. 1984;13(2):131–148. doi: 10.1002/jmv.1890130204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese GM, John R. COVID-19 in India: Moving from containment to mitigation. Indian J Med Res. 2020;151(2 & 3):136–139. doi: 10.4103/ijmr.IJMR_860_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Peterhoff D, Beileke S, Günther F, Berr M, Einhauser S, et al. Estimates and determinants of SARS-Cov-2 seroprevalence and infection fatality ratio using latent class analysis: the population-based Tirschenreuth study in the hardest-hit German county in spring 2020. Viruses. 2021;13(6):1118. doi: 10.3390/v13061118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–1034. doi: 10.1016/S0140-6736(21)00306-8. Epub 2021 Feb 12. PMID: 33587887PMCID: PMC7906643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. Epub 2020 Mar 12. PMID: 32171952PMCID: PMC7102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Kandula S, Huynh M, Greene SK, Van Wye G, Li W, et al. Estimating the infection-fatality risk of SARS-CoV-2 in New York City during the spring 2020 pandemic wave: a model-based analysis. Lancet Infect Dis. 2021;21(2):203–212. doi: 10.1016/S1473-3099(20)30769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]