Abstract
Clinical research for infants born with a congenital diaphragmatic hernia (CDH) has until recently mainly focused on advances in prenatal and postnatal treatment. However, during the early perinatal transition period there are major physiological adaptations. For most infants these changes will happen uneventfully, but for CDH infants this marks the beginning of serious respiratory complications. In recent years, there is emerging evidence that the clinical management during the perinatal stabilization period in the delivery room may influence postnatal outcomes. Herein, we discuss major knowledge gaps and novel concepts that aim to optimize fetal to neonatal transition for infants with CDH. One such novel and interesting approach is performing resuscitation with an intact umbilical cord, the efficacy of this procedure is currently being investigated in several clinical trials. Furthermore, close evaluation of neonatal physiological parameters in the first 24 h of life might provide early clues concerning the severity of lung hypoplasia and the risk of adverse outcomes. We will provide an overview of trending concepts and discuss potential areas for future research.
Keywords: congenital diaphragmatic hernia, birth, cord clamping, neonatal transition, oxygen, respiratory monitoring
Introduction
The management of infants with a congenital diaphragmatic hernia (CDH) is continuously evolving with major improvements in prenatal and postnatal care. Most advances are based on solid scientific evidence using available animal models of CDH prior to translating it into the clinical setting (1). For many of the in vivo experiments done in small animals (rabbit, rat and mice models), the endpoint is birth given the lethality of the condition without intensive care. To investigate novel concepts in early postnatal care large animal models (such as the ovine model) are often necessary, yet these experiments are costly and require a dedicated research facility.
Until recently, the transition period defined as the time immediately after birth, has been relatively overlooked. In fact, for a long time the main intervention was to clamp the cord and transfer the infant to the resuscitation table for further stabilization as soon as possible (2–4). On the other hand, major physiological adaptations occur during this immediate postnatal period and a complicated course may effect long term outcomes (5).
In the past decades there has been tremendous effort invested in optimizing the perinatal stabilization period for infants born preterm with immature lungs or those that may undergo problematic fetal to neonatal transition; such as due to birth asphyxia or in case of an elective cesarean section (6, 7). Our knowledge of the physiology underpinning the changes at birth has dramatically improved and novel concepts concerning the timing of cord clamping, oxygen management and the type and level of respiratory support required were introduced to clinical practice (7, 8).
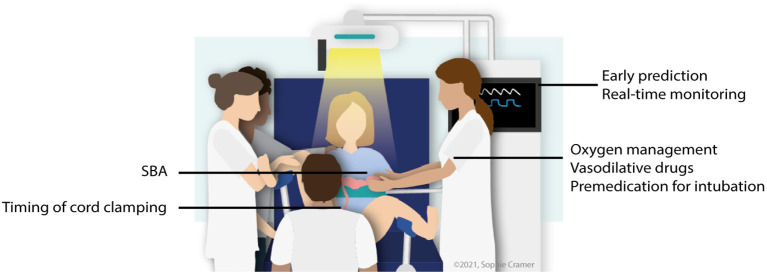
Some of these approaches are now being evaluated in large clinical trials, but the promising preliminary results have also inspired researchers to investigate their effectiveness for conditions that affect in-utero lung development, such as CDH (9–14). Research about neonatal transition for infants with a CDH is rapidly developing, in this literature review we describe new insights and we discuss knowledge gaps for future research (Figure 1).
Figure 1.
Overview of potential research areas concerning perinatal stabilization of CDH infants. SBA, spontaneous breathing approach. Adapted from Knol et al., (15) with permission of the illustrator.
Intact Cord Resuscitation
For most infants, adequate gas-exchange is promptly established after birth, i.e., within the first breaths, by rapid clearance of lung liquid resulting in aeration of the lungs. However, infants born with lung hypoplasia have a reduced liquid clearance rate, which is proportional to the lung size and thus reduces the infant's ability to aerate its lungs (16). This problem is likely a reflection of a simplified distal airway architecture and as such a reduced cross sectional area for moving lung liquid into the interstitial tissues. Furthermore, hypoplastic lungs generally have a higher elastic recoil (stiffer) demonstrated by a lower dynamic lung compliance (10, 16, 17).
Lung aeration is considered a key factor in driving vasodilation of the pulmonary vasculature and thereby increasing pulmonary blood flow (18). Apart from establishing adequate gas-exchange, lung aeration is essential for a smooth cardiovascular transition from a fetus to a newborn. Immediately after umbilical cord clamping there is a sudden increase in peripheral vascular resistance and at the same instant venous return to the left atrium via the ductus venosus and foramen ovale stops. In the hypoplastic lung, pulmonary vascular resistance remains high and therefore adequate left venous return is not rapidly restored, whereas in normally developed lungs venous return is established within the first breaths (10, 18). A delayed restoration of venous return translates in a sudden decrease in cardiac output (30–50% reduction) and neonatal hypoxemia, which is considered a risk factor for developing persistent pulmonary hypertension (9). Furthermore, the impaired vascular relaxation forces higher pulmonary perfusion pressures to maintain adequate pulmonary blood flow. We have recently shown that in a lamb CDH model, after an initial improvement in pulmonary vascular resistance, this short period of exposure to higher driving pressures may be a trigger for developing pulmonary hypertension at a later stage (9). This observation could be the physiological explanation of the so-called ‘honeymoon’ period, a transient time of clinical stability, that is observed in some infants with CDH (19).
In recent years, the importance of delaying cord clamping until after lung aeration (and adequate left venous return) has gained momentum, specifically in preterm infants born with immature lungs. Likewise, there have recently been two feasibility studies evaluating this approach for infants born with CDH (11, 12). An important consideration is the need to provide mechanical ventilation to the neonate in close proximity to the mother whilst the integrity of the umbilical cord remains intact. A mobile resuscitation trolley is required for this approach to be successful and several alternatives are currently commercially available (20). These trolleys have inherent limitations and advantages, which are important to consider when implementing intact cord resuscitation, as well as the financial aspect given considerable differences in acquisition costs.
Both studies, although small sample sizes (n = 20), reported good feasibility of 85% and 100%, respectively (11, 12). It is obviously not possible to draw firm conclusions, however both found improved cardiovascular adaptation, resulting in higher blood pressures, less need for cardiac resuscitation and higher Apgar scores (11, 12). These promising findings led to the initiation of two large randomized trials: Congenital Hernia Intact Cord (CHIC, NCT04429750) and Physiological-based cord clamping for infants with a Congenital Diaphragmatic Hernia (PinC, NCT04373902) (21).
These two trials aim to defer cord clamping until after the infant's lungs are aerated, which is challenging to determine. CO2 detectors, respiratory monitors or bedside echocardiography (ductus arteriosus evaluation) could be used for this purpose, but they have inherent technical limitations or are logistically not always feasible in the immediate postnatal setting. Hence, physiological parameters such as heart rate, oxygen saturation and the level of oxygen supplementation are considered as a good alternate proxies for determining lung aeration and the state of the infant's cardiovascular adaptation (9, 21). In the future, with the rapid improvement of bedside respiratory monitors, real-time evaluation of tidal volumes or other lung mechanics might be another way to ascertain adequate lung aeration.
The other challenge is to define a clinically relevant primary outcome. The ultimate endpoint is survival to discharge, however despite efforts to standardize postnatal management, considerable bias due to variations in local management make it difficult to determine the actual benefit of performing intact cord resuscitation. The concern of bias is even more pronounced in multicenter trials, however given the incidence of CDH and the required sample sizes it is almost impossible to investigate this in a single center setting. Consequently, short term outcomes such as Apgar scores (CHIC) and pulmonary hypertension (PinC) were chosen as alternative primary outcomes.
The results of these clinical trials are expected in the next two to three years. Despite differences in methodology, a subsequent meta-analysis using individual participant data might strengthen the scientific evidence physiologically based cord clamping even further.
Spontaneous Breathing Approach
For most infants with CDH, mechanical ventilation is a double-edged sword: it is essential for survival, but prolonged respiratory support also poses a risk of iatrogenic complications such as ventilator-induced lung injury. On the other hand, a small subset of infants born with a very small diaphragmatic defect, hence mild lung hypoplasia, may not develop severe respiratory insufficiency immediately after birth and thus mechanical ventilation may not be necessary. In fact, prior to the routine use of ultrasound this was probably the group that survived the neonatal period with minimal care and that was only diagnosed at childhood age. Moreover, prompt intubation after birth potentially causes stress and pain for the infant, thereby triggering the development of pulmonary hypertension and impacting neonatal transition. Regardless, the main purpose of routine intubation at birth is to avoid transient hypoxia, which is considered an even more important trigger for pulmonary hypertension (22).
We have recently published our experience of adopting a gentler approach during the initial perinatal stabilization phase by allowing spontaneous breathing in a select subset of patients (23). This approach was only offered for infants with an isolated left-sided CDH, born >35 weeks' gestation and predicted to have a very mild degree of lung hypoplasia. The latter was determined by an observed/expected lung-to-head ratio above 50% and an intra-abdominal position of the liver. In this small study (n = 15%), 40% of cases were only intubated at the time of postnatal correction of the diaphragmatic defect, thus required limited respiratory support and were discharged earlier from the intensive care unit. But more importantly, a trial of spontaneous breathing, even unsuccessful, did not appear to impact survival or short-term morbidity (23).
In attempt to improve the success rate of this SBA we have drafted a consensus protocol after several meetings with international experts on CDH management, neonatal resuscitation and fetal/neonatal physiology. One of the recommendations is to start non-invasive respiratory support in these infants, as many of the infants in the above-mentioned series required low flow oxygen supplementation or continuous positive airway pressure (CPAP). However, it is not clear whether the key component to facilitate neonatal transition is either oxygen supplementation, distending airway pressure or a combination of both. The use of positive pressure ventilation is certainly controversial as the main concern is insufflation of the digestive tract, which may impair and limit lung expansion. Therefore, nasal high flow therapy could be an interesting option, because it is relatively easy to position the device, well-tolerated and provides a vehicle for oxygen delivery but also generates distending airway pressure. The level of respiratory support can be adjusted based on the individual needs by changing the flow rate and/or concentration of oxygen delivered. An alternative could be the use of a CPAP mask rather than nasal prongs because it is easier to position. Regardless of the treatment modality used, early insertion of a naso- or orogastric tube with continuous section is advised to avoid stomach distention. The result of these consensus meetings is a proposed algorithm for SBA comparable to what is used in the current neonatal resuscitation guidelines, and this protocol will be published soon.
There is an increasing number of centers that are considering or already have started attempting a trial of spontaneous breathing for infants with mild lung hypoplasia. In the absence of a randomized trial, it is essential to collect the outcome data and given the rareness of the abnormality, a multicenter and international collaboration is the most logical step. To accommodate this, a research consortium was founded consisting of partners all over Europe and Australia: Very mild CDH–Spontaneous Breathing Approach; VeSBA. Outcome data will be collected prospectively in a web-based registry.
Sedate or Not Sedate?
The majority of infants with a CDH will be intubated immediately after birth. Given the urgency to commence respiratory support in these infants and usually the lack of intravenous access intubation is often done without administering any sedation. The physiological responses to awake intubation of neonates are well-described (24, 25). It can be painful for the infant, translating into markers of acute stress such as increased intracranial and systemic blood pressure, bradycardia and reduced transcutaneous oxygen saturation (24, 25). Furthermore, mediastinal shift and neonatal movements can complicate intubation resulting in a higher stress level for the infant. Therefore, in (semi) elective intubation premedication is considered good standard of care. For CDH infants, the priority is on establishing a secure airway for mechanical ventilation and thus vascular access is often obtained later in the stabilization phase. Alternative options to administer drugs are via the umbilical vein (direct puncture, not via a catheter), buccal or intranasal, however the interval to the onset of effect is potentially longer with the latter two (26, 27). In addition, there is an important knowledge gap when it comes to the optimal treatment regimen (type, dosage) of the premedication (28).
Oxygen Management
Oxygen supplementation is an essential part of perinatal stabilization of an infant with CDH. The aim is to avoid arterial hypoxemia as it may trigger a vasoactive response and many clinicians will initiate oxygen supplementation with 100% oxygen. After the initial stabilization in the delivery room, oxygen administration is titrated based on the infant's needs targeting a pre-ductal saturation of between 80 and 95% (2). In any case, hyperoxia should be avoided because it also has adverse effects by producing oxygen free radicals. This consideration is certainly important for infants with a relative mild degree of lung hypoplasia, as using 100% oxygen supplementation might be counter effective. Oxidative stress and oxygen free radicals are not only associated with short-term neonatal morbidity but may have long lasting influence on development (29, 30). It has been recently demonstrated that even a brief period of high oxygen exposure may attenuate vasoactive response of the pulmonary vessels to treatments such as inhaled nitric oxide (31).
An alternative approach would be to start stabilization with a reduced oxygen concentration and a stepwise increase or decrease guided by the infant's saturation values (13). This approach is comparable to the resuscitation guidelines for preterm infants (8). In a recent series, the safety of such an alternative approach was evaluated, observing comparable rates of perinatal survival, ECMO use and duration of mechanical ventilation compared to historical CDH controls (13). Moreover, the need of 100% oxygen during the perinatal stabilization period provided an early indication of disease severity and subsequent adverse outcomes (13).
Another important knowledge gap is the use of supplemental oxygen during resuscitation of CDH infants whilst the umbilical cord is still intact. A recent study showed that in preterm lambs, pulmonary blood flow was considerably higher to controls when using 100% oxygen during delayed cord clamping (32). Interestingly, this was not causing systemic hyperoxygenation and hypothetically, the placenta may act as a buffer to reduce the arterial oxygen saturation (32).
The degree of supplemental oxygen exposure at birth may also be diminished by initiating vasodilative treatments already during neonatal resuscitation, such as inhaled nitric oxide, as was recently observed in a small series of preterm infants (33). We speculate that using such an approach for CDH infants may facilitate decreasing pulmonary vascular resistance after birth, thereby preventing high perfusion pressures and potentially avoiding a dysregulated vascular tone of the lung vessels (34). Hypothetically, combining this approach with deferring cord clamping until the lungs are aerated, both appear to have a protective effect on the lung vessels, may have a synergistic effect (9).
Early Predictors of Adverse Outcome
Infants born with CDH will only face respiratory challenges after birth and consequently it is only at that moment that clinicians can determine the true impact on lung development. Prenatal ultrasound and fetal MRI have proven to be very useful in the individual prediction of prognosis, yet it remains challenging to perform a functional assessment of the lungs (35). More specifically, the occurrence and severity of pulmonary hypertension or cardiac dysfunction are difficult to predict given the differences between the fetal and the neonatal circulation.
The immediate postpartum period provides clinicians a first glance of the infant's respiratory capacity. Consequently, this period also enables clinicians a chance to determine the severity of the congenital abnormality by monitoring physiological parameters and/or ventilatory requirements. There are already several scoring systems to determine the risk of adverse outcomes available, such as the Score for Neonatal Acute Physiology–II (SNAP-II) score, Wilford Hall/Santa Rosa prediction model and the Brindle scoring model (36–40). Most of these scoring systems are combining several clinical parameters (such as blood pressure, serum pH, fraction of inspired oxygen FiO2) yet the majority of these scoring systems use the worst values of the parameters within the first 12–24 h (37–39).
We speculate that the level of respiratory support required during the initial neonatal resuscitation may provide important information of possible adverse outcomes. For instance, as described above, the need to supplement with a high FiO2 concentration in the delivery room appears to be associated with higher morbidity and mortality for CDH infants (13, 41). Likewise, similar observations were reported regarding expiratory tidal volumes, end-tidal carbon dioxide levels and dynamic lung compliance (41, 42). Respiratory monitors now allow real-time measurements of several lung function parameters. Recording these parameters gives an opportunity to gather large datasets by aggregating individual patient data within a framework of multicenter collaborations, which can be used for prediction modeling to identify early signs of deterioration. In addition, combining physiological and ventilatory outcome measures, such as is done with the oxygen saturation index (OSI), may improve the predictive value of these models for adverse models even further (36).
Conclusion
Herein, we have described some of the trending new concepts regarding interventions in the early perinatal stabilization phase for infants with CDH. Our understanding of the physiological adaptations immediately after birth has certainly grown in recent decades, but considerable knowledge gaps are remaining. Regardless, thorough investigation using appropriate preclinical models is essential prior to translating novel concepts into clinical practice. Importantly, optimizing the fetal to neonatal transition will not only improve postnatal outcomes for infants born with CDH, but also for those born with abnormal lung development caused by a broad range of other conditions, such as prolonged anhydramnios.
Author Contributions
PD and IR were involved in the conception of this paper and wrote the first draft, which was critically reviewed by all authors. The final version was approved by all authors.
Funding
PD and EH-O were supported by a grant from Sophia Children's Hospital Foundation (SSWO, Grant S19-12). This research was supported by the National Health and Medical Research Council (NHMRC) Project Grant APP1187580 (KC and PD).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Sophie J. E. Cramer for drawing the illustration.
References
- 1.van Loenhout RB, Tibboel D, Post M, Keijzer R. Congenital diaphragmatic hernia: comparison of animal models and relevance to the human situation. Neonatology. (2009) 96:137–49. 10.1159/000209850 [DOI] [PubMed] [Google Scholar]
- 2.Snoek KG, Reiss IKMM, Greenough A, Capolupo I, Urlesberger B, Wessel L, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium Consensus−2015 Update. Neonatology. (2016) 110:66–74. 10.1159/000444210 [DOI] [PubMed] [Google Scholar]
- 3.Puligandla PS, Skarsgard ED, Offringa M, Adatia I, Baird R, Bailey JAM, et al. Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. CMAJ. (2018) 190:E103–12. 10.1503/cmaj.170206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jancelewicz T, Brindle ME, Guner YS, Lally PA, Lally KP, Harting MT. Toward standardized management of congenital diaphragmatic hernia: an analysis of practice guidelines. J Surg Res. (2019) 243:229–35. 10.1016/j.jss.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 5.Horn-Oudshoorn EJJ, Knol R, Te Pas AB, Hooper SB, Cochius-Den Otter SCM, Wijnen RMH, et al. Perinatal stabilisation of infants born with congenital diaphragmatic hernia: a review of current concepts. Arch Dis Child Fetal Neonatal Ed. (2020) 105:449–54. 10.1136/archdischild-2019-318606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuart Hooper BB, Binder-Heschl C, Polglase R, Gill W, Kluckow M, Wallace EM, et al. Maternal health, neonatology and perinatology the timing of umbilical cord clamping at birth: physiological considerations. Matern Heal Neonatol Perinatol. (2016) 2:1–9. 10.1186/s40748-016-0032-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.te Pas A, Roehr CC, Foglia EE, Hooper S. Neonatal resuscitation research: closing the gap. Pediatr Res. (2021) 1–3. 10.1038/s41390-021-01403-y [DOI] [PubMed] [Google Scholar]
- 8.Wyckoff MH, Wyllie J, Aziz K, de Almeida MF, Fabres JW, Fawke J, et al. Neonatal life support 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. (2020) 156:A156–87. 10.1016/j.resuscitation.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 9.Kashyap AJ, Hodges RJ, Thio M, Rodgers KA, Amberg BJ, McGillick E V, et al. Physiologically based cord clamping improves cardiopulmonary haemodynamics in lambs with a diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. (2019) 105:18–25. 10.1016/j.ajog.2018.11.1016 [DOI] [PubMed] [Google Scholar]
- 10.Kashyap AJ, Crossley KJ, DeKoninck PLJ, Rodgers KA, Thio M, Skinner SM, et al. Neonatal cardiopulmonary transition in an ovine model of congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. (2019) 104:F617–23. 10.1136/archdischild-2018-316045 [DOI] [PubMed] [Google Scholar]
- 11.Lefebvre C, Rakza T, Weslinck N, Vaast P. Houfflin-debarge V, Mur S, Storme L. Feasibility and safety of intact cord resuscitation in newborn infants with congenital diaphragmatic hernia (CDH). Resuscitation. (2017) 120:20–5. 10.1016/j.resuscitation.2017.08.233 [DOI] [PubMed] [Google Scholar]
- 12.Foglia EE, Ades A, Hedrick HL, Rintoul N, Munson DA, Moldenhauer J, et al. Initiating resuscitation before umbilical cord clamping in infants with congenital diaphragmatic hernia: a pilot feasibility trial. Arch Dis Child Fetal Neonatal Ed. (2019) 105:322–6. 10.1136/archdischild-2019-317477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley JS, Antiel RM, Rintoul NE, Ades AM, Waqar LN, Lin N, et al. Reduced oxygen concentration for the resuscitation of infants with congenital diaphragmatic hernia. J Perinatol. (2018) 38:834–43. 10.1038/s41372-017-0031-5 [DOI] [PubMed] [Google Scholar]
- 14.Snoek KG, Capolupo I, Van Rosmalen J, De Jongste-Van Den Hout L, Vijfhuize S, Greenough A, et al. Conventional mechanical ventilation versus high-frequency oscillatory ventilation for congenital diaphragmatic hernia. A randomized clinical trial (The VICI-trial). Ann Surg. (2016) 263:867–74. 10.1097/SLA.0000000000001533 [DOI] [PubMed] [Google Scholar]
- 15.Knol R, Brouwer E, Akker TV, DeKoninck P, Geloven NV, Polglase GR, et al. Physiological-based cord clamping in very preterm infants - randomised controlled trial on effectiveness of stabilisation. Resuscitation. (2020) 147:26–33. 10.1016/j.resuscitation.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 16.Flemmer AW, Thio M, Wallace MJ, Lee K, Kitchen MJ, Kerr L, et al. Lung hypoplasia in newborn rabbits with a diaphragmatic hernia affects pulmonary ventilation but not perfusion. Pediatr Res. (2017) 82:536–43. 10.1038/pr.2017.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGillick E V, Davies IM, Hooper SB, Kerr LT, Thio M, DeKoninck P, et al. Effect of lung hypoplasia on the cardiorespiratory transition in newborn lambs. J Appl Physiol. (2019) 127:568–78. 10.1152/japplphysiol.00760.2018 [DOI] [PubMed] [Google Scholar]
- 18.Hooper SB, Te Pas AB, Lang J, Van Vonderen JJ, Roehr CC, Kluckow M, et al. Cardiovascular transition at birth: a physiological sequence. Pediatr Res. (2015) 77:608–14. 10.1038/pr.2015.21 [DOI] [PubMed] [Google Scholar]
- 19.Geggel RL, Murphy JD, Langleben D, Crone RK, Vacanti JP, Reid LM. Congenital diaphragmatic hernia: arterial structural changes and persistent pulmonary hypertension after surgical repair. J Pediatr. (1985) 107:457–64. 10.1016/S0022-3476(85)80534-5 [DOI] [PubMed] [Google Scholar]
- 20.Katheria A, Lee HC, Knol R, Irvine L, Thomas SA. review of different resuscitation platforms during delayed cord clamping. J Perinatol. (2021) 41:1540–8. 10.1038/s41372-021-01052-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duc L, Mur S, Rakza T, Boukhris MR, Rousset C, Vaast P, et al. Efficacy of intact cord resuscitation compared to immediate cord clamping on cardiorespiratory adaptation at birth in infants with isolated congenital diaphragmatic hernia (CHIC). Children (Basel). (2021) 8:339. 10.3390/children8050339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Storme L, Aubry E, Rakza T, Houeijeh A, Debarge V, Tourneux P, et al. Pathophysiology of persistent pulmonary hypertension of the newborn: impact of the perinatal environment. Arch Cardiovasc Dis. (2013) 106:169–77. 10.1016/j.acvd.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 23.Cochius-den Otter SCM, Horn-Oudshoorn EJJ, Allegaert K, DeKoninck PLJ, Peters NCJ, Cohen-Overbeek TE, et al. Routine intubation in newborns with congenital diaphragmatic hernia. Pediatrics. (2020) 146:e20201258. 10.1542/peds.2020-1258 [DOI] [PubMed] [Google Scholar]
- 24.Carbajal R, Eble B, Anand KJS. Premedication for tracheal intubation in neonates: confusion or controversy? Semin Perinatol. (2007) 31:309–17. 10.1053/j.semperi.2007.07.006 [DOI] [PubMed] [Google Scholar]
- 25.Caldwell CD, Watterberg KL. Effect of premedication regimen on infant pain and stress response to endotracheal intubation. J Perinatol. (2015) 35:415–8. 10.1038/jp.2014.227 [DOI] [PubMed] [Google Scholar]
- 26.McPherson C, Ortinau CM, Vesoulis Z. Practical approaches to sedation and analgesia in the newborn. J Perinatol. (2021) 41:383–95. 10.1038/s41372-020-00878-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borrhomée S, Merbouche S, Kern-Duciau N, Boize P. Umbilical vein catheterization through Wharton's jelly: a possibility for a fast and safe way to deliver treatments in the delivery room? Arch Pediatr. (2019) 26:381–4. 10.1016/j.arcped.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 28.Shay R, Weikel BW, Grover T, Barry JS. Standardizing premedication for non-emergent neonatal tracheal intubations improves compliance and patient outcomes. J Perinatol. (2021) 1–7. 10.1038/s41372-021-01215-2 [DOI] [PubMed] [Google Scholar]
- 29.Andresen JH, Saugstad OD. Oxygen metabolism and oxygenation of the newborn. Semin Fetal Neonatal Med. (2020) 25:101078. 10.1016/j.siny.2020.101078 [DOI] [PubMed] [Google Scholar]
- 30.Perrone S, Bracciali C, Di Virgilio N, Buonocore G. Oxygen use in neonatal care: a two-edged sword. Front Pediatr. (2017) 4:143. 10.3389/fped.2016.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res. (2007) 62:313–8. 10.1203/PDR.0b013e3180db29fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakshminrusimha S, Vali P, Chandrasekharan P, Rich W, Katheria A. Differential alveolar and systemic oxygenation during preterm resuscitation with 100% oxygen during delayed cord clamping. Am J Perinatol. (2021) 10.1055/s-0041-1730362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekar K, Szyld E, McCoy M, Wlodaver A, Dannaway D, Helmbrecht A, et al. Inhaled nitric oxide as an adjunct to neonatal resuscitation in premature infants: a pilot, double blind, randomized controlled trial. Pediatr Res. (2020) 87:523–8. 10.1038/s41390-019-0643-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mous DS, Kool HM, Wijnen R, Tibboel D, Rottier RJ. Pulmonary vascular development in congenital diaphragmatic hernia. Eur Respir Rev. (2018) 27:1–6. 10.1183/16000617.0104-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordier AG, Russo FM, Deprest J, Benachi A. Prenatal diagnosis, imaging, and prognosis in Congenital Diaphragmatic Hernia. Semin Perinatol. (2020) 44:51163. 10.1053/j.semperi.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 36.Rawat M, Chandrasekharan PK, Williams A, Gugino S, Koenigsknecht C, Swartz D, et al. Oxygen saturation index and severity of hypoxic respiratory failure. Neonatology. (2015) 107:161–6. 10.1159/000369774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snoek KG, Capolupo I, Morini F, Van Rosmalen J, Greenough A, Van Heijst A, et al. Score for neonatal acute physiology-II predicts outcome in congenital diaphragmatic hernia patients*. Pediatr Crit Care Med. (2016) 17:540–6. 10.1097/PCC.0000000000000738 [DOI] [PubMed] [Google Scholar]
- 38.Schultz CM, DiGeronimo RJ, Yoder BA. Congenital diaphragmatic hernia: a simplified postnatal predictor of outcome. J Pediatr Surg. (2007) 42:510–6. 10.1016/j.jpedsurg.2006.10.043 [DOI] [PubMed] [Google Scholar]
- 39.Brindle ME, Cook EF, Tibboel D, Lally PA, Lally KP. A clinical prediction rule for the severity of congenital diaphragmatic hernias in newborns. Pediatrics. (2014) 134:e413–9. 10.1542/peds.2013-3367 [DOI] [PubMed] [Google Scholar]
- 40.Cochius-Den Otter SCM, Erdem Ö, van Rosmalen J, Schaible T, Peters NCJ, Cohen-Overbeek TE, et al. Validation of a prediction rule for mortality in congenital diaphragmatic hernia. Pediatrics. (2020) 145:e20192379. 10.1542/peds.2019-2379 [DOI] [PubMed] [Google Scholar]
- 41.O'Rourke-Potocki A, Ali K, Murthy V, Milner A, Greenough A. Resuscitation of infants with congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F320–3. 10.1136/archdischild-2016-311432 [DOI] [PubMed] [Google Scholar]
- 42.Mank A, Carrasco Carrasco C, Thio M, Clotet J, Pauws SC, DeKoninck P, et al. Tidal volumes at birth as predictor for adverse outcome in congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. (2019) 105:248–52. 10.1136/archdischild-2018-316504 [DOI] [PubMed] [Google Scholar]