Abstract
Aims
The aim of the LAICA study was to evaluate the long‐term effectiveness and safety of intermittent levosimendan infusion in patients with advanced heart failure (AdHF).
Methods and results
This was a multicentre, randomized, double‐blind, placebo‐controlled clinical trial of intermittent levosimendan 0.1 μg/kg/min as a continuous 24‐h intravenous infusion administered once monthly for 1 year in patients with AdHF. The primary endpoint [incidence of rehospitalization (admission to the emergency department or hospital ward for >12 h) for acute decompensated HF or clinical deterioration of the underlying HF] occurred in 23/70 (33%) of the levosimendan group (Group I) and 12/27 (44%) of the placebo group (Group II) (P = 0.286). The incidence of hospital readmissions for acute decompensated HF (Group I vs. Group II) at 1, 3, 6, and 12 months was 4.2% vs. 18.2% (P = 0.036); 12.8% vs. 33.3% (P = 0.02); 25.7% vs. 40.7% (P = 0.147); 32.8% vs. 44.4% (P = 0.28), respectively. In a secondary pre‐specified time‐to‐event analysis no differences were observed in admission for acute decompensated HF between patients treated with levosimendan compared with placebo (hazard ratio 0.66; 95% CI, 0.32–1.32; P = 0.24). Cumulative incidence for the aggregated endpoint of acute decompensation of HF and/or death at 1 and 3 months were significatively lower in the levosimendan group than in placebo group [5.7% vs. 25.9% (P = 0.004) and 17.1% vs. 48.1% (P = 0.001), respectively], but not at 6 and 12 months [34.2% vs. 59.2% (P = 0.025); 41.4% vs. 66.6% (P = 0.022), respectively]. Survival probability was significantly higher in patients who received levosimendan compared with those who received placebo (log rank: 4.06; P = 0.044). There were no clinically relevant differences in tolerability between levosimendan and placebo and no new safety signals were observed.
Conclusions
In our study, intermittent levosimendan in patients with AdHF produced a statistically non‐significant reduction in the incidence of hospital readmissions for acute decompensated HF, a significantly lower cumulative incidence of acute decompensation of HF and/or death at 1 and 3 month of treatment and a significant improvement in survival during 12 months of treatment.
Keywords: Advanced heart failure, Inodilator, Intermittent administration, Levosimendan, Rehospitalization
Introduction
Heart failure (HF) is a major public health concern. 1 , 2 The magnitude of the public health problem is highlighted by the costs of care for patients with HF. 3 , 4 This is despite significant therapeutic advances made in recent years. 5 Advanced HF (AdHF) is a debilitating stage of the disease characterized by poor quality of life (QoL) and frequent hospitalizations, and patients with AdHF are at high risk of readmission, morbidity, and mortality. 4 , 5 , 7 , 8 It is differentiated from end‐stage HF in that the cardiac dysfunction and symptoms are still potentially reversible. 8 , 9 Selected patients may benefit from therapeutic measures with known impact on survival and QoL, such as the implantation of mechanical circulatory assist devices and heart transplantation. 10 , 11 However, in many cases, they may be delayed due to shortage of donor organs or are contraindicated due to host factors including advanced age and/or comorbidities. 11 , 12
Inotropic drugs such as beta‐adrenergic agents and phosphodiesterase inhibitors are often used in patients with AdHF for the treatment of acute severe decompensation because they can rapidly improve the clinical and haemodynamic status of the patient. 13 However, it has been shown that inotropic agents can have a negative effect on both short‐term and long‐term prognosis in patients with HF. 14 Levosimendan is a calcium‐sensitizing agent with inotropic and vasodilator (inodilator) effects exerted by a triple mechanism of action. First, it increases the calcium sensitivity of troponin C, without increasing the release of calcium into the cytosol or modifying intracellular cyclic AMP levels; second, it activates ATP‐sensitive sarcolemmal K+ channels in the smooth muscle cells of the vasculature; and third, it activates ATP‐sensitive mitochondrial K+ channels in cardiomyocytes. 15 , 16 Several studies have demonstrated its effectiveness in treating acute decompensated HF from a clinical, haemodynamic, and prognostic standpoint, compared with other treatments. 17 , 18 , 19 , 20 The efficacy and safety of intermittent outpatient treatment with levosimendan has been evaluated in a number of studies. 20 In individual studies, levosimendan has been reported to improve functional capacity, QoL, and event‐free survival in patients with acute decompensated HF. 20 , 21 , 22
The ‘Long‐Term Intermittent Administration of Levosimendan in Patients with Advanced Heart Failure’ (LAICA) study (ClinicalTrials.gov Identifier: NCT00988806/Number EudraCT: 2009‐011441‐11) is a clinical trial designed to evaluate the efficacy and safety of intermittent administration of levosimendan as a continuous 24 h intravenous (IV) infusion in patients with AdHF. The infusion was administered once monthly for 12 months, and efficacy was evaluated in terms of the reduction in the incidence of hospital readmissions for acute decompensated HF, time from randomization to first hospitalization for acute decompensated HF and/or death, the composite of cardiac and non‐cardiac mortality, and safety/tolerability.
Methods
Study design and patients
The LAICA study is a Spanish, independent, multicentre (seven hospitals), randomized, double‐blind, placebo‐controlled, parallel group trial. Refer to Supporting information, Table S1 for a full list of study committees, participating centres, and investigators. The study design has been described previously. 23 Briefly, eligible patients had AdHF [New York Heart Association (NYHA) Class III or IV]; evidence of severe left ventricular dysfunction 5 of any aetiology, with at least one episode of acute decompensation requiring hospital admission within the previous 6 months (refer to Table S2 for a comprehensive list of inclusion and exclusion criteria).
All patients received optimal standard treatment for HF in accordance with current recommendations for the management of chronic HF, including patients with implantable defibrillators or cardiac resynchronization therapy devices. 5
Randomization was performed centrally. Eligible patients were assigned to one of two therapeutic strategies, in a double‐blind manner, using a random number table generated using an Excel® for Windows® spreadsheet. Patients were randomly assigned (3:1), in double‐blind manner, to receive an infusion of levosimendan 0.1 μg/kg/min (Group I) without a loading dose, or placebo of the same colour (Group II); both over a 24 h period once every 30 days. The dose was established based on previous studies, researcher experience and the usual practice in many of the centres. The absence of a loading dose was for safety reasons, to avoid the risk of episodes of hypotension. The infusion rate could be reduced to 0.05 μg/kg/min or suspended, according to the patient's response to the drug (hypotension or tachycardia), in which case it was recorded as a serious adverse event. These 24‐h treatments were repeated every 30 days for 12 months. Both patients and investigators were blinded to which treatment had been administered to ensure the study was maintained double‐blind. If symptomatic hypotension or tachycardia occurred, the infusion rate could be reduced to 0.05 μg/kg/min or suspended, and it was recorded as a serious adverse event. All infusions were performed under medical supervision with electrocardiography and non‐invasive haemodynamic monitoring. The clinical status of each participant was evaluated 15 days after the infusion had been administered. If adequate compensation of cardiac function (defined as subjective improvement of HF symptoms and/or improvement by at least one NYHA functional class, and/or objective disappearance of signs of systemic venous congestion, pulmonary congestion and/or objective improvement in signs of peripheral perfusion) had not been achieved or if the clinical status worsened before the 30 day deadline for the next infusion, then the infusion timetable was shortened to every 15 days with clinical assessments at 7 days. In cases of persistent decompensation after two consecutive infusions every 15 days, the randomization code was broken and the patient received the medical treatment deemed most appropriate by the attending physician, including heart transplantation when indicated. Study medication was also discontinued in cases of serious adverse events, when the primary endpoint of the trial was attained or if patients withdrew their consent to continue.
The study was approved by the local clinical research ethics committee of each participating center and by the Spanish Agency of Medicines and Health Products, Ministry of Health. It was conducted in accordance with the Declaration of Helsinki concerning medical research in human subjects and in‐line with the standards of good clinical practice for trials with medical products in the European community. 24
All patients were required to sign an informed consent form before they could enter the trial.
An independent ad hoc clinical events committee, blinded to which treatment each patient had received, supervised the study with respect to efficacy and safety. The committee received reports on all events relating to the primary objective, secondary objectives, and adverse reactions that occurred during the study.
Main and secondary objectives of the study
Readmissions for acute decompensation of HF and survival or mortality are the most relevant clinical events in patients with AdHF and, following the publication of the initial methodological manuscript, 23 the executive committee and the researchers modified the study design accordingly. The primary endpoint was modified to ‘the incidence of rehospitalization (admission to the emergency department or a hospital ward for >12‐h) for acute decompensated HF or clinical deterioration of the underlying HF’ ( Table S3).
Secondary endpoints included time from randomization to first hospitalization for acute decompensated HF and/or death, cumulative incidence of hospitalization due to decompensation of HF, death or both, changes in NYHA functional class from randomization to 1, 3, 6 and 12 months, NT‐proBNP changes before and after treatment, and QoL assessment at 1, 3, 6, and 12 months using the Kansas City Cardiomyopathy Questionnaire (KCCQ). Adverse events were recorded throughout the trial ( Table S3).
Study endpoints were evaluated at patient visits between each administration of the study drug, based on history, physical signs, and laboratory tests [blood counts, renal function, haematological analysis, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP)] at baseline, and at 3, 6, and 12 months during the study.
Sample size and statistical analysis
Based on an earlier study, we assumed an 18% reduction in the incidence of the primary endpoint and calculated that 213 patients (163 patients to receive levosimendan treatment and 50 patients to receive placebo) would provide a statistical power of 80% and an alpha risk of 0.05 (one‐tailed contrast). 25 Statistical analyses were performed according to the intention‐to‐treat principle. A descriptive analysis of all recorded data was performed for both treatment groups. Continuous variables were analysed as sample size, mean, standard deviation, median, inter‐quartile range, and maximum and minimum values. Differences between treatment groups were tested by means of the parametric Student t test and the nonparametric Wilcoxon test as appropriate. Categorical variables were presented through the distribution of absolute and relative frequencies; 95% confidence intervals (95% CI) were calculated as necessary. To test the independence of two categorical variables we used the χ 2 test or Fisher's exact test, selecting the most appropriate in each case. P values for multiple comparisons were adjusted with Bonferroni correction (for P values = 0.05 and for multiple contrast corrected P values = 0.0125). Time‐to‐event analysis was performed to compare cumulative event‐free survival between study groups using the Kaplan–Meier method and log‐rank test. We used Cox proportional risk model analysis to obtain hazard ratios, 95% CIs and P values using levosimendan vs. placebo as an independent variable, time to event as dependent variable and cumulative incidence of HF decompensation and/or death as event. We assumed that acute decompensation of HF and death are events with competing risk effects. To check for competitive risk between the events HF decompensation and death, we used Grey's and Pepe and Mori's tests comparing levosimendan and placebo groups using the subdistribution hazards. The presence of both in each patient was added as an aggregated endpoint for acute decompensation of HF and/or death. To estimate the superiority of levosimendan treatment versus placebo treatment to reduce this endpoint, a ratio contrasts analysis using the χ 2 test with a one‐sided hypothesis test was performed. A P value of <0.05 was considered statistically significant for all tests. For missing data (partial and complete), statistical imputation with ‘last observation carried forward’ was used. All analyses were performed with SAS software v9.4.
Results
Between November 2009 and October 2014, when the study was stopped, 99 patients had been recruited. The study was prematurely stopped due to lack of funding and difficulties with patient recruitment. This report represents the findings from this cohort of 99 patients, with two patients excluded pre‐randomization (one screening failure and one death). A complete summary of the protocol and study flow chart is depicted in Figure 1 . Seventy patients were assigned to the levosimendan group and 27 to the placebo group. Demographic and clinical characteristics of the study patients are shown in Table 1 . Baseline therapy was in accordance with current guidelines for HF management. 5 No statistically significant differences were detected between the two treatment groups in demographic, clinical or therapeutic baseline characteristics. However, a greater number of the levosimendan group patients had implanted automatic defibrillators.
Figure 1.
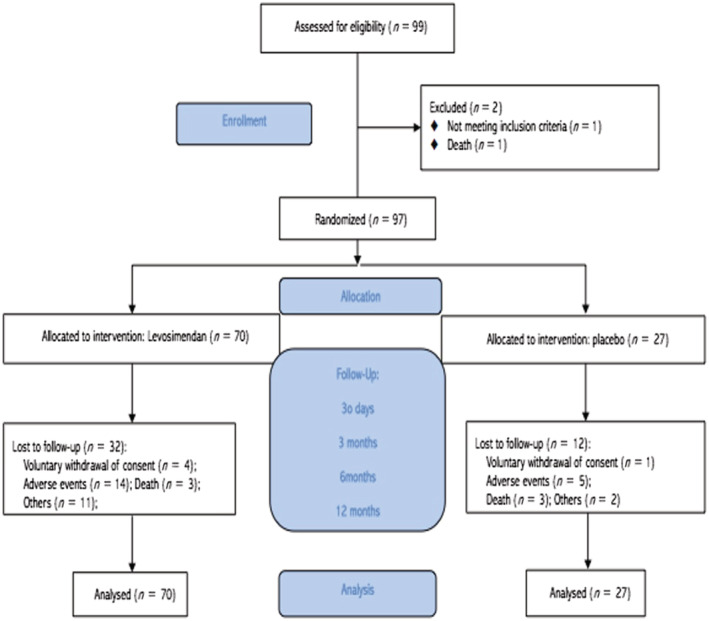
Study flow chart.
Table 1.
Baseline demographic data, clinical presentation, and concomitant drugs
| Levosimendan (N = 70) | Placebo (N = 27) | Total (N = 97) | P value | |
|---|---|---|---|---|
| Age (years) | 68.10 ± 11.09 | 71.33 ± 8.98 | 69.00 ± 10.60 | 0.2330 |
| Gender male n (%) | 62 (88.57) | 20 (74.07) | 82 (84.54) | 0.1145 |
| Caucasian n (%) | 69 (98.57) | 27 (100.0) | 96 (98.97) | 1.0000 |
| Diabetes n (%) | 36 (51.43) | 14 (51.85) | 50 (51.55) | 0.3585 |
| Dyslipidaemia n (%) | 38 (54.29) | 17 (62.96) | 55 (56.70) | 0.4395 |
| HTA n (%) | 47 (67.14) | 17 (62.96) | 64 (65.98) | 0.6970 |
| Smoking n (%) | 25 (35.71) | 6 (22.22) | 31 (31.96) | 0.3259 |
| Coronary disease n (%) | 33 (47.14) | 13 (48.15) | 46 (47.42) | 0.3090 |
| PAD n (%) | 9 (12.86) | 4 (14.81) | 13 (13.40) | 1.0000 |
| Stroke n (%) | 2 (2.86) | 2 (7.41) | 4 (4.12) | 0.2327 |
| CKD n (%) | 18 (25.71) | 6 (22.22) | 24 (24.74) | 0.8536 |
| COPD n (%) | 9 (12.86) | 4 (14.81) | 13 (13.40) | 1.0000 |
| LVEF n (%) | 24.63 (7.86) | 25.97 (9.93) | 25.00 (8.45) | 0.7297 |
| Mean NT‐proBNP and range (pg/mL) | 7963 (4835, 11,092) | 14,232 (3486, 24,978) | 9700 (6078, 13,321) | 0.6669 |
| NYHA functional class n (%) | ||||
| III | 64 (91.43) | 25 (92.59) | 89 (91.75) | 0.6614 |
| IV | 5 (7.14) | 1 (3.70) | 6 (6.19) | |
| Digoxin n (%) | 41 (58.57) | 11 (40.74) | 52 (53.61) | 0.1145 |
| Diuretics n (%) | 67 (95.71) | 27 (100.00) | 94 (96.91) | 0.5578 |
| ACEI n (%) | 45 (64.29) | 20 (74.07) | 65 (67.01) | 0.3581 |
| ARA II n (%) | 16 (22.86) | 3 (11.11) | 19 (19.59) | 0.1914 |
| Beta‐blockers n (%) | 58 (82.86) | 23 (85.19) | 81 (83.51) | 1.0000 |
| Mineralocorticoid antagonist (%) | 52 (74.30) | 18 (67.70) | 70 (72,16) | 0.4666 |
| ASA n (%) | 24 (34.29) | 12 (44.44) | 36 (37.11) | 0.3533 |
| Statins n (%) | 30 (43.48) | 16 (61.54) | 46 (48.42) | 0.1163 |
| Nitrates n (%) | 12 (17.14) | 7 (25.93) | 19 (19.59) | 0.3286 |
| Amiodarone n (%) | 15 (21.74) | 3 (11.54) | 18 (18.95) | 0.3806 |
| Oral antidiabetics n (%) | 17 (24.64) | 7 (26.92) | 24 (25.26) | 0.8192 |
| Insulin n (%) | 13 (18.84) | 5 (19.23) | 18 (18.95) | 1.0000 |
| NSAIDs n (%) | 1 (1.45) | 0 (0.00) | 1 (1.05) | 1.0000 |
| CRT n (%) | 15 (21.74) | 1 (3.85) | 16 (16.84) | 0.0613 |
| IAD n (%) | 37 (53.62) | 6 (23.08) | 43 (45.26) | 0.0077 |
ACEI, angiotensin converting enzyme inhibitors; ARA II, angiotensin‐II receptor antagonists; ASA, acetylsalicylic acid; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization device; HTA, arterial hypertension; IAD, implantable automatic defibrillator; LVEF, left ventricular ejection fraction; NSAIDs, non‐steroidal anti‐inflammatory drugs; PAD, peripheral artery disease.
The total number of infusions was 730 in the levosimendan group and 227 in the placebo group. The median [inter‐quartile range Q1, Q3] number of the infusions per patient was 12 [5, 13] for levosimendan and 13 [3, 13] for placebo. The mean cumulative dose of levosimendan per patient during the study was 110 ± 79 mg.
Primary endpoint
The primary endpoint [incidence of rehospitalization (admission to the emergency department or a hospital ward for >12 h) for acute decompensated HF or clinical deterioration of the underlying HF] occurred in 23/70 (33%) of patients receiving levosimendan and 12/27 (44%) of patients receiving placebo (P = 0.286). Admission for acute decompensated HF (levosimendan group vs. placebo) at 1, 3, 6, and 12 months was 4.2% vs. 18.5% (P = 0.036); 12.8% vs. 33.3% (P = 0.02); 25.7% vs. 40.7% (P = 0.147); 32.8% vs. 44.4% (P = 0.28), respectively. After applying the Bonferroni correction no significant differences were observed (Figure 2 A ).
Figure 2.
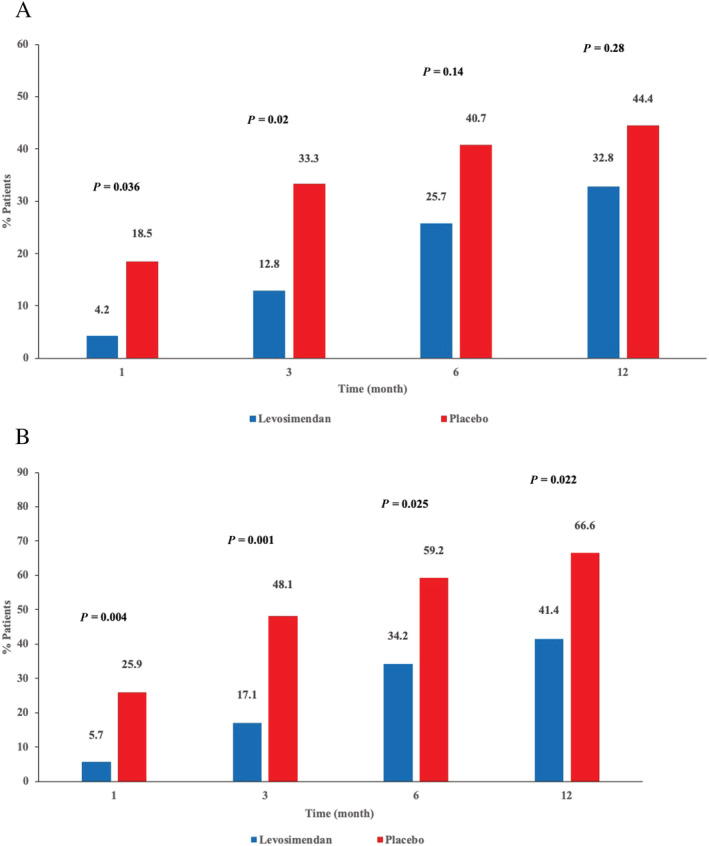
Cumulative incidence of (A) hospital admission for acute decompensated heart failure (HF) or HF worsening; and (B) hospital admission for acute decompensated HF or HF worsening and death.
Secondary and other endpoints
In a secondary pre‐specified time‐to‐event analysis no differences were observed in admission for acute decompensated HF between patients treated with levosimendan compared with placebo (hazard ratio [HR] 0.66; 95% CI, 0.32–1.32; P = 0.24) (Figure 3 A ). However, survival probability was significantly higher in patients who received levosimendan compared to those who received placebo (log rank: 4.06; P = 0.044) (Figure 3 B ). Overall mortality (8.5% vs. 22.2%; P = 0.08) showed a tendency towards reduction with levosimendan (6 of 70 patients: 4 patients HF/cardiogenic shock; 1 patient cardiac arrhythmia; 1 patient non‐cardiac death) than in the placebo group (6 of 27 patients: all HF/cardiogenic shock). The cumulative incidence of deaths during the course of the study was lower in the levosimendan group than in the placebo group but did not achieve statistical significance (Figure 4 ).
Figure 3.
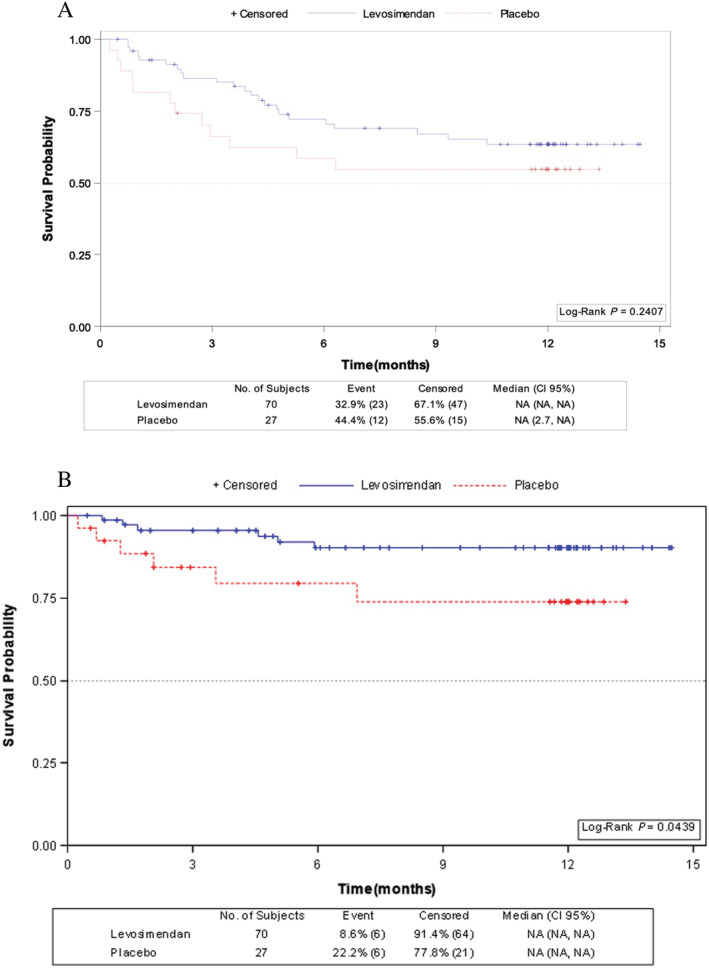
Kaplan–Meier survival probability for (A) time from randomization to first hospitalization for acute decompensated heart failure (HF); and (B) time from randomization to death.
Figure 4.
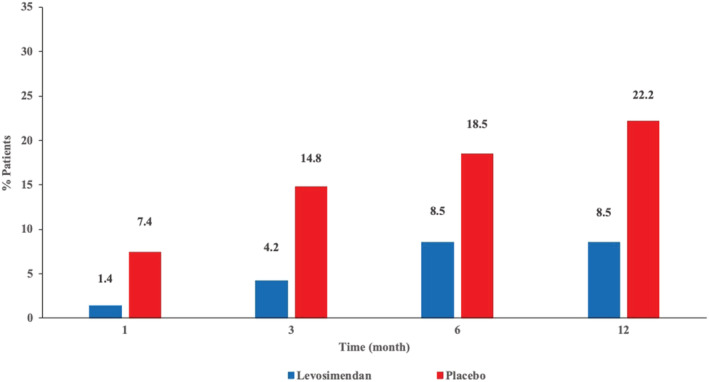
Cumulative incidence of death.
We did not find a competing risks effect in admission for acute decompensated HF [Grey's test: χ 2 DF:1 = 1.44; P = 0.23 and Pepe and Mori's test: χ 2 DF:1 = 2.01; P = 0.16], or in death [Grey's test: χ 2 DF:1 = 0.82; P = 0.37 and Pepe and Mori's test: χ 2 DF:1 = 1.96; P = 0.16]. For this reason, survival competitive risk analysis was not carried out.
After Bonferroni correction, cumulative incidence for acute decompensation of heart failure and/or death after 1 and 3 months were significatively lower in the levosimendan group than in placebo group [5.7% vs. 25.9% (P = 0.004) and 17.1% vs. 48.1% (P = 0.001) respectively], but not after 6 and 12 months [34.2% vs. 59.2% (P = 0.025); 41.4% vs. 66.6% (P = 0.022) respectively] (Figure 2 B ).
Cox proportional risk model analysis did not show a significant hazard ratio for levosimendan vs. placebo using time to event as dependent variable and cumulative incidence of acute decompensation of HF and/or death as event (HR = 0.66; 95% CI, 0.33–1.33; P = 0,24).
No significant changes were found in NYHA functional class between the two groups throughout the study (Figure 5 ). Data collection on variables such as NT‐proBNP and QoL assessment using the KCCQ throughout the study was inconsistent; therefore, an adequate analysis could not be performed.
Figure 5.
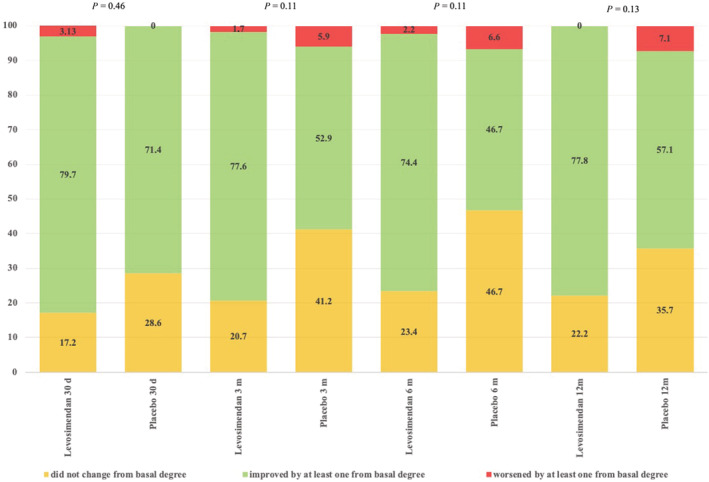
Improvement of New York Heart Association (NYHA) scale throughout the study: column results are expressed as frequencies (percentages).
Tolerability and safety
Adverse events were recorded throughout the study. There were 34 (48.6%) patients treated with levosimendan and 14 (52%) patients with placebo that had at least one adverse event (P = 0.77). In 22 (31.4%) patients assigned to levosimendan and 9 (33.3%) patients assigned to placebo, the adverse event caused premature discontinuation from the study (P = 0.8). In accordance with the intention‐to‐treat principle, these patients were included in all efficacy and safety analyses. Adverse drug‐related reactions reported for levosimendan included acute HF (1 patient), hypotension (5 patients), ventricular tachycardia (1 patient), nausea (1 patient), vomiting (1 patient), and hypothyroidism (1 patient). Three patients who developed hypotension during the levosimendan infusions continued the study at a reduced infusion rate. In the placebo group two adverse drug‐related reactions were reported: HF (1 patient) and hypotension (1 patient).
Discussion
The LAICA study evaluated the efficacy and safety of intermittent 24 h IV infusions of levosimendan as a therapeutic strategy to reduce the incidence of admission for acute decompensation in patients with AdHF. A lower incidence of admission for acute decompensated HF was observed between levosimendan and placebo after 12 m of treatment, but after applying the Bonferroni correction, it was shown that these differences were not significant. The lack of a sustained effect over time on the reduction in the number of events related to HF in our study requires further examination.
Previous studies have examined whether intermittent administration of levosimendan exerts clinical beneficial effects in patients with AdHF. 20 Clinical benefits of intermittent levosimendan administration in patients with AdHF has been demonstrated in terms of improved cardiac function/haemodynamic status; 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 improved symptomatology; 28 improved functionality; 30 , 31 improved QoL; 29 , 30 and improved survival. 21 , 26 , 32 Tasal and colleagues compared the effects of single and repeated infusions of levosimendan on left ventricular performance, biomarkers and neurohormonal activation in 29 consecutive HF patients. 31 In contrast to our study, these authors recorded statistically significant improvements in NYHA functional status and myocardial performance in the treatment group at 6 months compared with pre‐treatment values (P = 0.03 and P < 0.001, respectively). Additionally, a significant decrease in brain natriuretic peptide and IL‐6 was observed in patients receiving repeated doses of levosimendan (P < 0.01 and P < 0.05, respectively), but not in a single‐dose group. This was a small population of patients who were not randomized to treatment, the study was not controlled, and no comparator group (placebo or active) was included.
Two prospective, randomized, double‐blind, placebo‐controlled, multicentre, parallel‐group trials investigated repetitive therapy with levosimendan compared with placebo in similar patient populations to our study. These were the LevoRep (levosimendan in outpatients with advanced heart failure) study 21 and the LION‐HEART (Intermittent Intravenous Levosimendan in Ambulatory Advanced Chronic Heart Failure Patients) study. 34 The trial design in these two studies differs from ours in terms of dose schedules and cycle durations. The dose per cycle was identical in both (0.2 mg/kg/min for 6 h at 2 week intervals). The LevoRep protocol specified four cycles of IV levosimendan therapy while in the LION‐HEART study, two additional cycles of levosimendan therapy were administered. In our study, a lower dose was administered for longer (0.1 mg/kg/min intravenously for 24 h at 30 day intervals for up to 12 months). The LevoRep study failed to demonstrate a significant positive effect on a pre‐specified secondary endpoint, short‐term (8 weeks from randomization) and long‐term (24 weeks from randomization) event‐free survival (death, heart transplant, or decompensated HF admission). However, the LION‐HEART study reported a significant reduction in the number of decompensated HF hospitalizations. Consistent with the results of this study, the multicentre RELEVANT‐HF registry also reported a statistically significant reduction in the number and duration of HF‐related hospitalizations in the 6 months after starting intermittent levosimendan therapy. 35
It has been hypothesized that intermittent exposure to levosimendan results in a greater cumulative dosage. Through its cardioprotective effect levosimendan helps to preserve cardiac function and slows the progression of HF, thus preventing haemodynamic deterioration. This should translate clinically into a reduction in the number of HF‐related events. 34 Although in our study we have not observed this effect, despite delete of the mean cumulative dose of levosimendan per patient being considerably higher in comparison with other studies (110 ± 79 mg versus 30.3 ± 8.9 mg in LION‐HEART study and 14.3 ± 44.7 mg the LevoRep study), 21 , 33 the positive effects of treatment during the first 3 months should be noted. The insufficient number of recruited patients undoubtedly influenced the overall results, and we could assume that, for this reason, our study is underpowered. Our study was prematurely stopped due to lack of funding associated with poor patient recruitment.
It is also important to note that in the secondary pre‐specified time‐to‐event analysis, we found intermittent 24 h IV infusion of levosimendan resulted in a significantly higher survival rate compared with placebo. These findings are consistent with the results of other researchers. For example, in a meta‐analysis investigating the effects of repeated or intermittent levosimendan on mortality in patients with AdHF, Silvetti and Nieminen found that levosimendan was associated with a significant reduction in mortality at the longest available follow‐up [16% vs. 21.5%; odds ratio 0.54 (95% CI 0.32–0.91), P = 0.02]. 36 In this analysis, data from 438 patients (257 received levosimendan) in seven randomized clinical trials were included and the average follow‐up period was 8 months. The dosage varied between 6 and 12 μg/kg as an IV bolus, and between 0.1 and 0.4 μg/kg/min as a continuous IV infusion. Infusion duration was between 6 and 24 h, and the interval between administrations was 1 week (1 study), every 2 weeks (3 studies) or monthly (3 studies). Likewise, in the LION‐HEART study the composite secondary endpoint including hospitalization (all‐cause, CV or HF) and death or other terminal events was also significantly lower in patients receiving levosimendan compared with those receiving placebo [81% vs. 46% at 100 days, P = 0.015 (log‐rank test)]. 34 In the LevoRep study, assignment to levosimendan was associated with a 50% lower risk of death, heart transplant, or acute HF compared with placebo, but this difference did not quite reach statistical significance (HR 0.50, 95% CI 0.24–1.025; P = 0.069). 21
It is important to highlight that in our study, repetitive levosimendan administration was safe and well tolerated: the adverse events rate was comparable between the levosimendan and placebo groups, including a similar incidence of patients with an adverse event leading to discontinuation of the therapy. The proportion of patients with death as an outcome was lower in the levosimendan group than in the placebo group, and none were considered to be related to treatment. A similar level of safety and tolerability was reported in the LevoRep and LION‐HEART studies. 21 , 33 In the LevoRep study, levosimendan‐treated patients were more likely to experience arterial hypotension compared with placebo; however, tachycardia and arrhythmias were infrequent and did not differ between groups. Additionally, in the LION‐HEART study, no differences were found in the levosimendan and placebo groups with respect to serious and non‐serious adverse event rates. 34 The proportion of deaths tended to be lower in the levosimendan group than in the placebo group (31% vs. 38%), although this trend was not statistically significant.
Overall, clinical experience in these three larger trials of intermittent levosimendan therapy indicates that this treatment approach is well tolerated, in a patient population (AdHF), which is generally considered to be high risk.
Limitations
The aim of the LAICA study was to ascertain the usefulness of intermittent levosimendan as a continuous 24 h IV infusion administered once monthly for 12 months in patients with AdHF. Based on the findings of an earlier study, we calculated that 213 patients would provide the necessary statistical power to investigate the primary endpoint (the incidence of rehospitalization: admission to the emergency department or a hospital ward for >12 h for acute decompensated HF or clinical deterioration of the underlying HF). 23 However, within the timeframe set for the clinical trial (November 2009 to October 2014), due to logistical reasons already mentioned, only 99 patients were recruited. Consequently, the study was prematurely stopped, and this report represents the findings from these 99 patients. This limited the study power to reach its primary endpoint and our ability to further analyse the data according to certain patient sub‐groups (e.g. patients requiring dosage reductions) and to identify any possible less common or rare adverse events. Nevertheless, we should point out that this still represents one of the largest trials of its type in this clinical setting. Despite the limited sample size, we did find a significantly lower cumulative incidence of acute decompensation of heart failure and/or death at 1 and 3 month of treatment and a statistically significant higher survival probability in patients who received intermittent levosimendan treatment. However, this finding should be interpreted with caution because it is a secondary endpoint in an underpowered study.
The inability to provide important information on NT‐proBNP changes before and after treatment and QoL assessments at 1, 6, and 12 months (using KCCQ) should be mentioned as additional limitations and prevented timely analysis of these variables.
Conclusions
Long‐term intermittent administration of levosimendan in patients with AdHF produced a statistically non‐significant reduction in the incidence of hospital readmissions for acute decompensated HF, a significantly lower cumulative incidence of acute decompensation of HF and/or death at 1 and 3 month of treatment and a significant improvement in survival during 12 months of treatment. There were no clinically relevant differences in tolerability between levosimendan and placebo. Our findings are in‐line with those from a number of other research groups and there is a strong rationale for an adequately powered trial to confirm the effects of levosimendan on hospital readmissions for HF and mortality in patients with AdHF.
Conflict of interest
The authors declare no conflicts of interest.
Funding
Department of Pharmacy and Health Products. Ministry of Health, Social Services and Equality, Government of Spain 2009 (TRA‐058).
Department of Health. Canary Islands Government. Fundación Canaria de Investigación y Salud (FUNCIS) 2010.
Additional research unrestricted grant from Orion Pharma Laboratory.
This study has received the ‘2019 Santa Cruz de Tenerife City Hall Award’ from the Royal Academy of Medicine of Canary Islands.
Supporting information
Data S1. Supporting Information.
Acknowledgements
All authors wish to express their special thanks to Matti Kivikko for advice on study design and Dr Steve Clissold (Content Ed Net, Madrid, Spain) for editorial assistance. They also thank Orion Pharma for funding the editorial assistance and providing samples of drug and placebo.
García‐González, M. J. , Aldea Perona, A. , Lara Padron, A. , Morales Rull, J. L. , Martínez‐Sellés, M. , de Mora Martin, M. , López Díaz, J. , López Fernandez, S. , Ortiz Oficialdegui, P. , and Jiménez Sosa, A. (2021) Efficacy and safety of intermittent repeated levosimendan infusions in advanced heart failure patients: the LAICA study. ESC Heart Failure, 8: 4820–4831. 10.1002/ehf2.13670.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association statistics committeestroke statistics subcommittee . Heart disease and stroke Statistics‐2016 update: American Heart Association statistics committee; stroke statistics subcommittee. Circulation 2016; 133: e38–e360. [DOI] [PubMed] [Google Scholar]
- 2. Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep 2014; 11: 404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anguita Sánchez M, Crespo Leiro MG, De Teresa GE, Jiménez Navarro M, Alonso‐Pulpón L, Muñiz García J, PRICE study investigators . Prevalence of heart failure in the spanish general population aged over 45 years. the PRICE study. Rev Esp Cardiol 2008; 61: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 4. Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJ. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail 2002; 4: 361–371. [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, ESC Committee for Practice Guidelines (CPG) , Document Reviewers . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 6. Nieminen MS, Dickstein K, Fonseca C, Serrano JM, Parissis J, Fedele F, Wikström G, Agostoni P, Atar S, Baholli L, Brito D, Colet JC, Édes I, Gómez Mesa JE, Gorjup V, Garza EH, González Juanatey JR, Karanovic N, Karavidas A, Katsytadze I, Kivikko M, Matskeplishvili S, Merkely B, Morandi F, Novoa A, Oliva F, Ostadal P, Pereira‐Barretto A, Pollesello P, Rudiger A, Schwinger RH, Wieser M, Yavelov I, Zymliński R. The patient perspective: Quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol 2015; 191: 256–264. [DOI] [PubMed] [Google Scholar]
- 7. Braunwald E. Heart failure. JACCHeart Fail 2013; 1: 1–20. [DOI] [PubMed] [Google Scholar]
- 8. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, College A, of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 9. Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Böhm M, Anker S, Dargie H, Brutsaert D, Komajda M, Heart failure Association of the European Society of cardiology . Advanced chronic heart failure: a position statement from the study group on advanced heart failure of the heart failure Association of the European Society of cardiology. Eur J Heart Fail 2007; 9: 684–694. [DOI] [PubMed] [Google Scholar]
- 10. Slaughter MS, Singh R. The role of ventricular assist devices in advanced heart failure. Rev Esp Cardiol 2012; 65: 982–985. [DOI] [PubMed] [Google Scholar]
- 11. Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates ‐ 2006. J Heart Lung Transplant 2006; 25: 1024–1042. [DOI] [PubMed] [Google Scholar]
- 12. de Mora‐Martín M, Pérez‐Ruiz JM, Delgado‐Prieto JL, Urbano‐Carrillo CA. Comorbidity in patients admitted to a department of cardiology due to heart failure. Rev Esp Cardiol 2011; 64: 75–83. [DOI] [PubMed] [Google Scholar]
- 13. Bayram M, De Luca L, Massie MB, Gheorghiade M. Reassessment of dobutamine, dopamine, and milrinone in the management of acute heart failure syndromes. Am J Cardiol 2005; 96: G47–G58. [DOI] [PubMed] [Google Scholar]
- 14. Thackray S, Easthaugh J, Freemantle N, Cleland JG. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure—a meta‐regression analysis. Eur J Heart Fail 2002; 4: 515–529. [DOI] [PubMed] [Google Scholar]
- 15. Papp Z, Édes I, Fruhwald S, De Hert SG, Salmenperä M, Leppikangas H, Mebazaa A, Landoni G, Grossini E, Caimmi P, Morelli A, Guarracino F, Schwinger RH, Meyer S, Algotsson L, Wikström BG, Jörgensen K, Filippatos G, Parissis JT, González MJ, Parkhomenko A, Yilmaz MB, Kivikko M, Pollesello P, Follath F. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol 2012; 159: 82–87. [DOI] [PubMed] [Google Scholar]
- 16. Pollesello P, Papp Z. The cardioprotective effects of levosimendan: preclinical and clinical evidence. J Cardiovasc Pharmacol 2007; 50: 257–263. [DOI] [PubMed] [Google Scholar]
- 17. Delaney A, Bradford C, McCaffrey J, Bagshaw SM, Lee R. Levosimendan for the treatment of acute severe heart failure: a meta‐analysis of randomized controlled trials. Int J Cardiol 2010; 138: 281–289. [DOI] [PubMed] [Google Scholar]
- 18. Landoni G, Biondi‐Zoccai G, Greco M, Greco T, Bignami E, Morelli A, Guarracino F, Zangrillo A. Effects of levosimendan on mortality and hospitalization. a meta‐analysis of randomized controlled studies. Crit Care Med 2012; 40: 634–646. [DOI] [PubMed] [Google Scholar]
- 19. Pollesello P, Parissis J, Kivikko M, Harjola VP. Levosimendan meta‐analyses: is there a pattern in the effect on mortality? Int J Cardiol 2016; 209: 77–83. [DOI] [PubMed] [Google Scholar]
- 20. Pölzl G, Altenberger J, Baholli L, Beltrán P, Borbély A, Comin‐Colet J. Repetitive use of levosimendan in advanced heart failure: need for stronger evidence in a field in dire need of a useful therapy. Int J Cardiol 2017; 243: 389–395. [DOI] [PubMed] [Google Scholar]
- 21. Altenberger J, Parissis JT, Costard‐Jaeckle A, Winter A, Ebner C, Karavidas A, Sihorsch K, Avgeropoulou E, Weber T, Dimopoulos L, Ulmer H, Poelzl G. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advance heart failure (LevoREp) study: a multicentre randomized trial. Eur J Heart Fail 2014; 16: 898–906. [DOI] [PubMed] [Google Scholar]
- 22. Pellicori P, Clark AL. Clinical trials update from the european Society of Cardiology‐Heart Failure meeting 2015: AUGMENT‐HF, TITRATION, STOP‐HF, HARMONIZE, LION HEART, MOOD‐HF, and renin‐angiotensin inhibitors in patients with heart and renal failure. Eur J Heart Fail 2015; 17: 979–983. [DOI] [PubMed] [Google Scholar]
- 23. García‐González MJ, de Mora‐Martín M, López‐Fernández S, López‐Díaz J, Martínez‐Sellés M, Romero‐García J, Cordero M, Lara‐Padrón A, Marrero‐Rodríguez F, del Mar García‐Saiz M, Aldea‐Perona A, LAICA study investigators . Rationale and design of a randomized, double‐blind, placebo controlled multicenter trial to study efficacy, security, and long‐term effects of intermittent repeated levosimendan administration in patients with advanced heart failure: LAICA study. Cardiovasc Drugs Ther 2013; 27: 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eudralex ‐Vol 10 – Clinical trials guidelines. Public Health. Guidelines on good clinical practice specific to advance therapy medicinal products [Internet] European Commission. 2019. [accessed August 28, 2021] Available from: (http://www.ec.europa.eu/health/documents/eudralex/vol-10/index_en.html)
- 25. Mullens W, Abrahams Z, Skouri HN, Taylor DO, Starling RC, Francis GS, Young JB, Tang W. Prognostic evaluation of ambulatory patients with advanced heart failure. Am J Cardiol 2008; 101: 1297–1302. [DOI] [PubMed] [Google Scholar]
- 26. Nanas JN, Papazoglou P, Tsagalou EP, Ntalianis A, Tsolakis E, Terrovitis JV, Kanakakis J, Nanas SN, Alexopoulos GP, Anastasiou‐Nana MI. Efficacy and safety of intermittent, long‐term, concomitant dobutamine and levosimendan infusions in severe heart failure refractory to dobutamine alone. Am J Cardiol 2005; 95: 768–771. [DOI] [PubMed] [Google Scholar]
- 27. Parissis J, Adamopoulos S, Farmakis D, Filippatos G, Paraskevaidis I, Panou F, Iliodromitis E, Kremastinos DT. Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure. Heart 2006; 92: 1768–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mavrogeni S, Giamouzis G, Papadopoulou E, Thomopoulou S, Dritsas A, Athanasopoulos G, Adreanides E, Vassiliadis I, Spargias K, Panagiotakos D, Cokkinos DV. A 6‐month follow‐up of intermittent levosimendan administration effect on systolic function, specific activity questionnaire, and arrhythmia in advanced heart failure. J Card Fail 2007; 13: 556–559. [DOI] [PubMed] [Google Scholar]
- 29. Papadopoulou EF, Mavrogeni SI, Dritsas A, Cokkinos DV. Assessment of quality of life using three different activity questionnaires in heart failure patients after monthly, intermittent administration of levosimendan during a six‐month period. Hell J Cardiol 2009; 50: 269–274. [PubMed] [Google Scholar]
- 30. Malfatto G, Della Rosa F, Villani A, Rella V, Branzi G, Facchini M, Parati G. Intermittent levosimendan infusions in advanced heart failure: favourable effects on left ventricular function, neurohormonal balance, and one‐year survival. J Cardiovasc Pharmacol 2012; 60: 450–455. [DOI] [PubMed] [Google Scholar]
- 31. Tasal A, Demir M, Kanadasi M, Bacaksiz A, Vatankulu MA, Sahin DY. Comparison of single‐dose and repeated levosimendan infusion in patients with acute exacerbation of advanced heart failure. Med Sci Monit 2014; 20: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parle NM, Thomas MD, Dembo L, Best M, Driscoll GO. Repeated infusions of levosimendan: well tolerated and improves functional capacity in decompensated heart failure ‐ a single‐Centre experience. Heart Lung Circ 2008; 17: 206–210. [DOI] [PubMed] [Google Scholar]
- 33. Bonios MJ, Terrovitis JV, Drakos SG, Katsaros F, Pantsios C, Nanas SN. Comparison of three different regimens of intermittent inotrope infusions for end stage heart failure. Int J Cardiol 2012; 159: 225–229. [DOI] [PubMed] [Google Scholar]
- 34. Comín‐Colet J, Manito N, Segovia‐Cubero J, Delgado J, , García Pinilla JM, Almenar L, Crespo‐Leiro MG, Sionis A, Blasco T, Pascual‐Figal D, Gonzalez‐Vilchez F, Lambert‐Rodríguez JL, Grau M, Bruguera J, LION‐HEART Study Investigators . Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION‐HEART multicentre randomised trial. Eur J Heart Fail 2018; 20: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 35. Oliva F, Perna E, Marini M, Nassiacos D, Cirò A, Malfatto G, Morandi F, Caico I, Perna G, Meloni S, Vincenzi A, Villani A, Vecchi AL, Minoia C, Verde A, De Maria R, RELEVANT‐HF study group . Scheduled intermittent inotropes for ambulatory advanced heart failure. The RELEVANT‐HF multicentre collaboration. Int J Cardiol 2018; 272: 255–259. [DOI] [PubMed] [Google Scholar]
- 36. Silvetti S, Nieminen MS. Repeated or intermittent levosimendan treatment in advanced heart failure: an updated meta‐analysis. Int J Cardiol 2016; 202: 138–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


