Abstract
Aims
The kinocardiograph (KCG) is an unobtrusive device, consisting of a chest sensor, which records local thoracic vibrations produced in result of cardiac contraction and ejection of blood into the great vessels [seismocardiography (SCG)], and a lower back sensor, which records micromovements of the body in reaction to blood flowing through the vasculature [ballistocardiography (BCG)]. SCG and BCG signals are translated to the integral of cardiac kinetic energy (iK) and cardiac maximum power (Pmax), which might be promising metrics for future telemonitoring purposes in heart failure (HF). As a first step of validation, this study aimed to determine whether iK and Pmax are responsive to exercise‐induced changes in the haemodynamic load of the heart in HF patients.
Methods and results
Fifteen patients with stable HF with reduced ejection fraction performed a submaximal exercise protocol. KCG and cardiac ultrasound measurements were obtained both at rest and at submaximal exercise. BCG iK over the cardiac cycle (CC) increased significantly (0.0026 ± 0.0017 to 0.0052 ± 0.0061 mJ.s.; P = 0.01) during exercise, in contrast to a non‐significant increase in SCG iK CC. BCG Pmax CC increased significantly (0.92 ± 0.89 to 2.03 ± 1.95 mJ/s; P = 0.02), in contrast to a non‐significant increase in SCG Pmax CC. When analysing the systolic phase of the CC, similar patterns were found. Cardiac output (CO) ratio (i.e. CO exercise/CO rest) showed a moderate, significant correlation with BCG Pmax CC ratio (r = +0.65; P = 0.008) and with SCG Pmax CC ratio (r = +0.54; P = 0.04).
Conclusions
iK and Pmax measured with the KCG, preferentially using BCG, are responsive to changes in the haemodynamic load of the heart in HF patients. The combination of the BCG and SCG sensor might be of added value to fully understand changes in haemodynamics and to discriminate between an HF patient and a healthy individual.
Keywords: Chronic heart failure, Ballistocardiography, Seismocardiography, Kinetic energy, Hemodynamic load
Introduction
Heart failure (HF) is a major healthcare problem, affecting over 26 million people worldwide. 1 The prevalence is increasing because of ageing of the population. HF is characterized by a chronic and progressive course, with frequent exacerbations and hospital admissions, resulting in an impaired quality of life and posing a major economic burden. 2 Remote monitoring solutions have been proposed to improve HF care efficacy and to reduce HF readmissions. 3 , 4 Typically, telemonitoring services consist of a set of monitoring techniques to assess changes in vital signs and physical complaints related to HF decompensation. However, as these parameters change relatively late in the cascade of pathophysiological events leading to decompensated HF, there is a need for monitoring techniques, which are more closely related to cardiac function and intracardiac filling pressures, such as invasive monitoring of pulmonary artery pressures, which already proved to be successful for prevention of hospital admissions. 5 Despite the rapid evolution of telemedical services, accurate non‐invasive methods for monitoring of cardiac function and filling pressures are not yet available.
Seismocardiography (SCG) and ballistocardiography (BCG) are non‐invasive techniques for cardio‐mechanical assessment, which have the potential to be used in a remote setting. SCG measures local thoracic vibrations produced in result of cardiac contraction and ejection of blood into the great vessels, while BCG measures micromovements of the body in reaction to blood flowing through the vasculature. 6 Preliminary studies with wearable SCG and BCG systems showed the potential of these techniques for application in patients with cardiovascular diseases. In HF, a triaxial (linear accelerations) SCG patch placed on the sternum was able to estimate clinical status in HF patients; however, this method requires patients to perform a 6 min walk test on a daily basis, which is less feasible for remote monitoring of patients with severe HF. 7 Another study using a modified weighing scale‐based BCG system (head‐to‐foot axis) also showed promising results in differentiating decompensated from compensated HF patients. 8 , 9
In the present study, we introduce the kinocardiograph (KCG), an unobtrusive wearable device, which combines SCG and BCG. Unique to this device is that it records triaxial SCG and BCG signals (i.e. dorsoventral, lateral, and head‐to‐foot) in both the linear and rotational dimensions rather than using single‐dimension measurements. In this way, accelerations produced by cardiac contraction and flowing blood are not limited to a single direction or angle, thereby potentially improving its responsiveness to changes in cardiac function and filling pressures. The KCG measurements are translated to cardiac kinetic energy and its temporal integration (iK) as well as cardiac maximum power (Pmax). Both are indicators of cardiac function, where iK represents the integral of kinetic energy during the cardiac cycle (CC) and Pmax represents the maximum power generated by the myocardium mainly linked to the systolic phase of the CC. An important step for validation of the KCG is to evaluate whether the parameters measured with the KCG are responsive to changes in cardiac haemodynamic load (e.g. exercise or inotropic agents) in HF patients. In healthy individuals, iK showed a high correlation with dobutamine‐induced changes in stroke volume (SV) and cardiac output (CO). 10 Moreover, cardiovascular deconditioning in healthy people, resulting from long‐term head‐down bed rest, was made visible using KCG metrics. 11 In particular, the evolution of BCG metrics followed changes in SV. However, it is not known whether these results can be applied to HF patients. A study in patients with HF with reduced ejection fraction (HFrEF) showed that the average systolic cardiac kinetic energy, measured with magnetic resonance imaging, is higher in these patients as compared with healthy subjects, suggesting an altered blood flow. 12 Moreover, exercise haemodynamics are altered in patients with HFrEF as compared with healthy subjects, being characterized by an impaired increase in SV caused by a dilated left ventricle with impaired intrinsic contractility, a lower maximum heart rate (HR), and higher systemic vascular resistance. 13
The aim of the present study was to determine whether iK and Pmax, measured with the KCG, are responsive to exercise‐induced changes in the haemodynamic load of the heart in patients with stable HFrEF.
Methods
Study population
Patients (age ≥16 years), diagnosed with HFrEF regardless of aetiology, were included. HFrEF was defined in line with the European Society of Cardiology guidelines as a condition with HF symptoms (with or without signs) and a left ventricular (LV) ejection fraction of <40%. 14 Additional inclusion criteria were optimal medical treatment and a stable clinical condition.
Exclusion criteria were permanent atrial heart rhythm disturbances; haemodynamic significant valvular disease; documented cardiac ischaemia or chest pain when performing submaximal exercise; and neurological, orthopaedic, or vascular conditions preventing the patient from performing exercise. Patients were recruited at the outpatient cardiology clinic of Máxima Medical Centre, the Netherlands. All participants provided written informed consent. The study protocol was reviewed by the medical ethics committee of Máxima Medical Centre and received a waiver for formal ethical approval. The study conforms to the principles outlined in the Declaration of Helsinki and was registered in the Netherlands Trial Register (NL9101).
Protocol
Each participant underwent cardiac ultrasound and KCG measurements both on a bicycle in the left supine position with a 30° angle (Lode BV, Groningen, the Netherlands). KCG and cardiac ultrasound measurements were obtained simultaneously, both before and directly after exercise. The submaximal exercise protocol consisted of cycling at a workload of 50 W for 5 min with a pedalling frequency of 60–80 rotations per minute.
Cardiac ultrasound measurements
Cardiac ultrasound measurements were performed by a cardiologist (R. F. S.), experienced in stress echocardiography, who was blinded for the KCG measurements. Ultrasound measurements were performed with a Philips Epiq 7C US machine and a Philips X5‐1 transducer (Eindhoven, the Netherlands). LV outflow tract diameter was obtained in the parasternal long‐axis view during systole. The LV outflow tract velocity time integral was measured in the apical five‐chamber view, using the pulsed‐wave Doppler signal. SV was calculated using the following equation 15 :
Cardiac output was calculated by multiplying HR by SV.
Kinocardiograph measurements
Kinocardiograph measurements were performed by the local investigator (C. H.), who was blinded for the cardiac ultrasound measurements. The KCG is an unobtrusive device consisting of two sensors placed at the body surface with standard electrocardiogram (ECG) patches (Figure 1 ). The first sensor (64 cm2, 104 g) was placed on the sternum right below the insertion of the clavicle to the sternum recording the SCG signal. The second sensor (24 cm2, 64 g) was placed at the middle of the lower back recording the BCG signal. Both sensors contain a three‐axis MEMS accelerometer and a three‐axis gyroscope. The sensors were connected with each other via two cables. The KCG was controlled with a tablet containing the corresponding software (via Bluetooth). The following KCG data were collected during a 90 s recording: three‐degree‐of‐freedom accelerations (sampling frequency 50 Hz) in the linear and rotational angle, measured both at the sternum (SCG) and at the lumbar region (BCG), and a single‐lead ECG measurement.
Figure 1.
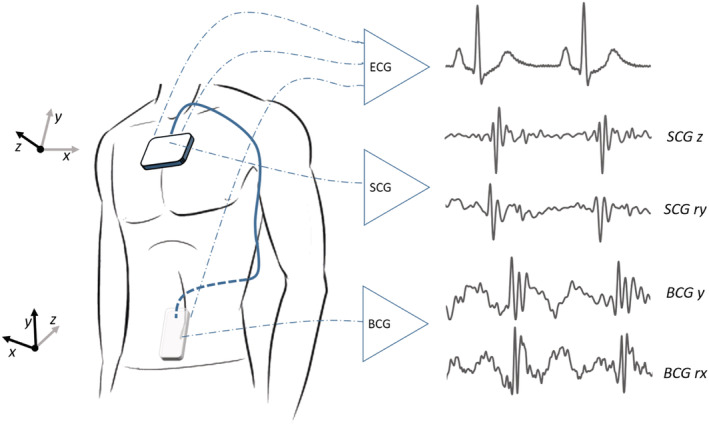
Localization of the seismocardiography (SCG) and ballistocardiography (BCG) sensor on the sternum and the lower back, respectively. ECG, electrocardiogram.
Kinocardiograph data processing
The aim of the KCG processing is to extract only a few scalar metrics from the 12 temporal signals of accelerations and angular rates acquired by the chest and lower back sensors. To do so, an ensemble average is performed for each signal to obtain the signal of a single heartbeat. This single heartbeat acts as a representative for all the heartbeats that happened during the record. The ensemble average method is described in a previous publication. 16 From the linear accelerations and angular rates average on one heartbeat, Newtonian laws are used to compute the instantaneous kinetic energies (K) and power (P) transmitted to the sensors by the cardiac activity. 10
The BCG sensor can therefore provide the linear kinetic energy (BCG K Lin ), the linear power (BCG P Lin ), the rotational kinetic energy (BCG K Rot ), and the rotational power (BCG P Rot ). In the same way, the SCG sensor can provide the linear kinetic energy (SCG K Lin ), the linear power (SCG P Lin ), the rotational kinetic energy (SCG K Rot ), and the rotational power (SCG P Rot ). These still are temporal signals. To obtain scalar metrics, the integral of a kinetic energy on a given CC interval (CCI) and the maximum of power on a given CCI respectively are computed as follows:
where CCI can be the whole CC and the systolic or diastolic phase. These phases are defined based on the ensemble‐averaged ECG signal: the systolic phase starts at the Q wave and finishes at the end of the T wave, while the diastolic phase corresponds to the rest of the heartbeat, from the end of the T wave until the next P wave. ECG is used to determine the systolic and diastolic periods, rather mechanical periods estimated from the SCG and BCG signal, to create more certainty as the SCG and BCG are still in research state. The delimitation of the systolic/diastolic phases has been both checked visually and corrected when needed.
The total iK and total Pmax were computed as the sum of the linear and rotational parts:
In the remainder of the article, the notation of iK represents iK total , and the notation of Pmax represents Pmax total .
Moreover, SCG iK and BCG iK were multiplied by HR, to compute two additional parameters, which correspond to the total kinetic energy generated by the heart per time unit. These better relate to CO, which is the volume of blood ejected by the heart per time unit.
The first 20 s of the KCG recordings was used to compute these metrics for both the baseline and the after‐exercise records, as this matches the time needed to obtain the cardiac ultrasound measurements.
The computing of KCG parameters was performed using a software toolbox in MATLAB (2019b, MathWorks, Natick, MA, USA). More detailed information on the signal processing of multidimensional BCG and SCG records has been described previously. 10 An example of SCG and BCG signals before and after exercise can be found in Figure 2 .
Figure 2.
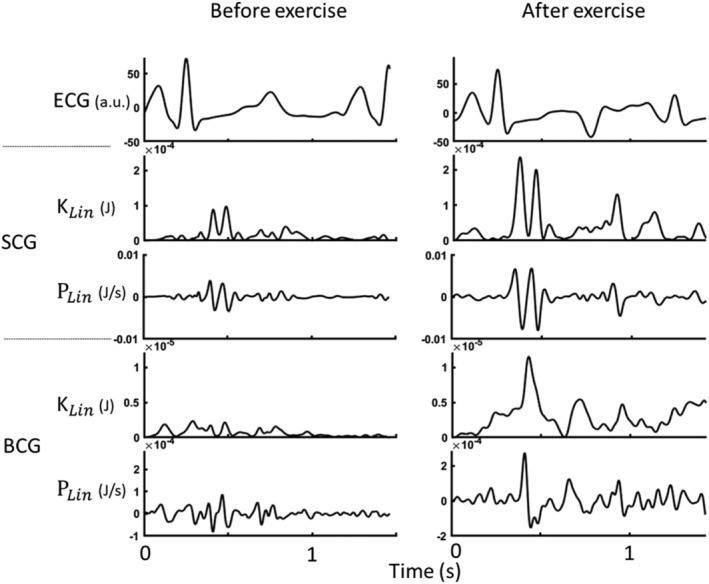
Ensemble‐averaged waveforms of (from top to bottom) seismocardiography (SCG) linear kinetic energy, SCG linear power, ballistocardiography (BCG) linear kinetic energy, and BCG linear power for a representative patient at baseline (left) and after exercise (right). ECG, electrocardiogram.
Statistical analysis
Descriptive statistics were used to describe the studied population regarding the patient characteristics, cardiac ultrasound measurements, and KCG measurements during rest and exercise. Because of the small sample size, non‐parametric tests were used. The Wilcoxon signed‐rank test was used to compare differences in SV, HR, CO, iK, and Pmax before and after exercise. In addition, effect sizes were calculated using the following standard response mean equation 17 : .
The Z score was based on the Wilcoxon signed‐rank test. An effect size of <0.2 represents a trivial effect, a value of ≥0.2 to <0.5 is considered a small effect, a value of ≥0.5 to <0.8 represents a moderate effect, and a value of ≥0.8 represents a large effect.
Ratios of SV, CO and iK, and Pmax were calculated (i.e. SVratio = ). Correlation coefficients between SV and CO ratio and iK and Pmax ratio were calculated using the Spearman rank correlation statistic. Statistical analysis was performed using SPSS software (Version 22, SPSS Inc.®). For all analyses, the significance level was set at P < 0.05.
Results
Patient characteristics
A total of 15 patients diagnosed with HFrEF completed the study protocol (age 67.4 ± 4.8 years; LV ejection fraction 29.2 ± 4.5%). Patient characteristics are summarized in Table 1 .
Table 1.
Patient characteristics (n = 15)
| Age (years) | 67.4 ± 4.8 |
| Gender | |
| Male | 10 (66.7) |
| Female | 5 (33.3) |
| Height (m) | 1.75 ± 0.08 |
| Weight (kg) | 84.3 ± 12.7 |
| BMI (kg/m2) | 27.6 ± 3.8 |
| LVEF (%) | 29.2 ± 4.5 |
| HF aetiology | |
| Ischaemic | 4 (26.7) |
| Non‐ischaemic | 11 (73.3) |
| NYHA classification | |
| I | 3 (20.0) |
| II | 7 (46.7) |
| III | 1 (6.7) |
| IV | 0 (0) |
| Unknown | 4 (26.7) |
BMI, body mass index; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
Values are presented as number (%) or mean ± SD.
Haemodynamic and iK parameters
Table 2 shows the changes in SV, HR, CO, and iK parameters during submaximal exercise. SV, HR, and CO all significantly increased during exercise (SV 62.8 ± 18.8 to 75.5 ± 20.5 mL, P = 0.009; HR 73 ± 14 to 94 ± 21 b.p.m., P = 0.001; and CO 4.4 ± 1.2 to 6.9 ± 1.8 L/min, P = 0.001).
Table 2.
Cardiac ultrasound and iK measurements before and after submaximal exercise
| Rest | Exercise | P‐value | Effect size | |
|---|---|---|---|---|
| SV (mL) | 62.8 ± 18.8 | 75.5 ± 20.5 | 0.009 | 0.68 |
| HR (b.p.m.) | 73 ± 14 | 94 ± 21 | 0.001 | 0.88 |
| CO (L/min) | 4.4 ± 1.2 | 6.9 ± 1.8 | 0.001 | 0.87 |
| Cardiac cycle (mJ.s) | ||||
| iK BCG | 0.0026 ± 0.0017 | 0.0052 ± 0.0061 | 0.01 | 0.65 |
| iK SCG | 0.32 ± 0.22 | 0.42 ± 0.39 | 0.31 | 0.26 |
| iK BCG * HR | 0.18 ± 0.10 | 0.48 ± 0.63 | 0.001 | 0.84 |
| iK SCG * HR | 24.2 ± 17.8 | 44.3 ± 56.0 | 0.07 | 0.47 |
| Systolic phase (mJ.s) | ||||
| iK BCG | 0.0014 ± 0.0012 | 0.0030 ± 0.0032 | 0.01 | 0.66 |
| iK SCG | 0.16 ± 0.12 | 0.27 ± 0.30 | 0.13 | 0.40 |
| Diastolic phase (mJ.s) | ||||
| iK BCG total | 0.0011 ± 0.0007 | 0.0022 ± 0.0030 | 0.26 | 0.29 |
| iK SCG total | 0.16 ± 0.15 | 0.15 ± 0.09 | 0.78 | 0.07 |
BCG, ballistocardiography; CO, cardiac output; HR, heart rate; iK, integrated kinetic energy; SCG, seismocardiography; SD, standard deviation; SV, stroke volume.
Values are presented as mean ± SD.
Ballistocardiography iK over the CC increased significantly (0.0026 ± 0.0017 to 0.0052 ± 0.0061 mJ.s; P = 0.01), in contrast to a non‐significant increase of SCG iK CC. The effect size of BCG iK CC was moderate (0.65). Moreover, BCG iK * HR also increased significantly (0.18 ± 0.10 to 0.48 ± 0.63 mJ.s; P = 0.001), in contrast to a non‐significant increase of SCG iK * HR.
A similar pattern was observed when analysing the systolic phase separately: BCG systolic iK increased significantly (0.0014 ± 0.0012 to 0.0030 ± 0.0032 mJ.s; P = 0.01), whereas SCG systolic iK showed a non‐significant increase. BCG systolic iK showed a moderate effect size (0.66). iK in the diastolic phase did not show a significant change during submaximal exercise (BCG diastolic iK: 0.0011 ± 0.0007 to 0.0022 ± 0.0030 mJ.s, P = 0.29; SCG diastolic iK: 0.16 ± 0.15 to 0.15 ± 0.09 mJ.s., P = 0.07).
iK ratios did not show a significant correlation with SV ratio, assessed over the total CC and in the systolic phase. Moreover, the linear and the rotational parts of both the SCG and BCG sensors were analysed separately. None of these iK parameter ratios showed a significant correlation with SV ratio.
Cardiac output ratio did not show a significant correlation with BCG iK * HR ratio (r = +0.51; P = 0.05) and with SCG iK * HR ratio (r = +0.47; P = 0.08).
Pmax parameters
Ballistocardiography Pmax CC increased significantly during submaximal exercise (0.92 ± 0.89 to 2.03 ± 1.95 mJ/s; P = 0.02), in contrast to a non‐significant increase of SCG Pmax CC. When splitting BCG Pmax in its linear and rotational component, BCG Pmaxlinear showed a large effect size (0.84). When analysing the systolic phase of the CC, a similar pattern is found. BCG systolic Pmax significantly increased (0.89 ± 0.90 to 1.98 ± 1.98 mJ/s; P = 0.01), in contrast to a non‐significant increase of SCG systolic Pmax. BCG Pmaxlinear in the systolic phase showed a large effect size (0.84). BCG Pmax and SCG Pmax in the diastolic phase both did not show significant differences during submaximal exercise (BCG Pmax: 0.03 ± 0.07 to 0.04 ± 0.10 mJ/s, P = 1.0; SCG Pmax: 11.2 ± 35.3 to 2.1 ± 3.6 mJ/s, P = 0.26). The Pmax results are summarized in Table 3 .
Table 3.
Pmax measurements before and after submaximal exercise
| Rest | Exercise | P‐value | Effect size | |
|---|---|---|---|---|
| Cardiac cycle (mJ/s) | ||||
| Pmax BCG | 0.92 ± 0.89 | 2.03 ± 1.95 | 0.02 | 0.63 |
| Pmax BCG linear | 0.16 ± 0.11 | 0.39 ± 0.28 | 0.001 | 0.84 |
| Pmax BCG rotational | 0.76 ± 0.82 | 1.64 ± 1.73 | 0.02 | 0.60 |
| Pmax SCG | 104.8 ± 85.9 | 134.9 ± 125.2 | 0.19 | 0.34 |
| Systolic phase (mJ/s) | ||||
| Pmax BCG | 0.89 ± 0.90 | 1.98 ± 1.98 | 0.01 | 0.65 |
| Pmax BCG linear | 0.16 ± 0.11 | 0.38 ± 0.28 | 0.001 | 0.84 |
| Pmax BCG rotational | 0.73 ± 0.83 | 1.61 ± 1.76 | 0.02 | 0.60 |
| Pmax SCG | 93.6 ± 85.7 | 132.8 ± 126.6 | 0.07 | 0.47 |
| Diastolic phase (mJ/s) | ||||
| Pmax BCG | 0.03 ± 0.07 | 0.04 ± 0.10 | 1.0 | 0.0 |
| Pmax SCG | 11.2 ± 35.3 | 2.1 ± 3.6 | 0.31 | 0.26 |
BCG, ballistocardiography; Pmax, maximum power; SCG, seismocardiography; SD, standard deviation.
Values are presented as mean ± SD.
We did not find a significant correlation between SV ratio and Pmax ratio. However, CO ratio showed a moderate and significant correlation with BCG Pmax CC ratio (r = +0.65; P = 0.008). CO ratio also showed a moderate, significant correlation with SCG Pmax CC ratio (r = +0.54; P = 0.04). Moreover, a moderate but significant correlation was found between CO ratio and BCG Pmax ratio in the systolic phase (r = +0.5, P = 0.04). SCG Pmax ratio in the systolic phase did not correlate with CO ratio.
Discussion
This study demonstrates that the iK and Pmax measured with the KCG, both during the total CC and the systolic phase, are responsive to changes in the haemodynamic load of the heart in patients with HFrEF. To our knowledge, this is the first study showing that multidimensional BCG and SCG are promising mobile and non‐invasive techniques for monitoring changes in haemodynamics in patients with HF.
A previous study using the same device in healthy individuals observed a significant increase of iK and Pmax in response to incremental doses of dobutamine, for both the SCG and BCG sensors. 10 This was in contrast to our study, which found that iK and Pmax measured with the BCG sensor showed better responsiveness than the SCG sensor, which can be expected based on HF pathology. This difference might be explained by the fact that HF is characterized by an attenuated cardiac power output (in Watts), which is defined as the energy generated by the heart to maintain the continuous circulation of blood. 18 Because SCG measures local thoracic vibrations due to myocardial contraction, an impaired cardiac power output in HF will likely result in a reduced kinetic energy amplitude assessed by SCG. On the other hand, BCG measures micromovements of the body in reaction to blood flowing to the vasculature (mainly the aorta). The exact physiological origin of the BCG signal is not fully understood, but it is generally regarded as an integration of forces produced not only by the heart but also by the motion of blood in the great vasculature. 19 In young and healthy people, the vascular adaptations to exercise include an increased rate of skeletal blood flow in expense of blood flow to the viscera. 20 , 21 The local aortic response to exercise includes a significant decrease of compliance and distensibility of the aorta, in correlation with an increase of pulse wave velocity. 22 In (elderly) patients with HF, the vascular response to exercise is altered. This results in an increased leg vascular resistance and decreased leg blood flow, although central arterial blood flow and its changes during exercise are maintained and comparable with healthy subjects. 23 , 24 These considerations might explain that BCG changes are significantly more pronounced than SCG changes in HF patients. However, the two sensors together can be used to fully understand haemodynamic status and thereby discriminate between an HF patient and a healthy person, which might not be doable with BCG alone.
A second remarkable finding of this study is that iK and Pmax measured by BCG changed significantly in reaction to changes in the haemodynamic load only when measured during the total cardiac and the systolic phase. These findings are in contrast with a study in healthy people, which showed an increase in both systolic and diastolic kinetics in healthy subjects during an exercise protocol on a supine bicycle. 25 That study also revealed that the LV apex contributed to a greater extend to diastolic KE during exercise, while the LV base contributed more to systolic KE. The fact that diastolic KE did not change significantly during exercise in HF patients might therefore be explained by regional wall motion disturbances, which are often more prominent in the LV apex in HF patients. In fact, Sunnerhagen et al. showed that diastolic basal wall motion is relatively preserved as compared with apical wall motion in these patients. 26 Therefore, we recommend monitoring iK and Pmax using the systolic phase or the total CC in future studies, with a slight preference for using the entire cycle because of practical advantages (e.g. no need for dividing the systolic and diastolic phase in case of bad‐quality ECG reading).
A third finding of this study is that we found a moderate, but significant correlation between exercise‐induced changes in CO and BCG Pmax CC. Similar results were found for changes in CO and SCG Pmax CC. However, we did not observe correlations between exercise‐induced changes in SV and CO and changes in iK and iK * HR, respectively, which is in contrast with a study in healthy individuals receiving incremental doses of dobutamine. 10 These differences might be explained by the fact that kinetic energy (and thus iK) depends not only on CO but also on mean arterial pressure and that both parameters are not affected in the same way in HF patients. In fact, Sullivan et al. showed that although CO is lower in patients with HF as compared with healthy subjects, both at rest and at submaximal exercise, mean arterial pressure was comparable between HF patients and healthy persons at rest and during submaximal exercise. 23 Also, resting HR can be increased in HF patients and reaches a lower maximum value upon exercise.
Strengths and limitations
This is the first study to evaluate the responsiveness of kinetic energy parameters obtained by multidimensional SCG and BCG, in response to changes in the haemodynamic load of the heart due to exercise in HF patients. By using a submaximal exercise protocol instead of infusion of inotropic agents, we aimed to mimic daily life conditions. However, some limitation should be considered. First, the exercise protocol was not tailored to the patients' maximal exercise capacity (i.e. 50 W for every patient). Therefore, patients exercising at a higher relative intensity may have shown more breathing and movement artefacts. Nevertheless, measurements were successfully obtained in all included patients.
Second, ideally, both cardiac ultrasound and KCG measurements should be performed while exercising and not directly after, because of the expected quick haemodynamic changes when the patient stops exercising. However, to obtain high‐quality measurements, the KCG should not be used when the patient is extensively moving his body. Therefore, we chose to perform both measurements simultaneously, directly after completing the exercise protocol. As exercise haemodynamics change quite rapid after cessation of exercise, the actual change in SV, CO, and KCG parameters might have been underestimated.
Finally, the results of this study are not applicable to the entire HF population, as we included patients with HFrEF, having sinus rhythm, and absence of significant valvular disease. The KCG signals and translation to iK and Pmax could be influenced by heart rhythm disturbances and valvular pathology.
Conclusions and future perspectives
This study demonstrated that Pmax and iK, measured with the KCG, are highly responsive to changes in exercise haemodynamics in patients with HFrEF. The measurements are most responsive when obtained from the BCG sensor during the systolic phase or total CC; however, the combination of the BCG and SCG sensor is of added value to fully understand changes in haemodynamics in order to discriminate between an HF patient and a healthy individual.
This is a first step in the validation of the KCG, showing it can serve as a non‐invasive, unobtrusive device for monitoring changes in the haemodynamic load of the heart in patients with HFrEF. Further research is needed to evaluate whether the device is responsive to changes in filling pressures related to fluid status in these patients. Moreover, usability and accuracy need to be evaluated in the home environment, rather than in‐hospital conditions.
Conflict of interest
P.‐F.M. and A.H. are minority shareholders at HeartKinetics. The other authors declare no conflicts of interest.
Funding
This research received no external funding.
Author contributions
C.H., H.M.C.K., P.‐F.M., and A.H. contributed to the conception and design of the study. C.H. and R.F.S. contributed to the acquisition of data. P.‐F.M. and A.H. prepared the data for statistical analysis. C.H. performed the analysis of data. All authors contributed to interpretation of data. C.H. drafted the manuscript, except for the Kinocardiograph data processing section, which was drafted by A.H. All authors critically revised the manuscript. All gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Herkert, C. , Migeotte, P.‐F. , Hossein, A. , Spee, R. F. , and Kemps, H. M. C. (2021) The kinocardiograph for assessment of changes in haemodynamic load in patients with chronic heart failure with reduced ejection fraction. ESC Heart Failure, 8: 4925–4932. 10.1002/ehf2.13522.
References
- 1. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017; 3: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lesyuk W, Kriza C, Kolominsky‐Rabas P. Cost‐of‐illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord 2018; 18: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bashi N, Karunanithi M, Fatehi F, Ding H, Walters D. Remote monitoring of patients with heart failure: an overview of systematic reviews. J Med Internet Res 2017; 19: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan B‐A, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Störk S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM‐HF 2): a randomised controlled parallel‐group, unmasked trial. Lancet 2018; 392: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 5. Veenis JF, Brugts JJ. Remote monitoring of chronic heart failure patients: invasive versus non‐invasive tools for optimising patient management. Netherlands Hear J 2020; 28: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inan OT, Migeotte P‐F, Park K‐S, Etemadi M, Tavakolian K, Casanella R, Zanetti J, Tank J, Funtova I, Prisk GK, di Rienzo M. Ballistocardiography and seismocardiography: a review of recent advances. IEEE J Biomed Heal Informatics 2015; 19: 1414–1427. [DOI] [PubMed] [Google Scholar]
- 7. Inan OT, Pouyan MB, Javaid AQ, Dowling S, Etemadi M, Dorier A, Heller JA, Bicen AO, Roy S, de Marco T, Klein L. Novel wearable seismocardiography and machine learning algorithms can assess clinical status of heart failure patients. Circ Heart Fail 2018; 11: e004313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Etemadi M, Hersek S, Tsjeng JM, Rabbani N, Heller JA, Roy S, Klein L, Inan OT. Tracking clinical status for heart failure patients using ballistocardiography and electrocardiography signal features. Conf Proc IEEE Eng Med Biol Soc 2014; 5188: 5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aydemir VB, Nagesh S, Shandhi MMH, Fan J, Klein L, Etemadi M, Heller JA, Inan OT, Rehg JM. Classification of decompensated heart failure from clinical and home ballistocardiography. IEEE Trans Biomed Eng 2020; 67: 1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hossein A, Mirica DC, Rabineau J, del Rio JI, Morra S, Gorlier D, Nonclerq A, van de Borne P, Migeotte P‐F. Accurate detection of dobutamine‐induced haemodynamic changes by kino‐cardiography: a randomised double‐blind placebo‐controlled validation study. Sci Rep 2019; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rabineau J, Hossein A, Landreani F, Haut B, Mulder E, Luchitskaya E, Tank J, Caiani EG, van de Borne P, Migeotte P‐F. Cardiovascular adaptation to simulated microgravity and countermeasure efficacy assessed by ballistocardiography and seismocardiography. Sci Rep 2020; 10: 17694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanski M, Arvidsson PM, Töger J, Borgquist R, Heiberg E, Carlsson M, Arheden H. Left ventricular fluid kinetic energy time curves in heart failure from cardiovascular magnetic resonance 4D flow data. J Cardiovasc Magn Reson 2015; 17: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 2003; 107: 1210–1225. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 15. Galiuto L, Badano L, Fox K, Sicari R, Zamorano JL. Assessment of systolic function. In The EAE Textbook of Echocardiography. New York: Oxford University Press; 2011. p 117–121. [Google Scholar]
- 16. Hossein A, Rabineau J, Gorlier D, Pinki F, van de Borne P, Nonclerq A, Migeotte P. Effects of acquisition device, sampling rate, and record length on kinocardiography during position‐induced heamodynamic changes. Biomed Eng Online 2021; 20: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol 2000; 53: 459–468. [DOI] [PubMed] [Google Scholar]
- 18. Cotter G, Williams SG, Vered Z, Tan LB. Role of cardiac power in heart failure. Curr Opin Cardiol 2003; 18: 215–222. [DOI] [PubMed] [Google Scholar]
- 19. Giovangrandi L, Inan OT, Wiard RM, Etemadi M, Kovacs GTA. Ballistocardiography—a method worth revisiting. Conf Proc IEEE Eng Med Biol Soc 2011: 4279–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiner DA. Normal hemodynamic, ventilatory, and metabolic response to exercise. Arch Intern Med 1983; 143: 2173–2175. [PubMed] [Google Scholar]
- 21. Osada T, Katsumura T, Hamaoka T, Inoue S, Esaki K, Sakamoto A, Murase N, Kajiyama J, Shimomitsu T, Iwane H. Reduced blood flow in abdominal viscera measured by Doppler ultrasound during one‐legged knee extension. J Appl Physiol 1999; 86: 709–719. [DOI] [PubMed] [Google Scholar]
- 22. Bal‐Theoleyre L, Lalande A, Kober F, Giorgi R, Collart F, Piquet P, Habib G, Avierinos J‐F, Bernard M, Guye M, Jacquier A. Aortic function's adaptation in response to exercise‐induced stress assessing by 1.5 T MRI: a pilot study in healthy volunteers. PLoS ONE 2016; 11: e0157704. 10.1371/journal.pone.0157704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure: muscle blood is reduced with maintenance of arterial perfusion pressure. Circulation 1989; 80: 769–781. [DOI] [PubMed] [Google Scholar]
- 24. Puntawangkoon C, Kitzman DW, Kritchevsky SB, Hamilton CA, Nicklas B, Leng X, Brubaker PH, Hundley WG. Reduced peripheral arterial blood flow with preserved cardiac output during submaximal bicycle exercise in elderly heart failure. J Cardiovasc Magn Reson 2009; 11: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stöhr EJ, González‐Alonso J, Bezodis IN, Shave R. Left ventricular energetics: new insight into the plasticity of regional contributions at rest and during exercise. Am J Physiol Heart Circ Physiol 2014; 306: 225–232. [DOI] [PubMed] [Google Scholar]
- 26. Sunnerhagen KS, Bhargava V, Shabetai R. Regional left ventricular wall motion abnormalities in idiopathic dilated cardiomyopathy. Am J Cardiol 1990; 65: 364–370. [DOI] [PubMed] [Google Scholar]


