Abstract
Aims
Although soluble interleukin 2 receptor (sIL‐2R) is a potentially useful biomarker in the diagnosis and evaluation of disease severity in patients with sarcoidosis, its prognostic implication in patients with cardiac sarcoidosis (CS) is unclear. We sought to investigate whether sIL‐2R was associated with clinical outcomes and to clarify the relationship between sIL‐2R levels and disease activity in patients with CS.
Methods and results
We examined 83 consecutive patients with CS in our hospital who had available serum sIL‐2R data between May 2003 and February 2020. The primary outcome was a composite of advanced atrioventricular block, ventricular tachycardia or ventricular fibrillation, heart failure hospitalization, and all‐cause death. Inflammatory activity in the myocardium and lymph nodes was assessed by 18F‐fluorideoxyglucose positron emission tomography/computed tomography. During a median follow‐up period of 2.96 (IQR 2.24–4.27) years, the primary outcome occurred in 24 patients (29%). Higher serum sIL‐2R levels (>538 U/mL, the median) were significantly related to increased incidence of primary outcome (P = 0.037). Multivariable Cox regression analysis showed that a higher sIL‐2R was independently associated with an increased subsequent risk of adverse events (HR 3.71, 95% CI 1.63–8.44, P = 0.002), even after adjustment for significant covariates. sIL‐2R levels were significantly correlated to inflammatory activity in lymph nodes (r = 0.346, P = 0.003) but not the myocardium (r = 0.131, P = 0.27).
Conclusions
Increased sIL‐2R is associated with worse long‐term clinical outcomes accompanied by increased systemic inflammatory activity in CS patients.
Keywords: Cardiac sarcoidosis, Soluble interleukin 2 receptor, Prognosis, Positron emission tomography
Introduction
Sarcoidosis is a multisystemic inflammatory granulomatous disease, 1 and the presence of cardiac involvement can lead to congestive heart failure, atrioventricular block (AVB), ventricular tachycardia/ventricular fibrillation (VT/VF), and sudden cardiac death. Therefore, it is recognized as a key determinant of worse clinical outcomes in patients with sarcoidosis. 2 , 3 Although several reliable indicators of clinical outcome are available, such as left ventricular systolic function, and the extent of late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) imaging in patients with cardiac sarcoidosis (CS) has been reported, 4 , 5 there are few serological biomarkers with unequivocally demonstrated prognostic value.
Several studies have identified that biomarkers such as serum angiotensin converting enzyme (ACE), soluble interleukin 2 receptor (sIL‐2R) and lysozyme, and serum amyloid A (SAA) are useful in the diagnosis of sarcoidosis. 6 ACE is derived from epithelioid granulomas; therefore, its activity is useful in supporting a diagnosis and monitoring disease activity in patients with systemic sarcoidosis; however, there is actually little evidence available that ACE activity is significantly different between active and inactive sarcoidosis or that it reliably correlates with disease severity and clinical outcomes. 7 , 8 , 9 On the other hand, SAA and lysozyme have been found to be elevated in patients with sarcoidosis and have been reported to be more sensitive markers than ACE for the diagnosis of sarcoidosis; however, these have a major problem of low diagnostic specificity. 10
Serum sIL‐2R is presumed to be able to serve as a marker of activity on T lymphocytes in patients with sarcoidosis. 11 Notably, sIL‐2R is a more useful biomarker than ACE, C‐reactive protein (CRP), and SAA for estimating the severity of lung lesions in patients with sarcoidosis. 12 Furthermore, sIL‐2R is associated with the abnormal uptake of 18F‐fluorideoxyglucose in positron emission tomography/computed tomography (18F‐FDG PET/CT) in patients with sarcoidosis. 13 However, it remains to be seen whether sIL‐2R is associated with the severity and activity of disease in patients with CS. Accordingly, the aims of this study were to investigate whether sIL‐2R was associated with clinical outcomes and to clarify the relationship between sIL‐2R levels and disease activity evaluated by 18F‐FDG PET/CT in patients with CS.
Methods
Study design
This was a single‐centre, observational, retrospective study that included consecutive patients who were admitted for a work‐up of suspected CS and finally diagnosed with CS during the indexed hospitalization. CS was diagnosed based on the criteria specified in the guidelines of the Japanese Circulation Society. 14 Briefly, according to the guidelines, a definite diagnosis of CS was made on the basis of histopathological endomyocardial biopsy findings or clinical and/or histopathological findings meeting clinical cardiac criteria, with extracardiac involvement of at least one organ. The study protocol was approved by the Institutional Review Board of Hokkaido University Hospital for Clinical Research (018‐0225). The investigation was carried out in accordance with the principles outlined in the Declaration of Helsinki.
Study population
We examined 101 consecutive patients with CS who were admitted to the Hokkaido University Hospital between May 2003 and February 2020. Patients who had no data of serum sIL‐2R levels before initiation of immunosuppressive therapy (n = 18) were excluded. Ultimately, 83 patients were examined in this study (Figure 1 ).
Figure 1.

Flow diagram of the present study. CS, cardiac sarcoidosis; sIL‐2R, soluble interleukin 2 receptor
Echocardiography
Echocardiographic examinations were performed during hospitalization. Left ventricular (LV) end‐diastolic dimensions, LV end‐systolic dimensions, and LV ejection fraction (LVEF), as calculated from apical 2‐chamber and 4‐chamber views using the biplane method of disks, were measured. The data were evaluated by two experienced cardiologists.
Blood tests
Venous blood samples were obtained to measure serum ACE, sIL‐2R, CRP, and plasma B‐type natriuretic peptide (BNP). Serum was obtained after at least 30 min of clotting by centrifugation at 3500 g for 5 min and was stored at −80°C until analysis. Serum sIL‐2R levels were measured with an enzyme‐linked immunosorbent assay (until November 2009: Kyowa Medex Co., Tokyo, Japan, from November 2009: Hitachi Chemical Diagnostics Systems Co., Tokyo, Japan). The normal ranges in both assays were 200–459 U/mL.
Cardiac magnetic resonance imaging procedure and analysis
Cardiac magnetic resonance imaging was carried out using a 1.5T whole‐body scanner (Achieva, Philips Medical Systems, Best, the Netherlands) with a cardiac five‐channel phased‐array cardiac coil or a 3‐T whole‐body scanner (Achieva Tx, Philips Medical Systems) with a 32‐channel phased‐array receiver torso‐cardiac coil. Gadolinium‐enhanced CMR imaging was performed 10–15 min after intravenous administration of 0.1 mmol/kg gadolinium diethylenetriamine penta‐acetic acid (Magnevist, Bayer Yakuhin, Osaka, Japan) or gadobutrol (Gadovist, Bayer Yakuhin, Osaka, Japan). The LV short‐axis images were obtained using a fast‐field echo pulse sequence with inversion recovery with fat saturation or a phase‐sensitive inversion recovery sequence. The optimal inversion time was selected to null the signal from normal myocardium using a Look‐Locker sequence. Although 64 patients underwent CMR, we analysed 50 patients after excluding those without sufficient quality of imaging data to analyse CMR (n = 14).
We used commercially available software Ziostation2® (Ziosoft Inc., Tokyo, Japan) for quantitative analysis of LGE. The LGE was defined as the region with signal intensity ≥5 standard deviations (SD) above the mean CMR signal intensity of the normal remote myocardium. 15 The total amount of LGE was calculated as a percentage of the LV mass (%LGE).
Positron emission tomography imaging procedure and analysis
Positron emission tomography/CT imaging was acquired using a Gemini TF® PET/CT scanner (Philips Healthcare, Cleveland, OH) or a Biograph 64 TruePoint with TrueV® PET/CT scanner (Siemens Japan, Tokyo, Japan). Despite all patients undergoing a 18F‐FDG PET/CT scan, we analysed data from 73 patients after excluding those without sufficient quality of imaging data to analyse 18F‐FDG PET/CT (n = 10). All studied patients fasted for at least 18 h before 18F‐FDG PET/CT to suppress physiological myocardial 18F‐FDG uptake. 16 Eighteen patients received an injection with unfractionated heparin (UFH; 50 IU/kg, Mochida, Tokyo, Japan) alone, and 30 patients received a low‐carbohydrate diet (LCD) alone. The remaining 25 patients received both UFH and LCD.
We used the METAVOL® software system (Department of Diagnostic Imaging, Hokkaido University, Sapporo, Japan) to analyse the maximal standard uptake value (SUVmax), cardiac metabolic volume (CMV), cardiac metabolic activity (CMA), lymph node metabolic volume (LyMV), and total lymph node glycolysis (TLyG) (Figure 2 ), as described previously. 17 , 18 The CMV was defined as the myocardium volume within the boundary determined by the FDG uptake threshold (SUV mean of blood pool × 1.5). The blood pool activity was measured in the descending aorta using a 1 cm spherical volume‐of‐interest. CMA was calculated by multiplying CMV by the SUVmean. 19 The lymph node sarcoidosis lesions were evaluated using LyMV and TLyG. The LyMV was defined as the volume of the lymph node within the boundary determined by the FDG uptake threshold (SUV mean of blood pool × 1.5). TLyG was calculated by multiplying LyMV by the SUVmean.
Figure 2.
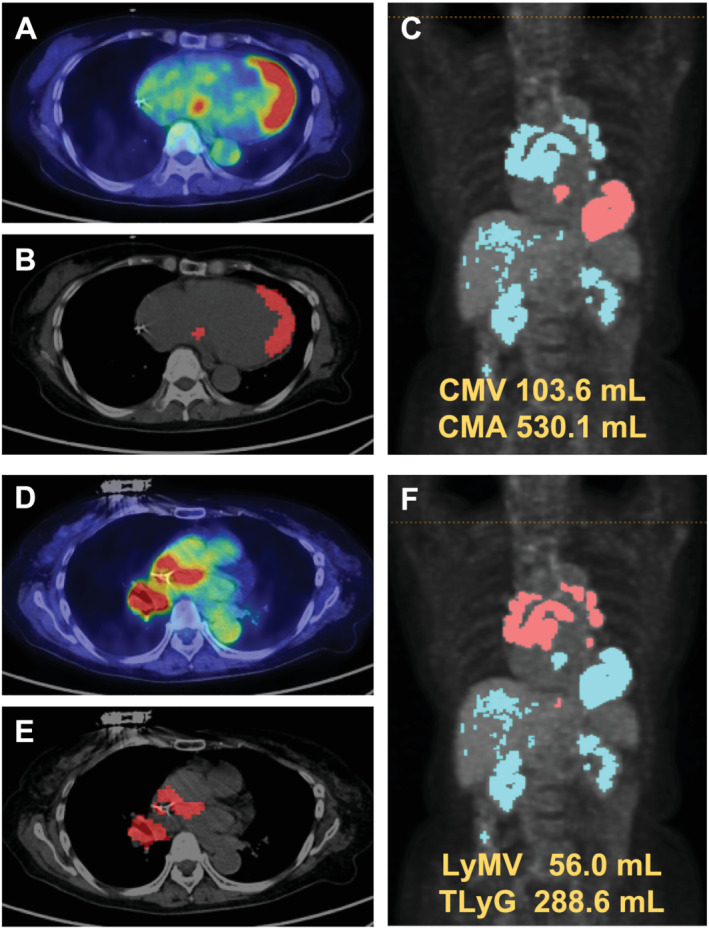
Representative case of FDG PET/CT. (A) Original image of abnormal myocardial FDG uptake. (B) Voxel image of abnormal myocardial FDG uptake. (C) Volume‐based image of abnormal myocardial FDG uptake. (D) Original image of lymph node FDG uptake. (E) Voxel image of lymph node FDG uptake. (F) Volume‐based image of lymph node FDG uptake. CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography
Clinical outcome
The primary outcome of interest was a composite of adverse outcomes, such as advanced AVB, VT/VF, heart failure hospitalization, and all‐cause death. Ventricular arrhythmic events were defined as either emergency treatments for VF or sustained VT including appropriate implantable cardioverter defibrillator therapies (shock or anti‐tachycardia pacing) or sustained VT with clinical symptoms and requiring admission. Advanced AVB was defined as AVB requiring permanent pacemaker implantation.
Statistical analyses
Continuous variables were presented as means ± standard deviations when normally distributed and as medians and interquartile ranges (IQR) when non‐normally distributed. Comparisons of differences between the two groups (high and low sIL‐2R levels) were performed using unpaired t‐tests or Mann–Whitney U tests for continuous variables and by χ 2 tests or Fisher's exact tests for dichotomous variables, when appropriate.
The long‐term cumulative incidence of the primary outcome was estimated using Kaplan–Meier curves, and a log‐rank test was performed to assess significance according to sIL‐2R value. To evaluate the influence of sIL‐2R on the primary outcome, we constructed multivariable Cox proportional hazard models. Several studies have reported that multicollinearity exists among LVEF, %LGE, and other strong determinants of clinical outcomes in patients with CS. 5 Correspondingly, we observed a multicollinearity between CMV and %LGE (r = 0.64, P < 0.0001), and CMV and BNP (r = 0.50, P < 0.0001) in this study. Thus, we constructed three multivariable Cox proportional hazard models. Adjustments for age, LVEF, estimated glomerular filtration rate (eGFR), and BNP; age, eGFR, and %LGE; and age, LVEF, eGFR, and CMV were made in models 1, 2, and 3, respectively. Complete case analyses were applied in all multivariable models.
We performed a receiver operating characteristic (ROC) analysis to determine the optimal cut‐off value of sIL‐2R for adverse events.
Multivariable linear regression analysis was performed based on the variables achieving P < 0.10 in an univariable linear regression analysis, to explore the strongest independent determinants of sIL‐2R. All tests were two tailed, and a value of P < .05 was considered statistically significant. All analyses were performed with Stata MP64 version 15 (StataCorp, College Station, TX).
Results
Baseline characteristics
Baseline characteristics of all 83 studied patients are summarized in Table 1 . Patients with a higher sIL‐2R (>538 U/mL, the median) had a higher prevalence of diabetes mellitus, serum CRP, and ACE levels. There were no significant differences between the groups in terms of age, body mass index, prevalence of coronary artery disease, LVEF, plasma BNP levels, and use of oral medications.
Table 1.
Baseline characteristics
| Variable | Overall | Low sIL‐2R | High sIL‐2R | P value |
|---|---|---|---|---|
| ≤538 U/mL | >538 U/mL | |||
| Number | 83 | 42 | 41 | |
| Age (years) | 61 (52–68) | 60 (51–66) | 63 (55–70) | 0.91 |
| Female | 63 (76) | 33 (79) | 30 (73) | 0.57 |
| Body mass index (kg/m2) | 21.8 (20.2–24.2) | 21.9 (21.1–24.2) | 21.5 (20.1–23.9) | 0.54 |
| Organ involvement | ||||
| Lung | 67 (81) | 35 (83) | 32 (78) | 0.54 |
| Skin | 20 (24) | 7 (16) | 13 (31) | 0.11 |
| Eye | 38 (46) | 16 (38) | 22 (54) | 0.16 |
| Number of involved organs | 3.2 ± 1.5 | 3.0 ± 1.4 | 3.3 ± 1.6 | 0.50 |
| Past history | ||||
| CAD | 3 (4) | 2 (5) | 1 (2) | 0.57 |
| VT | 7 (8) | 6 (14) | 1 (2) | 0.052 |
| Echocardiography | ||||
| LVEF (%) missing 6/83 | 44 (38–62) | 43 (37–61) | 50 (38–63) | 0.46 |
| LVDD (mm) missing 1/83 | 50 (45–57) | 51 (46–58) | 49 (45–55) | 0.49 |
| LVDS (mm) missing 2/83 | 34 (29–47) | 36 (30–47) | 34 (29–47) | 0.82 |
| IVS wall thinning missing 1/83 | 34 (41) | 19 (46) | 15 (37) | 0.37 |
| Laboratory data | ||||
| Haemoglobin (g/dL) | 13.5 ± 1.5 | 13.3 ± 1.6 | 13.7 ± 1.5 | 0.24 |
| CRP (mg/dL) | 0.06 (0.04–0.19) | 0.05 (0.02–0.08) | 0.14 (0.06–0.34) | <0.001 |
| eGFR (mL/min/1/73 m2) | 69.6 ± 17.9 | 69.3 ± 17.1 | 70.0 ± 18.8 | 0.86 |
| BNP (pg/mL) missing 14/83 | 74 (28–179) | 78 (28–180) | 72 (18–170) | 0.97 |
| ACE (U/L) missing 1/83 | 15.2 (11.2–18.4) | 12.6 (9.1–16) | 17.4 (14.1–21.4) | <0.001 |
| sIL2‐R (U/mL) | 538 (381–751) | 389 (317–464) | 751 (660–911) | <0.001 |
| Medications | ||||
| ACE‐inhibitors/ARBs | 48 (58) | 25 (60) | 23 (56) | 0.75 |
| Beta‐blockers | 44 (53) | 26 (62) | 18 (44) | 0.10 |
| Immunosuppressive therapy | 70 (84) | 34 (81) | 36 (89) | 0.39 |
| Amiodarone | 11 (13) | 9 (21) | 2 (5) | 0.03 |
| CMR | ||||
| Missing 33/83 | ||||
| LGE burden (%) | 14.8 (4.4–24.6) | 15.2 (3.3–25.4) | 13.2 (5.3–22.5) | 0.94 |
| 18F‐FDG PET/CT | ||||
| Missing 10/83 | ||||
| FDG dose (MBq) | 245.5 (216.6–276.3) | 243.2 (217.5–292) | 245.7 (215–268.9) | 0.51 |
| SUVmax | 10.8 (7.9–13.6) | 10.2 (6.8–13.6) | 11.0 (8.8–13.8) | 0.23 |
| CMV (mL) | 56.1 (10.4–104.5) | 29.5 (8.1–95.0) | 66.6 (23.5–134.4) | 0.14 |
| CMA (mL) | 174.3 (41.2–411.1) | 133.5 (28.2–411.1) | 259.6 (75.9–411.5) | 0.19 |
| LyMV (mL) | 56.0 (20.5–119.4) | 30.8 (3.5–91.5) | 80.1 (44.2–123.1) | 0.004 |
| TLyG (mL) | 215.1 (78.0–500.8) | 165.5 (13.1–411.9) | 351.9 (162.5–552.9) | 0.01 |
ACE, angiotensin converting enzyme; ARBs, angiotensin II receptor blockers; BNP, B‐type natriuretic peptide; CAD, coronary artery disease; CMA, cardiac metabolic activity; CMR, cardiac magnetic resonance; CMV, cardiac metabolic volume; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; FDG, fluorodeoxyglucose; IVS, interventricular septum; LGE, late gadolinium enhancement; LyMV, lymph node metabolic volume; LVDD, left ventricular end‐diastolic diameter; LVDS, left ventricular end‐systolic diameter; LVEF, left ventricular ejection fraction; PET, positron emission tomography; sIL2‐R, soluble interleukin‐2 receptor; SUV, standardized uptake value; TLyG, total lymph node glycolysis; VT, ventricular tachycardia.
Continuous variables are presented as mean ± standard deviation if normally distributed and median (interquartile range) if not normally distributed. Categorical variables are presented as number of patients (%).
Soluble interleukin 2 receptor levels and clinical outcomes
During a median follow‐up period of 2.96 (IQR 2.24–4.27) years, the primary outcome occurred in 24 patients (29%), including 1 advanced AVB, 13 VT/VF, 5 hospitalizations for heart failure, and 5 all‐cause deaths. Kaplan–Meier analyses showed that the primary outcome occurred more frequently in patients with higher sIL‐2R levels than in those with lower sIL‐2R levels (P = 0.037) (Figure 3 ). Notably, among the 24 patients with primary outcome, 12 patients developed an adverse event during within 1 year (10 VT/VF, 1 hospitalizations for heart failure, and 1 all cause death). Kaplan–Meier analysis showed that the primary outcome occurred more frequently in patients with higher sIL‐2R levels than those with lower sIL‐2R levels (P = 0.01). A multivariable Cox regression analysis revealed that a higher sIL‐2R level was independently associated with an increased subsequent risk of adverse events, even after adjustments for age, BNP, eGFR, CMV, LVEF, and %LGE, which are known to be strong determinants of clinical outcomes in patients with CS (Model 1 [n = 65, Event = 19], HR 3.71, 95% CI 1.63–8.44, P = 0.002; Model 2 [n = 50, Event = 12], HR 3.48, 95% CI 1.34–9.08, P = 0.01; Model 3 [n = 68, Event = 20], HR 3.04, 95% CI 1.18–7.82, P = 0.02) (Table 2 ). Harrell's c‐indices of the models 1, 2, and 3 were 0.79, 0.85, and 0.80, respectively. Based on ROC analysis, the optimal cut‐off value of sIL‐2R for the incidence of adverse events was 811 U/mL (sensitivity 45.8%, specificity 89.8%), and c‐index was 0.65 (95% CI 0.50–0.80). The hazard ratio was 4.90 (95% CI 2.17–11.04, P < 0.001) when the sIL‐2R was above 811 U/mL.
Figure 3.
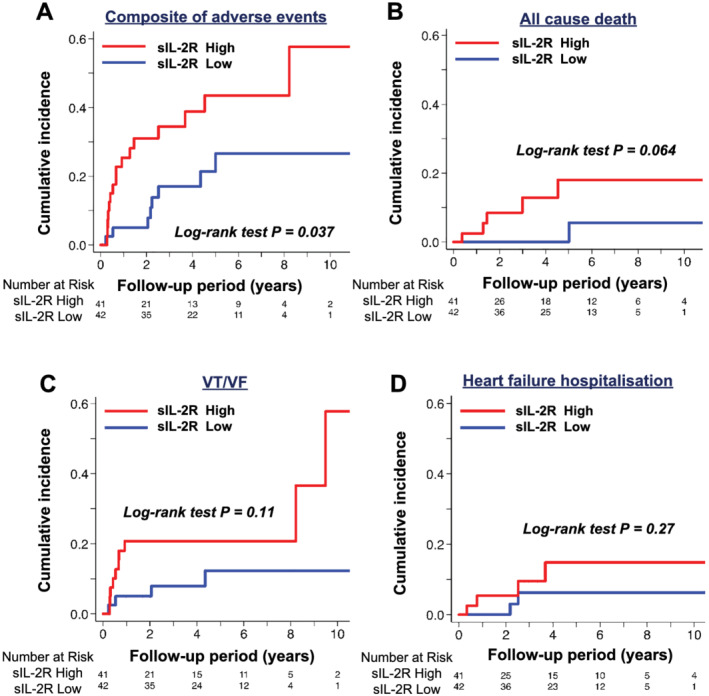
Survival analyses of long‐term clinical outcomes of patients with cardiac sarcoidosis categorized by sIL‐2R. (A) Composite of advanced atrioventricular block, VT/VF, heart failure hospitalization, and all‐cause death. (B) All‐cause death. (C) VT/VF. (D) Heart failure hospitalization. sIL‐2R, soluble interleukin 2 receptor; VF, ventricular fibrillation; VT, ventricular tachycardia
Table 2.
Cox proportional hazard model for composite of adverse events
| Univariable | Multivariable | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||||||
| Variable | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value |
| Age | 0.99 | 0.96–1.02 | 0.55 | 0.94 | 0.89–1.00 | 0.07 | 0.92 | 0.86–1.01 | 0.06 | 0.97 | 0.91–1.02 | 0.20 |
| Female | 1.02 | 0.40–2.58 | 0.97 | |||||||||
| History of VT | 1.82 | 0.42–7.94 | 0.42 | |||||||||
| Amiodarone | 1.88 | 0.70–5.06 | 0.21 | |||||||||
| Immunosuppressive therapy | 2.35 | 0.87–6.35 | 0.09 | |||||||||
| LVEF (%) | 0.97 | 0.94–0.99 | 0.02 | 0.97 | 0.92–1.01 | 0.13 | 0.98 | 0.95–1.01 | 0.23 | |||
| eGFR (mL/min/1.73 m2) | 0.97 | 0.95–0998 | 0.03 | 0.94 | 0.91–0.99 | 0.008 | 0.93 | 0.88–0.98 | 0.01 | 0.94 | 0.90–0.98 | 0.002 |
| CRP (mg/dL) | 1.06 | 0.58–1.95 | 0.84 | |||||||||
| Log BNP (pg/mL) | 1.48 | 1.07–2.05 | 0.02 | 1.32 | 0.83–2.12 | 0.24 | ||||||
| Log sIL‐2R (U/mL) | 3.02 | 1.51–6.06 | 0.002 | 3.71 | 1.63–8.44 | 0.002 | 3.48 | 1.34–9.08 | 0.01 | 3.04 | 1.18–7.82 | 0.02 |
| LGE burden (%) | 1.05 | 1.02–1.09 | 0.003 | 1.06 | 1.02–1.10 | 0.005 | ||||||
| SUVmax | 1.01 | 0.92–1.12 | 0.76 | |||||||||
| CMV (mL) | 1.01 | 1.00–1.01 | 0.02 | 1.01 | 1.00–1.01 | 0.01 | ||||||
| CMA (mL) | 1.00 | 1.00–1.002 | 0.07 | |||||||||
| LyMV (mL) | 1,00 | 1.00–1.01 | 0.04 | |||||||||
| TLyG (mL) | 1.00 | 1.00–1.002 | 0.17 | |||||||||
BNP, B‐type natriuretic peptide; CI, confidence interval; CMA, cardiac metabolic activity; CMV, cardiac metabolic volume; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LGE, late gadolinium enhancement; LyMV, lymph node metabolic volume; LVEF, left ventricular ejection fraction; SUV, standardized uptake value; TLyG, total lymph node glycolysis; sIL2‐R, soluble interleukin‐2 receptor; VT, ventricular tachycardia.
Model 1: adjusted for age, left ventricular ejection fraction, estimated glomerular filtration rate and B‐type natriuretic peptide. Model 2: adjusted for age, estimated glomerular filtration rate and late gadolinium enhancement burden. Model 3: adjusted for age, left ventricular ejection fraction, estimated glomerular filtration rate, cardiac metabolic volume.
Relationships between serum soluble interleukin 2 receptor levels and clinical variables
The association between sIL‐2R and variables of PET is shown in Figure 4 . There was a modest positive correlation between sIL‐2R levels and LyMV or TLyG (r = 0.438, P = 0.00001; r = 0.346, P = 0.003, respectively), whereas there was no significant correlation between sIL‐2R and CMV or CMA (r = 0.224, P = 0.06; r = 0.131, P = 0.271, respectively). In the multivariable linear regression analyses, LyMV and TLyG were independent determinants of sIL‐2R (β = 2.50, P = 0.002, β = 0.41, P = 0.04, respectively), even after adjustment for age and SUVmax (Table 3 ).
Figure 4.
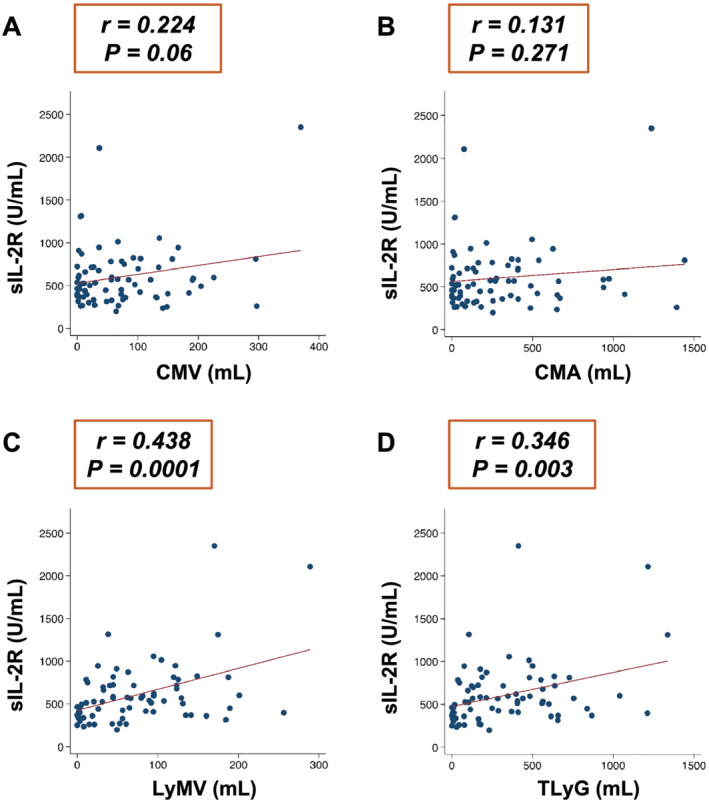
Relationship between the respective covariates and sIL‐2R. (A) CMV. (B) CMA. (C) LyMV. (D) TLyG. CMA, cardiac metabolic activity; CMV, cardiac metabolic volume; LyMV, lymph node metabolic volume; TLyG, total lymph node glycolysis
Table 3.
Linear regression analyses for sIL‐2R levels
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Model 1: LyMV | Model 2: TLyG | |||||
| Variable | β coefficient | P value | β coefficient | P value | β coefficient | P value |
| Age | −16.79 | 0.006 | −4.96 | 0.13 | −5.88 | 0.09 |
| LVEF (%) | 3.22 | 0.56 | Not selected | Not selected | ||
| Haemoglobin (g/dL) | 30.79 | 0.56 | Not selected | Not selected | ||
| CRP (mg/mL) | 168.55 | 0.21 | Not selected | Not selected | ||
| eGFR (mL/min/1.73 m2) | 5.54 | 0.21 | Not selected | Not selected | ||
| BNP (pg/mL) | −0.14 | 0.74 | Not selected | Not selected | ||
| LGE (%) | −4.64 | 0.61 | Not selected | Not selected | ||
| SUVmax | 16.19 | 0.048 | −3.40 | 0.72 | −2.77 | 0.81 |
| CMV (mL) | 1.03 | 0.06 | Not selected | Not selected | ||
| CMA (mL) | 0.13 | 0.27 | Not selected | Not selected | ||
| LyMV (mL) | 2.46 | <0.001 | 2.50 | 0.002 | ‐ | |
| TLyG (mL) | 0.39 | 0.003 | ‐ | 0.41 | 0.04 | |
BNP, B‐type natriuretic peptide; CMA, cardiac metabolic activity; CMV, cardiac metabolic volume; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; LGE, late gadolinium enhancement; LyMV, lymph node metabolic volume; LVEF, left ventricular ejection fraction; SUV, standardized uptake value; TLyG, total lymph node glycolysis; sIL2‐R, soluble interleukin‐2 receptor.
Model 1: adjusted for age, maximal standard uptake value and lymph node metabolic volume. Model 2: adjusted for age, maximal standard uptake value and total lymph node glycolysis.
Discussion
In the present study, we demonstrated that a higher serum sIL‐2R level was significantly associated with subsequent worse clinical outcomes in patients with CS. Furthermore, sIL‐2R levels were correlated to disease activities in lymph nodes rather than in the myocardium, as assessed by 18F‐FDG PET/CT, reflecting the systemic inflammatory activities of sarcoidosis.
Although the aetiology of sarcoidosis is unclear, it is thought that some antigens cause inflammation, which affects multiple organs. 20 This inflammation leads to the activation of monocyte‐macrophages, which in turn release cytokines, such as tumour necrosis factor‐α, interleukin‐1 (IL‐1), and interleukin‐6 (IL‐6). 21 These cytokines, mainly IL‐1 and IL‐6, stimulate the production of IL‐2, which promotes T cell activation. 22 Finally, activated T cells release the 55‐kDa protein, sIL‐2R. 23 Eurelings et al. reported that sIL‐2R was superior to ACE in screening for sarcoidosis (c‐index = 0.91 vs. 0.68, sensitivity 88%, specificity 85%, cut‐off value 3550 pg/mL) according to the analysis of 189 patients with suspected but unproven or unexcluded sarcoidosis. 24 Moreover, sIL‐2R could be a more sensitive marker than lysozyme and ACE in patients with sarcoidosis who visited the dermatology clinic. 25 Notably, sIL‐2R was significantly higher in patients with multiple organ involvements and parenchymal infiltrates, suggesting that sIL‐2R could be an indicator of systemic disease activity. 25 In fact, there are several reports that sIL‐2R is useful in assessing the severity of sarcoidosis, especially in pulmonary sarcoidosis. 12 , 26 , 27 Rothkrantz‐Kos et al. revealed that sIL‐2R appeared to be the best marker for estimating the severity of disease compared to ACE, high‐sensitivity CRP, and SAA in patients with untreated pulmonary sarcoidosis. 12 Given its value in outcome prediction, sIL‐2R would serve as a prognostic biomarker for progression and relapse in patients with sarcoidosis. 26 , 27
Among sarcoidosis patients with no clinical indication for steroid treatment at the time of diagnosis, approximately 40% of those with a high level of sIL‐2R had subsequent progression of disease, whereas those with a normal level of sIL‐2R had no progression. 26 In addition, a high level of sIL‐2R before initiation of infliximab, which is considered as a third‐line therapeutic option for severe sarcoidosis, is significant predictor of relapse (HR 2.24, 95% CI 1.07 to 4.68). 27 Taking these results together, sIL‐2R may further aid in the diagnosis and risk stratification of sarcoidosis compared with other markers such as ACE, lysozyme, and SAA.
To the best of our knowledge, this is the first report to examine the association between serum sIL‐2R levels and adverse outcomes in patients with CS. A possible explanation for this association includes a significant correlation between levels of sIL‐2R and LyMV or TLyG rather than CMV or CMA, as assessed by 18F‐FDG PET/CT. 18F‐FDG PET/CT is a useful modality in the diagnosis of CS and may be further associated with prognosis in patients with CS. Several studies have reported the usefulness of FDG uptake on PET/CT for risk stratification among patients with CS. 28 , 29 Blankstein et al. showed that the presence of abnormal FDG uptake on PET/CT, especially in the right ventricle, was significantly associated with VT/VF or death. 28 However, isolated sarcoid lesions in the right ventricle are relatively rare, suggesting that sarcoid lesions in the right ventricle follow left ventricular lesions and may be a more advanced stage of CS. 30 , 31 Ahmadian et al. demonstrated that CMA was independently associated with adverse events in patients with CS. 32 In contrast, Manabe et al. have reported that CMV and CMA, assessed with quantitative analysis of 18F‐FDG PET/CT, were not significantly related to adverse events in such patients. 33 Therefore, whether quantitative measures of FDG volume intensity, such as CMV and CMA, are associated with adverse outcomes in patients with CS remains controversial. In addition, it should be noted that LGE on CMR imaging is associated with VT/VF, whereas FDG uptake in the myocardium was not associated with VT/VF. 34 In a study with 50 CS patients, 19% of the studied patients with positive findings in both LGE and PET and 21% of those with positive in LGE and negative in PET had subsequent VTVF events, whereas none of those with negative in LGE and positive in PET experienced VTVF events, indicating FDG uptake in the myocardium may not have additional prognostic value if CMR is available. 34 In addition, the most common mechanism of VT/VF is likely to be re‐entrant arrhythmias associated with areas of scar tissue. 35 These findings indicate the limited prognostic implication of local inflammatory activity on the myocardium. In our study, we highlighted that sIL‐2R was significantly associated with inflammation in the lymph nodes throughout the body as a reflection of systemic inflammatory activity but not with inflammation in the myocardium. Given the diagnostic and prognostic value of inflammatory activities in patients with CS, it would be important to evaluate systemic inflammation as well as inflammation in the myocardium. Collectively, elevated sIL‐2R levels, which have an additive prognostic value to the extent of LGE, reflect a high level of systemic inflammation with advanced stage of CS.
Study limitations
There are several potential limitations of the present study that should be acknowledged. First, this was a single‐centre study with a relatively small sample size, thereby limiting the ability to generalize the findings and the statistical power for detecting differences in negative data. Therefore, a larger‐scale multicentre prospective study is warranted to confirm the relationship between sIL‐2R levels and the subsequent adverse outcomes. Second, there was unavoidable bias in this study because 10 and 33 patients were excluded from the analyses of PET and CMR imaging, respectively. Third, we have constructed multivariable models with complete case analyses. Although Harrell's c‐indices showed sufficient discriminative value in the multivariable models, the stability of the models might not be guaranteed. Fourth, because the optimal cut‐off value of sIL‐2R for the adverse events was derived from a relatively small number of study subjects, this cut‐off value might not be able to generalize. Fifth, we did not assess the serial changes of sIL‐2R levels and PET findings after immunosuppressive therapy; therefore, the association between the changes in sIL‐2R levels and adverse events after immunosuppressive therapy is unclear. Sixth, although the Kaplan–Meier curve spreads quite early, suggesting that higher sIL‐2R levels were associated with adverse events within a year rather than long‐term events, we could not assess the association of higher sIL‐2R levels with adverse events within a year because the number of adverse events in this duration were limited to 12. Finally, because patients without cardiac implantable electronic devices were not always monitored by 24 h electrocardiography, several ventricular arrhythmias might have been underestimated. Hence, the study endpoints regarding arrhythmic events were defined either as treatments for termination of VF or sustained VT including appropriate treatments with implantable cardioverter defibrillators or bradyarrhythmias requiring permanent pacemaker implantation.
Conclusions
A higher serum sIL‐2R level before initiation of immunosuppressive therapy was associated with worse long‐term clinical outcomes reflecting elevated systemic inflammatory activities in patients with CS. These findings suggest the importance of assessing sIL‐2R as a surrogate marker for further risk stratification in these patients.
Conflict of interest
None declared.
Funding
This work was supported by a Grant‐in‐Aid for Young Scientists from the Japan Society for the Promotion of Science (T.N., 15K19402) and a Grant from the Japan Heart Foundation (T.N.).
Acknowledgements
The authors are grateful for the contributions of all the investigators, clinical research coordinators, and data managers.
Kobayashi, Y. , Sato, T. , Nagai, T. , Hirata, K. , Tsuneta, S. , Kato, Y. , Komoriyama, H. , Kamiya, K. , Konishi, T. , Omote, K. , Ohira, H. , Kudo, K. , Konno, S. , and Anzai, T. (2021) Association of high serum soluble interleukin 2 receptor levels with risk of adverse events in cardiac sarcoidosis. ESC Heart Failure, 8: 5282–5292. 10.1002/ehf2.13614.
References
- 1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357: 2153–2165. [DOI] [PubMed] [Google Scholar]
- 2. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller‐Quernheim J. Sarcoidosis. Lancet 2014; 383: 1155–1167. [DOI] [PubMed] [Google Scholar]
- 3. Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, Kokkonen J, Pelkonen M, Pietila‐Effati P, Utrianen S, Kupari M. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015; 131: 624–632. [DOI] [PubMed] [Google Scholar]
- 4. Nagai T, Kohsaka S, Okuda S, Anzai T, Asano K, Fukuda K. Incidence and prognostic significance of myocardial late gadolinium enhancement in patients with sarcoidosis without cardiac manifestation. Chest. 2014; 146: 1064–1072. [DOI] [PubMed] [Google Scholar]
- 5. Coleman GC, Shaw PW, Balfour PC Jr, Gonzalez JA, Kramer CM, Patel AR, Salerno M. Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017; 10: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casanova N, Zhou T, Knox KS, Garcia JGN. Identifying novel biomarkers in sarcoidosis using genome‐based approaches. Clin Chest Med. 2015; 36: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmadzai H, Cameron B, Chui J, Lloyd A, Wakefield D, Thomas PS. Measurement of neopterin, TGF‐beta1 and ACE in the exhaled breath condensate of patients with sarcoidosis. J Breath Res. 2013; 7: 046003. [DOI] [PubMed] [Google Scholar]
- 8. Gungor S, Ozseker F, Yalcinsoy M, Akkaya E, Can G, Eroglu H, Genc NS. Conventional markers in determination of activity of sarcoidosis. Int Immunopharmacol. 2015; 25: 174–179. [DOI] [PubMed] [Google Scholar]
- 9. Jain R, Yadav D, Puranik N, Guleria R, Jin JO. Sarcoidosis: Causes, Diagnosis, Clinical Features, and Treatments. J Clin Med. 2020; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bons JA, Drent M, Bouwman FG, Mariman EC, van Dieijen‐Visser MP, Wodzig WK. Potential biomarkers for diagnosis of sarcoidosis using proteomics in serum. Respir Med. 2007; 101: 1687–1695. [DOI] [PubMed] [Google Scholar]
- 11. Grutters JC, Fellrath JM, Mulder L, Janssen R, van den Bosch JM, van Velzen‐Blad H. Serum soluble interleukin‐2 receptor measurement in patients with sarcoidosis: a clinical evaluation. Chest. 2003; 124: 186–195. [DOI] [PubMed] [Google Scholar]
- 12. Rothkrantz‐Kos S, van Dieijen‐Visser MP, Mulder PG, Drent M. Potential usefulness of inflammatory markers to monitor respiratory functional impairment in sarcoidosis. Clin Chem. 2003; 49: 1510–1517. [DOI] [PubMed] [Google Scholar]
- 13. Mostard RL, Voo S, van Kroonenburgh MJ, Verschakelen JA, Wijnen PA, Nelemans PJ, Erckens RJ, Drent M. Inflammatory activity assessment by F18 FDG‐PET/CT in persistent symptomatic sarcoidosis. Respir Med. 2011; 105: 1917–1924. [DOI] [PubMed] [Google Scholar]
- 14. Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, Inomata T, Ishibashi Ueda H, Eishi Y, Kitakaze M, Kusano K, Sakata Y, Shijubo N, Tsuchida A, Tsutsui H, Nakajima T, Nakatani S, Horii T, Yazaki Y, Yamaguchi E, Yamaguchi T, Ide T, Okamura H, Kato Y, Goya M, Sakakibara M, Soejima K, Nagai T, Nakamura H, Noda T, Hasegawa T, Morita H, Ohe T, Kihara Y, Saito Y, Sugiyama Y, Morimoto SI, Yamashina A, Japanese Circulation Society Joint Working G . JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis‐digest version. Circ J. 2019; 83: 2329–2388. [DOI] [PubMed] [Google Scholar]
- 15. Murtagh G, Laffin LJ, Beshai JF, Maffessanti F, Bonham CA, Patel AV, Yu Z, Addetia K, Mor‐Avi V, Moss JD, Hogarth DK, Sweiss NJ, Lang RM, Patel AR. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2016; 9: e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manabe O, Yoshinaga K, Ohira H, Masuda A, Sato T, Tsujino I, Yamada A, Oyama‐Manabe N, Hirata K, Nishimura M, Tamaki N. The effects of 18‐h fasting with low‐carbohydrate diet preparation on suppressed physiological myocardial (18)F‐fluorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis. J Nucl Cardiol. 2016; 23: 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirata K, Kobayashi K, Wong KP, Manabe O, Surmak A, Tamaki N, Huang SC. A semi‐automated technique determining the liver standardized uptake value reference for tumor delineation in FDG PET‐CT. PLoS One. 2014; 9: e105682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller RJH, Cadet S, Pournazari P, Pope A, Kransdorf E, Hamilton MA, Patel J, Hayes S, Friedman J, Thomson L, Tamarappoo B, Berman DS, Slomka PJ. Quantitative assessment of cardiac hypermetabolism and perfusion for diagnosis of cardiac sarcoidosis. J Nucl Cardiol. 2020. 10.1007/s12350-020-02201-5 [DOI] [PubMed] [Google Scholar]
- 19. Manabe O, Kroenke M, Aikawa T, Murayama A, Naya M, Masuda A, Oyama‐Manabe N, Hirata K, Watanabe S, Shiga T, Katoh C, Tamaki N. Volume‐based glucose metabolic analysis of FDG PET/CT: The optimum threshold and conditions to suppress physiological myocardial uptake. J Nucl Cardiol. 2019; 26: 909–918. [DOI] [PubMed] [Google Scholar]
- 20. Baughman RP, Lower EE, du Bois RM. Sarcoidosis. The Lancet. 2003; 361: 1111–1118. [DOI] [PubMed] [Google Scholar]
- 21. Authier FJ, Mhiri C, Chazaud B, Christov C, Cherin P, Barlovatz‐Meimon G, Gherardi RK. Interleukin‐1 expression in inflammatory myopathies: evidence of marked immunoreactivity in sarcoid granulomas and muscle fibres showing ischaemic and regenerative changes. Neuropathol Appl Neurobiol. 1997; 23: 132–140. [PubMed] [Google Scholar]
- 22. Vink A, Uyttenhove C, Wauters P, Van Snick J. Accessory factors involved in murine T cell activation. Distinct roles of interleukin 6, interleukin 1 and tumor necrosis factor. Eur J Immunol. 1990; 20: 1–6. [DOI] [PubMed] [Google Scholar]
- 23. Kumar A, Moreau JL, Gibert M, Theze J. Internalization of interleukin 2 (IL‐2) by high affinity IL‐2 receptors is required for the growth of IL‐2‐dependent T cell lines. J Immunol. 1987; 139: 3680–3684. [PubMed] [Google Scholar]
- 24. Eurelings LEM, Miedema JR, Dalm V, van Daele PLA, van Hagen PM, van Laar JAM, Dik WA. Sensitivity and specificity of serum soluble interleukin‐2 receptor for diagnosing sarcoidosis in a population of patients suspected of sarcoidosis. PLoS One. 2019; 14: e0223897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thi Hong Nguyen C, Kambe N, Kishimoto I, Ueda‐Hayakawa I, Okamoto H. Serum soluble interleukin‐2 receptor level is more sensitive than angiotensin‐converting enzyme or lysozyme for diagnosis of sarcoidosis and may be a marker of multiple organ involvement. J Dermatol. 2017; 44: 789–797. [DOI] [PubMed] [Google Scholar]
- 26. Ziegenhagen MW, Benner UK, Zissel G, Zabel P, Schlaak M, Muller‐Quernheim J. Sarcoidosis: TNF‐alpha release from alveolar macrophages and serum level of sIL‐2R are prognostic markers. Am J Respir Crit Care Med. 1997; 156: 1586–1592. [DOI] [PubMed] [Google Scholar]
- 27. Vorselaars AD, Verwoerd A, van Moorsel CH, Keijsers RG, Rijkers GT, Grutters JC. Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. Eur Respir J. 2014; 43: 602–609. [DOI] [PubMed] [Google Scholar]
- 28. Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014; 63: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mc Ardle BA, Birnie DH, Klein R, de Kemp RA, Leung E, Renaud J, DaSilva J, Wells GA, Beanlands RS, Nery PB. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by (1)(8)F‐ fluorodoexyglucose positron emission tomography? Circ Cardiovasc Imaging. 2013; 6: 617–626. [DOI] [PubMed] [Google Scholar]
- 30. Okada DR, Smith J, Derakhshan A, Gowani Z, Misra S, Berger RD, Calkins H, Tandri H, Chrispin J. Ventricular arrhythmias in cardiac sarcoidosis. Circulation. 2018; 138: 1253–1264. [DOI] [PubMed] [Google Scholar]
- 31. Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol. 2009; 104: 571–577. [DOI] [PubMed] [Google Scholar]
- 32. Ahmadian A, Brogan A, Berman J, Sverdlov AL, Mercier G, Mazzini M, Govender P, Ruberg FL, Miller EJ. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014; 21: 925–939. [DOI] [PubMed] [Google Scholar]
- 33. Manabe O, Koyanagawa K, Hirata K, Oyama‐Manabe N, Ohira H, Aikawa T, Furuya S, Naya M, Tsujino I, Tomiyama Y, Otaki Y, Anzai T, Tamaki N. Prognostic value of (18)F‐FDG PET using texture analysis in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2020; 13: 1096–1097. [DOI] [PubMed] [Google Scholar]
- 34. Gowani Z, Habibi M, Okada DR, Smith J, Derakhshan A, Zimmerman SL, Misra S, Gilotra NA, Berger RD, Calkins H, Tandri H, Chrispin J. Utility of cardiac magnetic resonance imaging versus cardiac positron emission tomography for risk stratification for ventricular arrhythmias in patients with cardiac sarcoidosis. Am J Cardiol. 2020; 134: 123–129. [DOI] [PubMed] [Google Scholar]
- 35. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014; 11: 1305–1323. [DOI] [PubMed] [Google Scholar]


