Abstract
Aims
In ambulatory patients with chronic heart failure (HF), congestion and decongestion assessment may be challenging. The aim of this study is to assess the value of lung ultrasound (LUS) in outpatients with HF in characterizing decompensation and recompensation, and in outcomes prediction.
Methods and results
Heart failure outpatients attended to establish HF decompensation were included. LUS was blindly performed at baseline (LUS1) and at clinical recompensation (LUS2). B‐lines were counted in eight scanned areas. Diagnosis of no HF decompensation vs. right‐sided, left‐sided, or global HF decompensation, and patients' management were performed by physicians blinded to LUS1. Outcome was the composite of all‐cause death or HF‐related hospitalization. Two hundred and thirty‐three suspicions of HF decompensation were included in 187 patients (71.4 ± 11.3 years, 66.8% men). Mean B‐line (LUS1) was 17.6 ± 11.2 vs. 3.7 ± 4.5 for episodes with and without HF decompensation, respectively (P < 0.001). Global HF decompensation showed the highest number of B‐lines (20.6 ± 11), followed by left‐sided (19.7 ± 11.6) and right‐sided (13.5 ± 9.8). B‐lines declined to 6.9 ± 6.7 (LUS2) (P < 0.001 vs. LUS1) after treatment, within a mean time of 24.2 ± 23.7 days [median 13.5 days (interquartile range 6–40)]. B‐lines were significantly associated with the composite endpoint at 30 days (hazard ratio [HR] 1.04 [95% confidence interval 1.01–1.07], P = 0.02), but not at 60 (P = 0.22) or 180 days (P = 0.54). In multivariable analysis, B‐line number remained as an independent predictor of the composite endpoint at 30 days, [HR 1.04 (1.01–1.07), P = 0.014], with a 4% increase risk per B‐line added. B‐lines correlated significantly with CA125 (R = 0.30, P = 0.001).
Conclusions
Lung ultrasound supports the diagnostic work‐up of congestion and decongestion in chronic HF outpatients and identifies patients at high risk of short‐term events.
Keywords: Heart failure, Lung ultrasound, Pulmonary congestion, Prognosis
Introduction
In outpatients with chronic heart failure (HF), an unplanned hospitalization is one of the strongest predictors of increased mortality. 1 Early detection of systemic congestion is key to improving patients' management, because it allows adjusting outpatient diuretic treatment and therefore avoid hospital admissions, but it might be difficult based only in clinical assessment and can be reliant on physicians' skills.
Clinical anamnesis and physical examination are cornerstones of congestion evaluation, but both have several limitations, showing high specificity but low sensitivity. 2 , 3 In this context, several decompensation risk scores to rule out congestion have been developed to improve patient's fluid status evaluation; however, they have built‐in subjective, highly variable measures that may not be applicable at initial stages and which diagnostic performance is at most intermediate. Therefore, diagnosing HF is a challenge, especially when based on clinical examination, as considerable diagnostic uncertainty may remain. Even in the absence of clinical findings of congestion, patients with HF may have elevated left ventricular filling pressures, and decompensation may not be timely recognized; on the other hand, symptoms such as fatigue, dizziness, or shortness of breath may appear in scenarios other than HF.
Complementary tests in addition to clinical evaluation can improve the diagnosis approach of patients with congestion, but not always are available in the outpatient setting (natriuretic peptides & echocardiography) or have limited accuracy (chest radiography).
Beyond clinical acumen, lung ultrasound (LUS) appears to be a reliable tool for the evaluation of interstitial syndromes including lung congestion detection and quantification, where the number and distribution of B‐lines indicate the amount of extravascular fluid in the lung. 4 B‐lines are a sonographic artefact caused by the interaction between air and water in the interstitial space. 5 Therefore, LUS may be an alternative method of lung fluid retention assessment, even in asymptomatic or mildly subclinical patients where lung congestion signs may go unnoticed. Advantages of LUS include bedside availability and feasibility, as its performance takes less than 15 min in experienced hands.
Lung ultrasound is already widely used in acute HF diagnosis and prognosis in the emergency setting, 6 , 7 reaching a sensitivity and specificity as high as 0.88 and 0.90, respectively 8 ; nevertheless, its value has not been described well in outpatients with suspected HF decompensation. The wet‐to‐dry HF study aimed to characterize LUS in the entire process of ambulatory HF decompensation management, including initial evaluation, pulmonary congestion changes, and predictive value for HF hospitalizations. We hypothesized that LUS, performed during the visit, would add value to the diagnosis, monitoring, and prognosis of congestion in outpatients with established HF.
Methods
Study design and patients
This is a prospective single‐centre observational cohort study of ambulatory patients examined at a specific HF clinic that they visited due to suspicion of HF decompensation. Criteria for referral to the HF unit have been reported elsewhere 9 , 10 ; briefly, the main criterion is having HF according to the ESC definition, with at least one hospitalization and/or reduced left ventricular ejection fraction (LVEF) < 40%, irrespective of aetiology. Less than 5% of patients are admitted to the HF unit for asymptomatic reduced LVEF after acute myocardial infarction. Since 2001, all patients have been seen regularly during follow‐up according to a set schedule and their clinical needs [REGI‐UNIC register (PI‐18‐037)]. Structured follow‐up includes quarterly nurse visits and one visit from a physician (cardiologist, internist, or family physician) every 6 months; a transthoracic Doppler‐echocardiogram is performed routinely by scheduled protocol at 1 year and every 2 years thereafter, and as many times as needed according to the clinical situation by experienced echocardiographers with a Philips iE33 system using a 3.5 MHz bandwidth sector transducer. For clinical needs, patients can contact the clinic spontaneously for an unscheduled visit in case of suspected HF decompensation and be reassessed as many times as necessary. The clinic has the infrastructure for short‐term diuretic and other intravenous drug administration.
For this study, we included consecutive patients older than 18 years evaluated due to suspicion of HF decompensation from 11 July 2016 to 31 July 2017. Exclusion criteria for the analysis were previous diagnosis of pulmonary fibrosis, lung cancer, congenital lung or heart disease, dialysis, or previous major thoracic surgery. We also excluded patients with radiological pachypleuritis by checking the annual chest x‐ray performed in our scheduled follow‐up protocol. The study was performed in accordance with the Guidelines of the Helsinki Declaration.
Data collection
Visits were performed by physicians of the HF clinic (MdA, PM, ES‐V, JS, & PC), blinded to LUS results and biomarker values. Clinical evaluation included decompensation assessment, using the HF clinical disease severity score (CDSS), 11 , 12 with a decompensated patient defined as having a score ≥ 2. Each of the major criteria counts for 1 point (paroxysmal nocturnal dyspnoea, pulmonary crackles, elevated jugular venous pressure, & third heart sound); each of the minor criteria counts for 0.5 point (orthopnoea, reduced exercise tolerance, resting sinus tachycardia, jugular venous pressure > 4 cm, hepatomegaly, & peripheral oedema). Demographic and clinical data were recorded from the computerized medical record review by one of the researchers (MD).
As a part of the study, peripheral venous blood samples were obtained for a biomarker panel performance. N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) was measured using Cobas Elecsys kits (Roche Diagnostics), cancer antigen 125 (CA125) by use of an Architect CA 125 II assay (Abbott Diagnostic) and soluble ST2 (sST2) by use of a human soluble neprilysin/CD10 ELISA kit (Aviscera Bioscience).
Lung ultrasound assessment
Lung ultrasound examination was performed in situ with a pocket portable device (V‐scan handheld single‐probe device, General Electric) by one of the two experienced investigators (MD& LC), blinded to clinical and visit data. Eight areas, established by a previous expert panel 13 and validated against other approach in an emergency setting, were examined (two upper and two lower areas of each hemithorax); patients were examined while in a semi‐supine position during the exam (Figure 1 ). LUS was performed using a phased‐array transducer, perpendicular to the ribs, with an imaging depth of 14 cm, and 2 s clip videos were recorded. LUS images were analysed offline by one of the two trained investigators (MD & LC), who recorded the number of B‐lines in the sagittal scan of every thoracic area. A B‐line was defined as a discrete laser‐like vertical hyperechoic reverberation artefact that arises from the pleural line, extends to the bottom of the screen without fading, and moves synchronously with lung sliding. 13 Pleural effusion was considered equivalent to 10 B‐lines. The sum of B‐lines across the eight lung zones was used for the main analyses, as previous studies have done in HF cohorts. 14 , 15 A simplified 4‐lung‐zones protocol (an upper anterior and a lower lateral area of each hemithorax) was also performed in a sensitivity analysis 16 (Figure 1 ).
Figure 1.
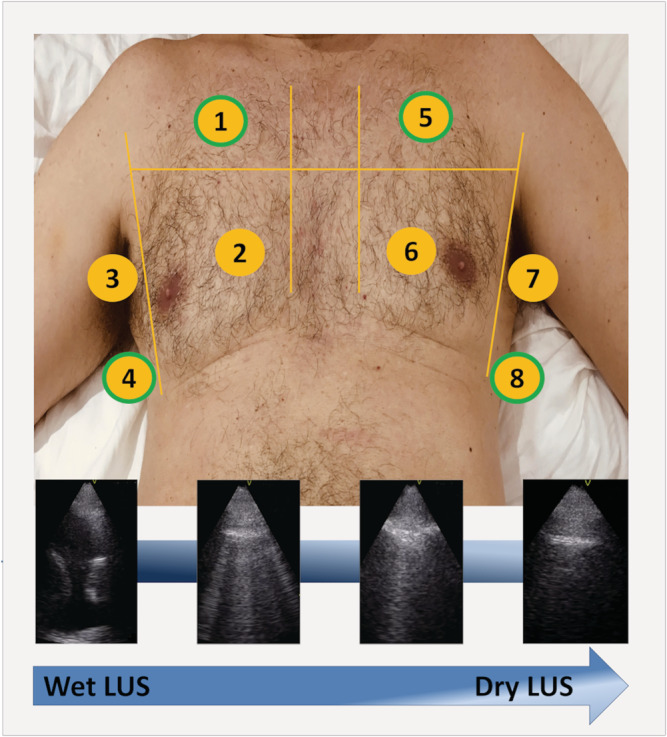
The eight thoracic areas scanned in the wet‐to‐dry HF study (upper panel): lung areas 1, 4, 5, and 8 (green border) were used for the simplified 4‐lung‐zones protocol; examples of LUS scans showing decreasing lung congestion (lower panel). LUS, lung ultrasound.
Follow‐up
Final diagnosis by the physician in charge of the initial clinical evaluation included four categories (no HF decompensation, right‐sided HF decompensation, left‐sided HF decompensation, and global HF decompensation), according to the HF guidelines of the European Society of Cardiology 17 [left‐sided: orthopnoea, paroxysmal nocturnal dyspnoea, pulmonary rales (bilateral), peripheral oedema (bilateral); right‐sided: jugular venous dilatation, peripheral oedema (bilateral), congested hepatomegaly, hepatojugular reflux, ascites, symptoms of gut congestion]; global HF decompensation included symptoms/signs of both left and right HF. As LUS data were blinded to treating physicians, complementary test for diagnostic purposes (blood test, chest x‐ray, and other explorations) and management of HF decompensation was based on the routine workup. Patients without HF decompensation were no longer followed for study purposes; those with diagnosed HF decompensation were visited for clinical assessment, and intravenous treatment if needed, as many times as necessary.
Blinded LUS follow‐up included a first thoracic scan at the initial visit (Wet‐LUS1), and a second study (Dry‐LUS2) at the time the patient was considered clinically recompensated. For prognostic purposes, the primary endpoints were the composite of all‐cause death or HF‐related hospitalization at 1, 2, and 6 months. All patients completed 6 months of follow‐up or until death.
Data analysis and statistics
Each episode of suspected or confirmed HF decompensation was treated as an individual independent data point in the analyses, although some of them occurred in the same patient. Categorical values are described as absolute numbers (percentages) and continuous variables as means (standard deviation) or medians (interquartile ranges), depending on whether data distribution was normal or non‐normal as assessed by normal Q–Q plots. All episodes of suspicion of HF decompensation were included, even if they occurred in the same patient, for the B‐lines group analyses. In contrast, only HF‐decompensation episodes were used for the prognosis analysis. Differences in clinical variables among groups were assessed by χ 2 test, t‐test, Mann Whitney U‐test, and means comparison (ANOVA) with Scheffe post‐hoc analysis, as appropriate. Differences in B‐lines [per lung area (LA) and total sum] between episodes with and without the diagnosis of HF decompensation were assessed by mean comparisons for independent data (t‐test). Differences in B‐lines (per LA and total sum) among episodes of the three different categories of HF were assessed by means comparison (ANOVA) with Scheffe post‐hoc analysis. Differences between LUS1 and LUS2 were assessed by paired t‐test. Correlation between the total B‐lines sum and biomarker concentrations was assessed using Pearson correlation. Univariable Cox regression analyses were performed using the composite endpoint as the dependent variable and the total sum of B‐lines as the independent variable. In a second step, multivariable Cox regression analysis (backward stepwise conditional) was performed for the composite endpoint at 30 days, adding age, sex, New York Heart Association (NYHA) functional class, LVEF, and NT‐proBNP at the decompensation visit as covariates given their clinical relevance. In a sensitivity analysis, this multivariable Cox regression was performed by patients and not by episodes (only the last episode per patient).
Results
Baseline demographics
Two hundred and thirty‐three suspected independent decompensations in 187 patients were prospectively evaluated (152 patients with one, 27 with two, 5 with three, and 3 with four episodes of suspicion of HF) (Supporting Information, Figure S1 ). Table 1 shows demographic and clinical characteristics of the 187 patients at the first episode of suspicion of HF decompensation. In summary, the mean age of the total cohort was 71 years; patients were predominantly male and of ischemic aetiology, and they were mainly in NYHA functional class III. Last previous LVEF was 42.5% ± 14.3 (the first LVEF at first clinic visit of the studied cohort was 34% ± 12.7). Patients were treated according to international guidelines and more than 75% received loop diuretics.
Table 1.
Demographic and clinical characteristics of the 187 patients at first decompensation
| Total, N = 187 | N | |
|---|---|---|
| Age | 71.4 ± 11.3 | 187 |
| Male sex, n (%) | 125 (66.8) | 187 |
| White | 183 (97.9) | 187 |
| Aetiology | 187 | |
| Ischaemic heart disease | 83 (44.4) | |
| Dilated CM | 32 (17.1) | |
| Hypertensive | 17 (9.1) | |
| Alcohol CM | 6 (3.2) | |
| Drug‐induced CM a | 5 (2.7) | |
| Valvular | 19 (10.2) | |
| Hypertrophic CM | 6 (3.2) | |
| Other | 19 (10.2) | |
| HF duration (months) | 61.0 (26.9–127.5) | 187 |
| NYHA class, n (%) | 187 | |
| I–II | 67 (35.8) | |
| III–IV | 120 (64.2) | |
| CDSS score | 1.75 ± 1.04 | 187 |
| LVEF (%) | 42.5 ± 14.3 | 187 |
| COPD, n (%) | 37 (19.8) | 187 |
| Hypertension | 134 (71.7) | 187 |
| Diabetes | 102 (54.5) | 187 |
| Atrial fibrillation/flutter, n (%) | 78 (41.7) | 187 |
| Anaemia b | 109 (58.3) | 182 |
| Renal insufficiency c , n (%) | 125 (66.8) | 187 |
| BMI (kg/m2) | 29.2 ± 6.2 | 181 |
| Systolic pressure (mm) | 122.8 ± 20.2 | 187 |
| Heart rate (bpm) | 75.4 ± 15.7 | 187 |
| Urea (mg/dL) | 69 (52–105) | 187 |
| Na (mEq/L) | 137.8 ± 3.2 | 186 |
| K (mEq/L) | 4.3 ± 0.5 | 185 |
| NT‐proBNP (ng/L) | 6,792 ± 8,478 | 183 |
| sST2 (ng/mL) | 75.4 ± 56.2 | 135 |
| CA125 (U/mL) | 61.1 ± 81.3 | 123 |
| Treatments | 187 | |
| ACEI or ARB | 132 (70.6) | |
| Sacubitril/Valsartan | 33 (17.6) | |
| Beta‐blockers | 165 (88.2) | |
| MRA | 112 (59.9) | |
| Loop diuretics | 157 (84.0) | |
| Digoxin | 34 (18.2) | |
| Ivabradine | 28 (15.0) | |
| CRT | 27 (14.4) | |
| ICD | 39 (20.9) |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CA125, cancer antigen 125; CDSS, HF clinical disease severity score; CM, cardiomyopathy; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ICD, implantable cardiac defibrillator; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; sST2, soluble interleukin‐1 receptor‐like 1.
Data in mean ± SD, median (Q1–Q3), or n (%).
Chemotherapy agents.
Haemoglobin < 120 g/L in women and <130 g/L in men.
Estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration equation) < 60 mL/min per 1.73 m2.
Mean CDSS of the cohort was 1.75 ± 1.04 points, being intrinsically higher in the independent data points classified as a HF decompensation (2.15 ± 0.97) (Table 2 ). Lung basal crackles, orthopnoea, paroxysmal nocturnal dyspnoea, and jugular ingurgitation were unusual in those episodes without the final diagnosis of HF decompensation (Table 2 ).
Table 2.
Clinical characteristics of patients in 233 episodes studied for possible decompensation
| No HF diagnosis, N = 57 | HF diagnosis, N = 176 | P value | |
|---|---|---|---|
| CDSS score | 0.68 ± 0.51 | 2.15 ± 0.97 | 0.001 |
| NYHA class III–IV, n (%) | 24 (42.1) | 129 (73.3) | <0.001 |
| Systolic BP (mmHg) | 121.7 ± 19.1 | 122 ± 20 | 0.92 |
| Heart rate (bpm) | 70 ± 12.6 | 77.0 ± 16.32 | 0.004 |
| Urea (mg/dL) | 55 (43.5–80) | 77 (55–122) | <0.001 |
| eGFR (mL/min/1.73 m2) | 59 ± 27.9 | 44.2 ± 22.4 | <0.001 |
| Haemoglobin (g/dL) | 12.9 ± 2.1 | 11.7 ± 1.9 | <0.001 |
| Na (mEq/L) | 137.6 ± 3.1 | 137.8 ± 3.3 | 0.69 |
| K (mEq/L) | 4.4 ± 0.5 | 4.2 ± 0.5 | 0.04 |
| NT‐proBNP (ng/L) a | 3145 ± 6425 | 8388 ± 8775 | <0.001 |
| sST2 (ng/mL) b | 46 ± 41.4 | 86.7 ± 53.9 | <0.001 |
| CA125 (U/mL) c | 15 (10–21.5) | 38 (18–38) | <0.001 |
| Clinical findings | |||
| Crackles | 1 (1.8) | 62 (35.2%) | <0.001 |
| Jugular venous distention | 4 (7.0) | 70 (39.8%) | <0.001 |
| Orthopnoea | 1 (1.8) | 49 (27.8%) | <0.001 |
| Paroxysmal nocturnal dyspnoea | 1 (1.8) | 28 (15.9%) | 0.003 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CA125, cancer antigen 125; CDSS, HF clinical disease severity score; eGFR, estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration equation); LUS, lung ultrasound; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; sST2, soluble Interleukin‐1 receptor‐like 1.
Data in mean ± SD, median (Q1–Q3), or n (%).
N = 225.
N = 171.
N = 159.
Acute respiratory infection (19.3%) and anaemia/iron deficiency (8.8%) were the most frequent diagnosis in the episodes considered non‐HF decompensation (N = 57). In 27 episodes (47.4%), the cause of symptoms was not found, and one patient was diagnosed with pulmonary embolism. Tiredness (38.6%) and dyspnoea (31.6%), followed by oedema and cough, were the most frequent symptoms in this group.
Lung ultrasound association with clinical data
The mean number of B‐lines in LUS1 assessment of all the HF suspicion episodes was 14.2 ± 11.6. Table 2 shows clinical and LUS1 data as well as biomarker profiles of the 233 episodes, according to the final HF diagnosis. Episodes without HF decompensation had significantly lower total B‐line counts (3.7 ± 4.5) than the 176 with HF decompensation (17.6 ± 11.2) (P < 0.001). Independent data points with right‐sided decompensation had significantly lower B‐lines count than left‐sided and global HF‐decompensation (13.49 ± 9.81 vs. 19.67 ± 11.58, P = 0.01 and 20.64 ± 11.09, P = 0.001 respectively, Table S1 ).
Regarding LA sites, the number of B‐lines was significantly higher in all LA in episodes with decompensated HF (Table 3 ). Lower areas, especially lateral zones (LA4 and LA8), were those with the highest number of B‐lines (P < 0.001 for both LA4 and LA8 vs. all the other LA; P = 0.61 between LA4 and LA8). This was specifically observed in episodes with the diagnosis of HF decompensation (again P < 0.001 for both LA4 and LA8 vs. all the other LA; P = 0.93 between LA4 and LA8). Table S 2 shows the number of B‐lines by LA according to the type of HF decompensation.
Table 3.
Total LUS1 data by lung areas and based on the diagnosis of HF decompensation
| Total suspicions of decompensation, N = 233 | No HF diagnosis, N = 57 | HF diagnosis, N = 176 | P value | |
|---|---|---|---|---|
| LA1 | 1.36 ± 2.02 | 0.33 ± 1.14 | 1.70 ± 2.13 | <0.001 |
| LA2 | 1.64 ± 2.07 | 0.39 ± 0.68 | 2.05 ± 2.20 | <0.001 |
| LA3 | 1.48 ± 2.21 | 0.47 ± 1.43 | 1.81 ± 2.32 | <0.001 |
| LA4 | 2.94 ± 3.26 | 0.89 ± 1.54 | 3.60 ± 3.39 | <0.001 |
| LA5 | 1.15 ± 1.53 | 0.25 ± 0.85 | 1.44 ± 1.59 | <0.001 |
| LA6 | 0.75 ± 1.20 | 0.11 ± 0.31 | 0.96 ± 1.30 | <0.001 |
| LA7 | 1.73 ± 1.92 | 0.40 ± 0.80 | 2.15 ± 1.99 | <0.001 |
| LA8 | 3.09 ± 3.14 | 0.86 ± 1.91 | 3.81 ± 3.13 | <0.001 |
| Total B‐lines sum | 14.2 ± 11.6 | 3.7 ± 4.5 | 17.6 ± 11.2 | <0.001 |
LA, lung area.
In HF decompensation episodes, the mean number of B‐lines moderately correlated with CDSS (R = 0.31, P < 0.001) and NYHA functional class at the moment of the visit (P for trend = 0.01), and inversely with LVEF (R = −0.29, P < 0.001). In contrast, we did not found a correlation with age (R = −0.05, P = 0.54), sex (men 17.4 ± 11.0 vs. women 17.9 ± 11.6, P = 0.76), or the presence of atrial fibrillation/flutter (present 18.9 ± 9 vs. absent 17.3 ± 11.2, P = 0.78).
Relative to biomarkers, NT‐proBNP was available in 171 out of the 176 episodes of HF decompensation, sST2 was available in 125, and CA125 in 113. The highest correlation of B‐line count was found with CA 125 (R = 0.30, P = 0.001), followed by NT‐proBNP (R = 0.21, P = 0.006), and finally sST2 (R = 0.22, P = 0.02).
Of the 176 HF decompensation episodes, a second LUS at the time of recompensation (LUS2) was available in 110, within a mean time of 24.2 ± 23.7 days [median 13.5 days (interquartile range 6–40)] (Figure S1 ). Interestingly, when patients were considered clinically compensated in every independent data point (LUS2), the total sum of B‐lines decreased from 17.3 ± 11.0 to 6.9 ± 6.7 (P < 0.001). This significant decrease in B‐lines occurred in all LA (all P < 0.001) (Table 4 ). Remarkably, the number of residual B‐lines in those recompensated episodes (dry) was still significantly higher than that observed in the initially considered non‐decompensated (6.9 ± 6.7 vs. 3.7 ± 4.5, P = 0.002).
Table 4.
LUS1 (wet) and LUS2 (dry) data of the 110 episodes diagnosed as HF decompensation with LUS2 study available
| Wet (LUS1) | Dry (LUS2) | P value | |
|---|---|---|---|
| Total B‐lines sum | 17.3 ± 11.0 | 6.9 ± 6.6 | <0001 |
| LA1 | 1.7 ± 2.0 | 0.4 ± 0.9 | <0.001 |
| LA2 | 1.9 ± 2.0 | 0.0 ± 1.5 | <0.001 |
| LA3 | 1.7 ± 2.1 | 0.7 ± 1.5 | <0.001 |
| LA4 | 3.5 ± 3.30 | 1.8 ± 2.8 | <0.001 |
| LA5 | 1.5 ± 1.6 | 0.5 ± 1.0 | <0.001 |
| LA6 | 1.0 ± 1.3 | 0.3 ± 0.6 | <0.001 |
| LA7 | 2.2 ± 2.0 | 0.7 ± 1.0 | <0.001 |
| LA8 | 3.8 ± 3.1 | 1.6 ± 2.2 | <0.001 |
LA, lung area.
Lung ultrasound and outcomes in HF decompensation episodes
During the 6 months of follow‐up, 66 primary composite endpoints were recorded in 61 decompensation episodes (53 HF‐related hospitalizations—4 patients had 2 hospitalizations—and 26 deaths). Twenty‐seven composite endpoints occurred during the first month (23 HF hospitalizations in 23 patients and 7 deaths) and 41 during the first 2 months (35 HF hospitalizations in 35 patients and 10 deaths). In the Cox regression analyses, total B‐line sum was significantly associated with the composite endpoint at 30 days (Table 5 ), but not at 60 days [HR 1.02 (95% CI 0.99–1.04), P = 0.22] nor at 6 months [HR 1.01 (95% CI 0.99–1.03), P = 0.54]. Figure 2 shows event‐free survival curves up to 30 days based on the B‐line sum, comparing first quartile (0–8 B‐lines) vs. quartiles second to fourth (≥9 B‐lines). In the multivariable analysis including age, sex, NYHA functional class, LVEF, and NT‐proBNP, the total B‐line sum remained statistically significantly associated with the composite endpoint at 30 days, carrying an increase of 4% in risk per every additional B‐line [HR 1.04 (1.00–1.07), P < 0.05], together with NYHA functional class (Table 5 ). Of note, NT‐proBNP was neutrally associated with outcome.
Table 5.
Cox regression analyses for the primary composite endpoint at 30 days by decompensation HF episodes
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Total B‐lines sum | 1.04 | 1.01–1.07 | 0.021 | 1.04 | 1.01–1.07 | 0.014 |
| Age | 0.98 | 0.95–1.02 | 0.35 | — | — | — |
| Female sex | 0.60 | 0.20–1.73 | 0.34 | — | — | — |
| NYHA class | 2.48 | 1.09–5.7 | 0.031 | — | — | — |
| LVEF | 1.00 | 0.98–1.03 | 0.91 | — | — | — |
| NT‐proBNP a | 1.00 | 1.00–1.00 | 0.57 | — | — | — |
LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Per 100 ng/L.
Figure 2.
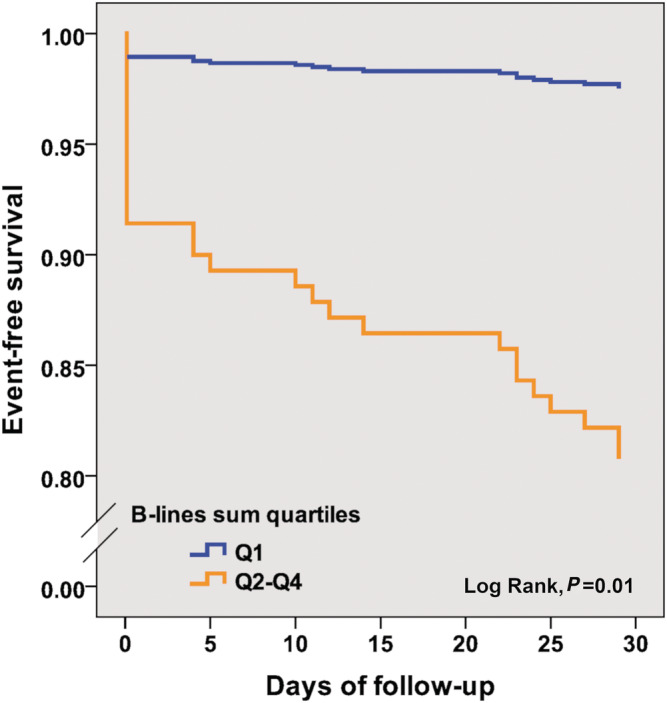
Event‐free survival at 30 days for the composite endpoint of all‐cause death or HF hospitalization, based on B‐lines sum quartiles [Q1 (≤8) vs. Q2–Q4 (9–52)].
Short‐term prognostic value of B‐lines sum was quite homogeneous among several subgroups based on demographic and clinical data (Figure 3 ).
Figure 3.
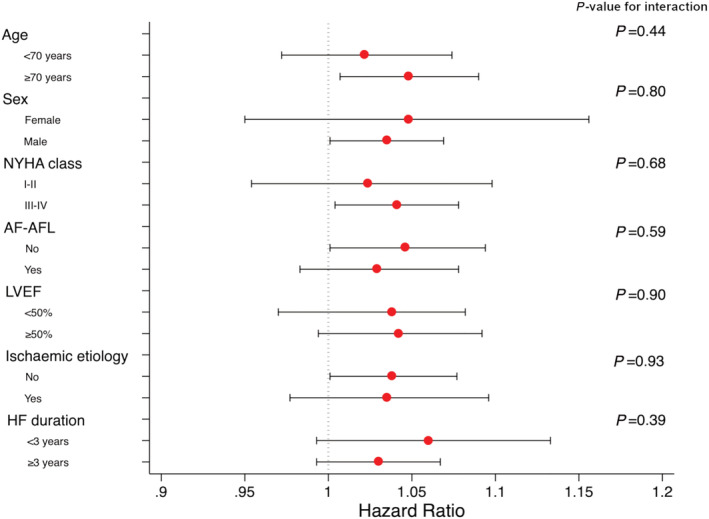
Forest plots for B‐lines sum for the composite endpoint of all‐cause death or HF hospitalization at 30 days according to several subgroups. Circles represent hazard ratio values, while the horizontal lines through the circles illustrate the length of the confidence interval.
In the sensitivity analysis performed by patients and not by episodes (only last episode per patient), B‐lines sum remained significantly associated with the composite endpoint at 30 days (Table 6 ). Figure S2 shows event‐free survival curves up to 30 days based on the B‐line sum, comparing first quartile vs. quartiles second to fourth based on patients and not on episodes (only last episode per patient).
Table 6.
Cox regression analyses for the primary composite endpoint at 30 days by patient (last HF episode, N = 145)
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Total B‐lines sum | 1.04 | 1.01–1.07 | 0.025 | 1.04 | 1.01–1.08 | 0.017 |
| Age | 0.98 | 0.94–1.02 | 0.26 | — | — | — |
| Female sex | 0.58 | 0.20–1.68 | 0.31 | — | — | — |
| NYHA class | 2.44 | 1.04–5.70 | 0.04 | — | — | — |
| LVEF | 1.01 | 0.98–1.03 | 0.71 | — | — | — |
| NT‐proBNP a | 1.00 | 1.00–1.00 | 0.85 | — | — | — |
CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Per 100 ng/L.
Sensitivity analysis of simplified 4‐area lung ultrasound protocol
Independent episodes with a clinical final diagnosis of HF decompensation had significantly more B‐lines than those without (10.5 ± 7.1 vs. 2.3 ± 3.2, P < 0.001) in the 4‐zone protocol. Global HF decompensation showed the highest number of B‐lines (12.8 ± 7.5), followed by left‐sided (10.6 ± 6.2) and right‐sided (8.5 ± 6.8); significant differences were only found between right‐sided and global HF decompensation (P = 0.002).
In the sensitivity multivariable analysis, the B‐line sum did not remain statistically significantly associated with the composite endpoint at 30 days [HR 1.06 (1.00–1.12), P = 0.07], although a 6% increase in risk per additional B‐line was found.
Discussion
Lung ultrasound has been widely evaluated in acute dyspnoea settings, especially in the emergency room and critical care wards, showing more diagnostic accuracy for HF than chest radiography or NT‐proBNP. 18 Nevertheless, few studies have addressed its value in chronic HF ambulatory patients to refine (less obvious and less acute) decompensated HF diagnosis. In our cohort of HF outpatients with mostly mild‐to‐moderate systolic dysfunction, our results show that (i) LUS is a useful tool in the diagnosis of HF decompensation and allows the monitoring of pulmonary congestion during follow‐up; (ii) pulmonary congestion by LUS is present in all HF clinical subtypes (i.e. right‐sided/left‐sided HF) but more importantly so in left‐sided and global HF; (iii) LUS significantly predicts short‐term outcomes in ambulatory patients with decompensated HF whereas NT‐proBNP did not; and (iv) the number of B‐lines correlates with known congestion biomarkers (Figure S3 ).
Lung ultrasound findings
This single‐centre study shows that in situ LUS can aid the diagnostic workup of acute congestion in patients with established HF, becoming a key element in the initial evaluation, because the number of B‐lines increases in the episodes with confirmed HF decompensation, similarly to what is reported in other smaller studies. 19 Specifically, episodes with HF decompensation have a significantly and markedly higher total count of B‐lines (17.6 ± 11.23) than those without congestion in our cohort (3.7 ± 4.5). Similarly, in the 4‐zone protocol, episodes with a final clinical diagnosis of HF decompensation also had significantly more B‐lines than those without (10.5 ± 7.1 vs. 2.3 ± 3.2), suggesting that this simplified protocol could also be useful in the initial evaluation in this setting, although 8‐zone protocol has been found to be superior in other studies. 20 Although all LA can be affected by pulmonary congestion, the lower zones are classically the first to exhibit extravascular fluid in all profiles of patients with HF. 21 As expected, data from our study also show that the highest number of B‐lines is found in lower lateral areas (LA4 and LA8).
Previous data from our group and from other studies performed in stable patients have demonstrated that subclinical pulmonary congestion is common in patients with chronic HF, 22 , 23 even in the absence of symptoms and clinical findings. Therefore, it is not surprising that, in this study, episodes without a clinical final diagnosis of HF decompensation showed 3.7 ± 4.5 B‐lines on LUS, quite comparable with the sum of B‐lines we found in HF stable patients. 22 As expected, episodes classified as having left or global HF decompensation had higher numbers of B‐lines, even taking into consideration the fact that pleural effusion, which can also be observed in right HF decompensation, accounted for 10 B‐lines.
Furthermore, LUS is a reliable tool for monitoring pulmonary fluid overload changes during outpatient follow‐up, because the B‐line profile varies according to the timing of assessment. In this context, we also performed a second LUS to evaluate residual pulmonary congestion after clinical improvement. In our cohort, B‐lines decreased significantly from 17.26 ± 11.0 to 6.85 ± 6.66, the latter number being very similar to that we have previously reported in stable patients (5.1 ± 6.1), 22 but still significantly higher than that in the episodes considered as non‐decompensation. That fact probably reflects that some pulmonary congestion still remains despite the observed clinical improvement. This result suggests that LUS could be useful for clinicians in tailoring HF diuretic therapy during decompensation; in fact, LUS‐guided treatment has been reported to be effective in both chronic and acute HF post discharge, 24 , 25 because LUS examination might be more sensitive in detecting congestion than clinical evaluation in several HF scenarios. 16 , 21 , 22 , 26
Other technologies are arising for early HF decompensation evaluation in outpatients such as impedance measurement, home telemonitoring, and wearable technology and devices that measure pulmonary artery or left atrial pressure wirelessly. Integrating LUS with some of these tools could become a novel approach that have to be investigated.
Lung ultrasound prognosis
Assessing pulmonary congestion accurately is critical to providing the optimal approach to hospitalized HF patients, because the number of B‐lines at admission and discharge predicts short‐term outcomes. 27 , 28 , 29 , 30 , 31 Our data further show that B‐lines assessment in an ambulatory setting also strongly predicted outcomes; specifically, each B‐line added increased by 4% the composite endpoint of all‐cause death or HF‐related hospitalization at 30 days. Furthermore, the sum of B‐lines across all LA, together with NYHA functional class, remained as an independent prognostic factor in the multivariable analysis, outperforming natriuretic peptides. Remarkably, patients with ≤8 B‐lines (quartile 1) showed significantly less events at 30 days. This added prognostic value was proved only in short‐term follow‐up, different from what has been observed in acute or stable chronic HF patients. We speculate that it may be due to various reasons. First, our clinic provides a structured follow‐up, including unscheduled visits. Similar to our results, Rivas‐Lasarte et al., 25 with a specified high intensity intervention, did not find significant differences in HF hospitalizations during a 6 month follow‐up. Second, we treated decompensated HF episodes with ambulatory IV diuretics within the first 30 days, thus decreasing the likelihood of HF admissions (and association with HF admissions) for longer follow‐ups.
Because a previous study by Platz et al. 16 reported that, in patients hospitalized for acute HF, a 4‐zone LUS is highly correlated for prognostic purposes with the 8‐zone LUS, we also evaluated this protocol in our cohort. However, our data show that in ambulatory decompensations, the B‐line count was not significantly associated with short‐term outcomes when using this simplified protocol.
Lung ultrasound and biomarkers
In our study, CA125 showed the best correlation between the number of B‐lines and established cardiac biomarkers. CA125 is a glycoprotein synthesized by epithelial serosal cells at sites such as the pericardium, pleura, and peritoneum, with proved usefulness for fluid overload assessment in patients with acute HF. 32 Moreover, as a risk stratification tool, serum CA125 levels have been associated with poor outcomes, and have even been used to guide diuretic treatment. 33 Although LUS and CA125 are both congestion and short‐term prognostic markers, 34 , 35 there is little evidence of their association in acute and chronic HF. Our finding supports hypothesized correlations between B‐lines and cardiac biomarkers other than CA125 in HF patients, as we have previous reported. 36
Limitations
This was a single‐centre study with patients treated at a specific multidisciplinary HF clinic at a day‐hospital in a tertiary‐care hospital. Most of our patients had high risk of unfavourable outcomes because they were referred after at least one hospital admission and partially recovered LVEF. We cannot disregard selection bias by disease severity and management. Each episode of suspected or confirmed HF decompensation was treated as an individual independent data point in the analyses, although some of them occurred in the same patient. This should be taken into account when analysing our results as they can be influenced by the existence co‐dependence in some variables. The number of B‐lines may be impacted by the clip duration 37 ; in our study, due to the characteristics of the device, we recorded 2 s video clips that may have underrepresented the number of B‐lines; However, such recording strategy is likely to be the one used in routine proactice. Finally, we did not perform other emerging types of point‐of‐care ultrasound for assessing venous congestion, such as the Venous Excess Ultrasound Score protocol. 38
Conclusions
Our data show that LUS is a valuable tool to identify HF decompensation in chronic HF outpatients. B‐lines count identifies clinical profile of congestion (right‐sided, left‐sided, or global) and appears to precisely assess pulmonary congestion changes during follow‐up. B‐line count added prognostic information regarding short‐term outcomes in ambulatory patients with confirmed HF decompensation. CA125 showed the best correlation between the B‐line count and several biomarkers.
Conflict of interest
N.G. reports honoraria from AstraZeneca, Bayer, Boehringer, Novartis, and Vifor. The rest of the authors have no conflict of interest regarding the present study.
Funding
This research was supported by Fundació La Marató de TV3 (PI 201510.10). The Nancy team is supported by the RHU Fight‐HF, a public grant overseen by the French National Research Agency (ANR) as part of the second ‘Investissements d'Avenir’ programme (reference: ANR‐15‐RHUS‐0004), by the French PIA project ‘Lorraine Université d'Excellence’ (reference: ANR‐15‐IDEX‐04‐LUE), the ANR FOCUS‐MR (reference: ANR‐15‐CE14‐0032‐01), ERA‐CVD EXPERT (reference: ANR‐16‐ECVD‐0002‐02), the Contrat de Plan Etat Lorraine IT2MP, and FEDER Lorraine.
Consent to participate
All participants provided written informed consent.
Consent for publication
the local ethical committee and all participants gave their written consent to publish the study.
Supporting information
Figure S1. Study flow‐chart.
Figure S2. Event‐free survival at 30 days for the composite end‐point of all‐cause death or HF hospitalization, comparing quartiles (Q1 [≤8] vs. Q2‐Q4 [9–52]) based on patients and not on episodes (only last episode per patient).
Figure S3. Summary of the study. LUS protocol performed in patients with HF decompensation suspicion (left panel) allows to confirm HF diagnosis, classify HF type and assess recopensation. B‐lines sum significantly difer according to HF situation (right‐upper panel) and showed short‐term prognostic value (right‐lower panel).
Table S1. Clinical characteristics of patients in the 176 diagnosed HF decompensations based on type of HF
Table S2. LUS1 (Wet) data by lung areas based on the type of HF decompensation
Domingo, M. , Lupón, J. , Girerd, N. , Conangla, L. , de Antonio, M. , Moliner, P. , Santiago‐Vacas, E. , Codina, P. , Cediel, G. , Spitaleri, G. , González, B. , Diaz, V. , Rivas, C. , Velayos, P. , Núñez, J. , and Bayes‐Genís, A. (2021) Lung ultrasound in outpatients with heart failure: the wet‐to‐dry HF study. ESC Heart Failure, 8: 4506–4516. 10.1002/ehf2.13660.
References
- 1. Huusko J, Tuominen S, Studer R, Corda S, Proudfoot C, Lassenius M, Ukkonen H. Recurrent hospitalizations are associated with increased mortality across the ejection fraction range in heart failure. ESC Heart Fail 2020; 7: 2406–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 1989; 261: 884–888. [PubMed] [Google Scholar]
- 3. Thibodeau JT, Drazner MH. The role of the clinical examination in patients with heart failure. JACC Heart Fail 2018; 6: 543–551. [DOI] [PubMed] [Google Scholar]
- 4. Platz E, Jhund PS, Campbell RT, McMurray JJ. Assessment and prevalence of pulmonary oedema in contemporary acute heart failure trials: a systematic review. Eur J Heart Fail 2015; 17: 906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Volpicelli G, Mussa A, Garofalo G, Cardinale L, Casoli G, Perotto F, Fava C, Frascisco M. Bedside lung ultrasound in the assessment of alveolar‐interstitial syndrome. Am J Emerg Med 2006; 24: 689–696. [DOI] [PubMed] [Google Scholar]
- 6. Maw AM, Hassanin A, Ho PM, McInnes MDF, Moss A, Juarez‐Colunga E, Soni NJ, Miglioranza MH, Platz E, DeSanto K, Sertich AP, Salame G, Daugherty SL. Diagnostic accuracy of point‐of‐care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta‐analysis. JAMA Netw Open 2019; 2: e190703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail 2017; 19: 1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maw AM, Hassanin A, Ho PM, McInnes MDF, Moss A, Juarez‐Colunga E, Soni NJ, Miglioranza MH, Platz E, DeSanto K, Sertich AP, Salame G, Daugherty SL. Diagnostic accuracy of point‐of‐care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta‐analysis. JAMA Netw Open 2019; 2: e190703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zamora E, Lupón J, Vila J, Urrutia A, de Antonio M, Sanz H, Grau M, Ara J, Bayés‐Genís A. Estimated glomerular filtration rate and prognosis in heart failure: value of the modification of diet in renal disease Study‐4, chronic kidney disease epidemiology collaboration, and cockroft‐gault formulas. J Am Coll Cardiol 2012; 59: 1709–1715. [DOI] [PubMed] [Google Scholar]
- 10. Díez‐López C, Lupón J, de Antonio M, Zamora E, Domingo M, Santesmases J, Troya MI, Boldó M, Bayes‐Genis A. Hemoglobin kinetics and long‐term prognosis in heart failure. Rev Esp Cardiol (Engl Ed) 2016; 69: 820–826. [DOI] [PubMed] [Google Scholar]
- 11. Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N‐BNP) concentrations. Lancet 2000; 355: 1126–1130. [DOI] [PubMed] [Google Scholar]
- 12. Pascual‐Figal DA, Domingo M, Casas T, Gich I, Ordoñez‐Llanos J, Martínez P, Cinca J, Valdés M, Januzzi JL, Bayes‐Genis A. Usefulness of clinical and NT‐proBNP monitoring for prognostic guidance in destabilized heart failure outpatients. Eur Heart J 2008; 29: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 13. Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T, International Liaison Committee on Lung Ultrasound (ILC‐LUS) for International Consensus Conference on Lung Ultrasound (ICC‐LUS) . International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012; 38: 577–591. [DOI] [PubMed] [Google Scholar]
- 14. Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Frasure SE, Jhund PS, Cheng S, Solomon SD. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 2016; 37: 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dwyer KH, Merz AA, Lewis EF, Claggett BL, Crousillat DR, Lau ES, Silverman MB, Peck J, Rivero J, Cheng S, Platz E. Pulmonary congestion by lung ultrasound in ambulatory patients with heart failure with reduced or preserved ejection fraction and hypertension. J Card Fail 2018; 24: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, Lee MMY, Merz AA, Silverman M, Swamy V, Lindner M, Rivero J, Solomon SD, McMurray JJV. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short‐ and long‐term outcomes. JACC Heart Fail 2019; 7: 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 18. Pivetta E, Goffi A, Nazerian P, Castagno D, Tozzetti C, Tizzani P, Tizzani M, Porrino G, Ferreri E, Busso V, Morello F, Paglieri C, Masoero M, Cassine E, Bovaro F, Grifoni S, Maule MM, Lupia E. Study group on lung ultrasound from the molinette and careggi hospitals (2019) lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: a randomized controlled trial. Eur J Heart Fail 2019; 21: 754–766. [DOI] [PubMed] [Google Scholar]
- 19. Miglioranza MH, Gargani L, Sant'Anna RT, Rover MM, Martins VM, Mantovani A, Weber C, Moraes MA, Feldman CJ, Kalil RA, Sicari R, Picano E, Leiria TL. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 2013; 6: 1141–1151. [DOI] [PubMed] [Google Scholar]
- 20. Buessler A, Chouihed T, Duarte K, Bassand A, Huot‐Marchand M, Gottwalles Y, Pénine A, André E, Nace L, Jaeger D, Kobayashi M, Coiro S, Rossignol P, Girerd N. Accuracy of several lung ultrasound methods for the diagnosis of acute heart failure in the ED: a multicenter prospective study. Chest 2020; 157: 99–110. [DOI] [PubMed] [Google Scholar]
- 21. Conangla L, Domingo M, Lupón J, Wilke A, Juncà G, Tejedor X, Volpicelli G, Evangelista L, Pera G, Toran P, Mas A, Cediel G, Verdú JM, Bayes‐Genis A. Lung ultrasound for heart failure diagnosis in primary care. J Card Fail 2020; 26: 824–831. [DOI] [PubMed] [Google Scholar]
- 22. Domingo M, Conangla L, Lupón J, de Antonio M, Moliner P, Santiago‐Vacas E, Codina P, Zamora E, Cediel G, González B, Díaz V, Rivas C, Velayos P, Santesmases J, Pulido A, Crespo E, Bayes‐Genís A. Prognostic value of lung ultrasound in chronic stable ambulatory heart failure patients. Rev Esp Cardiol (Engl Ed) 2020; S1885‐5857: 30335–30332. [DOI] [PubMed] [Google Scholar]
- 23. Pellicori P, Shah P, Cuthbert J, Urbinati A, Zhang J, Kallvikbacka‐Bennett A, Clark AL, Cleland JGF. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur J Heart Fail 2019; 21: 904–916. [DOI] [PubMed] [Google Scholar]
- 24. Marini C, Fragasso G, Italia L, Sisakian H, Tufaro V, Ingallina G, Stella S, Ancona F, Loiacono F, Innelli P, Costantino MF, Sahakyan L, Gabrielyan S, Avetisyan M, Margonato A, Agricola E. Lung ultrasound‐guided therapy reduces acute decompensation events in chronic heart failure. Heart 2020; 106: 1934–1939. [DOI] [PubMed] [Google Scholar]
- 25. Rivas‐Lasarte M, Álvarez‐García J, Fernández‐Martínez J, Maestro A, López‐López L, Solé‐González E, Pirla MJ, Mesado N, Mirabet S, Fluvià P, Brossa V, Sionis A, Roig E, Cinca J. Lung ultrasound‐guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS‐HF study). Eur J Heart Fail 2019; 21: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 26. Rivas‐Lasarte M, Maestro A, Fernández‐Martínez J, López‐López L, Solé‐González E, Vives‐Borrás M, Montero S, Mesado N, Pirla MJ, Mirabet S, Fluvià P, Brossa V, Sionis A, Roig E, Cinca J, Álvarez‐García J. Prevalence and prognostic impact of subclinical pulmonary congestion at discharge in patients with acute heart failure. ESC Heart Fail 2020; 7: 2621–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coiro S, Rossignol P, Ambrosio G, Carluccio E, Alunni G, Murrone A, Tritto I, Zannad F, Girerd N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail 2015; 17: 1172–1181. [DOI] [PubMed] [Google Scholar]
- 28. Coiro S, Porot G, Rossignol P, Ambrosio G, Carluccio E, Tritto I, Huttin O, Lemoine S, Sadoul N, Donal E, Zannad F, Girerd N. Prognostic value of pulmonary congestion assessed by lung ultrasound imaging during heart failure hospitalisation: a two‐Centre cohort study. Sci Rep 2016; 6: 39426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palazzuoli A, Ruocco G, Beltrami M, Nuti R, Cleland JG. Combined use of lung ultrasound, B‐type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin Res Cardiol 2018; 107: 586–596. [DOI] [PubMed] [Google Scholar]
- 30. Kobayashi M, Gargani L, Palazzuoli A, Ambrosio G, Bayés‐Genis A, Lupon J, Pellicori P, Pugliese NR, Reddy YNV, Ruocco G, Duarte K, Huttin O, Rossignol P, Coiro S, Girerd N. Association between right‐sided cardiac function and ultrasound‐based pulmonary congestion on acutely decompensated heart failure: findings from a pooled analysis of four cohort studies. Clin Res Cardiol 2020; 110: 1181–1192. [DOI] [PubMed] [Google Scholar]
- 31. Palazzuoli A, Ruocco G, Franci B, Evangelista I, Lucani B, Nuti R, Pellicori P. Ultrasound indices of congestion in patients with acute heart failure according to body mass index. Clin Res Cardiol 2020; 109: 1423–1433. [DOI] [PubMed] [Google Scholar]
- 32. Núñez J, Miñana G, Núñez E, Chorro FJ, Bodí V, Sanchis J. Clinical utility of antigen carbohydrate 125 in heart failure. Heart Fail Rev 2014; 19: 575–584. [DOI] [PubMed] [Google Scholar]
- 33. Núñez J, Llàcer P, Bertomeu‐González V, Bosch MJ, Merlos P, García‐Blas S, Montagud V, Bodí V, Bertomeu‐Martínez V, Pedrosa V, Mendizábal A, Cordero A, Gallego J, Palau P, Miñana G, Santas E, Morell S, Llàcer A, Chorro FJ, Sanchis J, Fácila L, CHANCE‐HFInvestigators . Carbohydrate Antigen‐125‐guided therapy in acute heart failure: CHANCE‐HF: a randomized study. JACC Heart Fail 2016; 4: 833–843. [DOI] [PubMed] [Google Scholar]
- 34. Li KHC, Gong M, Li G, Baranchuk A, Liu T, Wong MCS, Jesuthasan A, Lai RWC, Lai JCL, Lee APW, Bayés‐Genis A, de la Espriella R, Sanchis J, Wu WKK, Tse G, Nuñez J, International Health Informatics Study (IHIS) Network . Cancer antigen‐125 and outcomes in acute heart failure: a systematic review and meta‐analysis. Heart Asia 2018; 10: e011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Núñez J, Núñez E, Consuegra L, Sanchis J, Bodí V, Martínez‐Brotons A, Bertomeu‐González V, Robles R, Bosch MJ, Fácila L, Darmofal H, Llàcer A. Carbohydrate antigen 125: an emerging prognostic risk factor in acute heart failure? Heart 2007; 93: 716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Domingo M, Conangla L, Lupón J, Wilke A, Juncà G, Revuelta‐López E, Tejedor X, Bayes‐Genis A. Lung ultrasound and biomarkers in primary care: partners for a better management of patients with heart failure? J Circ Biomark 2020; 9: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Platz E, Pivetta E, Merz AA, Peck J, Rivero J, Cheng S. Impact of device selection and clip duration on lung ultrasound assessment in patients with heart failure. Am J Emerg Med 2015; 33: 1552–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beaubien‐Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, Denault AY. Quantifying systemic congestion with point‐of‐care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J 2020; 12: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study flow‐chart.
Figure S2. Event‐free survival at 30 days for the composite end‐point of all‐cause death or HF hospitalization, comparing quartiles (Q1 [≤8] vs. Q2‐Q4 [9–52]) based on patients and not on episodes (only last episode per patient).
Figure S3. Summary of the study. LUS protocol performed in patients with HF decompensation suspicion (left panel) allows to confirm HF diagnosis, classify HF type and assess recopensation. B‐lines sum significantly difer according to HF situation (right‐upper panel) and showed short‐term prognostic value (right‐lower panel).
Table S1. Clinical characteristics of patients in the 176 diagnosed HF decompensations based on type of HF
Table S2. LUS1 (Wet) data by lung areas based on the type of HF decompensation


