Abstract
Aim
We aim to determine the cost‐effectiveness of dapagliflozin in addition to standard therapy versus standard therapy alone among patients with heart failure with reduced ejection fraction (HFrEF) using the public healthcare provider's perspective in the Philippines.
Methods and results
A thousand Filipino patients with HFrEF (with or without type 2 diabetes mellitus) were included in a simulation cohort using a lifetime Markov model. The model, which was developed based on the results of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial, was composed of three health states. These were ‘alive without an event’ (chronic heart failure state), ‘alive but was hospitalized for heart failure’ (worsening heart failure), and ‘dead’ (death from any cause). Data regarding costs and utilities were obtained from previous studies and local data. These were used to estimate the incremental cost per quality‐adjusted life‐year (ICER). A 3% annual discount rate was used for both costs and effects. One‐way (deterministic) and probabilistic sensitivity analyses as well as scenario analyses were performed. The ICER for the addition of dapagliflozin to standard therapy among HFrEF patients was PHP177 868 (US$3434) and PHP160 983 (US$3108), respectively, if the present price (PHP44.00) and possible negotiated unit cost of dapagliflozin 10 mg tablet (PHP40.00) were used. These were deemed cost‐effective because they were both below the threshold ICER which was equivalent to the gross domestic product per capita of the Philippines in 2019, PHP180 500 (US$3485). Using the unit costs of dapagliflozin previously mentioned, the ICERs among HFrEF patients with diabetes were PHP132 582 (US$2560) and PHP120 249 (US$2321), respectively. Doing PSA involving Monte Carlo simulation of 10 000 iterations and plotting the resulting ICERs against the threshold ICER in the cost‐effectiveness acceptability curves, these ICERs for HFrEF among diabetics were determined to be 72% and 76% cost‐effective.
Conclusion
Dapagliflozin added to standard therapy for HFrEF patients is likely to be cost‐effective using the perspective of the Philippine public healthcare provider.
Keywords: dapagliflozin, heart failure, incremental cost‐effectiveness ratio
Introduction
In recent years, research on therapeutic options for type 2 diabetes mellitus (T2DM) has yielded a number of new information. One of these is on sodium‐glucose cotransporter 2 inhibitor (SGLT2) class of molecules. Since 2008 and 2012, the United States Food and Drug Administration (USFDA) and the European Medicines Agency (EMA), respectively, have required cardiovascular (CV) safety trials for all antidiabetic medications. Beyond just showing proof of CV safety, SGLT2 inhibitors have recently been shown to have cardiovascular benefits. Currently, there are several SGLT2 inhibitors, and one of them is dapagliflozin. 1 , 2
The Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE‐TIMI 58) trial evaluated dapagliflozin compared with placebo among patients with T2DM and high CV risk or established CV disease. It showed a lower rate of heart failure (HF) hospitalization and mortality. 3 A post hoc analysis among patients with HF with reduced ejection fraction (HFrEF) showed similar results. 4
The Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients with HF with Reduced Ejection Fraction (DEFINE‐HF) trial included 263 patients with HFrEF and were randomized to dapagliflozin or placebo. It found that although NT‐proBNP levels were not significantly reduced by dapagliflozin as compared with placebo, there was an improvement in symptoms and reduction in worsening heart failure and hospitalization in patients with HFrEF with or without diabetes. 5
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA‐HF) study is the first outcome trial to assess SGLT2 inhibitor on hard endpoints among patients with HFrEF with and without T2DM. It showed a significant reduction in worsening HF or cardiovascular death. There was also a significant reduction in the levels of NT‐proBNP. 6 Its sub‐analysis showed similar effects independent of diabetes status, background therapy, and age group. 7 , 8 , 9 It also improved the patients' quality of life. 10
The Heart Failure Association of the European Society of Cardiology and the American College of Cardiology Expert Consensus Decision Pathway for Optimization of HF Treatment recommended dapagliflozin for HFrEF treatment in patients with and without diabetes. 11 , 12
Assessment of treatment efficiency through an economic evaluation is important apart from evaluation of efficacy or effectiveness. Such studies, which showed that dapagliflozin as add‐on therapy for HF is cost‐effective, have been carried out in Australia, United Kingdom, Germany, Spain, and Thailand. 13 , 14 , 15 However, unlike randomized controlled trials, the results of which can be applied in other settings, and economic evaluation study results cannot be readily generalized to another country. Variability in the results of cost‐effectiveness studies for drugs in Western Europe had been documented. 16 In addition, sacubitril‐valsartan, an HF medication proven to be cost‐effective in the United States and Europe, was determined to be not cost‐effective in Thailand. 15 In this regard, this study was undertaken. The primary objective is to determine the cost‐effectiveness of dapagliflozin in addition to standard therapy versus standard therapy alone among patients with HFrEF using the public healthcare provider's perspective in the Philippines. The secondary objectives are to determine the (i) cost‐effectiveness of dapagliflozin in addition to standard therapy versus standard therapy alone among patients with HFrEF and diabetes; and (ii) cost‐effectiveness of dapagliflozin in addition to standard therapy versus standard therapy alone among patients with HFrEF without diabetes.
Methods
Model composition and transition probabilities
The Markov model in this cost‐utility analysis (CUA) used DAPA‐HF6 as the basis of dapagliflozin's efficacy. Recent economic evaluation studies using the perspective of the public healthcare payer from several countries also utilized DAPA‐HF as the basis of their models. 13 , 14 , 15
The following states in the model were identified: (i) chronic HFrEF patient, ‘alive’ without an event (‘event‐free’), (ii) chronic HFrEF patient, ‘alive’ but hospitalized due to HF (worsening HF), and (iii) ‘dead’ (death from any cause). Patients with worsening HF are at risk for recurrent HF hospitalization or death. The baseline state of all patients was ‘alive’ with chronic HFrEF and the patients go through the model in yearly cycles until death or lifetime horizon. Figure 1 illustrates the Markov model with the three health states as mentioned and labelled as (i) event‐free, (ii) post‐hospitalization (due to HF), and (iii) dead.
Figure 1.
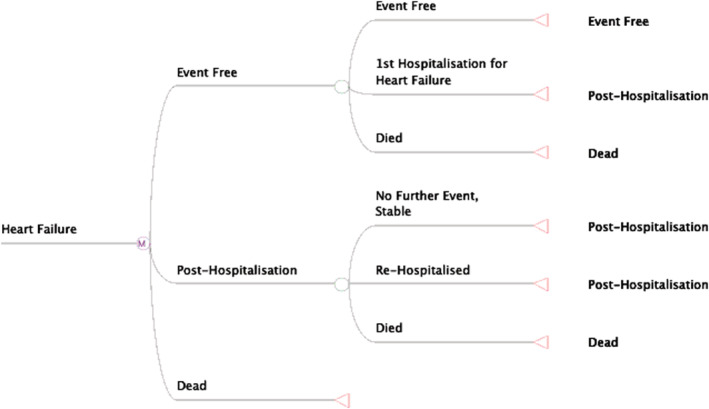
A Markov model for heart failure. This model illustrates the possible three health states for chronic heart failure patients with reduced ejection. This model is for both groups: the dapagliflozin plus standard of care and those given standard of care only.
The transition probabilities for the three Markov states were derived from the DAPA‐HF outcomes. The annual probabilities were estimated for the overall population of the study and separately for those with and without diabetes. The transition probability for death incorporated the latest Philippine age‐related mortality rate obtained from the global health data repository of the World Health Organization (WHO). 17
Population
The population in the model simulated the patients included in the DAPA‐HF study. These included HF patients with New York Heart Association class II, III, and IV whose ejection fraction was ≤40%. These patients either received standard treatment alone or dapagliflozin added to standard treatment for HF. Details of the patient characteristics in the DAPA‐HF trial are published together with the study results. 6
For this study, a cohort of 1000 Filipino patients with chronic HF with or without T2DM was included.
In the DAPA‐HF trial, the patient's mean age was 66 years, and this was used as the starting age the patients entered the model in the CEA by Savira et al. 13 On the other hand, an HF registry showed a lower mean age of HFrEF patients from Asia as compared with Europeans. 18 Those from the Philippines had a mean age of 54.3 years. 18 This was similar to the mean age (55 years) of the Filipino patients who were included in the pooled analysis of the two ‘largest and globally representative’ of HFrEF trials: PARADIGM‐HF and ATMOSPHERE trials. 19 Therefore, this study's starting age for the model was set at 55 years.
The Asian HF registry used the HFrEF definition of EF ≤ 40% similar to DAPA‐HF, while the pooled analysis defined HPrEF as EF ≤ 35%. 6 , 18 , 19
Utilities
The assessment of the quality of life among DAPA‐HF patients used the Kansas City Cardiomyopathy Questionnaire (KCCQ). KCCQ scores were found to correlate with the New York Heart Association (NYHA) Functional Class, whether among patients with HFrEF or HF with preserved ejection fraction (HFpEF). 20 In turn, utility estimates had been derived for each of the NYHA class. 21
In the pooled analysis of the two randomized trials, the left ventricular ejection fraction (LVEF) of Asians was lower than patients from Europe and the USA, but their KCCQ scores were higher. The mean KCCQ clinical summary score of patients from the Philippines was 80.2 with interquartile range of 69.4–96.4. 19 These might be related to the Asians' less severe functional limitation as per their NYHA functional classification (except for China and India). Using the utility inputs for the corresponding KCCQ total scores reported in the McEwan study, the aforementioned KCCQ scores of patients from the Philippines were equivalent to utility values of 0.705 to 0.833. The pooled analysis reported that majority of patients from the Philippines were mostly in NYHA Class I‐II (94.6%) and none in Class IV. 19 Using the Gohler et al. study, the utilities would be 0.714–0.785 (for Class I–II) and 0.624 (for Class III). 21 In consideration of these previous publications, this study's base case analysis used the utility value 0.769, which corresponds to the mean KCCQ score of Filipinos with HF. In terms of dis‐utilities for baseline comorbidities, for example, HF hospitalization or the presence of diabetes, the utility values reported by McEwan et al. 14 were used.
Costs
The study included HF hospitalization cost in the Philippines, cost of dapagliflozin, and cost of treating the adverse events—volume depletion and renal dysfunction.
The cost for HF hospitalization in both government and private hospitals in the country for 2014 was previously published. 22 The cost was converted to 2019 values, being the study's reference year. This was carried out through the use of the consumer price indices for 2014 and 2019. 23 The coverage provided by the Philippine Health Insurance Corporation (PhilHealth) for HF hospitalization (PHP15 700) 24 was used for HF hospitalization cost in the scenario analysis where adverse events were included in the model.
Regarding medication cost, the cost of standard care was not included because the options being compared were standard therapy alone versus standard therapy plus dapagliflozin among patients with HFrEF. The incremental cost would be equivalent to the cost of dapagliflozin. Assuming 100% compliance, this corresponded to the annual cost of giving a daily dose of 10 mg dapagliflozin. In the Philippines, the unit cost of 10 mg dapagliflozin corresponded to its unit price in the local market in 2019 which was PHP46.50 (US$0.90). In mid‐2020, the retail price in two drugstore chains decreased to PHP44.00 (US$0.85). This was because of the implementation of the maximum retail price (MRP) policy of the government. MRP represents the allowed maximum price of a drug in the retail market. 25 In connection with the study's perspective—that of the healthcare provider, the drug cost would be equivalent to the acquisition cost negotiated (without the mark‐up or profit of the retailers) by the government with the manufacturer. However, for dapagliflozin, none exist at present. Given this, a possible negotiated unit cost, PHP40.00, was assumed in the sensitivity analysis.
PhilHealth's coverage for volume depletion and renal dysfunction are PHP4000 and PHP19 300, 24 respectively. These costs were used for these adverse events in the specific scenario analysis mentioned earlier.
Outcomes
After considering the endpoints (reduction in HF hospitalization, mortality, and change in KCCQ scores) reported in the DAPA‐HF trial, incorporation of the Philippine age‐related mortality, and local costs, the outcomes are expressed in incremental cost‐effectiveness ratios (ICERs). The ICER represents the incremental cost for every additional quality‐adjusted life‐year gained from the addition of dapagliflozin to standard therapy among HFrEF patients. To determine whether these ICERs are cost‐effective in the local setting, a comparison with the threshold or ceiling ICER as determined by the local policymakers was performed. The threshold ICER had been reported to be equivalent to the gross domestic product (GDP) per capita. 26 With 2019 as the reference year, the GDP per capita of the Philippines at that time was US$3485.10. 27 This was equivalent to PHP180 514 (rounded off to PHP180 500) using the average foreign exchange rate of 1US$ to Philippine peso in 2019 (1 US$ = PHP51.7958). 28
Discount rates
Recommendations regarding discount rates in economic evaluation studies vary. One of them is to use 3% and 5% in the base‐case analysis and 0%, 3% and 5% for the sensitivity analysis, both for costs and effects. 29 The World Health Organization Guide for CEA recommends a discount rate of 3% for the base‐case analysis, both for costs and effects, while for sensitivity analysis, its advice is to use 6% for costs and 0% for effects. 30 On the other hand, the guidelines for the use of a reference case in the presentation of the results of an economic evaluation include discounting at the real rate of 3% per annum for both costs and health outcomes (effects) and 5% for comparison with existing studies. 31 In consideration of these recommendations, a discount rate of 3% for cost and effects was used in this study.
Sensitivity analyses
Several sensitivity analyses were performed. These included one‐way or univariate (deterministic) sensitivity analysis where the values of some parameters were changed one at a time. Using the lower and higher values of the parameters, the variations in the ICERs were estimated, and a tornado diagram was generated. In addition, scenario analyses, whereby the effect on the ICERs of changing the cost of dapagliflozin, HF hospitalization cost and the inclusion of the cost of adverse events were performed. Analyses for the scenarios involving different populations—‘all’ patients (whether with or without diabetes) or only those with or without diabetes were also performed. Lastly, a probabilistic sensitivity analysis (PSA) involving Monte Carlo simulation of 10 000 iterations was undertaken.
All analyses were performed using Excel 365.
Results
Table 1 shows the annual transition probabilities and the utilities. These values were estimated from the results of DAPA‐HF trial. Data regarding costs derived from other studies are also shown in this table. These data were used in the determination of the corresponding ICERs depending on the analyses undertaken.
Table 1.
Input parameters
| Parameter | Allocation | Base case | Range | Distribution | References |
|---|---|---|---|---|---|
|
Probabilities All Patients: All‐cause death |
Dapagliflozin a | 0.0783 | 0.0658–0.0912 | Beta | McMurray et al. |
| Control b | 0.0938 | 0.0803–0.1077 | Beta | McMurray et al. | |
| Hospitalization for HF | Dapagliflozin | 0.0653 | 0.0537–0.0772 | Beta | McMurray et al. |
| Control | 0.0906 | 0.0773–0.1043 | Beta | McMurray et al. | |
| Utilities | |||||
| Chronic HF (not hospitalized) | 0.7690 | 0.7386–0.8004 | Beta | McEwan et al. and Dewan et al. | |
| Hospitalized for HF | 0.7423 | 0.7119–0.7737 | Beta | McEwan et al. and Dewan et al. | |
|
Probabilities patients with diabetes: All‐cause death |
Dapagliflozin | 0.0898 | 0.0695–0.1101 | Beta | Petrie et al. |
| Control | 0.1137 | 0.0913–0.1361 | Beta | Petrie et al. | |
| Hospitalization for HF | Dapagliflozin | 0.0866 | 0.0666–0.1066 | Beta | Petrie et al. |
| Control | 0.1098 | 0.0876–0.1319 | Beta | Petrie et al. | |
| Utilities | |||||
| Chronic HF (not hospitalized) | 0.7520 | 0.7206–0.7834 | Beta | McEwan et al. and Dewan et al. | |
| Hospitalized for HF | 0.7253 | 0.6939–0.7567 | Beta | McEwan et al. and Dewan et al. | |
|
Probabilities patients without diabetes: All‐cause death |
Dapagliflozin | 0.0688 | 0.0523–0.0853 | Beta | Petrie et al. |
| Control | 0.0778 | 0.0604–0.0951 | Beta | Petrie et al. | |
| Hospitalization for HF | Dapagliflozin | 0.0478 | 0.0338–0.0619 | Beta | Petrie et al. |
| Control | 0.0751 | 0.0581–0.0922 | Beta | Petrie et al. | |
| Utilities | |||||
| Chronic HF (not hospitalized) | 0.7690 | 0.7376–0.8004 | Beta | McEwan et al. and Dewan et al. | |
| Hospitalized for HF | 0.7423 | 0.7109–0.7737 | Beta | McEwan et al. and Dewan et al. | |
| Cost of hospitalization for HF | PHP39 577 | PHP31 901–47 253 | Tumanan‐Mendoza et al. | ||
| Cost of hospitalization for HF | PHP15 700 | PhilHealth | |||
|
Cost of adverse events: Volume Depletion |
PHP4000 | PhilHealth | |||
|
Renal Dysfunction |
PHP19 300 | PhilHealth |
HF, heart failure; PhilHealth, Philippine Health Insurance Corporation; PHP, Philippine peso.
Dapagliflozin plus standard treatment.
Control = standard treatment alone.
Base case, deterministic sensitivity, and scenario analyses
Using the 2019 price of dapagliflozin as the base case, the incremental cost for an additional quality‐adjusted life year was computed to be PHP188 450 (US$3638). This amount is higher than the threshold ICER of PHP180 500 (US$3485); hence, dapagliflozin is deemed not cost‐effective in this scenario. However, the present unit price has gone down to PHP44.00; thus, analyses based on this unit price and a possible negotiated unit cost of dapagliflozin were performed. The resulting ICERs based on the different scenarios are shown in Table 2A . Dapagliflozin is cost‐effective among HFrEF patients if the unit cost is at least PHP44.00; however, it is more cost‐effective if its unit cost is PHP40 and if given to HFrEF patients with diabetes.
Table 2.
Scenario analyses
| A. Population and unit cost of dapagliflozin | ICERs (deterministic analysis) |
| All patients a | At 3% discount rate |
| Dapa @ PHP46.50 | PHP188 450 (US$3638) |
| Dapa @ PHP44.00 | PHP177 868 (US$3434) |
| Dapa @ PHP40.00 | PHP160 983 (US$3108) |
| Diabetics | |
| Dapa @ PHP46.50 | PHP140 290 (US$2708) |
| Dapa @ PHP44.00 | PHP132 582 (US$2560) |
| Dapa @ PHP40.00 | PHP120 249 (US$2321) |
| Non‐diabetics | |
| Dapa @ PHP46.50 | PHP295 131 (US$5698) |
| Dapa @ PHP44.00 | PHP278 286 (US$5372) |
| Dapa @ PHP40.00 | PHP251 333 (US$4852) |
| B. All patients a included in analyses | ICERs (deterministic analysis) |
| Dapagliflozin 10 mg @ PHP44.00 | |
| cHosp = PhilHealth case rate | PHP182 912 (US$3531) |
| cHosp = lower limit | PHP179 490 (US$3465) |
| cHosp = higher limit | PHP176 247 (US$3402) |
| Dapagliflozin 10 mg @ PHP40.00 | |
| cHosp = PhilHealth case rate | PHP165 982 (US$3204) |
| cHosp = lower limit | PHP162 560 (US$3138) |
| cHosp = higher limit | PHP159 317 (US$3076) |
Dapa, dapagliflozin 10 mg tablet; PHP, Philippine peso.
All patients in the DAPA‐HF trial with or without diabetes.
Variations in the HF hospitalization cost apart from variations in the unit cost of dapagliflozin, also resulted in varying ICERs. This is demonstrated in the other scenario analyses (Table 2B ). In these analyses, the population was set to ‘all patients’ (patients with and without diabetes mellitus). The ICERs become more cost‐effective if the lower unit cost of dapagliflozin is combined with the higher hospitalization cost of HF.
The last scenario analysis involved the adverse events—volume depletion and renal dysfunction—as reported in the DAPA‐HF study. Although there were no statistically significant difference between the rates (both less than 8%) in the dapagliflozin and control groups in either adverse event, the ICERs were determined. The population was again set at ‘all patients’. Using the case rates provided by PhilHealth, the resulting ICERs were PHP188 500 and PHP169 910 for the PHP44.00 and PHP40.00 unit cost of dapagliflozin 10 mg tablet, respectively.
The tornado diagram (Figure 2 ) demonstrates the effect of changing one parameter at a time. It shows that the parameter–probabilities of dying (in both control and dapagliflozin arms) had the biggest effect on the ICERs. Changes in these probabilities resulted in a large variation in the ICERs. The diagram also shows the effect of variations in the cost of dapagliflozin on the ICERs. This effect is greater as compared with variations in utilities and probabilities in hospitalization.
Figure 2.
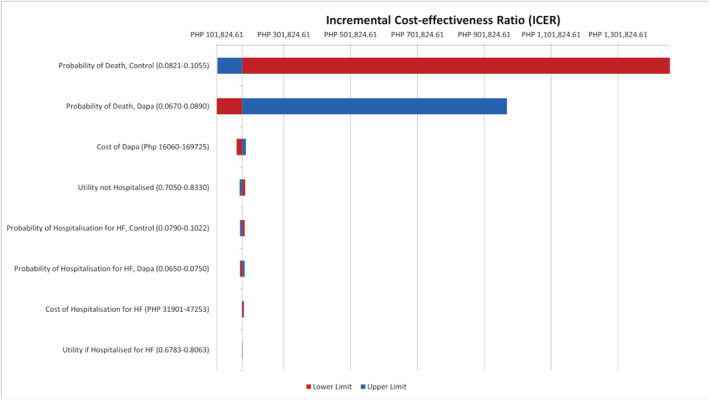
Tornado diagram (deterministic sensitivity analyses). Tornado diagram showing the resulting ICERs across the values of the parameters (one‐way sensitivity analysis). Red corresponds to the lower limit while blue corresponds to the upper limit of the parameter. Dapa, dapagliflozin; HF, heart failure.
Probabilistic sensitivity analysis (PSA)
Adding dapagliflozin to the standard regimen was 58% and 64% cost‐effective for ‘all’ HFrEF patients if the unit cost was PHP44.00 and PHP40.00, respectively. Among diabetics with HFrEF, it was 72% and 76% cost‐effective using the same unit costs. These per cent cost‐effectiveness were obtained by performing probabilistic sensitivity analysis (PSA). Using the population of HFrEF patients with diabetes and dapagliflozin's unit cost of PHP40.00, the scatterplot and corresponding cost‐effectiveness acceptability curve (CEAC) were obtained and are shown in Figures 3 and 4 . Figure 4 demonstrates the CEAC for the addition of dapagliflozin to standard therapy among HFrEF patients with diabetes. It shows that the ICERs below the threshold ICER of PHP180 500 constitute 76% of all the ICERs generated in the scatterplot in Figure 3 .
Figure 3.
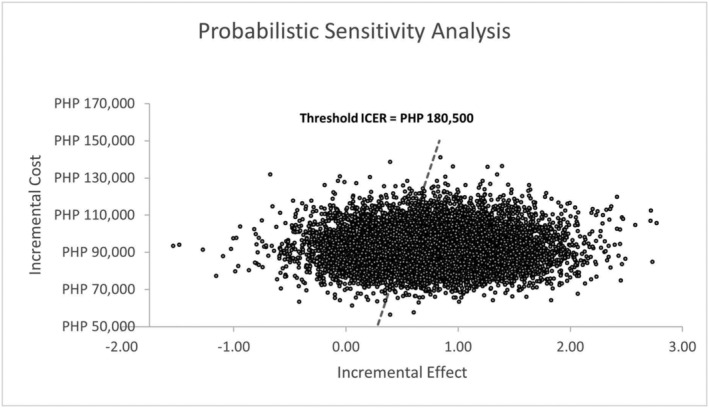
Scatterplot showing the incremental cost per quality‐adjusted life‐year for 10 000 simulations. Jagged line represents the threshold ICER or willingness to pay threshold (PHP180 500). Circles below the dashed line represent the ICERs below the threshold ICER. PHP, Philippine peso.
Figure 4.
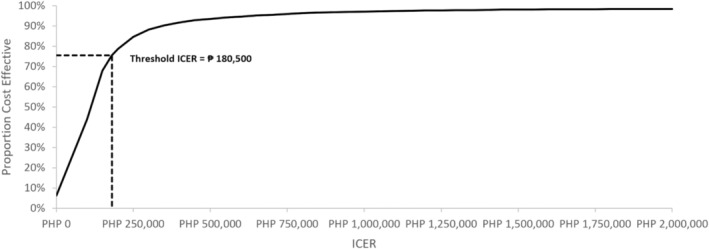
Cost‐effectiveness acceptability curve showing that 76% ICERs are below the threshold ICER of PHP180 500.
On the other hand, even though deterministic analyses have shown that dapagliflozin was not cost‐effective in some scenarios, PSA has shown that dapagliflozin among HFrEF patients who have no diabetes mellitus was cost‐effective 57%, 59%, and 63% of the time if the unit cost was PHP46.50, PHP44.00, and PHP40.00, respectively. If the unit price was PHP46.50, dapagliflozin was cost‐effective 54% of the time if given to ‘all’ patients, that is, irrespective of the presence or absence of diabetes.
Discussion
The DAPA‐HF trial established the efficacy of dapagliflozin on top of standard treatment for HFrEF patients, whether with or without diabetes. 6 , 7 In this CUA, the Philippines age‐adjusted mortality rate and local costs were incorporated to come up with the cost per quality‐adjusted life‐year if dapagliflozin will be added to standard therapy for Filipino patients with HFrEF. The results showed that the incremental cost per quality‐adjusted life‐year using the present unit price of dapagliflozin and possible negotiated unit cost are PHP177 868 (US$3434) and PHP169 983 (US$3108), respectively. These are deemed cost‐effective because they are below the ceiling ICER set by the local policy‐makers.
On the other hand, it was shown that the ICER was lower among HFrEF patients with T2DM; thus, dapagliflozin is more cost‐effective in this population. This can be traced to the higher efficacy rate of add‐on dapagliflozin among those with diabetes (particularly in all‐cause death). This higher efficacy rate translated to higher incremental effectiveness which then led to lower ICER among diabetics. This result of lower ICER among diabetics was also seen in the CUA that was carried out in Thailand. 15
This better efficacy and cost‐effectiveness among diabetics is relevant due to the increasing prevalence of diabetes in the Philippines. The Food and Nutrition Research Institute (FNRI) reported a prevalence of 5.6% in 2013 and 7.9% in 2018 of high fasting blood sugar (FBS ≥ 126) among Filipino aged ≥20 years old. 32
The WHO Choosing Interventions that are Cost‐effective project (WHO‐CHOICE) considers an intervention to be cost‐effective if the cost of preventing one ‘disability‐adjusted‐life‐year (DALY)’ is less than three times its annual GDP per capita, while it is very cost‐effective if it prevents one DALY at a cost of less than one GDP per capita. 33 This recommendation is followed in several countries especially those that do not have a specific ceiling or threshold ICER. In the Philippines, the local policy‐makers have chosen the cut‐off one GDP per capita per QALY as the country's willingness to pay or threshold ICER. 26 This may reflect a more stringent policy in terms of supporting a recommendation regarding the use of a new intervention which in turn may result in the provision of coverage by PhilHealth. On the other hand, some authors recommend the use of other approaches in addition to a threshold ICER in coming up with a decision to adopt or provide coverage for a new intervention. 34 , 35
The year 2019 was chosen as the reference year for the study because this was the time that the study protocol was developed. It was noted that the GDP for this year was higher as compared with the earlier years. This in turn resulted in a higher threshold ICER.
Several sensitivity analyses were performed in the study. These were performed to handle uncertainties in parameters that one encounters in doing an economic evaluation. 31 One such analysis is PSA. As shown in the results, ICERs obtained through deterministic analyses may be deemed outright cost‐effective or not cost‐effective when compared with the threshold ICER. Performing PSA showed the distribution of ICERs that were below the threshold ICER. Through a cost‐effectiveness acceptability curve, the percentage of ICERs below the threshold ICER was determined.
In keeping with the study's public healthcare provider perspective, the drug cost should not correspond to its retail price in the market or drugstore; instead, this should refer to the acquisition cost from the manufacturer by the healthcare provider (which may acquire the drug in bulk). This cost excludes mark‐ups or profits by drugstores in the sale of drugs. However, the study was constrained by the lack of this cost at present. In view of this, the study used the prevailing market price and estimated a possible acquisition cost. The lower possible acquisition cost resulted to lower ICERs, thus making the use of dapagliflozin for HFrEF patients more cost‐effective.
Cost‐utility analyses of the add‐on dapagliflozin had also been undertaken for several countries like Australia, United Kingdom, Germany, Spain, and Thailand. 13 , 14 , 15 Although these studies found that the add‐on dapagliflozin was cost‐effective, there is a need to undertake individual CUA in different countries. This is due to problems in applicability or generalizability of results of economic evaluation studies. Reasons for variability in the results include differences in the following: (i) resources used and their costs, (ii) unit cost of the drug, (iii) healthcare delivery system, (iv) treatment compliance, (v) health state valuations, (vi) perspective of the analysis, and (vii) threshold ICERs. In addition, variations in discount rates may also occur. This was demonstrated in the previous studies—5% was used for the CUA for Australia, 3% for Germany and Spain, 3.5% for UK, and 0–6% per annum for Thailand. 16 , 36 , 37 , 38 , 39 Lastly, it should be noted that ICERs reported in other studies cannot be simply converted to a specific country's currency through the use of foreign exchange rates.
In the Philippines, more than 50% of healthcare delivery is obtained through out‐of‐pocket expenses. PhilHealth provides hospitalization coverage; however, this does not cover the entire hospitalization cost as demonstrated in the study on hospitalization for HF. 22 In addition, for most diseases, provision for maintenance medications is through out‐of‐pocket expenses. This must be considered for chronic conditions like heart failure. The addition of dapagliflozin to the HF regimen in this study was shown to be cost‐effective using the perspective of the public healthcare provider. This has important implications in terms of healthcare policies because one of the requirements of a new drug to be listed in the national formulary is for it to be cost‐effective. Being listed in the formulary on the other hand is a requirement by PhilHealth. Cost‐effectiveness, however, does not translate to affordability. This problem is especially relevant and demonstrated in the individual patient setting. Patients need to shoulder the cost of medications not only for HF but also for other concomitant conditions. For the average Filipino, the daily cost of medications may be prohibitive leading to problems in compliance.
The study has several limitations. As mentioned in the earlier sections, the unit cost of dapagliflozin was based on the prevailing market price and an assumed acquisition cost between the government and the manufacturer. This assumption may be an over or under‐estimation of the cost that the two parties may agree on in the near future. Moreover, the utilities used were derived from the KCCQ scores and NYHA classification of HF patients obtained from the DAPA‐HF trial and other studies where some patients from the Philippines (although a relatively small percentage of the study population) were included.
Conflict of Interest
All authors received grants as mentioned in the section regarding funding. One author (F.E.R.P.) received honoraria as a lecturer regarding heart failure from AstraZeneca Pharmaceuticals (Phils), Inc.
Funding
This was funded by AstraZeneca Pharmaceuticals (Phils), Inc. through a tripartite agreement with De La Salle Medical Health Sciences Institute and the principal investigator. The protocol, data gathering, analysis, and writing of the manuscript were independently performed by the authors.
Mendoza, V. L. , Tumanan‐Mendoza, B. A. , and Punzalan, F. E. R. (2021) Cost‐utility analysis of add‐on dapagliflozin in heart failure with reduced ejection fraction in the Philippines. ESC Heart Failure, 8: 5132–5141. 10.1002/ehf2.13583.
References
- 1. Anderson S. Dapagliflozin efficacy and safety: a perspective review. Ther Adv Drug Saf 2014; 5: 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. SGLT2 inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health 2019; 16: 2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiviot SD, Raz I, Bonada MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde A‐M, Sabatine MS, for the DECLARE‐TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 4. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause‐Nilsson I, Johanson P, Langkilde A‐M, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019; 139:2528–2536. Circulation 2019; 139: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 5. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, Drazner MH, Fong MW, Givertz MM, Gordon RA, Jermyn R, Katz SD, Lamba S, Lanfear DE, LaRue SJ, Lindenfeld JA, Malone M, Margulies K, Mentz RJ, Mutharasan RK, Pursley M, Umpierrez G, Kosiborod M, On behalf of the DEFINE‐HF Investigators , Malik AO, Wenger N, Ogunniyi M, Vellanki P, Murphy B, Newman J, Hartupee J, Gupta C, Goldsmith M, Baweja P, Montero M, Gottlieb SS, Costanzo MR, Hoang T, Warnock A, Allen L, Tang W, Chen HH, Cox JM. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction the DEFINE‐HF trial. Circulation 2019; 140: 1463–1476. [DOI] [PubMed] [Google Scholar]
- 6. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bêlohlávek J, Böhm M, Chiang C‐E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A‐M, for the DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 7. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Bêlohlávek J, Böhm M, Chiang C‐E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, Katowa T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Vinh PN, Schou M, Tereshchenko S, Køber L, Kosiborod MN, Langkilde A‐M, Martinez FA, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD, Johnson P, Greasley PJ, Boulton D, Bengtsson O, Jhund PS, McMurray JJV. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. Published online 27 March 2020; 323: 1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Docherty KF, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, Sjöstrand M, Langkilde AM, Desai AS, Diez M, Howlett JG, Katova T, Ljungman CEA, O'Meara E, Petrie MC, Schou M, Verma S, Vinh PN, Solomon SD, McMurray JJV, the DAPA‐HF Investigators and Committees . Effects of dapagliflozin in DAPA‐HF according to background heart failure therapy. Eur Heart J 2020; 41: 2379–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang C‐E, Tereshchenko S, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA‐HF. Circulation 2020; 141: 100–111. [DOI] [PubMed] [Google Scholar]
- 10. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Køber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjöstrand M, Langkilde AM, McMurray JJV. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction results from the DAPA‐HF trial. Circulation 2020; 141: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seferovic PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, Anker SD, Ray R, Cavusoglu Y, Polovina M, Metra M, Ambrosio G, Prasad K, Seferovic J, Jhund PS, Dattilo G, Celutkiene J, Piepoli M, Moura B, Chioncel O, Gal TB, Heymans S, de Boer RA, Jaarsma T, Hill L, Lopatin Y, Lyon AR, Ponikowski P, Lainscak M, Jankowska E, Mueller C, Cosentino F, Lund L, Filippatos GS, Ruschitzka F, Coats AJS, Rosano GMC. Sodium‐glucose co‐transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 1495–1503. [DOI] [PubMed] [Google Scholar]
- 12. Maddox TM, Januzzi JL, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, Motiwala SR, Oliveros E, Patterson JH, Walsh MN, Wasserman A, Yancy CW, Youmans QR. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American college of cardiology solution set oversight committee. Expert consensus decision pathway. J Am Coll Cardiol 2021; 77: 772–210. [DOI] [PubMed] [Google Scholar]
- 13. Savira F, Wang BH, Kompa AR, Ademi Z, Owen AJ, Zoungas S, Tonkin A, Liew D, Zomer E. Cost‐effectiveness of dapagliflozin in chronic heart failure: an analysis from the Australian healthcare perspective. Eur J Prev Cardiol 2020; 0: 1–10. [DOI] [PubMed] [Google Scholar]
- 14. McEwan P, Darlington O, McMurray JJV, Jhund PS, Docherty KF, Böhm M, Petrie MC, Bergenheim K, Quin L. Cost‐effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health economic analysis of DAPA‐HF. Eur J Heart Fail 2020; 22: 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krittayaphong R, Permsuwan U. Cost‐utility analysis of add‐on dapagliflozin treatment in heart failure with reduced ejection fraction. Int J Cardiol 2020; 322: 183–190. [DOI] [PubMed] [Google Scholar]
- 16. Barbieri M, Drummond M, Wilke R, Chancellor J, Jolain B, Towse A. Variability of cost‐effectiveness estimates for pharmaceuticals in Western Europe: lessons for inferring generalizability. Value Health 2005; 8: 10–23. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . Global Health Observatory data repository. https://apps.who.int/gho/data/view.main.LT62150 (accessed 20 July 2020).
- 18. Lam CSP, Teng THK, Tay WT, Anand I, Zhang S, Shimizu W, Narasimhan C, Park SW, Yu C‐M, Ngarmukos T, Omar R, Reyes EB, Siswanto BB, Hung C‐L, Ling LH, Yap J, MacDonald M, Richards AM. Regional and ethnic differences among patients with heart failure in Asia; the Asian sudden cardiac death in heart failure registry. Eur Heart J 2016; 37: 3141–3153. [DOI] [PubMed] [Google Scholar]
- 19. Dewan P, Jhund PS, Shen L, Petrie MC, Abraham WT, Ali MA, Chen C‐H, Desai AS, Dickstein K, Huang J, Kiatchoosakun S, Kim K‐S, Køber L, Lai W‐T, Liao Y, Mogensen UM, Oh B‐H, Packer M, Rouleau JL, Shi V, Siburo AS Jr, Solomon SD, Sritara P, Swedberg K, Tsutsui H, Zile MR, McMurray JJV. Heart failure with reduced ejection fraction: comparison of patient characteristics and clinical outcomes within Asia and between Asia, Europe and the Americas. Eur J Heart Fail 2019; 21: 577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joseph SM, Novak E, Arnold SV, Jones PG, Khattak H, Platts AE, Dávila‐Román VG, Mann DL, Spertus JA. Comparable performance of the Kansas City cardiomyopathy questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail 2013; 6: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gohler A, Geisler BP, Manne JM, Kosiborod M, Zhang Z, Weintraub WS, Spertus JA, Gazelle GS, Siebert U, Cohen DJ. Utility estimates for decision‐analytic modeling in chronic heart failure–health states based on New York Heart Association classes and number of rehospitalizations. Value Health 2009; 12: 185–187. [DOI] [PubMed] [Google Scholar]
- 22. Tumanan‐Mendoza BA, Mendoza VL, Bermudez‐Delos Santos AAA, Punzalan FER, Pestano NS, Natividad RB, Shiu LA, Macabeo R, Lam HY. Economic burden of hospitalisation for congestive heart failure among adults in the Philippines. Heart Asia 2018; 10: e011039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The World Bank . Consumer price index (2010 = 100) Philippines. https://data.worldbank.org/indicator/FP.CPI.TOTL?locations+PH (accessed 19 November 2020).
- 24. PhilHealth . List of medical case rates (updated February 2017). https://www.philhealth.gov.ph/circulars/2017/annexes/0019/AnnexA‐MedicalCaseRates.pdf (accessed 29 November 2020).
- 25. Department of Health Republic of the Philippines . Implementation of the executive order Nol. 104 s. 2020: maximum retail price of drug and medicine. https://doh.gov.ph/implementation‐of‐eo104 (accessed 1 March 2021).
- 26. Genuino AJ, Chaikledkaew U, Guerrero AM, Reungwetwattana T, Thakkinstian A. Cost‐utility analysis of adjuvant trastuzumab therapy for HER2‐positive early‐stage breast cancer in the Philippines. BMC Health Serv Res 2019; 19: 874 (19 November 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The World Bank . GDP per capita (current US$) – Philippines. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=PH) (accessed 10 January 2021).
- 28. Bangko Sentral ng Pilipinas . Tab 12 Philippine peso per US dollar exchange rate. https://www.bsp.gov.ph/statistics/external/Table%2012.pdf (accessed 10 January 2021).
- 29. Drummond M. F., Sculpher M. J., Torrance G. W., O'Brien B. J., Stoddart G. L., eds. Cost Analysis. In Methods for the economic evaluation of health care programmes 3d ed. Oxford University Press; 2005. p 55–101. [Google Scholar]
- 30. Tan Torres‐Edejer T., Baltussen R., Adam T., Hutubessy R., Acharya A., Evans D. B., Murray C. J. L., eds. Discounting. In Making choices in health: WHO guide to cost‐effectiveness analysis. World Health Organization; 2003. p 67–71. [Google Scholar]
- 31. Drummond M. F., Sculpher M. J., Claxton K., Stoddart G. L., Torrance G. W., eds. Critical Assessment of Economic Evaluation. In Methods for the economic evaluation of health care programmes, 4th ed. Oxford University Press; 2015. p 41–76. [Google Scholar]
- 32. Department of Science and Technology Food and Nutrition Research Institute . Health and Nutritional Status of Filipino Adults, 20–59 years old. https://www.fnri.dost.gov.ph/images//sources/eNNS2018/Adults_and_Elderly.pdf (accessed 12 March 2021).
- 33.Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; 2001. http://apps.who.int/iris/bitstream/10665/42435/1/924154550X.pdf (accessed 13 March 2021).
- 34. Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost‐effectiveness of interventions: alternative approaches. Bull World Health Organ 2015; 93: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny M‐P, Hill SR. Cost‐effectiveness thresholds: pros and cons. Bull World Health Organ 2016; 94: 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ginsberg GM. Generalizability of cost‐utility analyses across countries and settings. Best Pract Res Clin Gastroenterol 2013; 27: 845–852. [DOI] [PubMed] [Google Scholar]
- 37. Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, Davies AM, Eastwood A. Generalisability in economic evaluation studies in health care: a review and case studies. Health Technol Assess 2004; 8: 1–192. [DOI] [PubMed] [Google Scholar]
- 38. Barbieri M, Drummond M, Rutten F, Cook J, Glick HA, Lis J, Reed SD, Sculpher M, Severens JL, the ISPOR Good Research Practices Economic Data Transferability Task Force . What do international pharmacoeconomic guidelines say about economic data transferability? Value Health 2010; 13: 1028–1037. [DOI] [PubMed] [Google Scholar]
- 39. Drummond M, Manca A, Sculpher M. Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int J Technol Assess Health Care 2005; 21: 165–171. [PubMed] [Google Scholar]


