Abstract
Aims
Rheumatic heart disease (RHD) remains a major global health problem. Renin–angiotensin–aldosterone system inhibitors (RAASi) are commonly administered in the treatment of cardiovascular disease, but its role in RHD patients is still limited. We performed a retrospective study to determine the effect of RAASi on long‐term outcomes for RHD patients.
Methods and results
A 1:1 propensity score matching was implemented to balance baseline characteristics between groups RAASi and non‐RAASi. Cox proportional hazards regression model was used to investigate the associations of RAASi with the risks of all‐cause mortality, cardiovascular death (CVD), and cerebrovascular death. Binary logistic regression analysis was used to evaluate the associations of RAASi with the risks of 1, 3, and 5 year heart failure (HF) rehospitalization, new‐onset atrial fibrillation (AF), and new‐onset stroke. A total of 734 RHD patients were enrolled as study participants; nearly half of these participants had combined valve damage (54.4%), worse New York Heart Association functional class status (III and IV, 55.2%), surgical treatment (54.2%), and AF (65.0%). After propensity score matching, 514 RHD patients were finally analysed. RAASi treatment was associated with decreased risks of all‐cause mortality [adjusted hazard ratio (HR) = 0.52, 95% confidence interval (CI): 0.37–0.73, P < 0.001], CVD (adjusted HR = 0.48, 95% CI: 0.30–0.76, P = 0.002), and cerebrovascular death (adjusted HR = 0.22, 95% CI: 0.08–0.60, P = 0.003). Further subgroup analysis showed that RAASi treatment was associated with decreased risks of all‐cause mortality (adjusted HR = 0.50, 95% CI: 0.31–0.79, P = 0.004), CVD (adjusted HR = 0.48, 95% CI: 0.25–0.91, P = 0.025), and cerebrovascular death (adjusted HR = 0.19, 95% CI: 0.05–0.65, P = 0.008) in RHD patients without surgical treatment, and better effect was observed in RHD patients with surgical treatment on the risks of all‐cause mortality (adjusted HR = 0.47, 95% CI: 0.26–0.85, P = 0.012) and CVD (adjusted HR = 0.43, 95% CI: 0.21–0.90, P = 0.024) except cerebrovascular death (adjusted HR = 0.52, 95% CI: 0.08–3.36, P = 0.491). RAASi treatment was associated with decreased HF rehospitalization risk of 1 year [adjusted odds ratio (OR) = 0.38, 95% CI: 0.23–0.61, P < 0.001], 3 year (adjusted OR = 0.43, 95% CI: 0.28–0.68, P < 0.001), and 5 year (adjusted OR = 0.48, 95% CI: 0.30–0.77, P = 0.002) as well as new‐onset AF risk (adjusted OR = 0.38, 95% CI: 0.21–0.68, P = 0.001). RAASi treatment had nothing to do with new‐onset stroke risk (adjusted OR = 0.80, 95% CI: 0.47–1.38, P = 0.428).
Conclusion
Renin–angiotensin–aldosterone system inhibitor treatment was significantly associated with decreased risks of mortality, HF rehospitalization, and new‐onset AF in RHD patients in median 5.9 year follow‐up.
Keywords: Renin–angiotensin system inhibitors, Rheumatic heart disease, Survival, Heart failure, Atrial fibrillation, Stroke
Introduction
Rheumatic heart disease (RHD) is virtually eliminated in most developed regions, but the burden of morbidity and mortality remains high in developing countries as well as underdeveloped regions of developed countries. 1 Reported cases likely underestimate the true global burden of the disease, based on accumulating data on subclinical RHD, and RHD remains a major global health problem. 2 Existing tertiary prevention involves surgical and medical treatment for complications of RHD, which remains the main interventions for reducing RHD mortality. 3
Surgical intervention to relieve valvular damage, mainly including the percutaneous balloon valvuloplasty, valvular commissurotomy, valve repair, and/or valve replacement, is the preferred treatment for RHD. 4 Surgical approaches improve the short‐term/long‐term prognosis of RHD patients, but it manifested the characteristics of timeliness effect. Compared with the improvement of short‐term prognosis on RHD patients, the benefits of long‐term prognosis are worse, and the mortality and valve‐related complications [e.g. heart failure (HF) rehospitalization, atrial fibrillation (AF), and thrombo‐embolism] are higher. 5 , 6 Despite this, it is largely unavailable to most RHD patients in the realistic clinical practice in developing countries because of social, educational, and economic conditions, especially for those patients with advanced stage or high surgical risk due to combination with other disease. Under these circumstances, it is necessary to explore alternative strategies in drug to surgical treatment for improving prognosis for RHD patients.
Rheumatic heart disease‐induced myocardial injury and left ventricular dysfunction are associated with mechanical damage of the valve itself, rather than direct involvement of myocardium. Thus, it cannot be eliminated or effectively alleviated without surgery. However, while standard antibiotics and surgical treatment eliminate clinical manifestations of rheumatic activity and subclinical persistent myocarditis, chronic non‐specific inflammation persists in myocardial, atrial, and valvular tissue. 7 Such damage leads to progressive development of myocardial, atrial, and valvular lesions and eventually results in poor surgical tolerance and a poor long‐term prognosis. The activation of local renin–angiotensin–aldosterone system (RAAS) plays an important role in this process. 8 Cong et al. 9 found that the expression of angiotensin II receptor type 1 in left atrium tissue samples was obviously increased in RHD patients with AF during cardiac surgery. Renin–angiotensin–aldosterone system inhibitors (RAASi), as RAAS antagonist [e.g. angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs)], not only reverse cardiac remodelling but also have anti‐inflammatory effects, which can theoretically improve the prognosis of RHD patients. The latest clinical guidelines recommend RAASi only for HF patients with valvular heart disease who are deemed unsuitable for surgical treatment and patients with persistent HF symptoms following valve surgery, 4 but the role of RAASi in the management of RHD patients is still poorly understood. A few previous studies enrolled small numbers of idealized RHD patients with any single valve–single lesion and reported subjective or surrogate outcomes (e.g. symptom relief, exercise capacity, and echocardiographic parameters). Neither of these studies were designed or powered to evaluate hard outcomes in relation to RAASi use in RHD patients with real and complex valve damage. In present retrospective real‐world study, we tried to assess the association of RAASi with long‐term outcomes in RHD patients, especially in combination with surgical treatment.
Methods
Study participants
The retrospective propensity‐matched study was reviewed and approved by the Ethics Committee of Guangzhou First People's Hospital, South China University of Technology, Guangzhou, China (K‐2018‐136‐1). All patients diagnosed with RHD were identified from the South China Cardiovascular related Disease Cohort between 1 January 1999 and 31 December 2018 (the final follow‐up date). Clinical data were collected from patient interviews, review of medical records, and contact with treating physicians. The diagnosis and severity of valve lesions were ascertained by using the standard criteria. 10 Patients were entered into the study from the date of first diagnosis of RHD with any locations (mitral, aortic, tricuspid, or combined valves) and conditions (stenosis or regurgitation), regardless of with or without RAASi and/or valve surgical treatment. Survival duration was measured from the date of enrolment to date of mortality or to date of last follow‐up. Patients missing/invalid clinical data and any contraindication of using RAASi were excluded from the study. Participants in RAASi group were those who had a prescription of any ACEIs or ARBs and can tolerate treatment via a 2 week initial titration according to the principle of minimum use of RAASi. The duration of RAASi treatment was calculated from the initial date of RAASi use to date of mortality or to date of last follow‐up. Participants in non‐RAASi group were those who were never prescribed with RAASi. RHD surgical treatment was defined as surgical or percutaneous intervention for valve repair or replacement of any affected valve(s) (e.g. mitral, aortic, and tricuspid) using tissue or mechanical prosthesis according to the updated guidelines. HF at baseline and during the study (recurrent HF) was diagnosed according to Framingham HF criteria. 11 At least one overnight admission to hospital was considered a rehospitalization event during follow‐up. AF was diagnosed according to prior history of AF or electrocardiographic findings at enrolment as well as during follow‐up. Coexisting medical conditions were evaluated according to relevant guidelines as follows: hypertension (HT), 12 coronary heart disease, 13 HF, 14 type 2 diabetes mellitus, 15 and stroke. 16 , 17 Cardiac standard chamber quantification was determined by echocardiography according to recommendations from the European Association of Echocardiography. 18 Biochemical tests were performed using standard chemical lab methods.
Propensity score matching
Because the RAASi group was smaller than the non‐RAASi group, an imbalance in crucial covariates related to outcomes could have biased the estimation of the RAASi treatment effect. To adjust for other baseline factors, we performed a 1:1 propensity score matching using SPSS Version 24 (SPSS, Chicago, IL, USA) referring to our previous method with minor modifications. 14 The propensity score was generated through logistic regression to predict the probability of effectiveness of RAASi use as a function of baseline factors as follows: age, sex, course, smoking, drinking, waiting time for surgery, cardiac valve damage, surgical intervention, New York Heart Association (NYHA) functional classification, medical condition, combined medication, and level of partial blood biochemical index. The calliper width for PSM was 0.1. After propensity score matching, a total of 514 RHD patients finally entered the study (Supporting Information, Figure S1 ).
Echocardiography
All patients underwent cardiac ultrasonic scanning at the time of first diagnosis and within 3 months before the end of follow‐up, respectively. Left ventricular end‐diastolic diameter, left atrial end‐systolic diameter (LAD), and right ventricular end‐diastolic diameter (RVD) measured from the parasternal long‐axis view and right atrial end‐systolic diameter measured from the apical four‐chamber view were determined using M‐mode or two‐dimensional echocardiography with a 1.7/3.4 MHz linear array transducer (Vivid 7, GE Healthcare, Chicago, IL, USA) over four cardiac cycles according to recommendations for chamber quantification from European Association of Echocardiography. 18 Left ventricular ejection fraction was determined using the biplane Simpson model.
Long‐term follow‐up endpoints
Primary endpoints were the risks of all‐cause mortality, cardiovascular death (CVD), and cerebrovascular death. The all‐cause mortality was defined as death from any cause. The CVD was defined as death resulting from acute myocardial infarction, HF, significant cardiac arrhythmia, sudden death, pulmonary arterial HT, death occurring during a cardiovascular‐related procedure, or other cardiovascular causes. The cerebrovascular death was defined as death due to cerebral infarction or cerebral haemorrhage. Secondary endpoints were the risks of HF rehospitalization, new‐onset AF, and new‐onset stroke. HF rehospitalization was defined as a readmission for which HF was the primary cause (including at least one overnight admission to emergency). The new‐onset AF was defined as those who present for the first time with persistent or paroxysmal AF during follow‐up period, regardless of whether the duration of the arrhythmia is known at the time of presentation. The new‐onset stroke was defined as the presence of a focal/global neurological event with symptoms and signs lasting >24 h including haemorrhagic stroke, ischaemic stroke, cardiogenic stroke, or transient ischaemic attack and determined by magnetic resonance image and/or computed tomography scanning of the brain. Three information sources were queried to identify primary and secondary endpoints: participants and their families, medical records, and the Center for Disease Control and Prevention.
Statistical analysis
All statistical analyses were performed using SPSS Version 24 (SPSS). Categorical variables were presented as numbers and percentages. Continuous variables were presented as mean ± standard deviation. The χ 2 test or Fisher's exact test was used to compare categorical variables, while two‐way ANOVA or independent‐sample t‐test was for continuous variables. The Cox proportional hazards regression model for survival analysis was fitted to estimate the crude hazard ratios (HRs), adjusted HRs, and their 95% confidence intervals (CIs) with adjustments for potential confounders. Binary logistic regression analysis was used to evaluate the odds ratios (ORs) of 1, 3, and 5 year HF rehospitalization, new‐onset AF, and new‐onset stroke. A P value less than 0.05 was considered statistically significant. All probabilities are two tailed.
Results
Characteristics of study participants
Baseline clinical and echocardiographic characteristics of the study patients before propensity score matching (PSM) are listed in Table 1 . Nearly half of these participants had combined valve damage (54.4%), worse NYHA functional class status (III and IV, 55.2%), surgical treatment (54.2%), and AF (65.0%). Of particular interest, subjects with or without RAASi showed significant differences on the constituent ratio of NYHA functional classification (P < 0.001), HT (P < 0.001), coronary heart disease (P = 0.041), HF (P = 0.040), type 2 diabetes mellitus (P < 0.001), beta‐receptor blockers (P = 0.002), mineralocorticoid receptor antagonist (P < 0.001), calcium channel blockers (P < 0.001), and statins (P < 0.001) as well as levels of LAD (P = 0.002) and RVD (P = 0.007). After PSM, there was no significant difference between RAASi and non‐RAASi groups on those baseline clinical characteristics (Table 1 ). A total of 514 RHD patients were finally included in the study with median follow‐up of 5.9 years, a median age of 51 years (inter‐quartile range: 44–58 years), and 66.5% female.
Table 1.
Baseline characteristics of study participants
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Non‐RAASi | RAASi | P value | Non‐RAASi | RAASi | P value | |
| N | 410 | 324 | — | 257 | 257 | — |
| Types of RAASi (ACEIs:ARBs) | — | 194:130 | — | — | 164:93 | — |
| ≥1/2 conventional target dose of RAASi (ACEIs:ARBs) | — | 128:88 | — | — | 125:62 | — |
| Duration of RAASi use (years) | — | 6.9 ± 4.4 | — | — | 6.7 ± 4.3 | — |
| Male:female | 127:283 | 104:220 | 0.745 | 88:169 | 84:173 | 0.708 |
| Age (years) | 51.8 ± 11.7 | 50.6 ± 8.6 | 0.122 | 51.7 ± 12.5 | 50.0 ± 8.9 | 0.076 |
| Course (years) | 9.9 ± 11.8 | 10.4 ± 9.3 | 0.539 | 10.4 ± 12.0 | 10.1 ± 8.8 | 0.721 |
| Smoking (%) | 48 (11.7) | 34 (10.5) | 0.604 | 32 (12.5) | 28 (10.9) | 0.583 |
| Drinking (%) | 48 (11.7) | 39 (12.0) | 0.891 | 35 (13.6) | 31 (12.1) | 0.598 |
| Waiting time for surgery (years) | 3.1 ± 5.7 | 3.4 ± 5.1 | 0.591 | 3.4 ± 6.6 | 3.7 ± 5.3 | 0.686 |
| SBP at initial diagnosis (mmHg) | 121.1 ± 12.0 | 120.4 ± 8.6 | 0.414 | 123.1 ± 16.4 | 125.5 ± 16.9 | 0.168 |
| DBP at initial diagnosis (mmHg) | 73.6 ± 11.3 | 74.0 ± 10.1 | 0.664 | 75.1 ± 11.8 | 75.5 ± 12.7 | 0.709 |
| HR at initial diagnosis (b.p.m.) | 85 ± 18 | 84 ± 19 | 0.236 | 85 ± 19 | 84 ± 19 | 0.124 |
| Cardiac valve damage | ||||||
| (A‐I) MS | 312 (76.1) | 230 (71.0) | 0.118 | 186 (72.4) | 189 (73.5) | 0.766 |
| (A‐II) MS degree | ||||||
| Mild | 163 (52.2) | 132 (57.4) | 0.245 | 93 (50.0) | 106 (56.1) | 0.493 |
| Moderate | 86 (27.6) | 64 (27.8) | 55 (29.6) | 50 (26.5) | ||
| Severe | 63 (20.2) | 34 (14.8) | 38 (20.4) | 33 (17.5) | ||
| (B‐I) MR | 230 (56.1) | 188 (58.0) | 0.601 | 159 (61.9) | 139 (54.1) | 0.074 |
| (B‐II) MR degree | ||||||
| Mild | 78 (33.9) | 74 (39.4) | 0.103 | 53 (33.3) | 54 (38.8) | 0.323 |
| Moderate | 54 (23.5) | 53 (28.2) | 40 (25.2) | 39 (28.1) | ||
| Severe | 98 (42.6) | 61 (32.4) | 66 (41.5) | 46 (33.1) | ||
| (C‐I) AS | 91 (22.2) | 87 (26.9) | 0.144 | 61 (23.7) | 72 (28.0) | 0.268 |
| (C‐II) AS degree | ||||||
| Mild | 52 (57.1) | 48 (55.2) | 0.938 | 38 (62.3) | 43 (59.7) | 0.722 |
| Moderate | 27 (29.7) | 26 (29.9) | 18 (29.5) | 20 (27.8) | ||
| Severe | 12 (13.2) | 13 (14.9) | 5 (8.2) | 9 (12.5) | ||
| (D‐I) AR | 138 (33.7) | 123 (38.0) | 0.226 | 95 (37.0) | 99 (38.5) | 0.716 |
| (D‐II) AR degree | ||||||
| Mild | 64 (46.4) | 58 (47.2) | 0.712 | 44 (46.3) | 45 (45.5) | 0.433 |
| Moderate | 60 (43.5) | 49 (39.8) | 43 (45.3) | 40 (40.4) | ||
| Severe | 14 (10.1) | 16 (13.0) | 8 (8.4) | 14 (14.1) | ||
| (E‐I) TR | 113 (27.6) | 86 (26.5) | 0.758 | 82 (31.9) | 64 (24.9) | 0.078 |
| (E‐II) TR degree | ||||||
| Mild | 36 (31.9) | 32 (37.2) | 0.478 | 27 (32.9) | 21 (32.8) | 0.565 |
| Moderate | 35 (31.0) | 29 (33.7) | 26 (31.7) | 25 (39.1) | ||
| Severe | 42 (37.1) | 25 (29.1) | 29 (35.4) | 18 (28.1) | ||
| (F) Single valve damage (stenosis or regurgitation) | ||||||
| MV | 159 (92.4) | 152 (93.3) | 0.944 | 90 (90.9) | 121 (93.1) | 0.833 |
| AV | 11 (6.4) | 9 (5.5) | 7 (7.1) | 7 (5.4) | ||
| TV | 2 (1.2) | 2 (1.2) | 2 (2.0) | 2 (1.5) | ||
| (G) Combined valve damage (stenosis or regurgitation) | ||||||
| MV + AV | 123 (51.7) | 81 (50.3) | 0.923 | 74 (46.8) | 65 (51.2) | 0.747 |
| MV + TV | 70 (29.4) | 47 (29.2) | 49 (31.0) | 35 (27.6) | ||
| MV + AV + TV | 45 (18.9) | 33 (20.5) | 35 (22.2) | 27 (21.2) | ||
| Surgical intervention | ||||||
| (A) Valve replacement (tissue or mechanical prosthesis) | ||||||
| MV | 145 (67.8) | 109 (69.0) | 0.569 | 84 (66.1) | 96 (69.6) | 0.896 |
| AV | 8 (3.7) | 9 (5.7) | 6 (4.7) | 6 (4.3) | ||
| MV + AV | 59 (27.6) | 37 (23.4) | 35 (27.6) | 33 (23.9) | ||
| MV + TV | 2 (0.9) | 3 (1.9) | 2 (1.6) | 3 (2.2) | ||
| (B) Valve repair | ||||||
| MV | 15 (50.0) | 15 (60.0) | 0.139 | 9 (50.0) | 14 (60.9) | 0.429 |
| TV | 15 (50.0) | 7 (28.0) | 9 (50.0) | 7 (30.4) | ||
| AV | 0 (0.0) | 2 (8.0) | 0 (0.0) | 1 (4.3) | ||
| MV + TV | 0 (0.0) | 1 (4.0) | 0 (0.0) | 1 (4.3) | ||
| NYHA | ||||||
| I | 40 (9.8) | 54 (16.7) | <0.001 | 36 (14.0) | 42 (16.3) | 0.380 |
| II | 117 (28.5) | 118 (36.4) | 82 (31.9) | 84 (32.7) | ||
| III | 194 (47.3) | 109 (33.6) | 108 (42.0) | 91 (35.4) | ||
| IV | 59 (14.4) | 43 (13.3) | 31 (12.1) | 40 (15.6) | ||
| Medical condition | ||||||
| HT | 52 (12.7) | 134 (41.4) | <0.001 | 52 (20.2) | 69 (26.8) | 0.077 |
| CHD | 22 (5.4) | 30 (9.3) | 0.041 | 13 (5.1) | 14 (5.4) | 0.843 |
| HF | 378 (92.2) | 284 (87.7) | 0.040 | 227 (88.3) | 222 (86.4) | 0.507 |
| T2D | 24 (5.9) | 53 (16.4) | <0.001 | 23 (8.9) | 22 (8.6) | 0.876 |
| AF | 155 (37.8) | 138 (42.6) | 0.188 | 97 (37.7) | 118 (45.9) | 0.060 |
| Stroke | 44 (10.7) | 37 (11.4) | 0.768 | 28 (10.9) | 33 (12.8) | 0.495 |
| Combined medication | ||||||
| Antiplatelet drugs | 69 (16.8) | 51 (15.7) | 0.692 | 44 (17.1) | 39 (15.2) | 0.549 |
| Warfarin | 230 (56.1) | 204 (63.0) | 0.060 | 146 (56.8) | 159 (61.9) | 0.243 |
| Diuretic | 247 (60.2) | 215 (66.4) | 0.089 | 164 (63.8) | 168 (65.4) | 0.712 |
| Digoxin | 240 (58.5) | 180 (55.6) | 0.418 | 156 (60.7) | 144 (56.0) | 0.418 |
| Nitrates | 46 (11.2) | 52 (16.0) | 0.056 | 33 (12.8) | 36 (14.0) | 0.698 |
| BBs | 118 (28.8) | 129 (39.8) | 0.002 | 88 (34.2) | 89 (34.6) | 0.926 |
| MRA | 208 (50.7) | 217 (67.0) | <0.001 | 154 (59.9) | 168 (65.4) | 0.202 |
| CCBs | 17 (4.1) | 54 (16.7) | <0.001 | 16 (6.2) | 21 (8.2) | 0.394 |
| Statins | 27 (6.6) | 54 (16.7) | <0.001 | 23 (8.9) | 24 (9.3) | 0.878 |
| Blood biochemical index | ||||||
| WBC (× 109/L) | 7.76 ± 3.49 | 7.77 ± 3.36 | 0.975 | 7.73 ± 3.22 | 7.78 ± 3.48 | 0.860 |
| HGB (g/L) | 123.4 ± 20.4 | 120.6 ± 19.1 | 0.056 | 123.8 ± 20.8 | 121.0 ± 19.4 | 0.114 |
| PLT (× 109/L) | 189.5 ± 71.6 | 187.0 ± 71.9 | 0.638 | 190.0 ± 72.5 | 185.7 ± 73.7 | 0.511 |
| FBG (mmol/L) | 5.70 ± 2.31 | 5.87 ± 2.22 | 0.321 | 5.83 ± 2.42 | 5.61 ± 1.85 | 0.253 |
| ALT (U/L) | 21.5 ± 16.3 | 24.6 ± 32.8 | 0.091 | 21.4 ± 15.1 | 25.7 ± 36.1 | 0.086 |
| AST (U/L) | 31.2 ± 20.5 | 33.7 ± 27.2 | 0.189 | 30.7 ± 19.7 | 35.0 ± 28.2 | 0.054 |
| Cr (μmol/L) | 86.1 ± 39.0 | 90.5 ± 36.8 | 0.120 | 87.8 ± 37.0 | 91.6 ± 38.2 | 0.256 |
| CRP (mg/L) | 14.5 ± 27.1 | 15.4 ± 27.1 | 0.711 | 13.9 ± 24.9 | 16.6 ± 29.5 | 0.352 |
| ASO (U/mL) | 53.2 ± 74.9 | 58.4 ± 81.1 | 0.623 | 54.0 ± 76.9 | 61.6 ± 84.3 | 0.533 |
| RF (U/mL) | 12.1 ± 25.4 | 11.1 ± 23.2 | 0.770 | 12.4 ± 29.8 | 10.4 ± 22.6 | 0.627 |
| ESR (mm/h) | 21.9 ± 17.9 | 24.5 ± 24.0 | 0.290 | 21.1 ± 17.1 | 24.0 ± 25.3 | 0.336 |
| BNP (pg/mL) | 1146.2 ± 3038.4 | 1099.9 ± 2716.3 | 0.832 | 1296.5 ± 3506.9 | 1228.9 ± 2919.7 | 0.814 |
| TRIG (mmol/L) | 1.21 ± 1.04 | 1.25 ± 1.01 | 0.630 | 1.18 ± 1.13 | 1.19 ± 1.01 | 0.926 |
| TC (mmol/L) | 4.36 ± 1.35 | 4.24 ± 1.10 | 0.172 | 4.37 ± 1.36 | 4.18 ± 1.08 | 0.077 |
| HDL‐C (mmol/L) | 1.16 ± 0.78 | 1.12 ± 0.35 | 0.358 | 1.14 ± 0.33 | 1.12 ± 0.34 | 0.490 |
| LDL‐C (mmol/L) | 2.49 ± 1.00 | 2.38 ± 0.82 | 0.096 | 2.45 ± 0.99 | 2.35 ± 0.82 | 0.221 |
| Na+ (mmol/L) | 139.5 ± 4.0 | 139.3 ± 4.5 | 0.502 | 139.4 ± 4.0 | 139.1 ± 4.3 | 0.493 |
| K+ (mmol/L) | 3.98 ± 0.49 | 3.95 ± 0.46 | 0.290 | 3.98 ± 0.50 | 3.95 ± 0.47 | 0.513 |
| Echocardiography | ||||||
| LVD (cm) | 4.65 ± 0.65 | 4.70 ± 0.87 | 0.515 | 4.73 ± 0.81 | 4.71 ± 0.85 | 0.749 |
| LAD (cm) | 4.94 ± 1.27 | 4.62 ± 0.97 | 0.002 | 4.77 ± 1.15 | 4.72 ± 1.08 | 0.604 |
| RVD (cm) | 2.15 ± 0.81 | 1.97 ± 0.61 | 0.007 | 2.18 ± 0.81 | 2.13 ± 0.67 | 0.428 |
| RAD (cm) | 3.78 ± 0.89 | 3.67 ± 0.71 | 0.129 | 3.76 ± 0.93 | 3.72 ± 0.71 | 0.535 |
| LVEF (%) | 57.6 ± 8.7 | 56.8 ± 9.0 | 0.330 | 57.2 ± 9.1 | 57.1 ± 9.2 | 0.859 |
ACEIs, angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ALT, alanine aminotransferase; AR, aortic regurgitation; ARBs, angiotensin receptor blockers; AS, aortic stenosis; ASO, antistreptolysin O; AST, aspartate aminotransferase; AV, aortic valve; BBs, beta‐receptor blockers; BNP, B‐type natriuretic peptide; CCBs, calcium channel blockers; CHD, coronary heart disease; Cr, creatinine; CRP, C‐reactive protein; DBP, diastolic blood pressure; ESR, erythrocyte sedimentation rate; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; HGB, haemoglobin; HR, heart rate; HT, hypertension; LAD, left atrial end‐systolic diameter; LDL‐C, low‐density lipoprotein cholesterol; LVD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; MS, mitral stenosis; MV, mitral valve; NYHA, New York Heart Association; PLT, platelet count; PSM, propensity score matching; RAASi, renin–angiotensin–aldosterone system inhibitors; RAD, right atrial end‐systolic diameter; RF, rheumatoid factor; RVD, right ventricular end‐diastolic diameter; SBP, systolic blood pressure; T2D, type 2 diabetes mellitus; TC, total cholesterol; TR, tricuspid regurgitation; TRIG, triglyceridaemia; TV, tricuspid valve; WBC, white blood cell count.
The bold values mean P value < 0.05.
Effect of renin–angiotensin–aldosterone system inhibitor treatment on all‐cause mortality among patients with rheumatic heart disease
Renin–angiotensin–aldosterone system inhibitor treatment was associated with decreased all‐cause mortality risk (adjusted HR = 0.52, 95% CI: 0.37–0.73, P < 0.001; Figure 1A ). Further subgroup analysis showed that the effect was observed in RHD patients without (adjusted HR = 0.50, 95% CI: 0.31–0.79, P = 0.004; Figure 1B ) and with (adjusted HR = 0.47, 95% CI: 0.26–0.85, P = 0.012; Figure 1C ) surgical treatment.
Figure 1.
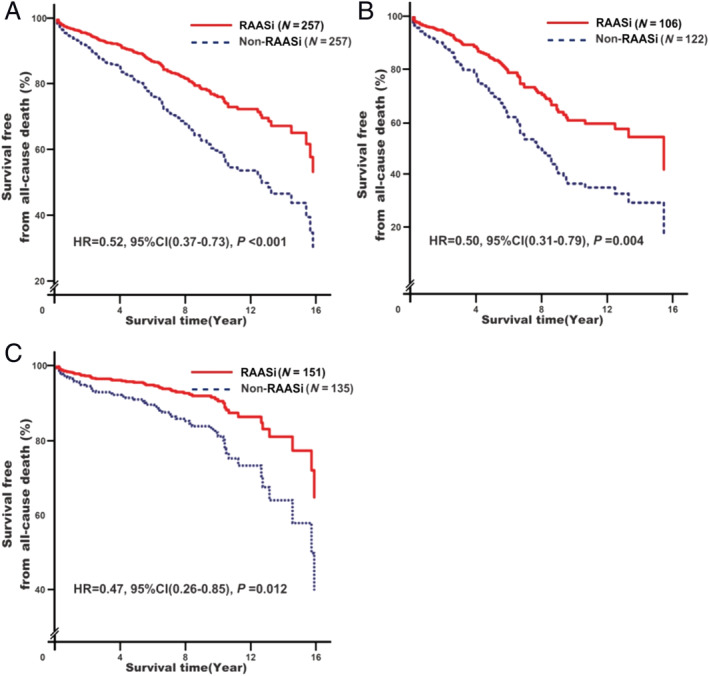
Associations of renin–angiotensin–aldosterone system inhibitor (RAASi) treatment with all‐cause mortality risk. Analyses were conducted among overall rheumatic heart disease (RHD) participants (A)a, among RHD participants without surgery (B)b, and among RHD participants with surgery (C)b. aModel 1: adjusting for baseline adjustment covariates, including age, gender, smoking, drinking, RHD course, New York Heart Association functional classification, cardiac valve damage, surgical intervention, medical condition (hypertension, coronary heart disease, type 2 diabetes mellitus, atrial fibrillation, and stroke), combined medication (antiplatelet drugs, warfarin, digoxin, nitrates, diuretic, beta‐receptor blockers, mineralocorticoid receptor antagonist, calcium channel blockers, and statins), blood biochemical index (white blood cell count, haemoglobin, serum sodium, serum potassium, creatinine, HbA1c, and C‐reactive protein), and echocardiography (left atrial end‐systolic diameter, left ventricular end‐diastolic diameter, right ventricular end‐diastolic diameter, and right atrial end‐systolic diameter). bModel 1s: it is the same as Model 1 with exception of surgical intervention. CI, confidence interval; HR, hazard ratio.
Effect of renin–angiotensin–aldosterone system inhibitor treatment on cardiovascular death among patients with rheumatic heart disease
Renin–angiotensin–aldosterone system inhibitor treatment was associated with decreased CVD risk (adjusted HR = 0.48, 95% CI: 0.30–0.76, P = 0.002; Figure 2A ). Further subgroup analysis showed that the effect was observed in RHD patients without (adjusted HR = 0.48, 95% CI: 0.25–0.91, P = 0.025; Figure 2B ) and with (adjusted HR = 0.43, 95% CI: 0.21–0.90, P = 0.024; Figure 2C ) surgical treatment.
Figure 2.
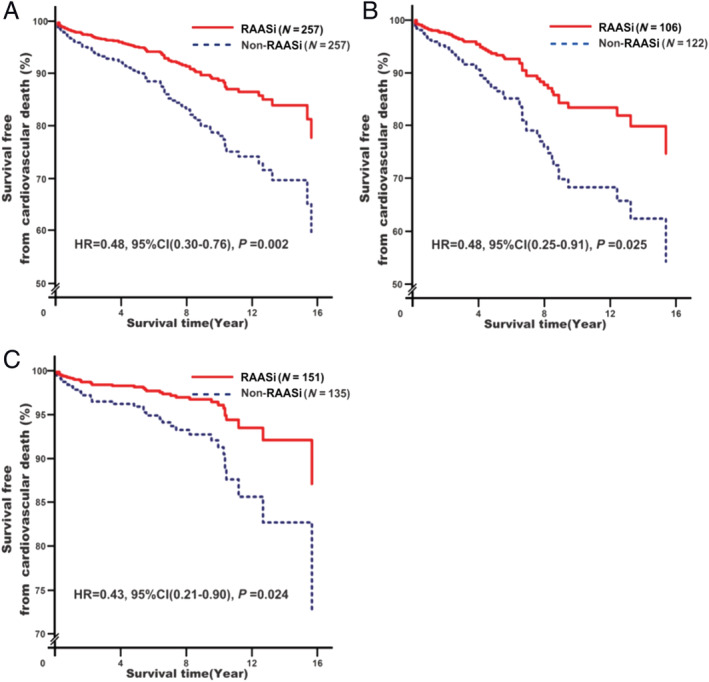
Associations of renin–angiotensin–aldosterone system inhibitor (RAASi) treatment with cardiovascular death risk. Analyses were conducted among overall rheumatic heart disease (RHD) participants (A)a, among RHD participants without surgery (B)b, and among RHD participants with surgery (C)b. aModel 2: adjusting for baseline adjustment covariates, including age, gender, smoking, drinking, RHD course, New York Heart Association functional classification, cardiac valve damage, surgical intervention, medical condition (hypertension, coronary heart disease, type 2 diabetes mellitus, atrial fibrillation, and stroke), combined medication (antiplatelet drugs, warfarin, digoxin, nitrates, diuretic, beta‐receptor blockers, mineralocorticoid receptor antagonist, calcium channel blockers, and statins), blood biochemical index (white blood cell count, haemoglobin, serum sodium, serum potassium, creatinine, HbA1c, and C‐reactive protein), and echocardiography (left atrial end‐systolic diameter, left ventricular end‐diastolic diameter, right ventricular end‐diastolic diameter, and right atrial end‐systolic diameter). bModel 2s: it is the same as Model 1 with exception of surgical intervention. CI, confidence interval; HR, hazard ratio.
Effect of renin–angiotensin–aldosterone system inhibitor treatment on cerebrovascular death among patients with rheumatic heart disease
Renin–angiotensin–aldosterone system inhibitor treatment was associated with decreased cerebrovascular death risk (adjusted HR = 0.22, 95% CI: 0.08–0.60, P = 0.003; Figure 3A ). However, further subgroup analysis showed that the effect was only observed in RHD patients without surgical treatment (adjusted HR = 0.19, 95% CI: 0.05–0.65, P = 0.008; Figure 3B ) rather than those with surgical treatment (adjusted HR = 0.52, 95% CI: 0.08–3.36, P = 0.491; Figure 3C ).
Figure 3.
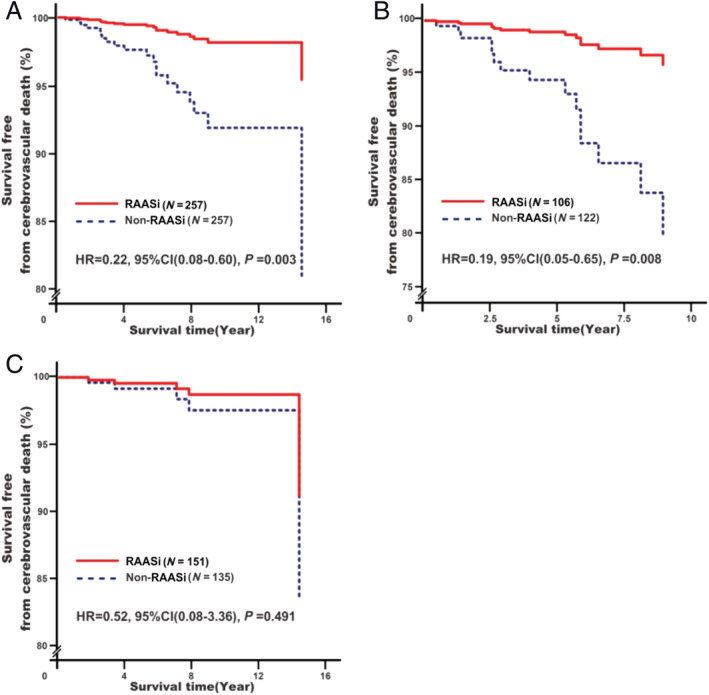
Associations of renin–angiotensin–aldosterone system inhibitor (RAASi) treatment with cerebrovascular death risk. Analyses were conducted among overall rheumatic heart disease (RHD) participants (A)a, among RHD participants without surgery (B)b, and among RHD participants with surgery (C)b. aModel 3: adjusting for baseline adjustment covariates, including age, gender, smoking, drinking, RHD course, New York Heart Association functional classification, cardiac valve damage, surgical intervention, medical condition (hypertension, coronary heart disease, type 2 diabetes mellitus, atrial fibrillation, and stroke), combined medication (antiplatelet drugs, warfarin, digoxin, nitrates, diuretic, beta‐receptor blockers, mineralocorticoid receptor antagonist, calcium channel blockers, and statins), blood biochemical index (white blood cell count, haemoglobin, serum sodium, serum potassium, creatinine, HbA1c, and C‐reactive protein), and echocardiography (left atrial end‐systolic diameter, left ventricular end‐diastolic diameter, right ventricular end‐diastolic diameter, and right atrial end‐systolic diameter). bModel 3s: it is the same as Model 1 with exception of surgical intervention. CI, confidence interval; HR, hazard ratio.
Effects of renin–angiotensin–aldosterone system inhibitors on the risks of heart failure rehospitalization, new‐onset atrial fibrillation, and new‐onset stroke
As shown in Table 2 , RAASi treatment was associated with decreased HF rehospitalization risks of 1 year (adjusted OR = 0.38, 95% CI: 0.23–0.61, P < 0.001), 3 year (adjusted OR = 0.43, 95% CI: 0.28–0.68, P < 0.001), and 5 year (adjusted OR = 0.48, 95% CI: 0.30–0.77, P = 0.002) as well as new‐onset AF risk (adjusted OR = 0.38, 95% CI: 0.21–0.68, P = 0.001). In addition, RAASi treatment had nothing to do with new‐onset stroke risk (adjusted OR = 0.80, 95% CI: 0.47–1.38, P = 0.428).
Table 2.
Effects of RAASi on the risks of HF rehospitalization, new‐onset AF, and new‐onset stroke
| Clinical outcomes | Non‐RAASi, N (%) | RAASi, N (%) | Crude OR (95% CI) | Crude P value | Adjusted OR (95% CI) | Adjusted P value |
|---|---|---|---|---|---|---|
| HF rehospitalization a | ||||||
| 1 year | ||||||
| No | 167 (68.4) | 210 (83.7) | 0.42 (0.28–0.65) | <0.001 | 0.38 (0.23–0.61) | <0.001 |
| Yes | 77 (31.6) | 41 (16.3) | ||||
| 3 year | ||||||
| No | 103 (48.6) | 144 (65.2) | 0.51 (0.34–0.74) | 0.001 | 0.43 (0.28–0.68) | <0.001 |
| Yes | 109 (51.4) | 77 (34.8) | ||||
| 5 year | ||||||
| No | 68 (34.3) | 94 (47.0) | 0.59 (0.39–0.88) | 0.010 | 0.48 (0.30–0.77) | 0.002 |
| Yes | 130 (65.7) | 106 (53.0) | ||||
| New‐onset AF b | ||||||
| No | 66 (41.3) | 86 (61.9) | 0.43 (0.27–0.69) | <0.001 | 0.38 (0.21–0.68) | 0.001 |
| Yes | 94 (58.8) | 53 (38.1) | ||||
| New‐onset stroke c | ||||||
| No | 193 (84.3) | 192 (85.7) | 0.89 (0.53–1.50) | 0.669 | 0.80 (0.47–1.38) | 0.428 |
| Yes | 36 (15.7) | 32 (14.3) | ||||
AF, atrial fibrillation; CI, confidence interval; HF, heart failure; OR, odds ratio; RAASi, renin–angiotensin–aldosterone system inhibitors.
Model 4: adjusting for baseline adjustment covariates, including age, gender, smoking, drinking, rheumatic heart disease course, New York Heart Association functional classification, cardiac valve damage, surgical intervention, medical condition (hypertension, coronary heart disease, type 2 diabetes mellitus, AF, and stroke), combined medication (antiplatelet drugs, warfarin, digoxin, nitrates, diuretic, beta‐receptor blockers, mineralocorticoid receptor antagonist, calcium channel blockers, and statins), blood biochemical index (white blood cell count, haemoglobin, serum sodium, serum potassium, Cr, HbA1c, and C‐reactive protein), and echocardiography (left atrial end‐systolic diameter, left ventricular end‐diastolic diameter, right ventricular end‐diastolic diameter, and right atrial end‐systolic diameter) and also including new‐onset AF.
Model 5: it is the same as Model 4 with exception of those covariates such as new‐onset AF, left ventricular end‐diastolic diameter, right ventricular end‐diastolic diameter, and right atrial end‐systolic diameter.
Model 6: it is the same as Model 4 with exception of those covariates such as left ventricular end‐diastolic diameter, right ventricular end‐diastolic diameter, and right atrial end‐systolic diameter.
The bold values mean P value < 0.05.
Discussion
Effect of renin–angiotensin–aldosterone system inhibitor treatment on all‐cause death and cardiovascular death in rheumatic heart disease patients
To our knowledge, this is the only study relating RAASi treatment to long‐term clinical hard outcomes in RHD patients with real and complex valve damage. The all‐cause death and CVD were respectively reduced by 48% and 52% in RAASi group compared with the control group. Further subgroup analysis showed that RHD patients without surgical intervention had similar benefits from RAASi treatment with a corresponding reduction of 50% and 52% (Figures 1B and 2B ). As expected, the risks of all‐cause death and CVD in RHD patients by combined treatment with surgery and RAASi were further reduced respectively by 6.0% and 9.6% (Figures 1C and 2C ). Our results are partially consistent with those studies on the effect of RAASi prescribed after percutaneous mitral valve repair, 19 aortic valve replacement, 20 and concomitant aortic and mitral valve replacement, 21 which RAASi treatment was significantly associated with a lower risk of mortality for those patients.
Indeed, Chockalingam et al. 22 found that enalapril not only was well tolerated but also improved functional status and exercise capacity in RHD patients with moderate and severe mitral stenosis (MS), especially in patients with concomitant regurgitant valvular heart disease. Sekuri et al. 23 found that losartan can improve the exercise tolerance and echocardiographic parameters in patients with rheumatic mitral regurgitation (MR) disease. Compared with rheumatic mitral valve disease, rheumatic aortic or tricuspid valve diseases are rare. From the perspective of inflammation, there is no essential difference between degenerative aortic valve damage and rheumatic aortic valve damage. 24 Chockalingam et al. 25 also found that enalapril was a well‐tolerable agent in patients with symptomatic severe aortic stenosis, and Goh et al. 26 had further shown that RAASi can delay the occurrence of cardiovascular complications in aortic stenosis patients, related to less left ventricular remodelling. Elder et al. 27 also found that RAASi in patients with aortic regurgitation were associated with significantly reduced all‐cause mortality. Besides degenerative aortic valve disease, a recent study showed that RAASi may confer a survival benefit in patients with degenerative MS. 28 In addition, tricuspid valve may be directly involved in the rheumatic inflammatory process, but much less is known about the effects of RAASi in tricuspid valve disease. 29 In a dog model of tricuspid valve regurgitation, benazepril markedly reduced the condition of valve regurgitation, which was further indirectly confirmed in albuminuric homozygous sickle cell patients with RAASi treatment. 30 These results suggested that RHD patients may benefit from RAASi therapy.
Effect of renin–angiotensin–aldosterone system inhibitor treatment on heart failure rehospitalization in rheumatic heart disease patients
Heart failure is recognized as the leading cause of complication and rehospitalization in RHD patients, especially with advanced RHD that may not be amenable to surgery. In this study, we found that RAASi treatment reduced HF rehospitalization risk within 5 years and had tendency to decreasing with increasing of RHD course by 5% per 2 years. However, it is worth noting that the reduction of HF rehospitalization risk on RAASi use in RHD patients without surgical intervention lasted only 3 years, while this effect in those with surgical intervention lasted at least for 5 years (Supporting Information, Table S1 ). The effect in RHD patients by combined treatment with surgery and RAASi was greater than that by RAASi treatment alone. Similarly, Michler et al. 6 reported that surgical intervention alone for patients with moderate ischaemic MR undergoing coronary artery bypass grafting did not significantly improve survival or reduce readmissions, even increased 2 year HF‐related readmission in patients with severe ischaemic MR, 31 which was consistent with the observation by Russell et al. 32 who found that surgical procedures for RHD had more readmissions to hospital. These results suggest that the benefits of RAASi or surgical treatment alone for RHD manifested characteristics of time limited on HF rehospitalization risk, relating to the duration cardiac remodelling reversal. 33 There could be two possible explanations for this timeliness. First, the left ventricular and atrial remodelling were very advanced at this late stage 34 due to long‐term progression of interstitial fibrosis and thus difficult to reverse by RAASi. In our study, we found that RAASi had no effect on reversing LAD and left ventricular end‐diastolic diameter sizes (Supporting Information, Table S2 ), which was partial similar to previously reported association of RAASi therapy with left heart remodelling in patients with chronic moderate–severe aortic regurgitation. 35 Second, surgical treatment only afforded short‐term easement of mechanical damage from cardiomyocyte hypertrophy caused by reduction of preload or afterload in the early post‐operative period. In patients with rheumatic MS undergoing percutaneous mitral balloon valvuloplasty, improved ventricular function only lasted ~1 year, and this was also observed for patients undergoing aortic valve replacement. 20
In addition to the left heart dysfunction, recent studies indicate that right heart dysfunction is also associated with poor prognosis. 36 RHD related right ventricular dysfunction is characterized by right atrium and ventricular dilatation. This condition exists even prior to the occurrence of pulmonary HT. 37 Right heart effects may be independent of improvement in left heart remodelling or reflect preferential improvement of right ventricular function and related phenotypes. 38 In our study, RAASi use did improve right heart remodelling (Supporting Information, Table S2 ) in RHD patients (smaller RVD and right atrial end‐systolic diameter), which was consistent with a pilot trial that showed small but significant changes in right ventricular volumes and mass after valsartan treatment, although no significant improvement of right ventricular ejection fraction, exercise capacity, or quality of life was observed. 39
Effect of renin–angiotensin–aldosterone system inhibitor treatment on atrial fibrillation, stroke, and its related cerebrovascular death in rheumatic heart disease patients
After HF, AF is the second most common complication of RHD, generally portending a poor prognosis. Restoration and maintenance of sinus rhythm are preferred in management of AF in RHD patients, but there are limited data on long‐term efficacy, because it may not be possible in cases of chronic persistent disease and huge left atrium. Consequently, primary AF prevention with RAASi as early as possible is a possible strategy among RHD patients. Existing evidence on RAASi reducing risk of new‐onset AF was based on non‐valvular AF (non‐VAF), there is as yet no evidence of RAASi benefits for VAF, especially on RHD‐related AF. Recently, Hsieh et al. 40 found that RAASi reduced new‐onset AF risk by ~50% in hypertensive patients with multiple risk factors (including ~2.1% of patients with valvular heart disease) for AF, which was similar to findings of the GISSI‐AF and ANTIPAF studies of RAASi treatment and non‐VAF. 41 In contrast, we found that RAASi treatment reduced the risk of new‐onset VAF in RHD patients, but this benefit was only observed in those subparticipants of RHD with surgery rather than in those without surgery (Supporting Information, Table S1 ). Possible explanations are as follows: the main driving factor of AF is left atrial structural and electrical remodelling. In this study, we found that left atrial remodelling is difficult to reverse by RAASi in RHD patients without surgery (Supporting Information, Table S3 ). Consistent with this, the GISSI‐AF study reported a low success rate for surgical radiofrequency ablation in persistent AF patients undergoing cardiac valve surgery, due to severe left atrial remodelling (larger LAD). 42 By contrast, improvement LAD size in RHD patients receiving surgery and RAASi treatment (Supporting Information, Table S3 ) was obvious and linked to lower risk of new‐onset AF.
Valvular atrial fibrillation is associated with increased risk of stroke, especially involving AF in patients with RHD. However, there are limited prospective data to assess the effect of RAASi on risk of stroke from RHD. In this study, RAASi treatment reduced the risk of new‐onset AF in RHD patients, but it did not reduce stroke risk even with a significant reduction on left atrium size by combined with surgery. This may be related to the following factors: first, unlike in non‐VAF where left atrial thrombus is mostly formed, RHD patients with AF are at much higher risk of thrombo‐embolism and the formation of the thrombus occurs mostly outside the left atrium for unclear reasons. This risk is not only related to the degree of valve damage, but it is also affected by those CHA2DS2‐VASc risk factors. 43 Second, left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with AF or in sinus rhythm. 44 , 45 Despite left atrial size reduction, this was insufficient to reduce risk of atrial mural thrombosis and its related stroke. Once left atrial remodelling occurs, it is difficult to reverse completely, even after valve surgery or AF cardioversion, especially in intermediate/advanced stages of RHD. Preventing remodelling is thus the only effective measure that requires sustained RAASi treatment starting as early as possible. In addition, mechanisms other than structural and electrical remodelling, including microvascular dysfunction, 46 epigenetic change 47 and genetic polymorphism, 48 , 49 also possibly play roles in triggering RHD‐related AF. Thus, it may not be sufficient to suppress a particular pathway (e.g. RAAS). However, even so, our results suggest that RAASi use combined with surgery is a feasible strategy for primary prevention of RHD‐related AF and its related stroke under current conditions.
We also found that RAASi treatment can reduce the cerebrovascular death, but the effect was only seen in RHD patients without surgery (Figure 3B ) while the risk of new‐onset AF and stroke was not reduced in this group (Supporting Information, Table S1 ). Conversely, RAASi treatment was associated with the decreased new‐onset AF risk in RHD patients with surgery, but there was no effect on the risk of stroke (Supporting Information, Table S1 ) and cerebrovascular death (Figure 3C ). These results suggested that the cerebrovascular death benefit was related to the other factors other than AF. HT is the most important risk factor for cerebrovascular death. 50 Indeed, we found that there was a higher proportion of HT in RHD patients with RAASi treatment in surgery subgroup rather than those without it (Supporting Information, Table S4 ). The reduction of systolic blood pressure level after RAASi use was significantly lower in both subparticipants without or with surgery, but the reduction of diastolic blood pressure level was not significant in surgery subgroup (Supporting Information, Table S4 ), related to the failure of cerebrovascular benefit at least partially. These results suggested that comprehensive management of cardiovascular risk factors is an important matter that cannot be ignored in RHD patients with prognosis improvement after surgery.
Limitations
This study was hurdled by some limitations. Firstly, the design of our study did not incorporate randomization, and selection bias may have influenced the results. Secondly, the sample size was not sufficiently large. Therefore, there was no reported separately for ACEIs and ARBs (e.g. drugs and dosage), which will make group sizes smaller and some comparisons may no longer be significant. This matter was also based on the following considerations: regardless of the types of RAASi, the haemodynamic status (e.g. systolic blood pressure levels) of RHD patients may not afford RAASi treatment at high dose, so the use strategy was crafted around the principle of minimum use of RAASi in patients with RHD. Besides RAASi, the effect of beta‐receptor blockers and mineralocorticoid receptor antagonists in patients with RHD also needs to be evaluated in the future based on this retrospective cohort. In addition, there was a high proportion of RHD participant combined with HF. The PSM was performed to decrease the bias related to HF on RAASi, but it is still uncertain whether this observed relationship was causal because of RAASi therapy or just reflects the severity of HF. Importantly, these results need to be interpreted with caution, and additional prospective studies of larger size will be required to validate our findings among RHD patients without HF, especially in developing countries with high incidence of RHD.
Conclusions
Rheumatic heart disease patients receiving RAASi treatment had better long‐term clinical outcomes and reduced incidences of cardiovascular events especially combined with surgical treatment, which can be a useful supplement to current surgical‐based treatment options, providing a reference point for prevention and treatment of RHD in developing countries and regions. This finding requires further investigation in randomized trials.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This study was funded by the National Natural Science Foundation of China (81100235), the Natural Science Foundation of Guangdong Province (S2011040004458), the Science and Technology Planning Project of Guangdong Province (2014A020212372), the Guangzhou Municipal Science and Technology Project (2012J4100035 and 201804010214), and the Natural Science Foundation of Xinjiang Province (201318101‐12).
Author contributions
C.L. contributed in the literature search; study format; writing of the protocol; collection, processing, interpretation, and analysis of the data; and writing of the manuscript. Yanxian Lai and R.F. contributed in the literature search and study format. D.W., R.F., and Yanfang Li contributed in the processing, interpretation, and analysis of the data. Yanfang Li, T.G., R.F., H.L., and Y.S. contributed in recruiting and following up the patients and in collecting the data. All authors read and approved the final manuscript.
Supporting information
Figure S1. Causes of death among study participants before PSM.
Table S1. Effect of RAASi on the risks of HF rehospitalizationa, new‐onset AFb and new‐onset strokec. aModel 2s: It is the same as Model S2, and also including new‐onset AF. bModel 3s: It is the same as Model 2s with exception of those covariates, including: new‐onset AF, LVD, RVD, and RAD. cModel 4s: It is the same as Model 2s with exception of those covariates, including: LVD, RVD, and RAD.
Table S2. Effect of RAASi treatment on heart remodeling in RHD participants at the end of follow‐up.
Table S3. Effect of RAASi and Surgical treatment on heart remodeling in RHD participants at the end of follow‐up.
Table S4. Effect of RAASi treatment on blood pressure levels in RHD participants at the end of follow‐up.
Acknowledgements
The authors wish to thank all the study participants from the South China Cardiovascular related Disease Cohort (SCCDC), research staff, and students who participated in this work.
Liu, C. , Lai, Y. , Wu, D. , Fu, R. , Li, Y. , Li, H. , Guan, T. , and Shen, Y. (2021) Impact of renin‐angiotensin system inhibitors on long‐term clinical outcomes of patients with rheumatic heart disease. ESC Heart Failure, 8: 5338–5351. 10.1002/ehf2.13623.
References
- 1. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR, Ribeiro ALP, Sable CA, Steer AC, Naghavi M, Mokdad AH, Murray CJL, Vos T, Carapetis JR, Roth GA. Global, regional, and national burden of rheumatic heart disease, 1990‐2015. N Engl J Med 2017; 377: 713–722. [DOI] [PubMed] [Google Scholar]
- 2. Remenyi B, ElGuindy A, Smith SC Jr, Yacoub M, Holmes DR Jr. Valvular aspects of rheumatic heart disease. Lancet 2016; 387: 1335–1346. [DOI] [PubMed] [Google Scholar]
- 3. Remenyi B, Carapetis J, Wyber R, Taubert K, Mayosi BM, World Heart F . Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat Rev Cardiol 2013; 10: 284–292. [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL, Group ESCSD . 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–2791. [DOI] [PubMed] [Google Scholar]
- 5. Steer AC, Carapetis JR. Prevention and treatment of rheumatic heart disease in the developing world. Nat Rev Cardiol 2009; 6: 689–698. [DOI] [PubMed] [Google Scholar]
- 6. Michler RE, Smith PK, Parides MK, Ailawadi G, Thourani V, Moskowitz AJ, Acker MA, Hung JW, Chang HL, Perrault LP, Gillinov AM, Argenziano M, Bagiella E, Overbey JR, Moquete EG, Gupta LN, Miller MA, Taddei‐Peters WC, Jeffries N, Weisel RD, Rose EA, Gammie JS, DeRose JJ Jr, Puskas JD, Dagenais F, Burks SG, El‐Hamamsy I, Milano CA, Atluri P, Voisine P, O'Gara PT, Gelijns AC. Two‐year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2016; 374: 1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saikia UN, Kumar RM, Pandian VK, Gupta S, Dhaliwal RS, Talwar KK. Adhesion molecule expression and ventricular remodeling in chronic rheumatic heart disease: a cause or effect in the disease progression‐‐a pilot study. Cardiovasc Pathol 2012; 21: 83–88. [DOI] [PubMed] [Google Scholar]
- 8. Sari I, Davutoglu V. Association of chronic subclinical inflammation with severity and progression of rheumatic valve disease. Int J Cardiol 2008; 124: 263. [DOI] [PubMed] [Google Scholar]
- 9. Cong H, Li X, Ma L, Jiang H, Mao Y, Xu M. Angiotensin II receptor type 1 is upregulated in atrial tissue of patients with rheumatic valvular disease with atrial fibrillation. J Thorac Cardiovasc Surg 2010; 140: 298–304. [DOI] [PubMed] [Google Scholar]
- 10. Remenyi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, Mocumbi AO, Mota C, Paar J, Saxena A, Scheel J, Stirling J, Viali S, Balekundri VI, Wheaton G, Zuhlke L, Carapetis J. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease‐‐an evidence‐based guideline. Nat Rev Cardiol 2012; 9: 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu C, Lai Y, Pei J, Huang H, Zhan J, Ying S, Shen Y. Clinical and genetic analysis of KATP variants with heart failure risk in patients with decreased serum ApoA‐I levels. J Clin Endocrinol Metab 2021; 106: 2264–2278. [DOI] [PubMed] [Google Scholar]
- 12. Carey RM, Whelton PK, Committee AAHGW . Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann Intern Med 2018; 168: 351–358. [DOI] [PubMed] [Google Scholar]
- 13. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P, Group ESCSD . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. [DOI] [PubMed] [Google Scholar]
- 14. Liu C, Lai Y, Guan T, Shen Y, Pan Y, Wu D. Outcomes of diuretics in rheumatic heart disease with compensated chronic heart failure: a retrospective study. ESC Heart Fail 2020; 7: 3929–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J 2012; 27: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu C, Guan T, Lai Y, Shen Y. Association of KATP Gene Polymorphisms with Dyslipidemia and Ischemic Stroke Risks Among Hypertensive Patients in South China. J Mol Neurosci 2021. [DOI] [PubMed] [Google Scholar]
- 17. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA, American Heart Association Stroke Council CoC , Stroke Nursing CoCC , Council on Peripheral Vascular D . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the european Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 19. Geis N, Raake P, Lewening M, Mereles D, Chorianopoulos E, Frankenstein L, Katus HA, Bekeredjian R, Pleger ST. Percutaneous repair of mitral valve regurgitation in patients with severe heart failure: comparison with optimal medical treatment. Acta Cardiol 2018; 73: 378–386. [DOI] [PubMed] [Google Scholar]
- 20. Inohara T, Manandhar P, Kosinski AS, Matsouaka RA, Kohsaka S, Mentz RJ, Thourani VH, Carroll JD, Kirtane AJ, Bavaria JE, Cohen DJ, Kiefer TL, Gaca JG, Kapadia SR, Peterson ED, Vemulapalli S. Association of renin‐angiotensin inhibitor treatment with mortality and Heart Failure Readmission in patients with transcatheter aortic valve replacement. JAMA 2018; 320: 2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yiu KH, Ng WS, Chan D, Sit KY, Wong A, Lee CW, Chum HL, Cheng WY, Pun CT, Ho KL, Chen Y, Ho LM, Kumana CR, Cheung HL, Chung MC, Lau CP, Au WK, Tse HF. Improved prognosis following renin‐angiotensin‐aldosterone system blockade in patients undergoing concomitant aortic and mitral valve replacement. Int J Cardiol 2014; 177: 680–682. [DOI] [PubMed] [Google Scholar]
- 22. Chockalingam A, Venkatesan S, Dorairajan S, Chockalingam V, Subramaniam T, Jaganathan V, Elangovan S, Alagesan R, Gnanavelu G, Arul AS. Safety and efficacy of enalapril in multivalvular heart disease with significant mitral stenosis‐‐SCOPE‐MS. Angiology 2005; 56: 151–158. [DOI] [PubMed] [Google Scholar]
- 23. Sekuri C, Utuk O, Bayturan O, Bilge A, Kurhan Z, Tavli T. Effect of losartan on exercise tolerance and echocardiographic parameters in patients with mitral regurgitation. J Renin Angiotensin Aldosterone Syst 2008; 9: 107–111. [DOI] [PubMed] [Google Scholar]
- 24. Wallby L, Steffensen T, Jonasson L, Broqvist M. Inflammatory characteristics of stenotic aortic valves: a comparison between rheumatic and nonrheumatic aortic stenosis. Cardiol Res Pract 2013; 2013: 895215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chockalingam A, Venkatesan S, Subramaniam T, Jagannathan V, Elangovan S, Alagesan R, Gnanavelu G, Dorairajan S, Krishna BP, Chockalingam V. Symptomatic Cardiac Obstruction‐Pilot Study of Enalapril in Aortic S. Safety and efficacy of angiotensin‐converting enzyme inhibitors in symptomatic severe aortic stenosis: Symptomatic Cardiac Obstruction‐Pilot Study of Enalapril in Aortic Stenosis (SCOPE‐AS). Am Heart J 2004; 147: e19. [DOI] [PubMed] [Google Scholar]
- 26. Goh SS, Sia CH, Ngiam NJ, Tan BY, Lee PS, Tay EL, Kong WK, Yeo TC, Poh KK. Effect of renin‐angiotensin blockers on left ventricular remodeling in severe aortic stenosis. Am J Cardiol 2017; 119: 1839–1845. [DOI] [PubMed] [Google Scholar]
- 27. Elder DH, Wei L, Szwejkowski BR, Libianto R, Nadir A, Pauriah M, Rekhraj S, Lim TK, George J, Doney A, Pringle SD, Choy AM, Struthers AD, Lang CC. The impact of renin‐angiotensin‐aldosterone system blockade on heart failure outcomes and mortality in patients identified to have aortic regurgitation: a large population cohort study. J Am Coll Cardiol 2011; 58: 2084–2091. [DOI] [PubMed] [Google Scholar]
- 28. Pasca I, Dang P, Tyagi G, Pai RG. Survival in patients with degenerative mitral stenosis: results from a large retrospective cohort study. J Am Soc Echocardiogr 2016; 29: 461–469. [DOI] [PubMed] [Google Scholar]
- 29. Borer JS, Sharma A. Drug therapy for heart valve diseases. Circulation 2015; 132: 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haymann JP, Hammoudi N, Stankovic Stojanovic K, Galacteros F, Habibi A, Avellino V, Bartolucci P, Benzerara Y, Arlet JB, Djebbar M, Letavernier E, Grateau G, Tabibzadeh N, Girshovich A, Chaignon M, Girot R, Levy P, Lionnet F. Renin‐angiotensin system blockade promotes a cardio‐renal protection in albuminuric homozygous sickle cell patients. Br J Haematol 2017; 179: 820–828. [DOI] [PubMed] [Google Scholar]
- 31. Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, Hung JW, Voisine P, Dagenais F, Gillinov AM, Thourani V, Argenziano M, Gammie JS, Mack M, Demers P, Atluri P, Rose EA, O'Sullivan K, Williams DL, Bagiella E, Michler RE, Weisel RD, Miller MA, Geller NL, Taddei‐Peters WC, Smith PK, Moquete E, Overbey JR, Kron IL, O'Gara PT, Acker MA. Two‐year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med 2016; 374: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russell EA, Tran L, Baker RA, Bennetts JS, Brown A, Reid CM, Tam R, Walsh WF, Maguire GP. A review of outcome following valve surgery for rheumatic heart disease in Australia. BMC Cardiovasc Disord 2015; 15: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lindman BR, Stewart WJ, Pibarot P, Hahn RT, Otto CM, Xu K, Devereux RB, Weissman NJ, Enriquez‐Sarano M, Szeto WY, Makkar R, Miller DC, Lerakis S, Kapadia S, Bowers B, Greason KL, McAndrew TC, Lei Y, Leon MB, Douglas PS. Early regression of severe left ventricular hypertrophy after transcatheter aortic valve replacement is associated with decreased hospitalizations. JACC Cardiovasc Interv 2014; 7: 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gaasch WH, Aurigemma GP. Inhibition of the renin‐angiotensin system and the left ventricular adaptation to mitral regurgitation. J Am Coll Cardiol 2002; 39: 1380–1383. [DOI] [PubMed] [Google Scholar]
- 35. Shah RM, Singh M, Bhuriya R, Molnar J, Arora RR, Khosla S. Favorable effects of vasodilators on left ventricular remodeling in asymptomatic patients with chronic moderate‐severe aortic regurgitation and normal ejection fraction: a meta‐analysis of clinical trials. Clin Cardiol 2012; 35: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Demirkol S, Ozturk C, Balta S, Unlu M. Right ventricular function in patients undergoing surgical or transcatheter aortic valve replacement. Eur J Cardiothorac Surg 2016; 49: 1296. [DOI] [PubMed] [Google Scholar]
- 37. Pande S, Agarwal SK, Dhir U, Chaudhary A, Kumar S, Agarwal V. Pulmonary arterial hypertension in rheumatic mitral stenosis: does it affect right ventricular function and outcome after mitral valve replacement? . Interact Cardiovasc Thorac Surg 2009; 9: 421–425. [DOI] [PubMed] [Google Scholar]
- 38. Perez‐Calvo JI, Torralba MA. Right ventricular ejection fraction and enalapril. Int J Cardiol 2010; 138: 211. [DOI] [PubMed] [Google Scholar]
- 39. van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos‐Hesselink JW, Zwinderman AH, Mulder BJ. Effect of valsartan on systemic right ventricular function: a double‐blind, randomized, placebo‐controlled pilot trial. Circulation 2013; 127: 322–330. [DOI] [PubMed] [Google Scholar]
- 40. Hsieh YC, Hung CY, Li CH, Liao YC, Huang JL, Lin CH, Wu TJ. Angiotensin‐receptor blocker, angiotensin‐converting enzyme inhibitor, and risks of atrial fibrillation: a Nationwide cohort study. Med (Baltimore) 2016; 95: e3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016; 18: 1609–1678. [DOI] [PubMed] [Google Scholar]
- 42. Cao H, Xue Y, Zhou Q, Yu M, Tang C, Wang D. Late outcome of surgical radiofrequency ablation for persistent valvular atrial fibrillation in China: a single‐center study. J Cardiothorac Surg 2017; 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lip GYH, Collet JP, Caterina R, Fauchier L, Lane DA, Larsen TB, Marin F, Morais J, Narasimhan C, Olshansky B, Pierard L, Potpara T, Sarrafzadegan N, Sliwa K, Varela G, Vilahur G, Weiss T, Boriani G, Rocca B, Group ESCSD . Antithrombotic therapy in atrial fibrillation associated with valvular heart disease: a joint consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology Working Group on Thrombosis, endorsed by the ESC Working Group on Valvular Heart Disease, Cardiac Arrhythmia Society of Southern Africa (CASSA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), South African Heart (SA Heart) Association and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace 2017; 19: 1757–1758. [DOI] [PubMed] [Google Scholar]
- 44. Hamatani Y, Ogawa H, Takabayashi K, Yamashita Y, Takagi D, Esato M, Chun YH, Tsuji H, Wada H, Hasegawa K, Abe M, Lip GY, Akao M. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non‐valvular atrial fibrillation. Sci Rep 2016; 6: 31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Overvad TF, Nielsen PB, Larsen TB, Sogaard P. Left atrial size and risk of stroke in patients in sinus rhythm. a systematic review. Thromb Haemost 2016; 116: 206–219. [DOI] [PubMed] [Google Scholar]
- 46. Wijesurendra RS, Casadei B. Atrial fibrillation: effects beyond the atrium? Cardiovasc Res 2015; 105: 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen K, Tu T, Yuan Z, Yi J, Zhou Y, Liao X, Liu Q, Zhou X. DNA methylation dysregulations in valvular atrial fibrillation. Clin Cardiol 2017; 40: 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roberts JD, Dewland TA, Glidden DV, Hoffmann TJ, Arking DE, Chen LY, Psaty BM, Olgin JE, Alonso A, Heckbert SR, Marcus GM. Impact of genetic variants on the upstream efficacy of renin‐angiotensin system inhibitors for the prevention of atrial fibrillation. Am Heart J 2016; 175: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu C, Pei J, Lai Y, Guan T, Zeyaweiding A, Maimaiti T, Zhao H, Shen Y. Association of ACE2 variant rs4646188 with the risks of atrial fibrillation and cardioembolic stroke in uygur patients with type 2 diabetes. BMC Cardiovasc Disord 2021; 21: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res 2017; 120: 472–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Causes of death among study participants before PSM.
Table S1. Effect of RAASi on the risks of HF rehospitalizationa, new‐onset AFb and new‐onset strokec. aModel 2s: It is the same as Model S2, and also including new‐onset AF. bModel 3s: It is the same as Model 2s with exception of those covariates, including: new‐onset AF, LVD, RVD, and RAD. cModel 4s: It is the same as Model 2s with exception of those covariates, including: LVD, RVD, and RAD.
Table S2. Effect of RAASi treatment on heart remodeling in RHD participants at the end of follow‐up.
Table S3. Effect of RAASi and Surgical treatment on heart remodeling in RHD participants at the end of follow‐up.
Table S4. Effect of RAASi treatment on blood pressure levels in RHD participants at the end of follow‐up.


