Abstract
Aims
Both left atrial strain (LAS) and skeletal muscle endurance demonstrate a linear relationship to peak VO2. Less is known about the relationship between central (cardiac) and peripheral (muscle endurance) limitations of exercise capacity in patients with heart failure (HF). We investigated this relationship using novel cardiac markers such as LAS and left atrial emptying fraction (LAEF).
Methods and results
We analysed echocardiographic measurements, cardiopulmonary exercise testing (CPET), and isokinetic muscle function in 55 subjects with HF and controls [17 heart failure with preserved ejection fraction (HFpEF), 18 heart failure with reduced ejection fraction (HFrEF), and 20 healthy controls]. Patients with reduced LAEF showed reduced peak VO2: 14.3 ± 3.5 vs. 18.5 ± 3.5 mL/min/kg, P = 0.003, and reduced muscle endurance (RME): 64.3 ± 23.9 vs. 88.5 ± 32.3 Nm/kg, P = 0.028. Patients with reduced LAS showed similar results. Neither left ventricular global longitudinal strain (LVGLS) nor left atrial volume index (LAVI) was associated with RME. The area under the curve of LAS and LAEF in patients with HF in association with RME were (0.76 vs. 0.80) with 95% confidence interval (CI) (0.59–0.96, P = 0.012 vs. 0.63–0.98, P = 0.006, respectively). In a multiple linear regression, LAEF and working load measured during CPET (watt) were independent factors for RME after adjusting for age, LVGLS, and 6 min walk test (6MWT) [LAEF (B: 0.09, 95% CI: 1.01; 1.18, P = 0.024), working load (B: 0.05, 95% CI: 1.01; 1.08, P = 0.006)]. Peak torque of the left leg was associated with E/LAS (E: early diastolic) in patients with HFpEF (r = −0.6, P = 0.020). Endurance of the left leg was associated with LAEF (r = 0.79, P = 0.001) in patients with HFrEF.
Conclusions
LAS/LAEF are potential cardiac markers in demonstrating the link between cardiac and peripheral limitations of exercise capacity. Thus, integrating LAS/LAEF in the evaluation of exercise intolerance in patients with HF could be useful.
Keywords: Heart failure, Left atrial strain, Skeletal muscle function
Introduction
Patients with heart failure with preserved (HFpEF) and reduced (HFrEF) ejection fraction present mainly with dyspnoea and reduced exercise capacity. 1 These manifestations could be explained with central (cardiac) or peripheral (skeletal muscle) factors. One of the suggested mechanisms is elevated left ventricular (LV) filling pressure. 2 Several studies showed that left atrial strain (LAS) measured using two‐dimensional speckle tracking echocardiography (2D‐STE) is a surrogate of elevated LV filling pressure. 3 , 4 , 5 Recently, left atrial (LA) function has gained attention due to the pivotal role of the left atrium in the resting and exercising cardiovascular system. Studies found a linear relationship between LA function and maximal oxygen uptake (peak VO2) during cardiopulmonary exercise testing (CPET) in different disease states such as HFpEF, diabetes mellitus, ischaemic, and dilated cardiomyopathies. 6 , 7 , 8 , 9 , 10
Similarly, reduced exercise capacity measured as reduced peak VO2 has been shown in several studies to be linked to peripheral factors such as skeletal muscle dysfunction both in patients with HFpEF and HFrEF. 11 , 12 , 13 In fact, about 20% of patients with HF suffer from skeletal muscle wasting, which is associated with reduced exercise and functional capacity (peak VO2). 11 , 12 We found recently that molecular, mitochondrial, and metabolic abnormalities in skeletal muscle in patients with HFpEF and HFrEF were associated clinically with reduced exercise capacity and reduced muscle function. 14
As mentioned earlier, reduced peak VO2 is associated with central and peripheral limitations. However, no direct relationship between central and peripheral factors could be proven yet. On the contrary, exercise training was shown to improve exercise tolerance in patients with HF independent of improving cardiac function measured with left ventricular ejection fraction (LVEF). 15
One of the possible explanations for the failure in demonstrating a link between central and peripheral limitations of exercise capacity is likely not using more sensitive and novel cardiac measurement such as left atrial emptying fraction (LAEF) and LAS.
We hypothesized, as the result to the multi‐organ involvement in HF syndrome, an association between central and peripheral factors involved in the reduced exercise capacity in HF and searched for a sensitive cardiac parameter to demonstrate this relationship. Therefore, we investigated the association between LAS, LAEF, and left ventricular global longitudinal strain (LVGLS), on one hand, and muscle endurance as surrogate of skeletal muscle function in patients with HFpEF, HFrEF, and healthy controls (HC), on the other hand. We hypothesized that central novel parameters (LAEF and LAS) are capable to detect the peripheral limiting factors (reduced skeletal muscle function).
Methods
Study population
Heart failure patients were recruited from the HF outpatient clinic at the University Hospital Jena from September 2016 until June 2017. Age‐matched HC were recruited from the general healthy population in Jena, Germany.
Heart failure inclusion criteria
Clinically stable men and women's outpatients with age > 55 years both with HFpEF and HFrEF (LVEF < 40%) with New York Heart Association (NYHA) class II or III were recruited. Definitions of HFpEF and HFrEF were according the European Society of Cardiology‐HF (ESC‐HF) 2016. LVEF cut‐off for HFpEF was 50%, BNP > 35 pg/mL. In addition, one of the following criteria was met: relevant structural heart disease (LV hypertrophy and/or LA enlargement) or diastolic dysfunction as defined in the ESC‐HF 2016. 16 Patients were on standard and stable HF medication for the last 3 months.
Heart failure exclusion criteria
Patients with major cardiovascular event or procedure in the last 6 weeks, or patients with HF secondary to severe uncorrected valvular disease as well as patients with uncontrolled diabetes mellitus, progressive renal dysfunction (glomerular filtration rate < 60 mL/min), and those with primary muscle disorder, for example, muscular dystrophies, were excluded.
Definition of heart failure comorbidities
Arterial hypertension was defined as blood pressure > 140/90 mmHg and/or receiving antihypertensive medication. Diabetes mellitus was defined as HbA1C ≥ 6.5% and/or taking oral hypoglycaemic agents and/or receiving insulin injection. Atrial fibrillation was diagnosed based on electrocardiogram (ECG) or on the patient's medical records. Acute myocardial infarction was defined by a history of an acute presentation with acute chest discomfort described as pain, pressure, tightness, and burning or with chest pain‐equivalent symptoms such as dyspnoea, epigastric pain, and pain in the left arm and either with ST‐segment elevation in ECG or non‐ST‐segment elevation but with increase or decrease in sensitive troponin. 17
Control subjects
Healthy controls with a history of cardiovascular disease or other diseases except arterial hypertension and diabetes mellitus were excluded.
Study protocol
All subjects (HFpEF, HFrEF, and HC) underwent a standardized series of assessments over two visits.
Dual‐energy X‐ray absorptiometry scan
We performed a whole‐body dual‐energy X‐ray absorptiometry (DEXA) scan in order to characterize the different compartments of soft tissue in the body. DEXA scan uses a low radiation dosage and is an established method to characterize the body composition in patients with advanced HF. 18 We used the lean mass of the extremities.
Muscle strength and endurance
The muscle function of the lower extremity was assessed by the isokinetic dynamometry (CSMi Cybex HumacNorm®). A standardized measurement protocol was used to detect the following parameters: (1) maximum muscle strength and (2) muscle strength endurance in the knee extension and knee flexion. The test protocol of the lower extremity included different angular velocities in the concentric (60 and 180°/s). All values of the isokinetic measurement of the lower extremities were related to the muscle mass of legs unless mentioned otherwise.
-
1
Maximum muscle strength
The participants were asked to perform five repetitions with the maximum speed with the velocity of 60°/s (concentric knee extension and flexion). The best single attempt was defined as peak torque muscle strength. The higher the value, the better is the muscle strength.
-
2
Muscle strength endurance
The participants were asked to perform 15 repetitions with the maximum speed and to maintain it across the required performance. The velocity of the dynamometer was defined as 180°/s (knee extension and flexion). To detect the muscular endurance, the areas under the curves of every single attempt were summed. This outcome equals total physical work during the 15 repetitions. 19 , 20
Analysis of left atrial function and strain using two‐dimensional speckle tracking
Left atrial strain, LAEF, and left atrial fractional area change (LAFAC) were measured in all patients and HC both in four‐chamber and two‐chamber dedicated views by a blinded reviewer. LVGLS was presented as the average of the measurements in two‐chamber, three‐chamber, and four‐chamber views. Image quality in six patients was reduced. Therefore, we did not include these patients in the final analysis. LAS was analysed using as zero point the R wave of the ECG. The recordings were processed using an acoustic‐tracking dedicated software (Image‐Arena™ Version 4.6; TomTec Imaging Systems, Unterschleissheim, Germany), which allowed for an off‐line semi‐automated analysis of speckle‐based strain. All data were analysed by one observer (S. I.).
Left atrial strain represented the average from the peak positive longitudinal strain curve from all LA segments in four‐chamber and two‐chamber views. We performed our measurement as recommended from the European Association of Cardiovascular Imaging (EACVI). 21 Normal values of LAS and LAEF were measured in a large European cohort and were published recently. 22 Accordingly, an abnormal LAS was defined as LAS < 26% and an abnormal LAEF was defined as LAEF < 48%. To show the profile of patients with reduced LAEF (< mean value of the cohort = 34.5%), we divided the cohort of patients into two groups according to the mean value of LAEF.
Cardiopulmonary exercise testing
The non‐invasive measurement of ventilatory gas exchange during exercise is the main principle of CPET. This involves the acquisition of expired ventilation and concentrations of oxygen (O2) and carbon dioxide (CO2) during exercise. Exercise testing in association with air–gas exchange using breath‐by‐breath method of analysis is considered to be an optimal gauge of functional capacity. We performed CPET in all participants using incremental biking exercise on an electronically braked cycle ergometer as described elsewhere. 23 , 24 CPET was performed on a bicycle ergometer. Ramp protocol (15 W/min) was used. The results were read by an experienced physician in CPET.
Six‐minute walk test
The 6 min walk test (6MWT) is a commonly used clinical tool, which allows testing of the functional capacity in HF patients in a ‘real‐life’ setting. Using standard methodology, 25 patients were asked to walk as fast as possible on a 25 m course for 6 min. The test was scored in rounded metres walked in 6 min.
All subjects provided written informed consent at enrolment, and the protocol was approved by the responsible ethical review boards and fulfilled all principles of the Declaration of Helsinki. The study was funded by a grant from the interdisciplinary centre for clinical research at the University Hospital in Jena.
Statistical analysis
All data and statistics are reported as mean ± standard deviation (n ± SD) for continuous data. Categorical data were summarized by percentages. The χ 2 test was used to compare categorical variables, and the Kruskal–Wallis test was applied for not normally distributed data. Analysis of variance (ANOVA) was used as appropriate. Variables perceived as clinically important and those with P < 0.2 in univariate analyses were included in a multivariable regression model. Final model selection was based on stepwise regression. A two‐tailed P‐value < 0.05 indicates statistical significance. The Statistical Package for Social Sciences software (SPSS 26, IBM, Armonk, USA) was used for statistical analysis.
Results
We recruited 62 patients and HC. Altogether, 55 subjects fulfilled our inclusion and exclusion criteria and were included in this study: 17 HFpEF patients, 18 HFrEF patients, and 20 HC. Seven patients, who did not fulfil the criteria for HFpEF, were excluded.
Basic characteristics of HF patients and HC are summarized in Table 1 . 2D echocardiographic data are shown in Table 2 . Patients with HFpEF and HFrEF showed reduced LAEF compared with HC (LAEF: 42.9 ± 14.0 vs. 28.0 ± 11.4 vs. 52.2 ± 10.7%, P < 0.001) (Table 2 and Figure 1 ).
Table 1.
Basic characteristics data in patients with heart failure with preserved and reduced ejection fraction and healthy controls
| HFpEF N = 17 | HFrEF N = 18 | HC N = 20 | P‐value | |
|---|---|---|---|---|
| Age (years) | 71 ± 6 | 68 ± 9 | 66 ± 7 | 0.17 |
| Sex (m/f) f% | 8/9 (53%) § | 15/3 (17%) # | 7/13 (65%) | 0.009 |
| BMI (kg/m2) | 28.7 ± 4.6 | 27.9 ± 5.3 | 26.4 ± 4.2 | 0.18 |
| NYHA (II/III) % | (76.5/23.5)* | (83.3/16.7) # | (0/0) | <0.001 |
| GFR (mL/min) | 71.8 ± 13.9 | 72.2 ± 22.6 | 82.4 ± 14.1 | 0.11 |
| BNP (pg/mL) | 168 ± 167* , § | 374 ± 290 # | 45.7 ± 35.5 | <0.001 |
| AMI % | 5 (29%)* | 6 (33%) # | 0 (0%) | 0.019 |
| Hypertension % | 15 (88%)* | 15 (83%) # | 10 (50%) | 0.016 |
| Diabetes mellitus % | 6 (35%) | 7 (39%) # | 2 (10%) | 0.091 |
| Atrial fibrillation % | 9 (53%)* | 5 (28%) | 1 (5%) | 0.005 |
| ASS % | 7 (29%)* | 5 (39%) # | 2 (10%) | 0.004 |
| Oral anticoagulation% | 9 (53%)* | 7 (39%) | 2 (10%) | 0.017 |
| Beta‐blocker % | 13 (77%)* | 17 (94%) # | 4 (20%) | <0.001 |
| ACEI/ARB/neprilysin inhibitor % | 12 (71%)* , § | 18 (100%) # | 8 (40%) | <0.001 |
| Aldosterone antagonist % | 3 (18%) § | 12 (67%) # | 0 (0%) | <0.001 |
| Diuretics % | 9 (53%) § | 16 (89%) # | 6 (30%) | <0.001 |
| Statins % | 11 (65%)* | 13 (72%) # | 2 (10%) | <0.001 |
| Oral antidiabetic therapy % | 5 (29%)* | 4 (22%) | 0 (0%) | 0.039 |
ACEI/ARB, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; AMI, acute myocardial infarction; ASS, aspirin; BMI, body mass index; BNP, brain natriuretic peptide; GFR, glomerular filtration rate; HC, healthy controls; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association.
P < 0.05 in comparison between HFpEF and HC.
P < 0.05 in comparison between HFrEF and HC.
P < 0.05 in comparison between HFpEF and HFrEF.
Table 2.
2D echocardiographic data in patients with heart failure with preserved and reduced ejection fraction and healthy controls
| HFpEF N = 17 | HFrEF N = 13 | HC N = 19 | P‐value | |
|---|---|---|---|---|
| IVSD (mm) | 13.8 ± 1.7* , § | 11.2 ± 2.9 | 11.3 ± 2.1 | 0.001 |
| PWD (mm) | 12.0 ± 1.7* | 11.5 ± 2.3 # | 9.9 ± 1.6 | 0.003 |
| LVED (mm) | 47.7 ± 6.5* , § | 61.7 ± 8.5 # | 42.7 ± 4.2 | <0.001 |
| LVEDVI (mL/m2) | 24.9 ± 3.2 § | 28.6 ± 7.0 # | 23.3 ± 2.6 | 0.004 |
| LVESVI (mL/m2) | 16.8 ± 3.6 § | 25.8 ± 4.8 # | 14.6 ± 2.9 | <0.001 |
| LV mass (g) | 295 ± 79.5* | 341 ± 126 # | 179 ± 46.7 | <0.001 |
| LVMI (g/m2) | 152 ± 30.8* | 165 ± 53.2 # | 97.3 ± 22.7 | <0.001 |
| LVEF % | 59.7 ± 10.2 § | 28.4 ± 5.9 # | 61.9 ± 6.0 | <0.001 |
| LVGLS (average) % | −18.9 ± 5.5 § | −6.8 ± 2.9 # | −19.8 ± 3.3 | <0.001 |
| TAPSE (mm) | 21.3 ± 4.5 | 18.3 ± 4.6 # | 23.2 ± 3.2 | <0.01 |
| E/A | 1.4 ± 0.7 | 1.1 ± 0.7 | 1.0 ± 0.2 | 0.3 |
| E′ (average) (cm) | 0.06 ± 0.01* , § | 0.05 ± 0.02 # | 0.08 ± 0.02 | <0.001 |
| E/E′ | 12.8 ± 3.2 § | 18.1 ± 7.5 # | 10.0 ± 3.1 | 0.001 |
| LAVI (mL/m2) | 34.1 ± 7.1* , § | 44.9 ± 19.0 # | 17.2 ± 8.3 | <0.001 |
| LAS (average) % | 26.0 ± 19.1 § | 11.8 ± 5.8 # | 31.3 ± 12.0 | 0.001 |
| LAEF (average) % | 42.9 ± 14.0* , § | 28.0 ± 11.4 # | 52.2 ± 10.7 | <0.001 |
| LAFAC (average) | 31.6 ± 11.2* , § | 19.7 ± 8.1 # | 39.9 ± 9.1 | <0.001 |
| LAEDV (average) | 70.4 ± 84.7* | 89.5 ± 41.6 # | 25.2 ± 11.9 | 0.005 |
| LAESV (average) | 87.6 ± 25.2* , § | 123 ± 46.2 # | 51.6 ± 18.3 | <0.001 |
HC, healthy controls; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IVSD, interventricular septum thickness at end‐diastole; LAEDV, left atrial end‐diastolic volume; LAEF, left atrial emptying fraction; LAESV, left atrial end‐systolic volume; LAFAC, left atrial fractional area change; LAS, left atrial strain; LAVI, left atrial volume index; LVED, left ventricular diameter at end‐diastole; LVEDVI, left ventricular volume index at end‐diastole; LVEF, left ventricular ejection fraction; LVESVI, left ventricular volume index at end‐systole; LVGLS, left ventricular global longitudinal strain; LVMI, left ventricular mass index; PWD, posterior wall thickness at end‐diastole; TAPSE, tricuspid annular plane systolic excursion.
P < 0.05 in comparison between HFpEF and HC.
P < 0.05 in comparison between HFrEF and HC.
P < 0.05 in comparison between HFpEF and HFrEF.
Figure 1.
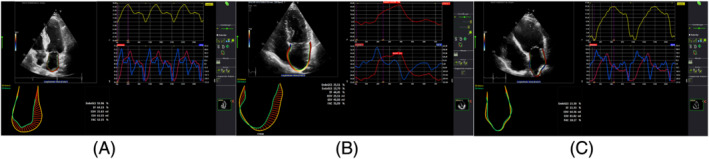
(A–C) Left atrial strain in healthy controls, heart failure with preserved ejection fraction, and heart failure with reduced ejection fraction, respectively.
We analysed the area under the curve (AUC) of LAS and LAEF in patients with HF in association with reduced muscle endurance (RME) and found AUC (0.76 vs. 0.80) with 95% confidence interval (CI) (0.59–0.96, P = 0.012 vs. 0.63–0.98, P = 0.006, respectively) (Figure 2 ).
Figure 2.
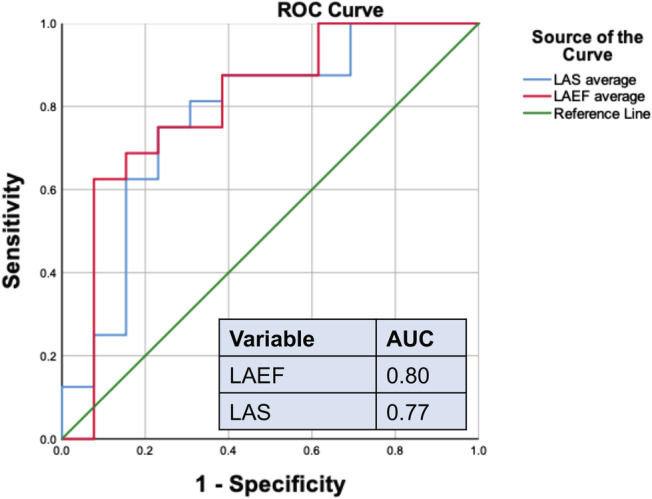
The receiver operator characteristic (ROC) curve of left atrial emptying fraction (LAEF) and left atrial global longitudinal strain to distinguish patients with reduced muscle endurance of left leg in extension/muscle mass of left leg (< mean value of the cohort of patients with heart failure). Area under the curve (AUC) is 0.80 vs. 0.76, respectively. LAS, left atrial strain.
To show the profile of patients with reduced LAEF (< mean value of the cohort = 34.5%), we divided the cohort of patients into two groups according to the mean value of LAEF. Accordingly, patients with reduced LAEF had reduced exercise capacity (peak VO2: 14.3 + 3.5 vs. 18.5 ± 3.5 mL/min/kg, P = 0.003, VE/VCO2: 39.3 ± 8.7 vs. 31.8 ± 4.9, P = 0.007) and reduced muscle function measured as peak torque and endurance (all P < 0.05) (Table 3 ). The atrioventricular coupling was reflected in reduced LVGLS in patients with reduced LAEF (−8.5 ± 5 vs. −19.6 ± 5.3%, P < 0.0001) (Figure 3 ). Considering the recommended cut‐off value of 48% for LAEF, we divided the cohort into two groups (21 patients with reduced LAEF vs. 9 patients with preserved LAEF) and found that two‐thirds of patients with RME of left leg in extension had reduced LAEF vs. one‐third without reduced LAEF [14/21 (66.7%) vs. 7/21 (33.3%), P = 0.03]. We were able to show similar results by dividing the cohort according to the mean value of LAS (Table 4 ).
Table 3.
Comparison between heart failure patients with reduced and relatively normal left atrial emptying fraction regarding exercise capacity, skeletal muscle function, atrophy‐related genes, and inflammatory biomarkers
| LAEF < 36.5% (mean value) | LAEF ≥ 36.5% (mean value) | P‐value | |
|---|---|---|---|
| N = 15 | N = 15 | ||
| Age (years) | 73 ± 7 | 69 ± 7 | 0.1 |
| Sex (m/f) f % | (10/5) 33.3 | (9/6) 40.0 | 1.00 |
| LVGLS % | −8.5 ± 5.0 | −19.6 ± 5.3 | <0.0001 |
| Peak VO2 (mL/min/kg) | 14.3 + 3.5 | 18.5 ± 3.5 | 0.003 |
| VE/VCO2 | 39.3 ± 8.7 | 31.8 ± 4.9 | 0.007 |
| Blood pressure at rest (mmHg) | 106 ± 17 | 123 ± 12 | 0.002 |
| Blood pressure at peak VO2 (mmHg) | 133 ± 34 | 184 ± 37 | 0.007 |
| BNP (pg/mL) | 435 ± 313 | 124 ± 64.2 | 0.001 |
| GFR (mL/min) | 61.1 ± 20.4 | 77.1 ± 11.0 | 0.012 |
| Peak torque of left leg in flexion (Nm/kg) | 6.0 ± 2.1 | 7.6 ± 2.2 | 0.038 |
| Peak torque of right leg in extension (Nm/kg) | 9.2 ± 2.3 | 11.0 ± 2.7 | 0.055 |
| Muscle endurance of the right leg in extension (Nm/kg) | 67.2 ± 24.9 | 90.4 ± 34.8 | 0.045 |
| Muscle endurance of the right leg in flexion (Nm/kg) | 57.1 ± 22.1 | 81.8 ± 29.3 | 0.015 |
| Muscle endurance of the left leg in extension (Nm/kg) | 64.3 ± 23.9 | 88.5 ± 32.3 | 0.028 |
| Muscle endurance of the left leg in flexion (Nm/kg) | 56.0 ± 23.1 | 76.1 ± 28.3 | 0.045 |
BNP, brain natriuretic peptide; GFR, glomerular filtration rate; LAEF, left atrial emptying fraction; LVGLS, left ventricular global longitudinal strain; PVO2, maximal oxygen uptake; VE/VCO2, ventilatory efficiency slope.
Figure 3.
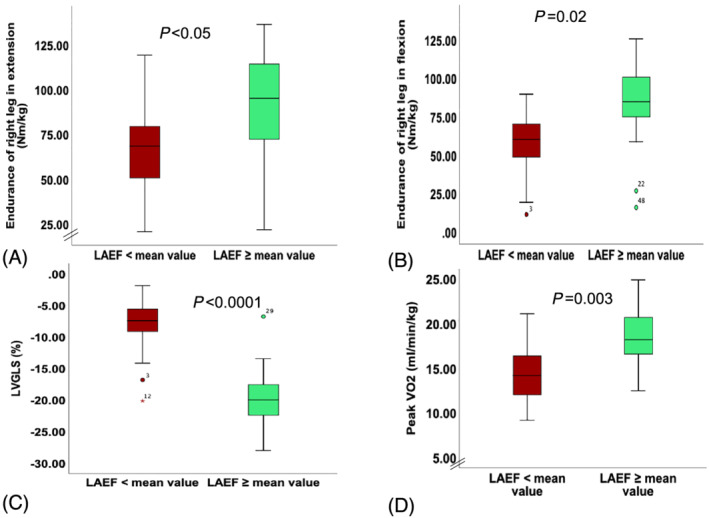
Comparison between patients with reduced left atrial emptying fraction (LAEF) and LAEF > mean value of the cohort of patients with heart failure. (A–D) Muscle endurance of right leg in extension and flexion divided by muscle mass of the right leg as well as left ventricular global longitudinal strain (LVGLS), peak VO2.
Table 4.
Comparison between heart failure patients with reduced and relatively normal left atrial strain regarding exercise capacity, skeletal muscle function, atrophy‐related genes, and inflammatory biomarkers
| LAS < 19.8% (mean value) | LAS ≥ 19.8% (mean value) | P‐value | |
|---|---|---|---|
| N = 17 | N = 13 | ||
| Age (years) | 72 ± 7 | 69 ± 7 | 0.4 |
| Sex (m/f) f % | (11/6) 35.3 | (8/5) 38.5 | 1.00 |
| LVGLS % | −9.0 ± 5.3 | −19.9 ± 5.3 | <0.0001 |
| Peak VO2 (mL/min/kg) | 14.7 ± 3.6 | 18.6 ± 3.6 | 0.006 |
| Blood pressure at rest (mmHg) | 108 ± 19 | 123 ± 8 | 0.012 |
| Blood pressure at peak VO2 (mmHg) | 143 ± 46 | 179 ± 31 | 0.022 |
| BNP (pg/mL) | 386 ± 309 | 133 ± 105 | 0.009 |
| GFR (mL/min) | 65.4 ± 21.9 | 74.01 ± 10.1 | 0.20 |
| Reduced muscle endurance of the right leg in extension (Nm/kg) (yes/no) % | 12/17 (70.6) | 5/13 (38.5) | 0.028 |
BNP, brain natriuretic peptide; GFR, glomerular filtration rate; LAS, left atrial strain; LVGLS, left ventricular global longitudinal strain; PVO2, maximal oxygen uptake.
In a multiple linear regression, LAEF and working load measured during CPET (watt) were independent factors for RME after adjusting for age, LVGLS, and 6MWT [LAEF (B: 0.09, 95% CI: 1.01; 1.18, P = 0.024), exercise capacity (watt) (B: 0.05, 95% CI: 1.01; 1.08, P = 0.006)] (Table 5 ).
Table 5.
Linear regression model with muscle endurance in extension of the left leg/skeletal muscle mass serving as the dependent variable
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| B | 95% CI | P‐value | B | 95% CI | P‐value | |
| Age | 0.07 | 0.87–1.01 | 0.08 | 0.09 | 0.95–1.29 | 0.21 |
| Sex (male/female) | 0.34 | 0.45–4.13 | 0.54 | |||
| LVGLS | −0.10 | 0.82–0.99 | 0.041 | 0.05 | 0.88–1.26 | 0.62 |
| LAEF | 0.08 | 1.03–1.14 | 0.004 | 0.09 | 1.01–1.18 | 0.024 |
| Working load (W) | 0.04 | 1.01–1.06 | 0.002 | 0.05 | 1.01–1.08 | 0.006 |
| 6MWT | 0.01 | 1.00–1.02 | 0.008 | 0.01 | 0.99–1.02 | 0.21 |
| LVEF | 0.03 | 0.99–1.07 | 0.08 | |||
| LAVI | −0.02 | 0.94–1.01 | 0.20 | |||
| LAS | 0.09 | 1.03–1.17 | 0.004 | |||
6MWT, 6 min walk test; CI, confidence interval; LAEF, left atrial emptying fraction; LAS, left atrial strain; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain.
In a simple regression analysis in the whole cohort, LAEF and LAS were associated with LVGLS (r = 0.7, P < 0.0001; r = 0.6, P < 0.0001), as well as with peak VO2 (r = 0.7, P < 0.0001; r = 0.5, P < 0.0001), and with VE/VCO2 (r = −0.6, P < 0.001; r = 0.4, P = 0.004), respectively. LAEF was similarly associated with muscle endurance (Figure 4 ). LVGLS and left atrial volume index (LAVI) were not associated with muscle function (P = 0.13, P = 0.20, respectively). Peak torque of the left leg in extension was associated with E/LAS in patients with HFpEF (r = −0.6, P = 0.020). LAEF and LAS were associated with endurance of left leg in extension and with peak VO2 in HFrEF patients (Tables 6A, 6B, 6C).
Figure 4.
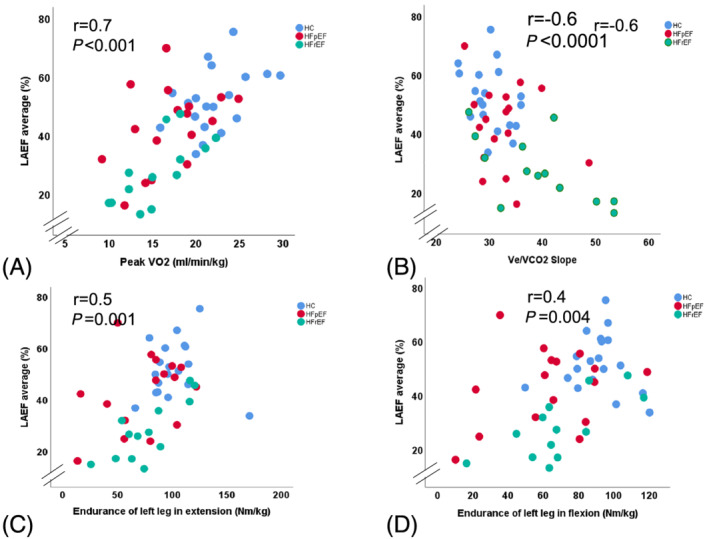
(A–D) Simple regression analysis between left atrial emptying fraction (LAEF) and peak VO2, VE/VCO2, muscle endurance of left leg in extension and flexion. HC, healthy controls; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Table 6A.
Simple regression analysis showing the relationship of cardiac measurements to peripheral factors (muscle endurance) as well as to BNP and peak VO2 in the whole cohort
| BNP | Muscle endurance | Peak VO2 | ||||
|---|---|---|---|---|---|---|
| P‐value | r‐value | P‐value | r‐value | P‐value | r‐value | |
| LAVI | 0.007 | 0.4 | 0.20 | 0.16 | <0.0001 | 0.52 |
| LAEF | <0.0001 | 0.6 | 0.001 | 0.46 | <0.0001 | 0.65 |
| LAS | 0.002 | 0.4 | 0.002 | 0.43 | <0.0001 | 0.53 |
| LVGLS | <0.0001 | 0.5 | 0.13 | 0.22 | <0.0001 | 0.49 |
P‐values < 0.05 and their associated r‐values were marked in bold.
Table 6B.
Simple regression analysis showing the relationship of cardiac measurements to peripheral factors (muscle endurance) as well as to BNP and peak VO2 patients with heart failure with preserved ejection fraction
| BNP | Muscle endurance | Peak VO2 | ||||
|---|---|---|---|---|---|---|
| P‐value | r‐value | P‐value | r‐value | P‐value | r‐value | |
| LAVI | 0.46 | 0.19 | 0.05 | 0.49 | 0.51 | 0.17 |
| LAEF | 0.05 | 0.48 | 0.18 | 0.35 | 0.09 | 0.42 |
| LAS | 0.19 | 0.33 | 0.09 | 0.44 | 0.045 | 0.49 |
| LVGLS | 0.68 | 0.11 | 0.64 | 0.13 | 0.06 | 0.46 |
P‐values < 0.05 and their associated r‐values were marked in bold.
Table 6C.
Simple regression analysis showing the relationship of cardiac measurements to peripheral factors (muscle endurance) as well as to BNP and peak VO2 patients with heart failure with reduced ejection fraction
| BNP | Muscle endurance | Peak VO2 | ||||
|---|---|---|---|---|---|---|
| P‐value | r‐value | P‐value | r‐value | P‐value | r‐value | |
| LAVI | 0.99 | 0.01 | 0.99 | 0.01 | 0.46 | 0.19 |
| LAEF | 0.18 | 0.44 | 0.001 | 0.79 | 0.01 | 0.67 |
| LAS | 0.35 | 0.32 | 0.02 | 0.64 | 0.02 | 0.62 |
| LVGLS | 0.81 | 0.01 | 0.39 | 0.27 | 0.64 | 0.15 |
BNP, brain natriuretic peptide; LAEF, left atrial emptying fraction; LAS, left atrial strain; LAVI, left atrial volume index; LVGLS, left ventricular global longitudinal strain.
P‐values < 0.05 and their associated r‐values were marked in bold.
Measurements of LAS (LAVI: P = 0.01, r = 0.36; E/e′: P = 0.03, r = 0.34) and LAEF (LAVI: P < 0.0001, r = 0.49; E/e′: P = 0.003, r = 0.50) correlated significantly to traditional echocardiographic measurements such as LAVI and E/e′.
In a sub‐analysis, by excluding patients with atrial fibrillation (a. fib), the results of our study remained unchanged. However, by focusing on patients with a. fib (excluding sinus rhythm), we found occasionally significant correlations between LAEF and peak VO2 (r = 0.57, P = 0.03) and between LAEF and LVGLS (r = 0.79, P = 0.001). All other results turned insignificant.
Discussion
To our knowledge, this is the first study that shows a link between central (LAEF and LAS) and peripheral factors (skeletal muscle function) involved in the pathophysiology of reduced exercise capacity in patients with HF. We found that patients with HFpEF and HFrEF have reduced LAEF and LAS compared with HC. Furthermore, we showed a high AUC of both LAEF and LAS in association with RME. In a multiple linear regression, LAEF and working load measured during CPET (watt) were independent factors for predicting RME after adjusting for age, LVGLS, and 6MWT.
To describe the profile of patients with reduced LAEF, we divided the cohort into two groups according to the mean value of LAEF and found that patients with reduced LAEF have reduced exercise capacity measured as peak VO2 and elevated VE/VCO2, as well as reduced muscle function measured as peak torque and muscle endurance of legs both in flexion and in extension. Similar results were shown by dividing the cohort according to the mean value of LAS. In other words, we showed for the first time a relationship between central and peripheral limitations of exercise capacity in patients with HF both with HFpEF and with HFrEF. Neither LVGLS nor LAVI was as sensitive and did not show any relation to muscle endurance.
Peak torque of the left leg in patients with HFpEF was inversely associated with E/LAS. The elevated novel LA filling index (E/LAS ratio) was recently shown to be an effective and useful parameter to determine the elevated LV filling pressure in patients with HFpEF. 26 Accordingly, our findings show that HFpEF patients with elevated LV filling pressure (elevated E/LAS ratio) correlated with reduced muscle strength of legs.
Patients with HF suffer mainly from dyspnoea and reduced exercise capacity measured in the CPET as reduced peak VO2. 1 , 27 , 28 The pathophysiology beyond dyspnoea and exercise intolerance in patients with HF is multifactorial and includes both central (cardiac) and peripheral (skeletal muscle) factors. 11 , 29 , 30 A link between cardiac and muscular function contributing to the reduced peak VO2 in HF, as a result to the systemic involvement of HF, is expected. In other words, we hypothesized that central novel parameters (LAEF and LAS) are capable to detect the peripheral limiting factors (reduced skeletal muscle function).
The role of peripheral factors such as skeletal muscle mass and function in explaining the reduced exercise capacity in patients with HF has been shown in several studies. 11 , 12 , 14
Centrally, elevated filling pressure of the left ventricle was suggested as an important mechanism in explaining dyspnoea and reduced exercise capacity. 1 , 29 LAS is a surrogate of elevated LV filling pressure and an indicator of cardiovascular performance through regulating pulmonary venous return and LV filling. 5 , 31 Recent studies and guidelines have defined the normal values of LAEF (>48%) and LAS (>26%). 3 , 21
Additionally, recent studies showed a link between LA function measured by 2D‐STE and reduced exercise capacity with CPET. 6 , 8 , 32 The latest relationship could be explained by the anatomical location and function of the LA. The LA functions as a reservoir during systole, conduit during early diastole, and a blood pump in the late diastole. 33 The harmony of all of these three phases is very important to keep the atrioventricular coupling intact during exercise and therefore maintaining the best possible cardiac output and exercise capacity. One of the adaptive mechanisms of the LA to maintain the atrioventricular coupling is to increase LA volume through LA dilation and keeping as a result the LV filing pressure optimally as low as possible, 34 which leads finally to increase LA volume and reduce LA function. HF guidelines recommend the evaluation of LAVI. 16 However, the relationship between LA function using 2D‐STE and exercise capacity (peak VO2) is stronger than LAVI. 8 Furthermore, LA dysfunction was documented in patients with hypertension or diabetes even with normal LA size. 35 Recently, LA function has also been shown to be an independent predictor for HF hospitalization. 36 Frydas et al. found recently in an analysis in 300 patients with HF that LAS is more sensitive than LAVI in detecting LA impairment in HF and that LAS is superior to LAEF, LAVI, or E/e′ in predicting the presence elevated LV filling pressures. Furthermore, the diagnostic value of LAS was independent from LVEF. 37
In spite of the strong correlation shown in our results between LVGLS and LAS/LAEF, LVGLS failed to predict the reduced exercise capacity measured evaluated both as peak VO2 and as RME. Lundberg et al. found in a simultaneous echocardiography and invasive haemodynamic measurements that LAS correlates with pulmonary capillary wedge pressure (PCWP) but not LVGLS. 38 This all emphasizes the importance of using LAS/LAEF and not LAVI or LVGLS in evaluating LA function and LV filling pressure. This was explained by the fact that LAS quantifies mechanical events at the LA level associated with PCWP, as opposed to LVGLS, which might better reflect LV end‐diastolic pressure. Furthermore, previous experimental studies have shown distinct cellular responses with more pronounced pro‐fibrotic changes detected in the LA as compared with the LV wall, 39 which supports the diagnostic importance of LAS/LAEF independent of the LVGLS.
Limitations
We investigated a small group of patients with HFpEF and HFrEF. Future studies should focus on cohorts of patients either with HFpEF or with HFrEF alone, because these two groups of patients present different cohorts with different pathophysiology. Larger and perspective studies are required to confirm and validate our results and prove the causality between LAS/LAEF and skeletal muscle function.
Although we mainly performed the majority of the echocardiography tests using GE technology, just few of these tests were performed with the Philips technology. Ideally, all investigations should be performed with a machine from one manufacture. Furthermore, LAS is not widely used yet and requires special training and experience. Image quality is very essential requirement for performing 2D‐STE. Furthermore, the assessment of LA mechanical function was performed at rest and subsequently associated with measures performed during physical activity (muscle function and peak VO2). Data on LA function during exercise would be more relevant and should be measured in future research parallel to CPET or during the measurement of muscle endurance. Additionally, strain studies on patients with a. fib need to be further validated and different cut‐off values might be required. Further, missing nutritional data present a limitation, as these might influence muscle mass and muscle function. An additional challenge was recruiting elderly HC without any diseases such arterial hypertension and diabetes.
Conclusions
Deteriorations on different levels of exercise capacity and skeletal muscle function in patients with HF are detectable efficiently centrally by measuring changes taking place in the LAEF and LAS. Using a sensitive cardiac measurement (LAEF/LAS), we proved a link between central and peripheral limitations in exercise capacity. Our findings might have diagnostic and therapeutic impact on the management of HF and its comorbidities. Developing scores that integrate central and peripheral factors in evaluating and staging the exercise intolerance in patients with HF could be very helpful. Accordingly, integrating the evaluation of LAEF and LAS in the assessment of functional capacity in patients with HF could be of special importance for staging the reduced exercise capacity and might be a potential therapeutic target or a marker to control the success of HF therapies.
Acknowledgement
Open Access funding enabled and organized by Projekt DEAL.
Bekfani, T. , Hamadanchi, A. , Ijuin, S. , Bekhite, M. , Nisser, J. , Derlien, S. , Westphal, J. , Bogoviku, J. , Morris, D. A. , Fudim, M. , Braun‐Dullaeus, R. C. , Möbius‐Winkler, S. , and Schulze, P. C. (2021) Relation of left atrial function with exercise capacity and muscle endurance in patients with heart failure. ESC Heart Failure, 8: 4528–4538. 10.1002/ehf2.13656.
Contributor Information
Tarek Bekfani, Email: tarek.bekfani@yahoo.com.
P. Christian Schulze, Email: christian.schulze@med.uni-jena.de.
References
- 1. Maeder MT, Thompson BR, Brunner‐la Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol 2010; 56: 855–863. [DOI] [PubMed] [Google Scholar]
- 2. Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the frank‐Starling mechanism. J Am Coll Cardiol 1991; 17: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 3. Morris DA, Takeuchi M, Krisper M, Kohncke C, Bekfani T, Carstensen T, Hassfeld S, Dorenkamp M, Otani K, Takigiku K, Izumi C, Yuda S, Sakata K, Ohte N, Tanabe K, Osmanoglou E, Kuhnle Y, Dungen HD, Nakatani S, Otsuji Y, Haverkamp W, Boldt LH. Normal values and clinical relevance of left atrial myocardial function analysed by speckle‐tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging 2015; 16: 364–372. [DOI] [PubMed] [Google Scholar]
- 4. Morris DA, Belyavskiy E, Aravind‐Kumar R, Kropf M, Frydas A, Braunauer K, Marquez E, Krisper M, Lindhorst R, Osmanoglou E, Boldt LH, Blaschke F, Haverkamp W, Tschöpe C, Edelmann F, Pieske B, Pieske‐Kraigher E. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging 2018; 11: 1405–1415. [DOI] [PubMed] [Google Scholar]
- 5. Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, D'Ascenzi F, Focardi M, Favilli R, Pierli C, Fineschi M, Mondillo S. Correlation of left atrial strain and doppler measurements with invasive measurement of left ventricular end‐diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography 2016; 33: 398–405. [DOI] [PubMed] [Google Scholar]
- 6. von Roeder M, Rommel KP, Kowallick JT, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuß G, Lücke C, Gutberlet M, Schuler G, Schuster A, Lurz P. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2017; 10:e005467. [DOI] [PubMed] [Google Scholar]
- 7. D'Andrea A, Caso P, Romano S, Scarafile R, Cuomo S, Salerno G, Riegler L, Limongelli G, di Salvo G, Romano M, Liccardo B, Iengo R, Ascione L, del Viscovo L, Calabrò P, Calabrò R. Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: a two‐dimensional speckle strain study. Int J Cardiol 2009; 132: 354–363. [DOI] [PubMed] [Google Scholar]
- 8. Leite L, Mendes SL, Baptista R, Teixeira R, Oliveira‐Santos M, Ribeiro N, Coutinho R, Monteiro V, Martins R, Castro G, Ferreira MJ, Pego M. Left atrial mechanics strongly predict functional capacity assessed by cardiopulmonary exercise testing in subjects without structural heart disease. Int J Cardiovasc Imaging 2017; 33: 635–642. [DOI] [PubMed] [Google Scholar]
- 9. Chien CY, Chen CW, Lin TK, Lin Y, Lin JW, Li YD, Chen CH, Tsai WC. Atrial deformation correlated with functional capacity in mitral stenosis patients. Echocardiography 2018; 35: 190–195. [DOI] [PubMed] [Google Scholar]
- 10. Vukomanovic V, Suzic‐Lazic J, Celic V, Cuspidi C, Grassi G, Galderisi M, Djukic V, Tadic M. Is there association between left atrial function and functional capacity in patients with uncomplicated type 2 diabetes? Int J Cardiovasc Imaging 2020; 36: 15–22. [DOI] [PubMed] [Google Scholar]
- 11. Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V, Schefold JC, Sandek A, Doehner W, Cleland JG, Lainscak M, Anker SD, von Haehling S. Sarcopenia in patients with heart failure with preserved ejection fraction: impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 2016; 222: 41–46. [DOI] [PubMed] [Google Scholar]
- 12. Fulster S, Tacke M, Sandek A, Ebner N, Tschope C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur Heart J 2013; 34: 512–519. [DOI] [PubMed] [Google Scholar]
- 13. Zizola C, Schulze PC. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail Rev. 2013;18: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bekfani T, Bekhite Elsaied M, Derlien S, Nisser J, Westermann M, Nietzsche S, Hamadanchi A, Fröb E, Westphal J, Haase D, Kretzschmar T, Schlattmann P, Smolenski UC, Lichtenauer M, Wernly B, Jirak P, Lehmann G, Möbius‐Winkler S, Schulze PC. Skeletal muscle function, structure, and metabolism in patients with heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. Circ Heart Fail 2020; 13: e007198. [DOI] [PubMed] [Google Scholar]
- 15. Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol 2012; 60: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the european Society of Cardiology (ESC)Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 17. Ibanez B, Ibánez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Rev Esp Cardiol (Engl Ed) 2017; 70: 1082. [DOI] [PubMed] [Google Scholar]
- 18. Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual‐energy X‐ray absorptiometry body composition model: review of physical concepts. Am J Physiol 1996; 271: E941–E951. [DOI] [PubMed] [Google Scholar]
- 19. Drouin JM, Valovich‐mcLeod T, Shultz SJ, Gansneder BM, Perrin DH. Reliability and validity of the biodex system 3 pro isokinetic dynamometer velocity, torque and position measurements. Eur J Appl Physiol 2004; 91: 22–29. [DOI] [PubMed] [Google Scholar]
- 20. Osternig LR. Isokinetic dynamometry: implications for muscle testing and rehabilitation. Exerc Sport Sci Rev 1986; 14: 45–80. [PubMed] [Google Scholar]
- 21. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, Mertens L, Popescu BA, Sengupta PP, Lancellotti P, Thomas JD, Voigt JU, Industry representatives , Prater D, Chono T, Mumm B, Houle H, Healthineers S, Hansen G, Abe Y, Pedri S, Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee , Delgado V, Gimelli A, Cosyns B, Gerber B, Flachskampf F, Haugaa K, Galderisi M, Cardim N, Kaufmann P, Masci PG, Marsan NA, Rosca M, Cameli M, Sade LE. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two‐dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018; 19: 591–600. [DOI] [PubMed] [Google Scholar]
- 22. Sugimoto T, Robinet S, Dulgheru R, Bernard A, Ilardi F, Contu L, Addetia K, Caballero L, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Hagendorff A, Hristova K, Lopez T, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, van de Veire N, von Bardeleben RS, Vinereanu D, Zamorano JL, Go YY, Marchetta S, Nchimi A, Rosca M, Calin A, Moonen M, Cimino S, Magne J, Cosyns B, Galli E, Donal E, Habib G, Esposito R, Galderisi M, Badano LP, Lang RM, Lancellotti P, NORRE Study , Lancellotti P, Dulgheru R, Kou S, Sugimoto T, Bernard A, Ilardi F, Marchetta S, Nchimi A, Robinet S, Go YY, Barone D, Baroni M, de Diego JJG, Hagendorff A, Hristova K, de la Morena G, Lopez T, Zamorano JL, Cardim N, Popescu BA, Kacharava G, Gonjilashvili N, Kurashvili L, Akhaladze N, Mgaloblishvili Z, Oliva MJ, González‐Carrillo J, Athanassopoulos GD, Vinereanu D, Rimbas R, Ciobanu AO, Badano LP, Peluso D, Jose SP, van de Veire N, de Sutter J, Penicka M, Kotrc M, Voigt JU, Ozyigit T, Carbonero JDR, Salustri A, von Bardeleben RS, Lang RM, Addetia K. Echocardiographic reference ranges for normal left atrial function parameters: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging 2018; 19: 630–638. [DOI] [PubMed] [Google Scholar]
- 23. Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J, EACPR. , AHA. EACPR/AHA joint scientific statement. clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J 2012; 33: 2917–2927. [DOI] [PubMed] [Google Scholar]
- 24. Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016; 133: e694–e711. [DOI] [PubMed] [Google Scholar]
- 25. Bittner V, Weiner DH, Yusuf S, Rogers WJ, Mcintyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M, Greenberg B. Prediction of mortality and morbidity with a 6‐minute walk test in patients with left ventricular dysfunction. JAMA 1993; 270: 1702–1707. [PubMed] [Google Scholar]
- 26. Braunauer K, Düngen HD, Belyavskiy E, Aravind‐Kumar R, Frydas A, Kropf M, Huang F, Marquez E, Tadic M, Osmanoglou E, Edelmann F, Tschöpe C, Boldt LH, Pieske B, Pieske‐Kraigher E, Morris DA. Potential usefulness and clinical relevance of a novel left atrial filling index to estimate left ventricular filling pressures in patients with preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging 2020; 21: 260–269. [DOI] [PubMed] [Google Scholar]
- 27. Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci 2013; 68: 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann‐Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the ex‐DHF (Exercise training in diastolic heart Failure) pilot study. J Am Coll Cardiol 2011; 58: 1780–1791. [DOI] [PubMed] [Google Scholar]
- 29. Pellicori P, Kallvikbacka‐Bennett A, Khaleva O, Carubelli V, Costanzo P, Castiello T, Wong K, Zhang J, Cleland JGF, Clark AL. Global longitudinal strain in patients with suspected heart failure and a normal ejection fraction: does it improve diagnosis and risk stratification? Int J Cardiovasc Imaging 2014; 30: 69–79. [DOI] [PubMed] [Google Scholar]
- 30. Katz SD, Maskin C, Jondeau G, Cocke T, Berkowitz R, LeJemtel T. Near‐maximal fractional oxygen extraction by active skeletal muscle in patients with chronic heart failure. J Appl Physiol 2000; 88: 2138–2142. [DOI] [PubMed] [Google Scholar]
- 31. Brecht A, Oertelt‐Prigione S, Seeland U, Rücke M, Hättasch R, Wagelöhner T, Regitz‐Zagrosek V, Baumann G, Knebel F, Stangl V. Left atrial function in preclinical diastolic dysfunction: two‐dimensional speckle‐tracking echocardiography‐derived results from the BEFRI trial. J Am Soc Echocardiogr 2016; 29: 750–758. [DOI] [PubMed] [Google Scholar]
- 32. Fontes‐Carvalho R, Sampaio F, Teixeira M, Ruivo C, Ribeiro J, Azevedo A, Leite‐Moreira A, Ribeiro VG. Left atrial deformation analysis by speckle tracking echocardiography to predict exercise capacity after myocardial infarction. Rev Port Cardiol 2018; 37: 821–830. [DOI] [PubMed] [Google Scholar]
- 33. Blume GG, Mcleod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, Tsang TSM. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 2011; 12: 421–430. [DOI] [PubMed] [Google Scholar]
- 34. Kusunose K, Motoki H, Popovic ZB, Thomas JD, Klein AL, Marwick TH. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart 2012; 98: 1311–1317. [DOI] [PubMed] [Google Scholar]
- 35. Mondillo S, Cameli M, Caputo ML, Lisi M, Palmerini E, Padeletti M, Ballo P. Early detection of left atrial strain abnormalities by speckle‐tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr 2011; 24: 898–908. [DOI] [PubMed] [Google Scholar]
- 36. Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the heart and soul study. J Am Coll Cardiol 2012; 59: 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frydas A, Morris DA, Belyavskiy E, Radhakrishnan AK, Kropf M, Tadic M, Roessig L, Lam CSP, Shah SJ, Solomon SD, Pieske B, Pieske‐Kraigher E. Left atrial strain as sensitive marker of left ventricular diastolic dysfunction in heart failure. ESC Heart Fail 2020; 7: 1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lundberg A, Johnson J, Hage C, Bäck M, Merkely B, Venkateshvaran A, Lund LH, Nagy AI, Manouras A. Left atrial strain improves estimation of filling pressures in heart failure: a simultaneous echocardiographic and invasive haemodynamic study. Clin Res Cardiol 2019; 108: 703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing‐induced congestive heart failure. Cardiovasc Res 2004; 63: 236–244. [DOI] [PubMed] [Google Scholar]


