Abstract
Aims
Despite regularly updated guidelines, there is still a delay in referral of advanced heart failure patients to mechanical circulatory support and transplant centres. We aimed to analyse characteristics and outcome of non‐inotrope‐dependent patients implanted with a left ventricular assist device (LVAD).
Methods and results
The ASSIST‐ICD registry collected LVAD data in 19 centres in France between February 2006 and December 2016. We used data of patients in Interagency Registry for Mechanically Assisted Circulatory Support Classes 4–7. The primary endpoint was survival analysis. Predictors of mortality were searched with multivariable analyses. A total of 303 patients (mean age 61.0 ± 9.9 years, male sex 86.8%) were included in the present analysis. Ischaemic cardiomyopathy was the leading heart failure aetiology (64%), and bridge to transplantation was the main implantation strategy (56.1%). The overall likelihood of being alive while on LVAD support or having a transplant at 1, 2, 3, and 5 years was 66%, 61.7%, 58.7%, and 55.1%, respectively. Age [hazard ratio (HR) 1.03, 95% confidence interval (CI) 1.00–1.05; P = 0.02], a concomitant procedure (HR 2.32, 95% CI 1.52–3.53; P < 0.0001), and temporary mechanical right ventricular support during LVAD implantation (HR 2.94, 95% CI 1.49–5.77; P = 0.002) were the only independent variables associated with mortality. Heart failure medications before or after LVAD implantation were not associated with survival.
Conclusion
Ambulatory heart failure patients displayed unsatisfactory survival rates after LVAD implantation. A better selection of patients who can benefit from LVAD may help improving outcomes.
Keywords: Advanced heart failure, Heart failure medications, Mechanical circulatory support, Left ventricular assist device, Outcome measures
Introduction
Heart failure (HF) is a major public health concern affecting approximately 1–2% of the adult population with an incidence of 100–900 cases per 100 000 person‐years. 1 , 2 Advanced HF proves refractory to guidelines‐directed optimal medical management and is not amenable to conventional cardiac surgery. The burden associated with advanced HF is very important because of its short‐term, high morbidity and mortality. 3 Despite regularly updated American and European position papers, 3 , 4 the diagnosis remains challenging and advanced HF is still underestimated leading to a suboptimal referral to tertiary centres. This is even more striking for the less severe patients in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) Classes 4–7 due to a broad clinical presentation.
Left ventricular assist devices (LVADs) are an established treatment option for end‐stage HF as a bridge to transplantation or a destination therapy. 5 Moreover, the advantages of LVAD over medical therapies in advanced HF patients have been previously demonstrated. 6 However, the index population was represented by advanced age, critically ill and inotrope‐dependent patients with at least one contraindication to heart transplantation. Despite a previous attempt to evaluate the clinical benefits of LVAD in a less critically ill population, 7 there are no randomized studies comparing LVAD and medical treatment in non‐inotrope‐dependent patients. Therefore, a thorough analysis of the ambulatory population of advanced HF patients receiving LVAD is of utmost importance in order to best define the role of mechanical circulatory support in this specific setting.
The purpose of this multicentre, observational study was to define the contemporary preoperative characteristics and long‐term outcome of an ambulatory advanced HF population supported with an LVAD in the ASSIST‐ICD registry.
Methods
ASSIST‐ICD registry
The ASSIST‐ICD study is a retrospective, multicentre, observational study of durable mechanical circulatory support implanted in 19 French tertiary centres. The study methods as well as the characteristics and overall results of the entire cohort have been previously reported. 8 , 9 Adult patients (aged 18 or older), who were implanted between February 2006 and December 2016 with axial HeartMate II (Abbott, Chicago, IL), Jarvik 2000 (Jarvik Heart, New York, NY), or centrifugal HeartWare (Medtronic, Columbia Heights, MN) continuous‐flow LVAD, were included independently of the therapeutic strategy (bridge to transplantation or destination therapy). The indication to LVAD implantation, the therapeutic strategy, and the type of pump implanted depended on the local Heart Team of each participating centre. The registry excluded patients who were supported with (i) a total artificial heart, (ii) a pulsatile LVAD, or (iii) any device other than those mentioned previously and had heart transplantation before LVAD support.
The ASSIST‐ICD registry was approved by local ethics committees, the French Advisory Committee on the Treatment of Research Data in Healthcare, and the French National Commission of Informatics and Civil Liberties. It was recorded as NCT02873169 in the ClinicalTrials.gov database. Owing to national regulations for retrospective studies, we collected non‐opposition letters from patients.
Study population
We included in the present analysis patients enrolled in the ASSIST‐ICD registry and assigned to INTERMACS Classes 4–7. The INTERMACS class was not reported as a pre‐specified item in the ASSIST‐ICD registry. Patients were dichotomized in ‘inotrope‐dependent’ corresponding to INTERMACS Classes 1–3 or ‘non‐inotrope‐dependent’ corresponding to INTERMACS Classes 4–7.
Study endpoints
The primary endpoint was survival analysis using being alive while on LVAD support or receiving a transplant (i.e. not dying while on LVAD support), as heart transplantation competes with survival on an LVAD. Secondary endpoints were complications' rate, intensive care unit length of stay, and total hospital length of stay.
Statistical analysis
Categorical and ordinal variables were presented as count and percentages while continuous variables as mean ± standard deviation or median with inter‐quartile range, depending on their distribution, which was assessed using the Kolmogorov–Smirnov test. Factors associated with mortality were assessed using univariable and multivariable Cox regression. Variables with P‐value <0.10 in univariate analysis were included in multivariable analysis. Effect of medical treatment prior to LVAD implantation was evaluated on 6 month post‐operative mortality with logistic regression. Effect of medical treatment after LVAD implantation was evaluated in patients alive at 1 month after surgery with logistic regression. The statistical analysis was performed with Stata 15 software (StataCorp, College Station, TX).
Results
Baseline characteristics
Between February 2006 and December 2016, 659 patients were implanted with a continuous‐flow LVAD. Among these, seven patients were excluded (three received a VentrAssist device, and four died during the LVAD surgery). Of the 652 patients included in the final analysis and followed for 9.1 (2.5–22.1) months, 303 (46.4%) were in INTERMACS Classes 4–7 before LVAD implantation and met our inclusion criteria. Table 1 shows the baseline characteristics of the study population. The mean age was 61.0 ± 9.9 years, and 86.8% were male. Ischaemic cardiomyopathy was the leading HF aetiology (64%), while 20 (6.6%) patients experienced at least one previous cardiac surgery operation before LVAD implantation. Many baseline parameters were characteristic of a patient population with advanced HF, in particular low cardiac index [2.0 L/min/m2 (1.7–2.4)] and high pulmonary capillary wedge pressure [36 mmHg (26–43)]. Beta‐blockers and angiotensin‐converting enzyme/angiotensin II receptor blocker were not used in the medical treatment of 14.4% and 18.2% of patients, respectively. Biological, echocardiographic, and haemodynamic parameters were typical of advanced HF patients.
Table 1.
Baseline characteristics of the study population
| Patients (n = 303) | |
|---|---|
| Age (years) | 61.0 ± 9.9 |
| Male sex, n (%) | 263 (86.8) |
| Body mass index (kg/m2) | 26.3 ± 5.0 |
| Body mass index ≥30 kg/m2, n (%) | 62 (20.5) |
| Familial history of cardiovascular disease, n (%) | 64 (21.2) |
| Cardiovascular risk factors, n (%) | |
| Hypertension | 124 (40.9) |
| Diabetes mellitus | 74 (24.4) |
| Dyslipidaemia | 147 (48.7) |
| History of smoking | 189 (62.4) |
| Heart failure aetiology, n (%) | |
| Ischaemic | 194 (64.0) |
| Idiopathic | 82 (27.1) |
| Other | 27 (8.9) |
| Previous sternotomy, n (%) | 20 (6.6) |
| History of supraventricular arrhythmia, n (%) | 148 (48.8) |
| History of ventricular arrhythmia, n (%) | 128 (42.2) |
| ICD before LVAD, n (%) | 250 (82.5) |
| Medical treatment before LVAD, n (%) | |
| Beta‐blocker | 256 (85.6) |
| ACE inhibitor | 211 (70.6) |
| ARB | 38 (12.9) |
| Loop diuretic | 277 (92.6) |
| MRA | 220 (73.6) |
| Serum biology | |
| Sodium (mEq/L) | 136 (132–138) |
| Creatinine (μmol/L) | 122 (91–151) |
| Total bilirubin (μmol/L) | 14 (10–24) |
| NT‐proBNP (ng/L) | 3622 (2045–5084) |
| BNP (ng/L) | 738 (363–1220) |
| Echocardiography | |
| LVEDD (mm) | 71 (65–77) |
| LVEF (%) | 20 (16–25) |
| Severe mitral regurgitation (%) | 40 (14.4) |
| TAPSE (mm) | 17 (13–20) |
| S′ (cm/s) | 10 (8–12) |
| Severe tricuspid regurgitation, n (%) | 13 (5.1) |
| Systolic pulmonary artery pressure (mmHg) | 52 (42–62) |
| Right heart catheterization | |
| CVP (mmHg) | 8.0 (5.0–15.0) |
| Mean pulmonary artery pressure (mmHg) | 36 (26–43) |
| PCWP (mmHg) | 25 (18–29) |
| Cardiac index (L/min/m2) | 2.0 (1.7–2.4) |
| Pulmonary vascular resistance (Wood units) | 2.9 (2.0–4.2) |
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; CVP, central venous pressure; ICD, implantable cardioverter defibrillator; LVAD, left ventricular assist device; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PCWP, pulmonary capillary wedge pressure; S′, pulsed‐wave tissue Doppler‐derived systolic myocardial velocity of the tricuspid lateral annulus; TAPSE, tricuspid annular plane systolic excursion.
Operative and post‐operative outcomes
A HeartMate II was implanted in 224 (73.9%) patients, and bridge to transplantation was the main strategy adopted in our patient population (56.1%). Forty‐six (15.2%) patients underwent a concomitant procedure during the index operation, and 18 (5.9%) received a temporary femoro‐pulmonary extracorporeal membrane oxygenation (ECMO) to support the right ventricle during the LVAD implantation surgery. Table 2 displays the operative and post‐operative outcomes of our study population. During the follow‐up period, 141 (46.5%) patients died while 87 (28.7%) were transplanted after a median waiting list time of 10.3 (6.0–22.0) months (Figure 1 ). We did not find a statistical association between medical treatment before LVAD implantation and 6 month survival as well as medical treatment after LVAD implantation and long‐term survival (Table 3 ).
Table 2.
Operative and post‐operative outcomes
| Patients (n = 303) | |
|---|---|
| LVAD type, n (%) | |
| HeartMate II | 224 (73.9) |
| HeartWare | 52 (17.2) |
| Jarvik 2000 | 27 (8.9) |
| LVAD strategy, n (%) | |
| Bridge to transplantation | 170 (56.1) |
| Destination therapy | 132 (43.6) |
| Bridge to candidacy | 1 (0.3) |
| Complications during follow‐up, n (%) | |
| Driveline infection | 80 (26.4) |
| Mechanical right ventricular support | 18 (5.9) |
| Septic shock | 22 (16.3) |
| Stroke | 19 (14.1) |
| Haemorrhagic | 11 (8.2) |
| Ischaemic | 8 (5.9) |
| LVAD thrombosis | 18 (13.3) |
| Bleeding | 47 (11.7) |
| Electrical storm | 9 (6.7) |
| Overall mortality, n (%) | 141 (46.5) |
| Cause of death, n (%) | |
| Cardiovascular | 68 (22.4) |
| Non‐cardiovascular | 73 (24.1) |
LVAD, left ventricular assist device.
Figure 1.
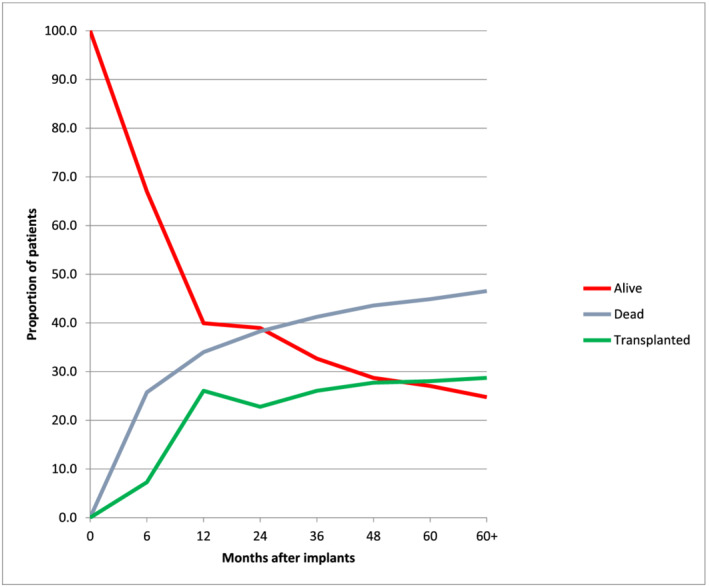
Competing events after left ventricular assist device (LVAD) implantation. Mutually exclusive endpoints of death, alive on ventricular assist device, or transplant were tracked through 5 years, with the cumulative percentage of events at any given time point equal to 100%.
Table 3.
Association between medical treatment before and after LVAD implantation and survival
| Variable | Medical treatment before LVAD implantation and 6 month post‐operative survival | Medical treatment after LVAD implantation and long‐term survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Beta‐blocker | 0.64 | 0.37–1.11 | 0.11 | 0.99 | 0.58–1.69 | 0.97 |
| ACEI | 1.06 | 0.65–1.73 | 0.82 | 1.15 | 0.69–1.91 | 0.59 |
| ARB | 1.28 | 0.69–2.37 | 0.43 | 0.34 | 0.05–2.46 | 0.28 |
| 1.19 | 0.70–2.02 | 0.51 | 0.95 | 0.56–1.62 | 0.86 | |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; HR, hazard ratio; LVAD, left ventricular assist device.
Short‐term and long‐term survival
The overall likelihood of being alive while on LVAD support or having a transplant at 1, 2, 3, and 5 years after implantation was 66%, 61.7%, 58.7%, and 55.1%, respectively. At the end of follow‐up, 87 patients (28.7%) had heart transplantation. Mortality in INTERMACS Class 4–7 patients was not different compared with INTERMACS Class 1–3 patients, P(log‐rank) = 0.994 (Supporting Information, Figure S1 ).
Risk factors of mortality
The univariate analysis identified the following risk factors of mortality: age, hypertension, familial history of cardiomyopathy, destination therapy as LVAD strategy, concomitant procedure during the index operation, and temporary femoro‐pulmonary ECMO. Age [hazard ratio (HR) 1.03, 95% confidence interval (CI) 1.00–1.05; P = 0.02], a concomitant procedure (HR 2.32, 95% CI 1.52–3.53; P < 0.0001), and temporary femoro‐pulmonary ECMO during LVAD implantation (HR 2.94, 95% CI 1.49–5.77; P = 0.002) were identified as the only independent variables associated with mortality. Table 4 summarizes the univariate and multivariable analysis of the predictors of mortality.
Table 4.
Univariate and multivariable analysis of predictors associated with mortality
| Variable | HR | 95% CI | P‐value |
|---|---|---|---|
| Univariate analysis | |||
| Age (years) | 1.03 | 1.01–1.05 | 0.001 |
| Male sex | 0.89 | 0.55–1.44 | 0.63 |
| Body mass index (kg/m2) | 0.99 | 0.95–1.02 | 0.55 |
| Hypertension | 1.46 | 1.05–2.03 | 0.03 |
| Diabetes mellitus | 1.42 | 0.98–2.06 | 0.06 |
| Dyslipidaemia | 1.18 | 0.85–1.65 | 0.32 |
| History of smoking | 0.96 | 0.68–1.35 | 0.80 |
| ICD before LVAD | 1.35 | 0.84–2.17 | 0.21 |
| NT‐proBNP (1000 U) | 0.96 | 0.91–1.01 | 0.15 |
| GFR (mL/min) | |||
| 15–30 | 1.45 | 0.68–3.12 | 0.34 |
| 31–60 | 1.44 | 0.87–2.39 | 0.16 |
| 61–90 | 1.53 | 0.90–2.60 | 0.12 |
| Total bilirubin (10 U) | 1.03 | 0.90–1.17 | 0.62 |
| Previous sternotomy | 1.08 | 0.55–2.13 | 0.82 |
| Destination therapy | 1.88 | 1.34–2.63 | 0.003 |
| Concomitant procedure | 2.25 | 1.49–3.39 | 0.0001 |
| Temporary mechanical right ventricular support | 2.01 | 1.08–3.74 | 0.03 |
| Ischaemic stroke | 1.33 | 0.87–2.02 | 0.19 |
| Bleeding | 0.73 | 0.47–1.13 | 0.15 |
| Multivariable analysis | |||
| Age (years) | 1.03 | 1.00–1.05 | 0.02 |
| Male sex | 0.63 | 0.37–1.08 | 0.09 |
| Hypertension | 1.39 | 0.97–2.00 | 0.08 |
| Diabetes mellitus | 1.37 | 0.92–2.02 | 0.12 |
| Destination therapy | 1.40 | 0.91–2.16 | 0.13 |
| Concomitant procedure | 2.32 | 1.52–3.53 | <0.0001 |
| Temporary mechanical right ventricular support | 2.94 | 1.49–5.77 | 0.002 |
CI, confidence interval; GFR, glomerular filtration rate; HR, hazard ratio; ICD, implantable cardioverter defibrillator; LVAD, left ventricular assist device; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Discussion
We described and reported herein the results of LVAD in the population of INTERMACS Class 4–7 patients from the ASSIST‐ICD registry. We presented a real‐life cohort of patients implanted with LVAD for advanced ambulatory HF over a 10 year period. Implantation in non‐inotrope‐dependent patients accounted for almost half of the LVAD activity in France during the study period, and 43% of these patients were implanted with a destination therapy strategy. At 5 year follow‐up, 27% of patients were still alive on LVAD while 28% had heart transplantation.
Contemporary trend of patients with ambulatory advanced heart failure
The rate of patients treated with beta‐blocker, renin–angiotensin–aldosterone system inhibitors, and mineralocorticoid receptor antagonists was comparable with prospective cohorts of advanced HF patients 10 , 11 , 12 and higher than that published by Greene et al. 13 We could speculate that this rate reflects the expertise in HF management of participating centres as well as their efforts to optimize medical treatment before LVAD implantation. Despite an ambulatory profile, patients had a severe HF phenotype with high levels of natriuretic peptide and critical haemodynamic parameters including elevated pulmonary capillary wedge pressure, pulmonary artery systolic pressure, and right atrial pressure. Patients in this current real‐life cohort seem as severe or slightly more than those included in prospective studies on LVAD. 10 , 11 , 12 There is an emerging literature on the importance for early diagnosis and referral of advanced HF patients in tertiary centres, although delay is still a reality in daily practice. 14 , 15 , 16 Delay is also possibly explained by patients and physicians resistance to surgery in non‐inotrope‐dependent patients. Indeed, acceptance of mechanical circulatory support increases proportionally with the severity of HF. 17 , 18
High early mortality rate opposite to long‐term good prognosis
Perioperative and short‐term mortality was high, reaching one‐third of patients at 1 year follow‐up. Several factors could explain these findings. Firstly, the era of implantation and associated devices are consistent with a 1 year mortality of 20%. 19 Secondly, all the participating centres in this study are considered as very low (≤10 implants/year) or low (11–30 implants/year) volume centres, shifting the volume–outcome relationship towards worse survival. 20 Thirdly, ambulatory advanced HF patients have a long history of HF characterized by a relative haemodynamic stability, leading to frailty and sarcopenia and explaining the comparable prognosis of these patients with in INTERMACS Class 2 or 3 patients. 21 The annual mortality rate varies between 2% and 4% after the first year and is much lower compared with the annual mortality rate of advanced HF patients. 10
Prognostic factors of mortality
Older age, concomitant surgery, and temporary femoro‐pulmonary ECMO during LVAD implantation were the only independent variables associated with worse outcome. This is coherent with previous reports. 9 , 19 The rate of a concomitant procedure was 15% and comparable between ambulatory advanced HF patients and the more severe patients of the population. 9 The complexity of the procedure was associated with a two‐fold increase in the early risk of death in our study, and this is consistent with the results of the 2019 INTERMACS report. 21
The need for a temporary right ventricular support was the variable with the highest HR of death. The 20% rate of right ventricular dysfunction is similar to a previous report with the HeartMate II LVAD. 22 These observations highlight the importance of a meticulous evaluation of patient history and anticipated surgical procedure with a special attention on right ventricular function and valvular disease. Heart transplantation or biventricular assist device could be a better option for patients at high risk of RV failure after LVAD implantation. 23 , 24
Impact of medical treatment on post‐operative and long‐term survival
Beta‐blocker, renin–angiotensin–aldosterone system inhibitors, and mineralocorticoid receptor antagonist are the cornerstone of HF treatment in the American and European guidelines, 1 , 25 but data about their effect on post‐operative survival are sparse. In our study, we found no relationship between preoperative treatment and dose (data not shown) and short‐term survival after surgery. To the best of our knowledge, there are no data validating a beneficial effect of preoperative medical treatment on LVAD survival.
Conversely, McCullough et al. found an association between medical use at 6 months and long‐term survival. 26 These results were not found in our study owing probably to the small sample size of our population of patients and a shorter follow‐up. Finally, we found no dose effect in our cohort as well (data not shown).
Implications for clinical practice
The results of this study yield three main implications for our daily clinical practice:
INTERMACS Class 4–7 patients referred for LVAD implantation are highly severe despite an ambulatory presentation resulting in high post‐operative mortality similar with INTERMACS Classes 1–3. We thought that an early referral, rehabilitation, and implantation of ambulatory patients before surgery could improve outcome. A prospective trial is ongoing to answer this question (NCT04768322).
The 1 year mortality rate after LVAD implantation is extremely low compared with the annual mortality rate of advanced HF patients and should represent another aspect favouring referral especially in patients with recurrent hospitalizations (INTERMACS Class 4).
Age, a concomitant procedure, and temporary mechanical right ventricular support during LVAD implantation were the only independent variables associated with mortality; preoperative thorough evaluation is consequently of utmost importance. A better characterization of ‘good candidates’ is likely necessary to improve outcome. Heart transplantation or biventricular support could be an alternative option in selected patients.
Limitations
Despite a wide participation in this registry of tertiary HF centres in France, this report is not totally exhaustive but describes the results of LVAD in a population of ambulatory advanced HF patients. Duration of post‐operative inotrope treatment and impact on survival was not assessed in our registry. Because of era of implantation, full magnetic levitation devices were not assessed in this registry and outcome of patients is not generalizable to outcome with new generation of device. Most centres in France are low‐volume centres corresponding to the organization in many European countries, leading to a limitation in generalization of the results in high‐volume centres. The stratification of individual risk in this population of less severely ill patients—in whom destination therapy accounts for approximately half of the implantation strategies—should represent the objective of future research. Unfortunately, frailty was not assessed in a standardized way across centres and not reported in the registry. Moreover, the lack of a control group prevents comparison with the medical treatment strategy in this population. Because of the small sample size, the statistical power of our analysis could not show an association between preoperative medical treatment and survival after LVAD implantation. Moreover, sodium–glucose cotransporter‐2 inhibitor and neprilysin inhibitor were not part of the standard treatment. Timing of systematic follow‐up visits and local protocols of post‐operative medical treatment differed among each participating centre and may have affected the results of the present study.
Despite the key role of early referral in the setting of ambulatory advanced HF, the results of the ASSIST‐ICD registry pointed out that our patients were severely ill at time of LVAD implantation. Age, concomitant surgery, and need for a temporary right ventricular support were independent predictors of mortality. Medical treatment before or after LVAD surgery was not associated with improved outcomes.
Conflict of interest
G.B. is a consultant for Boehringer Ingelheim, AstraZeneca, and Abbott outside the submitted work. The remaining authors have nothing to disclose.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Supporting information
Figure S1. Kaplan–Meier survival curves by Intermacs class, 1–3 versus 4–7.
Acknowledgement
The authors thank Julien Berthiller for his help on statistical analyses.
Baudry, G. , Nesseler, N. , Flecher, E. , Vincentelli, A. , Goeminne, C. , Delmas, C. , Porterie, J. , Nubret, K. , Pernot, M. , Kindo, M. , Hoang Minh, T. , Rouvière, P. , Gaudard, P. , Michel, M. , Senage, T. , Boignard, A. , Chavanon, O. , Para, M. , Verdonk, C. , Pelcé, E. , Gariboldi, V. , Anselme, F. , Litzler, P.‐Y. , Blanchart, K. , Babatasi, G. , Bielefeld, M. , Bouchot, O. , Hamon, D. , Lellouche, N. , Bailleul, X. , Genet, T. , Eschalier, R. , d'Ostrevy, N. , Bories, M.‐C. , Akar, R. A. , Blangy, H. , Vanhuyse, F. , Obadia, J. F. , Galand, V. , and Pozzi, M. (2021) Characteristics and outcome of ambulatory heart failure patients receiving a left ventricular assist device. ESC Heart Failure, 8: 5159–5167. 10.1002/ehf2.13592.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016; 37: 2129–2200.27206819 [Google Scholar]
- 2. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016; 13: 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge‐Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska‐Migaj E, McDonagh T, Seferovic P, Ruschitzka F. Advanced heart failure: a position statement of the heart failure Association of the European Society of cardiology. Eur J Heart Fail. 2018; 20: 1505–1535. [DOI] [PubMed] [Google Scholar]
- 4. Fang JC, Ewald GA, Allen LA, Butler J, Westlake Canary CA, Colvin‐Adams M, Dickinson MG, Levy P, Stough WG, Sweitzer NK, Teerlink JR, Whellan DJ, Albert NM, Krishnamani R, Rich MW, Walsh MN, Bonnell MR, Carson PE, Chan MC, Dries DL, Hernandez AF, Hershberger RE, Katz SD, Moore S, Rodgers JE, Rogers JG, Vest AR, Givertz MM, Heart Failure Society of America Guidelines Committee . Advanced (stage D) heart failure: a statement from the heart failure society of america guidelines committee. J Card Fail. 2015; 21: 519–534. [DOI] [PubMed] [Google Scholar]
- 5. Estep JD, Starling RC, Horstmanshof DA, Milano CA, Selzman CH, Shah KB, Loebe M, Moazami N, Long JW, Stehlik J, Kasirajan V, Haas DC, O'Connell JB, Boyle AJ, Farrar DJ, Rogers JG, ROADMAP Study Investigators . Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients results from the ROADMAP Study. J Am Coll Cardiol. 2015; 66: 1747–1761. [DOI] [PubMed] [Google Scholar]
- 6. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne‐Nickens P, Oz MC, Poirier VL, Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group . Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med. 2001; 345: 1435–1443. [DOI] [PubMed] [Google Scholar]
- 7. Pagani FD, Aaronson KD, Kormos R, Mann DL, Spino C, Jeffries N, Taddei‐Peters WC, Mancini DM, McNamara DM, Grady KL, Gorcsan J 3rd, Petrucci R, Anderson AS, Glick HA, Acker MA, Eduardo Rame J, Goldstein DJ, Pamboukian SV, Miller MA, Timothy BJ, REVIVE‐IT Investigators . The NHLBI REVIVE‐IT study: Understanding its discontinuation in the context of current left ventricular assist device therapy. J Hear Lung Transplant. 2016; 35: 1277–1283. [DOI] [PubMed] [Google Scholar]
- 8. Galand V, Flécher E, Auffret V, Boulé S, Vincentelli A, Dambrin C, Mondoly P, Sacher F, Nubret K, Kindo M, Cardi T, Gaudard P, Rouvière P, Michel M, Gourraud JB, Defaye P, Chavanon O, Verdonk C, Ghodbane W, Pelcé E, Gariboldi V, Pozzi M, Obadia JF, Litzler PY, Anselme F, Babatasi G, Belin A, Garnier F, Bielefeld M, Hamon D, Radu C, Pierre B, Bourguignon T, Eschalier R, D'Ostrevy N, Bories MC, Marijon E, Vanhuyse F, Blangy H, Verhoye JP, Leclercq C, Martins RP, ASSIST‐ICD Investigators . Predictors and clinical impact of late ventricular arrhythmias in patients with continuous‐flow left ventricular assist devices. JACC Clin Electrophysiol. 2018; 4: 1166–1175. [DOI] [PubMed] [Google Scholar]
- 9. Anselmi A, Galand V, Vincentelli A, Boule S, Dambrin C, Delmas C, Barandon L, Pernot M, Kindo M, Tam HM, Gaudard P, Rouviere P, Senage T, Michel M, Boignard A, Chavanon O, Verdonk C, Para M, Gariboldi V, Pelce E, Pozzi M, Obadia JF, Anselme F, Litzler PY, Babatasi G, Belin A, Garnier F, Bielefeld M, Guihaire J, Kloeckner M, Radu C, Lellouche N, Bourguignon T, Genet T, D'Ostrevy N, Duband B, Jouan J, Bories MC, Vanhuyse F, Blangy H, Colas F, Verhoye JP, Martins R, Flecher E. Current results of left ventricular assist device therapy in France: the ASSIST‐ICD registry. Eur J Cardiothorac Surg. 2020; 58: 112–120. [DOI] [PubMed] [Google Scholar]
- 10. Stewart GC, Kittleson MM, Patel PC, Cowger JA, Patel CB, Mountis MM, Johnson FL, Guglin ME, Rame JE, Teuteberg JJ, Stevenson LW. INTERMACS (Interagency registry for mechanically assisted circulatory Support) profiling identifies ambulatory patients at high risk on medical therapy after hospitalizations for heart failure. Circ Hear Fail. 2016; 9: 1–9. [DOI] [PubMed] [Google Scholar]
- 11. Kittleson MM, Shah P, Lala A, McLean RC, Pamboukian S, Horstmanshof DA, Thibodeau J, Shah K, Teuteberg J, Gilotra NA, Taddei‐Peters WC, Cascino TM, Richards B, Khalatbari S, Jeffries N, Stevenson LW, Mann D, Aaronson KD, Stewart GC, REVIVAL Investigators . INTERMACS profiles and outcomes of ambulatory advanced heart failure patients: a report from the REVIVAL registry. J Hear Lung Transplant. 2020; 39: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samman‐Tahhan A, Hedley JS, McCue AA, Bjork JB, Georgiopoulou VV, Morris AA, Butler J, Kalogeropoulos AP. INTERMACS profiles and outcomes among Non–Inotrope‐dependent outpatients with heart failure and reduced ejection fraction. JACC Hear Fail. 2018; 6: 743–753. [DOI] [PubMed] [Google Scholar]
- 13. Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Butler J. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019; 73: 2365–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guglin M, Zucker MJ, Borlaug BA, Breen E, Cleveland J, Johnson MR, Panjrath GS, Patel JK, Starling RC, Bozkurt B, ACC Heart Failure and Transplant Member Section and Leadership Council . Evaluation for heart transplantation and LVAD implantation: JACC council perspectives. J Am Coll Cardiol. 2020; 75: 1471–1487. [DOI] [PubMed] [Google Scholar]
- 15. Baumwol J. “I need Help”—A mnemonic to aid timely referral in advanced heart failure. J Hear Lung Transplant. 2017; 36: 593–594. [DOI] [PubMed] [Google Scholar]
- 16. Thorvaldsen T, Lund LH. Focusing on referral rather than selection for advanced heart failure therapies. Card Fail Rev. 2019; 5: 24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Estep JD, Starling RC, Horstmanshof DA, Milano CA, Selzman CH, Shah KB, Loebe M, Moazami N, Long JW, Stehlik J, Kasirajan V, Haas DC, O'Connell JB, Boyle AJ, Farrar DJ, Rogers JG, ROADMAP Study Investigators . Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients results from the ROADMAP study. J Am Coll Cardiol. 2015; 66: 1747–1761. [DOI] [PubMed] [Google Scholar]
- 18. Stewart GC, Kittleson MM, Cowger JA, Johnson FL, Patel CB, Mountis MM, Patel PC, Rame JE, Testani J, Guglin ME, Teuteberg JJ, Stevenson LW. Who wants a left ventricular assist device for ambulatory heart failure? Early insights from the MEDAMACS screening pilot. J Heart Lung Transplant. 2015; 34: 1630–1633. [DOI] [PubMed] [Google Scholar]
- 19. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Hear Lung Transplant. 2017; 36: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 20. Cowger JA, Stulak JM, Shah P, Dardas TF, Pagani FD, Dunlay SM, Maltais S, Aaronson KD, Singh R, Mokadam NA, Kirklin JK, Salerno CT. Impact of center left ventricular assist device volume on outcomes after implantation: an INTERMACS analysis. JACC Hear Fail. 2017; 5: 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, Grady KL, Kirklin JK. The society of thoracic surgeons intermacs database annual report: evolving indications, outcomes, and scientific partnerships. Ann Thorac Surg. 2019; 107: 341–353. [DOI] [PubMed] [Google Scholar]
- 22. Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, Farrar DJ, HeartMate II Clinical Investigators . Right ventricular failure in patients with the HeartMate II continuous‐flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010; 139: 1316–1324. [DOI] [PubMed] [Google Scholar]
- 23. Takeda K, Naka Y, Yang JA, Uriel N, Colombo PC, Jorde UP, Takayama H. Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J Hear Lung Transplant. 2014; 33: 141–148. [DOI] [PubMed] [Google Scholar]
- 24. Khush KK, Potena L, Cherikh WS, Chambers DC, Harhay MO, Hayes D, Hsich E, Sadavarte A, Singh TP, Zuckermann A, Stehlik J, International Society for Heart and Lung Transplantation . The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: 37th adult heart transplantation report—2020; focus on deceased donor characteristics. J Hear Lung Transplant. 2020; 39: 1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the american College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart failure Society of America. J Am Coll Cardiol. 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 26. McCullough M, Caraballo C, Ravindra NG, Miller PE, Mezzacappa C, Levin A, Gruen J, Rodwin B, Reinhardt S, van Dijk D, Ali A, Ahmad T, Desai NR. Neurohormonal blockade and clinical outcomes in patients with heart failure supported by left ventricular assist devices. JAMA Cardiol. 2020; 5: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan–Meier survival curves by Intermacs class, 1–3 versus 4–7.


