Abstract
Aims
Surveillance imaging is often used to detect remodelling, a change in cardiac geometry, and/or function; however, there are limited data in patients with chronic heart failure (HF). We sought to characterize cardiac remodelling in patients with chronic HF and evaluate its association with outcome.
Methods and results
A prospective cohort of patients at risk for HF or with chronic HF underwent cardiac magnetic resonance (CMR) at baseline and 1 year. Ventricular function, volumes, mass, left atrial volume, global longitudinal strain, and myocardial scar were measured. The primary outcome was a composite of death or cardiovascular hospitalization up to 5 years from the 1 year scan. Cox regression was used to identify 1 year CMR predictors of outcome after adjusting for baseline risk. A total of 262 patients (median age 68 years, 57% males) including 96 at risk for HF, 97 with HF and preserved ejection fraction, and 69 with HF and reduced ejection fraction were included. In the patients with HF, 55 events were identified during follow‐up. After adjustment for baseline clinical risk, Cox proportion hazard regressions only identified 1 year change in left ventricular (LV) mass index as a CMR predictor of outcome, adjusted hazard ratio 1.21 (1.02, 1.44) per 10% increase, P = 0.031. Cardiac remodelling defined as a 1 year change in LV mass index ≥15% was observed in 35% of patients with HF. Patients with adverse remodelling of LV mass index had more events on Kaplan–Meier analyses compared to those with no remodelling, log‐rank P = 0.004 for overall cohort, P = 0.035 for heart failure with preserved ejection fraction and P = 0.035 for heart failure and reduced ejection fraction.
Conclusions
Cardiac remodelling is common during serial CMR assessment of patients with chronic HF. Change in LV mass predicted long‐term outcomes whereas change in left ventricular ejection fraction did not.
Keywords: Chronic heart failure, Cardiac remodelling
Introduction
Heart failure (HF) is a progressive and complex syndrome with poor prognosis. 1 Cardiac remodelling clinically manifests as a change in size, shape, and function of the heart and plays a crucial role in the development 2 and progression of HF. 3 Changes in cardiac geometry have been shown to predict outcome in pre‐clinical 4 and clinical HF 5 and can be used to assess response to therapy. 6 , 7 , 8 , 9 , 10 , 11 Routine cardiac imaging is a common surveillance strategy for patients with HF 12 ; however, this approach is not supported by expert opinion, principally due to concerns of cost, access, and measurable impact on patient care. 13 Also, there are limited data characterizing temporal changes in cardiac structure and function and their clinical relevance in patients with chronic HF. To date, imaging studies of cardiac remodelling have largely been limited to patients with heart failure and reduced ejection fraction and are complicated by variable definitions of remodelling. 14
Cardiac magnetic resonance (CMR) is well suited for longitudinal study of remodelling due to high reproducibility of cardiac volumes and function. To date, no observational CMR studies have evaluated serial changes in cardiac geometry and function in patients with chronic HF. We hypothesized that cardiac remodelling assessed longitudinally by serial CMR is common among patients with chronic HF and is predictive of clinical outcomes.
Methods
Study population
This study was conducted with institutional approval from the Health Research Ethics Boards at the University of Alberta and University of Calgary and was registered on clinicaltrials.gov (NCT02052804). Written informed consent was obtained from all study participants. Recruitment and examination procedures have been previously described. 15 In brief, patients with HF and those at‐risk for HF were prospectively and consecutively recruited from adult ambulatory clinics from 2010 to 2014 and underwent comprehensive phenotyping that included a detailed medical history and physical examination, serum biomarkers, and a multi‐parametric CMR exam. Individuals at‐risk for HF had a history of coronary artery disease, diabetes mellitus, hypertension, atrial fibrillation, and/or obesity without a diagnosis of HF (AHA/ACC Classes A and B). Patients with HF (AHA/ACC Class C), were sub‐grouped into those with preserved [heart failure and preserved ejection fraction (HFpEF), left ventricular ejection fraction (LVEF) ≥50%] or reduced ejection fraction (HFrEF, LVEF <50%). 1 Baseline clinical parameters were used to calculate the MAGGIC risk score 16 as a measure of HF burden. This risk score is derived from 13 clinical elements that include age, gender, diabetes mellitus, current smoker, chronic obstructive pulmonary disease, time since heart failure diagnosis, New York Heart Association class, beta‐blocker use, angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use, body mass index, systolic blood pressure, LVEF, and serum creatinine. Patients with HF < 6 months duration or with a contraindication to magnetic resonance imaging were excluded.
Cardiac magnetic resonance protocol
All subjects underwent a baseline and 1 year CMR scan on Siemens Sonata or Avanto 1.5 T system (Siemens Healthcare, Erlangen, Germany). Imaging sequences included steady‐state free precession cine imaging in long‐axis and short‐axis projections to determine ventricular volumes and function as well as late gadolinium enhancement (cardiac magnetic resonance) imaging with 0.15 mmol/kg of gadolinium contrast to assess for the presence of myocardial scar. Typical imaging parameters for cines: repetition time/echo time 2.8 ms/1.4 ms, 50°–70° flip angle, 8 mm slice thickness with a 2 mm gap for short axis slices, 256 × 192 matrix, 380 × 285 mm field of view, 10 views per segment with 25 or 30 reconstructed cardiac phases per cardiac cycle and for LGE imaging: 380 × 285 mm field of view, 256 × 173 matrix, repetition time/echo time 14.7 ms/4.2 ms, flip angle 25° and inversion time of 300 ms. All cardiac images were acquired with electrocardiographic gating, using 8 mm slice thickness and 2 mm gap within 8–12 s breath‐holds.
Image analysis
Ventricular volumes and mass were quantified by a single interpreter (DIP) from short‐axis cines using commercially available image analysis software: Syngo Argus, (Siemens Healthcare, Erlangen, Germany) or CVI42 (Circle, Calgary, Canada). Volumes and mass were normalized to body surface area. Myocardial trabeculations were included in RV and LV end‐diastolic volumes and were excluded from LV mass. Left atrial volume was calculated by the area‐length biplane method. Strain was measured at a mid‐wall contour generated as the mid‐point of endocardial and epicardial borders, both of which were traced at end‐diastole and propagated to all image frames over the full cardiac cycle using the calculated feature tracking displacement fields, similar to previous reports. 17 Strain in each slice was calculated as the fractional change of the mid‐wall contour in length relative to the end‐diastolic cardiac phase using customized analysis software (MATLAB 2017.a). Global circumferential systolic strain (GCS) was calculated as the average of the peak strains from two‐mid‐ventricular short‐axis slices. Similarly, global longitudinal systolic strain (GLS) was calculated as the average of the peak strains from the three long‐axis slices. LV volumes and mass were remeasured in 20 patients from the overall cohort selected at random to determine intra‐observer variability and coefficient of variation.
Myocardial scar quantification was measured from LGE magnitude images using commercially available software (CVI42, Circle Cardiovascular Inc., Calgary, Canada). A threshold of 5 standard deviations from the mean signal of a reference normal region of interest was used to define the scar signal. 18 , 19 Total scar mass was expressed as the absolute value in grams and the relative value as a percentage of the LV mass. Furthermore, baseline myocardial scar was classified into five categories: no scar, ischaemic scar, minor non‐ischaemic scar, major non‐ischaemic scar or no contrast given. 20
Clinical outcomes
Clinical events were identified in the subgroup of patients with HF from electronic health records (International Classification of Diseases codes version 10) and direct patient contact during 5 year follow‐up from 1 year scan. The primary outcome was time to first composite of all‐cause mortality or cardiovascular disease related hospitalization. Time to first composite of all‐cause mortality or HF‐related hospitalization was evaluated as a secondary outcome.
Statistical approach
Continuous variables were expressed as mean ± standard deviation or median (25th, 75th percentile), as appropriate. Categorical variables were expressed as frequency and percentage. Missing data were assumed to occur at random. Multiple imputation with chained equation was used to generate missing data by taking the average of 50 imputations. 21
χ 2 testing was used to compare categorical variables at baseline and McNemar's test was used to compare medication use at baseline and 1 year. The normal distribution of continuous variables was tested by Shapiro–Wilk normality test. A logarithmic transformation was applied to N‐terminal prohormone of b‐type brain natriuretic peptide (NT‐proBNP) and creatinine. Two sample t test (or Mann–Whitney U test) or one‐way analysis of variances with post‐hoc correction (or Dunn's test) was used to compare continuous variables among groups of patients, as appropriate. Paired t test (or Wilcoxon signed‐rank test) was used to compare continuous CMR measures at baseline and 1 year.
Univariable Cox proportional regression of outcome was performed in all clinical and CMR‐derived imaging metrics at baseline and stepwise forward selection of parameters with P value <0.2 was used to identify the best predictors of outcome. In the multivariable Cox proportional hazard analysis, all non‐collinear CMR parameters of remodelling with univariable P value <0.2 were independently tested for their association with outcome after adjustment for baseline risk. The Kaplan–Meier method was used to plot time to clinical events for significant CMR parameters from multivariable analysis. Cardiac remodelling was defined as the optimal cut‐off of CMR metric(s) on receiver operating characteristic analyses identifying the greatest number of events.
Restricted cubic spline based on the Cox regression was computed to illustrate the relationship between continuous CMR parameters of interest and composite clinical outcome. To further assess the association of CMR metrics with clinical outcomes, we applied likelihood ratio testing.
A P value less than 0.05 was considered significant for all tests. Statistical analyses were performed using STATA version 16.0 software (StataCorp LP, College Station, Texas, USA).
Results
Clinical findings
The study cohort comprised 262 patients (median age: 68 years, 57% male) and included 96 at‐risk for HF, 97 with HFpEF, and 69 with HFrEF (see CONSORT flow diagram, Figure 1 ). Patients with HFpEF were older and had higher serum creatinine compared with those with HFrEF but otherwise had similar disease burden, mean MAGGIC score 19 vs. 17 respectively, P = 0.08. In terms of aetiology, the HFrEF group included 28 with ischaemic cardiomyopathy, 22 with dilated cardiomyopathy, 7 with myocarditis, 2 with valvular disease and 10 with other causes. Only 5/262 patients had been hospitalized or visited the emergency department within 30 days of the baseline scan (Table 1 ). Between the baseline and 1 year scan, seven patients with HFpEF and three with HFrEF had an HF‐related hospitalization. Medication use at 1 year for the overall cohort was similar to baseline with 80% on an angiotensin converting enzyme inhibitor or angiotensin receptor blocker, 62% on a beta blocker and 17% on a mineralocorticoid antagonist, P > 0.05 for paired comparison in each case.
Figure 1.
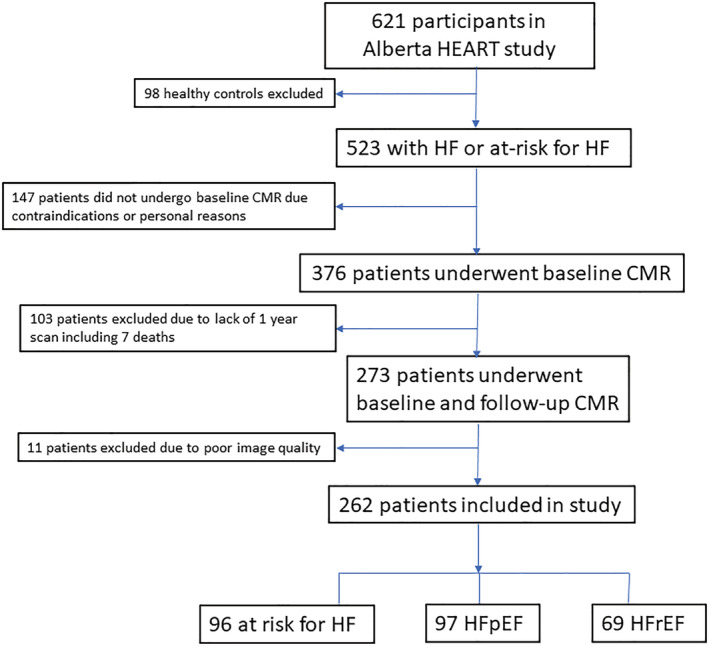
CONSORT flow diagram of patient disposition. Abbreviations: HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; CMR, cardiac magnetic resonance.
Table 1.
Baseline clinical characteristics of heart failure cohort
| Overall cohort (n = 262) | At risk (n = 96) | HFpEF (n = 97) | HFrEF (n = 69) | P value | |
|---|---|---|---|---|---|
| Vital statistics | |||||
| Age, years | 68 (61, 76) | 64 (59, 72)* | 72 (64, 80)** | 66 (59, 76) | <0.001 |
| Male | 150 (57%) | 50 (52%) | 52 (54%) | 48 (70%) | 0.05 |
| BMI, kg/m2 | 29.9 ± 5.3 | 29.9 ± 5.3 | 30.5 ± 5.5 | 29.0 ± 4.9 | 0.19 |
| Systolic BP, mmHg | 130 (118, 142) | 136 (120, 151)* ** | 128 (118, 142) | 128 (116, 134) | 0.002 |
| Heart rate, /min | 65 (60, 76) | 68 (60, 76) | 64 (60, 72) | 65 (60, 73) | 0.58 |
| Medical history | |||||
| HF duration, years | 3 (1.5, 5) | NA | 2.8 (1.5, 5) | 4 (2, 8) | 0.14 |
| New York Heart Association class | 1.9 ± 0.7 | NA | 1.8 ± 0.7 | 2.0 ± 0.7 | 0.23 |
| Hypertension | 194 (74%) | 77 (80%)** | 75 (77%) | 42 (61%) | 0.01 |
| Diabetes mellitus | 88 (33%) | 28 (29%) | 35 (36%) | 25 (36%) | 0.52 |
| Coronary artery disease | 88 (34%) | 20 (21%)* ** | 42 (43%) | 26 (38%) | 0.009 |
| Atrial fibrillation | 83 (32%) | 18 (19%)* ** | 38 (39%) | 27 (39%) | 0.003 |
| Current smoker | 25 (10%) | 10 (10%) | 10 (10%) | 5 (7%) | 0.75 |
| COPD | 34 (13%) | 5 (5%)* | 18 (19%) | 11 (16%) | 0.02 |
| Renal insufficiency | 31 (12%) | 1 (1%)* ** | 17 (18%) | 13 (19%) | <0.001 |
| ACEI or ARB use | 209 (80%) | 71 (74%) | 82 (85%) | 56 (81%) | 0.18 |
| Beta blocker use | 165 (63%) | 31 (32%)* ** | 75 (77%) | 59 (86%) | <0.001 |
| MRA use | 45 (17%) | 3 (3%)* ** | 14 (14%) | 28 (41%) | <0.001 |
| CV hospitalization/ED visit in last 30 days | 5 (2%) | 1 (1%) | 2 (2%) | 2 (3%) | 0.85 |
| Laboratory test | |||||
| Creatinine, umol/L | 89 (76, 108) | 81 (72, 92)* ** | 101 (80, 125)** | 90.0 (78, 109) | <0.001 |
| NT‐proBNP, pg/mL | 203 (59, 746) | 59 (25, 152)* ** | 559 (186, 1263) | 364 (169, 1034) | <0.001 |
| MAGGIC score | 16 ± 7 | 13 ± 5* ** | 19 ± 7 | 17 ± 8 | <0.001 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; ED, emergency department; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; MRA, mineralocorticoid antagonist; NA, not applicable; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide.
Continuous variables expressed as mean ± standard deviation or median (25–75th percentile) as appropriate. P value for comparison of three groups.
P < 0.05 compared with HFpEF.
P < 0.05 compared with HFrEF.
Note: NT‐proBNP and creatinine was missing for 31 (12%) and 26 (10%) participants respectively.
Cardiac magnetic resonance parameters at baseline and 1 year
Baseline CMR findings are reported in Table 2 . LGE imaging was acquired at baseline in 205/262 patients. Major non‐ischaemic scar was found in 14 patients, minor non‐ischaemic scar in 33, ischaemic scar in 30 and no scar in 128.
Table 2.
Baseline vs. 1 year cardiac magnetic resonance measurements in patient groups
| Variable | At risk (baseline) | At risk (1 year) | P value | HFpEF (Baseline) | HFpEF (1 year) | P value | HFrEF (Baseline) | HFrEF (1 year) | P value |
|---|---|---|---|---|---|---|---|---|---|
| LVEF, % | 64 (59, 70) | 65 (60, 70) | 0.08 | 61 (55, 65) | 62 (55, 67) | 0.43 | 42 (35, 45) | 46 (39, 51) | <0.001 |
| LVEDVi, mL/m2 | 65 (58, 78) | 68 (56, 77) | 0.27 | 66 (58, 77) | 67 (56, 77) | 0.37 | 98 (79, 116) | 87 (72, 111) | 0.07 |
| LVESVi, mL/m2 | 24 (18, 31) | 23 (18, 29) | 0.06 | 26 (20, 32) | 25 (19, 32) | 0.11 | 55 (44, 72) | 45 (38, 63) | <0.001 |
| LV massi, g/m2 | 54 (45, 69) | 56 (48, 64) | 0.32 | 56 (48, 67) | 58 (50, 70) | 0.05 | 73 (61, 82) | 70 (57, 82) | 0.09 |
| LV mass/LVEDV | 0.81 (0.72, 0.91) | 0.83 (0.75, 0.90) | 0.55 | 0.85 (0.71, 0.96) | 0.85 (0.75, 0.99) | 0.13 | 0.74 (0.64, 0.88) | 0.76 (0.66, 0.87) | 0.37 |
| RVEF, % | 60 (55, 66) | 60 (54, 66) | 0.76 | 57 (51, 62) | 57 (50, 65) | 0.29 | 52 (45, 57) | 53 (45, 57) | 0.72 |
| RVEDVi, mL/m2 | 69 (55, 76) | 62 (53, 71) | <0.001 | 64 (53, 77) | 62 (50, 75) | 0.009 | 73 (64, 92) | 72 (54, 84) | 0.005 |
| RVESVi, mL/m2 | 26 (20, 33) | 25 (19, 31) | 0.002 | 28 (22, 35) | 26 (20, 35) | 0.01 | 33 (27, 43) | 33 (25, 40) | 0.12 |
| LAVi, mL/m2 | 37 (29, 48) | 40 (28, 48) | 0.99 | 51 (36, 71) | 51 (38, 66) | 0.35 | 53 (43, 63) | 52 (49, 62) | 0.44 |
| GLS, % | −18.9 ± 3.6 | −19.1 ± 3.1 | 0.34 | −18.0 ± 3.3 | −17.2 ± 4.0 | 0.03 | −11.9 ± 2.8 | −12.8 ± 3.9 | 0.01 |
| GCS, % | −19.2 ± 3.2 | −19.1 ± 3.6 | 0.66 | −18.5 ± 3.6 | −18.0 ± 4.0 | 0.18 | −11.2 ± 3.0 | −11.9 ± 3.2 | 0.03 |
| Scar prevalence, %* | 20 (24%) | 20 (24%) | 1.0 | 16 (37%) | 21 (41%) | 0.18 | 28 (51%) | 36 (73%) | 0.008 |
| Scar mass, g* | 0 (0, 0) | 0 (0, 0) | 0.65 | 0 (0, 5.8) | 0 (0, 6.1) | 0.44 | 7.0 (0, 24.2) | 8.8 (0, 25) | 0.002 |
| Scar %LV* | 0 (0, 0) | 0 (0, 0) | 0.36 | 0 (0, 5.3) | 0 (0, 5.6) | 0.89 | 6 (0, 16.2) | 6.8 (0, 16) | 0.01 |
Abbreviations: GCS, global circumferential strain; GLS, global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LAVi, left atrial volume index; LV massi, left ventricular mass index; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end‐systolic volume index; RVEDVi, right ventricular end‐diastolic volume index; RVEF, right ventricular ejection fraction; RVESVi, right ventricular end‐systolic volume index.
Continuous variables expressed as mean ± SD or median (25–75th percentile), as appropriate. P values for comparison between baseline and 1 year measurement.
183/262 patients underwent late gadolinium enhancement imaging at both baseline and 1 year.
Median time to the 1‐year scan was 372 days. At 1 year, right ventricular volumes decreased in all 3 patient groups (Table 2 ). Otherwise, cardiac volumes and function remained stable at 1 year in patients at risk for HF. Comparatively at 1 year, patients with HFpEF had more impaired global longitudinal strain, mean −17.2% vs.‐18.0% at baseline, P = 0.03, and a borderline increase in LV mass index, median 58 g/m2 vs. 56 g/m2 at baseline, P = 0.05. Conversely, at 1 year, patients with HFrEF showed improved cardiac function with a median LVEF 46% vs. 42% at baseline, P < 0.001, mean GLS −12.8% vs. −11.9% at baseline, P = 0.01, and median LV end‐systolic volume index 45 mL/m2 vs. 55 mL/m2 at baseline, P < 0.001. Refer to supporting information Figure S1 for examples of cardiac remodelling at 1 year.
Coefficient of variation was excellent for all CMR measures including 7% for LVEF, 6% for LV mass, 5% for LV end‐diastolic volume, 5% for LV end‐systolic volume, 9% for RVEF, 7% for RV end‐diastolic volume, 21% for RV end‐systolic volume, 11% for left atrial volume, 4% for GLS, and 4% for GCS.
Cardiac magnetic resonance predictors of outcome
In the patients with HF (N = 166), after 5 years of follow‐up from the 1 year scan there were 55 primary outcome events, including 19 deaths (9 for HFpEF and 10 for HFrEF) and 44 cardiovascular disease related hospitalizations (25 for HFpEF and 19 for HFrEF). There were also 31 secondary outcome events at 5 years including 18 HF related hospitalizations (13 for HFpEF and 5 for HFrEF).
Age, heart rate, New York Heart Association classification, coronary artery disease, diabetes mellitus, renal insufficiency, MAGGIC score, log (NT‐proBNP), log (creatinine), LVEF, LV mass/LVEDV, left atrial volume index, and scar pattern were identified as clinical and imaging parameters at baseline predicting the primary outcome (death or cardiovascular related hospitalization at 5 years) (Table S1). However, subsequent stepwise forward selection identified only MAGGIC score, log (NT‐proBNP), LV mass/LVEDV, and scar pattern as the best predictors. Similarly, only MAGGIC score and log (NT‐proBNP) at baseline were identified as significant predictors of secondary outcome (death or HF‐related hospitalization at 5 years). After adjusting for these baseline predictors, the only cardiac remodelling parameter on CMR that independently predicted both the primary and secondary outcome was % Δ LV mass index, HR 1.21, 95% confidence interval (1.02, 1.44) per 10% increase, P = 0.031, and HR 1.37, 95% CI (1.11, 1.69) per 10% increase, P = 0.003, respectively (Table 3 ). On univariate analysis, change in LV mass index was also associated with the primary outcome for patients with HFpEF, HR 1.27, 95% CI (1.03, 1.56) per 10% increase, P = 0.026, (Table 4 ) and HFrEF, HR 1.27, 95% CI (1.01, 1.59) per 10% increase, P = 0.037 (Table 5 ).
Table 3.
Regression analysis of remodelling for predicting outcomes during 5‐year follow‐up from 1‐year CMR scan in 166 patients with heart failure
| Primary outcome: death or cardiovascular hospitalization, N = 55 | ||||
|---|---|---|---|---|
| Univariable Cox analysis | Multivariable Cox analysis | |||
| Hazard ratio | P value | Hazard ratio | P value | |
| % ∆ LVEF, per 10% increase | 1.00 (0.87, 1.14) | 0.96 | ||
| % ∆ LVEDVi, per 10% increase | 1.02 (0.87, 1.20) | 0.83 | ||
| % ∆ LVESVi, per 10% increase | 1.05 (0.95, 1.15) | 0.37 | ||
| % ∆ LV massi, per 10% increase | 1.26 (1.08, 1.46) | 0.003 | 1.21 (1.02, 1.44) | 0.031 |
| % ∆ LV mass/LVEDV, per 0.1 increase | 1.15 (1.03, 1.27) | 0.009 | 1.01 (1.00, 1.02) | 0.17 |
| % ∆ RVEF, per 10% increase | 0.97 (0.88, 1.06) | 0.47 | ||
| % ∆ RVEDVi, per 10% increase | 1.05 (0.93, 1.19) | 0.42 | ||
| % ∆ RVESVi, per 10% increase | 1.05 (0.98, 1.14) | 0.18 | 1.02 (0.95, 1.10) | 0.54 |
| % ∆ LAVi, per 10% increase | 1.04 (0.98, 1.10) | 0.23 | ||
| % ∆ GLS, per 1% increase | 1.00 (0.99, 1.01) | 0.66 | ||
| % ∆ GCS, per 1% increase | 1.00 (0.99, 1.01) | 0.75 | ||
| ∆ Scar mass, per 10 g increase a | 1.16 (0.55, 2.45) | 0.70 | ||
| ∆ Scar % LV, per 10% increase a | 1.11 (0.41, 3.01) | 0.84 | ||
| Secondary outcome: death or heart failure hospitalization, N = 31 | ||||
|---|---|---|---|---|
| % ∆ LVEF, per 10% increase | 0.93 (0.76, 1.13) | 0.45 | ||
| % ∆ LVEDV, per 10% increase | 0.97 (0.79, 1.18) | 0.73 | ||
| % ∆ LVESV, per 10% increase | 1.02 (0.90, 1.15) | 0.81 | ||
| % ∆ LV mass, per 10% increase | 1.31 (1.07, 1.60) | 0.008 | 1.37 (1.11, 1.69) | 0.003 |
| % ∆ LV mass/LVEDV, per 0.1 increase | 1.16 (1.02, 1.33) | 0.022 | 1.23 (1.06, 1.44) | 0.008 |
| % ∆ RVEF, per 10% increase | 1.00 (0.90, 1.11) | 0.94 | ||
| % ∆ RVEDV, per 10% increase | 1.14 (0.98, 1.33) | 0.10 | 1.09 (0.94, 1.26) | 0.28 |
| % ∆ RVESV, per 10% increase | 1.06 (0.96, 1.17) | 0.28 | ||
| % ∆ LAV, per 10% increase | 0.98 (0.90, 1.08) | 0.73 | ||
| % ∆ GLS, per 1% increase | 1.00 (0.98, 1.01) | 0.58 | ||
| % ∆ GCS, per 1% increase | 0.99 (0.97, 1.00) | 0.16 | 0.99 (0.98, 1.01) | 0.28 |
| ∆ Scar mass, per 10 g increase b | 0.99 (0.37, 2.64) | 0.99 | ||
| ∆ Scar % LV, per 10% increase b | 0.72 (0.18, 2.67) | 0.62 | ||
Definitions: Multivariable analyses were performed in variables with univariable P value <0.2 and were adjusted for MAGGIC score + log (NT‐proBNP) + baseline LV mass/LVEDV + baseline scar pattern in upper table and adjusted for MAGGIC score + log (NT‐proBNP) in lower table.
100/166 patients had late gadolinium enhancement imaging at both baseline and 1 year (26 events).
100/166 patients had late gadolinium enhancement imaging at both baseline and 1 year (17 events).
Abbreviations: see Table 2 .
Table 4.
Regression analysis of remodelling for predicting outcomes during 5 year follow‐up from 1 year CMR scan in 97 patients with heart failure and preserved ejection fraction
| Univariable Cox analysis | ||
|---|---|---|
| Hazard ratio | P value | |
| % ∆ LVEF, per 10% increase | 0.85 (0.64, 1.13) | 0.26 |
| % ∆ LVEDVi, per 10% increase | 1.07 (0.85, 1.34) | 0.58 |
| % ∆ LVESVi, per 10% increase | 1.09 (0.96, 1.22) | 0.20 |
| % ∆ LV massi, per 10% increase | 1.27 (1.03, 1.56) | 0.026 |
| % ∆ LV mass/LVEDV, per 0.1 increase | 1.17 (0.99, 1.39) | 0.062 |
| % ∆ RVEF, per 10% increase | 0.99 (0.89, 1.10) | 0.86 |
| % ∆ RVEDVi, per 10% increase | 1.01 (0.86, 1.19) | 0.87 |
| % ∆ RVESVi, per 10% increase | 1.07 (0.96, 1.20) | 0.22 |
| % ∆ LAVi, per 10% increase | 1.05 (0.95, 1.15) | 0.34 |
| % ∆ GLS, per 1% increase | 1.00 (0.98, 1.02) | 0.93 |
| % ∆ GCS, per 1% increase | 0.99 (0.97, 1.01) | 0.34 |
| ∆ Scar mass, per 10 g increase a | 2.34 (0.55, 10.4) | 0.24 |
| ∆ Scar % LV, per 10% increase a | 2.24 (0.34, 14.7) | 0.40 |
51/97 patients had late gadolinium enhancement imaging at both baseline and 1 year (9 events).
Table 5.
Regression analysis of remodelling for predicting outcomes during 5 year follow‐up from 1 year CMR scan in 69 patients with heart failure and reduced ejection fraction
| Univariable Cox analysis | ||
|---|---|---|
| Hazard ratio | P value | |
| % ∆ LVEF, per 10% increase | 1.10 (0.93, 1.30) | 0.25 |
| % ∆ LVEDVi, per 10% increase | 0.99 (0.78, 1.24) | 0.92 |
| % ∆ LVESVi, per 10% increase | 0.97 (0.79, 1.19) | 0.77 |
| % ∆ LV massi, per 10% increase | 1.27 (1.01, 1.59) | 0.037 |
| % ∆ LV mass/LVEDV, per 0.1 increase | 1.14 (0.99, 1.32) | 0.071 |
| % ∆ RVEF, per 10% increase | 0.93 (0.82, 1.07) | 0.33 |
| % ∆ RVEDVi, per 10% increase | 1.14 (0.95, 1.37) | 0.20 |
| % ∆ RVESVi, per 10% increase | 1.05 (0.94, 1.17) | 0.42 |
| % ∆ LAVi, per 10% increase | 1.03 (0.96, 1.12) | 0.39 |
| % ∆ GLS, per 1% increase | 1.00 (0.99, 1.02) | 0.62 |
| % ∆ GCS, per 1% increase | 1.00 (0.98, 1.01) | 0.87 |
| ∆ Scar mass, per 10 g increase a | 0.94 (0.41, 2.16) | 0.88 |
| ∆ Scar % LV, per 10% increase a | 0.81 (0.25, 2.62) | 0.72 |
49/69 patients had late gadolinium enhancement imaging at both baseline and 1 year (17 events).
From receiver operating characteristic analyses, the optimal cut‐off for change in LV mass index for predicting the primary outcome was calculated as 15%. In the overall 262 patient cohort, reverse remodelling, defined as a ≥15% 1 year decrease in LV mass index, was seen in 35 patients (13%), including 11 with HFpEF and 10 with HFrEF. Adverse remodelling, defined as a ≥15% 1 year increase in LV mass index, was seen in 57 patients (22%), including 25 with HFpEF and 11 with HFrEF. On Kaplan–Meier analysis, HF patients with adverse remodelling of LV mass index had more events compared with those without adverse remodelling, log‐rank P = 0.004 for overall cohort, P = 0.035 for HFpEF and P = 0.035 for HFrEF (Figure 2 ). Reverse remodelling was also associated with fewer events in the overall cohort, log‐rank P = 0.04, but not in the HFpEF or HFrEF subgroups. Restricted cubic spline demonstrated an increased risk for the primary outcome (death or cardiovascular hospitalization) and secondary outcome (death or HF hospitalization) in the overall cohort with increasing % ∆ LV mass, even after adjusting for baseline risk (Figure 3 ).
Figure 2.
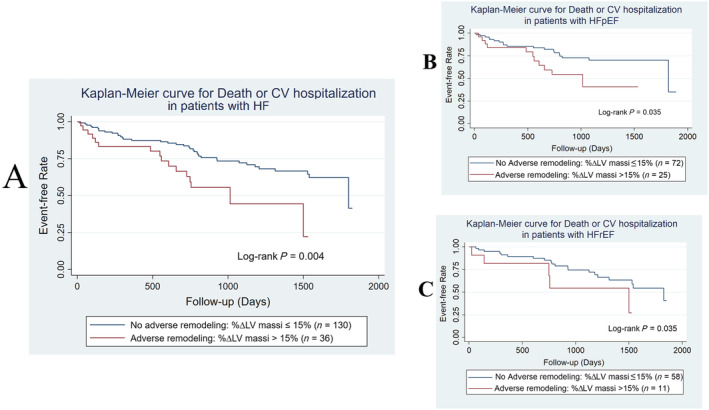
(A–C) Kaplan–Meier analyses of cardiac remodelling for predicting outcome in patients with heart failure. Definitions: Outcome = death, or cardiovascular hospitalization at 5 years from 1 year scan; adverse remodelling = 1 year increase in left ventricular mass index ≥15%.
Figure 3.
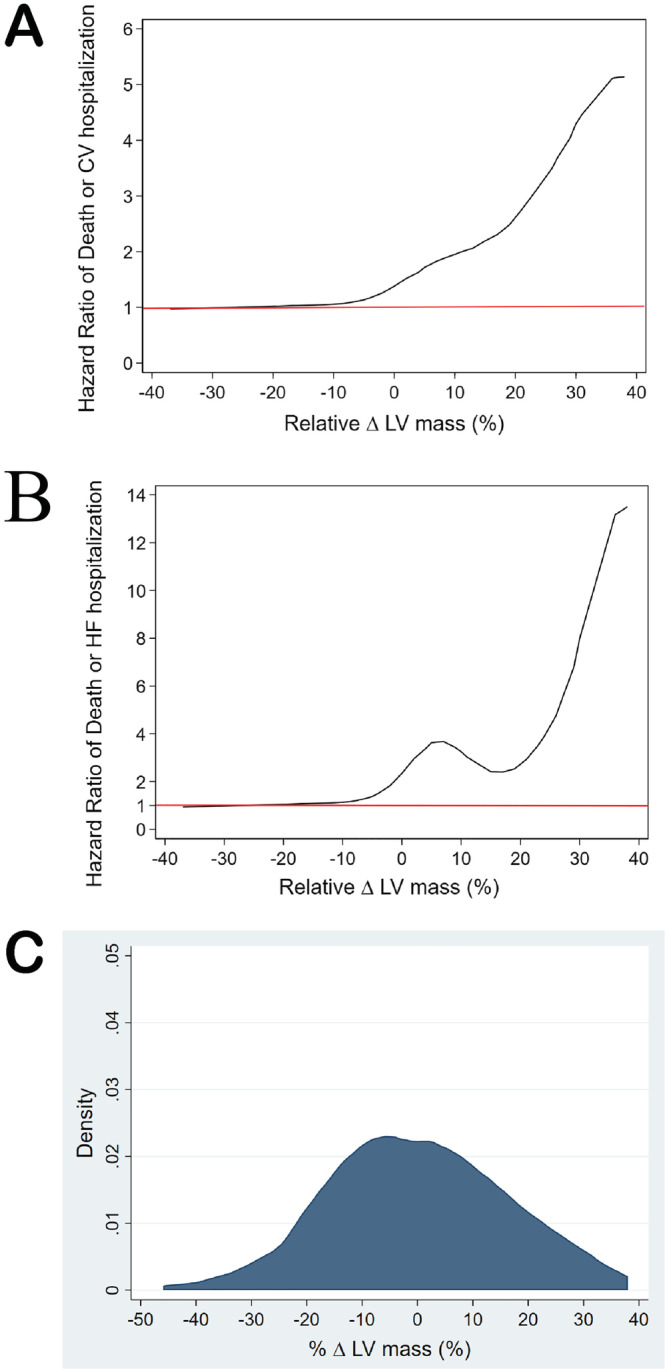
(A–C) Central figure. Cubic spline modelling of the relationship between outcome and change in left ventricular mass index in patients with heart failure.
Discrimination performance of dynamic remodelling
To identify the incremental prognostic performance of CMR measures of remodelling, significant predictors of primary outcome from Table 3 were each modelled with baseline predictors. % Δ LV mass index demonstrated added value over baseline predictors on likelihood ratio testing (Figure S2).
Discussion
In this prospective cohort study of patients with stable, chronic HF, and those at risk, we found a high proportion with cardiac remodelling, expressed as a 15% change in LV mass index during 1 year follow‐up. Reverse or adverse remodelling was observed in 30% of patients with HFrEF and 37% of patients with HFpEF. More importantly, a change in LV mass index predicted long‐term outcomes for these patients, even after adjustment for baseline clinical risk.
Limitations of prior imaging studies
To our knowledge, this is the first study to comprehensively investigate longitudinal changes in cardiac structure and function in individuals across the HF spectrum. In a 5 year study of patients with incident HF, Dunlay et al. found that those with HFpEF had a decrease in LVEF and those with HFrEF had increased LVEF. 22 However, other echocardiographic measures of cardiac structure and function were not reported.
Prior imaging studies of patients with HF have primarily evaluated the prognostic potential of cardiac measures at a single time‐point and have identified cardiac function and/or geometry as the best predictors of outcome. In cross‐sectional imaging studies, LV mass predicts outcome for patients with HFpEF 23 , 24 , 25 ; however, its prognostic utility in HFrEF is less well established. In our cohort after correcting for baseline clinical risk using the MAGGIC risk score and NT‐proBNP, the only CMR measures at baseline predictive of outcome were LV mass/LVEDV and the presence of major non‐ischaemic scar. Similarly, Shanbhag et al. found that major non‐ischaemic scar was the best CMR predictor of adverse outcome in a well‐characterized cross‐sectional study of patients without HF. 20
Optimally defining cardiac remodelling
Longitudinal imaging studies of HF have typically evaluated changes in LV volume and/or ejection fraction and have defined LV remodelling arbitrarily using a threshold of 10–15%. 7 , 9 , 14 , 26 In our study, LV remodelling was defined through statistical analyses. We did not find a change in LV volume or ejection fraction to be associated with outcome in our well‐characterized cohort. However, a 1 year change in LV mass index of ≥15% strongly predicted event free survival. Compared with other cardiac structural and functional parameters, LV mass is less susceptible to transient changes in loading conditions and is therefore a potentially more reliable interstudy measure of remodelling. LV mass can also be obtained from echocardiography although the threshold for significant remodelling will likely be greater than CMR due to a lower reproducibility of measures.
Our study results confirm that HF is a dynamic process, even in patients with chronic disease on stable medical therapy. In our overall cohort, reverse remodelling occurred in 13%, and adverse remodelling was present in 22% at 1 year follow‐up. Adverse remodelling of LV mass index was also strongly predictive of outcome. The utility of imaging guided care has not been evaluated in ambulatory HF. The GUIDE‐IT HF trial did not find a survival advantage for patients with chronic HFrEF undergoing serial measures of NT‐proBNP during a mean of 15 months follow‐up. 27 However, our results suggest that serial imaging measures provide long‐term (>1 year) prognostic information even after adjustment for baseline clinical risk and NT‐proBNP.
Study limitations
This study's sample size may limit our subgroup analyses of survival. Nevertheless, we did find that change in LV mass predicted clinical outcomes in the HFpEF and HFrEF subgroups as well as the overall cohort. Another important limitation was our definition of HFrEF which allowed for patients with LVEF <50% given existing heart failure knowledge at that time. Furthermore, cardiac electronic implantable devices are common in patients with HF and was an exclusion criterion for CMR in our study. Consequently, in our HFrEF group, 28 patients (41%) had an LVEF <40% and 41 (59%) had an LVEF of 40–49%. Future studies should therefore confirm these results in patients with HFrEF and in HF mid‐range EF using current definitions. In our study, LGE was not available in 30% of patients at both time points, thus limiting the evaluation of scar remodelling in HF. Our finding of cardiac remodelling in 37% of patients at risk for HF is an intriguing result; however, its relationship to downstream incident heart failure is beyond the scope of our study. Ultimately, the utility of routine surveillance imaging for ambulatory patients with HF (and those at risk) should be evaluated in a randomized controlled trial.
Conclusions
Our study confirms that cardiac remodelling is common in patients with chronic HF, even those with clinically stable disease. One‐year change in LV mass strongly predicts outcome, even after adjustment for baseline clinical risk, whereas change in LVEF was not predictive. Future studies should evaluate mechanisms of adverse and reverse remodelling and if surveillance cardiac imaging can guide care and improve outcomes for patients with chronic HF.
Conflict of interest
K. C. is currently an employee of Siemens Healthcare but was a graduate student at the time of the study. D. I. P. reports funding from Alnylam and Akcea. J. E. reports study funding from Novartis and Servier as well as grants from Merck, Bayer, Trevena, and Amgen. G. O. reports study funding from Amgen. All other authors have no conflicts of interest to disclose apart from sources of funding listed in the funding section.
Funding
Funding was provided by Alberta Innovates‐Health Solutions (AI‐HS) (grant #AHFMR ITG 200801018). In‐kind contributions were received from Capital Health Regional Authority (now Alberta Health Services) and the Alberta HEART investigators. L. X. received a scholarship from the Sino‐Li Ka Shing foundation. J. E., J. D., and G. O. are supported by AI‐HS, the Canadian Institutes of Health Research, and/or the Heart and Stroke Foundation of Canada. M. H. is supported by the University of Alberta Nursing research chair in ageing and quality of life.
Supporting information
Table S1. Univariate analysis of the baseline clinical and imaging parameters for the prediction of the primary outcome.
Figure S1. Examples of reverse remodelling and adverse remodelling for patients at risk (panel A), with HFpEF (panel B) and with HFrEF (panel C).
Abbreviations: HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; LVEDVi = left ventricular end‐diastolic volume index; LV massi = left ventricular mass index; GLS = global longitudinal strain.
Figure S2. Incremental value of the change in left ventricular mass index for predicting outcome by global model χ2 test.
Abbreviations: BM = Base Model; LV massi = left ventricular mass index.
Definitions:
Outcome = death or cardiovascular hospitalization at 5 years from 1‐year scan.
Base Model = MAGGIC score + log (NT‐proBNP) + baseline left ventricular mass/left ventricular end‐diastolic volume + baseline scar pattern.
Xu, L. , Pagano, J. , Chow, K. , Oudit, G. Y. , Haykowsky, M. J. , Mikami, Y. , Howarth, A. G. , White, J. A. , Howlett, J. G. , Dyck, J. R. B. , Anderson, T. J. , Ezekowitz, J. A. , Thompson, R. B. , and Paterson, D. I. (2021) Cardiac remodelling predicts outcome in patients with chronic heart failure. ESC Heart Failure, 8: 5352–5362. 10.1002/ehf2.13626.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 2. Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med 1997; 336: 1350–1355. [DOI] [PubMed] [Google Scholar]
- 3. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling‐‐concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000; 35: 569–582. [DOI] [PubMed] [Google Scholar]
- 4. Pugliese NR, Fabiani I, La Carrubba S, Conte L, Antonini‐Canterin F, Colonna P, Caso P, Benedetto F, Santini V, Carerj S, Romano MF, Citro R, Di Bello V, Italian Society of Cardiovascular Echography . Classification and prognostic evaluation of left ventricular remodeling in patients with asymptomatic heart failure. Am J Cardiol. 2017; 119: 71–77. [DOI] [PubMed] [Google Scholar]
- 5. Lee TH, Hamilton MA, Stevenson LW, Moriguchi JD, Fonarow GC, Child JS, Laks H, Walden JA. Impact of left ventricular cavity size on survival in advanced heart failure. Am J Cardiol. 1993; 72: 672–676. [DOI] [PubMed] [Google Scholar]
- 6. Wong M, Staszewsky L, Latini R, Barlera S, Volpi A, Chiang YT, Benza RL, Gottlieb SO, Kleemann TD, Rosconi F, Vandervoort PM, Cohn JN, Val‐HeFT Heart Failure Trial Investigators . Valsartan benefits left ventricular structure and function in heart failure: Val‐HeFT echocardiographic study. J Am Coll Cardiol. 2002; 40: 970–975. [DOI] [PubMed] [Google Scholar]
- 7. Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, Chan YS, Kong SL, Bax JJ. Left ventricular reverse remodeling but not clinical improvement predicts long‐term survival after cardiac resynchronization therapy. Circulation. 2005; 112: 1580–1586. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Q, Fung JW, Auricchio A, Chan JY, Kum LC, Wu LW, Yu CM. Differential change in left ventricular mass and regional wall thickness after cardiac resynchronization therapy for heart failure. Eur Heart J. 2006; 27: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 9. Linde C, Gold MR, Abraham WT, St John Sutton M, Ghio S, Cerkvenik J, Daubert C, REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction Study Group . Long‐term impact of cardiac resynchronization therapy in mild heart failure: 5‐year results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Eur Heart J. 2013; 34: 2592–2599. [DOI] [PubMed] [Google Scholar]
- 10. Menet A, Guyomar Y, Ennezat PV, Graux P, Castel AL, Delelis F, Heuls S, Cuvelier E, Gevaert C, Le Goffic C, Tribouilloy C, Maréchaux S. Prognostic value of left ventricular reverse remodeling and performance improvement after cardiac resynchronization therapy: A prospective study. Int J Cardiol. 2016; 204: 6–11. [DOI] [PubMed] [Google Scholar]
- 11. Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM, Solomon SD, PROVE‐HF Investigators . Association of change in N‐Terminal pro‐B‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019; 322: 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braga JR, Leong‐Poi H, Rac VE, Austin PC, Ross HJ, Lee DS. Trends in the use of cardiac imaging for patients with heart failure in Canada. JAMA Netw Open. 2019; 2: e198766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, Picard MH, Polk DM, Ragosta M, Ward RP, Weiner RB, American College of Cardiology Foundation Appropriate Use Criteria Task Force , American Society of Echocardiography , American Heart Association , American Society of Nuclear Cardiology , Heart Failure Society of America , Heart Rhythm Society , Society for Cardiovascular Angiography and Interventions , Society of Critical Care Medicine , Society of Cardiovascular Computed Tomography , Society for Cardiovascular Magnetic Resonance . ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011; 57: 1126–1166. [DOI] [PubMed] [Google Scholar]
- 14. Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL Jr. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. 2019; 7: 782–794. [DOI] [PubMed] [Google Scholar]
- 15. Ezekowitz JA, Becher H, Belenkie I, Clark AM, Duff HJ, Friedrich MG, Haykowsky MJ, Howlett JG, Kassiri Z, Kaul P, Kim DH, Knudtson ML, Light PE, Lopaschuk GD, McAlister FA, Noga ML, Oudit GY, Paterson DI, Quan H, Schulz R, Thompson RB, Weeks SG, Anderson TJ, Dyck JR. The Alberta heart failure etiology and analysis research team (HEART) study. BMC Cardiovasc Disord. 2014; 14: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN, Meta‐Analysis Global Group in Chronic Heart Failure . Predicting survival in heart failure: a risk score based on 39,372 patients from 39 studies. Eur Heart J. 2013; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 17. Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson 2016; 18: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mikami Y, Kolman L, Joncas SX, Stirrat J, Scholl D, Rajchl M, Lydell CP, Weeks SG, Howarth AG, White JA. Accuracy and reproducibility of semi‐automated late gadolinium enhancement quantification techniques in patients with hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2014; 16: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raman B, Ariga R, Spartera M, Sivalokanathan S, Chan K, Dass S, Petersen SE, Daniels MJ, Francis J, Smillie R, Lewandowski AJ, Ohuma EO, Rodgers C, Kramer CM, Mahmod M, Watkins H, Neubauer S. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: mechanisms and clinical implications. Eur Heart J Cardiovasc Imaging. 2019; 20: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shanbhag SM, Greve AM, Aspelund T, Schelbert EB, Cao JJ, Danielsen R, Þorgeirsson G, Sigurðsson S, Eiríksdóttir G, Harris TB, Launer LJ, Guðnason V, Arai AE. Prevalence and prognosis of ischaemic and non‐ischaemic myocardial fibrosis in older adults. Eur Heart J. 2019; 40: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Royston P, White IR. Multiple imputation by chained equations (MICE): implementation in stata. J Stat Softw 2011; 45: 1–20. [Google Scholar]
- 22. Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail 2012; 5: 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, Rizkala A, Lukashevich I, O'Meara E, Ryan JJ, Shah SJ, Mullens W, Zile MR, Lam CSP, McMurray JJV, Solomon SD, PARAGON‐HF Investigators . Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2019; 74: 2858–2873. [DOI] [PubMed] [Google Scholar]
- 24. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circ Heart Fail. 2014; 7: 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garg P, Assadi H, Jones R, Chan WB, Metherall P, Thomas R, van der Geest R, Swift AJ, Al‐Mohammad A. Left ventricular fibrosis and hypertrophy are associated with mortality in heart failure with preserved ejection fraction. Sci Rep. 2021; 11: 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghimire A, Fine N, Ezekowitz JA, Howlett J, Youngson E, McAlister FA. Frequency, predictors, and prognosis of ejection fraction improvement in heart failure: an echocardiogram‐based registry study. Eur Heart J 2019; 40: 2110–2117. [DOI] [PubMed] [Google Scholar]
- 27. Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston‐Miller N, Januzzi JL Jr, Mark DB, Piña IL, Passmore G, Whellan DJ, Yang H, Cooper LS, Leifer ES, Desvigne‐Nickens P, O'Connor CM. Effect of natriuretic peptide‐guided therapy on hospitalization or cardiovascular mortality in high‐risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017; 318: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate analysis of the baseline clinical and imaging parameters for the prediction of the primary outcome.
Figure S1. Examples of reverse remodelling and adverse remodelling for patients at risk (panel A), with HFpEF (panel B) and with HFrEF (panel C).
Abbreviations: HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; LVEDVi = left ventricular end‐diastolic volume index; LV massi = left ventricular mass index; GLS = global longitudinal strain.
Figure S2. Incremental value of the change in left ventricular mass index for predicting outcome by global model χ2 test.
Abbreviations: BM = Base Model; LV massi = left ventricular mass index.
Definitions:
Outcome = death or cardiovascular hospitalization at 5 years from 1‐year scan.
Base Model = MAGGIC score + log (NT‐proBNP) + baseline left ventricular mass/left ventricular end‐diastolic volume + baseline scar pattern.


