Abstract
Aims
Recent evidence has demonstrated that ketone bodies, particularly β‐hydroxybutyrate (BHB), are beneficial to the failing heart due to their potential as an alternative energy substrate as well as their anti‐inflammatory and anti‐oxidative properties. Exogenous supplementation of ketones also helps prevent heart failure (HF) development in rodent models, but whether ketones can be used to treat HF remains unexplored. Herein, we investigated whether chronic supplementation of ketones is beneficial for the heart in a mouse model of established HF.
Methods and results
To elevate circulating ketone levels, we utilized (R)‐3‐hydroxybutyl‐(R)‐3‐hydroxybutyrate [ketone ester (KE)]. C57Bl/6N male mice were subjected to transverse aortic constriction (TAC) surgery. After developing HF, mice were treated with either 20% KE or vehicle via drinking water for 2 weeks. In another cohort, mice 3–4 weeks post‐TAC received acute intravenous infusions of BHB or saline for 1 h and their cardiac function was measured. 20% KE significantly elevated blood BHB in mice (P < 0.01) without inducing ketoacidosis or altering other metabolic parameters. Mice with overt HF (30–45% ejection fraction) treated with 20% KE displayed significantly elevated circulating ketone levels compared with vehicle‐treated mice (P < 0.05). The significant cardiac dysfunction in mice with HF continued to worsen after 2 weeks of vehicle treatment, whereas this decline was absent in KE‐treated mice (mean difference 4.7% ejection fraction; P < 0.01). KE treatment also alleviated TAC‐induced cardiomyocyte hypertrophy (P < 0.05) and reduced the TAC‐induced elevated cardiac periostin (P < 0.05), a marker of activated fibroblasts. Cardiac fibrosis was also significantly reduced with KE treatment in TAC mice (P < 0.01). In another cohort, acute BHB infusion significantly increased the cardiac output of mice with HF (P < 0.05), providing further support that ketone therapy can be used to treat HF.
Conclusions
We show that chronic treatment of exogenous ketones is of benefit to the failing heart and that chronic ketone elevation may be a therapeutic option for HF. Further investigations to elucidate the underlying mechanism(s) are warranted.
Keywords: Ketone ester, β‐Hydroxybutyrate, Heart failure
Background
During states of glucose deprivation, ketone bodies, particularly β‐hydroxybutyrate (BHB), serve as an important metabolic fuel for extra‐hepatic organs, including the brain, skeletal muscle, and the heart. Furthermore, it has been suggested that the failing heart relies more on ketone bodies as a fuel than previously appreciated. 1 However, BHB also functions as an anti‐inflammatory and anti‐oxidative stress signalling molecule. 2 , 3 , 4 Thus, it has been proposed that elevating circulating BHB could be of benefit to the failing heart.
Recently, it has been demonstrated that the acute infusion and elevation of BHB increases cardiac output in patients with heart failure (HF) with reduced ejection fraction (HFrEF). 5 Given that infusions are not a practical approach for chronically elevating blood ketone levels, an alternative method is to promote repeated ingestion of exogenous ketones that will avoid a short and transient BHB elevation 5 , 6 and excessive salt intake. 7 One feasible and sustainable approach is to use the ketone monoester [(R)‐3‐hydroxybutyl‐(R)‐3‐hydroxybutyrate] (KE) via oral supplementation. 6 This orally ingested KE is rapidly metabolized to elevate blood BHB levels and achieve ketosis for ~3–4 h in humans. 6 In athletes, this KE‐induced increase in blood BHB levels has shown to improve muscle metabolism and enhance exercise performance. 8 However, whether the chronic elevation of circulating ketones with exogenous supplementation is beneficial for the failing heart remains unknown.
Aims
The aim of the present study was to test whether chronic elevation of circulating ketones is beneficial in treating HF. To do this, we provided KE in drinking water in a murine model of HF and examined cardiac function, structure, and molecular parameters.
Methods
All protocols involving mice were approved by the University of Alberta Institutional Animal Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (eighth edition; revised 2011). The University of Alberta adheres to the principles for biomedical research involving animals developed by the Council for International Organizations of Medical Sciences and complies with the Canadian Council on Animal Care guidelines.
7‐week‐old C57Bl/6N male mice (Charles River Laboratories) were housed individually under standard conditions (25°C, 12:12 h light/dark cycle) with ad libitum access to standard chow and water. To induce pressure‐overload HF, 8‐week‐old mice were subjected to either sham or transverse aortic constriction (TAC) surgery as previously described. 4 After a 2–3 week period to allow the development of HF, all mice were subjected to echocardiography using a Vevo 3100 high‐resolution imaging system (RMV‐707B, VisualSonics) under isoflurane (1.5–3%), 9 , 10 and mice with systolic dysfunction [30–45% ejection fraction (EF)] were allocated into two groups, supplemented with ad libitum vehicle [0.4% Rebaudioside A (Sigma‐Aldrich) dissolved in water] or with ketone ester (KE) [20% KE (0.38 g ketones/mL) diluted in water (HVMN, San Francisco, CA)] for 2 weeks, after which they underwent echocardiography again. After 1–2 days recovery from anaesthesia, mice were euthanized by decapitation after a 16 h fast for subsequent analysis. In another cohort, mice 3–4 weeks post‐TAC were intravenously infused with BHB (Sigma‐Aldrich) or saline for 60 min to examine acute effects on cardiac function. Detailed methods are available in Supporting Information.
Statistical analysis
Before analysis, outliers were detected by the ROUTE method (Q = 1%) and excluded. Comparisons between two groups were performed by the Student's or paired t‐tests, and between three or more groups using repeated two‐way ANOVA followed by Sidak's post hoc multiple comparison, appropriately. Results are expressed as mean ± standard deviation. P < 0.05 was considered significant. GraphPad Prism 8.0 was used for statistical analyses.
Results
Ketone ester drink efficiently induces ketosis in mice
We tested how effectively the KE could elevate blood BHB levels in mice. Because the average water intake at one time was ~90–100 μL (data not shown), 100 μL of different concentrations of KE (10%, 20%, 50%, and 100%) was orally administered to mice to examine blood BHB levels. Similar to humans, 6 most dilutions of KE elevated blood ketone levels at 20 min after intake in mice (Figure 1 A ). Because ketosis may cause ketoacidosis, 11 we further analysed the correlation between blood BHB levels elevated by KE and arterial blood pH (Figure 1 B). Of note, while the drop iin arterial blood pH by KE is moderate in humans, 12 BHB elevation by KE induced acidosis in mice (Figure 1 B ). The significant correlation between blood BHB and arterial blood pH in mice (Figure 1 B ) suggests that pH buffering capacity in mice is different from humans. Since 1.5 mM BHB is within the physiological range in mice 13 and does not cause acidosis, we surmised that 20% KE would be useful in our studies.
Figure 1.
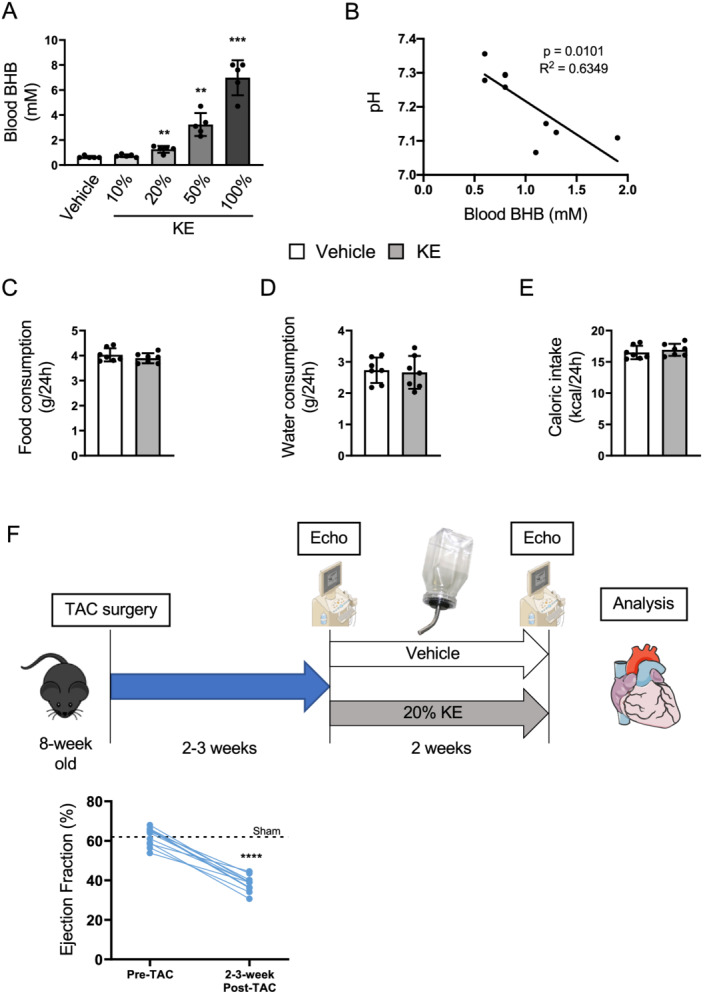
Ketone ester supplementation efficiently elevates circulating ketone levels in mice. (A) Circulating BHB levels at 20 min after oral administration of 100 μL of ketone ester (KE) with different concentrations in water (n = 5 per group). Comparisons were made with the vehicle group. (B) Arterial blood pH in the fed state following a 20 h supplementation of 50% KE drink. Pearson's correlation coefficient r with P‐value was calculated to determine correlation between pH and blood BHB levels. (C and D) Food and water consumption were measured with or without 20% KE supplementation in water (n = 7 per group). (E) Caloric intake was calculated based on the food and water consumption (n = 7 per group). (F) Schematic of the study design. C56Bl/6N male mice undergo transverse aortic constriction (TAC) surgery at 8 weeks old. Two to three weeks after surgery, mice in heart failure (30–45% EF) are treated with vehicle or 20% KE in their drinking water for another 2 weeks. After the treatment period, the cardiac function is assessed by echocardiography and mice are euthanized for further analysis of heart. (The figure was created using materials provided by Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License; http://smart.servier.com/.) Dots represent individual values. Results are expressed as the mean ± standard deviation. Comparisons were made by the Student's t‐test in (A) and (C–E) and by paired t‐test in (F). **P < 0.01, ***P < 0.001, ****P < 0.0001. BHB, β‐hydroxybutyrate; EF, ejection fraction.
To further confirm that 20% KE could be used for our studies, we measured food and water (vehicle and 20% KE) intake in healthy mice. Because KE can suppress appetite 14 and its bitterness could affect water intake, 15 both these parameters were measured as changes in these could influence cardiac function. 16 , 17 However, the food and water consumption and caloric intake were comparable in the KE and vehicle groups (Figure 1 C–E ). Thus, we used 20% KE to treat mice with established HF in the present study.
Eight‐week‐old male mice underwent TAC surgery to induce HF at approximately 2–3 weeks post‐surgery (30–45% EF) and were then treated with 20% KE (~8.0 g ketones/kg body weight/day) or vehicle for 2 additional weeks (Figure 1 F ), as established previously. 9 , 10 , 18
Chronic ketone ester treatment of mice with heart failure blunted a further decline in cardiac function, ameliorated cardiomyocyte hypertrophy, and reduced activation of cardiac fibroblasts
All KE‐treated mice demonstrated significantly elevated fed state blood BHB levels (Figure 2 A ), confirming that 20% KE supplementation elevates circulating ketones without alterations in other metabolic parameters, such as glucose, fatty acid, and insulin (Table 1 ). Most notably, while vehicle‐treated mice exhibited a significant decline in %EF following the 2 week treatment, KE treatment modestly but significantly blunted this decline in EF (Figure 2 B ). As expected with this modest protection, there were not any other significant decreases in TAC‐induced adverse cardiac remodelling, such as left ventricular wall thickness, by KE (Table 2 ). However, at the molecular level, we observed that the ratio of Myh7/Myh6 transcripts (indicators of HF) was increased in the TAC‐vehicle group but was significantly decreased by KE treatment (Figure 2 C ). Consistent with this, KE ameliorated the TAC‐induced cardiomyocyte hypertrophy (Figure 2 D ). In addition, while cardiac periostin, a marker of activated fibroblasts, was significantly elevated in vehicle‐treated TAC mice, its expression was significantly blunted in TAC mice that received KE treatment (Figure 2 E,F and Supporting Information, Figure S1 ). Consistent with this, KE treatment also significantly reduced cardiac collagen deposition (Figure 2 G ). While previous reports have characterized some mechanisms by which BHB reduces inflammation and oxidative stress, 2 , 3 , 4 this model of HF is known to have transient inflammation and oxidative stress that occurs soon after TAC surgery. Therefore, we were not able to observe any protective effects of ketones on inflammation and oxidative stress in these mice (Supporting Information, Figure S2 ). Nevertheless, our data show that chronically elevated circulating ketones blunted activation of cardiac fibroblasts, which can eventually lead to adverse cardiac remodelling. 19
Figure 2.
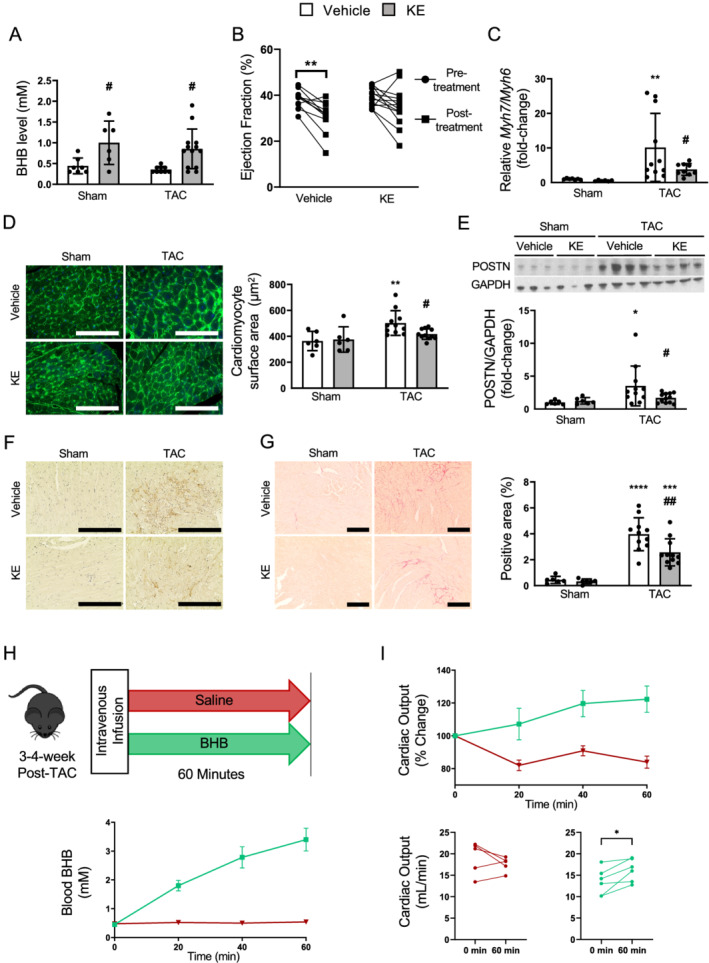
Chronic ketone ester treatment of mice with heart failure blunted a further decline in cardiac function, ameliorated cardiomyocyte hypertrophy, and reduced activation of cardiac fibroblasts. (A) Circulating BHB levels at random fed states (n = 7, 6, 11, 13). (B) Change in % ejection fraction during the treatment period in TAC groups (n = 11, 13). (C) The ratio of transcript levels of Myh7 and Myh6 in the heart (n = 7, 6, 11, 13). (D) Representative images and quantification of cardiomyocyte surface area using wheat‐germ‐agglutinin staining conjugated with Alexa Fluor 488 (n = 6, 6, 11, 12; 170–298 cells/mouse). The scale bars indicate 100 μm. (E) Representative immunoblots and semi‐quantification of POSTN (periostin)/GAPDH in the hearts (n = 7, 6, 11, 13). (F) Representative images of immunostaining with anti‐POSTN antibody, where the positive is indicated by the brown stain. The scale bars indicate 100 μm. (G) Representative images and quantification of formalin‐fixed LV heart sections stained with picrosirius red (n = 6, 6, 11, 12). The scale bars indicate 200 μm. (H) Schematic of acute intravenous infusions. Blood ketone levels of mice 3–4 weeks post‐TAC undergoing either intravenous BHB or saline infusions for 60 min, and (I) the corresponding changes in cardiac output (n = 5 saline, 6 BHB). Dots represent individual values. Results are expressed as the mean ± standard deviation. * indicates the comparison with its sham group in (A) and (C–G). # indicates the comparison between vehicle and ketone in either sham or TAC group. *, # P < 0.05, **, ## P < 0.01. P‐values were derived by repeated two‐way ANOVA in (B) and two‐way ANOVA followed by Sidak's multiple comparisons in (A) and (C–G), and by paired t‐test in (I). BHB, β‐hydroxybutyrate; KE, ketone ester; TAC, transverse aortic constriction.
Table 1.
Metabolic parameters after 2 weeks of treatment with either vehicle or 20% ketone ester drink
| Parameter | Sham | TAC | ||
|---|---|---|---|---|
| Vehicle (n = 7) | Ketone ester (n = 6) | Vehicle (n = 11) | Ketone ester (n = 13) | |
| Free fatty acid (mEq/L) | 1.214 ± 0.171 | 1.058 ± 0.191 | 1.129 ± 0.246 | 1.016 ± 0.250 |
| Triglyceride (mg/dL) | 80.160 ± 15.600 | 72.710 ± 9.435 | 80.000 ± 7.374 | 72.980 ± 12.660 |
| Glucose (mmol/L) | 5.357 ± 0.789 | 5.550 ± 0.686 | 5.100 ± 0.956 | 5.342 ± 0.613 |
| Insulin (pg/mL) | 0.214 ± 0.050 | 0.338 ± 0.306 | 0.196 ± 0.044 | 0.345 ± 0.217 |
TAC, transverse aorta constriction.
Results are expressed as the mean ± standard deviation. Comparisons were made with two‐way ANOVA followed by Sidak's multiple comparisons.
Table 2.
Cardiac function and structure after 2 weeks of treatment with either vehicle or 20% ketone ester drink
| Parameter | Sham | TAC | ||
|---|---|---|---|---|
| Vehicle (n = 7) | Ketone ester (n = 6) | Vehicle (n = 11) | Ketone ester (n = 13) | |
| HR, b.p.m. | 387.00 ± 34.74 | 397.08 ± 28.01 | 454.10 ± 64.74 a | 465.41 ± 61.37 a |
| LVEF, % | 53.08 ± 1.99 | 52.90 ± 4.95 | 30.78 ± 6.83 a | 35.15 ± 8.68 a |
| FS, % | 26.92 ± 1.27 | 26.90 ± 3.24 | 14.36 ± 3.45 a | 16.78 ± 4.54 a |
| SV, μL | 36.90 ± 5.81 | 37.47 ± 2.53 | 27.33 ± 5.65 a | 29.61 ± 7.61 a |
| CO, mL/min | 14.32 ± 2.78 | 14.92 ± 1.98 | 12.32 ± 2.49 | 13.50 ± 4.76 |
| IVSTd, mm | 0.63 ± 0.04 | 0.68 ± 0.04 | 0.93 ± 0.09 a | 0.91 ± 0.09 a |
| LVPWTd, mm | 0.64 ± 0.05 | 0.69 ± 0.03 | 0.92 ± 0.08 a | 0.93 ± 0.07 a |
| LV mass (corrected), mg | 64.36 ± 10.75 | 76.08 ± 6.73 | 139.60 ± 23.77 a | 133.00 ± 24.98 a |
| LVIDd, mm | 3.83 ± 0.38 | 4.03 ± 0.05 | 4.51 ± 0.29 a | 4.38 ± 0.52 |
| LVIDs, mm | 2.87 ± 0.21 | 2.91 ± 0.19 | 3.78 ± 0.35 a | 3.63 ± 0.57 a |
| E/A | 1.91 ± 0.38 | 1.87 ± 0.45 | 1.64 ± 0.57 | 1.59 ± 0.27 |
| E/e′ | −30.60 ± 4.98 | −28.13 ± 3.77 | −32.80 ± 14.24 | −33.49 ± 7.63 |
| Tei index | 0.38 ± 0.07 | 0.39 ± 0.07 | 0.74 ± 0.19 a | 0.74 ± 0.18 a |
A, peak velocity mitral flow in late diastole; CO, cardiac output; E, peak velocity mitral flow in early diastole; e′, peak velocity of mitral annulus in early diastole; FS, fractional shortening; HR, heart rate; IVSTd, interventricular septal thickness at end‐diastole; LVEF, left ventricular ejection fraction; LVIDd/s, left ventricular internal diameter at end‐diastole/systole; LVPWTd, left ventricular posterior wall thickness at end‐diastole; SV, stroke volume; TAC, transverse aortic constriction.
Results are expressed as the mean ± standard deviation. Comparisons were made with two‐way ANOVA followed by Sidak's multiple comparisons.
indicates the comparison with its sham group.
While we cannot explain why we do not observe a reversal of some of these molecular markers of HF with ketone supplementation, we speculate that reversing these effects in our study may be more of a challenge than preventing them as observed in a previous study. 20 Indeed, we still observed improvement in EF in KE‐treated mice, demonstrating that there is still a benefit of exogenously supplied ketones to the failing heart. To further confirm this, we conducted another set of experiments to test if ketones have beneficial in vivo effects in mice with HF. Using this approach, we show that TAC mice acutely infused with BHB exhibited significantly greater cardiac output compared with those infused with saline vehicle, suggesting that the elevation of blood BHB is directly associated with improved cardiac function (Figure 2 H,I ). Together, these data support the notion that a mild elevation of blood BHB can contribute to improving cardiac function both acutely and chronically.
Lastly, while these effects occur in male mice, this treatment remains to be investigated in female mice. However, this may be more difficult in our model as female mice that have undergone TAC surgery are resistant to developing HF. 21 Nevertheless, our findings indicated that KE treatment modestly but significantly ameliorated cardiac damage by TAC and prevented worsening functional decline in HF in male mice.
Conclusions
We provide evidence that chronic elevation of circulating ketone levels by exogenous KE supplementation may be of benefit for treating HF. Although the observed effect on cardiac pathophysiology was not profound, the ketone therapy may be a promising option for preventing the further worsening of HF. 22 Further investigations to elucidate the underlying mechanisms by which chronic elevation of circulating ketones could be beneficial for the failing heart, with respect to inflammation, oxidative stress, or otherwise, are warranted. In addition, these effects remain to be determined in female mice with HF. Nevertheless, our findings highlight the possibility of ketone supplementation as a potential therapy for HF patients.
Conflict of interest
The authors have no conflicts to disclose.
Funding
This work was supported by grants from Canadian Institutes of Health Research (CIHR) and the Heart and Stroke Foundation of Canada (HSF) to J.R.B.D. J.R.B.D. is a Canada Research Chair in Molecular Medicine. S.T. is supported by a postdoctoral fellowship from CIHR and Japan Society for the Promotion of Science Overseas Challenge Program for Young Researchers. S.S. is supported by the CIHR Canadian Graduate Doctoral Scholarship and an Alberta Innovates Graduate Student Scholarship. Z.H.M. is supported by Alberta Innovates Health Solution post‐doctoral and CIHR fellowship awards.
Supporting information
Table S1. Primer sequences.
Figure S1. Images of the original blots and periostin quantification.
A, periostin. B, GAPDH. C, quantification of periostin staining.
Figure S2. Transcripts of cardiac inflammation and oxidative stress. Relative transcript levels of Lgals3, Lcn2, IL‐6, F4/80, Mt2, Cat, Sod1, and Nrf2. All transcript levels are normalized to cyclophilin A.
Takahara, S. , Soni, S. , Phaterpekar, K. , Kim, T. T. , Maayah, Z. H. , Levasseur, J. L. , Silver, H. L. , Freed, D. H. , Ferdaoussi, M. , and Dyck, J. R. B. (2021) Chronic exogenous ketone supplementation blunts the decline of cardiac function in the failing heart. ESC Heart Failure, 8: 5606–5612. 10.1002/ehf2.13634.
References
- 1. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation 2016; 133: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by beta‐hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013; 339: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite beta‐hydroxybutyrate blocks NLRP3 inflammasome‐mediated inflammatory disease. Nat Med. 2015; 21: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Byrne NJ, Soni S, Takahara S, Ferdaoussi M, Al Batran R, Darwesh AM, Levasseur JL, Beker D, Vos DY, Schmidt MA, Alam AS, Maayah ZH, Schertzer JD, Seubert JM, Ussher JR, Dyck JRB. Chronically elevating circulating ketones can reduce cardiac inflammation and blunt the development of heart failure. Circ Heart Fail. 2020; 13: e006573. [DOI] [PubMed] [Google Scholar]
- 5. Nielsen R, Moller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frokiaer J, Eiskjaer H, Jespersen NR, Mellemkjaer S, Lassen TR, Pryds K, Botker HE, Wiggers H. Cardiovascular effects of treatment with the ketone body 3‐hydroxybutyrate in chronic heart failure patients. Circulation 2019; 139: 2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa‐Veloso K, Ho M, Roberts A, Robertson J, Vanitallie TB, Veech RL. Kinetics, safety and tolerability of (R)‐3‐hydroxybutyl (R)‐3‐hydroxybutyrate in healthy adult subjects. Reg Toxicol Pharmacol: RTP. 2012; 63: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation 2020; 141: 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J, McLure SW, King MT, Dodd MS, Holloway C, Neubauer S, Drawer S, Veech RL, Griffin JL, Clarke K. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab 2016; 24: 256–268. [DOI] [PubMed] [Google Scholar]
- 9. Byrne NJ, Levasseur J, Sung MM, Masson G, Boisvenue J, Young ME, Dyck JR. Normalization of cardiac substrate utilization and left ventricular hypertrophy precede functional recovery in heart failure regression. Cardiovasc Res 2016; 110: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Byrne NJ, Matsumura N, Maayah ZH, Ferdaoussi M, Takahara S, Darwesh AM, Levasseur JL, Jahng JWS, Vos D, Parajuli N, El‐Kadi AOS, Braam B, Young ME, Verma S, Light PE, Sweeney G, Seubert JM, Dyck JRB. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (Nucleotide‐binding domain‐like receptor protein 3) inflammasome activation in heart failure. Circ Heart Fail 2020; 13: e006277. [DOI] [PubMed] [Google Scholar]
- 11. Green A, Bishop RE. Ketoacidosis ‐ where do the protons come from? Trends Biochem Sci. 2019; 44: 484–489. [DOI] [PubMed] [Google Scholar]
- 12. Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, Magor‐Elliott S, Hiyama S, Stirling M, Clarke K. On the metabolism of exogenous ketones in humans. Front Physiol 2017; 8: 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahoney LB, Denny CA, Seyfried TN. Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis 2006; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stubbs BJ, Cox PJ, Evans RD, Cyranka M, Clarke K, de Wet H. A ketone Ester drink lowers human ghrelin and appetite. Obesity 2018; 26: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soto‐Mota A, Norwitz NG, Clarke K. Why a d‐beta‐hydroxybutyrate monoester? Biochem Soc Trans. 2020; 48: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watanabe T, Takemura G, Kanamori H, Goto K, Tsujimoto A, Okada H, Kawamura I, Ogino A, Takeyama T, Kawaguchi T, Morishita K, Ushikoshi H, Kawasaki M, Mikami A, Fujiwara T, Fujiwara H, Minatoguchi S. Restriction of food intake prevents postinfarction heart failure by enhancing autophagy in the surviving cardiomyocytes. Am J Pathol 2014; 184: 1384–1394. [DOI] [PubMed] [Google Scholar]
- 17. Philipson H, Ekman I, Forslund HB, Swedberg K, Schaufelberger M. Salt and fluid restriction is effective in patients with chronic heart failure. Eur J Heart Fail 2013; 15: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 18. Sung MM, Das SK, Levasseur J, Byrne NJ, Fung D, Kim TT, Masson G, Boisvenue J, Soltys CL, Oudit GY, Dyck JR. Resveratrol treatment of mice with pressure‐overload‐induced heart failure improves diastolic function and cardiac energy metabolism. Circ Heart Fail 2015; 8: 128–137. [DOI] [PubMed] [Google Scholar]
- 19. Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 2007; 101: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yurista SR, Matsuura TR, Sillje HHW, Nijholt KT, McDaid KS, Shewale SV, Leone TC, Newman JC, Verdin E, van Veldhuisen DJ, de Boer RA, Kelly DP, Westenbrink BD. Ketone Ester treatment improves cardiac function and reduces pathologic remodeling in preclinical models of heart failure. Circ Heart Fail 2021; 14: e007684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, Kintscher U, Gustafsson JA, Regitz‐Zagrosek V. Female sex and estrogen receptor‐beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 2010; 298: R1597–R1606. [DOI] [PubMed] [Google Scholar]
- 22. Takahara S, Soni S, Maayah ZH, Ferdaoussi M, Dyck JRB. Ketone therapy for heart failure: current evidence for clinical use. Cardiovasc Res 2021. 10.1093/cvr/cvab068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer sequences.
Figure S1. Images of the original blots and periostin quantification.
A, periostin. B, GAPDH. C, quantification of periostin staining.
Figure S2. Transcripts of cardiac inflammation and oxidative stress. Relative transcript levels of Lgals3, Lcn2, IL‐6, F4/80, Mt2, Cat, Sod1, and Nrf2. All transcript levels are normalized to cyclophilin A.


