Abstract
Unique vascular responses adhere to the cardiovascular efficacy of the inodilator levosimendan. In particular, selective venodilation appears to explain its clinical benefit during pulmonary hypertension complicated by heart failure with preserved ejection fraction. Vasodilators increase vessel diameter in various parts of the vascular system to different degrees and thereby influence blood pressure, its distribution, and organ perfusion depending on their mechanisms of action. Levosimendan and its long‐lived active metabolite OR‐1896 mobilize a set of vasodilatory mechanisms, that is, the opening of the ATP‐sensitive K+ channels and other K+ channels on top of a highly selective inhibition of the phosphodiesterase III enzyme. A vessel‐specific combination of the above vasodilator mechanisms—in concert with cardiac effects and cardiovascular reflex regulations—illustrates the pharmacological profile of levosimendan in various cardiovascular disorders. While levosimendan has been known to be an inotrope, its properties as an activator of ATP‐sensitive K+ channels have gone largely ignored with respect to clinical applications. Here, we provide a summary of what is known about the ATP‐sensitive K+ channel properties in preclinical studies and now for the first time, its ATP‐sensitive K+ channel properties in a clinical trial.
Keywords: Pulmonary hypertension, Heart failure with preserved ejection fraction, Therapy, Venodilation, Pharmacology, Levosimendan
Introduction
Tissue‐specific and vessel‐specific differences in the availability of drug‐responsive vasodilating mechanisms explain distinct pharmacological responses upon vasodilator administrations. Vasodilation affects peripheral and central circulation, target organ function, and clinical outcomes in a way specific for the employed drug and its concentration. The above factors set the basis for the applicability of different vasoactive agents in cardiovascular diseases.
Levosimendan is an inodilator used to restore haemodynamic balance in acute cardiac care. After extensive studies, the emerging consensus is that the vasodilatory properties of levosimendan are due to ATP‐sensitive K+ (K(ATP)) channel activation. 1 , 2 , 3 In general, K+ outflux (upon K+ channel activation/opening) shifts vascular smooth muscle cell membrane potential to more negative values (i.e. hyperpolarization) with a consequent vasodilatation because of Ca2+ channel closure. 4 Nonetheless, due to its complex pharmacodynamic and pharmacokinetic profile, levosimendan‐induced vasodilation requires additional consideration and, in particular, when its region‐specific vasodilatory properties are evaluated throughout the cardiovascular system.
Marked reductions in pulmonary capillary wedge pressure (PCWP) and improvements in pulmonary circulation have been recognized long ago as hallmarks of levosimendan during intravenous administrations in acute and advanced heart failure (HF). 5 Moreover, results of preclinical investigations suggested that levosimendan might reduce right ventricular afterload by relaxing pulmonary arteries and alleviate pulmonary oedema by pulmonary venodilation. 6 , 7 The safety and efficacy of a repeated weekly intravenous infusion of levosimendan formulation has been recently tested in patients with stable pulmonary hypertension (PH) and heart failure with preserved ejection fraction (HFpEF), where initial data also implicated favourable vascular and clinical responses. Interestingly, reductions of PCWP and central venous pressure (CVP) were demonstrated in the absence of systemic or pulmonary arterial vasodilation, or changes in cardiac index. 8 Accordingly, selective venodilation leading to the redistribution of stressed blood volume (SBV) to the splanchnic venous reservoir has been proposed to explain these findings. 9
Here, we provide an overview on the pharmacological mechanisms that can form the basis of the pulmonary‐selective and veno‐selective effect of levosimendan and evaluate the related pharmacologic, physiologic, and clinical implications.
The complex mechanism of levosimendan‐induced vasodilation
Levosimendan‐induced K(ATP) channel activation was first reported in rat mesenteric arterial myocytes and ventricular cardiomyocytes on the basis of whole‐cell and single channel patch‐clamp experiments under in vitro conditions. 10 , 11 These findings were subsequently corroborated in ex vivo and in vivo experiments in the coronary circulation of isolated guinea pig hearts, in isolated small mesenteric arteries of the rat, in the renal circulation of mice, and in human internal thoracic arteries. 12 , 13 , 14 , 15 Interestingly, in porcine endothelium denuded epicardial coronary arteries and in human umbilical arteries, the vasodilator effects of levosimendan were associated with the combined activation of voltage‐gated K+ channels (KV channels) and large conductance Ca2+‐activated K+ channels (BKCa channels). 16 , 17 Moreover, in human internal thoracic arteries levosimendan‐evoked relaxations were explained by the simultaneous activation of K(ATP) and BKCa channels. 18 Of note, results of levosimendan administrations in human internal thoracic arteries implicated sex‐specific differences, whereby the vasodilatory effects of levosimendan were more pronounced in male participants than in female participants. 19
Results of parallel preclinical studies using porcine coronary arteries extended the above observations towards additional effector mechanisms and suggested the involvement of intracellular cyclic adenosine monophosphate (cAMP) accumulation in the framework of β‐adrenergic signalling. 20 An increase in cAMP concentration would activate the protein kinase A enzyme to phosphorylate and inhibit the myosin light chain kinase enzyme thereby leading to vasorelaxation. The involvement of cAMP in the levosimendan‐evoked vascular responses, and at relatively high drug concentrations in particular, is not surprising in view of its highly selective inhibitory effect on the phosphodiesterase (PDE) III isozyme (i.e. without PDE IV inhibition at low levosimendan concentrations). 21
Interestingly, in an in vitro experimental study on porcine coronary endothelial cells an additional, nitric oxide‐(NO) dependent vasodilating mechanism (with the potential involvement of the cyclic guanosine monophosphate (cGMP)—protein kinase G—myosin light chain kinase enzyme axis) has been also suggested for levosimendan. 22 Moreover, an interaction between K(ATP) channel activations and NO signalling in reducing cell death has also been implicated upon intracoronary levosimendan administrations in pigs. 23
K+ channel openings were also linked to the vasodilating effects of OR‐1896, the long‐acting metabolite of levosimendan. OR‐1896‐elicited vasodilation largely depended on BKCa channel activations in isolated coronary microvessels, and K(ATP) channel activation in skeletal muscle arterioles of the rat. 24 In an in vivo follow‐up investigation on real resistance arterioles (that are pertinent to the regulation of microcirculation), levosimendan and OR‐1896‐induced dilation were similarly effective and were both dominated by K(ATP) channel activation. 25
The involvement of K(ATP) channel activations were also demonstrated with levosimendan administrations in isolated human portal vein preparations. 26 In human saphenous vein preparations, levosimendan‐evoked vasodilation was explained by the combined K(ATP) and BKCa channel activation. 27
Taken together, results of preclinical investigations (using sulfonylureas and other types of K+ channel blockers) pointed to the involvement of more than a single mechanism for the explanation of levosimendan‐induced vasodilation in several vascular beds (Table 1 ). Accordingly, activation of K(ATP) channels and other types of K+ channels together with a variable degree of PDE inhibition can all be involved in this effect depending on the characteristics of the vascular bed and the nature of the experimental conditions (e.g. levosimendan dose) (Figure 1 ).
Table 1.
Levosimendan‐ (LS) and OR‐1896‐ (OR) induced vasodilating mechanisms
| Effector | Drug | Vascular bed (species) | Reference |
|---|---|---|---|
| K(ATP) | LS | Mesenteric artery (rat) | Yokosihiki et al. 10 |
| LS | Coronary circulation (guinea pig) | Kaheinen et al. 13 | |
| LS | Renal circulation (mice) | Zager et al. 15 | |
| LS | Internal thoracic artery (human) | Yildiz, Seyrek, et al. 12 | |
| OR | Skeletal muscle arteriole (rat) | Erdei et al. 24 | |
| LS/OR | Resistance arteriole (rat) | Gödény et al. 25 | |
| LS | Portal vein (human) | Pataricza, Hõhn, et al. 26 | |
| KV + BKCa | LS | Coronary artery (pig) | Pataricza, Krassói, et al. 16 |
| LS | Umbilical cord artery (human) | Yildiz, Nacitarhan, et al. 17 | |
| BKCa | OR | Coronary arteriole (rat) | Erdei et al. 24 |
| K(ATP) + BKCa | LS | Internal thoracic artery (human) | Usta et al. 18 |
| LS | Saphenous vein (human) | Höhn et al. 27 | |
| cAMP | LS | Coronary artery (pig) | Gruhn et al. 20 |
| K(ATP) + cAMP + cGMP | LS | Pulmonary circulation (cat) | De Witt et al. 28 |
| Pulmonary artery (guinea pig) | Rieg, Rossaint, et al. 6 | ||
| K(ATP) + BKCa + cAMP + cGMP | LS | Pulmonary vein (guinea pig) | Rieg, Rossaint, et al. 6 |
| K(ATP) + KV + cAMP + cGMP | LS | Pulmonary circulation (human) | Rieg, Suleiman, et al. 7 |
| NO | LS | Coronary endothelial cells (pig) | Grossini et al. 22 |
BKCa, large conductance Ca2+‐activated K+ channels; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; K(ATP), ATP‐sensitive K+ channel; KV channels, voltage‐gated K+ channels; NO, nitric oxide.
Figure 1.
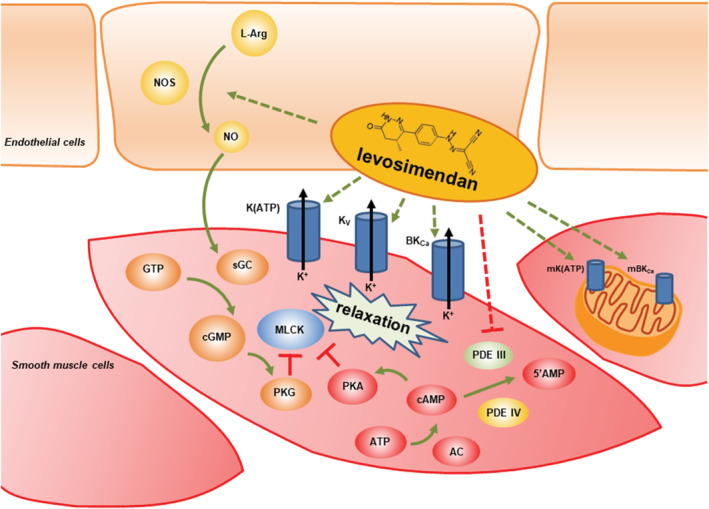
Schematic illustration of levosimendan‐induced putative vasodilating mechanisms. Levosimendan is capable to mobilize a set of vasodilatory mechanisms. Stimulatory and inhibitory effects are illustrated by green and red arrows, respectively. Effects of levosimendan are highlighted by dashed arrows. 5′AMP: 5′ adenosine monophosphate; AC, adenylate cyclase; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; cGMP, cylic guanosine monophosphate; GTP, guanosine triphosphate; l‐Arg: l‐arginine; MLCK, myosin light chain kinase; NO, nitric oxide; NOS, nitric oxide synthase; PDE III, phosphodiesterase III; PDE IV, phosphodiesterase IV; PKA: protein kinase A; PKG, protein kinase G; sGC, soluble guanylate cyclase. See the text for further abbreviations and details.
The mechanism of levosimendan‐induced vasodilation in the pulmonary circulation
The involvement of K(ATP) channel activation in levosimendan‐induced pulmonary vasodilation was clearly demonstrated under sophisticated experimental conditions in cats in vivo. 28 Moreover, in precision‐cut lung slices from guinea pigs, complex signalling pathways were associated with levosimendan‐evoked vascular smooth muscle relaxation. While in pulmonary arteries, the involvement of K(ATP) channel activation and cAMP/cGMP‐dependent processes were stressed, in pulmonary veins, additional roles for KV and BKCa channel activation was postulated. 6 Subsequent investigations confirmed and extended these observations for the human lung whereby levosimendan‐induced vascular smooth muscle relaxation was explained by a combination of K(ATP) and KV channel activation, as well as by increased intracellular cAMP and cGMP. 7 Interestingly, levosimendan also attenuated the vascular remodelling process in a rat model of pulmonary hypertension suggestive for K(ATP) channel‐dependent long‐term anti‐proliferative and anti‐inflammatory effects. 29
The role of mitochondrial K(ATP) channels in the levosimendan‐evoked cardiovascular effects
In addition to the effects on vascular smooth muscle cells, levosimendan was also shown to open mitochondrial K(ATP) channels (mK(ATP) channels) in cardiomyocytes, 30 , 31 , 32 which has been associated—in both ex vivo and in vivo models—with pharmacological pre‐conditioning 33 , 34 and post‐conditioning. 33 , 35 Interestingly, activation of another mitochondrial K+ channel, the mitochondrial BKCa channel (mBKCa channel), has been also demonstrated during levosimendan‐evoked cardiac pre‐conditioning and post‐conditioning in rats. 36 , 37
Overall, the cardiovascular significance of K(ATP) and/or mK(ATP) (and possibly other K+) channel activations has been supported by repeated observations related to levosimendan‐evoked clinical benefits. 38 , 39 For example, K(ATP) channel activation was a prerequisite for improved survival following cardiopulmonary resuscitation in levosimendan‐treated rats following ventricular fibrillation. 40 In line with preclinical studies of these kinds, pharmacological pre‐treatment with levosimendan significantly improved outcomes in patients undergoing coronary artery bypass graft surgery. 41 Of note, the administration of sulfonylureas did not attenuate the haemodynamic or other effects of levosimendan under clinical conditions suggestive for the significance of the several times higher concentrations of sulfonylureas used in experimental settings than in clinical conditions. 42 It is also a matter of importance that levosimendan shares its K(ATP) channel agonist property with the anti‐angina medication nicorandil. 43 Nicorandil, however, has a pronounced negative inotropic effect while levosimendan—in line with its unique dual inodilator mechanism ‐ is a positive inotrope. 44
Importantly, the sum of levosimendan‐evoked effects on cardiac mitochondria, cardiomyocytes and coronary circulation preserves the overall energy balance of cardiac function, which has not been shown for any other inodilator. 45
Levosimendan‐evoked K(ATP) channel activation beyond the cardiovascular system
Levosimendan‐induced K(ATP) channel activation in combination with BKCa channel activation has been identified in tracheal ring preparations of guinea pigs. 46 Importantly, levosimendan prevented bronchoconstriction via K(ATP) channel activation in rabbits in vivo, which can be of importance for patients with decreased cardiorespiratory reserve. 47 Additionally, lung tissue integrity was protected in a rat model of pulmonary ischaemia and reperfusion through mitigated levels of apoptosis by levosimendan‐evoked post‐conditioning. 48
Levosimendan also induced relaxation of human myometrial strip preparations via K(ATP) channel activation. 49 Moreover, the anticonvulsant effects of levosimendan (and thus its influence on the central nervous system) has also been associated with K(ATP) channel activation. 50
An effect of levosimendan on liver mitochondria has been documented, 30 which—in association with the levosimendan‐induced increase in liver blood flood 51 —could explain the protective effect elicited by the drug against liver ischaemia–reperfusion. 52 Similar observations in a pig model, where the protective effects of levosimendan against ischaemia/reperfusion injury on kidney function were demonstrated, support the activation of mK(ATP) channels in organ‐protective effects. 53
Characteristics of levosimendan‐induced vasodilation in view of other vasodilators
Results detailed above illustrate a complex mechanism of action for levosimendan‐induced vasodilation that involve the activation of sarcolemmal and mitochondrial K+ channels and the modulation of cyclic nucleotide levels in cardiovascular and other tissues. Here, we postulate that the combination of these effector mechanisms, together with its other cardiovascular effects, form the foundation for the unique organ‐selective and veno‐selective characteristics of levosimendan that cannot be reproduced by single drug‐target interactions (Figure 2 ).
Figure 2.
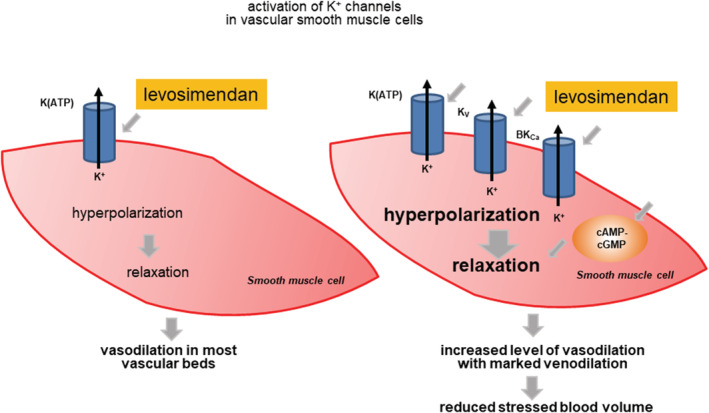
Hypothetical explanation for increased tissue sensitivity of levosimendan‐evoked vasodilation with marked venodilation. An increased level of levosimendan‐induced vasodilation can relate to more than a single vasodilator effector mechanism.
Indeed, in porcine coronary vascular smooth muscle cells, levosimendan‐evoked vasodilation was paralleled by an apparent Ca2+‐desensitization (thus giving space for K+ channel activation), an effect that was not seen upon the administration of milrinone, a PDE III inhibitor. 54 Likewise, in contrast to levosimendan, dobutamine (a β‐mimetic) evoked frequent arrhythmias and suggested distinct effects on [Ca2+]i in a model of ischaemia and reperfusion of Langendorff‐perfused guinea pig hearts. 55 Moreover, while the concentration dependencies of levosimendan and OR‐1896 on systemic cardiovascular haemodynamic responses (e.g. blood pressure, pulse pressure, rate‐pressure product, cardiac output, & peripheral resistance) were similar, they were different from those evoked by dobutamine or milrinone in rats in vivo. 56
In a canine model, Pagel et al. 51 showed that levosimendan and milrinone cause different alterations in regional tissue perfusion while producing similar systemic haemodynamic effects. Importantly, levosimendan decreased vascular resistance in the renal and splanchnic circulation while milrinone increased it. Moreover, in that model, milrinone did not reduce pressure work index (an estimate of myocardial‐oxygen consumption) in contrast to levosimendan. 57 In a dog model, Schwarte et al. 58 showed that levosimendan was superior to milrinone and dobutamine in selectively increasing microvascular gastric mucosal oxygenation.
In an experimental study conducted in instrumented cats, levosimendan was significantly more potent in decreasing lobar pulmonary arterial pressure than either of the type III or IV PDE inhibitors, or the K(ATP) channel agonist pinacidil. 28
In summary, cardiovascular responses following levosimendan administration are distinguishable from those evoked by activators of the β‐adrenergic system and consequently from sole intracellular cyclic nucleotide changes (Table 2 ).
Table 2.
Cardiovascular profiles of levosimendan (LS) and other inodilators in comparative studies
| Drug | Examined parameter | Effect | Preparation (species) | Ref. |
|---|---|---|---|---|
| LS | [Ca2+]i‐force relationship | Desensitization | Coronary arteries (pig) | Bowman et al. 54 |
| Milrinone | " | No desensitization | ||
| LS | Post‐ischaemic arrhythmia | None | Isolated hearts (guinea pig) | Du Toit et al. 55 |
| Dobutamine | " | Frequent | ||
| LS/OR‐1896 | Mean arterial pressure | Decrease (high potential) | Instrumented animals (rats) | Segreti et al. 56 |
| Pulse pressure | Decrease (high potential) | |||
| Rate‐pressure product | Decrease (low potential) | |||
| Cardiac output (LS) | Increase (high potential) | |||
| Peripheral resistance | Decrease (high potential) | |||
| Milrinone | Mean arterial pressure | Decrease (low potential) | ||
| Pulse pressure | Decrease (low potential) | |||
| Rate‐pressure product | Decrease (low potential) | |||
| Cardiac output | Small increase (low potential) | |||
| Peripheral resistance | Decrease (low potential) | |||
| Dobutamine | Blood pressure | No effect | ||
| Pulse pressure | Increase | |||
| Rate‐pressure product | Increase (high potential) | |||
| Cardiac output | No effect | |||
| Peripheral resistance | No effect | |||
| LS | Pulmonary lobar pressure decrease | High potency | Instrumented animals (cats) | De Witt et al. 28 |
| Siguazodan | " | Low potency | ||
| Rolipram | " | Low potency | ||
| Pinacidil | " | Low potency | ||
| LS | Regional distribution of cardiac output | LS‐specific combination at comparable systemic effects | Anaesthetized animals (dogs) | Pagel et al. 51 , 57 |
| Renal vascular resistance | Decrease | |||
| Splanchnic vascular resistance | Decrease | |||
| Pressure work index | Decrease | |||
| Milrinone | Regional distribution of cardiac output | Milrinone‐specific combination at comparable systemic effects | ||
| Renal vascular resistance | Increase | |||
| Splanchnic vascular resistance | Increase | |||
| Pressure work index no effect | ||||
| Pimobendan | Regional distribution of cardiac output | Pimobendan‐specific combination at comparable systemic effects | ||
| Renal vascular resistance | Increase | |||
| Splanchnic vascular resistance | Increase | |||
| Pressure work index | No effect | |||
| LS | Oxygenation of gastric mucosa | Selective increase | Anaesthetized animals (dogs) | Schwarte et al. 58 |
| Milrinone | " | No effect | ||
| Dobutamine | " | Non‐selective increase |
Milrinone, pimobendan, and siguazodan are inhibitors of the PDE III enzyme, rolipram is an inhibitor of the PDE IV enzyme, and pinacidil is an activator of K(ATP) channels.
Levosimendan in pulmonary hypertension from heart failure with preserved ejection fraction
Recent investigations into the mechanisms of elevated PCWP in HF have drawn attention to increased SBV as playing an important role. 9 Total blood volume of the body is divided into two functional compartments: SBV and unstressed blood volume (UBV). UBV is the amount of blood required to fill the vascular system just to the point wall stress and mean circulatory filling pressure start to rise; SBV is the blood volume about UBV. Thus, TBP = SBV + UBV (Figure 3 ). The splanchnic circulation constitutes the body's largest reservoir of UBV, predominantly in the veins, which can be recruited rapidly to the SBV pool. Importantly, the splanchnic reservoir is responsive to changes in sympathetic tone due to the large concentration of adrenergic receptors in the walls of venous vessel that regulate their smooth muscle tone and thus the capacity of the venous system. SBV plays a critical role in determining venous pressure 61 and is an adaptive mechanism that regulates systemic and pulmonary venous pressures during exercise, times of stress, and in response to haemorrhage. Chronic increases in SBV become maladaptive from the sustained elevated activity of the sympathetic nervous system that occurs in HF and in pulmonary hypertension.
Figure 3.
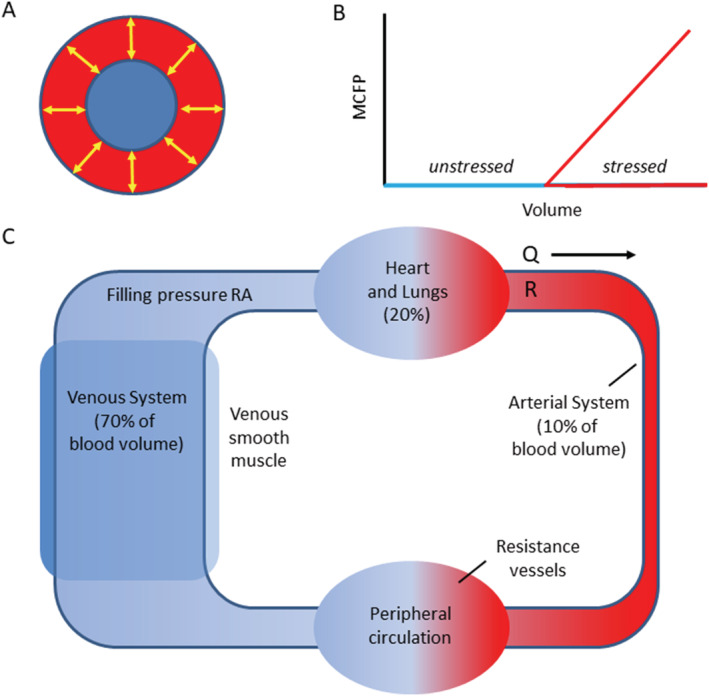
Stressed and unstressed vascular volume. The volume inside a vessel at near zero transmural pressure is termed ‘unstressed volume’ (blue). It fills the system without exerting tension in the vessel wall. The blood volume that creates positive transmural pressure via the elastic recoil of the vessel wall is termed ‘stressed volume’ (red). Mean circulatory filling pressure (MCFP) is a function of stressed volume and vascular compliance; compliance is the slope of the pressure–volume curve above the unstressed volume. (A) Cross section of a blood vessel. (B) The relationship between blood volume and MCFP. (C) The venous system contains approximately 70% of the blood volume. The splanchnic vascular bed serves as a reservoir and will adjust the amount of venous return based on signalling from the autonomic nervous system. In chronic HF, the increased sympathetic tone associated with activation of the renin–angiotensin–aldosterone system will also activate the splanchnic circulation to increase venous return, referred to SBV. This will increase the CVP and the pulmonary capillary wedge pressure. Q, cardiac output; R, systemic vascular resistance; RA: right atrium. Panels (A) and (B) freely adapted from Grübler et al. 59 Panel (C) is freely adapted from Noel‐Morgan and Muir. 60
The importance of SBV in HF was recently validated by experiments in patients with chronic HF and elevated PCWP. A percutaneous splanchnic ganglion nerve block that temporarily dilated the splanchnic veins was shown to markedly lower CVP and PCWP at rest and during exercise with a reduction in estimated SBV. 62 The splanchnic veins have also been shown to dilate in response to K(ATP) activators. 26
The Hemodynamic Evaluation of Levosimendan in Patients With PH‐HFpEF (HELP) trial was a mechanistic trial designed to understand the mechanisms behind a potential benefit of levosimendan in patients with PH‐HFpEF. 8 PH‐HFpEF was chosen as the disease to study because of the following 63 : (1) it is a progressive and fatal disease with no effective treatment and a high unmet medical need; (2) it has become increasingly common in pulmonary hypertension specialty clinics; (3) a chronic elevation in PCWP at rest which worsens with exercise has been identified as an important target to achieve clinical benefit; and (4) the inotropic properties of levosimendan could prove helpful in patients with coexistent right HF. 64
Levosimendan has demonstrated to be consistently effective in lowering PCWP in a broad spectrum of acute HF trials. 65 The classical teaching was that the fall in PCWP was attributed to its inotropic effect which would, theoretically, increase LV ejection and thereby result in greater ‘emptying’ of the pulmonary venous system; potential concomitant lusitropic effects have also been postulated to improve relaxation to enhance diastolic filling. However, the haemodynamic studies of levosimendan in HF patients show a rapid and marked reduction in PCWP before meaningful increases in cardiac output occur, which raised questions about a different mechanism of action. 66
The HELP trial had two phases. Phase 1 was an open‐label lead‐in where patients would undergo a rest and exercise right heart catheterization to set a baseline haemodynamic profile of the PH‐HFpEF. The patients then received a 24 h intravenous infusion of levosimendan and returned to the lab the following day for a repeat haemodynamic study. Those patients who demonstrated a >4 mmHg reduction in exercise PCWP were characterized as levosimendan responders and were enrolled into Phase 2, which was a 6 week outpatient, randomized, placebo‐controlled, blinded study design. Phase 1 allowed the evaluation of the mechanism of action of levosimendan with patients serving as their own control. It showed significant reductions in PCWP and CVP at rest and exercise with no change in cardiac output, supporting effects on both venous pressure independent of any inotropic property of levosimendan, which were similar to the effect of splanchnic ganglion blockade. Phase 2 tested the durability of weekly levosimendan infusions over 6 weeks, followed by an end‐of‐study right heart catheterization at rest and exercise. The 24 h haemodynamic changes persisted over the 6 weeks, but importantly were associated with a significant increase in exercise capacity. An analysis of the data supports that the mechanism of action to be a reduction in SBV, with no evidence for an inotropic effect. 67 This is consistent with the vasodilator effects of levosimendan as a K(ATP) channel activator on the splanchnic bed. 26
There are also considerable data that support the downregulation of K+ channels as one of the fundamental processes that underlies the development of pulmonary hypertensive vascular disease. 68 Whether a long‐term reduction in pulmonary arterial pressure due to the K(ATP) channel activation from levosimendan is also possible in these patients is unknown, but needs to be studied to see if this additional property of levosimendan could reverse the underlying pulmonary vascular disease.
Conclusions
Preclinical investigations with various vascular (arterial and venous) and non‐vascular preparations identified K+ channel (most frequently K(ATP) channel) activation as a mediator of the levosimendan‐mediated smooth muscle relaxing effects. The pharmacological profile of levosimendan and of its long‐acting metabolite, OR‐1896, is very similar in this respect. Methodological and tissue‐specific characteristics may explain part of the discrepancies among the observed combinations of additional effector mechanisms (including KV and BKCa channels, cAMP and NO—cGMP). Of note, the expression levels of K+ channel subtypes depend on the type of vascular bed and cardiovascular diseases. 69 Intuitively, the number of levosimendan‐mobilized targets may correlate with the tissue sensitivity for vasodilation (e.g. in the pulmonary circulation and in peripheral veins) and can form the basis of its favourable haemodynamic profile with low‐dose levosimendan administrations (Figure 2 ).
Undoubtedly, levosimendan affects a host of vasodilatory mechanisms and its smooth muscle relaxing effects extend beyond the cardiovascular system. The vasodilation is exerted in many vascular beds: in arteries and veins and in the peripheral and central circulations. Levosimendan mobilizes vasodilating mechanisms in combinations allowing for a differential and region‐specific regulation in vascular beds and for the promotion of venodilation in the absence of systemic effects on the cardiovascular system as a whole at relatively low levosimendan concentrations. This feature differentiates this drug from other vasodilators or inodilators.
Conflicts of interest
S.R. is Chief Medical Officer at Tenax Therapeutics. P.P. is full time employee at Orion Pharma. Z.P. and D.B. have nothing to declare.
Funding
This work was supported by project TKP2020‐IKA‐04 and TKP2020‐NKA‐04 from the National Research, Development and Innovation Fund of Hungary, under the 2020–4.1.1‐TKP2020 funding scheme. This work was also supported by the GINOP‐2.3.2‐15‐2016‐00043 project. The project is co‐financed by the European Union and the European Regional Development Fund. The research group of Z.P. is supported by the Hungarian Academy of Sciences.
Compliance with ethical standards
Ethics approval and consent to participate
The submitted work is original and has not been published elsewhere in any form or language.
Consent for publication
All listed authors have approved the manuscript before submission, including the names and order of authors.
Research involving human participants and/or animals
This review paper does not report on original data. Nevertheless, this review paper refers to the results of a completed clinical trial [Hemodynamic Evaluation of Levosimendan in Patients With PH‐HFpEF (HELP); NCT03541603] published elsewhere (JACC Heart Fail. 2021 May;9(5):360‐370. https://doi.org/10.1016/j.jchf.2021.01.015.).
Burkhoff, D. , Rich, S. , Pollesello, P. , and Papp, Z. (2021) Levosimendan‐induced venodilation is mediated by opening of potassium channels. ESC Heart Failure, 8: 4454–4464. 10.1002/ehf2.13669.
References
- 1. Papp Z, Agostoni P, Alvarez J, Bettex D, Bouchez S, Brito D, Černý V, Comin‐Colet J, Crespo‐Leiro MG, Delgado JF, Édes I, Eremenko AA, Farmakis D, Fedele F, Fonseca C, Fruhwald S, Girardis M, Guarracino F, Harjola VP, Heringlake M, Herpain A, Heunks LMA, Husebye T, Ivancan V, Karason K, Kaul S, Kivikko M, Kubica J, Masip J, Matskeplishvili S, Mebazaa A, Nieminen MS, Oliva F, Papp JG, Parissis J, Parkhomenko A, Põder P, Pölzl G, Reinecke A, Ricksten SE, Riha H, Rudiger A, Sarapohja T, Schwinger RHG, Toller W, Tritapepe L, Tschöpe C, Wikström G, Lewinski DV, Vrtovec B, Pollesello P. Levosimendan efficacy and safety: 20 years of SIMDAX in clinical use. J Cardiovasc Pharmacol 2020; 76: 4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papp Z, Édes I, Fruhwald S, De Hert SG, Salmenperä M, Leppikangas H, Mebazaa A, Landoni G, Grossini E, Caimmi P, Morelli A, Guarracino F, Schwinger RH, Meyer S, Algotsson L, Wikström BG, Jörgensen K, Filippatos G, Parissis JT, González MJ, Parkhomenko A, Yilmaz MB, Kivikko M, Pollesello P, Follath F. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol 2012; 159: 82–87. [DOI] [PubMed] [Google Scholar]
- 3. Yildiz O. Vasodilating mechanisms of levosimendan: involvement of K channels. J Pharmacol Sci 2007; 104: 1–5. [DOI] [PubMed] [Google Scholar]
- 4. Siegel G, Emden J, Wenzel K, Mironneau J, Stock G. Potassium channel activation in vascular smooth muscle. Adv Exp Med Biol 1992; 311: 53–72. [DOI] [PubMed] [Google Scholar]
- 5. Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low‐output heart failure (the LIDO study): a randomised double‐blind trial. Lancet 2002; 360: 196–202. [DOI] [PubMed] [Google Scholar]
- 6. Rieg AD, Rossaint R, Verjans E, Maihöfer NA, Uhlig S, Martin C. Levosimendan relaxes pulmonary arteries and veins in precision‐cut lung slices ‐ the role of KATP‐channels, cAMP and cGMP. PLoS One. 2013; 8: e66195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rieg AD, Suleiman S, Bünting NA, Verjans E, Spillner J, Schnöring H, Kalverkamp S, Schröder T, von Stillfried S, Braunschweig T, Schälte G, Uhlig S, Martin C. Levosimendan reduces segmental pulmonary vascular resistance in isolated perfused rat lungs and relaxes human pulmonary vessels. PLoS One. 2020; 15: e0233176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burkhoff D, Borlaug BA, Shah SJ, Zolty R, Tedford RJ, Thenappan T, Zamanian RT, Mazurek JA, Rich JD, Simon MA, Chung ES, Raza F, Majure DT, Lewis GD, Preston IR, Rich S. Levosimendan improves hemodynamics and exercise tolerance in PH‐HFpEF: results of the randomized placebo‐controlled HELP trial. JACC. Heart Fail. 2021. Apr 3; 9: 360–370. [DOI] [PubMed] [Google Scholar]
- 9. Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail 2011; 4: 669–675. [DOI] [PubMed] [Google Scholar]
- 10. Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. Levosimendan, a novel Ca2 sensitizer, activates the glibenclamide‐sensitive K channel in rat arterial myocytes. Eur J Pharmacol 1997; 333: 249–259. [DOI] [PubMed] [Google Scholar]
- 11. Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. The novel calcium sensitizer levosimendan activates the ATP‐sensitive K channel in rat ventricular cells. J Pharmacol Exp Ther 1997; 283: 375–383. [PubMed] [Google Scholar]
- 12. Yildiz O, Seyrek M, Yildirim V, Demirkilic U, Nacitarhan C. Potassium channel‐related relaxation by levosimendan in the human internal mammary artery. Ann Thorac Surg 2006; 81: 1715–1719. [DOI] [PubMed] [Google Scholar]
- 13. Kaheinen P, Pollesello P, Levijoki J, Haikala H. Levosimendan increases diastolic coronary flow in isolated Guinea‐pig heart by opening ATP‐sensitive potassium channels. J Cardiovasc Pharmacol 2001; 37: 367–374. [DOI] [PubMed] [Google Scholar]
- 14. Ozdem SS, Yalcin O, Meiselman HJ, Baskurt OK, Usta C. The role of potassium channels in relaxant effect of levosimendan in rat small mesenteric arteries. Cardiovasc Drugs Ther 2006; 20: 123–127. [DOI] [PubMed] [Google Scholar]
- 15. Zager RA, Johnson AC, Lund S, Hanson SY, Abrass CK. Levosimendan protects against experimental endotoxemic acute renal failure. Am J Physiol Renal Physiol 2006; 290: F1453–F1462. [DOI] [PubMed] [Google Scholar]
- 16. Pataricza J, Krassói I, Höhn J, Kun A, Papp JG. Functional role of potassium channels in the vasodilating mechanism of levosimendan in porcine isolated coronary artery. Cardiovasc Drugs Ther 2003; 17: 115–121. [DOI] [PubMed] [Google Scholar]
- 17. Yildiz O, Nacitarhan C, Seyrek M. Potassium channels in the vasodilating action of levosimendan on the human umbilical artery. J Soc Gynecol Investig 2006; 13: 312–315. [DOI] [PubMed] [Google Scholar]
- 18. Usta C, Eksert B, Gölbasi I, Bigat Z, Ozdem SS. The role of potassium channels in the vasodilatory effect of levosimendan in human internal thoracic arteries. Eur J Cardiothorac Surg 2006; 30: 329–332. [DOI] [PubMed] [Google Scholar]
- 19. Akar F, Manavbasi Y, Parlar AI, Ulus AT, Katircioglu SF. The gender differences in the relaxation to levosimendan of human internal mammary artery. Cardiovasc Drugs Ther 2007; 21: 331–338. [DOI] [PubMed] [Google Scholar]
- 20. Gruhn N, Nielsen‐Kudsk JE, Theilgaard S, Bang L, Olesen SP, Aldershvile J. Coronary vasorelaxant effect of levosimendan, a new inodilator with calcium‐sensitizing properties. J Cardiovasc Pharmacol 1998; 31: 741–749. [DOI] [PubMed] [Google Scholar]
- 21. Szilágyi S, Pollesello P, Levijoki J, Kaheinen P, Haikala H, Edes I, Papp Z. The effects of levosimendan and OR‐1896 on isolated hearts, myocyte‐sized preparations and phosphodiesterase enzymes of the Guinea pig. Eur J Pharmacol 2004; 486: 67–74. [DOI] [PubMed] [Google Scholar]
- 22. Grossini E, Molinari C, Caimmi PP, Uberti F, Vacca G. Levosimendan induces NO production through p38 MAPK, ERK and akt in porcine coronary endothelial cells: role for mitochondrial K (ATP) channel. Br J Pharmacol 2009; 156: 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caimmi PP, Molinari C, Uberti F, Micalizzi E, Valente G, Mary DA, Vacca G, Grossini E. Intracoronary levosimendan prevents myocardial ischemic damages and activates survival signaling through ATP‐sensitive potassium channel and nitric oxide. Eur J Cardiothorac Surg 2011; 39: e59–e67. [DOI] [PubMed] [Google Scholar]
- 24. Erdei N, Papp Z, Pollesello P, Edes I, Bagi Z. The levosimendan metabolite OR‐1896 elicits vasodilation by activating the K (ATP) and BK (Ca) channels in rat isolated arterioles. Br J Pharmacol 2006; 148: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gödény I, Pollesello P, Edes I, Papp Z, Bagi Z. Levosimendan and its metabolite OR‐1896 elicit KATP channel‐dependent dilation in resistance arteries in vivo. Pharmacol Rep 2013; 65: 1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pataricza J, Hõhn J, Petri A, Balogh A, Papp JG. Comparison of the vasorelaxing effect of cromakalim and the new inodilator, levosimendan, in human isolated portal vein. J Pharm Pharmacol 2000; 52: 213–217. [DOI] [PubMed] [Google Scholar]
- 27. Höhn J, Pataricza J, Petri A, Tóth GK, Balogh A, Varró A, Papp JG. Levosimendan interacts with potassium channel blockers in human saphenous veins. Basic Clin Pharmacol Toxicol 2004; 94: 271–273. [DOI] [PubMed] [Google Scholar]
- 28. De Witt BJ, Ibrahim IN, Bayer E, Fields AM, Richards TA, Banister RE, Kaye AD. An analysis of responses to levosimendan in the pulmonary vascular bed of the cat. Anesth Analg 2002; 94: 1427–1433 table of contents. [DOI] [PubMed] [Google Scholar]
- 29. Revermann M, Schloss M, Mieth A, Babelova A, Schröder K, Neofitidou S, Buerkl J, Kirschning T, Schermuly RT, Hofstetter C, Brandes RP. Levosimendan attenuates pulmonary vascular remodeling. Intensive Care Med 2011; 37: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 30. Kopustinskiene DM, Pollesello P, Saris NE. Levosimendan is a mitochondrial K (ATP) channel opener. Eur J Pharmacol 2001; 428: 311–314. [DOI] [PubMed] [Google Scholar]
- 31. Kopustinskiene DM, Pollesello P, Saris NE. Potassium‐specific effects of levosimendan on heart mitochondria. Biochem Pharmacol 2004; 68: 807–812. [DOI] [PubMed] [Google Scholar]
- 32. Kersten JR, Montgomery MW, Pagel PS, Warltier DC. Levosimendan, a new positive inotropic drug, decreases myocardial infarct size via activation of K (ATP) channels. Anesth Analg 2000; 90: 5–11. [DOI] [PubMed] [Google Scholar]
- 33. du Toit EF, Genis A, Opie LH, Pollesello P, Lochner A. A role for the RISK pathway and K (ATP) channels in pre‐ and post‐conditioning induced by levosimendan in the isolated Guinea pig heart. Br J Pharmacol 2008; 154: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papp JG, Pollesello P, Varró AF, Végh AS. Effect of levosimendan and milrinone on regional myocardial ischemia/reperfusion‐induced arrhythmias in dogs. J Cardiovasc Pharmacol Ther 2006; 11: 129–135. [DOI] [PubMed] [Google Scholar]
- 35. Hönisch A, Theuring N, Ebner B, Wagner C, Strasser RH, Weinbrenner C. Postconditioning with levosimendan reduces the infarct size involving the PI3K pathway and KATP‐channel activation but is independent of PDE‐III inhibition. Basic Res Cardiol 2010; 105: 155–167. [DOI] [PubMed] [Google Scholar]
- 36. Bunte S, Behmenburg F, Bongartz A, Stroethoff M, Raupach A, Heinen A, Minol JP, Hollmann MW, Huhn R, Sixt SU. Preconditioning by levosimendan is mediated by activation of mitochondrial Ca(2 )‐sensitive potassium (mBK (Ca)) channels. Cardiovasc Drugs Ther 2018; 32: 427–434. [DOI] [PubMed] [Google Scholar]
- 37. Stroethoff M, Bunte S, Raupach A, van de Snepscheut M, Torregroza C, Heinen A, Mathes A, Hollmann MW, Huhn R, Sixt SU. Impact of Ca(2 )‐sensitive potassium channels in levosimendan‐induced postconditioning. Cardiovasc Drugs Ther 2019; 33: 581–588. [DOI] [PubMed] [Google Scholar]
- 38. Pollesello P, Mebazaa A. ATP‐dependent potassium channels as a key target for the treatment of myocardial and vascular dysfunction. Curr Opin Crit Care 2004; 10: 436–441. [DOI] [PubMed] [Google Scholar]
- 39. Pollesello P, Papp Z. The cardioprotective effects of levosimendan: preclinical and clinical evidence. J Cardiovasc Pharmacol 2007; 50: 257–263. [DOI] [PubMed] [Google Scholar]
- 40. Cammarata GA, Weil MH, Sun S, Huang L, Fang X, Tang W. Levosimendan improves cardiopulmonary resuscitation and survival by K (ATP) channel activation. J Am Coll Cardiol 2006; 47: 1083–1085. [DOI] [PubMed] [Google Scholar]
- 41. Tritapepe L, De Santis V, Vitale D, Guarracino F, Pellegrini F, Pietropaoli P, Singer M. Levosimendan pre‐treatment improves outcomes in patients undergoing coronary artery bypass graft surgery. Br J Anaesth 2009; 102: 198–204. [DOI] [PubMed] [Google Scholar]
- 42. Kivikko M, Nieminen MS, Pollesello P, Pohjanjousi P, Colucci WS, Teerlink JR, Mebazaa A. The clinical effects of levosimendan are not attenuated by sulfonylureas. Scand Cardiovasc J 2012; 46: 330–338. [DOI] [PubMed] [Google Scholar]
- 43. Patel DJ, Purcell HJ, Fox KM. Cardioprotection by opening of the K (ATP) channel in unstable angina. is this a clinical manifestation of myocardial preconditioning? Results of a randomized study with nicorandil. CESAR 2 investigation. clinical european studies in angina and revascularization. Eur Heart J 1999; 20: 51–57. [DOI] [PubMed] [Google Scholar]
- 44. Farmakis D, Alvarez J, Gal TB, Brito D, Fedele F, Fonseca C, Gordon AC, Gotsman I, Grossini E, Guarracino F, Harjola VP, Hellman Y, Heunks L, Ivancan V, Karavidas A, Kivikko M, Lomivorotov V, Longrois D, Masip J, Metra M, Morelli A, Nikolaou M, Papp Z, Parkhomenko A, Poelzl G, Pollesello P, Ravn HB, Rex S, Riha H, Ricksten SE, Schwinger RHG, Vrtovec B, Yilmaz MB, Zielinska M, Parissis J. Levosimendan beyond inotropy and acute heart failure: evidence of pleiotropic effects on the heart and other organs: an expert panel position paper. Int J Cardiol 2016; 222: 303–312. [DOI] [PubMed] [Google Scholar]
- 45. Nieminen MS, Pollesello P, Vajda G, Papp Z. Effects of levosimendan on the energy balance: preclinical and clinical evidence. J Cardiovasc Pharmacol 2009; 53: 302–310. [DOI] [PubMed] [Google Scholar]
- 46. Eksert B, Usta C. Role of potassium channels in the relaxant effect of levosimendan in Guinea pig tracheal preparations. Pharmacol Rep 2009; 61: 275–280. [DOI] [PubMed] [Google Scholar]
- 47. Babik B, Balogh AL, Sudy R, Ivankovitsne‐Kiss O, Fodor GH, Petak F. Levosimendan prevents bronchoconstriction and adverse respiratory tissue mechanical changes in rabbits. Am J Physiol Lung Cell Mol Physiol 2017; 313: L950–L956. [DOI] [PubMed] [Google Scholar]
- 48. Zhang C, Guo Z, Liu H, Shi Y, Ge S. Influence of levosimendan postconditioning on apoptosis of rat lung cells in a model of ischemia‐reperfusion injury. PLoS One. 2015; 10: e0114963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hehir MP, Moynihan AT, Morrison JJ. Relaxant effect of levosimendan on human uterine contractility in vitro. Am J Obstet Gynecol 2010; 203: 184 e187–184 e112. [DOI] [PubMed] [Google Scholar]
- 50. Gooshe M, Tabaeizadeh M, Aleyasin AR, Mojahedi P, Ghasemi K, Yousefi F, Vafaei A, Amini‐Khoei H, Amiri S, Dehpour AR. Levosimendan exerts anticonvulsant properties against PTZ‐induced seizures in mice through activation of nNOS/NO pathway: role for K (ATP) channel. Life Sci 2017; 168: 38–46. [DOI] [PubMed] [Google Scholar]
- 51. Pagel PS, Hettrick DA, Warltier DC. Influence of levosimendan, pimobendan, and milrinone on the regional distribution of cardiac output in anaesthetized dogs. Br J Pharmacol 1996; 119: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grossini E, Pollesello P, Bellofatto K, Sigaudo L, Farruggio S, Origlia V, Mombello C, Mary DA, Valente G, Vacca G. Protective effects elicited by levosimendan against liver ischemia/reperfusion injury in anesthetized rats. Liver Transpl 2014; 20: 361–375. [DOI] [PubMed] [Google Scholar]
- 53. Grossini E, Molinari C, Pollesello P, Bellomo G, Valente G, Mary D, Vacca G, Caimmi P. Levosimendan protection against kidney ischemia/reperfusion injuries in anesthetized pigs. J Pharmacol Exp Ther 2012; 342: 376–388. [DOI] [PubMed] [Google Scholar]
- 54. Bowman P, Haikala H, Paul RJ. Levosimendan, a calcium sensitizer in cardiac muscle, induces relaxation in coronary smooth muscle through calcium desensitization. J Pharmacol Exp Ther 1999; 288: 316–325. [PubMed] [Google Scholar]
- 55. Du Toit EF, Muller CA, McCarthy J, Opie LH. Levosimendan: effects of a calcium sensitizer on function and arrhythmias and cyclic nucleotide levels during ischemia/reperfusion in the langendorff‐perfused Guinea pig heart. J Pharmacol Exp Ther 1999; 290: 505–514. [PubMed] [Google Scholar]
- 56. Segreti JA, Marsh KC, Polakowski JS, Fryer RM. Evoked changes in cardiovascular function in rats by infusion of levosimendan, OR‐1896 [(R)‐N‐(4‐(4‐methyl‐6‐oxo‐1,4,5,6‐tetrahydropyridazin‐3‐yl)phenyl)acetamide], OR‐1855 [(R)‐6‐(4‐aminophenyl)‐5‐methyl‐4,5‐dihydropyridazin‐3(2H)‐one], dobutamine, and milrinone: comparative effects on peripheral resistance, cardiac output, dP/dt, pulse rate, and blood pressure. J Pharmacol Exp Ther 2008; 325: 331–340. [DOI] [PubMed] [Google Scholar]
- 57. Pagel PS, Hettrick DA, Warltier DC. Comparison of the effects of levosimendan, pimobendan, and milrinone on canine left ventricular‐arterial coupling and mechanical efficiency. Basic Res Cardiol 1996; 91: 296–307. [DOI] [PubMed] [Google Scholar]
- 58. Schwarte LA, Picker O, Bornstein SR, Fournell A, Scheeren TW. Levosimendan is superior to milrinone and dobutamine in selectively increasing microvascular gastric mucosal oxygenation in dogs. Crit Care Med 2005; 33: 135–142 discussion 246‐137. [DOI] [PubMed] [Google Scholar]
- 59. Grübler MR, Wigger O, Berger D, Blöchlinger S. Basic concepts of heart‐lung interactions during mechanical ventilation. Swiss Med Wkly 2017; 147: w14491. [DOI] [PubMed] [Google Scholar]
- 60. Noel‐Morgan J, Muir WW. Anesthesia‐associated relative hypovolemia: mechanisms, monitoring, and treatment considerations. Front Vet Sci. 2018; 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Uemura K, Sugimachi M, Kawada T, Kamiya A, Jin Y, Kashihara K, Sunagawa K. A novel framework of circulatory equilibrium. Am J Physiol Heart Circ Physiol 2004; 286: H2376–H2385. [DOI] [PubMed] [Google Scholar]
- 62. Fudim M, Boortz‐Marx RL, Ganesh A, DeVore AD, Patel CB, Rogers JG, Coburn A, Johnson I, Paul A, Coyne BJ, Rao SV, Gutierrez JA, Kiefer TL, Kong DF, Green CL, Jones WS, Felker GM, Hernandez AF, Patel MR. Splanchnic nerve block for chronic heart failure. JACC Heart Fail. 2020; 8: 742–752. [DOI] [PubMed] [Google Scholar]
- 63. Levine AR, Simon MA, Gladwin MT. Pulmonary vascular disease in the setting of heart failure with preserved ejection fraction. Trends Cardiovasc Med 2019; 29: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hansen MS, Andersen A, Nielsen‐Kudsk JE. Levosimendan in pulmonary hypertension and right heart failure. Pulm Circ. 2018; 8: 2045894018790905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cleland JG, Nikitin N, McGowan J. Levosimendan: first in a new class of inodilator for acute and chronic severe heart failure. Expert Rev Cardiovasc Ther 2004; 2: 9–19. [DOI] [PubMed] [Google Scholar]
- 66. Lilleberg J, Laine M, Palkama T, Kivikko M, Pohjanjousi P, Kupari M. Duration of the haemodynamic action of a 24‐h infusion of levosimendan in patients with congestive heart failure. Eur J Heart Fail 2007; 9: 75–82. [DOI] [PubMed] [Google Scholar]
- 67. Brener MI, Hamid NB, Sunagawa K, Borlaug BA, Shah SJ, Rich S, Burkhoff D. Changes in stressed blood volume with levosimendan in pulmonary hypertension from heart failure with preserved ejection fraction: insights regarding mechanism of action from the HELP trial. J Card Fail 2021. Jun 15; 27: 1023–1026. [DOI] [PubMed] [Google Scholar]
- 68. Mondéjar‐Parreño G, Cogolludo A, Perez‐Vizcaino F. Potassium (K(+)) channels in the pulmonary vasculature: implications in pulmonary hypertension physiological, pathophysiological and pharmacological regulation. Pharmacol Ther 2021; 225: 107835. [DOI] [PubMed] [Google Scholar]
- 69. Dogan MF, Yildiz O, Arslan SO, Ulusoy KG. Potassium channels in vascular smooth muscle: a pathophysiological and pharmacological perspective. Fundam Clin Pharmacol 2019; 33: 504–523. [DOI] [PubMed] [Google Scholar]


