Abstract
In heart failure (HF), acute decompensation can occur quickly and unexpectedly because of worsening of chronic HF or to new‐onset HF diagnosed for the first time (‘de novo’). Patients presenting with acute HF (AHF) have a poor prognosis comparable with those with acute myocardial infarction, and any delay of treatment initiation is associated with worse outcomes. Recent HF guidelines and recommendations have highlighted the importance of a timely diagnosis and immediate treatment for patients presenting with AHF to decrease disease progression and improve prognosis. However, based on the available data, there is still uncertainty regarding the optimal ‘time‐to‐treatment’ effect in AHF. Furthermore, the immediate post‐worsening HF period plays an important role in clinical outcomes in HF patients after hospitalization and is known as the ‘vulnerable phase’ characterized by high risk of readmission and early death. Early and intensive treatment for HF patients in the ‘vulnerable phase’ might be associated with lower rates of early readmission and mortality. Additionally, in the chronic stable HF outpatient, treatments are often delayed or not initiated when symptoms are stable, ignoring the risk for adverse outcomes such as sudden death. Consequently, there is a dire need to better identify HF patients during hospitalization and after discharge and treating them adequately to improve their prognosis. HF is an urgent clinical scenario along all its stages and disease conditions. Therefore, time plays a significant role throughout the entire patient's journey. Therapy should be optimized as soon as possible, because this is beneficial regardless of severity or duration of HF. Time lavished before treatment initiation is recognized as important modifiable risk factor in HF.
Keywords: Heart failure, Treatment, Prognosis
Introduction
Despite remarkable improvements in the management of cardiovascular diseases including heart failure (HF) during the last few decades, death due to cardiovascular diseases remains the leading global cause of mortality. 1 Cardiac events occur rapidly, and a significant number of patients die, rendering the prompt diagnosis and treatment into a challenging task to improve outcome. 1 , 2 Before the 1980s, ST‐elevation myocardial infarction (STEMI) management was based on relieving pain and heart rate reduction to decrease cardiac work and oxygen consumption. 3 , 4 Subsequently, a revolutionary concept known as ‘Time Is Muscle’ was proposed by Eugene Braunwald. 4 When this concept was introduced, it provided a paradigm shift in the management of acute coronary syndromes with STEMI. 3 , 4 , 5 , 6 Based on the concept that the delay between symptom onset and first balloon inflation affects myocardial perfusion and clinical outcomes, 4 , 5 , 6 many trials were prompted to judge quality of care as ‘door‐to‐needle time’ (thrombolysis) or ‘door‐to‐balloon time’ (coronary interventions). 7 This approach is widely accepted as standard of care of STEMI with timely treatment linked to improved outcomes. 7
Chronic HF (CHF) is widely regarded as a chronic condition, 8 with particularly high and consistent increase in all epidemiological parameters making it as one of the most rapidly growing cardiovascular (CV) conditions. This results in a substantial burden on healthcare systems worldwide. 8 , 9 The connotation of the term ‘chronic’ HF might suggest that this syndrome does not require urgent treatment and there could be time to weigh up benefit and potential risks of outcome‐improving drugs, to observe the natural course of symptoms and signs and to delay treatment initiation to the outpatient setting in a stable HF patient. 2 , 10 This approach does not take into consideration that an acute decompensation is associated with acute and potentially irreversible myocardial damage. 11 , 12 , 13 , 14 , 15 This damage is indicated by elevation of injury markers as seen in acute coronary syndromes, 13 , 16 , 17 , 18 with greater levels associated with worse outcomes. 18 After discharge following an episode of acute HF (AHF) worsening, high rates of rehospitalization and death have been recognized and the term ‘vulnerable phase’ of the HF syndrome was coined. 19 , 20 In these patients, outcome‐modifying and guideline‐recommended treatments often have not been introduced. This might be related to concerns, but not scrutiny, of intolerance to treatments or due to the ‘evidentio‐centric’ view that outcome trials are only performed in stable outpatients following a definite time after discharge from the hospital and because post‐discharge follow‐up has been delayed, neglected, and/or not occurred in setting with adequate HF expertise. Furthermore, in the stable CHF outpatient, with ‘provider inertia’, treatments are often not maximized when symptoms are stable, despite evidence supporting ongoing titration. 20 The earlier after an episode of decompensation, the higher risk of hospitalization or death. Herein, we focus on the time factor of treatment initiation from acute decompensation to the treatment of the stable CHF patient. Although the clinical status is on a continuum, we identify three distinct phases: AHF, vulnerable phase (before and early after discharge), and CHF.
In‐hospital treatment of patients with acute worsening of heart failure
Worsening of CHF accounts for 70–80% of those hospitalized with AHF, while only 20–30% have new‐onset or advanced HF. 8 , 9 Presently, hospitalization due to AHF is still associated with poor outcomes, with in‐hospital mortality of 4–6% and rehospitalization rates and 1 year mortality of 10–30% 8 , 9 (Figure 1 ).
Figure 1.
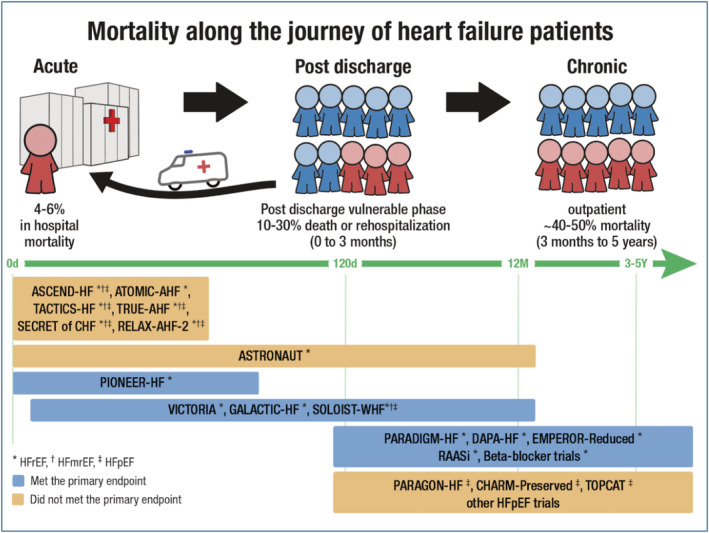
In‐hospital mortality is high after acute decompensation (left, 4–6%). In the post‐discharge phase, rehospitalization rate (depicted by arrow) and death are higher (10–30% death or rehospitalization) than in the chronic outpatient heart failure population (40–50% 5 year mortality). The figure shows landmark heart failure trials during the period where patients were included in those studies (in‐hospital and pre‐discharge, early post‐hospitalization—vulnerable phase, and in the chronic phase). Also, this figure shows which trial did or did not meet its primary endpoints. Data taken from several studies. 8 , 9 , 21 , 22 , 23 , 24 , 25 , 26 , 27
In AHF, pathophysiological pathways are activated, which may initially be compensatory to maintain haemodynamics acutely, but with time become maladaptive. 19 Among those, neurohormonal activation such as activation of the sympathetic nervous and renin–angiotensin–aldosterone system leading to congestion, myocardial inflammation, and cellular interstitial and myocyte remodelling might be important. 19 , 28 Volume overload and congestion cause signs and symptoms of AHF 9 , 28 but impose wall stress on the heart, thereby damaging cardiomyocytes directly in concert with neurohormonal activation, leading to extracellular matrix accumulation and myocyte apoptosis and necrosis. 17 , 18 , 19 Thus, recent HF guidelines and recommendations highlighted the importance of urgent diagnosis and immediate treatment of AHF; however, specific timelines were not mentioned. 12 , 13 , 14 , 15
Although an appropriate implementation is lacking, the concept of ‘door‐to‐diuretic’ (D2D) time is not novel. Around a decade ago, Maisel et al. first introduced the D2D time by analysing decompensated HF patients in the ED from the Acute Decompensated Heart Failure National Registry (ADHERE). 29 , 30 They demonstrated a significant association between each 4 h delay in initiation of diuretics and an increase in mortality. Another analysis from the same registry showed that hospital stay, need for intensive care unit, and symptoms at discharge were more prevalent in patients receiving late treatment. 10 , 31 However, these analyses were observational retrospective studies with no uniform estimation of the delay in treatment and of the therapy applied. 10 , 29 Approximately 20 years later, the first prospective registry to evaluate the association between time‐to‐diuretic treatment and clinical outcome was conducted. 11 In this analysis, 1291 patients with AHF presenting to the ED were included. The study assessed the association between D2D treatment and all‐cause in‐hospital mortality. The median time to intravenous diuretic was 90 min. The investigators then divided the subjects into early (<60 min) and later D2D times. Patients in the early treatment group presented with more prominent congestion and higher blood pressure than the later treated patients. In‐hospital mortality was significantly lower in the early treatment group (2.3% vs. 6.0% in the late treatment group; P = 0.002). In multivariate analysis, earlier treatment remained significantly associated with lower in‐hospital mortality (odds ratio: 0.39; 95% confidence interval: 0.20–0.76; P = 0.006). An optimal cut‐off of 100 min from ED arrival was determined for predicted mortality, which steeply increased in the first 100 min and levelled off afterwards. The same question was addressed later among 5625 consecutive patients enrolled in the KorAHF [Registry (Prospective Cohort) for Heart Failure in Korea] registry hospitalized for AHF. 32 Patients were divided into early (D2D time <60 min) and delayed (D2D time >60 min) groups. The primary outcomes were in‐hospital death and post‐discharge death at 1 month and 1 year on the basis of D2D time. Both groups had similar in‐hospital and post‐discharge outcomes. These results were not substantially changed by risk adjustment or propensity score matching to control for visible potential confounders. Very recently, the observational FAST‐FURO study showed that early intravenous furosemide administration by emergency medical services during the prehospital phase in AHF patients who will require intravenous furosemide treatment at the ED was not associated with changes in short‐term mortality or length of hospitalization after adjustment for several confounders. 33 However, the results of the previous studies should be evaluated carefully because of unavoidable confounding of observational data. Additionally, the complexity of HF diagnosis and different pathobiological mechanisms may lead to diverse presentation pictures. Notably, differences between studies results might be related to different medical practice and to varying densities of HF units and hospitals in different countries, and even in different regions within one country might affect mortality 2 , 34 , 35 (Figure 2 ).
Figure 2.
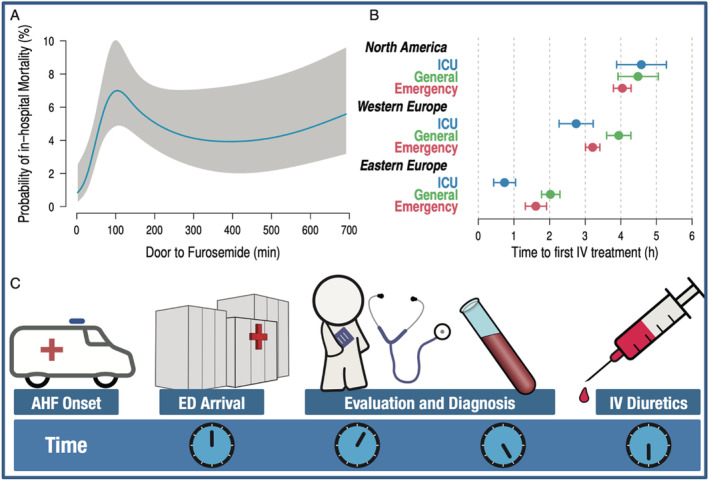
(A) Association of door‐to‐furosemide time with probability of in‐hospital mortality. (B) Duration to time‐to‐first intravenous (IV) diuretic treatment on the intensive care unit (ICU), general ward, or emergency ward in North America (upper panel) Western Europe (middle panel) and Eastern Europe (lower panel). (C) Recommendation for a timely evaluation, diagnosis, and IV start of diuretic treatments after arrival in the emergency department (ED) after onset of acute heart failure (AHF) symptoms. Data taken from Matsue et al. and Filippatos et al. 11 , 34
Hitherto, no specific therapy was established to target the pathophysiological process in AHF beyond timely decongestion. This was also shown by the neutral mortality result from TRUE‐AHF trial with ularitide 36 as well as from the RELAX‐HF trial with serelaxin, 37 where effects beyond decongestion with diuretics could not be scrutinized.
All these data make it hard to believe that shorter D2D time is analogue to door‐to‐balloon time in STEMI. In brief, there is still uncertainty regarding the optimal D2D effect in AHF and whether rapid clinical decongestion might improve prognosis besides improving symptoms.
Pre‐discharge and post‐hospitalization vulnerable phase: time matters!
As HF represents a huge cost burden for healthcare systems globally, there is a huge need to reduce hospitalizations due to readmissions. 1 , 9 The ASCEND‐HF registry studied patients with AHF according to variations of length of stay across countries. 38 It showed that readmission rates were significantly lower between patients treated in countries with longer lengths of stay for HF hospitalizations. After multivariable adjustment, each additional 1 day in the mean length of stay was independently associated with a lower risk of all‐cause readmission by 14% and HF readmission by 21%. 38 This is in line with the strategy to take the time to decongest the patients as a priority in order to reduce readmission and mortality. 39 Many analyses have shown that patients with severe symptoms (New York Heart Association IV) have a 50% mortality risk within 1–2 years compared with 20–30% for Class III symptoms. 39 , 40 Patients who were well decongested at discharge had an improved survival despite previous Class IV symptoms at admission. 39
Moreover, another strong predictor of mortality in HF patients is the number of previous HF hospitalizations. For example, 50% of patients die by 1 year after three hospitalizations. 41 , 42 This simple predictor of mortality in HF patients might help to triage patients to guide management and predict prognosis. 41 This in turn suggests that prevented hospitalizations could have an impact on subsequent mortality/hospitalizations. Although this concept needs to be scrutinized in adequately designed clinical trials, preventing HF hospitalization is an important therapeutic objective particularly in patients with CHF with reduced ejection fraction (HFrEF) as they still represent the majority of the HF population. 43 The risk of mortality and rehospitalization is clearly high in the early phase of hospitalization and remains elevated even after 18 months of discharge. 43 Based on the earlier, the immediate post‐discharge period plays an important role in clinical outcomes in HF patients after hospitalization and has been suggested to be the vulnerable phase, including patients at high risk of readmission and early death. 20 , 44 The most likely potential mechanism for the poor prognosis is the persistent subclinical or manifested congestion at discharge. 44 , 45 , 46 , 47 , 48 Therefore, there is a need to better identify these patients during hospitalization, to treat them intensively and early, on the basis this might be associated with lower rates of early readmission and mortality. 20 , 42 , 43 , 44 , 45
Initiation and optimization of guideline‐directed CHF therapy might be important for patients already at, or before discharge after hospitalization for HF worsening, to reduce early death and rehospitalization. However, randomized controlled clinical trials are usually performed in stable outpatients, thus not covering the vulnerable early post‐discharge phase with few exceptions 21 , 22 , 23 (Figure 1 ). The majority of clinical trials performed during pre‐discharge and post‐hospitalization phase showed neutral results that did not meet its primary endpoints (Figure 1 ). These are sometimes used to argue that early intervention may not be helpful in the setting of AHF. However, this is a misused argument and should not be extrapolated to argue that therapies with strong evidence for CHF that may not have been studied in AHF should not be started as early as possible. Undertreatment at discharge might be also due to the reluctance of physicians to introduce guideline‐recommended treatments as they reduce blood pressure and heart rate and as HF patients with low blood pressure have worse outcomes than those with higher blood pressure levels. 49 , 50 , 51 , 52 However, withholding of beta‐blocker therapy after discharge in decompensated HF patients has been shown to be associated with an increased risk of post‐discharge mortality. 53 , 54 Additionally, in euvolemic patients with symptoms at rest or on minimal exertion (New York Heart Association III–IV), the addition of carvedilol to conventional therapy ameliorates the severity of HF and reduces the risk of clinical deterioration, hospitalization, and other serious adverse clinical events. 55 Moreover, starting with HF oral therapy at hospital discharge is associated with a significantly better outcome of AHF patients regardless of associated co‐morbidities. 48 The GREAT registry 48 demonstrated that treatment with oral HF therapy including beta‐blockers, renin–angiotensin system inhibitors, and mineralocorticoid receptor antagonist in the hospital and before discharge provided better outcomes compared with deferring the treatment initiation in post‐AHF patients.
Furthermore, optimizing HF therapy during hospitalization covering the vulnerable phase early after discharge improves outcomes. 20 CV death or hospitalizations for worsening HF increased by 3% with every beat per minute (b.p.m.) increase from baseline heart rate and 16% for every 5 b.p.m. increase with a direct association between lower heart rate achieved after treatment initiation at 28 days and subsequently reduced cardiac outcomes. 56 In agreement with this, initiation of ivabradine and beta‐blockers during AHF hospitalization leads to improved haemodynamics by a sufficient decrease in heart rate 56 , 57 , 58 and subsequently improves clinical parameters of HF patients at short term. 57 , 58 The PIONEER‐HF trial included patients during hospitalization for acute decompensated HF. Sacubitril/valsartan led to a greater unloading of the heart suggested by a stronger reduction of N‐terminal pro‐brain natriuretic peptide concentration and a reduction of exploratory outcomes (HF rehospitalizations, death, and heart transplantation) compared with enalapril therapy without safety concerns. 22 Risk reduction was present as early as 1 week after drug initiation in enalapril‐pretreated and naïve patients. 22 Notably, this trial was not powered for clinical endpoints but still provides signals for risk reduction. Data pointing in the same direction are available for other HF drugs. Very recently, the SOLOIST‐WHF trial showed that patients with diabetes and recent worsening HF were treated with sotagliflozin, which was initiated before or shortly after discharge resulting in a significantly lower total number of CV deaths and HF hospitalizations compared with placebo. 23 All these data suggest to initiate medical therapy early and probably before discharge, or as soon as possible during the following outpatient visits. However, as other large trials did not explore treatments starting in the hospital patients, most recent HF guidelines and recommendations are reluctant to recommend introduction of newly introduced oral HF therapies shortly after decompensation and during hospitalization, although continuation of introduced drugs during hospitalization and at hospital discharge is recommended. 12 , 15 , 53 This creates sometimes uncertainties of when to start in recompensated patients on incomplete therapies.
Chronicity of heart failure and outcomes
At the present time, optimization of guideline‐directed chronic HF therapy remains the only definitive therapy for HFrEF patients to reduce early death and hospitalization. It is a matter of debate whether patients with longer duration of CHF are those who are better tolerating this condition or respond better to therapy and have prognosis or whether they are representing patients with long‐standing clinical signs and symptoms with more advanced disease, higher non‐cardiac co‐morbidity load, and poorer outcomes. Data from the SHIFT trial have shown that the duration between the onset of HF signs and symptoms to treatment initiation has a substantial effect on clinical outcomes. 59 Patients with worsening CHF and long‐standing symptoms, requiring mechanical circulatory support, had worse outcomes compared with acute or sub‐acute HF. 60 , 61 In turn, better clinical outcomes were observed in HF patients receiving cardiac resynchronization therapy early after developing symptoms compared with those with longer symptoms duration. 62
Heart failure is associated with a high burden of cardiac and non‐cardiac co‐morbidities, 8 , 16 and HF patients with co‐morbidities such as chronic kidney disease or diabetes are at higher risk. 8 , 12 It has been demonstrated that a longer duration of CHF was associated with a higher burden of co‐morbidities 59 and the number of co‐morbidities was associated with worse outcomes. 60 This question has recently been confirmed in a post hoc analysis from DAPA‐HF trial. 24 , 63 In this analysis, patients with longer HF duration had higher rates of worsening HF and death. Additionally, these patients were older with more co‐morbidities. Interestingly, patients with a longer HF duration in SHIFT 59 and DAPA‐HF 24 had a similar relative risk reduction with ivabradine and dapagliflozin, respectively, and a greater absolute risk reduction, compared with those with more recently diagnosed HF. 24 , 59 For these reasons, HF therapy should be optimized as soon as possible, as those with a longer HF duration might benefit at least as well. This might apply not only to drug therapies but also to implantable cardioverter defibrillator as even in later stages the risk of sudden cardiac death remained the same with shorter or longer duration of HF. 64
There is an emerging discipline of ‘implementation science’ founded on the idea that advances and positive trials in HF have little meaning if not widely implemented. Treatment of HFrEF has become complex, and few patients get the opportunity to benefit from the wide array of treatments indicated in all or some patients. These include the four foundational HFrEF drug classes that were effective in reducing morbidity and mortality even at low starting doses, namely, angiotensin receptor neprilysin inhibitor, beta‐blockers, mineralocorticoid receptor antagonist, and sodium–glucose co‐transporter‐2 inhibitors. 65 Notably, a significant benefit of these foundational treatments was apparent within the first 30 days after randomization, 66 , 67 , 68 and subsequent therapy with all four drug classes should therefore be achieved within 4 weeks. 65 Furthermore, an additional approach, which might play an important role in saving time in all HF outpatients, is hemodynamically guided pharmacotherapy with devices such as the pulmonary artery pressure sensor, which has been shown to reduced HF hospitalization risk, irrespective of left ventricular ejection fraction. 65 , 69 , 70
This complex array of therapy combined of medications and devices is at odds with how HF care is organized in many countries, with delays in follow‐up and poor access to cardiology specialists. 71 , 72 Indeed, emerging studies are showing that the main factors associated with non‐use of evidence‐based HFrEF therapy include not only higher age and co‐morbidities but also acute care and chronic follow‐up by non‐cardiologists, lower socio‐economic status as measured by education, income and marital status, and care provided in smaller town, rural, and non‐university medical centre settings. 71 , 72
Conclusion and future directions
Heart failure is an urgent clinical scenario along all its stages and disease conditions. Therefore, rapid management plays a significant role throughout the entire patient's journey. Prompt recognition of AHF patients in the ED and early initiation of therapy is in our view a mandatory step for improving in‐hospital outcomes in acutely decompensated patients (Figure 3 ). Moreover, oral HF therapy should be started and optimized as early as possible after recompensation of patients and likely even before discharge, regardless of severity or duration of HF. Improving strategies to identify patients at risk, optimize HF therapy, and develop follow‐up methods is a beneficial, productive, and cost‐effective approach to reduce economic burden of HF and improve outcomes. Time lavished before treatment initiation is recognized as important modifiable risk factor in HF! This is not really a very novel wisdom:
Figure 3.
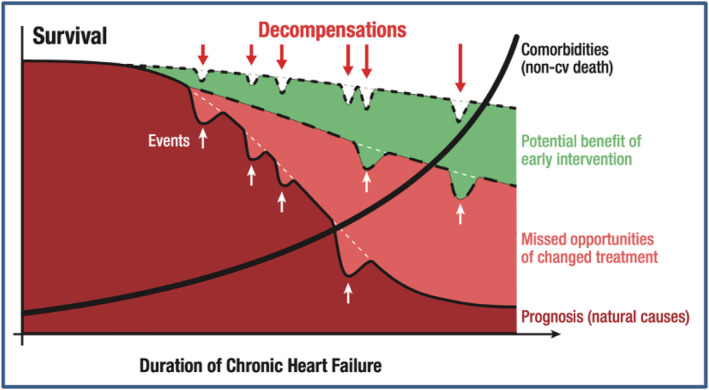
A schematic scheme of the association of outcomes, treatment initiation, missed opportunities, and potential benefits of early intervention in heart failure patients. Data taken from Böhm et al. 59
Defer no time, delays have dangerous ends. 25
Conflict of interest
A.A. received speaker's honoraria from Novartis and travel grants from Biotronik and Novartis. S.D.A. received honoraria for lectures and scientific advice from Vifor, Bayer, Boehringer Ingelheim, Novartis, Servier, Abbott, Actimed, Cardiac Dimensions, and Impulse Dynamics. J.B. received honoraria for lectures and scientific advice from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Novartis, BI‐Lilly, and Janssen. A.J.S.C. received grants and personal fees from Vifor International and personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Gore, Impulse Dynamics, and Respicardia. I.K. reports personal fees from Akcea Therapeutics Germany GmbH, AstraZeneca, Bayer Vital GmbH, Boehringer Ingelheim, Bristol Myers Squibb Company, Hexal AG, Novartis, Pfizer Pharma GmbH, Servier Deutschland GmbH, Vifor, and Daiichi Sankyo Deutschland GmbH. M.L. reports personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Vifor Pharma, and Novartis. L.H.L. received grants and personal fees from Relypsa, Boehringer Ingelheim, and Novartis, grants from Boston Scientific, and personal fees from Merck, Vifor Fresenius, AstraZeneca, Bayer, Pharmacosmos, Abbott, Medscape, Myokardia, Sanofi, Lexicon, and Mundipharma. M.M. received personal fees and non‐financial support from Amgen, Abbott Vascular, and Bayer and personal fees from Servier, AstraZeneca, Edwards Therapeutics, Vifor Pharma, Actelion, LivaNova, and Windtree Therapeutics. W.M. received research grants from Novartis, Vifor, Medtronic, Biotronik, Abbott, and Boston Scientific. M.B. reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Medtronic, Novartis, Servier, and Vifor.
Funding
I.K. and M.B. are supported by the German Research Foundation (Deutsche Forschungsgemeinschaft; TTR 219, Project No. 322900939). M.L. was funded by the Slovenian Research Agency (Javna Agencija za Raziskovalno Dejavnost RS; Research Grant Nos. J3‐9292 and J3‐9284).
Acknowledgement
We are grateful to Armin Schweitzer for his technical and artwork help.
Open access funding enabled and organized by Projekt DEAL.
Abdin, A. , Anker, S. D. , Butler, J. , Coats, A. J. S. , Kindermann, I. , Lainscak, M. , Lund, L. H. , Metra, M. , Mullens, W. , Rosano, G. , Slawik, J. , Wintrich, J. , and Böhm, M. (2021) ‘Time is prognosis’ in heart failure: time‐to‐treatment initiation as a modifiable risk factor. ESC Heart Failure, 8: 4444–4453. 10.1002/ehf2.13646.
References
- 1. McClellan M, Brown N, Califf RM, Warner JJ. Call to action: urgent challenges in cardiovascular disease: a presidential advisory from the American heart association. Circulation 2019; 139: e44–e54. [DOI] [PubMed] [Google Scholar]
- 2. Felker GM, Januzzi JL. "Time is muscle" in acute heart failure: critical concept or fake news? ACC Heart Fail 2018; 6: 295–297. [DOI] [PubMed] [Google Scholar]
- 3. Gibson M, De Lemos J, Antman E, TIMI Study Group . Time is muscle in primary PCI: the strength of the evidence grows. Eur Heart J 2004; 25: 1001–1002. [DOI] [PubMed] [Google Scholar]
- 4. Braunwald E. Evolution of the management of acute myocardial infarction: a 20th century saga. Lancet 1998; 352: 1771–1774. [DOI] [PubMed] [Google Scholar]
- 5. DeWood MA, Spores J, Notske R, Golden MS, Lang HT. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N England J Med 1980; 303: 897–902. [DOI] [PubMed] [Google Scholar]
- 6. Maroko PR, Kjekshus JK, Sobel BE, Watanabe T, Covell JW, Ross J Jr, Braunwald E. Factors influencing infarct size following experimental coronary artery occlusions. Circulation 1971; 43: 67–82. [DOI] [PubMed] [Google Scholar]
- 7. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio A, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J 2018; 39: 119–177.28886621 [Google Scholar]
- 8. Metra M, Teerlink J. Heart failure. Lancet. 2017; 390: 1981–1995. [DOI] [PubMed] [Google Scholar]
- 9. Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, Mebazaa A. Acute heart failure. Nat Rev Dis Primers 2020; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piazza V. ‘Door‐to‐furosemide’ timing: early treatment of heart failure. Eur Heart J Supplements 2019; 21: B69–B70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, Okumura T, Kida K, Mizuno A, Oishi S, Inuzuka Y, Akiyama E, Matsukawa R, Kato K, Suzuki S, Naruke T, Yoshioka K, Miyoshi T, Baba Y, Yamamoto M, Murai K, Mizutani K, Yoshida K, Kitai T. Time‐to‐furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017; 69: 3042–3051. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anke SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano G, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members, Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, deFilippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray J, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite‐Moreira AF, Bellou A, Anker SD, Filippatos G. Recommendations on pre‐hospital & early hospital management of acute heart failure: a consensus paper from the heart failure association of the European society of cardiology, the European society of emergency medicine and the society of academic emergency medicine. Eur J Heart Fail 2015; 17: 544–558. [DOI] [PubMed] [Google Scholar]
- 14. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐La Rocca HP, Martens P, Testani JM, Tang W, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJS. The use of diuretics in heart failure with congestion ‐ a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 137–155. [DOI] [PubMed] [Google Scholar]
- 15. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, De Boer DA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 1169–1186. [DOI] [PubMed] [Google Scholar]
- 16. Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L, Heart Failure Association of the European Society of Cardiology (HFA) . EURObservational research programme: the heart failure pilot survey (ESC‐HF Pilot). Eur J Heart Fail 2010; 12: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 17. Perna ER, Macin SM, Canella JP, Augier N, Stival JLR, Cialzeta JR, Pitzus AE, Garcia EH, Obregón R, Brizuela M, Barbagelata A. Ongoing myocardial injury in stable severe heart failure: value of cardiac troponin T monitoring for high‐risk patient identification. Circulation 2004; 110: 2376–2382. [DOI] [PubMed] [Google Scholar]
- 18. Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner‐La Rocca HP, Chioncel O, Collins SP, Doehner W, Filippatos GS, Flammer AJ, Fuhrmann V, Lainscak M, Lassus J, Legrand M, Masip J, Mueller C, Papp Z, Parissis J, Platz E, Rudiger A, Ruschitzka F, Schäfer A, Seferovic PM, Skouri H, Yilmaz MB, Mebazaa A. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the acute heart Failure committee of the heart failure association (HFA) of the European society of cardiology (ESC). Eur J Heart Fail 2017; 19: 821–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol 2012; 21: 365–371. [DOI] [PubMed] [Google Scholar]
- 20. Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015; 12: 220–229. [DOI] [PubMed] [Google Scholar]
- 21. Gheorghiade M, Böhm M, Greene MDSJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP, ASTRONAUT Investigators and Coordinators . Effect of Aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure. The ASTRONAUT randomized trial. JAMA 2013; 309: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 22. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER‐HF Investigators . Angiotensin–Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 23. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B, SOLOIST‐WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 24. Yeoh SE, Dewan P, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Sjöstrand M, Langkilde AM, McMurray JJ, DAPA‐HF Investigators and Committees . Patient characteristics, clinical outcomes, and Effect of dapagliflozin in relation to duration of heart failure: is it ever too late to start a new therapy? Circ Heart Fail. 2020; 13: e007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shakespeare W, Alexander P, Henry VI. Part 1. London: British broadcasting co, 1983. [Google Scholar]
- 26. Hamo CE, O'Connor C, Metra M, Udelson JE, Gheorghiade M, Butler J. A Critical Appraisal of Short‐Term End Points in Acute Heart Failure Clinical Trials. J Card Fail 2018; 24: 783–792. [DOI] [PubMed] [Google Scholar]
- 27. Upadhya B, Kitzman DW. Heart failure with preserved ejection fraction: New approaches to diagnosis and management. Clin Cardiol 2020; 43: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mentz RJ, O'Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol 2016; 13: 28–35. [DOI] [PubMed] [Google Scholar]
- 29. Januzzi JL, Felker GM. Door‐to‐furosemide therapy in the ED: new quality metric or just a piece of the puzzle? J Am Coll Cardiol 2017; 69: 3052–3054. [DOI] [PubMed] [Google Scholar]
- 30. Maisel AS, Peacock WF, McMullin N, Jessie R, Fonarow GC, Wynne J, Mills RM. Timing of immunoreactive B‐type natriuretic peptide levels and treatment delay in acute decompensated heart failure. J Am Coll Cardiol 2008; 52: 534–540. [DOI] [PubMed] [Google Scholar]
- 31. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail 2009; 15: 256–264. [DOI] [PubMed] [Google Scholar]
- 32. Park JJ, Kim S‐H, Oh I‐Y, Choi DJ, Park HA, Cho HJ, Lee HY, Cho JY, Kim KH, Son JW, Yoo BS, Oh J, Kang SM, Baek SH, Lee GY, Choi JO, Jeon ES, Lee SE, Kim JJ, Lee JH, Cho MC, Jang SY, Chae SC, Oh BH. The effect of door‐to‐ diuretic time on clinical outcomes in acute heart failure patients. J Am Coll Cardiol HF 2018; 6: 286–294. [DOI] [PubMed] [Google Scholar]
- 33. Miró O, Harjola P, Rossello X, Gil V, Jacob J, Llorens P, Martín‐Sánchez FJ, Herrero P, Martínez‐Nadal G, Aguiló S, López‐Grima ML, Fuentes M, Álvarez Pérez JM, Rodríguez‐Adrada E, Mir M, Tost J, Llauger L, Ruschitzka F, Harjola VP, Mullens W, Masip J, Chioncel O, Peacock WP, Müller C, Mebazaa A, ICA‐SEMES Research Group . The FAST‐FURO study: effect of very early administration of intravenous furosemide in the prehospital setting to patients with acute heart failure attending the emergency department. Eur Heart J Acute Cardiovasc Care. 2021: zuaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filippatos G, Angermann CE, Cleland JG, Lam CSP, Dahlström U, Dickstein K, Ertl G, Hassanein M, Hart KW, Lindsell CJ, Perrone SV, Guerin T, Ghadanfar M, Schweizer A, Obergfell A, Collins SP. Global differences in characteristics, precipitants, and initial management of patients presenting with acute heart failure. JAMA Cardiol 2020; 5: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holstiege J, Akmatov MK, Störk SA, Bätzing J. Higher prevalence of heart failure in rural regions: a population‐based study covering 87% of German inhabitants. Clin Res Cardiol 2019; 108: 1102–1106. [DOI] [PubMed] [Google Scholar]
- 36. Packer M, O'Connor C, McMurray JJ, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister H, TRUE‐AHF Investigators . Effect of Ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 2017; 376: 1956–1964. [DOI] [PubMed] [Google Scholar]
- 37. Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Voors AA, Adams KF, Anker SD, Arias‐Mendoza A, Avendaño P, Bacal F, Böhm M, Bortman G, Cleland JGF, Cohen‐Solal A, Crespo‐Leiro MG, Dorobantu M, Echeverría LE, Ferrari R, Goland S, Goncalvesová E, Goudev A, Køber L, Lema‐Osores J, Levy PD, McDonald K, Manga P, Merkely B, Mueller C, Pieske B, Silva‐Cardoso J, Špinar J, Squire I, Stępińska J, Van Mieghem W, von Lewinski D, Wikström G, Yilmaz MB, Hagner N, Holbro T, Hua TA, Sabarwal SV, Severin T, Szecsödy P, Gimpelewicz C, RELAX‐AHF‐2 Committees Investigators . Effects of Serelaxin in patients with acute heart failure. N Engl J Med 2019; 381: 716–726.31433919 [Google Scholar]
- 38. Eapen ZJ, Reed SD, Li Y, Kociol RD, Armstrong PW, Starling RC, McMurray JJ, Massie BM, Swedberg K, Ezekowitz JA, Fonarow GC, Teerlink JR, Metra M, Whellan DJ, O'Connor CM, Califf RM, Hernandez AF. Do countries or hospitals with longer hospital stays for acute heart failure have lower readmission rates? findings from ASCEND‐HF. Circ Heart Fail 2013; 6: 727–732. [DOI] [PubMed] [Google Scholar]
- 39. Lucas C, Johnson W, Hamilton MA, Woo MA, Flavell CM, Creaser JA, Stevenson LW. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J 2000; 140: 840–847. [DOI] [PubMed] [Google Scholar]
- 40. Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M. A comparison of enalapril with hydralazine‐isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325: 303–310. [DOI] [PubMed] [Google Scholar]
- 41. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007; 154: 260–266. [DOI] [PubMed] [Google Scholar]
- 42. Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow‐up and 30‐day readmission among medicare beneficiaries hospitalized for heart failure. JAMA 2010; 303: 1716–1722. [DOI] [PubMed] [Google Scholar]
- 43. Abrahamsson P, Swedberg K, Borer JS, Böhm M, Kober L, Komajda M, Lloyd SM, Metra M, Tavazzi L, Ford I. Risk following hospitalization in stable chronic systolic heart failure. Eur J Heart Fail 2013; 15: 885–891. [DOI] [PubMed] [Google Scholar]
- 44. Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation 2012; 126: 501–506. [DOI] [PubMed] [Google Scholar]
- 45. O'Connor CM, Miller AB, Blair JE, Konstam MA, Wedge P, Bahit MC, Carson P, Haass M, Hauptman PJ, Metra M, Oren RM, Patten R, Piña I, Roth S, Sackner‐Bernstein JD, Traver B, Cook T, Gheorghiade M. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J 2010; 159: 841–849. [DOI] [PubMed] [Google Scholar]
- 46. Yancy CW, Lopatin M, Stevenson LW, Marco TD, Fonarow GC, ADHERE Scientific Advisory Committee and Investigators . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the acute decompensated heart failure national registry (ADHERE) database. J Am Coll Cardiol 2006; 47: 76–84. [DOI] [PubMed] [Google Scholar]
- 47. Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J, ADHERE Scientific Advisory Committee and Investigators , ADHERE Study Group . In‐hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the acute decompensated heart failure national registry (ADHERE). J Am Coll Cardiol 2005; 46: 57–64. [DOI] [PubMed] [Google Scholar]
- 48. Gayat E, Arrigo M, Littnerova S, Sato N, Parenica J, Ishihara S, Spinar J, Müller C, Harjola VP, Lassus J, Miró O, Maggioni AP, AlHabib KF, Choi DJ, Park JJ, Zhang Y, Zhang J, Januzzi JL Jr, Kajimoto K, Cohen‐Solal A, Mebazaa A, GREAT Network . Heart failure oral therapies at discharge are associated with better outcome in acute heart failure: a propensity‐score matched study. Eur J Heart Fail. 2018; 20: 345–354. [DOI] [PubMed] [Google Scholar]
- 49. Böhm M, Ewen S. Blood pressure risk associations in heart failure: True effects or inverse causality? JACC Heart Fail 2017; 5: 820–822. [DOI] [PubMed] [Google Scholar]
- 50. Ambrosy AP, Vaduganathan M, Mentz RJ, Greene SJ, Subačius H, Konstam MA, Maggioni AP, Swedberg K, Gheorghiade M. Clinical profile and prognostic value of low systolic blood pressure in patients hospitalized for heart failure with reduced ejection fraction: insights from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial. Am Heart J 2013; 165: 216–225. [DOI] [PubMed] [Google Scholar]
- 51. Gheorghiade M, Vaduganathan M, Ambrosy A, Böhm M, Campia U, Cleland JC, Fedele F, Fonarow GC, Maggioni AP, Mebazaa A, Mehra M, Metra M, Nodari S, Pang PS, Ponikowski P, Sabbah HN, Komajda M, Butler J. Current management and future directions for the treatment of patients hospitalized for heart failure with low blood pressure. Heart Fail Rev. 2013; 18: 107–122. [DOI] [PubMed] [Google Scholar]
- 52. Komajda M, Böhm M, Borer JS, Ford I, Robertson M, Manolis AJ, Tavazzi L, Swedberg K, Investigators SHIFT. Efficacy and safety of ivabradine in patients with chronic systolic heart failure according to blood pressure level in SHIFT. Eur J Heart Fail 2014; 16: 810–816. [DOI] [PubMed] [Google Scholar]
- 53. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Am Coll Cardiol 2016; 68: 1476–1488. [DOI] [PubMed] [Google Scholar]
- 54. Böhm M, Link A, Cai D, Nieminen MS, Filippatos GS, Salem R, Solal AC, Huang B, Padley RJ, Kivikko M, Mebazaa A. Beneficial association of β‐blocker therapy on recovery from severe acute heart failure treatment: data from the survival of patients with acute heart failure in need of intravenous inotropic support trial. Crit Care Med 2011; 39: 940–944. [DOI] [PubMed] [Google Scholar]
- 55. Packer M, Fowler MB, Roecker EB, Coats AJS, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann‐Zalan I, DeMets DL, Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group . Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002; 106: 2194–2199. [DOI] [PubMed] [Google Scholar]
- 56. Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L, SHIFT Investigators . Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet. 2010; 376: 886–894. [DOI] [PubMed] [Google Scholar]
- 57. Komajda M, Tavazzi L, Swedberg K, Böhm M, Borer JS, Moyne A, Ford I, SHIFT Investigators . Chronic exposure to ivabradine reduces readmissions in the vulnerable phase after hospitalization for worsening systolic heart failure: a post‐hoc analysis of SHIFT. Eur J Heart Fail. 2016; 18: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 58. Hidalgo FJ, Anguita M, Castillo JC, Rodríguez S, Pardo L, Durán E, Sánchez JJ, Ferreiro C, Pan M, Mesa D, Delgado M, Ruiz M. Effect of early treatment with ivabradine combined with beta‐blockers versus beta‐blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC‐AHF): A randomised study. Int J Cardiol 2016; 217: 7–11. [DOI] [PubMed] [Google Scholar]
- 59. Böhm M, Komajda M, Borer JS, Ford I, Maack C, Tavazzi L, Moyne A, Swedberg K, SHIFT Investigators . Duration of chronic heart failure affects outcomes with preserved effects of heart rate reduction with ivabradine: findings from SHIFT. Eur J Heart Fail. 2018; 20: 373–381. [DOI] [PubMed] [Google Scholar]
- 60. Böhm M, Robertson M, Ford I, Borer JS, Komajda M, Kindermann I, Maack C, Lainscak M, Swedberg K, Tavazzi L. Influence of cardiovascular and noncardiovascular co‐morbidities on outcomes and treatment effect of heart rate reduction with ivabradine in stable heart failure (from the SHIFT Trial). Am J Cardiol 2015; 116: 1890–1897. [DOI] [PubMed] [Google Scholar]
- 61. Loyaga‐Rendon RY, Acharya D, Pamboukian SV, Tallaj JA, Cantor R, Starling RC, Naftel DC, Kirklin JK. Duration of heart failure is an important predictor of outcomes after mechanical circulatory support. Circ Heart Fail 2015; 8: 953–959. [DOI] [PubMed] [Google Scholar]
- 62. Verbrugge FH, Dupont M, Vercammen J, Jacobs L, Verhaert D, Vandervoort P, Tang WHW, Mullens W. Time from emerging heart failure symptoms to cardiac resynchronization therapy: impact on clinical response. Heart 2013; 99: 314–319. [DOI] [PubMed] [Google Scholar]
- 63. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA‐HF Trial Committees and Investigators , DAPA‐HF trial committees and investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 64. Elming MB, Thøgersen AM, Videbæk L, Bruun NE, Eiskjær H, Haarbo J, Egstrup K, Gustafsson F, Hastrup Svendsen J, Høfsten DE, Pehrson S, Nielsen JC, Køber L, Thune JJ. Duration of heart failure and effect of defibrillator implantation in patients with nonischemic systolic heart failure. Circ Heart Fail 2019; 12: e006022. [DOI] [PubMed] [Google Scholar]
- 65. McMurray JJ, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction? A redefinition of evidence‐based medicine. Circulation 2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 66. Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, Aschermann M, van Veldhuisen DJ, Zannad F, Krum H, Mukherjee R, Vincent J, EPHESUS Investigators . Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005; 46: 425–431. [DOI] [PubMed] [Google Scholar]
- 67. Packer M, McMurray JJ, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Bělohlávek J, Böhm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzàlez‐Medina A, Hagège AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan Ö, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz‐Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr, Silva‐Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC, PARADIGM‐HF Investigators and Coordinators . Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131: 54–61. [DOI] [PubMed] [Google Scholar]
- 68. Berg DD, Jhund PS, Docherty KF, Murphy SA, Verma S, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sjöstrand M, Solomon SD, McMurray JJ, Sabatine MS. Time to clinical benefit of dapagliflozin and significance of prior heart failure hospitalization in patients with heart failure with reduced ejection fraction. JAMA Cardiol 2021. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet 2016; 387: 453–461. [DOI] [PubMed] [Google Scholar]
- 70. Angermann CE, Assmus B, Anker SD, Anker SD, Asselbergs FW, Brachmann J, Brett ME, Brugts JJ, Ertl G, Ginn G, Hilker L, Koehler F, Rosenkranz S, Zhou Q, Adamson PB, Böhm M, MEMS‐HF Investigators . Pulmonary artery pressure‐guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European Monitoring Study for Heart Failure (MEMS‐HF). Eur J Heart Fail 2020; 22: 1891–1901. [DOI] [PubMed] [Google Scholar]
- 71. Fu M, Vedin O, Svennblad B, Lampa E, Johansson D, Dahlström U, Lindmark K, Vasko P, Lundberg A, Costa‐Scharplatz M, Lund LH. Implementation of sacubitril/valsartan in Sweden: clinical characteristics, titration patterns, and determinants. ESC Heart Fail 2020; 7: 3633–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano G, Dahlström U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2018; 20: 1326–1334. [DOI] [PubMed] [Google Scholar]


