Abstract
Acanthamoeba keratitis is a vision-threatening infection caused by pathogenic species of the genus Acanthamoeba. Although not all Acanthamoeba spp. can cause keratitis, it is important to differentiate pathogenic species and isolates from nonpathogens. Since extracellular proteases may play a role in ocular pathology, we used colorimetric, cytopathic, and zymographic assays to assess extracellular protease activity in pathogenic and nonpathogenic Acanthamoeba. Colorimetric assays, using azo-linked protein as a substrate, showed extracellular protease activity in Acanthamoeba-conditioned medium and differentiated pathogenic and nonpathogenic Acanthamoeba. Monolayers of immortalized corneal epithelial cells in four-well plates were used for cytopathic effect (CPE) assays. Pathogenic Acanthamoeba isolates exhibited marked CPE on immortalized corneal epithelial cells, while nonpathogenic isolates did not exhibit CPE. Protease zymography was performed with Acanthamoeba-conditioned medium as well as with Acanthamoeba- plus epithelial-cell-conditioned medium. The zymographic protease assays showed various banding patterns for different strains of Acanthamoeba. In pathogenic Acanthamoeba isolates, all protease bands were inhibited by phenylmethylsulfonyl fluoride (PMSF), suggesting serine type proteases, while in nonpathogenic strains only partial inhibition was observed by using PMSF. The pathogenic Acanthamoeba strains grown under typical laboratory conditions without epithelial cells exhibited one overexpressed protease band of 107 kDa in common; this protease was not observed in nonpathogenic Acanthamoeba strains. The 107-kDa protease exhibited activity over a pH range of 5 to 9.5.
Acanthamoeba spp. are a group of free-living amoebae which are widely distributed in soil, fresh water, marine environments, swimming pools, heating and cooling ducts, water supplies, and even eye wash stations (1). These amoebae can survive adverse conditions by forming resistant cysts which are impervious to inorganic chlorine up to 50 ppm (14). Trophozoites (vegetative cells) are sensitive to chlorine at 2 ppm, but even this concentration is well in excess of that achieved in public water supplies (<1 ppm) (14). Given access, many Acanthamoeba species will colonize human tissue. Most cases of acanthamoebiasis are associated with eye keratitis principally related to contact lens wear through application of lenses contaminated with Acanthamoeba (7, 10). Acanthamoeba infections are relatively infrequent; over the past 15 years an increase in the number of Acanthamoeba infections has been observed. This likely reflects the increased use of contact lenses (16, 17); estimates suggest that there are over 25,000,000 contact lens wearers in the United States alone (15).
A classification of Acanthamoeba based on morphological characteristics is still in use (16). Attempts to correlate pathogenicity with species by using this scheme have proven difficult and thus presents a problem for clinical diagnosis. For this reason, there is a need to develop assays that can be used to differentiate between pathogenic and nonpathogenic isolates of Acanthamoeba.
Intracellular and extracellular proteases have been reported from a number of other protozoa (8). Amoebic proteases are important in tissue invasion, migration, and host pathology (9, 10). Extracellular proteases from trophozoites of Acanthamoeba induced damage to collagen shields in an in vitro and an in vivo rat cornea model (5).
In this study, we have attempted to show that extracellular proteases of Acanthamoeba are markers for differentiating pathogenic from nonpathogenic organisms.
MATERIALS AND METHODS
Culture of amoeba.
All reagents were obtained from Sigma (St. Louis, Mo.). All species and isolates of Acanthamoeba (Table 1) were grown axenically in PYG medium (Proteose Peptone, 0.75% [wt/vol]; yeast extract, 0.75% [wt/vol]; glucose, 1.5% [wt/vol]) at 30°C without shaking. Immortalized corneal epithelial cells, kindly provided by D. Walton (Department of Medicine, University of Hull, Hull, HU6 7RX, United Kingdom), were grown in minimum essential medium (MEM; Sigma catalog no. M3786) without serum at 37°C in 5% CO2.
TABLE 1.
Acanthamoeba strains tested in the present study
| Designation | Species | Straina | Sourceb | Pathogenicity tested by CPE assays |
|---|---|---|---|---|
| Sp1 | Acanthamoeba sp. | PHLS | Keratitis, U.K. | Pathogen |
| Sp2 | Acanthamoeba sp. | PHLS | Keratitis, U.K. | Pathogen |
| Sp3 | A. castellanii | ATCC 30234 | Fresh water, U.S. | Pathogen |
| Sp4 | Acanthamoeba sp. | PHLS | Keratitis, U.K. | Pathogen |
| Sp5 | A. palestinensis | CCAP 1547/1 | Soil, Israel (1933) | Nonpathogen |
| Sp6 | A. astronyxis | CCAP 1534/1 | Fresh water, U.S. (1944) | Nonpathogen |
| Sp7 | A. polyphaga | CCAP 1501/3C | Fresh water, U.S. (1967) | Nonpathogen |
| Sp8 | A. royreba | CCAP 1501/7 | BeWo tissue culture, U.S. (1977) | Nonpathogen |
| Sp9 | A. griffini | ATCC 30731 | Marine, U.S. | Unknown |
PHLS, Public Health Laboratory Services, Leicester, United Kingdom; CCAP, Culture Collection of Algae and Protozoa, Ambleside, Cumbria, United Kingdom; UK. ATCC, American Type Culture Collection, Manassas, Va.
U.K., United Kingdom; U.S., United States.
For the assays of extracellular protease activity, cytopathic effect (CPE), and zymography, Acanthamoeba organisms (106) were incubated in MEM without serum at 37°C in a 5% CO2 incubator for 24 h. Acanthamoeba organisms were removed from the medium by centrifugation (100 × g for 5 min), and the supernatant, termed Acanthamoeba-conditioned medium (ACM), was used for the assays. UV-killed Acanthamoeba organisms (Acanthamoeba organisms treated with UV for 1 h) were used to determine if internal proteases were released from cells during the centrifugation process. The viability of UV-killed Acanthamoeba organisms was assayed by culturing them in PYG medium.
Protease assays.
Protease activity in conditioned medium was determined by a colorimetric method (13). Briefly, 200 μl of 1-mg ml−1 azocasein or azoalbumin was incubated with 100 μl of ACM for 60 min. Reactions were stopped by adding 10% trichloroacetic acid (TCA) and shaking, mixtures were left for 15 min and then centrifuged, and finally, 1 ml of supernatant was added to 1 ml of 1 M NaOH. Absorbance of this solution was determined at 440 nm and then converted to units of protease activity by the equation (absorbance/extinction coefficient) × 103 = micromoles of dye, and micromoles of dye are converted to units of enzyme by the equation 1 U of enzyme activity = 1 μmol of substrate converted per min. In negative controls, ACM was added immediately prior to the addition of TCA.
CPE assays.
The pathogenicity of intact Acanthamoeba cells was assayed by observing degradation of epithelial-cell monolayers essentially as described elsewhere (3). Briefly, epithelial cells were grown to a monolayer in 4-well plates using MEM without serum. Either pathogenic or nonpathogenic intact Acanthamoeba cells (106) or ACM (10% or 30%) was added to these monolayers, and the plates were incubated at 37°C in a 5% CO2 incubator for 12 to 24 h. Monolayer CPE was assessed visually after eosin staining or by a cytotoxicity assay determined by using a cytotoxicity detection kit (lactate dehydrogenase [LDH]; catalog no. 1644793; Boehringer Mannheim, Indianapolis, Ind.) based on the measurement of LDH activity released from damaged cells using the 96-well plates. Briefly, cell supernatant containing LDH catalyzes the conversion of lactate (solution from kit) to pyruvate, generating NADH and H+. In the second step, the catalyst (diaphorase, solution from kit) transfers H and H+ from NADH and H+ to the tetrazolium salt p-iodo-nitrotetrazolium violet (INT), which is reduced to formazon (dye), and absorbance is read at 492 nm. For the cytotoxicity assay, hydrogen peroxide-treated epithelial-cell monolayers were used to give 100% cell death (high control). A control with untreated epithelial cells alone was used to give the zero value. Absorbance was converted to percent cytotoxicity as follows: (experimental absorbance/high-control absorbance) × 100 = percent cytotoxicity.
Zymography.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels containing gelatin (2 mg ml−1) were used for zymography of ACM from pathogenic and nonpathogenic species as well as from Acanthamoeba with epithelial-cell-conditioned medium essentially as described previously (3). Briefly, 5 μl of ACM was diluted (1:1) with electrophoresis sample loading buffer and then applied to the gels. After electrophoresis, gels were soaked in 2.5% Triton X-100 (wt/vol) solution for 60 min, incubated in a developing buffer (50 mM Tris-HCL, pH 7.5, containing 10 mM CaCl2) at 37°C overnight, rinsed, and stained with Coomassie brilliant blue. Areas of gelatin digestion were visualized as nonstaining regions in the gel. In some experiments, samples were pretreated with phenylmethylsulfonyl fluoride (PMSF, an inhibitor of serine protease; 1 mM), aprotonin (a serine protease inhibitor; 5 U/ml), and iodoacetamide (a cysteine protease inhibitor; 1 mM) for 30 min prior to electrophoresis, or 1,10-phenanthroline (a metalloprotease inhibitor; 1 mM) in the developing buffer. For pH profile studies, gels were incubated at different pH values using different buffers. The following developing buffers were used to assess the effect of pH on the protease: citrate-phosphate buffer (pH 4 to 5.5), N-(2-morpholino)ethanesulfonic acid (MES) (pH 5.5 to 6.7) and bis-Tris propane (pH 6.3 to 9.5).
RESULTS
Protease assays.
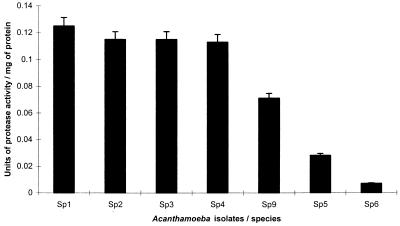
A clear distinction can be seen between protease (ACM) activity in pathogenic and nonpathogenic species of Acanthamoeba (Fig. 1). All pathogenic isolates (Sp1, Sp2, Sp3, Sp4) exhibited significantly higher protease activity than the nonpathogenic isolates (Sp5, Sp6) (P < 0.01; t-statistic, 13.0826, calculated using unpaired t-test using SlideWrite Plus, version 3 for Windows, for pathogenic and nonpathogenic isolates except for A. griffini (Sp9), which exhibited activity intermediate between the two groups. UV-killed Acanthamoeba organisms exhibited no extracellular protease activity and did not show any growth in the viability assays.
FIG. 1.
Extracellular protease activities of pathogenic and nonpathogenic isolates of Acanthamoeba obtained by colorimetric assay. Sp1, Acanthamoeba sp.; Sp2, Acanthamoeba sp.; Sp3, A. castellanii; Sp4, Acanthamoeba sp.; Sp9, A. griffini; Sp5, A. palestinensis; Sp6, A. astronyxis. Error bar, standard deviation of the mean. One unit of enzyme activity is 1 μmol of substrate converted per min.
CPE assays.
Results of the LDH assays showed (Table 2) that pathogenic species of Acanthamoeba produced marked cytotoxicity in epithelial cells after incubation for 24 h. No epithelial-cell cytotoxicity was detected in incubations with nonpathogenic amoebae or ACM. Staining the cell monolayers revealed that only intact pathogenic species of Acanthamoeba (Fig. 2A) and ACM from pathogenic amoebae (Fig. 2B) disrupted epithelial-cell monolayers after 12 h. These results were observed in all Acanthamoeba isolates tested. Although no epithelial-cell damage can be seen with the naked eye in the well with 10% ACM from pathogenic Acanthamoeba (Fig. 2B), small holes were observed microscopically which were not present in the well with 10% ACM from nonpathogenic Acanthamoeba strains. This CPE becomes apparent after 24 h. However, holes can be clearly observed in the well with 30% ACM from pathogenic Acanthamoeba isolates (Fig. 2B). This suggests that extracellular proteases in Acanthamoeba isolates play some role in epithelial-cell disaggregation.
TABLE 2.
Cytotoxicity of Acanthamoeba organisms on immortalized corneal epithelial cells
| Samplea | % Cytotoxicity |
|---|---|
| H2O2 treated epithelial cells | 100 |
| Acanthamoeba sp. (Sp1) (P) | 99 |
| Acanthamoeba sp. (Sp2) (P) | 95 |
| A. palestinensis (Sp5) (NP) | 0 |
| A. astronyxis (Sp6) (NP) | 0 |
| 30% (vol/vol) ACM from Acanthamoeba sp. (Sp1) (CO2) | 0 |
| 30% (vol/vol) ACM from Acanthamoeba sp. (Sp1) (O2) | 0 |
| Epithelial cells alone | 0 |
P, pathogenic species; NP, nonpathogenic species.
FIG. 2.
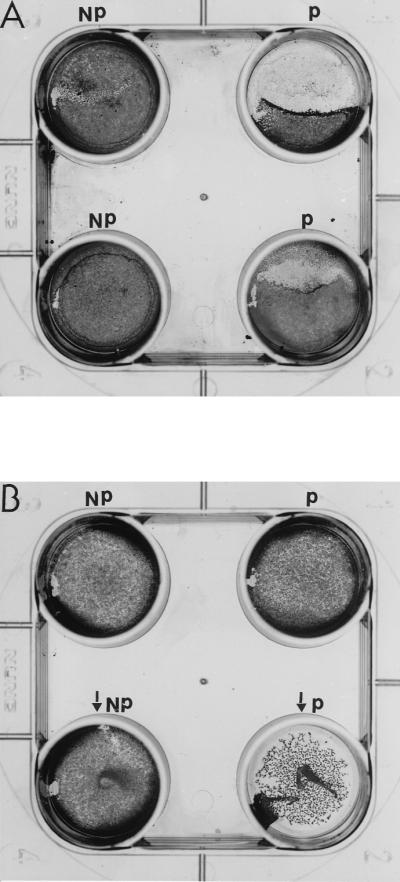
(A) Representative effects of intact Acanthamoeba cells on immortalized corneal epithelial cells after 12 h. P represents pathogenic and NP represents nonpathogenic species or strains. (B) Epithelial-cell monolayer disruption after 24 h using 10 or 30% ACM from Sp1 (P) or Sp6 (NP). Wells containing 30% ACM are indicated by arrows.
Zymography.
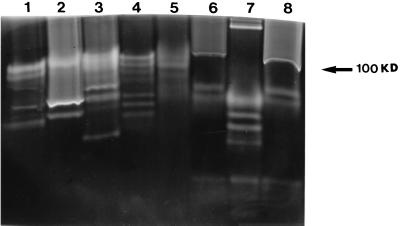
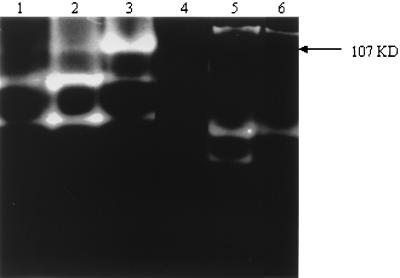
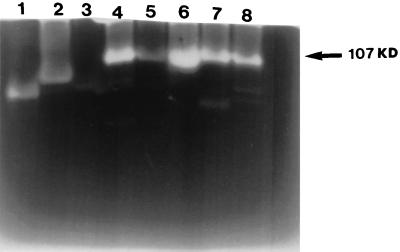
ACM from all Acanthamoeba strains tested showed different protease banding patterns on gelatin gels (Fig. 3), and these were unchanged when Acanthamoeba organisms were incubated with epithelial cells. Only one strain is shown as a representative (Fig. 4, lanes 1 and 2). ACM from UV-killed amoebae did not exhibit any protease activity in zymography assays (data not shown). However, ACM from pathogenic species, incubated in 5% CO2 or in an atmospheric concentration of CO2 (Fig. 4) showed a marked change. One protease becomes predominant when cells are incubated at atmospheric concentrations of CO2. This protease has a molecular mass of 107 kDa (Fig. 5) and was inhibited by either 1 mM aprotonin or 1 mM PMSF (Fig. 4, lane 4), suggesting that this may be a serine protease. The pH profile reveals that this protease is active in all strains between pH 5 and 9.5 (data not shown). Interestingly, this protease showed overexpression only in pathogenic species of Acanthamoeba (Fig. 5).
FIG. 3.
Different Acanthamoeba isolates exhibit different protease banding patterns. Acanthamoeba organisms (106) were incubated in 1 ml of MEM without serum at 37°C in a 5% CO2 incubator for 24 h. The parasites were removed from the medium by centrifugation (100 × g for 5 min). Five microliters of the supernatant was diluted (1:1) in sample buffer, loaded onto an SDS-PAGE gel, and electrophoresed. Following this, SDS was removed by washing in 2.5% Triton, and the gel was incubated overnight in developing buffer (pH 7.5) containing 10 mM CaCl2. Note that different isolates of Acanthamoeba exhibit different banding patterns. Lane 1, Acanthamoeba sp. (Sp2); lane 2, Acanthamoeba sp. (Sp1); lane 3, Acanthamoeba sp. (Sp4); lane 4, A. castellanii (Sp3); lane 5, Acanthamoeba sp. (Sp5); lane 6, A. polyphaga (Sp7); lane 7, A. astronyxis (Sp6); lane 8, A. palestinensis (Sp5).
FIG. 4.
Different culture conditions alter the protease banding patterns. Acanthamoeba organisms (Sp4; 106) were incubated in 1 ml of MEM without serum under different culture conditions. Lane 1, conditioned medium from Acanthamoeba organisms incubated with epithelial cells at 37°C in a 5% CO2 incubator; lane 2, Acanthamoeba organisms incubated without epithelial cells (ACM) at 37°C in a 5% CO2 incubator; lane 3, Acanthamoeba organisms incubated without epithelial cells (ACM) at 37°C in an atmospheric concentration of CO2; lane 4, conditioned medium from Acanthamoeba sp. (Sp4; pathogenic) treated with 1 mM PMSF, showing complete inhibition; lane 5, ACM from A. astronyxis (nonpathogenic [Sp6]); lane 6, ACM from A. astronyxis treated with 1 mM PMSF, showing partial inhibition. Note the change in the protease banding pattern with different culture conditions. Five microliters of ACM was used per lane.
FIG. 5.
Overexpression of a 107-kDa protease in pathogenic isolates of Acanthamoeba only. Acanthamoeba parasites (106) were incubated in 1 ml of MEM without serum at 37°C at an atmospheric concentration of CO2 for 24 h. The parasites were removed from the media by centrifugation (100 × g for 5 min). Five microliters of the supernatant was diluted (1:1) in sample buffer, loaded onto an SDS-PAGE gel, and electrophoresed. Following this, SDS was removed by washing in 2.5% Triton, and the gel was incubated overnight in developing buffer (pH 7.5) containing 10 mM CaCl2. Note that the 107-kDa protease is overexpressed only in pathogenic Acanthamoeba strains. Lane 1, A. astronyxis (Sp6); lane 2, A. palestinensis (Sp5); lane 3, A. polyphaga (Sp7); lane 4, Acanthamoeba sp. (Sp4); lane 5, Acanthamoeba sp. (Sp9); lane 6, A. castellanii (Sp3); lane 7, Acanthamoeba sp. (Sp2); lane 8, Acanthamoeba sp. (Sp1).
DISCUSSION
This study has shown that pathogenic and nonpathogenic Acanthamoeba strains can be differentiated on the basis of extracellular protease activity using a colorimetric assay. Also, cytotoxicity assays showed that pathogenic species produced significant levels of cytotoxicity, thus confirming the results shown by Cao et al. (3). However, it is interesting that disaggregation of epithelial cells occurred when ACM from pathogenic Acanthamoeba strains was added to immortalized corneal epithelial-cell monolayers. Although disaggregation of epithelial cells can be observed, no cytotoxicity was detected in these wells by using LDH assays. These data suggest that extracellular proteases may well be involved in separating the cells or breaking the epithelial-cell layer in in vivo infections. Interestingly, monolayers incubated with ACM treated with 1 mM PMSF did not exhibit CPE, suggesting the importance of total protease activity in comparison to any individual protease.
While no inducible protease was observed in this study, all tested Acanthamoeba isolates showed different protease banding patterns in zymography. Extracellular proteases from all pathogenic Acanthamoeba isolates tested could be inhibited by PMSF and aprotonin, suggesting serine type proteases. Other protease inhibitors tested at 1 mM did not inhibit extracellular proteases from pathogenic Acanthamoeba isolates. Pathogenic Acanthamoeba isolates showed higher protease activity in colorimetric assays than nonpathogenic Acanthamoeba isolates, suggesting the role of proteases in pathogenicity.
The mechanisms of Acanthamoeba-induced CPEs are not known, but the data presented here suggest that higher quantities of extracellular proteases from pathogenic compared to nonpathogenic strains may well be responsible for epithelial-cell destruction to some extent. Protease activity has already been reported for a number of protozoan parasites, including Entamoeba histolytica (6, 12), Giardia lamblia (4), Leishmania amazonensis (11), and Trypanosoma cruzi (2), and may well be involved in pathogenicity.
In summary, our data show higher extracellular protease activities in pathogens compared to nonpathogens, which could be used as a marker in the differentiation of Acanthamoeba spp. Moreover, overexpression of the 107-kDa protease in pathogenic Acanthamoeba isolates could be of diagnostic use in differentiation.
REFERENCES
- 1.Auran J D, Starr M B, Jacobiec F A. Acanthamoeba keratitis. Cornea. 1987;6:2–26. [PubMed] [Google Scholar]
- 2.Bonaldo M C, D'Escoffier L N, Salless J M, Goldenberg S. Characterization and expression of proteases during Trypanosoma cruzi metacyclogenesis. Exp Parasitol. 1991;73:44–51. doi: 10.1016/0014-4894(91)90006-i. [DOI] [PubMed] [Google Scholar]
- 3.Cao Z, Jefferson M D, Panjwani N. Role of carbohydrate-mediated adherence in cytopathogenic mechanisms of Acanthamoeba. J Biol Chem. 1998;273:15838–15845. doi: 10.1074/jbc.273.25.15838. [DOI] [PubMed] [Google Scholar]
- 4.Hare D F, Jarroll E L, Lindmark D G. Giardia lamblia: characterization of proteinase activity in trophozoites. Exp Parasitol. 1989;29:168–175. doi: 10.1016/0014-4894(89)90094-5. [DOI] [PubMed] [Google Scholar]
- 5.He Y G, Neiderkorn J Y, McCulley J P, Stewart G L, Meyer D R, Silvany R, Doughtery J. In vivo and in vitro collagenolytic activity of Acanthamoeba castellanii. Invest Ophthal Vis Sci. 1990;31:2235–2240. [PubMed] [Google Scholar]
- 6.Keene W E. Entamoeba histolytica: correlation of the cytopathic effect of virulent trophozoites with secretion of a cysteine proteinase. Exp Parasitol. 1990;71:199–206. doi: 10.1016/0014-4894(90)90022-5. [DOI] [PubMed] [Google Scholar]
- 7.Ma P, Visvesvara G S, Martinez A J, Theodore F H, Daggett P M, Sawyer T K. Naegleria and Acanthamoeba infections. Rev Infect Dis. 1991;13(Suppl. 5):369–372. doi: 10.1093/clinids/12.3.490. [DOI] [PubMed] [Google Scholar]
- 8.McKerrow J H, Sun E, Rosenthal P, Bouvier J. The proteases and the pathogenicity of parasitic protozoa. Annu Rev Microbiol. 1993;47:821–853. doi: 10.1146/annurev.mi.47.100193.004133. [DOI] [PubMed] [Google Scholar]
- 9.McKerrow J H. The role of proteases in the pathogenesis and immune response to parasitic disease. UCLA Symp Mol Cell Biol. 1987;42:553–557. [Google Scholar]
- 10.Mitro K, Bhagavathiammai A, Zhou O M, Bobbett G, McKerrow H J, Chokshi R, Chokshi B, James R E. Partial characterization of proteolytic secretions of Acanthamoeba polyphaga. Exp Parasitol. 1994;78:377–385. doi: 10.1006/expr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 11.Prina E, Antoine J C, Weideranders B, Kirschke H. Localization and activity of various lysosomal proteases in Leishmania amazonensis-infected macrophages. Infect Immun. 1990;58:1730–1737. doi: 10.1128/iai.58.6.1730-1737.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeds S L, Keene W E, McKerrow J H. Thiol proteinase expression and pathogenicity of Entamoeba histolytica. J Clin Microbiol. 1989;27:2772–2777. doi: 10.1128/jcm.27.12.2772-2777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarath G, de la Motte S R, Wagner W F. Protease methods. In: Beynon R J, Bond J S, editors. Proteolytic enzymes, a practical approach. 2nd ed. Oxford, United Kingdom: IRL; 1994. p. 28. [Google Scholar]
- 14.Seal V D. Acanthamoeba keratitis, contact lenses and the potential health implications of global marketing. Br Mol J. 1994;308:1116–1117. doi: 10.1016/s0035-9203(96)90276-x. [DOI] [PubMed] [Google Scholar]
- 15.Stehr-Green J K, Baily T M, Visvesvara G S. The epidemiology of Acanthamoeba keratitis in the United States. Am J Opthalmol. 1989;107:331–336. doi: 10.1016/0002-9394(89)90654-5. [DOI] [PubMed] [Google Scholar]
- 16.Visvesvara G S. Classification of Acanthamoeba. Rev Infect Dis. 1991;13(Suppl. 5):S369–S372. doi: 10.1093/clind/13.supplement_5.s369. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelmus K R. The increasing importance of Acanthamoeba. Rev Infect Dis. 1991;13(Suppl. 5):5367–5446. doi: 10.1093/clind/13.supplement_5.s367. [DOI] [PubMed] [Google Scholar]