Abstract
Aims
High‐sensitivity cardiac troponin T (hs‐cTnT) and B‐type natriuretic peptide (BNP) are associated with prognosis and severity in patients with heart failure (HF); however, their association with physical function is unclear. This study aimed to investigate whether hs‐cTnT and BNP levels are associated with physical function in patients with HF.
Methods and results
Hs‐cTnT, BNP, and physical function (maximal quadriceps isometric strength [QIS], usual gait speed, and 6‐min walk distance [6MWD]) were evaluated in 363 consecutive patients with HF (median age, 70 [60–78] years). Patients were divided into four groups according to their median hs‐cTnT and BNP levels. After adjusting for demographic characteristics, laboratory levels, and HF severity, higher hs‐cTnT and BNP levels were significantly associated with lower physical function (log hs‐cTnT, β = −0.162, P = 0.001, for maximal QIS; β = −0.175, P = 0.002, for usual gait speed, and β = −0.129, P = 0.004, for 6MWD; log BNP, β = −0.090, P = 0.092, for maximal QIS, β = 0.038, P = 0.516, for usual gait speed, and β = −0.108, P = 0.023, for 6MWD). In addition, the high hs‐cTnT and high BNP group had significantly lower physical function (all P < 0.05) than the low hs‐cTnT and low BNP group.
Conclusions
Higher hs‐cTnT and BNP levels are both associated with lower physical function in patients with HF, but hs‐cTnT levels showed a more consistent association. The combination of hs‐cTnT and BNP may be effective for the stratification of physical function in patients with HF.
Keywords: High‐sensitivity cardiac troponin T, Hs‐cTnT, B‐type natriuretic peptide, BNP, Physical function
Introduction
Heart failure (HF) is a major health problem, and numerous useful indicators have been reported to determine the effectiveness of treatment and disease management. 1 , 2 , 3 With advances in HF treatment and management, the target population is becoming progressively older; hence, sarcopenia and frailty are becoming major issues. 4 , 5 Therefore, if it is possible to evaluate physical dysfunction using indicators that are frequently used in clinical practice, the identification of patients with sarcopenia and frailty may become easier.
Recently, to understand the severity of HF and for appropriate treatment and intervention, the measurement of B‐type natriuretic peptide (BNP) and cardiac troponin T (cTnT) has been recommended by each country guidelines. 1 , 2 , 3 BNP is secreted primarily by cardiac ventricular myocytes in response to increased ventricular wall stress generated by volume or pressure overload or ischaemia 6 , 7 and has been established as a biomarker to assist in the diagnosis of HF and to predict prognosis in patients with HF. 7 , 8 , 9 On the other hand, high‐sensitivity cTnT (hs‐cTnT), which is elevated by various factors such as minor myocardial injury and skeletal muscle myopathy, has recently attracted attention. 10 , 11 , 12 In particular, minor elevations in hs‐cTnT levels have been identified as an independent poor prognostic factor in patients with HF. 13 , 14 , 15 , 16 Furthermore, the combination of hs‐cTnT and BNP has been reported to be highly effective for risk stratification in patients with HF. 14 , 15 , 16
It is said that the heart and skeletal muscles are bi‐directionally related via the ergoreflex system. 17 Recently, hs‐cTnT and BNP have been reported to be associated with skeletal muscle mass. 18 , 19 Skeletal muscle mass is an important factor that regulates physical function, and these two biomarkers may have additional value in screening for physical dysfunction; however, reports on this association in patients with HF are limited.
The present study sought to examine whether hs‐cTnT and BNP levels are associated with physical function in patients with HF and whether the combination of hs‐cTnT and BNP is effective for stratifying physical function in patients with HF.
Methods
Study population
We conducted a single‐centre cross‐sectional study. Among patients who were admitted in Kitasato University Hospital between April 2011 and January 2019, we identified HF patients who presented with acute or worsening HF symptoms. Acute decompensated HF (ADHF) diagnosis, based on the Framingham criteria, 20 was performed by experienced cardiologists.
In this study population, we investigated the association between hs‐cTnT level and physical function and between BNP level and physical function. We limited our study to HF patients for whom hs‐cTnT and BNP measurement was performed and who underwent the evaluation of physical function at hospital discharge. Patients with skeletal muscle disease (dystrophy) were excluded due to the high possibility of cross‐reactivity in looking at the relationship between skeletal muscle function and cardiac biomarkers. Furthermore, patients who had missing data on patient characteristics, left ventricular ejection fraction (LVEF), and New York Heart Association (NYHA) functional class were excluded. Consequently, 363 patients with HF were studied (Figure 1 ). The study was performed in accordance with the tenets of the Declaration of Helsinki and was approved by the ethics committee of Kitasato University Hospital (B18‐075).
Figure 1.

Flow chart of the study population.
Clinical data collection
Data on all variables, including age, sex, body mass index (BMI), clinical details at presentation (co‐morbidities and medication use) as well as demographic, echocardiographic, and biochemical data just before hospital discharge, were obtained from electronic medical records. Hs‐cTnT was measured using the high‐sensitivity Roche Elecsys assay (Roche Diagnostics, Tokyo, Japan), whereas BNP concentration was measured using a commercially available immunoradiometric assay (Shionogi, Osaka, Japan). The Simpson's method was used to estimate LVEF on two‐dimensional echocardiograms. The estimated glomerular filtration rate (eGFR) was defined according to the formula recommended by the Japanese Society of Nephrology as follows: 194 × (serum creatinine)1.094 × (age)0.287 in men and 194 × (serum creatinine)1.094 × (age)0.287 × 0.739 in women. 21
Physical function
For the assessment of physical function, we used maximal quadriceps isometric strength (QIS), usual gait speed, and 6‐min walk distance (6MWD) measured at hospital discharge.
Maximal QIS was measured using a handheld dynamometer (μ‐Tas, ANIMA, Tokyo, Japan). Patients sat in a chair with an inextensible strap connecting the ankle of their leg to a strain gauge. Five‐second maximal isometric voluntary contractions of the quadriceps were collected three successive times for both legs at 90° flexion and for the hip joint at approximately 90° flexion. The right and left quadriceps were tested consecutively. The greatest strength values on the right and left sides were averaged and expressed as absolute values (kg) and as relative values to body mass (%body mass, %BM). 22 , 23
To measure usual gait speed, the patients were asked to walk at their usual gait speed; their movement over the middle 10 m of a 16 m walkway (from 3 to 13 m) was timed. By measuring the time it took the patients to walk the 10 m distance, we calculated the usual gait speed (m/s) of each participant.
The 6MWD was measured according to the American Thoracic Society guidelines, 24 and the measurement was supervised by technicians. The measurement location was the flat in‐hospital hallway, which was marked at 1 m intervals. The patients were instructed to walk as far as possible along a straight line, and the distance in meters was recorded at the end of a 6 min period.
Statistical analysis
Continuous variables are presented as median (interquartile range), whereas categorical variables are expressed as number and percentage. To examine whether the combinations of hs‐cTnT and BNP levels are associated with physical function in patients with HF, the patients were divided into four groups according to their median hs‐cTnT and BNP levels. The groups were low hs‐cTnT and low BNP group, low hs‐cTnT and high BNP group, high hs‐cTnT and low BNP group, and high hs‐cTnT and high BNP group. Patient characteristics were compared between the groups using one‐way analysis of variance or the Kruskal–Wallis test for continuous variables, and χ 2 test was used for categorical variables. Spearman's rank correlation coefficient was calculated to evaluate the correlation between hs‐cTnT, BNP, and physical function. Multiple regression analyses were performed to test the independent associations between hs‐cTnT, BNP, and physical function, with adjustments for age, sex, BMI, ischaemic aetiology, LVEF, NYHA functional class, and eGFR. Moreover, hierarchical multiple linear regression analyses were performed to assess the contributions of hs‐cTnT and BNP in predicting physical function after adjusting for clinical model. The clinical model consisted of the variables used in multiple regression, excluding hs‐cTnT and BNP. Comparisons between the four groups were performed via analysis of covariance (ANCOVA), with adjustments for age, sex, BMI, ischaemic aetiology, LVEF, NYHA functional class, and eGFR. Furthermore, we modelled nonlinear associations between hs‐cTnT level and physical function and between BNP level and physical function, using restricted cubic splines with three knots in sensitivity analyses. Natural logarithmic (log) transformations were performed for hs‐cTnT and BNP levels with skewed distributions before the levels were inserted into Spearman's rank correlation coefficient, multiple regression analyses, hierarchical multiple linear regression analyses, and restricted cubic splines with three knots. Analyses were performed using R version 3.6.3 (R Project for Statistical Computing, Vienna, Austria), Stata version 16.0 (StataCorp, College Station, TX, USA), and JMP® Pro version 14.1 (SAS Institute Inc., Cary, NC, USA). In all analyses, a two‐tailed P‐value <0.05 was considered to indicate statistical significance.
Results
Patients characteristics
The baseline characteristics of all patients and the results of comparisons between the four groups are shown in Table 1 . The median age of the study population was 70 years, 62.2% of the patients were male, and HF in 44.6% of the patients was of an ischaemic aetiology. High hs‐cTnT and BNP levels were associated with older age, an ischaemic aetiology more than a non‐ischaemic aetiology, lower eGFR, and all physical functions. The median hs‐cTnT and BNP levels were 0.037 ng/mL and 286.0 pg/mL, respectively.
Table 1.
Patient characteristics
| Characteristics | All patients | hs‐cTnT < 0.037 ng/mL BNP < 286.0 pg/mL | hs‐cTnT < 0.037 ng/mL BNP ≥ 286.0 pg/mL | hs‐cTnT ≥ 0.037 ng/mL BNP < 286.0 pg/mL | hs‐cTnT ≥ 0.037 ng/mL BNP ≥ 286.0 pg/mL | P‐value |
|---|---|---|---|---|---|---|
| n = 363 | n = 113 | n = 67 | n = 68 | n = 115 | ||
| Age [year] | 70 [60–78] | 67 [50–74] | 71 [62–78] | 68 [58–78] | 74 [67–79] | <0.001 |
| Sex, male (%) | 226 (62.2) | 60 (53.1) | 40 (59.7) | 50 (73.5) | 76 (66.1) | 0.035 |
| BMI [kg/m2] | 21.7 [19.3–24.7] | 22.4 [20.0–25.1] | 21.4 [19.2–23.5] | 22.6 [20.2–25.8] | 20.8 [18.9–23.9] | 0.009 |
| Ischaemic aetiology (%) | 162 (44.6) | 37 (32.7) | 22 (32.8) | 38 (55.8) | 65 (56.5) | <0.001 |
| LVEF [%] | 37.0 [27.0–50.0] | 39.0 [28.0–53.0] | 34.0 [24.2–49.5] | 40.0 [30.0–50.4] | 34.0 [27.0–45.7] | 0.200 |
| NYHA functional class (%) | ||||||
| I | 14 (3.8) | 3 (2.7) | 2 (3.0) | 5 (7.3) | 4 (3.5) | 0.413 |
| II | 236 (65.0) | 88 (77.9) | 44 (65.7) | 43 (63.2) | 61 (53.0) | 0.001 |
| III | 107 (29.4) | 22 (19.5) | 21 (31.3) | 17 (25.0) | 47 (40.9) | 0.004 |
| IV | 6 (1.6) | 0 (0.0) | 0 (0.0) | 3 (4.4) | 3 (2.6) | 0.077 |
| Medications | ||||||
| ACE inhibitor or ARB (%) | 326 (89.8) | 101 (89.4) | 64 (95.5) | 62 (91.1) | 99 (86.1) | 0.200 |
| Beta‐blocker (%) | 299 (82.3) | 91 (80.5) | 63 (94.0) | 52 (76.4) | 93 (80.9) | 0.039 |
| Aldosterone blocker (%) | 206 (56.7) | 61 (54.0) | 44 (65.7) | 38 (55.8) | 63 (54.8) | 0.400 |
| Diuretic (%) | 314 (86.5) | 94 (83.2) | 64 (95.5) | 58 (85.2) | 98 (85.2) | 0.110 |
| Co‐morbidities | ||||||
| Hypertension (%) | 255 (70.2) | 86 (76.1) | 38 (56.7) | 48 (70.5) | 83 (72.2) | 0.047 |
| Diabetes (%) | 188 (51.7) | 49 (43.4) | 31 (46.3) | 40 (58.8) | 68 (59.1) | 0.049 |
| Dyslipidaemia (%) | 178 (50.4) | 51 (47.2) | 31 (47.7) | 33 (49.2) | 63 (55.8) | 0.600 |
| Current smoker (%) | 81 (23.1) | 26 (23.4) | 17 (27.0) | 17 (25.3) | 21 (19.3) | 0.700 |
| Laboratory data | ||||||
| Haemoglobin [g/dL] | 12.5 [10.8–14.1] | 13.0 [12.1–14.6] | 12.9 [11.3–15.1] | 13.0 [10.9–14.1] | 11.0 [9.8–12.7] | <0.001 |
| Sodium [mEq/L] | 139 [136–140] | 139 [138–141] | 139 [136–140] | 139 [136–140] | 138 [135–140] | 0.001 |
| Hs‐cTnT [ng/mL] | 0.037 [0.018–0.091] | 0.017 [0.012–0.023] | 0.021 [0.015–0.029] | 0.084 [0.047–0.180] | 0.107 [0.054–0.233] | <0.001 |
| BNP [pg/mL] | 286.0 [130.1–658.4] | 119.2 [58.8–181.5] | 510.2 [386.1–730.6] | 153.2 [97.9–199.6] | 831.9 [515.5–1653.5] | <0.001 |
| eGFR [mL/min/1.73 m2] | 49.3 [35.5–63.8] | 59.4 [47.1–70.8] | 53.6 [39.8–65.5] | 51.0 [37.6–64.2] | 35.2 [22.0–50.6] | <0.001 |
| Physical function | ||||||
| Maximal QIS [%BM] | 38.5 [29.2–49.5] | 41.7 [33.4–54.5] | 42.6 [31.9–49.9] | 39.3 [28.6–50.9] | 34.5 [24.6–43.4] | <0.001 |
| Usual gait speed [m/s] | 1.05 [0.82–1.23] | 1.11 [0.98–1.30] | 1.12 [0.93–1.32] | 1.07 [0.94–1.23] | 0.87 [0.64–1.09] | <0.001 |
| 6‐min walk distance [m] | 400 [310–470] | 436 [344–510] | 410 [313–470] | 416 [333–486] | 319 [167–388] | <0.001 |
Median [quartile1 − quartile3]. %BM, percentage body mass; ACE inhibitor, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; BMI, body mass index; BNP, B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; hs‐cTnT, high‐sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; Maximal QIS, maximal quadriceps isometric strength; NYHA functional class, New York Heart Association functional class.
Associations between hs‐cTnT, BNP, and physical function
Figure 2 shows the scatter plot of hs‐cTnT (A) and BNP (B) with physical function. The scatter diagram shows negative correlations between hs‐cTnT levels and all physical functions and between BNP and all physical functions. Spearman's rank correlation coefficient shows that both hs‐cTnT and BNP were significantly associated with all physical functions (log hs‐cTnT, rho = −0.265 for maximal QIS, rho = −0.290 for usual gait speed, and rho = −0.318 for 6MWD, all P < 0.001; log BNP, rho = −0.208 for maximal QIS, rho = −0.203 for usual gait speed, and rho = −0.310 for 6MWD, all P < 0.001).
Figure 2.
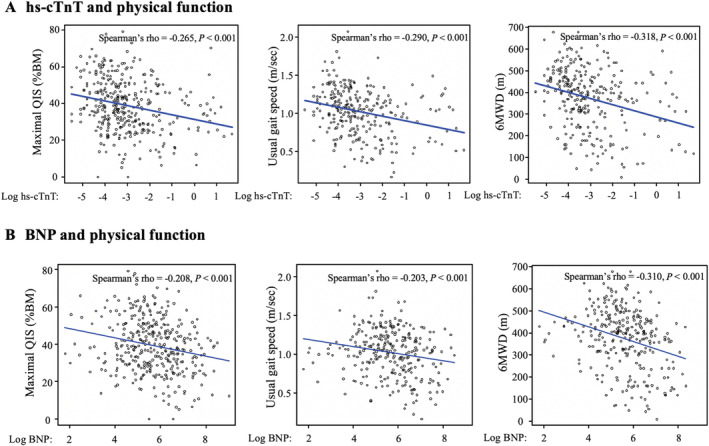
Correlations between hs‐cTnT and physical function (A) and between BNP and physical function (B). The regression lines for each plot are shown in blue. %BM, percentage body mass; 6MWD, 6‐min walk distance; BNP, B‐type natriuretic peptide; hs‐cTnT, high‐sensitivity cardiac troponin T; maximal QIS, maximal quadriceps isometric strength; Spearman's rho, Spearman's rank correlation coefficient.
Table 2 shows the results of multiple regression analyses for identifying the independent associations between hs‐cTnT, BNP, and physical function. After adjusting for age, sex, BMI, ischaemic aetiology, LVEF, NYHA functional class, and eGFR, a higher hs‐cTnT level was significantly associated with lower levels of all physical functions (log, β = −0.162, P = 0.001, for maximal QIS; β = −0.175, P = 0.002, for usual gait speed; and β = −0.129, P = 0.004, for 6MWD). Conversely, higher BNP was significantly associated with shorter 6MWD (log, β = −0.108, P = 0.023), but not with maximal QIS (log, β = −0.090, P = 0.092) and usual gait speed (log, β = 0.038, P = 0.516). Moreover, hierarchical multivariate linear regression analysis revealed that adding hs‐cTnT or BNP explained the greater amount of variance in the physical function measures. The clinical model with hs‐cTnT changed the amount of variance obtained in all physical function measures (maximal QIS, change in R 2: 0.030, change in F: 16.092, P < 0.001; usual gait speed, change in R 2: 0.022, change in F: 9.343, P = 0.002; and 6MWD, change in R 2: 0.021, change in F: 13.791, P < 0.001). Adding BNP levels to the clinical model changed the amount of variance in the physical function measures (maximal QIS, change in R 2: 0.016, change in F: 8.499, P = 0.003; and 6MWD, change in R 2: 0.017, change in F: 10.792, P = 0.001) but not in usual gait speed (change in R 2: <0.001, change in F: 0.069, P = 0.791).
Table 2.
Multiple regression analysis for association of physical function, hs‐cTnT, and BNP
| Variables | Maximal QIS | Usual gait speed | 6MWD | |||
|---|---|---|---|---|---|---|
| Standardized β | P‐value | Standardized β | P‐value | Standardized β | P‐value | |
| Log hs‐cTnT | −0.162 | 0.001 | −0.175 | 0.002 | −0.129 | 0.004 |
| Log BNP | −0.090 | 0.092 | 0.038 | 0.516 | −0.108 | 0.023 |
| Age | −0.159 | 0.004 | −0.167 | 0.011 | −0.279 | <0.001 |
| Male | 0.319 | <0.001 | 0.157 | 0.002 | 0.140 | <0.001 |
| BMI | −0.059 | 0.219 | 0.131 | 0.015 | 0.046 | 0.288 |
| Ischaemic aetiology | −0.066 | 0.162 | −0.038 | 0.478 | −0.020 | 0.630 |
| LVEF | −0.004 | 0.929 | −0.012 | 0.811 | 0.060 | 0.161 |
| NYHA functional class | −0.318 | <0.001 | −0.358 | <0.001 | −0.477 | <0.001 |
| eGFR | 0.0002 | 0.995 | 0.080 | 0.194 | −0.024 | 0.621 |
6MWD, 6‐min walk distance; BMI, body mass index; BNP, B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; hs‐cTnT, high‐sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; Maximal QIS, maximal quadriceps isometric strength; NYHA functional class, New York Heart Association functional class.
Figure 3 shows the results of ANCOVA for a comparison of physical function between the four groups, after adjustments for age, sex, BMI, ischaemic aetiology, LVEF, NYHA functional class, and eGFR. The high hs‐cTnT and high BNP group had significantly lower levels in all physical functions than the low hs‐cTnT
Figure 3.

Associations of hs‐cTnT and BNP with physical function determined by analysis of covariance. Adjusted for age, sex, BMI, ischaemic aetiology, LVEF, NYHA functional class, and eGFR. Bar graphs represent adjusted mean levels, with error bars representing 95% confidence intervals. %BM, percentage body mass; 6MWD, 6‐min walk distance; BMI, body mass index; BNP, B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; hs‐cTnT, high‐sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; maximal QIS, maximal quadriceps isometric strength; NYHA functional class, New York Heart Association functional class; low hs‐cTnT, hs‐cTnT < median; low BNP, BNP < median; high hs‐cTnT, hs‐cTnT ≥ median; high BNP, BNP ≥ median. *and ** represent a significant difference between the two groups (P < 0.05 and P < 0.001, respectively).
and low BNP group. The high hs‐cTnT and high BNP group had significantly slower usual gait speed and shorter 6MWD than the low hs‐cTnT and high BNP group. In addition, the high hs‐cTnT and high BNP group had a significantly shorter 6MWD compared with the high hs‐cTnT and low BNP group.
Figure 4 shows nonlinear associations between hs‐cTnT (A), BNP (B), and physical function using restricted cubic splines in sensitivity analyses. All physical functions declined with elevating hs‐cTnT levels. Similarly, all physical functions declined with elevating BNP levels. Further, compared with the tendency of physical function decline with BNP level elevation, minor elevation in hs‐cTnT levels tended to lead to a decline in physical function.
Figure 4.

Nonlinear associations between hs‐cTnT and physical function (A) and between BNP and physical function (B). Dotted line represents 95% confidence intervals. %BM, percentage body mass; 6MWD, 6‐min walk distance; BNP, B‐type natriuretic peptide; hs‐cTnT, high‐sensitivity cardiac troponin T; maximal QIS, maximal quadriceps isometric strength.
Discussions
The primary findings of our study were as follows: First, higher hs‐cTnT and BNP levels were associated with lower physical function in patients with HF; however, hs‐cTnT levels were more consistently associated with all physical function measures independent of clinical characteristics and HF severity. Second, the high hs‐cTnT and high BNP group had significantly lower levels in all physical functions than the low hs‐cTnT and low BNP group. These findings suggest that hs‐cTnT and BNP have additional value in screening for physical dysfunction in patients with HF. In addition, the combination of hs‐cTnT and BNP may be an effective stratification for low physical function in patients with HF.
The usefulness of some alternative indices to predict exercise capacity in patients with HF has been reported. The NYHA classification is frequently used in clinical practice to predict exercise tolerance in patients with HF. 25 However, the NYHA classification, while easy and rapid to perform, has been noted to have a low discriminatory power. 26 Moreover, there are large differences in terms of exercise tolerance within the same NYHA class. On the other hand, BNP is a well‐established biomarker in patients with HF and has been reported to be associated with peak exercise oxygen consumption (VO2) and self‐assessed physical function. 27 , 28 , 29 In previous studies, hs‐cTnT and BNP have been reported to be associated with skeletal muscle mass. 18 , 19 Moreover, it has been reported that the elevated hs‐cTnT and BNP are strongly and independently associated with a higher fall risk in older adults. 30 Therefore, these biomarkers may be related to physical function. To our knowledge, this is the first study to show that hs‐cTnT and BNP have additional value in screening for physical dysfunction in patients with HF.
Many factors may influence the relationship between cardiac biomarkers for detecting myocardial injury and left ventricular overload and skeletal muscle dysfunction. The hemodynamic disorders observed in ADHF may have elicited skeletal muscle circulatory insufficiency, the activation of neurohumoral factors, and an inflammatory response, leading to skeletal muscle atrophy and dysfunction. 31 Moreover, cardiac biomarkers are primarily secreted by cardiomyocytes upon excessive stretch, increased transmural pressure, or direct injury. These homodynamic changes stimulate cardiac biomarker secretion and were associated with the low skeletal muscle. 18 , 19 Furthermore, elevated circulatory BNP levels may contribute to increased energy dissipation and oxidative capacity by skeletal muscle. 32 It has been reported that skeletal muscle growth attenuated cardiac remodelling and dysfunction in a mouse myocardial infarction model. 33 Ponikowski et al. 17 reported that patients with HF with a more severe clinical condition (defined by the NYHA class, reduced peak VO2, or increased minute ventilation/carbon dioxide production slope) were characterized by a more marked ergoreflex component in the ventilatory and hemodynamic responses to exercise. Thus, these factors may also affect the association of cardiac biomarkers with physical function.
When BNP and hs‐cTnT were used in a combined biomarker strategy, these markers were more effective for identifying patients with lower physical function than when each marker was used alone. A previous study reported that the combination of increased troponin and increased BNP levels identified patients with HF and patients with a markedly increased mortality risk. 14 , 15 , 16 Our study further demonstrated the value of a multi‐biomarker strategy to differentiate a higher risk of physical dysfunction in HF. These findings support the utility of a multiple‐biomarker strategy involving hs‐cTnT and BNP in patients with HF.
In the present study, the association between both hs‐cTnT and BNP and physical function showed different dose–response associations. BNP did not affect physical function up to a certain level, but further increases tended to decrease physical function. Similarly, with respect to the relationship between BNP and prognosis, an increase in BNP above a certain level has been reported to be associated with poor prognosis. 8 , 9 On the other hand, even a minor elevation in hs‐cTnT levels tended to decrease physical function. Minor myocardial injury detected by hs‐cTnT has been shown to be an independent predictor of prognosis in patients with HF. 13 , 14 , 15 , 16 Accordingly, we suggest that the relationship between hs‐cTnT, BNP, and prognosis reported by previous studies bears a similar relevance to physical function.
With the recent aging of the population, the number of HF patients with low physical function such as frailty is increasing, and the importance of continued follow‐up after hospital discharge is becoming increasingly important. Kamiya et al. 34 reported that cardiac rehabilitation significantly reduced mortality and rehospitalization rates in patients with HF, even after adjusting for HF medications and other factors in addition to clinical characteristics and HF severity, and that these results were similar in HF patients with frailty. Further, it has been reported that cardiac rehabilitation, which continues until discharge from the hospital, improves physical function in CVD patients with frailty, physical function disability, and many co‐morbidities, 35 suggesting that it improves mortality and readmission rates. Therefore, participation in cardiac rehabilitation and its continuation from the hospital discharge are important. However, participation and referral rates for patients after hospitalization for HF to cardiac rehabilitation are low, with lack of physician referrals cited as the primary reason. 36 , 37 In this study, hs‐cTnT and BNP levels were useful in stratifying patients with low physical function. This may be beneficial in identifying patients who need more intensive rehabilitation, which in turn may help improve the referral rate to cardiac rehabilitation.
Study limitations
This study had several limitations. First, because this was a single‐centre observational study and external validity could not be guaranteed, we were unable to determine the optimal cut‐off values of biomarkers for predicting the decline in physical function. Further prospective multicentre studies are needed to inform clinical management. Second, factors such as age, sex, and renal function were adjusted as covariates because they are known to increase serum hs‐cTnT and BNP. It has been reported that cTnT is often elevated in patients with skeletal muscle disease without evidence of cardiac disease. 12 Therefore, patients with skeletal muscle disease were excluded due to the high possibility of cross‐reactivity in looking at the relationship between skeletal muscle function and cardiac biomarkers. However, we did not adjust for the influence of medications on hs‐cTnT and BNP levels. It was suggested that the risk of myocardial injury was likely to increase with the use of inotropic drugs. 38 Previous studies have demonstrated that BNP levels were reduced after treatment with beta‐blockers, 39 whereas BNP levels were increased after treatment with angiotensin‐converting enzyme inhibitors 40 or digitalis. 41 Third, our study used the patients' hs‐cTnT and BNP levels at hospital discharge, but the relationship between biomarkers and physical function would have been more reliable in patients with chronic HF and a more stable clinical status. Finally, our study aimed to determine whether cardiac biomarkers used to manage HF and determine the effectiveness of treatment have new aspects related to physical function. Further research is needed to determine (i) whether biomarkers, such as creatinine, creatine phosphokinase, and lactate dehydrogenase 42 , 43 reportedly associated with skeletal muscle atrophy or damage are useful in predicting physical function in patients with HF, (ii) which of them is more useful, and (iii) whether they can be used in conjunction with cardiac biomarkers.
Conclusions
Higher hs‐cTnT and BNP levels are associated with lower physical function in patients with HF, and hs‐cTnT levels are more consistently associated with physical function. Furthermore, the combination of hs‐cTnT and BNP seems highly effective for the stratification of physical function in patients with HF. These findings suggest that hs‐cTnT and BNP have additional value in screening for physical dysfunction in patients with HF.
Conflict of interest
The authors declare that there is no conflict of interest.
Author contributions
K.U., K.K., A.M., and J.A. contributed to the conception or design of the work. K.U., K.K., N.H., K.N., T.I., M.Y., S.U., N.Y., E.M., and M.T. contributed to the acquisition, analysis, or interpretation of data for the work. K.U. and K.K. drafted the manuscript. N.H., K.N., T.I., M.Y., S.U., N.Y., E.M., M.T., A.M., and J.A. critically revised the manuscript.
Acknowledgements
This work was partially supported by JSPS KAKENHI Grant Number 21H03309 and the Nakatomi Foundation grant.
Ueno, K. , Kamiya, K. , Hamazaki, N. , Nozaki, K. , Ichikawa, T. , Yamashita, M. , Uchida, S. , Yanagi, N. , Maekawa, E. , Yamaoka‐Tojo, M. , Matsunaga, A. , and Ako, J. (2021) Relationship between high‐sensitivity cardiac troponin T, B‐type natriuretic peptide, and physical function in patients with heart failure. ESC Heart Failure, 8: 5092–5101. 10.1002/ehf2.13577.
References
- 1. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 3. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Kitakaze M, Kinugawa K, Kihara Y, Goto Y, Komuro I, Saiki Y, Saito Y, Sakata Y, Sato N, Sawa Y, Shiose A, Shimizu W, Shimokawa H, Seino Y, Node K, Higo T, Hirayama A, Makaya M, Masuyama T, Murohara T, Momomura SI, Yano M, Yamazaki K, Yamamoto K, Yoshikawa T, Yoshimura M, Akiyama M, Anzai T, Ishihara S, Inomata T, Imamura T, Iwasaki YK, Ohtani T, Onishi K, Kasai T, Kato M, Kawai M, Kinugasa Y, Kinugawa S, Kuratani T, Kobayashi S, Sakata Y, Tanaka A, Toda K, Noda T, Nochioka K, Hatano M, Hidaka T, Fujino T, Makita S, Yamaguchi O, Ikeda U, Kimura T, Kohsaka S, Kosuge M, Yamagishi M, Yamashina A. Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group. Guidelines for diagnosis and treatment of acute and chronic heart failure. Circ J 2019; 83: 2084–2184. [DOI] [PubMed] [Google Scholar]
- 4. Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Heart Fail 2017; 4: 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reeves GR, Whellan DJ, O'Connor CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva A, Patel MJ, Kitzman DW. A novel rehabilitation intervention for older patients with acute decompensated heart failure: the REHAB‐HF pilot study. JACC Heart Fail 2017; 5: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Kinoshita M. High levels of plasma brain natriuretic peptide and interleukin‐6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol 2000; 36: 1587–1593. [DOI] [PubMed] [Google Scholar]
- 7. Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007; 50: 2357–2368. [DOI] [PubMed] [Google Scholar]
- 8. Hsich EM, Grau‐Sepulveda MV, Hernandez AF, Eapen ZJ, Xian Y, Schwamm LH, Bhatt DL, Fonarow GC. Relationship between sex, ejection fraction, and B‐type natriuretic peptide levels in patients hospitalized with heart failure and associations with inhospital outcomes: findings from the Get With The Guideline‐Heart Failure Registry. Am Heart J 2013; 166: 1063–1071.e3. [DOI] [PubMed] [Google Scholar]
- 9. Fonarow GC, Peacock WF, Phillips CO, Givertz MM, Lopatin M. ADHERE Scientific Advisory Committee and Investigators. Admission B‐type natriuretic peptide levels and in‐hospital mortality in acute decompensated heart failure. J Am Coll Cardiol 2007; 49: 1943–1950. [DOI] [PubMed] [Google Scholar]
- 10. Sato Y, Yamada T, Taniguchi R, Nagai K, Makiyama T, Okada H, Kataoka K, Ito H, Matsumori A, Sasayama S, Takatsu Y. Persistently increased serum concentrations of cardiac troponin T in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 2001; 103: 369–374. [DOI] [PubMed] [Google Scholar]
- 11. Mair J, Lindahl B, Müller C, Giannitsis E, Huber K, Möckel M, Plebani M, Thygesen K, Jaffe AS. What to do when you question cardiac troponin values. Eur Heart J Acute Cardiovasc Care 2018; 7: 577–586. [DOI] [PubMed] [Google Scholar]
- 12. Schmid J, Liesinger L, Birner‐Gruenberger R, Stojakovic T, Scharnagl H, Dieplinger B, Asslaber M, Radl R, Beer M, Polacin M, Mair J, Szolar D, Berghold A, Quasthoff S, Binder JS, Rainer PP. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol 2018; 71: 1540–1549. [DOI] [PubMed] [Google Scholar]
- 13. Koide K, Yoshikawa T, Nagatomo Y, Kohsaka S, Anzai T, Meguro T, Ogawa S. Elevated troponin T on discharge predicts poor outcome of decompensated heart failure. Heart Vessels 2010; 25: 217–222. [DOI] [PubMed] [Google Scholar]
- 14. Demissei BG, Cotter G, Prescott MF, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Severin TM, Wang Y, Qian M, Teerlink JR, Metra M, Davison BA, Voors AA. A multimarker multi‐time point‐based risk stratification strategy in acute heart failure: results from the RELAX‐AHF trial. Eur J Heart Fail 2017; 19: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 15. Jackson CE, Haig C, Welsh P, Dalzell JR, Tsorlalis IK, McConnachie A, Preiss D, Anker SD, Sattar N, Petrie MC, Gardner RS, McMurray JJ. The incremental prognostic and clinical value of multiple novel biomarkers in heart failure. Eur J Heart Fail 2016; 18: 1491–1498. [DOI] [PubMed] [Google Scholar]
- 16. Alehagen U, Dahlström U, Rehfeld JF, Goetze JP. Prognostic assessment of elderly patients with symptoms of heart failure by combining high‐sensitivity troponin T and N‐terminal pro‐B‐type natriuretic peptide measurements. Clin Chem 2010; 56: 1718–1724. [DOI] [PubMed] [Google Scholar]
- 17. Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation 2001; 104: 2324–2330. [DOI] [PubMed] [Google Scholar]
- 18. van Vugt JLA, Boerma D, Dijkstra IM, Bollen TL, Eefting FD, IJzermans JNM, Noordzij PG. Elevated high‐sensitive cardiac troponin T levels are associated with low skeletal muscle mass in abdominal surgical oncology patients at risk for coronary artery disease. Int J Cardiol 2015; 191: 229–230. [DOI] [PubMed] [Google Scholar]
- 19. Yamashita T, Kohara K, Tabara Y, Bollen TL, Eefting FD, IJzermans JN, Noordzij PG. Muscle mass, visceral fat, and plasma levels of B‐type natriuretic peptide in healthy individuals (from the J‐SHIPP Study). Am J Cardiol 2014; 114: 635–640. [DOI] [PubMed] [Google Scholar]
- 20. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 21. Ando Y, Ito S, Uemura O, Kato T, Kimura G, Nakao T, Hattori M, Fukagawa M, Horio M, Mitarai T. Japanese Society of Nephrology. CKD Clinical Practice Guidebook. The essence of treatment for 7CKD patients. Clin Exp Nephrol 2009; 13: 191–248. [DOI] [PubMed] [Google Scholar]
- 22. Kamiya K, Masuda T, Tanaka S, Hamazaki N, Matsue Y, Mezzani A, Matsuzawa R, Nozaki K, Maekawa E, Noda C, Yamaoka‐Tojo M, Arai Y, Matsunaga A, Izumi T, Ako J. Quadriceps strength as a predictor of mortality in coronary artery disease. Am J Med 2015; 128: 1212–1219. [DOI] [PubMed] [Google Scholar]
- 23. Bohannon N. Reference values for extremity muscle strength obtained by hand‐held 13dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil 1997; 78: 26–32. [DOI] [PubMed] [Google Scholar]
- 24. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 25. Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M, Greenberg B, Woods PA, Bourassa MG. Prediction of mortality and morbidity with a 6‐minute walk test in patients with left ventricular dysfunction. SOLVD Investigators JAMA 1993; 270: 1702–1707. [PubMed] [Google Scholar]
- 26. Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, Garten R, Rodriguez‐Miguelez P, Guazzi M, Lavie CJ, Abbate A. Exercise intolerance in patients with heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019; 73: 2209–2225. [DOI] [PubMed] [Google Scholar]
- 27. Krüger S, Graf J, Kunz D, Stickel T, Hanrath P, Janssens U. Brain natriuretic peptide levels predict functional capacity in patients with chronic heart failure. J Am Coll Cardiol 2002; 40: 718–722. [DOI] [PubMed] [Google Scholar]
- 28. Kato TS, Collado E, Khawaja T, Kawano Y, Kim M, Farr M, Mancini DM, Schulze PC. Value of peak exercise oxygen consumption combined with B‐type natriuretic peptide levels for optimal timing of cardiac transplantation. Circ Heart Fail 2013; 6: 6–14. [DOI] [PubMed] [Google Scholar]
- 29. Fox AA, Marcantonio ER, Collard CD, Thoma M, Perry TE, Shernan SK, Muehlschlegel JD, Body SC. Increased peak postoperative b‐type natriuretic peptide predicts decreased longer‐term physical function after primary coronary artery bypass graft surgery. Anesthesiology 2011; 114: 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Juraschek SP, Daya N, Appel LJ, Miller ER 3rd, Matsushita K, Michos ED, Windham BG, Ballantyne CM, Selvin E. Subclinical cardiovascular disease and fall risk in older adults: results from the atherosclerosis risk in communities study. Am Geriatr Soc 2019; 67: 1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Węgrzynowska‐Teodorczyk K, Siennicka A, Josiak K, Zymliński R, Kasztura M, Banasiak W, Ponikowski P, Woźniewski M. Evaluation of skeletal muscle function and effects of early rehabilitation during acute heart failure: rationale and study design. Biomed Res Int 2018; 2018: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moro C, Lafontan M. Natriuretic peptides and cGMP signaling control of energy homeostasis. Am J Physiol Heart Circ Physiol 2013; 304: H358–H368. [DOI] [PubMed] [Google Scholar]
- 33. Araki S, Izumiya Y, Hanatani S, Rokutanda T, Usuku H, Akasaki Y, Takeo T, Nakagata N, Walsh K, Ogawa H. Akt1‐mediated skeletal muscle growth attenuates cardiac dysfunction and remodeling after experimental myocardial infarction. Circ Heart Fail 2012; 5: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamiya K, Sato Y, Takahashi T, Tsuchihashi‐Makaya M, Kotooka N, Ikegame T, Takura T, Yamamoto T, Nagayama M, Goto Y, Makita S, Isobe M. Multidisciplinary cardiac rehabilitation and long‐term prognosis in patients with heart failure. Circ Heart Fail 2020; 13: e006798. [DOI] [PubMed] [Google Scholar]
- 35. Ushijima A, Morita N, Hama T, Yamamoto A, Yoshimachi F, Ikari Y, Kobayashi Y. Effects of cardiac rehabilitation on physical function and exercise capacity in elderly cardiovascular patients with frailty. J Cardiol 2020; S0914‐5087: 30372–30375. [DOI] [PubMed] [Google Scholar]
- 36. Park LG, Schopfer DW, Zhang N, Shen H, Whooley MA. Participation in cardiac rehabilitation among patients with heart failure. J Card Fail 2017; 23: 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Golwala H, Pandey A, Ju C, Butler J, Yancy C, Bhatt DL, Hernandez AF, Fonarow GC. Temporal trends and factors associated with cardiac rehabilitation referral among patients hospitalized with heart failure: findings from get with the guidelines‐heart failure registry. J Am Coll Cardiol 2015; 66: 917–926. [DOI] [PubMed] [Google Scholar]
- 38. Sato Y, Kuwabara Y, Taniguchi R, Nishio Y, Miyamoto T, Fujiwara H, Takatsu Y. Malignant link between chronic heart failure and acute cardiac decompensation in patients with persistently increased serum concentrations of cardiac troponin. Int J Cardiol 2008; 126: 171–176. [DOI] [PubMed] [Google Scholar]
- 39. Kawai K, Hata K, Takaoka H, Kawai H, Yokoyama M. Plasma brain natriuretic peptide as a novel therapeutic indicator in idiopathic dilated cardiomyopathy during beta‐blocker therapy: a potential of hormone‐guided treatment. Am Heart J 2001; 141: 925–932. [DOI] [PubMed] [Google Scholar]
- 40. Talwar S, Squire IB, Downie PF, Mccullough AM, Campton MC, Davies JE, Barnett DB, Ng LL. Profile of plasma N‐terminal proBNP following acute myocardial infarction; correlation with left ventricular systolic dysfunction. Eur Heart J 2000; 21: 1514–1521. [DOI] [PubMed] [Google Scholar]
- 41. Tsutamoto T, Wada A, Maeda K, Hisanaga T, Fukai D, Maeda Y, Ohnishi M, Mabuchi N, Kinoshita M. Digitalis increases brain natriuretic peptide in patients with severe congestive heart failure. Am Heart J 1997; 134: 910–916. [DOI] [PubMed] [Google Scholar]
- 42. ter Maaten JM, Damman K, Hillege HL, Bakker SJ, Anker SD, Navis G, Voors AA. Creatinine excretion rate, a marker of muscle mass, is related to clinical outcome in patients with chronic systolic heart failure. Clin Res Cardiol 2014; 103: 976–983. [DOI] [PubMed] [Google Scholar]
- 43. Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Exp Gerontol 2002; 37: 477–489. [DOI] [PubMed] [Google Scholar]


