Abstract
Aims
Cardiac resynchronization therapy (CRT) in appropriately selected patients with heart failure improves symptoms and survival. It is necessary to correctly identify patients who will benefit most from this therapy. We aimed to assess the predictive power of the multidisciplinary team's clinical judgement in the short‐term death after CRT implantation.
Methods and results
Patients with heart failure and referred for the first CRT implant were prospectively included. Prior to implantation, all patients underwent a systematic assessment with a team composed of social work, nurse, psychologist, nutritionist, and clinical cardiologist. Based on this assessment, patients could be contraindicated to CRT or referred to the procedure as favourable or unfavourable. All patients should complete 12 months of follow‐up; 172 patients were referred for CRT, 21 (12.2%) were contraindicated after the multidisciplinary team evaluation, 71 (47%) referred to CRT as non‐favourable implants, and 80 (53%) as favourable implants. All‐cause mortality occurred in only 2 (2.5%) patients in the favourable group and in 30 (42.3%) in the non‐favourable group, P < 0.001 (log rank). Among the 20 variables used as possible predictors of worse prognosis by the multidisciplinary team, four were independently associated with mortality in the follow‐up after the multivariate analysis: 1 year MAGGIC score between 40% and 49%, relative risk (RR) 5.0, 95% confidence interval (CI) 1.3–18.6, P = 0.016; poor pharmacological adherence, RR 4.9, 95% CI 1.6–15.6, P = 0.007; glomerular filtration rate <35 mL/min/1.73 m2, RR 3.0, 95% CI 1.1–8.5, P = 0.041; and right ventricular dysfunction, RR 2.6, 95% CI 1.2–5.7, P = 0.018.
Conclusions
The clinical judgement before the CRT implantation performed by a multidisciplinary team through the analysis of clinical and psychosocial variables is a strong predictor of short‐term mortality.
Keywords: Cardiac resynchronization therapy, Heart failure, Multidisciplinary care, Device team
Introduction
Cardiac resynchronization therapy (CRT) has a well‐established recommendation in guidelines for implantable electrical cardiac devices. When properly indicated, several studies have shown improvement in symptoms and survival. 1 , 2 However, 30% of patients do not respond favourably to this therapy, and complications related to the procedure can reach 9.5%. 3 , 4
Several variables are related to an unfavourable response to CRT, but clinical characteristics are generally the most taken into account when choosing the best candidates. 5 Although it has been observed that socio‐economic and psychosocial variables are associated with cardiovascular outcomes, it remains unclear if these variables addressed in a multidisciplinary team (MDT) can contribute as a predictor of events after CRT implantation. 6 , 7 This is even more relevant in developing countries, where socio‐economic status and poor access to health care have a significant impact on the health of the population.
Candidates for advanced therapies for heart failure (HF), such as CRT, are generally fragile and need an integrative approach between various medical and non‐medical specialties. Multidisciplinary approach post‐CRT implantation is associated with a better clinical outcome and reduced HF hospitalization and all‐cause mortality. 8 As such, the MDT usually aggregates relevant data that go beyond clinical judgement, which can help in selecting patients who are more favourable for CRT implantation.
The aim of this study was to perform an MDT assessment prior to CRT implantation and to evaluate the predictive power of MDT judgement in short‐term mortality.
Methods
Study design
This is a observational prospective, single‐centre cohort study carried out at the Hospital Ana Nery, Salvador (Brazil), a reference of cardiovascular diseases of Brazil's Public Health System. Patients were recruited between May 2017 and May 2019. The follow‐up time was at least 12 months.
Participants and protocol
We enrolled adults (>18 years old) diagnosed with HF and reduced ejection fraction, referred by their own cardiologist assistants for the first implantation of cardiac resynchronization therapy (CRT‐P) or CRT combined with cardioverter‐defibrillator (CRT‐D). Patients who refused to sign the consent form to enter the study were excluded.
All patients underwent a systematic evaluation with an MDT composed of a social worker, nurse, psychologist, nutritionist, and clinical cardiologist. Patients were evaluated for New York Heart Association (NYHA) functional classification, presence of co‐morbidities (hypertension, diabetes, obstructive pulmonary disease, stroke, coronary artery disease, atrial fibrillation, and chronic kidney disease), data regarding the echocardiogram and electrocardiogram of the last 6 months, and the MAGGIC score. 9
In addition, the MDT included data regarding mood disorders with the Hospital Anxiety and Depression Scale (HADS‐A and HADS‐D) and were considered with some degree of anxiety and depression when the sum of the points on the scale was >8 (HADS‐A > 8 or HADS‐D > 8). 10 Poor therapeutic adherence and autonomy for maintaining self‐care was assessed using the adapted Self Care Hf questionnaire. Self‐care deficits were considered to be those with <50% success in the proposals relevant to the participant. 11 Additionally, the presence of social vulnerability was investigated, which was considered by the team in the presence of family income below the minimum wage and to be illiterate.
Patients who had a guideline‐based contraindication for CRT implantation, after the MDT evaluation, had the procedure suspended and returned for our outpatient follow‐up with optimal medical treatment. These patients were allocated to the contraindicated group and completed the study follow‐up.
Patients who had no contraindication for CRT were finally evaluated with the MDT for a decision‐making meeting regarding whether the implant would be favourable or not. This decision was made subjectively by the MDT taking into account 20 variables associated with a worse prognosis for CRT: left ventricular diastolic diameter >65 mm, right ventricular (RV) dysfunction, severe mitral insufficiency, age ≥75 years, 1 year MAGGIC score between 40% and 49%, body mass index <20, glomerular filtration rate (GFR) <35 mL/min/1.73 m2, NYHA IV in previous 6 months, use of vasoactive drugs in previous 6 months, absence of pharmacological optimization, absence of left bundle branch block QRS morphology, atrial fibrillation, presence of ≥3 co‐morbidities, RV pacing—defined as RV pacing >40% of time, left bundle branch block with QRS duration between 120 and 130 ms, HADS‐A > 8 points, HADS‐D > 8 points, deficit in self‐care, poor pharmacological adherence, and social vulnerability.
After the MDT evaluation, the patients were referred to procedure as favourable or unfavourable for cardiac resynchronization therapy. The cardiologist responsible for the CRT implantation and follow‐up was blind to the MDT opinion.
Follow‐up and endpoints
All patients were followed up during the in‐hospital phase and in outpatient visits every 3 months. Those unable to attend face‐to‐face consultations were contacted by phone. All survivors completed a minimum of 12 months of follow‐up. The main outcome was mortality from all causes.
Statistical analysis
The Kolmogorov–Smirnov test, Shapiro–Wilk test, and graphical methods were used to verify the normal distribution of continuous variables. Variables with normal distribution were described by mean ± standard deviation and compared by Student's t‐test, while variables with non‐parametric distribution were described by median (inter‐quartile range 25–75th percentile) and compared using the Mann–Whitney U test. Categorical variables were described as absolute and relative frequencies and compared using the χ 2 test. Kaplan–Meier curve was used to estimate survival, and the log‐rank test was used to compare survival between groups. For multivariate analysis, the Cox model was used, including variables with possible association with the outcome (P < 0.1). A value of P < 0.05 was considered statistically significant. The Statistical Package for the Social Sciences (SPSS) Version 20.0 was used for the analysis of all data.
Ethics committee
The Ethics Committee of the Hospital Ana Nery, Salvador, Bahia, approved the study, and all procedures were performed in accordance with the Declaration of Helsinki.
Results
A total of 172 patients were referred for cardiac resynchronization therapy in the study period. Of these, 21 (12.2%) were contraindicated for CRT implantation and were followed up in a registry parallel to the main study. The main reasons that led to the contraindication of the procedure were as follows: patients were asymptomatic in 10 (47%), QRS duration <120 ms in 6 (28.5%), presence of malignant neoplasia with life expectancy of <1 year in 5 (23.8%), left ventricular ejection fraction >35% in 5 (23.8%), mortality estimated by the 1 year MAGGIC score >50% in 4 (19%), and 9 (38%) patients had more than one characteristic for contraindication.
Of the 151 patients who underwent resynchronization therapy, 76 (50.3%) received CRT‐P and 75 (49.7%) CRT‐D. The implants were mainly transvenous, and only 3 (1.9%) patients received an epicardial implantation. On the MDT evaluation, 71 (47%) patients were classified as non‐favourable implants and 80 (53%) as favourable implants. The clinical and demographic characteristics of two groups are shown in Table 1 . Patients in the favourable group compared with the non‐favourable group had a similar age (mean), 58 (±10.6) vs. 59 (±13.8) years, P = 0.085; similar left ventricular ejection fraction (mean), 24.3% (±6.2) vs. 23.3% (±7.2), P = 0.315; and implanted less CRT‐D, 33 (41.3%) vs. 42 (59.2%), P = 0.034, respectively.
Table 1.
Baseline characteristic
| Favourable group | Non‐favourable group | P a | Contraindicated group | |
|---|---|---|---|---|
| N = 80 | N = 71 | N = 21 | ||
| Male sex, n (%) | 45 (52.5%) | 43 (60%) | 0.319 | 12 (57%) |
| Age, mean (±SD) | 58 (±10.6) | 59 (±13.8) | 0.085 | 60 (±13) |
| Co‐morbidities | ||||
| Hypertension, n (%) | 60 (75%) | 61 (86%) | 0.093 | 19 (91%) |
| Diabetes, n (%) | 18 (22.5%) | 31 (46%) | 0.006 | 13 (62%) |
| COPD, n (%) | 8 (10%) | 11 (15.6%) | 0.310 | 2 (9.5%) |
| Stroke, n (%) | 10 (12.5%) | 23 (32%) | 0.003 | 12 (57%) |
| Coronary artery disease, n (%) | 13 (16.2%) | 11 (15.6%) | 0.899 | 2 (9.5%) |
| Chronic kidney failure, n (%) | 4 (5%) | 29 (40.8%) | <0.001 | 11 (52.4%) |
| Body mass index, mean (±SD) | 25.8 (±1.3) | 23.4 (±4.4) | 0.331 | 24.2 (±5.7) |
| GFR, mean (±SD) | 71 (±21) | 58.2 (±28.2) | 0.180 | 58 (±27.2) |
| NYHA III–IV, n (%) | 47 (58.8%) | 60 (84.5%) | <0.001 | 10 (47.6%) |
| CRT‐D implantation, n (%) | 33 (41.3%) | 42 (59.2%) | 0.034 | — |
| Aetiology of heart failure | ||||
| Chagas disease, n (%) | 11 (13.7%) | 37 (52%) | <0.001 | 9 (43%) |
| Ischaemic, n (%) | 12 (15%) | 8 (11.3%) | 0.499 | 2 (9.5%) |
| Myocarditis, n (%) | 28 (35%) | 3 (4.2%) | <0.001 | 4 (19%) |
| Hypertensive, n (%) | 5 (6.3%) | 6 (8.5%) | 0.417 | 1 (4.3%) |
| Idiopathic, n (%) | 13 (16.3%) | 3 (4.2%) | 0.015 | 3 (14.7%) |
| Other known aetiologies, n (%) | 10 (12.5%) | 14 (19.7%) | 0.268 | 2 (9.5%) |
| Other characteristic | ||||
| Optimized medical treatment, n (%) | 70 (87.5%) | 53 (74%) | 0.034 | 15 (71.4%) |
| LVEF, mean (±SD) | 24.3 (±6.2) | 23.3 (±7.2) | 0.315 | 26.3 (±10) |
| LBBB and QRS > 140 ms, n (%) | 63 (78.7%) | 19 (26.8%) | <0.001 | 6 (28.5%) |
| 1 year MAGGIC score, mean (±SD) | 13.3 (±6.2) | 19.9 (±10.2) | <0.001 | 28.3 (±19.9) |
COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy combined with cardioverter‐defibrillator; GFR, glomerular filtration rate; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
Between favourable vs. non‐favourable groups.
The median number of variables that indicated a worse prognosis assessed by the MDT before CRT implantation was higher in the non‐favourable group when compared with the favourable group, 6 (5–8) vs. 2 (1–3), P < 0.001. Of these variables, the patients classified as non‐favourable had higher prevalence of multiple co‐morbidities, self‐care deficit, RV dysfunction, atrial fibrillation, depression, severe mitral regurgitation, poor pharmacological adherence, extreme social vulnerability, body mass index, GFR < 35 mL/min/1.73m2, RV pacing, vasoactive drugs in previous 6 months, NYHA Class IV in previous 6 months, and age >75 years old (Table 2 ).
Table 2.
Variables assessed by the multidisciplinary team before CRT implantation
| Favourable group | Non‐favourable group | P | |
|---|---|---|---|
| N = 80 | N = 71 | ||
| Left ventricular diastolic diameter >65 mm, n (%) | 29 (36.3%) | 53 (74.6%) | 0.102 |
| Multiple co‐morbidities (3 or more), n (%) | 14 (17.5%) | 42 (59.2%) | <0.001 |
| Self‐care deficit (Self Care <50%), n (%) | 4 (5%) | 40 (56.3%) | <0.001 |
| Right ventricular dysfunction, n (%) | 14 (17.5%) | 34 (47.9%) | <0.001 |
| Atrial fibrillation, n (%) | 2 (2.5%) | 33 (46.5%) | <0.001 |
| Depression (HADS‐D > 8 points), n (%) | 9 (11.3%) | 27 (38%) | <0.001 |
| Severe mitral regurgitation, n (%) | 10 (12.5%) | 25 (35.2%) | 0.001 |
| Poor pharmacological adherence, n (%) | 1 (1.3%) | 25 (35.2%) | <0.001 |
| Extreme social vulnerability, n (%) | 3 (3.8%) | 24 (33.8%) | <0.001 |
| Body mass index <20, n (%) | 3 (3.8%) | 23 (32.4%) | <0.001 |
| GFR < 35 mL/min/1.73 m2, n (%) | 3 (3.8%) | 20 (28.2%) | <0.001 |
| RV pacing, n (%) | 7 (8.8%) | 20 (28%) | 0.008 |
| Vasoactive drugs in previous 6 months, n (%) | 3 (3.8%) | 18 (25.4%) | <0.001 |
| NYHA IV in previous 6 months, n (%) | 1 (1.3%) | 16 (22.6%) | <0.001 |
| Without LBBB, n (%) | 8 (10%) | 13 (18.3%) | 0.108 |
| HADS‐A > 8 or HADS‐D > 8, n (%) | 5 (6.3%) | 11 (15.5%) | 0.057 |
| Age ≥75 years, n (%) | 3 (3.8%) | 11 (15.5%) | 0.013 |
| Without optimized medical treatment, n (%) | 7 (8.8%) | 10 (14.1%) | 0.218 |
| LBBB and QRS duration between 120 and 130 ms, n (%) | 2 (2.5%) | 7 (9.9%) | 0.058 |
| 1 year MAGGIC score between 40% and 49%, n (%) | — | 6 (8.5%) | — |
CRT, cardiac resynchronization therapy; GFR, glomerular filtration rate; HADS, Hospital Anxiety and Depression Scale; LBBB, left bundle branch block; NYHA, New York Heart Association; RV, right ventricular.
All surviving patients completed at least 12 months of follow‐up, and there was no loss of follow‐up. The mean follow‐up was 444.6 (±233.6) days. Eight (5.3%) individuals were contacted by phone, as they were unable to travel for a visit. Among patients who underwent CRT, death from any cause occurred in 32 (21.2%) participants, with 2 (2.5%) in the favourable group and 30 (42.3%) in the non‐favourable group, P < 0.001 (log rank) (Figure 1 ). Among patients contraindicated to CRT and followed in the study registry, death from any cause occurred in 9 (42.8%) participants in the same period.
Figure 1.
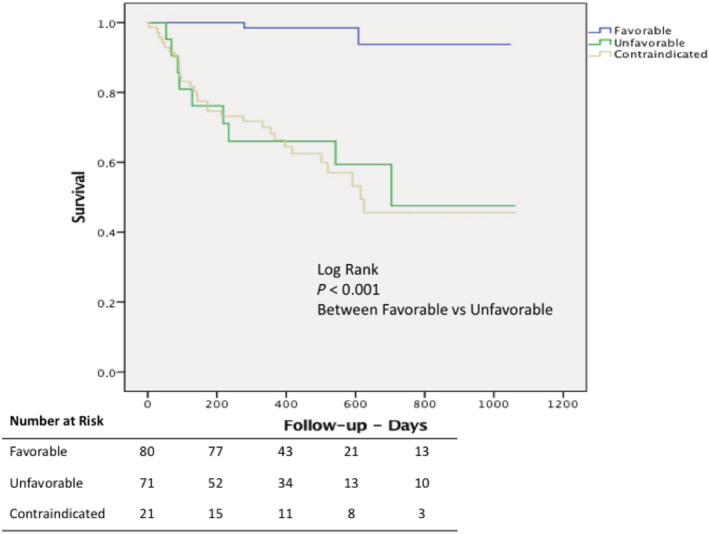
Kaplan–Meier estimates of survival in patients undergoing cardiac resynchronization therapy referenced as favourable by the multidisciplinary team (blue line), unfavourable (green line), and those contraindicated for cardiac resynchronization therapy (yellow line).
Among the 20 variables used as markers of worse prognosis by the MDT, four were independently associated with mortality in the follow‐up after the multivariate analysis: 1 year MAGGIC score between 40% and 49%, relative risk (RR) 5.0, 95% confidence interval (CI) 1.3–18.6, P = 0.016; poor pharmacological adherence, RR 4.9, 95% CI 1.6–15.6, P = 0.007; GFR < 35 mL/min/1.73 m2, RR 3.0, 95% CI 1.1–8.5, P = 0.041; and RV dysfunction, RR 2.6, 95% CI 1.2–5.7, P = 0.018 (Table 3 ).
Table 3.
Univariate and multivariate analysis for predictors of mortality
| Univariate analysis | P | Multivariate analysis | P | |
|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | |||
| Left ventricular diastolic diameter >65 mm, n (%) | 1.63 (0.7–3.9) | 0.286 | — | — |
| Multiple co‐morbidities (3 or more), n (%) | 1.3 (0.7–2.7) | 0.411 | — | — |
| Self‐care deficit (Self Care <50%), n (%) | 2.2 (1.1–4.5) | 0.026 | 0.4 (0.1–1.2) | 0.103 |
| Right ventricular dysfunction, n (%) | 2.9 (1.5–6.1) | 0.003 | 2.6 (1.2–5.7) | 0.018 |
| Atrial fibrillation, n (%) | 2.2 (1.1–4.4) | 0.036 | 1.6 (0.6–4.3) | 0.376 |
| Depression (HADS‐D > 8 points), n (%) | 2.4 (1.2–4.9) | 0.016 | 0.7 (0.2–2.2) | 0.530 |
| Severe mitral regurgitation, n (%) | 1.6 (0.8–3.5) | 0.182 | — | — |
| Poor pharmacological adherence, n (%) | 3.0 (1.4–6.3) | 0.003 | 4.9 (1.6–15.6) | 0.007 |
| Extreme social vulnerability, n (%) | 1.7 (0.7–3.9) | 0.239 | — | — |
| Body mass index <20, n (%) | 3.9 (1.9–7.9) | <0.001 | 1.8 (0.7–4.9) | 0.261 |
| GFR < 35 mL/min/1.73 m2, n (%) | 4.3 (2.0–9.2) | <0.001 | 3.0 (1.1–8.5) | 0.041 |
| RV pacing, n (%) | 3.2 (1.6–6.7) | 0.001 | 2.2 (0.9–5.0) | 0.067 |
| Vasoactive drugs in previous 6 months, n (%) | 2.3 (1.1–5.1) | 0.033 | 0.9 (0.3–2.9) | 0.816 |
| NYHA IV in previous 6 months, n (%) | 2.7 (1.3–5.9) | 0.010 | 1.2 (0.4–3.8) | 0.753 |
| Without LBBB, n (%) | 1.3 (0.4–4.4) | 0.630 | — | — |
| HADS‐A > 8 or HADS‐D > 8, n (%) | 1.3 (0.5–3.3) | 0.521 | — | — |
| Age ≥75 years, n (%) | 2.8 (1.1–7.0) | 0.025 | 1.8 (0.5–6.7) | 0.369 |
| Without optimized medical treatment, n (%) | 1.9 (0.7–5.4) | 0.242 | — | — |
| LBBB and QRS duration between 120 and 130 ms, n (%) | 1.6 (0.4–6.8) | 0.528 | — | — |
| 1 year MAGGIC score between 40% and 49%, n (%) | 11.9 (4.3–32.5) | <0.001 | 5.0 (1.3–18.6) | 0.016 |
CI, confidence interval; GFR, glomerular filtration rate; HADS, Hospital Anxiety and Depression Scale; LBBB, left bundle branch block; NYHA, New York Heart Association; RR, relative risk; RV, right ventricular.
Discussion
Our study demonstrated that patients identified as non‐favourable by the assessment of an MDT, in the pre‐implantation moment, had an increase in mortality in 1 year follow‐up after the procedure when compared with those classified as favourable. As the study design did not prevent the CRT implantation in these groups, we were able to demonstrate the predictive power of the MDT evaluation in patients referred for CRT.
Previous studies have already shown that in patients undergoing CRT, the performance of MDT after the procedure had an impact on reducing rehospitalization due to HF and all‐cause mortality when combined with conventional treatment. In addition, it is currently widely recommended by the HF guidelines to encourage multidisciplinary management of these patients. 8 This study corroborates the relevant role that the MDT has by showing how there was a better identification of the ideal candidate for CRT after its evaluation, reinforcing the importance and originality of the results presented. 12
The criteria for indicating CRT, as well as the type of device (CRT‐P or CRT‐D), are well recognized by cardiology societies, with a lower degree of recommendation for primary prevention of sudden cardiac death in subgroups of patients with non‐ischaemic HF. 13 However, the rate of non‐responders, despite the clinical indication according to the guidelines, can reach 40–50% according to a meta‐analysis that evaluated the largest studies in the area. 14 This stimulates the interest in searching for other variables that can interfere with the outcomes after CRT implantation, in addition to those already known. Furthermore, expanding the assessment to cover clinical socio‐economic and psychosocial issues can assist in choosing the ideal candidate. The inclusion of the MDT in the decision‐making process for CRT implantation could amplify the accountability of practitioners, yield superior cost‐effectiveness, and improve outcomes. 8
Another interesting aspect of this study was the similar mortality rates in the period among patients considered contraindicated and those classified as non‐favourable for CRT by the MDT. Although the contraindicated group was heterogeneous, most patients had poor prognostic variables. This result raises questions about the futility of CRT in specific populations and suggests a relevant role for the multidisciplinary care to better select the ideal candidate for CRT.
Despite the MDT decision‐making process being eminently subjective, it was based on 20 variables associated with a worse prognosis, four of which were independently associated with mortality in our study: 1 year MAGGIC score between 40% and 49%, poor pharmacological adherence, GFR < 35 mL/min/1.73 m2, and RV dysfunction.
The MAGGIC score is an easily applicable scale composed of 13 clinical variables, already validated in several cohorts in the Western world. This instrument helps to outline the guidelines regarding the so‐called minimum life expectancy of 1 year, which for a long time came from the field of subjectivity. In the cohort that assessed >20 000 patients with reduced ejection fraction, the MAGGIC score >50% was associated with higher mortality. This article positively reinforces the use of an objective instrument with high accuracy of prediction to assess the life expectancy of patients with HF. 9 , 15
Poor pharmacological adherence is undoubtedly one of the greatest challenges in care and patients with chronic diseases. Among patients with HF, low adherence affects negativelying morbidity and mortality, with increased risk of death and hospitalization due to HF decompensation. 16 In addition to behavioural aspects, socio‐economic status is a multifactorial problem that directly contributes to this phenomenon. We believe that the potential of an MDT to identify patients with poor therapeutic adherence, using specific instruments, should be encouraged.
Heart failure and chronic kidney disease (CKD) often coexist, and renal dysfunction is a predictor of poor prognosis in HF. 17 The symptomatic response to resynchronization does not differ between patients with and without CKD. However, the mortality in that with CKD is higher, despite the improvement in the functional class. 18
As already known, RV dysfunction is a worse prognosis variable in HF patients. Similarly, RV function significantly affects response to CRT with poor left ventricular reverse remodelling after CRT in patients with HF having severe RV dysfunction at baseline. 19 In our study, the RV function was an independent predictor of death on follow‐up suggesting its routine incorporation into the decision process of CRT.
This study has some limitations. We emphasize the unicentric design, the observational and subjective character of the MDT evaluation, which may impact its external validity. Additionally, it is a non‐randomized study that generates hypotheses and is exposed to confounding bias. Finally, the limited sample size makes the study vulnerable to Type 1 error.
The difference in mortality from all causes between the groups classified as favourable and unfavourable by the MDT highlights the reflection on the implementation of this type of assessment routinely in reference centres for implantation of devices through the performance of a Device Team. This assessment would help to identify patients who potentially do not respond favourably to CRT, adding other variables, mainly psychosocial, in addition to those already established in the guidelines.
Conclusions
Our data suggest that the evaluation by an MDT, using clinical and psychosocial variables, prior to the implantation of cardiac resynchronization therapy, is a strong predictor of short‐term mortality. The adoption of this strategy on a routine basis should be encouraged to better identify the patients who will benefit most from CRT. Additionally, these results serve as a reminder that subjective clinical judgement remains an important predictor of outcomes, in an era of evidence‐based medicine recommended by guidelines.
Conflict of interest
None declared.
Funding
None.
Passos, L. C. S. , Viana, T. T. , Carvalho, W. , Grimaldi, A. , Roriz, P. , Figueiredo, C. , Nascimento, T. , and Vieira de Melo, R. M. (2021) Judgement of the multidisciplinary team is an important predictor of mortality after cardiac resynchronization therapy. ESC Heart Failure, 8: 5275–5281. 10.1002/ehf2.13611.
References
- 1. Leyva F, Zegard A, Okafor O, De Bono J, McNulty D, Ahmed A, Marshall H, Ray D, Qiu T. Survival after cardiac resynchronization therapy: Results from 50 084 implantations. Europace 2019; 21: 754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Normand C, Linde C, Singh J, Dickstein K. Indications for cardiac resynchronization therapy: a comparison of the major international guidelines. JACC Hear Fail 2018; 6: 308–316. [DOI] [PubMed] [Google Scholar]
- 3. Hosseini SM, Moazzami K, Rozen G, Vaid J, Saleh A, Kevin Heist E, Vangel M, Ruskin JN. Utilization and in‐hospital complications of cardiac resynchronization therapy: Trends in the United States from 2003 to 2013. Eur. Heart J. 2017; 38: 2122–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014; 35: 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shanks M, Delgado V, Ng ACT, Auger D, Mooyaart EAQ, Bertini M, Marsan NA, Van Bommel RJ, Holman ER, Poldermans D, Schalij MJ, Bax JJ. Clinical and echocardiographic predictors of nonresponse to cardiac resynchronization therapy. Am Heart J 2011; 161: 552–557. [DOI] [PubMed] [Google Scholar]
- 6. Sokoreli I, Cleland JG, Pauws SC, Steyerberg EW, de Vries JJG, Riistama JM, Dobbs K, Bulemfu J, Clark AL. Added value of frailty and social support in predicting risk of 30‐day unplanned re‐admission or death for patients with heart failure: An analysis from OPERA‐HF. Int J Cardiol 2019; 278: 167–172. [DOI] [PubMed] [Google Scholar]
- 7. Wang SY, Tan ASL, Claggett B, Chandra A, Khatana SAM, Lutsey PL, Kucharska‐Newton A, Koton S, Solomon SD, Kawachi I. Longitudinal Associations between Income Changes and Incident Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. JAMA Cardiol 2019; 4: 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Altman RK, Parks KA, Schlett CL, Orencole M, Park MY, Truong QA, Deeprasertkul P, Moore SA, Barrett CD, Lewis GD, Das S, Upadhyay GA, Heist EK, Picard MH, Singh JP. Multidisciplinary care of patients receiving cardiac resynchronization therapy is associated with improved clinical outcomes. Eur Heart J 2012; 33: 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sartipy U, Dahlström U, Edner M, Lund LH. Predicting survival in heart failure: validation of the MAGGIC heart failure risk score in 51 043 patients from the swedish heart failure registry. Eur J Heart Fail 2014; 16: 173–179. [DOI] [PubMed] [Google Scholar]
- 10. Marcolino JÁM, Suzuki FM, Alli LAC, Gozzani JL, Mathias LADST. Measurement of anxiety and depression in preoperative patients. comparative study. Rev Bras Anestesiol 2007; 57: 157–166. [DOI] [PubMed] [Google Scholar]
- 11. Ferreira VMP, Silva LN, Furuya RK, Schmidt A, Rossi LA, Dantas RAS. Self‐care, sense of coherence and depression in patients hospitalized for decompensated heart failure. Rev da Esc Enferm 2015; 49: 387–393. [DOI] [PubMed] [Google Scholar]
- 12. Brignole M, Auricchio A, Baron‐Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2013; 15: 1070–1118. [DOI] [PubMed] [Google Scholar]
- 13. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez‐Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ, ESC Scientific Document Group . 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death the task force for the Management of Patients with ventricular arrhythmias and the prevention of sudden cardiac death of the european Society of Cardiology (ESC) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015; 36: 2793–2867. [DOI] [PubMed] [Google Scholar]
- 14. Birnie DH, Tang ASL. The problem of non‐response to cardiac resynchronization therapy. Curr Opin Cardiol 2006; 21: 20–26. [DOI] [PubMed] [Google Scholar]
- 15. Sawano M, Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, Sujino Y, Nagatomo Y, Kohno T, Anzai T, Fukuda K, Yoshikawa T. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Hear Fail 2018; 5: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitzgerald AA, Powers JD, Ho PM, Maddox TM, Peterson PN, Allen LA, Masoudi FA, Magid DJ, Havranek EP. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail 2011; 17: 664–669. [DOI] [PubMed] [Google Scholar]
- 17. Damman K, Valente MAE, Voors AA, O'Connor CM, Van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta‐analysis. Eur Heart J 2014; 35: 455–469. [DOI] [PubMed] [Google Scholar]
- 18. Bogdan S, Klempfner R, Sabbag A, Luria D, Gurevitz O, Bar‐Lev D, Lipchenca I, Nof E, Kuperstein R, Goldenberg I, Eldar M, Glikson M, Beinart R. Functional response to cardiac resynchronization therapy in patients with renal dysfunction and subsequent long‐term mortality. J Cardiovasc Electrophysiol 2014; 25: 1188–1195. [DOI] [PubMed] [Google Scholar]
- 19. Scuteri L, Rordorf R, Marsan NA, Landolina M, Magrini G, Klersy C, Frattini F, Petracci B, Vicentini A, Campana C, Tavazzi L, Ghio S. Relevance of echocardiographic evaluation of right ventricular function in patients undergoing cardiac resynchronization therapy. PACE ‐ Pacing Clin Electrophysiol 2009; 32: 1040–1049. [DOI] [PubMed] [Google Scholar]


