Abstract
Aims
The methodology to distinguish between the heart failure (HF) with recovered ejection fraction (HFrecEF) and those with continuously reduced ejection fraction (EF) (HFcrEF) on admission has not been established. We recently demonstrated that the ratio of plasma levels of pro‐B‐type natriuretic peptide (proBNP) to total BNP (proBNP plus mature BNP) is decreased on admission in patients with mild acute HF, but not in severe acute HF as a compensatory mechanism for activating cyclic GMP via increases of bioactive mature BNP. We aimed to test the hypothesis that the ratio of bioactive mature BNP to total BNP is associated with reverse remodelling capacity in patients with HF with reduced EF.
Methods and results
Plasma proBNP and total BNP were measured in patients with acute decompensated HF by using specific and sensitive enzyme immunochemiluminescent assay. Estimated percent mature BNP (%emBNP) was calculated as ([total BNP − proBNP]/total BNP) × 100. We retrospectively identified the patients with reduced EF (≤40%, on admission) who had echocardiographic data after discharge (n = 93). We defined patients with increased EF by >10% during the follow‐up term (median, 545 days) after the admission as HFrecEF group. We compared patient characteristics, %emBNP, and other biomarkers between HFrecEF and HFcrEF. Of the enrolled patients with HFrecEF (n = 32) and HFcrEF (n = 61), on admission, %emBNP was significantly higher in HFrecEF than in HFcrEF (44.1% vs. 36.9%; P < 0.05). There were no significant differences in left ventricular EF on admission between the two groups. The univariate analysis revealed that %emBNP on admission was associated with HFrecEF occurrence rate (P < 0.05), in contrast both total BNP and high‐sensitive cardiac troponin‐T levels were not associated with HFrecEF occurrence rate.
Conclusions
The ratio of mature BNP to total BNP in plasma at the time of hospital admission may be predictive of left ventricular contractile recovery. Preservation of the capacity to convert proBNP to mature BNP, but not myocardial injury itself, is associated with future ventricular contractile recovery.
Keywords: Heart failure, B‐type natriuretic peptide, Acute heart failure, Reverse remodelling
Background
In heart failure (HF) with reduced ejection fraction (HFrEF), recovery of left ventricular (LV) systolic function in response to medical therapy improves clinical outcomes. 1 Evaluation of an ability to predict a favourable myocardial response in cases of acute decompensated HF (ADHF) would be important for therapeutic management, particularly in clinical situations where inotropic agents and mechanical circulatory support have been applied as a bridge to recovery. Although B‐type natriuretic peptide (BNP) is utilized as a key indicator for risk stratification of HF patients, 2 no biomarkers measured during acute phases have yet been established as predictive of future ventricular contractile recovery.
Three molecular forms of BNP are present in the circulation: proBNP, the precursor of mature BNP with weak bioactivity, and two fragments cleaved from proBNP, bioactive mature BNP and the inactive N‐terminal fragment of proBNP (NT‐proBNP). 3 , 4 Mature BNP and NT‐proBNP are generated through enzymatic cleavage of proBNP, which is suppressed by glycosylation of proBNP and accelerated by activation of the cleavage enzymes. 5 We recently reported that a higher proBNP‐to‐total BNP ratio is frequently observed during acute phases in patients with severe HF. This suggests conversion of proBNP is attenuated in patients with severe ADHF. 6 Moreover, this attenuated conversion may be associated with further HF progression and reduction of the reverse remodelling effects of mature BNP due to its deficiency.
Aims
We hypothesized that lower proBNP levels, indicating a higher mature BNP‐to‐total BNP ratio, could be predictive of future reverse remodelling. To test that idea, we evaluated whether a higher mature BNP to total BNP ratio in plasma during acute phases of HF is associated with reverse remodelling in patients with HFrEF.
Methods
Patients admitted to our hospital with ADHF were enrolled prospectively (n = 183). Patients diagnosed with acute coronary syndrome, those receiving dialysis, those treated with carperitide before enrolment and those with preserved ejection fraction (EF > 40%) were excluded. This study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees. Written informed consent was obtained from all patients.
Venous blood was sampled within 48 h after admission, 3 days after admission, and 7 days after admission. Plasma total BNP (mature BNP + proBNP) and proBNP were measured using our recently developed chemiluminescent enzyme immunoassays. 7 Percent estimated mature BNP (%emBNP) was calculated as [(total BNP − proBNP)/total BNP] × 100. Two‐dimensional transthoracic echocardiography was performed on admission and 6 months or later after enrolment. Participants were classified into two groups based on their echocardiograms: the HF with recovered EF (HFrecEF) group was defined as patients whose EF was <40% on enrolment but increased by >10% from the baseline during the follow‐up, 8 and the HF with continuously reduced EF (HFcrEF) group was defined as patients whose EF was <40% on enrolment and did not increase more than 10% during the follow‐up. Categorical variables were compared between these two groups using the χ 2 test or Fisher's exact test, while continuous variables were compared using the Mann–Whitney U‐test. Linear regression was conducted to evaluate potential predictors of HFrecEF occurrence. One‐way repeated‐measures ANOVA was used to compare the changes in %mature BNP over 7 days.
Results
From a total of 111 consecutive patients with HFrEF, we excluded two patients who missed echocardiography examinations on admission and 16 patients who missed the examinations during the follow‐up. Ultimately, 93 patients were included and classified into the HFrecEF (n = 32) and HFcrEF (n = 61) groups (New York Heart Association functional class III–IV, 97.9%). The preadmission information was summarized in Supporting Information, Table S1 . The echocardiographically determined median tricuspid regurgitation evaluated by echocardiography was 3.0 m/s [interquartile range (IQR): 2, 5, & 3.3 m/s]. This indicates that substantial rate of patients had more than a mild degree of pulmonary hypertension on admission. The aetiologies of our patients' HF were ischaemic heart disease (n = 34, 36.6%), non‐ischaemic cardiomyopathy (n = 34, 36.6%), valvular heart disease (n = 8, 8.6%), and hypertensive heart disease (n = 17, 18.3%). Hypertensive heart disease was more frequently observed in patients with HFrecEF than with HFcrEF, while non‐ischaemic cardiomyopathy was observed less frequently in patients with HFrecEF than with HFcrEF. During the follow‐up period (median: 545 days), the change in EF from admission to the end of the follow‐up period was 22.5% (IQR, 15.4–27.6%) in the HFrecEF group and −0.5% (IQR, −5.0% to 3.8%) in the HFcrEF group (P < 0.0001).
There were no significant differences in age (median, 73 vs. 74 years old) or New York Heart Association functional class III–IV (96.7% vs. 100%) between the two groups (Table S1 ). As shown in Figure 1A–C , there was also no difference in EF on admission (median, 25% vs. 26%), but LV end‐diastolic diameter was larger (median, 64.0 mm vs. 58.5 mm) and total BNP levels were higher (median, 174.1 pM vs. 131.4 pM) in the HFcrEF group. As shown in the Table 1 , both total BNP and high‐sensitive cardiac troponin‐T (troponin‐T) were not associated with occurrence rate of HFrecEF. In contrast, %emBNP on admission was significantly associated with the HFrecEF occurrence rate. In addition, proBNP itself was not associated with clinical outcomes, while proBNP/estimated mature BNP ratio was statistically associated with the frequency of occurrences of HFrecEF even though it had borderline significance (P = 0.032). Through the course of treatment for HF, in the HFrecEF group, %emBNP was decreased at Day 7 from those on admission. In the HFcrEF group, by contrast, %emBNP remained unchanged (Figure 1 D ). On admission, %emBNP was significantly higher in HFrecEF than in HFcrEF (44.1% vs. 36.9%; P < 0.05). There was also a significant difference between the two groups with respect to the time‐course of %emBNP during the initial 7 days (P < 0.05). In patients with HFrecEF, a rate of those with ischaemic cardiomyopathy tended to be lower compared with those with HFcrEF (25.0% vs. 42.6%, respectively, P = 0.093). In a multivariate analysis, the statistical independence of the association between %emBNP and LV functional recovery from HF aetiologies was borderline significant (P = 0.081). There were statistically significant differences in %emBNP between the highest and lowest estimated glomerular filtration rate (eGFR) quartiles (P < 0.05). In addition, there was a statistically significant association between %emBNP and eGFR (P = 0.048) (Table S2 ). Percent emBNP was not associated with degree of pulmonary hypertension in this study.
Figure 1.
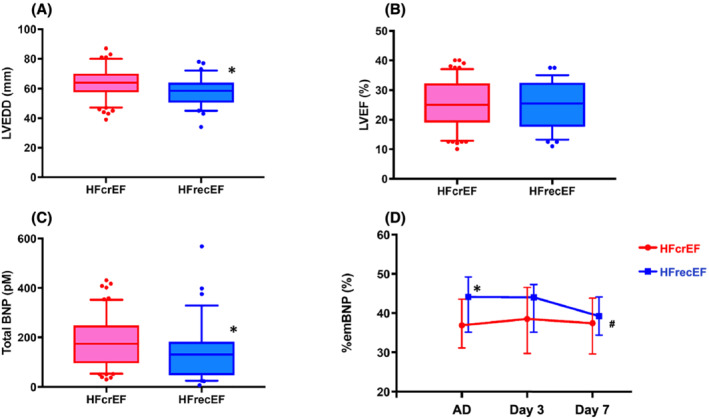
Differences in left ventricular (LV) end‐diastolic diameter (LVEDD) (A), LV ejection fraction (LVEF) (B), and total BNP levels (C) on admission between patients with heart failure (HF) with continuously reduced ejection fraction (HFcrEF, red) and HF with recovered ejection fraction (HFrecEF, blue). *P < 0.05, vs. HFcrEF. (D) Changes in the %estimate mature BNP (%emBNP) during the initial 7 days of hospitalization in patients with HFcrEF (red) or HFrecEF (blue). On admission to the hospital (AD), %emBNP was higher in patients with HFrecEF (*P < 0.05). #P = 0.045, one‐way repeated ANOVA between patients with HFcrEF and HFrecEF.
Table 1.
Relationship between variables with occurrences of HF with recovered EF
| Univariate analysis | Parameter estimate | 95%CI, lower | 95%CI, upper | P value |
|---|---|---|---|---|
| Total BNP (pM) | 0.003 | −0.001 | 0.008 | 0.131 |
| ProBNP (pM) | −0.070 | −0.015 | 0.000 | 0.056 |
| proBNP/emBNP | −0.008 | −0.017 | −0.002 | 0.032 |
| %emBNP | 6.084 | 1.231 | 11.476 | 0.019 |
| Troponin‐T (ng/mL) | 0.062 | −1.109 | 4.790 | 0.976 |
BNP, B‐type natriuretic peptide; CI, confidence interval; HF, heart failure.
Discussion
In the present study, we demonstrated that %emBNP, indicating the ratio of bioactive mature BNP to total BNP, on admission was significantly associated with the HFrecEF occurrence rate. In contrast, total BNP itself was not associated with its occurrence rate in patients with ADHF. This suggests that a higher plasma ratio of mature BNP to total BNP on admission is predictive of EF recovery. With that in mind, we suggest the following two hypotheses. First, elevated mature BNP levels contribute to LVEF recovery in HFrecEF patients. Second, HFrecEF patients capable of LV contractile recovery preserve a capacity for proBNP conversion to mature BNP, resulting in higher plasma levels of mature BNP. A smaller LV end‐diastolic diameter in HFrecEF patients, suggesting less advanced LV remodelling, which is also indicated by the low total BNP levels, may support the latter hypothesis. As shown in the Table 1 , the proBNP/emBNP ratio was also inversely associated with clinical outcomes. These findings suggest that enhanced O‐glycosylation of proBNP, which inhibits its processing and increases levels of proBNP and decreases levels of mature BNP, may be associated with poor LV functional recovery, as the physiological effects of proBNP are weaker than those of mature BNP. 9 , 10 In addition, troponin‐T levels during the acute phase were not associated with LVEF changes, which suggests the relationship between myocardial injury itself and LVEF recovery was weak in the present study.
These findings raise the question, does sacubitril/varsartan brings about cardiac reverse remodelling in clinical settings via elevation of mature natriuretic peptide? They also allow us to suggest that increases in the molar ratio of mature BNP to total BNP may be compatible with elevation of mature BNP in patients treated with sacubitril/valsartan. Further investigation will be necessary to verify whether elevation of mature BNP with sacubitril/valsartan brings about reverse remodelling in HFrEF patients.
Limitation
One limitation of this study is that although we excluded patients with acute coronary syndrome, a substantial number of patients with ischaemic cardiomyopathy were enrolled to this study. In a multivariate analysis, the statistical independence of the association between %emBNP and LV functional recovery from HF etiologies had only borderline significance. However, the number of patients enrolled this study was limited. Further large‐scale investigation will be necessary to confirm our findings in more detail. For example, it remains unclear whether differences in the underlying cardiac disease affect future LV functional recovery through the mature BNP‐cGMP cascade. Second, a statistically significant association between %emBNP and eGFR was observed, as shown in Table S2 . We need to carefully interpret the %emBNP results from patients with a low eGFR, since %emBNP is affected by renal function. Further large‐scale investigation will be required to confirm the eGFR cut‐off value when assessing the %emBNP.
Conclusions
In conclusion, the ratio of mature BNP to total BNP in plasma at the time of hospital admission may be predictive of LV contractile recovery. Preservation of the capacity to convert proBNP to mature BNP, but not myocardial injury itself, is associated with future ventricular contractile recovery.
Conflict of interest
The authors declare that they have no conflict of interest in this study.
Supporting information
Table S1. Preadmission information in the enrolled patients.
Table S2. BNP levels on admission in patients divided by estimated glomerular filtration rate (eGFR).
Kimura, A. , Takahama, H. , Nishikimi, T. , Takashio, S. , Hayashi, T. , Nagai‐Okatani, C. , Nakagawa, Y. , Yasuda, S. , Anzai, T. , Minamino, N. , and Izumi, C. (2021) Molecular ratio of mature B‐type natriuretic peptide in acute heart failure: an indicator for ventricular contractile recovery. ESC Heart Failure, 8: 5617–5621. 10.1002/ehf2.13684.
Naoto Minamino and Chisato Izumi equally contributed to this work.
References
- 1. Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction. Circulation 2014; 129: 2380–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL Jr, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR, American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research. Role of biomarkers for the prevention, assessment, and Management of Heart Failure: A scientific statement from the American Heart Association. Circulation 2017; 135: e1054–e1091. [DOI] [PubMed] [Google Scholar]
- 3. Weber M, Hamm C. Role of B‐type natriuretic peptide (BNP) and NT‐proBNP in clinical routine. Heart 2006; 92: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishikimi T, Kuwahara K, Nakao K. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J Cardiol 2011; 57: 131–140. [DOI] [PubMed] [Google Scholar]
- 5. Vodovar N, Séronde M‐F, Laribi S, Gayat E, Lassus J, Boukef R, Nouira S, Manivet P, Samuel JL, Logeart D, Ishihara S, Cohen Solal A, Januzzi JL Jr, Richards AM, Launay JM, Mebazaa A, GREAT Network. Post‐translational modifications enhance NT‐proBNP and BNP production in acute decompensated heart failure. Eur Heart J 2014; 35: 3434–3441. [DOI] [PubMed] [Google Scholar]
- 6. Takahama H, Takashio S, Nishikimi T, Hayashi T, Nagai‐Okatani C, Nakagawa Y, Amaki M, Ohara T, Hasegawa T, Sugano Y, Kanzaki H, Yasuda S, Kangawa K, Minamino N, Anzai T. Ratio of pro‐B‐type natriuretic peptide (BNP) to total BNP is decreased in mild, but not severe, acute decompensated heart failure patients: A novel compensatory mechanism for acute heart failure. Int J Cardiol 2018; 258: 165–171. [DOI] [PubMed] [Google Scholar]
- 7. Nishikimi T, Okamoto H, Nakamura M, Ogawa N, Horii K, Nagata K, Nakagawa Y, Kinoshita H, Yamada C, Nakao K, Minami T, Kuwabara Y, Kuwahara K, Masuda I, Kangawa K, Minamino N, Nakao K. Direct immunochemiluminescent assay for proBNP and total BNP in human plasma proBNP and total BNP levels in normal and heart failure. PLoS One. 2013; 8: e53233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilcox JE, Fang JC, Margulies KB, Mann DL. Heart failure with recovered left ventricular ejection fraction: JACC scientific expert panel. J Am Coll Cardiol 2020; 76: 719–734. [DOI] [PubMed] [Google Scholar]
- 9. Nakagawa Y, Nishikimi T, Kuwahara K, Fujishima A, Oka S, Tsutamoto T, Kinoshita H, Nakao K, Cho K, Inazumi H, Okamoto H, Nishida M, Kato T, Fukushima H, Yamashita JK, Wijnen WJ, Creemers EE, Kangawa K, Minamino N, Nakao K, Kimura T. MiR30‐GALNT1/2 Axis‐mediated glycosylation contributes to the increased secretion of inactive human prohormone for brain natriuretic peptide (proBNP) from failing hearts. J Am Heart Assoc 2017; 6: e003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang F, O'Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, O'Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, Apple FS, Maisel AS, Pollitt NS, Protter AA. Evidence for functional heterogeneity of circulating B‐type natriuretic peptide. J Am Coll Cardiol 2007; 49: 1071–1078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Preadmission information in the enrolled patients.
Table S2. BNP levels on admission in patients divided by estimated glomerular filtration rate (eGFR).


