Abstract
Aims
Abnormal renal function is a common feature in patients on heart transplant waiting lists. This study aimed to identify the haemodynamic parameters associated with decreased estimated glomerular filtration rate (eGFR) in patients listed for heart transplantation (HT) and renal function improvement following HT.
Methods and results
A total of 176 adults (52 years old, 81% men) with available right heart catheterization (RHC) listed in our centre for HT between 2014 and 2019 were studied. Cardiac catheterization measurements were obtained at time of HT listing evaluation. Changes in renal function were assessed between RHC and 6 months after HT. Median eGFR was 63 mL/min/1.73 m2 at time of RHC. Central venous pressure > 10 mmHg was associated with a two‐fold increase in the likelihood of eGFR < 60 mL/min/1.73 m2 at time of RHC (adjusted odd ratio, 2.2; 95% confidence interval, 1.1–4.7; P = 0.04). In the 134 patients (76%) who underwent HT during follow‐up, eGFR decreased by 7.9 ± 29.7 mL/min/1.73 m2 from RHC to 6 months after HT. In these patients, low cardiac index (<2.1 L/min/m2) at initial RHC was associated with a (adjusted) 6 month post‐HT eGFR improvement of 12.2 mL/min/1.73 m2 (P = 0.018). Patients with eGFR < 60 mL/min/1.73 m2 and low cardiac index at time of RHC exhibited the greatest eGFR improvement (delta eGFR = 18.3 mL/min/1.73 m2) while patients with eGFR ≥ 60 mL/min/1.73 m2 and normal cardiac index had a marked decrease in eGFR (delta eGFR = −27.7 mL/min/1.73 m2, P < 0.001).
Conclusions
Central venous pressure is the main haemodynamic parameter associated with eGFR < 60 mL/min/1.73 m2 in patients listed for HT. Low cardiac index prior to HT is associated with post‐transplant renal function recovery.
Keywords: Cardiorenal syndrome, Heart failure, Heart transplantation, Renal function, Cardiac index, Cardiovascular diseases, Cardiac oedema
Introduction
Cardiorenal syndrome is a well‐known phenomenon in acute and chronic settings leading to high morbidity and mortality in heart failure (HF) patients. 1 , 2 Glomerular filtration rate (GFR) below 60 mL/min/1.73 m2 is observed in 30–50% of patients with HF. 1 , 2 The pathophysiology of renal impairment in the setting of HF reportedly includes age, underlying co‐morbidities (especially hypertension and diabetes), and also specific HF factors such as reduced renal blood flow, ncreased central venous pressure (CVP), and reduced systemic blood pressure. 3
Central venous pressure has been deemed to be an independent key factor associated with impaired renal function in patients with a wide range of heart diseases and especially HF. 4 , 5 , 6 Surprisingly, low cardiac index, which has initially been emphasized as a central driver of cardiorenal interactions, 7 does not appear to be strongly associated with renal function 4 , 5 especially when competing with other haemodynamic variables. 5 Similarly, some uncertainty remains regarding the impact of low blood pressure on renal function.
Advanced HF is the end‐stage of all cardiomyopathies and is characterized by the severity of the symptoms becoming refractory to medical treatment. 8 , 9 In this advanced setting, episodes of low output and congestion are frequent, the latter of which can trigger organ dysfunction, including worsening renal function. The prevalence of estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 in advanced HF patients awaiting transplantation is considerably high when considering the relatively young age of these patients compared with the general HF population. Accordingly, between 2000 and 2017, 43% of heart transplant recipients in the USA had an eGFR < 60 mL/min/1.73 m2 at transplant listing. 10 Because a chronically low cardiac index is a predominant feature in patients with advanced HF, 8 this trait may be increasingly associated with renal dysfunction in this particular population as opposed to previous reports in which haemodynamic studies were primarily performed in less severely ill populations.
Renal function frequently drops during the first year after heart transplantation (HT), particularly in patients with prior renal dysfunction, co‐morbidities (age and diabetes), and receiving high‐dose cyclosporine. 11 , 12 International HT guidelines consider reduced eGFR < 40 mL/min/1.73 m2 as a relative contraindication for HT alone. 13 However, renal dysfunction in this advanced HF setting can be partially reversible 14 possibly due to the normalization of haemodynamics following cardiac transplantation. Notwithstanding, the association of haemodynamic parameters with renal function prior to cardiac transplantation as well as with changes in post‐transplant renal function remains poorly characterized.
The co‐primary objectives of the present study were (i) to assess the haemodynamic parameters associated with renal dysfunction in patients with advanced HF listed for HT and (ii) to determine whether pre‐transplant haemodynamic features are related to renal function recovery following HT.
Methods
Study population
This study consisted of a single‐centre cohort analysis using the French national CRISTAL database. The 201 newly registered adult patients (18 years or older) entered on the French national waiting list for HT between 1 January 2014 and 31 December 2019 in our heart transplant centre were included. Twenty‐five patients without right heart catheterization were excluded from the analysis. Overall, 134 patients (76%) ultimately underwent HT. The data of 99 patients who survived the HT process were included in the analysis of eGFR change at 6 months. Among them, two patients were on dialysis at 6 months, and an arbitrary value of 15 mL/min/1.73 m2 of eGFR was used in the analysis of eGFR changes.
Pulmonary artery catheterization
Cardiac catheterization measurements were obtained at the time of assessment for HT listing. The most recent catheterization was selected in those having undergone multiple exams before their listing. Serum creatinine was obtained within 24 h prior to right heart catheterization. Haemodynamic variables obtained during catheterization included systolic blood pressure (mmHg), diastolic blood pressure (mmHg), cardiac output (L/min), pulmonary capillary wedge pressure (mmHg), mean pulmonary arterial pressure (mmHg), and right atrial pressure as an indicator of CVP (mmHg). Cardiac index (L/min/m2) was determined as cardiac output divided by body surface area, which was calculated using the Boyd formula (BSA = 0.03330 × W(0.6157 − 0.0188 × log10(W)) × H(0.3)).
Data collection and variables
This single‐centre study was performed using the data of the French national registry (CRISTAL). The registry is administered by the Agence de la biomédecine and prospectively collects data on all organ transplant candidates in France along with their outcomes. A description of the CRISTAL registry has previously been published. 15 Selected data included the candidate's demographics, primary diagnosis, clinical status, device therapy, short‐term and long‐term mechanical circulatory support, and laboratory parameters at transplantation. Data at time of heart catheterization and at 6 months after HT were collected retrospectively. GFR was estimated using the Modification of Diet in Renal Disease (MDRD) formula (186.3 * (creatinine in μmol/L/88.4)−1.154 * Age−0.203 (* 0.742 for female patients)).
The study was conducted according to French legislation stipulating that anonymized research studies based on the national CRISTAL registry do not require institutional review board approval.
Statistical analysis
All analyses were performed using SPSS Version 27 (IBM SPSS Statistics for Windows, NY: IBM Corp). Continuous variables are expressed as medians with interquartile 25–75% (IQ25–75) range, categorical variables as frequencies (percentages), and odd ratios (ORs) as estimates and 95% confidence interval (95% CI). Comparisons of baseline characteristics were carried out using Student's t‐test and 𝜒2 test as required. The two‐tailed significance level was set at P < 0.05.
Factors associated with decreased eGFR were assessed using univariable and multivariable logistic regression. To this aim, a dichotomous categorization of catheterization parameters was used: CVP, mean pulmonary artery pressure, and pulmonary capillary wedge pressure were classified as normal if ≤10, ≤25, and ≤21 mmHg or increased if >10, >25, and >21 mmHg, respectively, and cardiac index and blood pressure were described as decreased if <2.1 L/min/m2 and <90 mmHg or normal if ≥2.1 L/min/m2 and ≥90 mmHg, respectively. 16 Models were adjusted for age, BNP Z‐score, and history of hypertension. In a supplementary analysis, models were further adjusted for renin–angiotensin system blockers and loop diuretics dose at transplant listing.
Factors associated with changes in eGFR between catheterization and 6 months after HT were assessed using linear regression in crude and adjusted models. The adjusted model included adjustment for age, BNP Z‐score, history of hypertension, eGFR at time of catheterization, treatment with renin–angiotensin system blockers, loop diuretics dose at transplant listing, and calcineurin inhibitor regimen after HT.
Results
Baseline characteristics
Among the 176 studied patients (mean age 52, 81% men), 40% had dilated cardiomyopathy and 34% had ischaemic cardiomyopathy (Table 1 ). Median HF duration was 15 months, and a minority of patients presented co‐morbidities (23% hypertension and 15% diabetes). All patients had severe left ventricular systolic dysfunction with a median ejection fraction of 25%. Median BNP and eGFR were 868 ng/L and 63 mL/min/1.73 m2, respectively. Cardiac index was severely reduced (1.97 L/min/m2) although pulmonary artery pressure, CVP, and pulmonary capillary wedge pressure were only moderately increased (Figure S1).
Table 1.
Baseline patient characteristics, medication use, and haemodynamic variables
| Missing (%) | Whole cohort | GFR < 60 mL/min/1.73 m2 | GFR > 60 mL/min/1.73 m2 | P‐value | |
|---|---|---|---|---|---|
| 176 | 83 (47) | 93 (53) | |||
| Demographic data | |||||
| Age, years | 0 (0) | 52 (44–61) | 58 (49–63) | 49 (38–57) | 0.001 |
| Male sex, n (%) | 0 (0) | 143 (81.3) | 70 (84.3) | 73 (78.5) | 0.234 |
| Body mass index, kg/m2 | 0 (0) | 24.6 (22.1–27.9) | 24.3 (22.3–28.4) | 24.9 (22–27.5) | 0.801 |
| Medical history | |||||
| Hypertension, n (%) | 0 (0) | 41 (23.3) | 28 (33.7) | 13 (14) | 0.002 |
| Diabetes, n (%) | 0 (0) | 26 (14.8) | 15 (18.1) | 11 (11.8) | 0.246 |
| Smoking history, n (%) | 0 (0) | 115 (65.3) | 53 (63.9) | 62 (66.7) | 0.698 |
| Heart failure duration, months | 23 (13) | 15 (8–48) | 15 (8–39) | 13 (6–51) | 0.188 |
| Heart failure aetiology, n (%) | 0.21 | ||||
| Ischaemic, n (%) | 0 (0) | 60 (34.1) | 32 (38.6) | 28 (30.1) | |
| Dilated cardiomyopathy, n (%) | 0 (0) | 71 (40.3) | 28 (33.7) | 43 (46.2) | |
| Hypertrophic cardiomyopathy, n (%) | 0 (0) | 13 (7.4) | 9 (10.8) | 4 (4.3) | |
| Heart transplant rejection, n (%) | 0 (0) | 6 (3.4) | 4 (4.8) | 2 (2.2) | |
| Other, n (%) | 0 (0) | 26 (14.8) | 10 (12) | 16 (17.2) | |
| Medical treatment, n (%) | |||||
| Beta‐blocker, n (%) | 0 (0) | 101 (57.4) | 49 (59) | 52 (55.9) | 0.678 |
| ACE inhibitor or ARB, n (%) | 0 (0) | 71 (40.3) | 31 (37.3) | 40 (43) | 0.448 |
| ARNI, n (%) | 0 (0) | 53 (30.1) | 25 (30.1) | 28 (30.1) | 0.999 |
| MRA, n (%) | 101 (57.4) | 42 (50.6) | 59 (63.4) | 0.99 | |
| Furosemide equivalent dose, mg | 0 (0) | 100 (40–250) | 80 (0–160) | 40 (0–160) | 0.93 |
| CRT, n (%) | 0 (0) | 56 (31.8) | 34 (41) | 22 (23.7) | 0.014 |
| ICD, n (%) | 0 (0) | 129 (73.3) | 61 (73.5) | 68 (73.1) | 0.955 |
| Inotropic support, n (%) | 0 (0) | 54 (30.7) | 29 (34.9) | 25 (26.9) | 0.25 |
| LVAD, n (%) | 0 (0) | 30 (17) | 13 (15.7) | 17 (18.3) | 0.647 |
| Biological data | |||||
| Creatinine, μmol/L | 0 (0) | 110 (86–142) | 144 (127–189) | 88 (75–101) | <0.001 |
| eGFR, mL/min/1.73 m2 | 11 (6) | 62.7 (46.9–83.7) | 44.9 (36.1–51.7) | 82.0 (68.0–100.3) | <0.001 |
| Total bilirubin, μmol/L | 0 (0) | 18 (12–26) | 20 (15–31) | 15 (12–22) | 0.011 |
| BNP, ng/L | 11 (6) | 868 (395–1696) | 1240 (640–2027) | 582 (244–1277) | <0.001 |
| Echocardiography | |||||
| LVEF, % | 9 (5) | 25 (20–30) | 25 (20–35) | 25 (20–30) | 0.185 |
| LVEF < 25%, n (%) | 9 (5) | 88 (50%) | 41 (49.4) | 47 (50.5) | 0.881 |
| LVEDD, mm | 24 (14) | 66 (57–74) | 64 (54–73) | 67 (59–74) | 0.521 |
| Right heart catheterization | |||||
| Ambulatory | 0 (0) | 93 (52.8) | 43 (65.1) | 50 (53.8) | 0.132 |
| Hospitalized | 33 (18.8) | 19 (22.9) | 14 (15.1) | ||
| ICU | 50 (28.4) | 21 (25.3) | 29 (31.2) | ||
| Haemodynamic variables | |||||
| CVP, mmHg | 0 (0) | 7 (3–12) | 10 (4–15) | 6 (3–10) | 0.003 |
| CVP > 10 mmHg | 0 (0) | 67 (38.1) | 42 (50.6) | 25 (26.9) | 0.001 |
| Mean PAP, mmHg | 0 (0) | 27 (21–35) | 28 (22–37) | 26 (19–34) | 0.069 |
| Mean PAP > 25 mmHg | 0 (0) | 102 (58) | 51 (61.4) | 51 (54.8) | 0.378 |
| PCWP, mmHg | 2 (1) | 19 (12–24) | 20 (13–26) | 19 (11–24) | 0.132 |
| PCWP > 22 mmHg, mmHg | 2 (1) | 61 (35) | 32 (39) | 29 (32) | 0.359 |
| CI, L/min/m2 | 6 (3) | 1.98 (1.66–2.31) | 1.95 (1.64–2.27) | 2.04 (1.70–2.41) | 0.458 |
| CI < 2.1 L/min/m2 | 6 (3) | 102 (58) | 52 (64) | 50 (56) | 0.289 |
| CVP/PCWP | 3 (1) | 0.39 (0.22–0.66) | 0.46 (0.24–0.71) | 0.33 (0.19–0.56) | 0.034 |
| PAPi | 21 (12) | 3.2 (1.8–5.4) | 2.6 (1.6–4.3) | 3.7 (2.2–6.7) | 0.274 |
| RVSWI, g/m/beat/m2 | 6 (3) | 6.4 (4.4–9.2) | 5.8 (4.2–9.2) | 6.8 (4.8–9.2) | 0.937 |
| Clinical and functional variables | |||||
| Pulmonary vascular resistance, Wood unit | 0 (0) | 2.38 (1.51–3.41) | 2.5 (1.63–3.56) | 2.3 (1.63–2.36) | 0.187 |
| Systolic blood pressure, mmHg | 0 (0) | 100 (90–110) | 100 (85–109) | 100 (91–110) | 0.203 |
| Diastolic blood pressure, mmHg | 0 (0) | 62 (56–70) | 61 (56–70) | 62 (57–70) | 0.612 |
| Heart rate, b.p.m. | 0 (0) | 77 (67–90) | 78 (70–90) | 76 (65–92) | 0.87 |
| NYHA | 1 (0.5) | 2.83 (0.63) | 2.89 (0.61) | 2.77 (0.64) | 0.224 |
| VO2 max | 82 (47) | 12 (9.6–15.5) | 10.8 (9.4–13.6) | 14 (10.5–15.8) | 0.137 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor; BNP, B‐type natriuretic peptide; CI, cardiac index; CRT, cardiac resynchronization therapy; CVP, central venous pressure; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; ICU, intensive care unit; LVAD, left ventricular assist device; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PAPi, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; RVSWI, right ventricular stroke work index; VO2 max, maximum oxygen consumption rate.
Factors associated with estimated glomerular filtration rate < 60 mL/min/1.73 m2 in patients awaiting heart transplantation
Approximately half of the population (47%) had an eGFR < 60 mL/min/1.73 m2; these patients were older, had a higher prevalence of hypertension, and had cardiac resynchronization therapy. CVP was higher in those patients with eGFR < 60 mL/min/1.73 m2 (10 mmHg vs. 6 mmHg, P = 0.003). Similarly, prevalence of eGFR < 60 mL/min/1.73 m2 was 38% and 63% in patients with CVP ≤ 10 and >10 mmHg, respectively (Figure 2 ).
Figure 2.
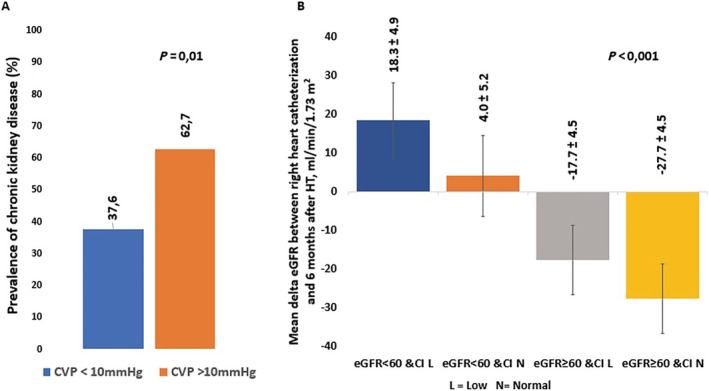
Prevalence of eGFR < 60 mL/min/1.73 m2 according to central venous pressure at right heart catheterization (Panel A) and mean delta eGFR between right heart catheterization and 6 months after heart transplantation according to eGFR and cardiac index at right heart catheterization (Panel B). CI, cardiac index; CVP, central venous pressure; eGFR, estimated glomerular filtration rate; HT, heart transplantation; RHC, right heart catheterization.
Associations between various pulmonary artery catheter parameters and chronic kidney disease using logistic regression are shown in Table 2 . Continuous and categorical CVPs were associated with decreased eGFR (OR per mmHg increase, 1.07; 95% CI, 1.02–1.12; P = 0.004 and OR for CVP > 10 mmHg, 2.79; 95% CI, 1.49–5.22; P = 0.001, respectively). This finding resulted in a correlation of eGFR and CVP on a continuous scale (ρ = −0.24, P = 0.001) (Figure 1 ). This association persisted after adjustment for age, history of hypertension, and BNP Z‐score. Patients with CVP > 10 mmHg doubled the likelihood of eGFR < 60 mL/min/1.73 m2 (OR in adjusted model = 2.21; 95% CI, 1.05–4.66; P = 0.036). The results remained unchanged when further adjusting for renin–angiotensin system blockers and loop diuretics dose (OR of CVP > 10 mmHg = 2.39; 95% CI, 1.09–5.25; P = 0.030, whereas all other haemodynamic variables had P > 0.05).
Table 2.
Univariable and multivariable models of the association between clinical or haemodynamic profiles and estimated glomerular filtration rate < 60 mL/min/1.73 m2
| Crude associations | Adjusted associations | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | |
| Haemodynamic variables | ||||||
| CVP per 1 mmHg | 1.07 | 1.02–1.12 | 0.004 | 1.05 | 1.00–1.11 | 0.065 |
| CVP > 10 mmHg | 2.79 | 1.49–5.22 | 0.001 | 2.21 | 1.05–4.66 | 0.036 |
| mPAP per mmHg | 1.03 | 1.00–1.06 | 0.072 | 1.00 | 0.97–1.04 | 0.881 |
| mPAP > 25 mmHg | 1.31 | 0.72–2.40 | 0.376 | 0.90 | 0.43–1.89 | 0.780 |
| PCWP per mmHg | 1.02 | 0.99–1.06 | 0.220 | 0.99 | 0.95–1.04 | 0.997 |
| PCWP > 22 mmHg | 1.34 | 0.72–2.50 | 0.356 | 0.91 | 0.43–1.90 | 0.797 |
| CI per 1 L/min/m2 | 0.67 | 0.38–1.19 | 0.171 | 1.18 | 0.67–2.08 | 0.560 |
| CI < 2.1 L/min/m2 | 1.40 | 0.75–2.60 | 0.287 | 1.11 | 0.54–2.30 | 0.769 |
| PVR per Wood unit | 1.15 | 0.93–1.41 | 0.188 | 1.01 | 0.81–1.27 | 0.906 |
| CVP/PCWP | 2.51 | 1.02–6.20 | 0.045 | 2.24 | 0.81–6.18 | 0.118 |
| PAPi | 0.96 | 0.90–1.03 | 0.278 | 0.99 | 0.91–1.07 | 0.772 |
| RVSWI | 1.00 | 0.92–1.07 | 0.936 | 0.97 | 0.88–1.06 | 0.483 |
| Clinical and functional variables | ||||||
| SBP per mmHg | 0.99 | 0.97–1.01 | 0.203 | 0.99 | 0.96–1.01 | 0.258 |
| SBP < 90 mmHg | 1.80 | 0.89–3.60 | 0.099 | 1.68 | 0.74–3.79 | 0.214 |
| DBP per mmHg | 0.99 | 0.97–1.02 | 0.610 | 1.00 | 0.97–1.03 | 0.918 |
| NYHA class per 1 unit | 1.35 | 0.83–2.18 | 0.224 | 1.09 | 0.62–1.92 | 0.768 |
| VO2 max per mL/kg/min | 0.93 | 0.84–1.02 | 0.140 | 0.99 | 0.88–1.12 | 0.905 |
95% CI, 95% confidence interval; CI, cardiac index; CVP, central venous pressure; DBP, diastolic blood pressure; HR, heart rate; mPAP, mean pulmonary arterial pressure; NYHA, New York Heart Association; OR, odds ratio; PAPi, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RVSWI, right ventricular stroke work index; SBP, systolic blood pressure, VO2 max, maximum oxygen consumption rate.
Model adjusted for age, BNP Z‐score, and history of hypertension.
Figure 1.
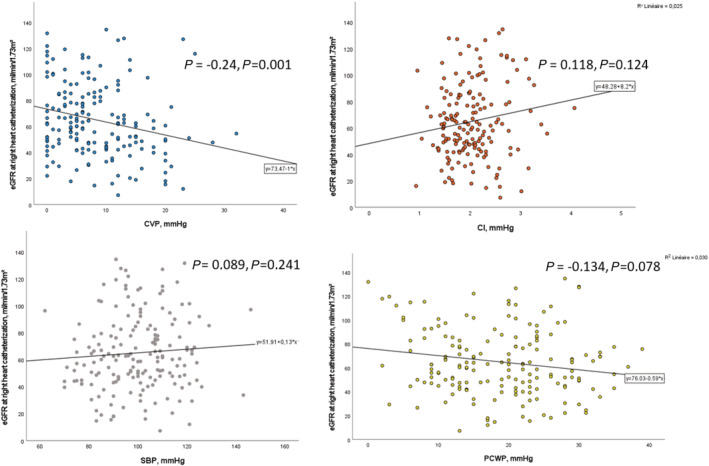
Correlation between CVP, CI, SBP, PCWP, and eGFR at right heart catheterization. CI, cardiac index; CVP, central venous pressure; eGFR, estimated glomerular filtration rate; HR, heart rate; PCWP, pulmonary capillary wedge pressure; SBP, systolic blood pressure.
Factors associated with favourable renal outcome at 6 months after heart transplantation
Median time between right heart catheterization and HT was 4.5 (1–11) months. Mean eGFR decreased by 7.9 ± 29.7 mL/min/1.73 m2 at 6 months after HT, with substantial intergroup differences according to eGFR and cardiac index at the time of heart catheterization (Table 3).
Table 3.
Evolution of renal parameters between right heart catheterization and 6 months after heart transplantation according to estimated glomerular filtration rate at the time of right heart catheterization
| Missing (%) | Whole cohort | GFR < 60 mL/min/1.73 m2 at the time of RHC | GFR > 60 mL/min/1.73 m2 at the time of RHC | P‐value | |
|---|---|---|---|---|---|
| Haemodialysis at time of transplantation, n (%) | 0 (0) | 11 (8) | 7 (5) | 4 (3) | 0.23 |
| Acute kidney injury in first 3 months after HT | 0 (0) | 0.17 | |||
| No acute kidney injury | 30 (22) | 18 (28) | 12 (17) | ||
| Stage 1 acute kidney injury | 16 (12) | 9 (14) | 7 (10) | ||
| Stage 2 acute kidney injury | 27 (20) | 9 (14) | 18 (26) | ||
| Stage 3 acute kidney injury | 60 (45) | 28 (44) | 33 (47) | ||
| Length of ICU stay, days | 0 | 11 (8–19) | 11 (8–21) | 11 (8–16) | 0.07 |
| Post‐operative mortality | 0 | 21 (16) | 14 (10) | 7 (5) | 0.06 |
| End‐stage renal dysfunction at 6 months after HT, n (%) | 0 (0) | 2 (1) | 1 (1) | 1 (1) | 0.79 |
| Creatinine at time of HT, μmol/L | 0 (0) | 113 (88–145) | 142 (118–180) | 92 (71–111) | <0.001 |
| Creatinine at 6 months after HT | 2 (1) | 113 (92–143) | 123 (94–139) | 105 (89–149) | 0.387 |
| Mean eGFR at time of HT, mL/min/1.73 m2 | 0 (0) | 65.7 (30.6) | 45.8 (17.4) | 82.8 (29.1) | <0.001 |
| Median eGFR at time of HT, mL/min/1.73 m2 | 0 (0) | 59.3 (42.8–80.3) | 44.7 (34.1–56.3) | 75.2 (60.6–108) | <0.001 |
| Mean eGFR at 6 months after HT, mL/min/1.73 m2 | 0 (0) | 62.3 (24.7) | 57.0 (23.4) | 65.8 (25.1) | 0.086 |
| Median eGFR at 6 months after HT, mL/min/1.73 m2 | 0 (0) | 59.3 (43.3–76.3) | 52.0 (43.5–68.8) | 65.5 (41.5–80.7) | 0.086 |
| Mean delta eGFR between RHC and HT, mL/min/1.73 m2 | 0 (0) | −1.0 (23.3) | 2.1 (16.0) | −3.6 (28.0) | 0.027 |
| Median delta eGFR between RHC and HT, mL/min/1.73 m2 | 0 (0) | −2.6 (−14.2 to 7.7) | −0.9 (−7.0 to 9.4) | −7.0 (−20.1 to 7.5) | 0.027 |
| Mean delta eGFR between RHC and M6 after HT, mL/min/1.73 m2 | 0 (0) | −7.9 (29.7) | 13.1 (23.6) | −22.2 (24.5) | <0.001 |
| Median delta eGFR between RHC and M6 after HT, mL/min/1.73 m2 | 0 (0) | −5.9 (−24.2 to 9.0) | 14.9 (−4.2 to 23.0) | −16.0 (−34.6 to −3.1) | <0.001 |
eGFR, estimated glomerular filtration rate; HT, heart transplantation; ICU, intensive care unit; M6, 6 months; RHC, right heart catheterization.
Linear regression analysis for delta eGFR between right heart catheterization and 6 months after HT is shown in Table 4 . Low cardiac index was the only parameter associated with an increase in eGFR between pulmonary artery catheterization and 6 months after HT in univariable analysis and after adjustment. Patients with cardiac index < 2.1 (over 60% of the study population) exhibited an increase in eGFR of 16.6 mL/min/1.73 m2 (P = 0.006) and 11.8 mL/min/1.73 m2 (P = 0.036) in univariable and multivariable analysis, respectively. The results remained unchanged after exclusion of the two patients on haemodialysis at 6 months after HT. Neither elevated venous pressure (P = 0.766) nor low systolic blood pressure (P = 0.526) was associated with changes in kidney function in multivariable analysis.
Table 4.
Linear regression analysis for delta estimated glomerular filtration rate between right heart catheterization and 6 months after heart transplantation
| Delta eGFR between RHC and M6 after HT | ||||
|---|---|---|---|---|
| Crude associations | Adjusted associations | |||
| β | P‐value | β | P‐value | |
| Haemodynamic variables | ||||
| CVP per 1 mmHg | −0.01 | 0.980 | −0.19 | 0.633 |
| CVP > 10 mmHg | −1.89 | 0.766 | −3.81 | 0.481 |
| mPAP per mmHg | 0.27 | 0.368 | −0.06 | 0.823 |
| mPAP > 25 mmHg | −1.16 | 0.855 | −6.64 | 0.257 |
| PCWP per mmHg | 0.10 | 0.210 | −0.23 | 0.491 |
| PCWP > 22 mmHg | −3.87 | 0.530 | −6.03 | 0.239 |
| CI per 1 L/min/m2 | −12.5 | 0.027 | −15.4 | 0.006 |
| CI < 2.1 L/min/m2 | 16.7 | 0.006 | 12.2 | 0.018 |
| PVR per Wood unit | 3.16 | 0.109 | 2.08 | 0.200 |
| Clinical variables | ||||
| SBP per mmHg | −0.08 | 0.706 | −0.04 | 0.842 |
| SBP < 90 mmHg | 4.38 | 0.526 | −3.90 | 0.503 |
| DBP per mmHg | −0.03 | 0.912 | 0.02 | 0.937 |
| NYHA class per 1 unit | −9.98 | 0.045 | −7.66 | 0.066 |
| VO2 max per mL/kg/min | 0.63 | 0.434 | 1.38 | 0.117 |
| Renal variable | ||||
| eGFR at the time of RHC, mL/min/1.73 m2 | −0.67 | <0.001 | ||
CI, cardiac index; CVP, central venous pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, heart rate; mPAP, mean pulmonary arterial pressure; NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RHC, right heart catheterization; SBP, systolic blood pressure; VO2 max, maximum oxygen consumption rate.
Model adjusted for age, BNP Z‐score, history of hypertension, eGFR at the time of RHC, treatment with renin–angiotensin system blockers and loop diuretics dose at transplant listing, and calcineurin inhibitor regimen in the 6 months after heart transplantation.
Interplay of estimated glomerular filtration rate and cardiac index or central venous pressure in predicting estimated glomerular filtration rate improvement following heart transplantation
Patients with eGFR < 60 mL/min/1.73 m2 and cardiac index < 2.1 L/min/m2 exhibited the greatest eGFR improvement (delta eGFR = 18.3 mL/min/1.73 m2) while patients with eGFR ≥ 60 mL/min/1.73 m2 and cardiac index ≥ 2.1 L/min/m2 had a marked decrease in eGFR (delta GFR = −27.7 mL/min/1.73 m2, P < 0.001) (Figure 2 ). When further considering the interplay of CVP and pre‐transplant eGFR, patients who had the most significant renal recovery were patients with low CVP and low eGFR, whereas eGFR changes were similar in patients with low CVP and elevated CVP when eGFR was normal at baseline (Supporting Information, Figure S2 ). In addition, patients who exhibited the greatest eGFR recovery following HT were patients with low eGFR at baseline, low CI, and normal CVP (Supporting Information, Figure S3 ).
Results according to estimated glomerular filtration rate estimated by the chronic kidney disease epidemiology collaboration formula
The association of CVP with decreased eGFR (adjusted OR for CVP > 10 mmHg = 2.59, 1.22–5.51, P = 0.01) and the association of CI with eGFR recovery (adjusted beta for CI < 2.1 L/min/m2 = 9.9, P = 0.04) remained significant when using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) formula in lieu of the MDRD formula.
Discussion
To the best of our knowledge, this is the first report describing the haemodynamic determinants of mid‐term renal function in advanced HF patients listed for cardiac transplantation. Our results highlight the association between elevated CVP and renal dysfunction in patients awaiting transplantation. Moreover, this is the first study to show that low cardiac index prior to HT is associated with long‐term renal improvement after transplantation, especially in patients with renal function impairment prior to transplantation.
Impact of central venous pressure and cardiac index on renal function in patients awaiting heart transplantation
Damman et al. previously showed that elevated CVP was associated with impaired renal function in a wide spectrum of cardiomyopathy patients who had undergone catheterization. 4 However, in this latter study, HF patients accounted for only 16% of the population and mean cardiac index was normal (2.9 L/min/m2). Importantly, our population also differed from the HF population studied in the ESCAPE trial in that all of our patients were listed for HT, thus identifying a very specific population of young patients with advanced HF and minimal co‐morbidities. 17
Central venous pressure elevation can represent a cause and also a consequence of chronic kidney dysfunction. Indeed, baseline renal dysfunction is an important predictor of diuretic response leading to congestion despite an increase in diuretic dose, described as diuretic resistance. 18 , 19 The mechanisms of renal toxicity secondary to congestion are multiple and not fully understood, although are reported to include haemodynamic effects, neurohormonal effects, endothelial activation, and proinflammatory cytokine secretion as well as iron deficiency. 20 Prior to the present study, the respective weight of elevated CVP and decreased cardiac index on eGFR in an advanced HF population with severely reduced cardiac index was uncertain. Indeed, renal blood flow is an important determinant of GFR, 5 , 7 with cardiac index and renal blood flow concomitantly decreasing in patients with advanced HF. 7 There is conversely some uncertainty regarding the association between cardiac index and eGFR. 7 , 21 Cardiac index and GFR have been reported not to be associated in acute decompensated severe HF populations, 6 , 22 although these studies lacked specific data regarding advanced HF populations with severely reduced cardiac index. In our pre‐transplant patients, we found no relationship between reduced cardiac index and eGFR, suggesting that these two parameters are not significantly related even in the lowest cardiac index values range.
Impact of cardiac index in post‐transplant kidney function
Solid organ transplantation is associated with a risk of decline in kidney function. 12 , 23 Common factors associated with chronic kidney dysfunction include patient age, race and sex, history of hypertension, reduced eGFR, and post‐transplant calcineurin regimen. 10 , 23 Similarly, acute kidney injury following HT is a common feature 24 that can have an impact on mid‐term renal function in certain patients. In the present study population, a decrease in eGFR (7.1 mL/min/1.73 m2) was observed between right heart catheterization and 6 months after HT. The benefit of restoring cardiac output could have been counterbalanced by acute perioperative renal toxicity and calcineurin nephropathy. Moreover, this relative stability masked a sharp disparity between individuals according to renal function and cardiac index at the time of right heart catheterization.
The greatest improvement in renal function in our transplanted population was observed in patients with eGFR < 60 mL/min/1.73 m2 and low cardiac index at the time of right heart catheterization. Decreased cardiac index was the only significant predictor of renal function recovery after transplantation. Improvement in renal function after restoring cardiac index is a well‐known phenomenon in patients implanted with a left ventricular assist device 25 , 26 in all stages of chronic kidney disease. 27 Early renal function improvement is mostly observed in this setting in younger patients with impaired baseline eGFRs, most often in patients implanted within a bridge‐to‐transplant strategy. 27 Similarly, Yalcin et al. emphasized that patients experiencing renal improvement had a lower cardiac index (2.5 L/min/m2 vs. 3 L/min/m2) and also increased CVP and pulmonary capillary wedge pressure. 27 These prior findings in patients with left ventricular assist device are in keeping with the present results in the setting of HT.
The International Society for Heart and Lung Transplantation guidelines recommend combined heart–kidney transplantation as opposed to HT alone in patients with irreversible renal dysfunction and eGFR < 40 mL/min/1.73 m2. 13 Nevertheless, standardized criteria for defining irreversible renal dysfunction are still lacking. There is furthermore no recommendation for systematic kidney biopsy given the procedural risk and the absence of strong evidence supporting this approach. Combined heart and kidney transplantation is recommended by some authors if tubular atrophy and interstitial fibrosis involve at least 25% of the sampled cortex. 28 In light of the present results, we suggest integrating low cardiac index to the clinical decision‐making process, in addition to currently considered criteria such as age, severity of HF, history of diabetes or hypertension, baseline eGFR, and kidney biopsy (if feasible or routinely performed on site in this setting).
Study limitations
The present analysis has several limitations. Some of the data were collected through a national registry and thus subject to missing data and potential coding errors. However, data are entered prospectively in order to limit these errors. The cross‐sectional and retrospective design of our study further precludes the assessment of a cause–effect relationship. The patient population comprised in the current study was limited to patients with advanced HF listed for HT and may not be generalizable to less severe or older HF patients. Haemodynamic variables are subject to measurement errors as well as intraoperator or interoperator variability; however, our centre is a tertiary centre with a vast experience in pulmonary artery catheterization limiting this bias. We did not routinely perform right heart catheterization following HT. We consequently cannot assess the association between CVP changes and renal function recovery. Glomerular function was not measured but rather estimated by the MDRD formula, and no patient underwent a renal biopsy prior to HT to assess cardiorenal syndrome reversibility. INTERMACS class at time of transplantation was not reported in the CRISTAL registry and thus not available for further investigation. Episodes of rejection and calcineurin dose were not assessed although severe rejections were quite rare due to induction with anti‐thymocyte globulin, and our routine protocol always included a calcineurin inhibitor without a dose reduction strategy in the first 6 months after HT.
Implications for clinical practice
Renal congestion is a common occurrence in patients with advanced HF and is associated with chronic kidney disease. Although there is no prospective study showing the benefit associated with the normalization of blood volume in patients with advanced HF, it would seem reasonable to endeavour achieving this goal even in patients with severely impaired renal or cardiac function. The present findings clearly highlight the association between low cardiac index and renal improvement after HT. In practice, while a decrease in renal function is likely to be observed following HT, clinicians should nevertheless be aware that patients with low cardiac output often experience an improvement in renal function. Low cardiac index should hence be considered as an additional good prognosis factor for renal function recovery in addition to young age, absence of diabetes or hypertension history, and baseline renal function when considering patients for isolated HT versus combined heart–kidney transplantation.
Conclusions
Central venous pressure is the main haemodynamic factor associated with decreased eGFR in advanced HF patients listed for HT. Low cardiac index prior to transplantation is associated with renal function recovery and should be assessed during the decision‐making process of combined heart–kidney transplantation versus isolated HT.
Conflict of interest
G.B. reports personal fees from AstraZeneca, Boehringer Ingelheim, and Abbott, outside the submitted work. L.S. reports personal fees from Novartis Pharma, AstraZeneca, and Vifor Pharma, outside the submitted work. J.B. has nothing to disclose. E.H.V. has nothing to disclose. A.J.D. reports personal fees from Boehringer Ingelheim, Novartis, Sanofi Genzyme, and Amicus. N.M. reports grants and personal fees from Novartis, personal fees from Bayer, grants from AstraZeneca, and personal fees from Vifor Pharma, outside the submitted work. M.P. has nothing to disclose. P.R. reports consulting fees from Bayer, G3P (stocks), Idorsia, and KBP; honoraria from Ablative Solutions, AstraZeneca, Bayer, Boehringer Ingelheim, Corvidia, CVRx, Fresenius, Grunenthal, Novartis, Novo Nordisk, Vifor Pharma, Inc., Sanofi, Sequana Medical, Servier, Stealth Peptides, and Vifor Fresenius Medical Care Renal Pharma; and cofounder: CardioRenal. N.G. reports personal fees from AstraZeneca, Bayer, Boehringer, Novartis, and Vifor.
Funding
The Nancy team is supported by the RHU Fight‐HF, a public grant overseen by the French National Research Agency (ANR) as part of the second ‘Investissements d'Avenir’ programme (reference: ANR‐15‐RHUS‐0004), the French PIA project ‘Lorraine Université d'Excellence’ (reference: ANR‐15‐IDEX‐04‐LUE), the ANR FOCUS‐MR (reference: ANR‐15‐CE14‐0032‐01), the ERA‐CVD EXPERT (reference: ANR‐16‐ECVD‐0002‐02), the Contrat de Plan Etat Lorraine IT2MP, and FEDER Lorraine.
Supporting information
Figure S1. Baseline hemodynamic values at time of right heart catheterization according to eGFR.
Figure S2. Mean delta eGFR between right heart catheterization and 6 months after heart transplantation according to eGFR and central venous pressure at right heart catheterization.
Figure S3. Mean delta eGFR between right heart catheterization and 6 months after heart transplantation according to eGFR, cardiac index and central venous pressure at right heart catheterization.
Baudry, G. , Sebbag, L. , Bourdin, J. , Hugon‐Vallet, E. , Jobbe Duval, A. , Mewton, N. , Pozzi, M. , Rossignol, P. , and Girerd, N. (2021) Haemodynamic parameters associated with renal function prior to and following heart transplantation. ESC Heart Failure, 8: 4944–4954. 10.1002/ehf2.13534.
References
- 1. Rossignol P, Hernandez AF, Solomon SD, Zannad F. Heart failure drug treatment. Lancet 2019; 393: 1034–1044. [DOI] [PubMed] [Google Scholar]
- 2. Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation 2018; 138: 929–944. [DOI] [PubMed] [Google Scholar]
- 3. Carubelli V, Metra M, Lund LH. Negotiating renal dysfunction when treating patients with heart failure. Expert Rev Cardiovasc Ther 2018; 16: 113–122. [DOI] [PubMed] [Google Scholar]
- 4. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009; 53: 582–588. [DOI] [PubMed] [Google Scholar]
- 5. Damman K, Navis G, Smilde TDJ, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 2007; 9: 872–878. [DOI] [PubMed] [Google Scholar]
- 6. Guglin M, Rivero A, Matar F, Garcia M. Renal dysfunction in heart failure is due to congestion but not low output. Clin Cardiol 2011; 34: 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure: relationship of cardiac index to kidney function. Drugs 1990; 39: 10–21. [DOI] [PubMed] [Google Scholar]
- 8. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge‐Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska‐Migaj E, McDonagh T, Seferovic P, Ruschitzka F. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 1505–1535. [DOI] [PubMed] [Google Scholar]
- 9. Metra M, Dinatolo E, Dasseni N. The new heart failure association definition of advanced heart failure. Card Fail Rev 2019; 5: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar A, Howard A, Thomas CP. Estimated glomerular filtration rate at transplant listing and other predictors of post‐heart transplant mortality and the development of ESRD. Transplantation 2020; 104: 2444–2452. [DOI] [PubMed] [Google Scholar]
- 11. Kolsrud O, Karason K, Holmberg E, Ricksten SE, Felldin M, Samuelsson O, Dellgren G. Renal function and outcome after heart transplantation. J Thorac Cardiovasc Surg 2018; 155: 1593–1604.e1. [DOI] [PubMed] [Google Scholar]
- 12. Rubel JR, Milford EL, McKay DB, Jarcho JA. Renal insufficiency and end‐stage renal disease in the heart transplant population. J Heart Lung Transplant 2004; 23: 289–300. [DOI] [PubMed] [Google Scholar]
- 13. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger‐Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EAM, Zuckermann A. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10‐year update. J Heart Lung Transplant 2016; 35: 1–23. [DOI] [PubMed] [Google Scholar]
- 14. Brisco MA, Coca SG, Chen J, Owens AT, McCauley BD, Kimmel SE, Testani JM. Blood urea nitrogen/creatinine ratio identifies a high‐risk but potentially reversible form of renal dysfunction in patients with decompensated heart failure. Circ Heart Fail 2013; 6: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jasseron C, Legeai C, Jacquelinet C, Leprince P, Cantrelle C, Audry B, Porcher R, Bastien O, Dorent R. Prediction of waitlist mortality in adult heart transplant candidates. Transplantation 2017; 101: 2175–2182. [DOI] [PubMed] [Google Scholar]
- 16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200.27206819 [Google Scholar]
- 17. Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW, ESCAPE Investigators and ESCAPE Study Coordinators . Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness. JAMA 2005; 294: 1625–1633 Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.294.13.1625 [DOI] [PubMed] [Google Scholar]
- 18. Damman K, Voors AA, Hillege HL, Navis G, Lechat P, Van Veldhuisen DJ, Dargie HJ, CIBIS‐2 Investigators and Committees . Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. Eur J Heart Fail 2010; 12: 974–982. [DOI] [PubMed] [Google Scholar]
- 19. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion — a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 137–155. [DOI] [PubMed] [Google Scholar]
- 20. Gnanaraj JF, Von Haehling S, Anker SD, Raj DS, Radhakrishnan J. The relevance of congestion in the cardio‐renal syndrome. Kidney Int 2013; 83: 384–391. [DOI] [PubMed] [Google Scholar]
- 21. Gilbert C, Cherney DZI, Parker AB, Mak S, Floras JS, Al‐Hesayen A, Parker JD. Hemodynamic and neurochemical determinates of renal function in chronic heart failure. Am J Physiol Regul Integr Comp Physiol 2016; 310: R167–R175. [DOI] [PubMed] [Google Scholar]
- 22. Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 2008; 51: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 23. Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349: 931–940. [DOI] [PubMed] [Google Scholar]
- 24. Guven G, Brankovic M, Constantinescu AA, Brugts JJ, Hesselink DA, Akin S, Struijs A, Birim O, Ince C, Manintveld OC, Caliskan K. Preoperative right heart hemodynamics predict postoperative acute kidney injury after heart transplantation. Intensive Care Med 2018; 44: 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hasin T, Topilsky Y, Schirger JA, Li Z, Zhao Y, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Joyce L, Daly R, Park SJ, Kushwaha SS. Changes in renal function after implantation of continuous‐flow left ventricular assist devices. J Am Coll Cardiol 2012; 59: 26–36. [DOI] [PubMed] [Google Scholar]
- 26. Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WWH, Parikh CR, Testani JM. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail 2014; 7: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yalcin YC, Muslem R, Veen KM, Soliman OI, Hesselink DA, Constantinescu AA, Brugts JJ, Manintveld OC, Fudim M, Russell SD, Tomashitis B, Houston BA, Hsu S, Tedford RJ, Bogers AJJC, Caliskan K. Impact of continuous flow left ventricular assist device therapy on chronic kidney disease: a longitudinal multicenter study. J Card Fail 2020; 26: 333–341. [DOI] [PubMed] [Google Scholar]
- 28. Labban B, Arora N, Restaino S, Markowitz G, Valeri A, Radhakrishnan J. The role of kidney biopsy in heart transplant candidates with kidney disease. Transplantation 2010; 89: 887–893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Baseline hemodynamic values at time of right heart catheterization according to eGFR.
Figure S2. Mean delta eGFR between right heart catheterization and 6 months after heart transplantation according to eGFR and central venous pressure at right heart catheterization.
Figure S3. Mean delta eGFR between right heart catheterization and 6 months after heart transplantation according to eGFR, cardiac index and central venous pressure at right heart catheterization.


