Abstract
Aims
The importance of iron deficiency (ID) in heart failure with preserved ejection fraction (HFpEF) is unknown. In HF with reduced ejection fraction (HFrEF), ID is reported as an independent predictor of mortality in HF although not all published studies agree. Different definitions of ID have been assessed, and the natural history of untreated ID not established, which may explain the conflicting results. This study aimed to assess the relationship between ID and mortality in HFpEF, clarify which definition of ID correlates best with outcomes in HFrEF, and determine the prognostic importance of change in ID status over time.
Methods and results
Analyses were conducted on data from 1563 patients participating in a prospective international cohort study comparing HFpEF with HFrEF. Plasma samples from baseline and 6 month visits were analysed for the presence of ID. Two ID definitions were evaluated: IDFerritin = ‘ferritin < 100 mcg/L or ferritin 100–300 mcg/L + transferrin saturation < 20%’ and IDTsat = ‘transferrin saturation < 20%’. The risk of all‐cause mortality and death/HF hospitalization associated with baseline ID (IDFerritin or IDTsat) and change in ID status at 6 months (persistent, resolving, developing, or never present) was estimated in multivariable Cox proportional hazards models. Of 1563 patients, 1115 (71%) had HFrEF and 448 (29%) HFpEF. Prevalence of ID was similar in HFpEF and HFrEF (58%). Patients with ID were more likely to be female, diabetic, and have a higher co‐morbid burden than patients without ID. ID by either definition did not confer independent risk for either all‐cause mortality or death/HF hospitalization for patients with HFpEF [IDFerritin hazard ratio (HR) 0.65 (95% confidence interval 0.40–1.05), P = 0.08; IDTsat HR 1.16 (0.72–1.87), P = 0.55]. In the overall study cohort (HFrEF + HFpEF) and HFrEF subgroup, IDFerritin was inferior to IDTsat in prediction of all‐cause mortality [overall cohort: HR 1.21 (0.95–1.53), P = 0.12 vs. HR 1.95 (1.52–2.51), P < 0.01; HFrEF: HR 1.12 (0.85–1.48), P = 0.43 vs. HR 1.57 (1.15–2.14), P < 0.01]. Persistence of IDTsat at 6 months was strongly associated with poor outcomes compared with never having IDTsat [HR 2.22 (1.42–3.46), P < 0.01] or having IDTsat at baseline self‐resolve by 6 months [HR 1.40 (1.06–1.86), P = 0.02].
Conclusions
Iron deficiency is equally prevalent in HFpEF and HFrEF but is negatively prognostic only in HFrEF. The natural history of ID is important; persistent ID is strongly associated with mortality whereas resolution is not. IDTsat is the superior definition of ID and should inform future trials investigating the efficacy of intravenous iron replacement in patients with HFrEF.
Keywords: Heart failure, Heart failure with preserved ejection fraction, Iron deficiency, Mortality, Rehospitalization
Introduction
Iron deficiency (ID) is recognized as an important co‐morbidity in patients with heart failure (HF). A high prevalence of ID has been found in patients with HF with reduced ejection fraction (HFrEF). 1 , 2 , 3 Whether this is true for patients with HF with preserved ejection fraction (HFpEF) is not well established, as studies published to date have either excluded HFpEF patients or included insufficient numbers for definitive conclusions. ID is reported as an independent predictor of mortality in HF although not all published studies agree. 1 , 2 , 3 , 4 , 5 , 6 Different definitions of ID have been used, which may explain the conflicting results. ID defined as ‘ferritin < 100 or 100–300 mcg/L with a transferrin saturation (tsat) < 20%’ are the criteria most widely studied and were used in the randomized controlled trials underpinning guideline recommendations for intravenous (IV) iron replacement in HF patients with ID. However, when correlated with the ‘gold standard’ definition of ID [bone marrow (BM) stores], ferritin < 100 or 100–300 mcg/L with a tsat < 20% was found to be inferior to other definitions of ID. 7 Furthermore, the natural history of untreated ID in HF and its prognostic implications have not been established.
Aims
Quantify prevalence of ID and determine whether ID predicts long‐term outcomes in HFpEF as compared with HFrEF.
Clarify which definition of ID is more closely related to all‐cause mortality and the secondary combined endpoint of death/HF hospitalization.
Determine whether change in ID status over time is an important prognostic factor.
Methods
Patient cohort
Patients were a subset of those enrolled in a prospective, longitudinal multicentre study carried out in New Zealand (four centres) and Singapore (six centres) to determine clinical outcomes and predictors of outcome in HFpEF compared with HFrEF. The study design and main outcomes have been previously published. 8 In brief, patients with clinically confirmed HF (both inpatients and outpatients) were prospectively identified between March 2010 and August 2014 for inclusion in this study, a clinical era pre‐dating the widespread use of IV iron replacement in HF. Patients were assessed at recruitment (baseline visit), 6 weeks, and 6 months after enrolment, and clinical outcomes were determined up to 2 years following enrolment. The diagnosis of HF satisfied European Society of Cardiology criteria at the time of data collection (2012 Guidelines). Left ventricular ejection fraction (LVEF) <50% and ≥50%, determined quantitatively on transthoracic echocardiogram, defined HFrEF and HFpEF, respectively. All patients who had serum samples stored at their baseline visit were included. Informed consent had been obtained from all patients for future studies on their stored blood samples. Our study complies with the Declaration of Helsinki, and our research protocol was approved by all local ethics committees.
Evaluation of iron deficiency
Blood samples were drawn at baseline and 6 month visits, plasma separated by 10 min centrifugation at 4000 rpm at 4°C, aliquoted into EDTA, and then frozen at −80°C. Haemoglobin (Hb) was measured using a standard Seismic assay, and iron, ferritin, and transferrin levels were measured on the Abbott Architect platform. Tsat was calculated as the ratio of serum iron (mg/L) to total iron binding capacity (mg/L) × 100.
Our primary definition of ID (IDFerritin) was a ‘ferritin level < 100 mcg/L’ (absolute ID) or ‘ferritin 100–300 mcg/L with a tsat < 20%’ (functional ID). This was chosen as, to date, it is the most widely assessed definition of ID in HF patients. 9 , 10 We also assessed a second definition of ID—‘tsat < 20%’ (IDTsat). This was chosen as it was the definition of ID most closely reflective of BM iron stores, the gold standard definition, that is also available in routine clinical care worldwide. 7
Patients who had ID at both their baseline and 6 month visits were designated as having ‘persistent ID’. Patients with ID at baseline but not at 6 months were labelled ‘resolved ID’. Patients without ID at baseline but with ID at 6 months had ‘developed ID’, and, finally, those free of ID at either time point were classified ‘never ID’.
Statistical analysis
Statistical analyses were performed using R software (R Core Team 2017). Statistical tests are two‐sided, and P < 0.05 was considered significant. Normally distributed data are presented as mean (standard deviation), whereas categorical variables are expressed as numbers (percentages). Comparisons of categorical variables were performed using a χ 2 or Fisher's exact test, as required. Multivariable Cox proportional hazards models were used to estimate the adjusted risk associated with baseline ID (IDFerritin or IDTsat) and outcomes including all‐cause mortality and death/rehospitalization. Covariates were selected a priori as the clinical and laboratory variables established as independent predictors of all‐cause mortality and death or HF readmission in the parent study. 8 These were age, gender, ischaemic aetiology, N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), New York Heart Association (NYHA) class, systolic blood pressure (SBP), atrial fibrillation (AF), and serum creatinine. 8 To assess the effect of change in ID status at 6 months (persistent, resolving, developing, or never present), we developed proportional hazard models that adjusted only for change in ID status (Model 1); for change in ID status and EF group (Model 2); for change in ID status, EF group, age, and gender (Model 3); and for change in ID status, age, gender, ΔlogCreat, and ΔlogNT‐proBNP (Model 4).
Results
Baseline characteristics
Baseline characteristics of participants according to LVEF category are given in Table 1 . There were 1565/2039 (77%) patients with adequate stored plasma samples of whom 1563 had LVEF measured; 1115 (71%) had HFrEF and 448 (29%) HFpEF. IDFerritin classified 900 (58%) patients as ID; of these, 546 patients had absolute ID and 354 functional ID. Prevalence of IDFerritin was similar in HFpEF and HFrEF patients (58%). Absolute ID was the cause of ID in 64% of HFpEF and 59% of HFrEF patients. When ID was defined as IDTsat, 902 (58%) patients were classified as ID. Prevalence of IDTsat was also similar in HFpEF and HFrEF patients (57% vs. 58%).
Table 1.
Baseline characteristics by ejection fraction and IDFerritin status
| HFpEF | HFrEF | Total cohort | ||||
|---|---|---|---|---|---|---|
| n = 448 | N = 1115 | N = 1563 | ||||
| IDFerritin | Not IDFerritin | IDFerritin | Not IDFerritin | IDFerritin | Not IDFerritin | |
| n = 258 | n = 190 | n = 642 | n = 473 | n = 900 | n = 663 | |
| Age, years | 74 (11) | 70 (11) | 63 (13) | 63 (14) | 66 (13) | 65 (13) |
| Female, n (%) | 143 (55) | 72 (38) | 154 (24) | 66 (14) | 297 (33) | 138 (21) |
| BMI, kg/m2 | 30 (8) | 29 (6) | 28 (7) | 28 (7) | 28 (7) | 28 (7) |
| AF/flutter, n (%) | 154 (60) | 86 (45) | 222 (35) | 180 (38) | 376 (42) | 266 (40) |
| DM, n (%) | 114 (44) | 80 (42) | 315 (49) | 174 (37) | 429 (48) | 254 (38) |
| HTN, n (%) | 206 (80) | 134 (71) | 427 (67) | 282 (60) | 633 (70) | 416 (63) |
| PUD, n (%) | 26 (10) | 11 (6) | 36 (6) | 17 (4) | 62 (7) | 28 (4) |
| NYHA III/IV, n (%) | 81 (31) | 36 (19) | 181 (28) | 126 (27) | 262 (29) | 162 (24) |
| Hx HF hosp < 6/12, n (%) | 50 (19) | 42 (22) | 221 (34) | 136 (29) | 271 (30) | 178 (27) |
| HR, b.p.m. | 70 (60, 80) | 68 (61, 78) | 76 (67, 86) | 74 (65, 83) | 75 (64, 84) | 72 (64, 81) |
| SBP, mmHg | 129 (115, 140) | 129 (110, 142) | 118 (104, 130) | 118 (105, 132) | 120 (107, 135) | 120 (108, 136) |
| DBP, mmHg | 70 (60, 78) | 70 (61, 80) | 70 (60, 79) | 70 (62, 80) | 70 (60, 79) | 70 (62, 80) |
| NT‐proBNP, pg/mL | 1453 (710, 2693) | 969 (375, 2376) | 2513 (1342, 5057) | 1756 (824, 3932) | 2203 (1054, 4264) | 1599 (606, 3597) |
| hsTnT, ng/L | 26 (16, 44) | 23 (13, 40) | 33 (20, 52) | 26 (16, 45) | 30 (19, 50) | 25 (15, 43) |
| LVEF (%) | 61 (7) | 60 (6) | 29 (9) | 30 (9) | 38 (17) | 38 (16) |
| E:E′ | 15 (8) | 13 (7) | 18 (8) | 17 (8) | 17 (8) | 16 (8) |
| Creatinine, μmol/L | 106 (84, 138) | 109 (84, 143) | 107 (88, 136) | 104 (87, 128) | 107 (87, 137) | 105 (86, 135) |
| eGFR, mL/min/1.73 m2 | 54 (40, 69) | 57 (40, 76) | 60 (45, 77) | 64 (48, 80) | 59 (44, 75) | 62 (46, 79) |
| Hb, g/dL | 121 (18) | 129 (22) | 131 (19) | 139 (19) | 128 (19) | 136 (21) |
| HCT | 0.38 (0.06) | 0.4 (0.06) | 0.4 (0.06) | 0.42 (0.06) | 0.4 (0.06) | 0.41 (0.06) |
| Ferritin, mcg/L | 78 (47, 126) | 310 (188, 459) | 85 (51, 139) | 315 (189, 461) | 81 (49, 136) | 313 (189, 460) |
| Iron, mg/L | 10 (5) | 14 (6) | 10 (5) | 16 (8) | 10 (5) | 16 (7) |
| % saturation | 15 (7) | 25 (9) | 15 (8) | 27 (13) | 15 (7) | 27 (12) |
| Anaemia, n (%) | 144 (56) | 74 (39) | 251 (39) | 119 (25) | 395 (44) | 193 (29) |
| Anti‐coag use, n (%) | 92 (36) | 57 (30) | 174 (27) | 131 (28) | 266 (30) | 188 (28) |
| Anti‐plt use, n (%) | 121 (47) | 63 (33) | 255 (40) | 192 (41) | 376 (42) | 255 (38) |
| Iron supp, n (%) | 54 (21) | 39 (21) | 158 (25) | 141 (30) | 212 (24) | 180 (27) |
AF, atrial fibrillation; BMI, body mass index; BP, blood pressure; coag, coagulant; DBP, diastolic blood pressure; DM, diabetes mellitus; E:E′, mitral Doppler early velocity/mitral annular early velocity; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; HCT, haematocrit; HR, heart rate; hsTnT, high‐sensitivity troponin T; HTN, hypertension; Hx HF hosp, history of heart failure hospitalization; ID, iron deficiency; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; plt, platelet; PUD, peptic ulcer disease; sat, saturation; SBP, systolic blood pressure; supp, supplement.
Values are mean (standard deviation) or median (25% and 75% quartiles).
Characteristics of iron deficiency compared with non‐iron deficiency patients
Within our study population, patients with IDFerritin were more likely to be female (33% vs. 21%, P < 0.0001), diabetic (48% vs. 38%, P = 0.0003), and have a higher co‐morbid burden than patients without IDFerritin. There was a small difference in clinical markers of HF including more patients with NYHA class III/IV symptoms (P = 0.046) and a higher average plasma NT‐proBNP in those with IDFerritin. The use of anti‐coagulant (30% IDFerritin, 28% non‐IDFerritin, P = 0.65), anti‐platelet (42% IDFerritin, 38% non‐IDFerritin, P = 0.20), and oral iron replacement medications (24% IDFerritin, 27% non‐IDFerritin, P = 0.12) was high and did not differ significantly by iron status. More patients with IDFerritin had a history of peptic ulceration (7% vs. 4%, P = 0.034).
There was no significant difference in baseline characteristics between patients defined as IDTsat vs. IDFerritin (Supporting Information, Table S1 ). Differences between IDTsat and non‐IDTsat were similar to between IDFerritin and non‐IDFerritin except a history of peptic ulceration was not more common in the ID group (6% vs. 5%, P = 0.43).
Characteristics of patients with heart failure with preserved ejection fraction (iron deficiency vs. non‐iron deficiency)
The difference in co‐morbid burden and clinical markers of HF between those with and without ID was more pronounced in HFpEF patients (Table 1 ). Patients with HFpEF and IDFerritin had higher prevalence of AF (60% vs. 45%, P = 0.003), hypertension (80% vs. 71%, P = 0.030), and NYHA III–IV HF (31% vs. 19%, P = 0.004) than HFpEF patients without IDFerritin. Proportionally, more HFpEF patients with IDFerritin were prescribed anti‐coagulant or anti‐platelet agents than HFpEF patients without IDFerritin and HFrEF patients with or without IDFerritin. Similar differences were present between ID and non‐ID HFpEF patients when ID was defined as IDTsat.
Iron deficiency as a predictor of all‐cause mortality
During 2 years of follow‐up, 293/1563 (19%) patients died from any cause. There were 179 deaths in those with IDFerritin compared with 114 deaths in those without. In a model adjusting for age and sex, there was no significant difference in all‐cause mortality between patients with IDFerritin at baseline vs. those without {hazard ratio [HR] 1.21 [confidence interval (CI) 0.95–1.53], P = 0.12} (Table 2 , Figure 1 ).
Table 2.
Multivariable associations with outcome [hazard ratio (95% confidence interval)]
| HFpEF | HFrEF | Total cohort | |
|---|---|---|---|
| N = 448 | N = 1115 | N = 1563 | |
| Hazard ratio for all‐cause mortality (age/gender) | |||
| IDFerritin | 0.65 (0.40–1.05) | 1.41 (1.07–1.86) | 1.21 (0.95–1.53) |
| Age | 1.09 (1.06–1.12) | 1.04 (1.03–1.05) | 1.04 (1.03–1.05) |
| Gender (F:M) | 0.83 (0.52–1.34) | 0.59 (0.40–0.86) | 0.59 (0.45–0.78) |
| IDTsat | 1.16 (0.72–1.87) | 2.31 (1.71–3.11) | 1.95 (1.52–2.5) |
| Age | 1.08 (1.05–1.11) | 1.04 (1.03–1.06) | 1.04 (1.03–1.05) |
| Gender (F:M) | 0.77 (0.48–1.23) | 0.58 (0.4–0.84) | 0.57 (0.43–0.75) |
| Hazard ratio for all‐cause mortality (all independent variables) | |||
| IDFerritin | 0.68 (0.41–1.12) | 1.12 (0.85–1.48) | 1.00 (0.78–1.27) |
| Age | 1.06 (1.03–1.11) | 1.02 (1.01–1.03) | 1.03 (1.02–1.04) |
| Gender (F:M) | 0.82 (0.49–1.40) | 0.75 (0.51–1.11) | 0.84 (0.62–1.12) |
| Ischaemic aetiology | 1.13 (0.69–1.86) | 1.82 (1.32–2.52) | 1.47 (1.13–1.90) |
| Log_NT‐proBNP | 1.80 (1.39–2.32) | 1.68 (1.45–1.93) | 1.69 (1.50–1.89) |
| NYHA | 1.47 (0.89–2.41) | 1.71 (1.29–2.26) | 1.69 (1.33–2.15) |
| SBP 10 mmHg | 0.90 (0.80–1.01) | 0.86 (0.80–0.93) | 0.88 (0.82–0.93) |
| AF | 0.75 (0.44–1.28) | 1.30 (0.97–1.74) | 1.11 (0.86–1.43) |
| logCreatinine | 1.22 (0.62–2.38) | 1.33 (0.88–2.0) | 1.36 (0.96–1.92) |
| IDTsat | 1.12 (0.69–1.84) | 1.57 (1.15–2.14) | 1.46 (1.12–1.89) |
| Age | 1.06 (1.03–1.09) | 1.02 (1.01–1.03) | 1.03 (1.02–1.04) |
| Gender (F:M) | 0.77 (0.46–1.29) | 0.74 (0.50–1.10) | 0.81 (0.6–1.08) |
| Ischaemic aetiology | 1.05 (0.65–1.71) | 1.78 (1.29–2.49) | 1.45 (1.12–1.88) |
| Log_NT‐proBNP | 1.82 (1.40–2.36) | 1.61 (1.40–1.86) | 1.65 (1.46–1.84) |
| NYHA | 1.47 (0.89–2.41) | 1.64 (1.24–2.18) | 1.65 (1.29–2.10) |
| SBP 10 mmHg | 0.89 (0.80–1.00) | 0.86 (0.80–0.93) | 0.88 (0.83–0.93) |
| AF | 0.70 (0.41–1.18) | 1.31 (0.98–1.76) | 1.12 (0.87–1.45) |
| logCreatinine | 1.22 (0.62–2.40) | 1.36 (0.90–2.06) | 1.37 (0.97–1.94) |
| Hazard ratio for death or HF hospitalization (age/gender) | |||
| IDFerritin | 1.11 (0.81–1.52) | 1.33 (1.11–1.60) | 1.29 (1.11–1.51) |
| Age | 1.03 (1.01–1.04) | 1.02 (1.01–1.03) | 1.01 (1.01–1.02) |
| Gender (F:M) | 0.90 (0.66–1.23) | 0.67 (0.53–0.85) | 0.69 (0.58–0.83) |
| IDTsat | 1.19 (0.87–1.63) | 1.66 (1.38–1.99) | 1.54 (1.31–1.80) |
| Age | 1.03 (1.01–1.04) | 1.02 (1.01–1.02) | 1.01 (1.01–1.02) |
| Gender (F:M) | 0.89 (0.65–1.22) | 0.68 (0.53–0.86) | 0.69 (0.57–0.82) |
| Hazard ratio for death or HF hospitalization (all independent variables) | |||
| IDFerritin | 0.99 (0.71–1.37) | 1.11 (0.92–1.33) | 1.07 (0.92–1.26) |
| Age | 1.00 (0.99–1.02) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) |
| Gender (F:M) | 0.97 (0.69–1.37) | 0.82 (0.64–1.05) | 0.89 (0.74–1.08) |
| Ischaemic aetiology | 1.33 (0.96–1.84) | 1.52 (1.24–1.86) | 1.44 (1.22–1.71) |
| Log_NT‐proBNP | 1.56 (1.33–1.83) | 1.37 (1.25–1.50) | 1.41 (1.31–1.51) |
| NYHA | 1.39 (1.00–1.95) | 1.54 (1.27–1.86) | 1.50 (1.27–1.77) |
| SBP 10 mmHg | 0.94 (0.87–1.01) | 0.96 (0.92–1.01) | 0.96 (0.93–1.00) |
| AF | 0.97 (0.69–1.38) | 1.04 (0.85–1.27) | 1.03 (0.87–1.22) |
| logCreatinine | 1.37 (0.89–2.10) | 1.41 (1.09–1.83) | 1.42 (1.15–1.77) |
| IDTsat | 1.06 (0.77–1.46) | 1.24 (1.02–1.50) | 1.19 (1.01–1.41) |
| Age | 1.00 (0.99–1.02) | 1.00 (0.99–1.01) | 1.00 (1.00–1.01) |
| Gender (F:M) | 0.96 (0.69–1.35) | 0.82 (0.64–1.05) | 0.89 (0.74–1.08) |
| Ischaemic aetiology | 1.32 (0.96–1.82) | 1.50 (1.23–1.84) | 1.44 (1.21–1.70) |
| Log_NT‐proBNP | 1.56 (1.33–1.82) | 1.35 (1.23–1.48) | 1.39 (1.29–1.50) |
| NYHA | 1.39 (1.00–1.95) | 1.51 (1.25–1.84) | 1.49 (1.26–1.75) |
| SBP 10 mmHg | 0.94 (0.87–1.01) | 0.96 (0.92–1.01) | 0.96 (0.93–0.99) |
| AF | 0.97 (0.69–1.37) | 1.04 (0.85–1.26) | 1.04 (0.87–1.23) |
| logCreatinine | 1.37 (0.90–2.10) | 1.43 (1.10–1.85) | 1.43 (1.15–1.77) |
AF, atrial fibrillation; Hb, haemoglobin; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ID, iron deficiency; NYHA, New York Heart Association functional class; SBP, systolic blood pressure; Δ, change.
Bold highlights the results for iron status—the focus of the article.
Figure 1.
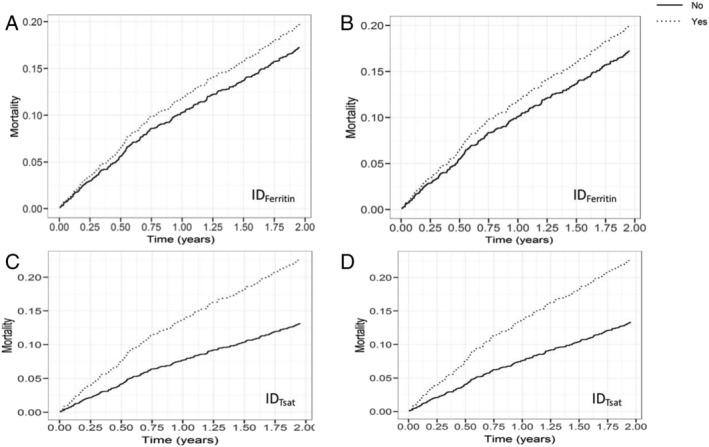
Cumulative event curves for all‐cause mortality in patients with IDFerritin and IDTsat compared with patients without ID during 2 years of follow‐up. (A and C) Models adjusted for age/sex; (B and D) models adjusted for age, sex, ischaemic aetiology, New York Heart Association class, systolic blood pressure, atrial fibrillation, log(N‐terminal pro‐brain natriuretic peptide), and log(creatinine). ID, iron deficiency.
When ID was defined by IDTsat, there were 206 (23%) deaths in those with IDTsat compared with 179 (20%) in the group identified as IDFerritin. In contrast to IDFerritin, IDTsat was an independent risk factor for all‐cause mortality in a model adjusted for age and sex [HR 1.95 (1.52–2.51), P < 0.01]. When age, sex, ischaemic aetiology, NYHA class, SBP, AF, log(NT‐proBNP), and log(creatinine) were included, IDTsat remained an independent predictor for all‐cause mortality [adjusted HR 1.46 (1.12–1.89), P < 0.01].
Iron deficiency as a predictor of death and heart failure hospitalization
During 2 years of follow‐up, 677 (43%) patients either died or incurred worsening HF requiring HF hospitalization, including 417 (46%) patients with IDFerritin and 260 (39%) without. Cox proportional hazard modelling including age and sex demonstrated a significant difference in the risk of death or hospitalization between those with and without IDFerritin, which was not maintained when ischaemic aetiology, NYHA class, SBP, AF, log(NT‐proBNP), and log(creatinine) were included in the model [HR 1.07 (0.92–1.26), P = 0.52] (Table 2 , Figure 2 ). In contrast, IDTsat remained a significant risk factor for death/HF hospitalization [HR 1.19 (1.01–1.41), P = 0.03] when the model was adjusted for age, sex, ischaemic aetiology, NYHA class, SBP, AF, log(NT‐proBNP), and log(creatinine).
Figure 2.
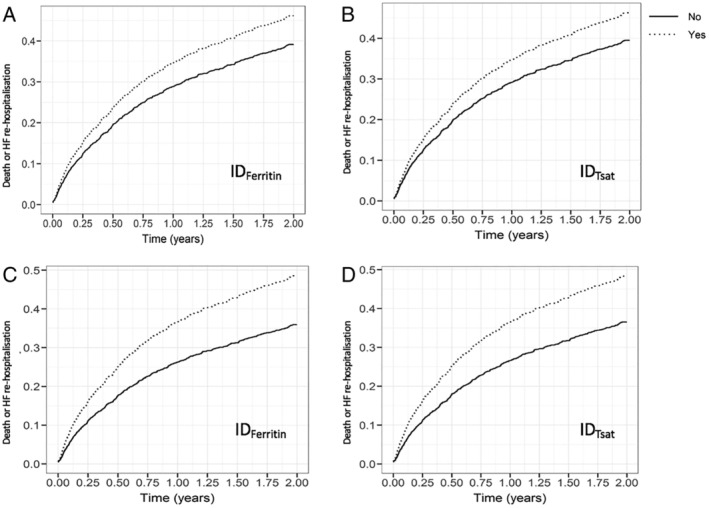
Cumulative event curves for death and HF hospitalization in patients with IDFerritin and IDTsat compared with patients without ID during 2 years of follow‐up. (A and C) Adjusted for age/sex; (B and D) adjusted for age, sex, ischaemic aetiology, New York Heart Association class, systolic blood pressure, atrial fibrillation, log(N‐terminal pro‐brain natriuretic peptide), and log(creatinine). HF, heart failure; ID, iron deficiency.
Relevance of iron deficiency in heart failure with preserved ejection fraction compared with heart failure with reduced ejection fraction sub‐cohorts
Among patients with IDFerritin, fewer HFpEF patients died from any cause compared with HFrEF patients [39/258 (15%) vs. 140/642 (22%), respectively, P = 0.03].
There were 72 deaths and 170 combined deaths or rehospitalization in the HFpEF subgroup. There were more deaths when ID was defined by IDTsat 45/257 (18%) compared with IDFerritin 39/358 (15%) but the same number of deaths/HF hospitalization [105/257 (41%) vs. 105/258 (41%), respectively]. ID by either definition did not confer independent risk for either all‐cause mortality [IDFerritin HR 0.65 (0.40–1.05), P = 0.08; IDTsat HR 1.16 (0.72–1.87), P = 0.55] or death/HF hospitalization [IDFerritin HR 1.11 (0.81–1.52), P = 0.52; IDTsat HR 1.19 (0.69–1.84), P = 0.28] (Table 2 ). Absolute ID was also not associated with increased rates of all‐cause mortality [0.72 (0.44–1.19), P = 0.20] or death/HF hospitalization [1.17 (0.86–1.50), P = 0.33] (Supporting Information, Table S2 ).
Within the HFrEF subgroup, there was a higher rate of all‐cause mortality in those with IDFerritin compared with those without IDFerritin [140/642 (22%) vs. 81/473 (17%), respectively, P = 0.063] although the difference did not reach statistical significance. In Cox proportional hazard modelling adjusted for age and sex, IDFerritin was independently predictive of all‐cause mortality [HR 1.41 (1.07–1.86), P = 0.01]. With adjustment for age, sex, ischaemic aetiology, NYHA class, SBP, AF, log(NT‐proBNP), and log(creatinine), IDFerritin was no longer an independent predictor of all‐cause mortality [HR 1.12 (0.85–1.48), P = 0.43] in patients with HFrEF. In contrast, within HFrEF, IDTsat was an independent risk factor for all‐cause mortality in both models [adjusted for age and sex: HR 2.31 (1.71–3.11), P < 0.01; adjusted for age, sex, ischaemic aetiology, NYHA class, SBP, AF, log(NT‐proBNP), and log(creatinine): HR 1.57 (1.15–2.14), P < 0.01]. For the combined endpoint of death or HF hospitalization in HFrEF patients, IDTsat but not IDFerritin was an independent predictor of outcome [HR 1.24 (1.02–1.50), P = 0.03 vs. HR 1.11 (0.92–1.33), P = 0.28].
Relevance of change in iron status during the first 6 months
As we found ID defined by tsat criteria more clearly associated with outcomes, we focused on this definition for assessing the prognostic relevance of change in ID status. There were 96 (6%) deaths within the first 6 months of the study. Of these, 73 (76%) had IDTsat at baseline (in comparison, 61 were identified by IDFerritin). Of the 1467 patients who survived to 6 months, 988 had a blood sample available from a 6 month visit. Of these, 537 patients had IDTsat at baseline, of whom 284 (53%) had persistent IDTsat at 6 months. Of the 451 patients without IDTsat at baseline, 128 (20%) had developed IDTsat by their 6 month follow‐up.
Patients with persistent IDTsat had an increased risk of all‐cause mortality compared with those who never had IDTsat [HR 2.22 (1.42–3.46), P < 0.01] (Table 3 , Figure 3 ). Persistent IDTsat remained a risk factor for all‐cause mortality regardless of EF group [Model 2: HR 2.26 (1.45–3.53), P < 0.01], when adjusted for age and sex [Model 3: HR 2.34 (1.50–3.67), P < 0.01], change in NT‐proBNP, and change in creatinine [Model 4: HR 2.16 (1.33–3.51), P < 0.01]. Persistent IDTsat was also an independent risk factor for death/HF hospitalization across all four models (Table 3 , Figure 3 ).
Table 3.
Association of change in IDTsat status over 6 months with outcome
| BL | 6/12 | Model 1 | Model 2 (Model 1 + EF) | Model 3 (Model 2 + age + gender) | Model 4 (age + gender + ΔlogCreat + ΔHb + ΔlogNT‐proBNP) | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Hazard ratio for all‐cause mortality | ||||||
| ID [reference] | No | No | ||||
| ID | No | Yes | 0.84 (0.41–1.72) | 0.85 (0.42–1.74) | 0.91 (0.44–1.86) | 0.82 (0.37–1.83) |
| ID | Yes | No | 1.21 (0.72–2.03) | 1.20 (0.72–2.01) | 1.38 (0.82–2.31) | 0.99 (0.52–1.88) |
| ID | Yes | Yes | 2.22 (1.42–3.46) | 2.26 (1.45–3.53) | 2.34 (1.50–3.67) | 2.16 (1.33–3.51) |
| HFrEF | 1.22 (0.82–1.81) | 1.52 (0.99–2.31) | ||||
| Age (years) | 1.05 (1.03–1.07) | 1.06 (1.04–1.08) | ||||
| Female | 0.51 (0.32–0.82) | 0.49 (0.30–0.81) | ||||
| ΔHb (g/dL) | 1.01 (0.99–1.02) | |||||
| ΔlogCreatinine (μmol/L) | 1.74 (0.81–3.72) | |||||
| ΔlogNT‐proBNP (pg/mL) | 1.34 (1.14–1.57) | |||||
| Hazard ratio for death or HF hospitalization | ||||||
| ID [reference] | No | No | ||||
| ID | No | Yes | 1.12 (0.78–1.61) | 1.13 (0.79–1.62) | 1.16 (0.81–1.66) | 1.11 (0.73–1.69) |
| ID | Yes | No | 1.4 (1.06–1.86) | 1.38 (1.04–1.83) | 1.43 (1.08–1.90) | 1.46 (1.04–2.06) |
| ID | Yes | Yes | 1.94 (1.49–2.51) | 1.99 (1.53–2.59) | 2.04 (1.57–2.65) | 1.75 (1.30–2.37) |
| HFrEF | 1.28 (1.02–1.61) | 1.29 (1.01–1.64) | ||||
| Age (years) | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) | ||||
| Female | 0.67 (0.52–0.86) | 0.63 (0.47–0.84) | ||||
| ΔHb (g/dL) | 1.01 (1.00–1.02) | |||||
| ΔlogCreatinine (μmol/L) | 2.21 (1.36–3.61) | |||||
| ΔlogNT‐proBNP (pg/mL) | 1.47 (1.34–1.62) | |||||
BL, baseline; CI, confidence interval; EF, ejection fraction; Hb, haemoglobin; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; ID, iron deficiency; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; Δ, change.
Reference group is NN (baseline no ID, 6/12 no ID).
Figure 3.
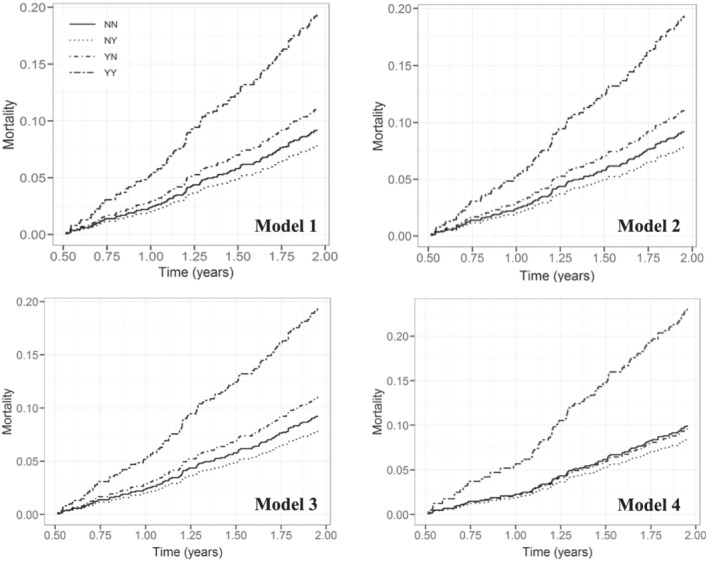
Cumulative event curves for all‐cause mortality by change in iron deficiency status over 6 months.
Patients with baseline IDTsat resolved by 6 month follow‐up had a similar risk of all‐cause mortality as those who never had IDTsat. However, these patients did have an increased risk of the composite endpoint of death/HF hospitalization [HR 1.40 (1.06–1.86), P = 0.02] evident in all four models [Model 2: HR 1.38 (1.04–1.83), P = 0.02; Model 3: HR 1.43 (1.08–1.90), P = 0.01; Model 4: HR 1.46 (1.04–2.06), P = 0.03]. Patients who developed IDTsat during the first 6 months of follow‐up did not have a higher mortality rate or risk of death/HF hospitalization compared with those who never had IDTsat.
Discussion
This study demonstrated that ID was not predictive of either all‐cause mortality or the combined endpoint of death/HF hospitalization in patients with HFpEF, despite a similar prevalence of ID as patients with HFrEF. Furthermore, the most widely studied definition of ID (‘ferritin < 100 or 100–300 mcg/L with a tsat < 20%’) was not an independent predictor of either mortality or the combined endpoint of death or HF admission in patients with HFrEF. In contrast, ID defined as ‘tsat < 20%’ was independently predictive of both endpoints in patients with HFrEF. Finally, this study demonstrated that the natural history of ID is important as persistence of IDTsat over 6 months was independently associated with poorer clinical outcomes than no ID, ID that resolves within 6 months, and newly developed ID.
Prevalence of iron deficiency in patients with heart failure with reduced ejection fraction and heart failure with preserved ejection fraction
The prevalence of ID in the current study is high (58%) consistent with previous reports. 1 , 2 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 The prevalence of ID has been widely reported in patients with HFrEF but not HFpEF. A recent meta‐analysis pooled the results of multiple small studies and found a prevalence for ID of 59% (95% CI 52–64%) in 1414 patients with HFpEF. 20 The prevalence (58%) of ID in patients with HFpEF in this study is consistent with this meta‐analysis and with data from 448 HFpEF patients is the largest single study of ID in patients with HFpEF to date.
Association between heart failure with preserved ejection fraction, iron deficiency, and outcomes
No association between ID (by either definition) and all‐cause mortality in patients with HFpEF was found despite equally high prevalence of ID as in patients with HFrEF. The overall mortality rate of 16% in the HFpEF subgroup is relatively low but comparable with event rates in HFrEF studies that have demonstrated an association between ID and mortality. 3 , 5 The addition of HF hospitalizations to our secondary outcome did not improve the prognostic power of ID by either definition in the HFpEF group. In comparison with patients with HFrEF, those with the phenotype of HFpEF did not have any distinguishing characteristics that would explain the difference in effect ID has on these two groups. Patients with HFpEF were not included in the prior studies comparing circulating biomarkers of iron and ID definitions to BM iron stores 7 ; thus, these definitions of ID may not be an accurate reflection of true ID in patients with HFpEF. Our results, although demanding independent corroboration, challenge any rationale for iron replacement in HFpEF at this time.
Importance of definition of iron deficiency on relationship between iron deficiency and mortality in patients with heart failure
It has been asserted that ID is an independent risk factor for mortality in HF despite published trials returning conflicting results. 1 , 2 , 3 , 4 , 5 , 6 As with other populations suffering chronic disease, the definition of ID applied in the general population is ‘adjusted’ for the HF population to allow for a raised ferritin caused by inflammation. The contrary results may be explained by a lack of applicability for this ‘adjustment’ across the spectrum of HF. When our population of HF patients is compared with HF populations where a positive association was found, there are notable differences. For example, in the European Iron Consortium study of 1506 patients, ID was associated with an HR for mortality of 1.42 (95% CI 1.14–1.77, P ≤ 0.01) on multivariable analysis (which included all univariate significant variables: age, sex, body mass index, diabetes, NYHA functional class, LVEF, estimated glomerular filtration rate, high‐sensitivity C‐reactive protein, NT‐proBNP, treatment with angiotensin‐converting enzyme inhibitor and/or angiotensin receptor blocker, statins, loop diuretics, and the presence of anaemia or ID) compared with an HR of 1.00 (95% CI 0.78–1.27, P = 0.97) on multivariable analysis [age, sex, ischaemic aetiology, NYHA class, SBP, AF, log(NT‐proBNP), and log(creatinine)] for ID in our study patients (Table 2 ). 1 The European population studied had more advanced HF than the current cohort, as reflected in higher NYHA class III/IV (61% vs. 29%) and 2 year mortality rates (29% vs. 19%). Of note, the European consortium cohort included a smaller proportion of patients with HFpEF (16% vs. 29%). A study of 574 patients with self‐reported HF (EF unknown) in the USA found no association between ID and mortality. 6 As in our study, the patients in this study had less advanced HF than the European cohort. In a study of 1684 patients (37% NYHA class III/IV), ID was found to be a predictor of mortality on univariate but not multivariable analysis incorporating adjustment for established predictors of mortality. 4 The difference in results cannot be explained by inadequate event rates as our cohort and other negative studies have reported higher event rates than studies that have found a positive association between ID and mortality. 2 In our study, there was also no difference in baseline characteristics between those with IDFerritin and those with IDTsat. Our study adds weight to the concern that IDFerritin, the most widely used definition of ID, does not best relate iron status to clinical outcomes across the spectrum of HF and in particular for patients with HFpEF. This may be because the ‘adjustment’ for inflammation is inaccurate. Definitions not requiring ferritin, or those using other inflammatory markers to guide the ‘adjustment’, may therefore be more suitable for HF patients who are less unwell (e.g. NYHA class I/II) or have HFpEF.
Until recently, the definitions of ID commonly used in patients with HF had not been correlated to BM iron stores, the gold standard definition of ID. Grote Beverborg et al. examined 42 patients undergoing coronary artery bypass graft with HF and EF < 45% and found that there was no strong correlation between ID defined as IDFerritin and BM iron stores (sensitivity 82.4%, specificity 75%). In contrast, a tsat of ≤19.8% strongly correlated with BM iron stores (sensitivity 94%, specificity 84%). 7 This was subsequently validated in a cohort of 387 outpatients with known HFrEF. 7 In a cohort of 1821 HFrEF patients, low tsat (<20%), but not low ferritin (<100 mcg/L), was associated with increased mortality. 21 We found that tsat < 20% was a significant risk factor for both all‐cause mortality and death or rehospitalizations in our 1563 patients. Our results further confirm tsat < 20% as the current definition of ID most closely related to adverse outcomes in HFrEF patients and therefore it should be considered as the preferred definition of ID in future trials assessing the effect of IV replacement therapy on morbidity and mortality in HFrEF.
Natural history of iron deficiency if left untreated
FAIR‐HF and CONFIRM‐HF established IV iron replacement therapy as effective at improving symptoms and reducing hospitalizations in IDFerritin patients with HFrEF, and a meta‐analysis suggested IV replacement may reduce mortality. 9 , 10 , 22 This has led to international guidelines recommending IV iron replacement in HF. 23 , 24 However, the natural history of ID in the HF population and its relevance to outcome has not been previously examined. The current study has demonstrated that the natural history of ID is important. Persistent IDTsat is associated with a 60% higher mortality rate compared with maintaining normal iron homeostasis. However, patients with ID at baseline that resolved within 6 months did not have a higher risk of mortality on multivariable analysis. Curiously, patients who had ID at baseline had a higher risk of morbidity and mortality, but those who ‘developed ID’ in the first 6 months did not. We hypothesize several explanations for this. Firstly, we do not know how long patients with ‘developed ID’ were iron deficient. If they were ID for a similar duration as patients with baseline ID that ‘resolved’ by 6 months, then one would expect a similar lack of independent risk for mortality. However, unlike patients with ‘resolved ID’, they did not have a higher risk of the composite endpoint of death/rehospitalization. This could be due to sample size as the number of patients who ‘developed ID’ was half the number with ‘resolved’ or ‘persistent’ ID or a difference in the severity of ID between these cohorts.
We did not find any baseline characteristics that could reliably predict those at risk of persistent ID despite patients with persistent ID being more likely to be co‐morbid than the other groups, with a higher rate of AF, diabetes mellitus, and hypertension (Supporting Information, Table S3 ). They had equivalent use of anti‐platelet, anti‐coagulant, and oral iron supplementation. Haematinic variables were potentially more predictive. Hb, ferritin, iron, and transferrin saturation levels were all lower in those with persistent ID compared with other groups. In persistent ID, the median ferritin level was 90 vs. 125 mcg/L and mean Hb level 123 vs. 133 g/dL compared with resolved ID; however, the difference in tsat was minimal (12% vs. 13%). Whether any cut‐off values could be used to decide IV replacement therapy would require further study. Given the high rate of persistent ID (53%) and the association of persistent ID with mortality, we would suggest that the most pragmatic approach is to treat ID in HFrEF unless a clear clinical discriminator between those who will experience resolution versus persistence of ID can be established.
Limitations
This study was a sub‐study of a prospective multicentre study that was powered for the primary outcome comparison of death from any cause between HFpEF and HFrEF. This sub‐study did not have an independent power calculation specifically for the purposes of the ID analyses, and therefore, despite similar event rates to positive studies in HFrEF patients, we recognize it could be underpowered to detect a true association for IDFerritin and all‐cause mortality or death/HF rehospitalization in patients with HFpEF. Not all patients could be included in this sub‐study as some did not have serum samples available from their baseline visit for analysis. This may have introduced a selection bias; however, we did not detect an appreciable difference in baseline characteristics or event rates in our subgroup compared with the parent study. This study enrolled patients in both the outpatient and inpatient settings (immediately prior to discharge). We recognize this could be a confounding factor as there is a likelihood of an interaction between a patients' acuity of disease and ferritin levels. However, we felt this was a strength from a practical perspective, as it would be easier for clinicians if one definition could accurately identify ID of prognostic importance in all stages of disease. Finally, whether IV iron will be of benefit in patients with HFpEF can only be determined from prospective randomized controlled trials.
Conclusions
Iron deficiency is common among patients with HF with similar prevalence in patients with HFpEF and HFrEF. However, among patients with HFpEF, ID is not independently associated with adverse outcomes. This suggests that targeting this co‐morbidity would not improve outcomes in this patient group. In contrast, ID is clearly associated with poor outcomes in HFrEF patients, particularly when it persists over time which occurs in more than half of ID patients if left untreated. In our patients with HFrEF, ID defined solely by ‘tsat < 20%’ was clearly a superior definition of ID than the more commonly used ‘ferritin < 100 mcg/L or ferritin 100–300 mcg/L + tsat < 20%’. To optimize treatment efficacy, this definition should inform future trials investigating the role of IV iron replacement in patients with HFrEF. As patients with persistent ID have no distinguishing baseline characteristics to determine who is likely to self‐resolve their ID, treatment with IV replacement should be given following initial diagnosis.
Conflict of interest
S.F. reports a grant from Green Lane Research and Educational Fund (NZ) during the conduct of the study. L.H.L. reports a grant from the National Medical Research Council of Singapore during the conduct of the study. K.P. reports grants from New Zealand Heart Foundation outside the submitted work. R.T. reports grants from Heart Foundation of NZ during the conduct of the study; grants and personal fees from Roche Diagnostics; and grants and personal fees from Merck, outside the submitted work. C.S.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, Astra Zeneca, Medtronic, and Vifor Pharma; has served as consultant or on the Advisory Board/Steering Committee Executive Committee for Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, Vifor Pharma, Novartis, Amgen, Merck, Janssen Research & Development LLC, Menarini, Boehringer Ingelheim, Novo Nordisk, Abbott Diagnostics, Corvia, Stealth BioTherapeutics, JanaCare, Biofourmis, Darma, Applied Therapeutics, MyoKardia, Cytokinetics, WebMD Global LLC, Radcliffe Group Ltd, and Corpus; and serves as co‐founder and non‐executive director of eKo.ai. A.M.R. reports grants from Health Research Council of New Zealand, grants from National Medical Research Council of Singapore, and other from Roche Diagnostics, during the conduct of the study; and grants, personal fees, non‐financial support, and other from Roche Diagnostics, outside the submitted work. R.N.D. reports grants from NZ Health Research Council, grants from NZ Heart Foundation, and grants from Green Lane Research and Educational Fund (NZ) during the conduct of the study. T.J.Y., D.S., K.T.G.L., P.S.D.Y., H.Y.O., F.J., T.P.N., M.L., and G.D. have no competing interests.
Funding
This research was enabled by a grant from the Green Lane Research and Educational Fund (14/49/4107) for the iron assays in New Zealand. Funding sources and acknowledgements for the main study are included in the publication. 8 R.N.D. is the recipient of the New Zealand Heart Foundation Chair of Heart Health. K.P. holds the New Zealand Heart Foundation Hynds Senior Fellowship.
Supporting information
Table S1. Baseline clinical characteristics IDFerritin vs IDTsat.
Table S2. Multivariable associations with outcome for absolute and functional ID.
Table S3. Baseline clinical characteristics by persistence or development of ID.
Fitzsimons, S. , Yeo, T. J. , Ling, L. H. , Sim, D. , Leong, K. T. G. , Yeo, P. S. D. , Ong, H. Y. , Jaufeerally, F. , Ng, T. P. , Poppe, K. , Lund, M. , Devlin, G. , Troughton, R. , Lam, C. S. P. , Richards, A. M. , and Doughty, R. N. (2021) Impact of change in iron status over time on clinical outcomes in heart failure according to ejection fraction phenotype. ESC Heart Failure, 8: 4572–4583. 10.1002/ehf2.13617.
References
- 1. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582.e3. [DOI] [PubMed] [Google Scholar]
- 2. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010; 31: 1872–1880. [DOI] [PubMed] [Google Scholar]
- 3. Okonko DO, Mandal AK, Missouris CG, Poole‐Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 2011; 58: 1241–1251. [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez‐Costello J, Comin‐Colet J, Lupon J, Enjuanes C, de Antonio M, Fuentes L, Moliner‐Borja P, Farre N, Zamora E, Manito N, Pujol R, Bayes‐Genis A. Importance of iron deficiency in patients with chronic heart failure as a predictor of mortality and hospitalizations: insights from an observational cohort study. BMC Cardiovasc Disord 2018; 18: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakano H, Nagai T, Sundaram V, Nakai M, Nishimura K, Honda Y, Honda S, Iwakami N, Sugano Y, Asaumi Y, Aiba T, Noguchi T, Kusano K, Yokoyama H, Ogawa H, Yasuda S, Chikamori T, Anzai T. Impact of iron deficiency on long‐term clinical outcomes of hospitalized patients with heart failure. Int J Cardiol 2018; 261: 114–118. [DOI] [PubMed] [Google Scholar]
- 6. Parikh A, Natarajan S, Lipsitz SR, Katz SD. Iron deficiency in community‐dwelling US adults with self‐reported heart failure in the National Health and nutrition examination survey III: prevalence and associations with anemia and inflammation. Circ Heart Fail 2011; 4: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grote Beverborg N, Klip IT, Meijers WC, Voors AA, Vegter EL, van der Wal HH, Swinkels DW, van Pelt J, Mulder AB, Bulstra SK, Vellenga E, Mariani MA, de Boer RA, van Veldhuisen DJ, van der Meer P. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail 2018; 11: e004519. [DOI] [PubMed] [Google Scholar]
- 8. Lam CSP, Gamble GD, Ling LH, Sim D, Leong KTG, Yeo PSD, Ong HY, Jaufeerally F, Ng TP, Cameron VA, Poppe K, Lund M, Devlin G, Troughton R, Richards AM, Doughty RN. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi‐ethnic cohort study. Eur Heart J 2018; 39: 1770–1780. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J 2015; 36: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole‐Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 11. Cohen‐Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, Zannad F, Laperche T, Leclercq C, Concas V, Duvillie L, Darne B, Anker S, Mebazaa A. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail 2014; 16: 984–991. [DOI] [PubMed] [Google Scholar]
- 12. Yeo TJ, Yeo PS, Ching‐Chiew Wong R, Ong HY, Leong KT, Jaufeerally F, Sim D, Santhanakrishnan R, Lim SL, Chan MMY, Chai P, Low AF, Ling LH, Ng TP, Richards AM, Lam CS. Iron deficiency in a multi‐ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail 2014; 16: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 13. Rangel I, Goncalves A, de Sousa C, Leite S, Campelo M, Martins E, Amorim S, Moura B, Silva Cardoso J, Maciel MJ. Iron deficiency status irrespective of anemia: a predictor of unfavorable outcome in chronic heart failure patients. Cardiology 2014; 128: 320–326. [DOI] [PubMed] [Google Scholar]
- 14. Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC‐HF: a randomized, controlled, observer‐blinded trial. J Am Coll Cardiol 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 15. Schou M, Bosselmann H, Gaborit F, Iversen K, Goetze JP, Soletomas G, Rasmussen J, Kistorp C, Kober L, Gustafsson F, Tonder N. Iron deficiency: prevalence and relation to cardiovascular biomarkers in heart failure outpatients. Int J Cardiol 2015; 195: 143–148. [DOI] [PubMed] [Google Scholar]
- 16. Makubi A, Hage C, Lwakatare J, Mmbando B, Kisenge P, Lund LH, Ryden L, Makani J. Prevalence and prognostic implications of anaemia and iron deficiency in tanzanian patients with heart failure. Heart 2015; 101: 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jankowska EA, Kasztura M, Sokolski M, Bronisz M, Nawrocka S, Oleskowska‐Florek W, Zymlinski R, Biegus J, Siwolowski P, Banasiak W, Anker SD, Filippatos G, Cleland JG, Ponikowski P. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35: 2468–2476. [DOI] [PubMed] [Google Scholar]
- 18. Jankowska EA, Malyszko J, Ardehali H, Koc‐Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJ, Anker SD, Gheorghiade M, Ponikowski P. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebner N, Jankowska EA, Ponikowski P, Lainscak M, Elsner S, Sliziuk V, Steinbeck L, Kube J, Bekfani T, Scherbakov N, Valentova M, Sandek A, Doehner W, Springer J, Anker SD, von Haehling S. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. results from the studies investigating co‐morbidities aggravating heart failure. Int J Cardiol 2016; 205: 6–12. [DOI] [PubMed] [Google Scholar]
- 20. Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D. Iron deficiency in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Open Heart 2019; 6: e001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moliner P, Jankowska EA, van Veldhuisen DJ, Farre N, Rozentryt P, Enjuanes C, Polonski L, Merono O, Voors AA, Ponikowski P, Van der Meer P, Comin‐Colet J. Clinical correlates and prognostic impact of impaired iron storage versus impaired iron transport in an international cohort of 1821 patients with chronic heart failure. Int J Cardiol 2017; 243: 360–366. [DOI] [PubMed] [Google Scholar]
- 22. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Luscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018; 20: 125–133. [DOI] [PubMed] [Google Scholar]
- 23. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 24. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline clinical characteristics IDFerritin vs IDTsat.
Table S2. Multivariable associations with outcome for absolute and functional ID.
Table S3. Baseline clinical characteristics by persistence or development of ID.


