Abstract
The growing population of left ventricular assist device (LVAD)‐supported patients increases the probability of an LVAD‐ supported patient hospitalized in the internal or surgical wards with certain expected device related, and patient‐device interaction complication as well as with any other comorbidities requiring hospitalization. In this third part of the trilogy on the management of LVAD‐supported patients for the non‐LVAD specialist healthcare provider, definitions and structured approach to the hospitalized LVAD‐supported patient are presented including blood pressure assessment, medical therapy of the LVAD supported patient, and challenges related to anaesthesia and non‐cardiac surgical interventions. Finally, important aspects to consider when discharging an LVAD patient home and palliative and end‐of‐life approaches are described.
Keywords: End of life, Internal Medicine, LVAD, Surgical departments
In the internal medicine ward
Blood pressure management in LVAD patients
The haemodynamics and blood flow patterns of the left ventricular assist device (LVAD) supported patients are unique to the valve‐less, continuous flow generated by the pump. Blood flow through the pump is inversely related to pump head pressure gradient [aortic pressure—left ventricular (LV) pressure]. In every heart beat the flow through the pump is increased on systole and decreased on diastole. The reduced native LV contractility of LVAD patients and the unloading of the LV by the LVAD, result in a diminished pulse pressure in these patients. 1
Pulse pressure of less than 15 mmHg is not palpable in physical examination and not detected by automated BP cuffs. Most LVAD patients have low pulse pressure, hence have no palpable pulse (‘non‐pulsatile’) and automated BP cuff would not be able to measure their BP. For that reason, the measured and reported BP value of LVAD patients is mean arterial pressure (MAP). 2
BP measurement
Direct measurement of the blood pressure BP with arterial line is the most accurate and reliable method but is available only at the inpatient setting. An automated BP cuff is effective in those LVAD patients that have palpable pulse (‘pulsatile’), which are minority of the patients. If that is the case, the MAP should be calculated [MAP = (2*DBP + SBP)/3].
For most LVAD patients with low pulsatility, the preferred method is using a vascular Doppler transducer with manual cuff (sphygmomanometer). The Doppler transducer is placed on either the brachial or radial artery or the arterial flow detected in the resting condition. The cuff is inflated and then deflated slowly. The BP recorded when an arterial flow sound is heard is approximately the MAP. It is important to note that when this method is used on a pulsatile LVAD patient, the value measured is the systolic BP (SBP), and the MAP would be lower.
If No palpable pulse—use Doppler method—measures MAP
Palpable pulse—use automated BP cuff and calculate MAP from systolic and diastolic BP
BP management
Elevated BP is associated with serious adverse events, including ischaemic stroke, intra‐cranial haemorrhage, pump thrombosis, aortic regurgitation, and ventricular arrhythmia. 3 , 4 The pathophysiologic process is complex and starts with elevated afterload on the pump, causing a decrease in pump flow. 5 The recommended BP for LVAD patients is MAP of ≤80 to 85 mmHg. 6 , 7 On the lower part of the scale, MAP of less than 60 mmHg is associated with hypoperfusion and therefore must be avoided. The optimal target for MAP in LVAD‐supported patients is in the range of 70–90 mmHg. 6
Hypertension treatment
Hypertension is common among LVAD patients and is preferably managed with heart failure medications, as angiotensin‐converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB's), beta‐blockers, and spironolactone. 6 , 7 Special caution is needed with the use of beta‐blockers in patients with RV failure, due to their negative inotropic effect. Second line medication includes calcium channel blockers, alpha‐blockers, hydralazine, and nitrates. Patients treated with phosphodiesterase 5 (PDE5) inhibitors for elevated pulmonary pressure, must not be concomitantly treated with nitrates due to risk of profound and life‐threatening hypotension.
Most LVAD patients do not have palpable pulse.
If there is palpable pulse, an automated cuff system will give reliable measurements; otherwise, use a Doppler probe.
Aim to reach mean arterial pressure of 70–90 mmHg.
Treat BP with HF medications but with caution when using beta‐blockers, in particular in the presence of RV failure.
Medical therapy during LVAD support
While there is a significant amount of clinical trial data to guide the use of traditional heart failure (HF) medications in patients with HF and reduced ejection fraction, similar data to guide the management of patients with LVADs are not available. The role of HF medications—ACE inhibitors, ARBs, β‐blockers, mineralocorticoid antagonists (MRAs), and diuretics—in the care of patients with an LVAD is largely unknown. Most of the recommended use of these HF medications in patients with LVADs is based only on consensus agreement.
Factors such as right heart failure, atrial and ventricular arrhythmias, renal dysfunction, and pulmonary hypertension can compromise the efficiency of an LVAD, resulting in ineffective cardiac support and necessitating medical therapy to improve cardiac function and optimize ventricular unloading.
Observational studies have reported that traditional HF therapies were moderately prescribed at discharge to patients with LVADs and were more frequently prescribed to patients with advanced HF without LVAD support. 8 Moderate prescription rates suggest clinical uncertainty in the use HF medication in this population.
The most recent ISHLT executive summary recommended use of HF medications in patients with LVADs mainly to control BP. Continuous flow LVADs are afterload sensitive and therefore pump performance is affected by hypertension. In addition, poorly controlled hypertension may increase the risk of aortic insufficiency (AI) and stroke.
The use of beta‐blockers is reserved for heart rate control in the setting of tachyarrhythmia, especially atrial arrhythmias which are common post‐LVAD implantation. Due to the potential negative inotropic effect of these agents on the right ventricular function, use those agents with caution in LVAD‐supported patients.
The use of neurohormonal blockade drug therapy (NHBDT) post‐LVAD may be beneficial as cardiac angiotensin generation does increase despite LV unloading, thus activating the sympathetic nervous system. NHBDT in LVAD‐supported patients is also associated with a significant reversal in adverse cardiac remodelling and a reduction in morbidity and mortality compared with LVAD support alone. The use of ACE inhibitors/ARB may also protect against angiogenesis and has been shown to be associated with reduced risk for major gastrointestinal bleeding (GIB) and AV malformation related GIB in patients with LVAD. There is no evidence to support the use of sacubitril/valsartan over ACE inhibitors after LVAD implantation. Recent observational data have demonstrated a large decrease in BP associated with sacubitril/valsartan in LVAD recipients but also improved clinical outcomes. 9 Results of ongoing studies are awaited.
Action:
Control the LVAD patient's BP preferably using HF drugs like ACEI and ARBs.
Use beta‐blocker in the case of tachyarrhythmia but be cautious in the presence of right ventricular failure.
Anticoagulation and left ventricular assist devices
Current guidelines recommend anticoagulation with vitamin K antagonist for all patients on left mechanical circulatory support. 6 , 7 The delicate balance in anticoagulation is mandatory for preventing both bleeding and thrombotic events.
Anticoagulant therapy may be evaluated in three different periods: (1) intraoperative and intensive care unit, (2) internal medicine ward, and (3) outpatient (home).
Intraoperative and intensive care unit: intraoperative full dose anticoagulation as in other cardiac surgeries is recommended. Early anticoagulation is needed to prevent thrombotic events. Intravenous heparin is commonly used as the primary choice. Intravenous direct thrombin inhibitors such as bivalirudin and argatroban may be used in patients with heparin induced thrombocytopenia. Start anticoagulation with oral vitamin K antagonist once the patient's condition is stable. 7
Internal medicine ward: Although centrifugal pumps have shown to be superior to axial flow devices regarding gastrointestinal (GI) bleeding and haemocompatibility related adverse events, the target International normalized ratio (INR) has not been changed and kept between 2.0 and 3.0. However, some centres have lowered their target INR to 2.0–2.5 for patients with centrifugal pumps, even if this strategy has not yet been proven effective. Higher values (INR up to 3.5) may be preferred for patients with biventricular support. Acetylsalicylic acid at the dose of 80–325 mg is routinely administered according to the device specifications.
Bridging with intravenous heparin is advised if the INR level is <2.0. Measurement of both the activated partial thromboplastin time and factor Xa is used for monitoring heparin dose. 6 , 7 It has been shown that there is high discordance between activated partial thromboplastin time (aPTT) and anti‐factor Xa (anti‐FXa). aPTT may be affected by warfarin use, liver disease, haemolysis and factor deficiencies. The most common observation in these patients was supratherapeutic aPTT value with a therapeutic anti‐FXa level. 10 Haemolysis and warfarin use may falsely elevate aPTT resulting in overestimation of heparin concentration and under anti‐coagulation. 10 Low‐molecular weight heparin (LMWH) may also be used although caution should be exerted in patients with renal dysfunction. Enoxaparin and dalteparin have shown to be effective in the mechanical circulatory support population. 11 No specific type of LMWH has been proposed in the guideline. 7
Outpatient: Routinely measure of INR level at least once a week. It has been shown that self‐testing of INR at home is possible and likely to increase the average time in therapeutic range. In one study, miscorrelation between laboratory and self‐testing INR levels were shown and suggested performing series of individual correlation tests on patients before instituting home INR monitoring and to exclude patients in whom the INR level difference is unacceptable.
Optimal long‐term regimen of anticoagulation must be tailored to the recipient and device type. During periods of under targeted INR or during periods of bridging with un‐fractionated heparin or LMWH, follow the patient closely for pump thrombosis or thromboembolic events.
Bleeding in LVAD recipients and its management is reviewed in detail in Part 2 of the position paper. Recommendations here also pertain to the outpatient phase.
Table 1 presents management of anticoagulation pre‐operative, peri‐operative, and postoperative of left MCS implantation.
Table 1.
Suggested management of anticoagulation pre‐operative, peri‐operative and postoperative of left MCS implantation 7
| Management of anticoagulation pre‐operative, peri‐operative, and postoperative of left MCS implantation | Class | Level |
|---|---|---|
| Early postoperative anticoagulation starting with IV anticoagulation, followed by vitamin K antagonists are recommended | I | C |
| The use of low‐molecular weight heparin as an early postoperative anticoagulation regimen should be considered | IIa | C |
| A postoperative INR target between 2.0 and 3.0 is recommended | I | C |
| The use of acetylsalicylic acid is recommended | I | C |
| The use of low‐molecular weight heparin for bridging during long term support is recommended | I | C |
| Re‐evaluation of antithrombotic therapy during bleeding episode is recommended | I | C |
| The use of novel oral anticoagulants is NOT recommended | III | B |
Anticoagulation is mandatory for all settings of care followed by close monitoring.
New oral anticoagulant drugs have not been studied in this population.
Heart failure exacerbation in LVAD‐supported patients
Although the LVAD provides continuous and steady flow from the left ventricle to the aorta, many factors may still compromise its function. Moreover, the function of the LVAD depends on adequate blood flow from the right ventricle. Therefore, any trigger interrupting RV function or the LVAD function might result in a clinical presentation of HF.
The assessment of the LVAD patient with HF is based on the usual evaluation of a non‐LVAD patient with HF. Several points need to be emphasized:
History taking—Evaluate the patient for whether left heart or right heart failure symptoms predominate and if their onset was abrupt or subtle (symptoms that started on a specific day will help when interrogating past events within the LVAD memory recordings). Ask if the medical treatment was changed recently and whether any device related alarms were noted prior to the symptom presentation.
Physical examination—Differentiate between right or left HF symptoms, and accurately measure BP. If MAP >100, treat with anti‐hypertensive drugs to a target MAP <80.
In cases of fluid overload, prescribe or increase the dose of diuretics.
Laboratory exams—Complete blood count, eGFR, liver enzymes, Lactic acid dehydrogenase (LDH) level and plasma free haemoglobin (elevation might suggest pump thrombosis), and brain natriuretic protein (BNP) might differentiate cardiac from non‐cardiac cause, and INR.
Electrocardiogram (ECG) and if appropriate ICD interrogation—Look for any tachy/brady arrhythmia that might explain symptoms.
Chest X ray—pulmonary congestion might point to pump failure, pleural effusion, or pneumonia might explain dyspnoea, mal‐positioning of the device might cause suction events—consult with an LVAD specialist regarding its positioning. 12
Echocardiography—must be performed by an experienced technician. RV function is at times difficult to assess technically but nevertheless must be determined. Meticulously search for new valvular dysfunction; specifically, new aortic regurgitation which might explain the acute decompensation. Assess the position of the interventricular and interatrial septum.
Referral to the LVAD specialist for LVAD parameters evaluation, LVAD history interrogation, and recording of the alarm's history.
General approach for evaluation of HF exacerbation in a LVAD patient for the non‐LVAD specialist
As in any other medical setting, meticulous history and physical examination are the first step. Try to locate a non‐cardiac event as a possible trigger for the decompensation such as use on non‐steroidal anti‐inflammatory drugs, infection, and anaemia, and treat it accordingly. If not found, the patient must be profiled into left HF, right HF, or both. 13
If left HF predominates look for possible triggers. If BP is high, treat the hypertension targeting MAP <80 mmHg. If there is significant AI on echo, manage BP and estimate the need for valve replacement. If there are signs of device malfunction, consult an LVAD specialist and treat the malfunction. If pump thrombosis is suspected, treat with IV heparin and thrombolysis, and even be ready for an emergent surgery. Increase cardiac output by elevating the device speed if the echo findings support the possibility of inadequate speed. Treat pulmonary congestion with vasodilators and diuretics.
If no specific trigger can be corrected, treat with inotropes, and if this fails, be prepared for pump replacement or high urgency cardiac transplantation.
If right heart failure predominates—rule out ‘normal’ causes of RV failure like pulmonary embolus, severe tricuspid regurgitation, cardiac tamponade, and RV infarction. If none of them exists, insert a Swan‐Ganz catheter, treat with IV diuretics and if applicable also pulmonary vasodilators and perform an echocardiogram. If the patient does not respond, treat with inotropes, and if this fails, assess for RV mechanical support or cardiac transplantation.
Assessment of the LVAD patient with HF is like the non‐LVAD with emphasis on interrogation of past events if possible.
Treating HF exacerbation starts by deciding whether left or right failure predominates. In most of the cases, it will be right‐sided heart failure.
In the cases of left‐sided heart failure, keep good control of the BP and fluid status and consult the heart failure specialist in order to evaluate the pump history.
Kidney dysfunction in LVAD‐supported patients
The vast majority of HF patients referred for LVAD therapy have had increasing symptoms and signs of congestion with volume overload. 14 Moreover, growing evidence has established the pathophysiological importance of congestion in HF disease progression. 15 The goal therefore is to relieve congestion through achieving a state of euvolemia, mainly through the use of judicious diuretic therapy. The appropriate use of diuretics however remains challenging, especially when shock, worsening renal function, diuretic resistance, and electrolyte disturbances occur. 16 The interdependence between heart and kidney has been a topic of extensive research for decades. Intuitively, progressive renal dysfunction is often attributed to hypoperfusion of the kidney due to progressive impairment of cardiac output. However, a drop in systemic BP, venous congestion and intra‐abdominal pressure are haemodynamic parameters strongly associated with worsening renal function in heterogenous populations of HF. 16
As LVAD use increases, so does the number of patients with LVADs who also have kidney disease, with limited data on how best to care for them. Following LVAD implantation, kidney function may improve in the short term, particularly if central venous congestion/low renal blood flow were responsible for the poor glomerular filtration rate. 17 , 18 Individuals with glomerular filtration rates chronically <30 mL/min/1.73 m2, including those treated with maintenance dialysis, are generally ineligible for destination LVADs as they have a very poor outcome. 18 As such, extra care is warranted to avoid the need for renal replacement therapy in LVAD patients.
Patients who develop acute kidney injury and require dialysis following LVAD implantation have high mortality rates. 18 Although haemodialysis is the most common modality for patients with LVADs, peritoneal dialysis might be an option in selected cases taking into consideration the risk for driveline infections. 19 However, peritoneal dialysis may be associated with lower risk for bloodstream infection and fewer haemodynamic shifts. 19
Patients who develop acute kidney injury requiring haemodialysis while on LVAD have poorer prognosis.
Peritoneal dialysis is preferred in the appropriate LVAD patient on the need for renal replacement therapy.
Liver dysfunction in LVAD‐supported patient
Liver dysfunction is common in chronic and acute decompensated HF. The cardio‐hepatic interaction in HF is related to both increased venous pressure and forward failure of the left ventricle. Increased venous pressure is associated with elevated cholestatic liver enzymes, while reduced cardiac output results in liver injury and subsequent elevated transaminases. 20 In patients with advanced HF, potential candidates for LVAD implantation, liver dysfunction is of important prognostic value.
The Model for End‐stage Liver Disease (MELD) score is predictive of outcome in patients with end‐stage liver disease. Similarly, the MELD score may be helpful in prediction of outcome in patients with advanced HF, who are screened for LVAD implantation. 21 Yang et al. 22 demonstrated that a MELD‐XI (MELD excluding INR) score <17 was associated with an improved outcome in 255 patients after continuous flow LVAD implantation. In addition, assessment of liver stiffness, as a marker of liver fibrosis, with ultrasound elastography may provide important prognostic information. In a study with 28 patients with continuous flow LVADs, it was demonstrated that liver stiffness correlated with invasively measured central venous pressure and extent of liver fibrosis from biopsies. Furthermore, an improvement in liver stiffness after LVAD implantation was noted. 23
Several studies have demonstrated that liver dysfunction is (partly) reversible after LVAD implantation. In a cohort of 309 continuous flow LVAD patients, Russell et al. 24 noted that 6 months after implantation, alanine transaminase (ALT), aspartate transaminase (AST), and total bilirubin had significantly decreased. In a more recent study, it was shown that after more than 3 years of LVAD support, hepatic function is still preserved. 25 However, worsening of liver function may occur as well after LVAD implantation. In a cohort of 270 LVAD patients, up to 47.8% developed some degree of post‐operative liver dysfunction. Patients with combined elevated transaminases and total bilirubin had a significant worse prognosis compared with patients without liver dysfunction after implantation. 26 Although the exact mechanism is most likely to be multi‐factorial, post‐operative liver dysfunction may be related to pre‐operative liver dysfunction and inflammatory state and post‐operative right ventricular failure and venous congestion.
Therefore, careful assessment of liver function during pre‐operative patient selection and during post‐operative monitoring is critical for good outcomes after LVAD implantation.
MELD = 3.78*Ln (Bili) + 11.2*Ln (INR) + 9.57*Ln (Cr) + 6.43.
MELD‐XI = 5.11*Ln (Bili) + 11.76*Ln (Cr) + 9.44.
In the MELD calculation, any variable with a value less than 1 is assigned a value of 1 to avoid negative scores.
In most patients after LVAD implantation liver, functions will be preserved and even improved.
Post‐LVAD liver dysfunction may be due to a pre‐operative inflammatory state and post‐operative right ventricular failure and venous congestion.
Management of infections in LVAD‐supported patients
Patients with LVAD comprise several risk factors rendering them more vulnerable for infection than other patient populations. Per definition, LVAD patients form a population with advanced HF, which is commonly associated with numerous comorbidities, such as diabetes mellitus or renal impairment, which increases the risk for infection. 27 Repetitive hospitalizations during acutely decompensated HF, often infection‐triggered, 28 in the months and years preceding LVAD implantation expose this patient population to hospital‐associated pathogens, allowing for potential colonization with frequently antibiotic‐resistant pathogens. Advanced disease in general is associated with a compromised immune system, a phenomenon described as immune‐senescence, highly prevalent in patients with advanced HF. 29 , 30 , 31 Noteworthy, infection is among the most frequent reasons for non‐cardiovascular death in HF patients. 32 Moreover, the yet obligatory transdermal driveline frequently serves as port of entry for pathogens. Once acquired, an infection weighs heavier in LVAD‐assisted patients than it does in other populations since it is associated with a significantly worse outcome. 32 Beyond the compromised immune system of this vulnerable patient population, two other major reasons account for the poor prognosis associated with infection in LVAD patients. One reason is the difficulty in diagnosing infection. Signs and symptoms of infection in LVAD patients are very versatile and range from scant erythema or malaise to high‐grade fever or sepsis; therefore, early consideration and identification of infection is of paramount importance. The second reason is the difficulty to successfully treat device infection. As with any other implanted foreign material, pathogens tend to form biofilms on the foreign surface of any component of the implanted LVAD. Within biofilms, pathogens are inherently resistant to the host immune system and extremely difficult to eradicate.
Biofilms
Biofilms are communities of microorganisms attached to any surface such as metals and minerals, plant tissues, dental plaque and on implanted medical devices. Biofilm formation plays a significant role in the persistence of bacterial infections and is a major contributor to and virulence determinant of LVAD‐specific infections. 33 , 34 Pathogens capable of forming biofilms are typically Staphylococcus aureus and Staphylococcus epidermidis; however, less common, Pseudomonas and Enterobacteriaceae species as well as Candida albicans also form biofilms on implanted foreign material. After irreversible attachment to the foreign surface, they produce a matrix of extracellular substances (composed of DNA, polysaccharides, and proteins) that surrounds the foreign material and protects biofilm‐producing pathogens both from the host immune system and from circulating antibiotics. Pathogens spread, on the one hand, by migration along the foreign surface and, on the other hand, by a process called dispersion, during which pathogens detach from their original colony and translocate to a new location. 35 This inherently protective behaviour makes it extremely difficult, if not impossible, to eradicate biofilm‐associated infections.
Uniform definition of infections in LVAD‐assisted patients
Infections in LVAD patients can be differentiated according to the standardized classification of infections, formulated by the ISHLT Infectious Disease Working Group in 2017. 27
LVAD‐specific infections: These infections only occur in LVAD patients and are directly related to the implanted hardware (i.e. pump, inflow cannula, outflow graft, driveline) or the body surfaces containing it (i.e. pump pocket, anastomoses, driveline tunnel). These infections are often difficult to diagnose conclusively, and, secondary to the common involvement of biofilm formation, difficult to eradicate.
LVAD‐related infections: These infections may also occur in non‐LVAD patients. However specific considerations apply, if present in LVAD patients. The following entities belong into this group: endocarditis, blood stream infections, mediastinitis, and sternal wound infection.
Non‐LVAD‐related infections: These infections are independent or not directly associated with the presence of an LVAD and may occur in any severely sick patient. Such infections are mainly lower respiratory tract infections like pneumonia, blood stream infections, urinary tract infections, and gastrointestinal infections.
Epidemiology of infections in LVAD‐assisted patients
With an incidence of up to 37% of all patients after LVAD implantation, infections are the most common adverse event in patients with mechanical circulatory support. Interestingly, the most frequent infections in LVAD patients are non‐LVAD‐related infections. 32 They occur predominantly within the first 3 months after LVAD implantation, indicating that these are nosocomial infections. Pneumonia and urinary tract infections are most common infections among non‐LVAD‐related infections with an incidence of up to 10.8% (0.45 events per patient‐year) and 10.6% (0.44 events per patient‐year), respectively. Non‐LVAD‐related infections are followed by VAD‐specific infections, among which driveline infections are by far the most frequent, occurring in 9.1% (1.28 events per patient year) of patients within 3 months after LVAD implantation, and in 29.3% (1.20 events per patient year) of patients thereafter.
Challenges in management of biofilm associated infection in LVAD patients
Whereas device removal is a routine therapeutic option in patients with device infection of devices other than LVADs (e.g. prosthetic joint material, cardiac pacemakers or implantable cardioverter defibrillators), a pump exchange or removal of an infected LVAD is usually associated with high mortality, often outweighing the benefit of such an intervention. Usually the only possibility in infected LVAD patients requiring LVAD removal is heart transplantation.
In summary,
Be aware of the vulnerability of this patient population.
Meticulous line and wound care may prevent infection.
Early consideration of the possibility of an infection allows prompt diagnostic work up and initiation of therapy, where indicated.
Remember that LVAD can delay the symptoms of sepsis so consider early use of antibiotics.
When in doubt, take swabs and blood cultures (ideally 3 sets of 2 in 24 h) and/or contact your LVAD centre.
Always involve your LVAD centre when suspecting an infection in your LVAD patient, irrespective of LVAD‐specific, LVAD‐related or non‐LVAD related.
Optimal management of suspected infection in an LVAD patient necessitates the collaboration of a multidisciplinary team of infectious disease specialist and/or clinical microbiologist, cardiologist, and cardiac surgeon.
Photo documentation is useful.
Diagnostic work‐up:
Careful history and review of symptoms may prompt early suspicion of infection.
A detailed physical exam, always including inspection of the driveline exit site and surgical wounds, is warranted.
Lab test include a complete blood count and serial assessment of C‐reactive protein (CRP) or erythrocyte sedimentation rate (ESR).
If purulent effusion is visible, always take swabs before initiation antibiotic treatment.
Take three sets of blood cultures at different time points within the first 24 h of suspicion, at least the first set before initiating antibiotic therapy.
If a central or peripheral line is present, take two sets of blood cultures simultaneously, one from the central or peripheral line, the other from a non‐related peripheral site. Differential time to positivity may allow differentiation between a LVAD‐related and a non‐LVAD‐related blood stream infection.
Imaging can help in diagnosing the source of infection. Routinely perform a chest X‐ray when in suspicion of an infection in your LVAD patient. Ultrasound can help detecting fluid collections and differentiate between local and extended infection. Single‐photon emission tomography (PET) in combination with computed tomography (PET‐CT) may be very useful in diagnosing and/or differentiating LVAD‐specific and LVAD‐related infections.
Patients with LVAD are precluded from MRI.
Therapeutic approach:
Avoid initiation of antibiotic therapy without prior swabs and blood cultures.
Involve the LVAD centre when initiating antibiotic therapy.
Even in cases of superficial infection without signs of systemic disease, initiation of antibiotic therapy is not to be deferred until the culture results are available but later modified accordingly.
In case of clinical signs of infection and negative culture results, start empirical antibiotic therapy and evaluation based on clinical response.
In case of systemic disease or sepsis, empirical IV antibiotic therapy must cover Staphylococcus, Pseudomonas, and Enterobacteriaceae species.
Take the local (institutional) resistance profile into account when initiating antibiotic therapy.
Avoid rifampicin because of its interference with vitamin K antagonists.
Signs and symptoms of infection in LVAD patients are very versatile and range from scant erythema or malaise which quickly deteriorates to high‐grade fever or sepsis.
Early recognition of infection is of paramount importance.
If an infection is suspected, the use of empiric antibiotic therapy is advised
Diabetes mellitus, obesity, and COPD as a major comorbidity in the LVAD patient
DM is common among LVAD patients, with an estimate prevalence of 40%. 36 There are conflicting data regarding the association of DM with increased rate of mortality and adverse events including stroke, pump thrombosis, and infection after LVAD implantation. 36 , 37 , 38 , 39 This uncertainty regarding the role of DM on outcomes after LVAD stands in contrast to the fact that LVAD implantation is associated with a significant improvement in DM status, manifested as lower Hb A1c, lower fasting glucose and decreased insulin consumption. 40 , 41 , 42 Current guidelines recommend screening for DM before LVAD implantation and consider excluding patients with DM and severe end‐organ complications from LVAD candidacy. 7 The data on DM in LVAD recipients generally apply to type 2 DM as there is little information on patients with type 1 diabetes.
Treatment of DM in LVAD patients should follow guidelines DM treatment in HF patients. In the new era of SGLT2 inhibitors, it is advised to use these new anti‐diabetic drugs carefully as there are no data regarding this drug in this group of patients.
Obesity
Obesity is not associated with increased mortality after LVAD implantation; however, it is associated with some serious complications including device related infections, right‐sided heart failure and pump thrombosis. Therefore, weight loss counselling is recommended as part of the evaluation before LVAD implantation and including morbidly obese patients as LVAD candidates must be performed cautiously. On the other hand, it is noteworthy that bariatric surgery after LVAD implantation is a safe and feasible option, 43 at times even enabling recovery from the LVAD. 44
COPD
Severe chronic obstructive pulmonary disease (COPD) patients are not eligible for LVAD implantation due to increased surgical risk and poor ability to recover after major chest surgery. On average, lung function does not improve after LVAD implantation. 45 Mild COPD is not a contra‐indication for LVAD if there is no secondary RV failure.
LVAD implantation is associated with a significant improvement in DM status.
Pre‐LVAD implantation weight loss is beneficial.
Pre‐LVAD implantation obesity is associated with complications including device‐related infections and right‐sided HF and pump thrombosis.
Mild COPD by itself is not a contra‐indication for LVAD and not complicated by secondary RV failure
Aortic insufficiency following LVAD implantation
One of the unique features that may develop following implantation of a continuous‐flow LVAD is AI. In the presence of an LVAD, AI leads to a circulatory loop, with a portion of LVAD output flow regurgitating through the aortic valve to the left ventricle and then back through the device, leading to ineffective forward flow, and resultant organ mal‐perfusion and increased LV diastolic pressures.
The aetiology of continuous‐flow LVAD‐associated AI is due to several factors, including reduced valve opening, altered blood flow dynamics, pan cyclic transvalvular gradients, high shear stress, and leaflet mal‐coaptation. These processes promote leaflet fusion, valve degeneration, and aortic wall remodelling, which ultimately lead to AI. 46
In a retrospective study of 184 LVAD cases from the Duke University Medical Center, LVAD implantation was associated with significant worsening of AI relative to the nonsurgical patients with end‐stage HF. 47
In a retrospectively analysed group of 237 patients implanted with HeartMate II LVAD at the Minneapolis Heart Institute, moderate or severe AI occurred in 32 (15.2%) patients. Freedom from moderate or severe AI was 94%, 76%, and 65% of patients at 1, 3, and 5 years, respectively, and there was no difference in survival between recipients who developed significant AI and those who did not. Patients who had an aortic valve that opened with every beat were less likely to develop AI compared with those having intermittent opening or none at all. 48
In a meta‐analysis following a systematic literature search, which found eight studies totalling 548 patients with continuous‐flow LVADs, the pooled incidence of de novo AI was 37%. Factors influencing the development of AI and its progression were older age, being female, duration of LVAD support and persistent aortic valve closure. 49
In patients going into LVAD surgery with a pre‐existing clinically significant AI, several surgical solutions have been tried and suggested. 49 , 50 , 51 These techniques were found effective in reducing AI and durable, with follow‐up extending into 2 years, although early survival favoured those without the need for aortic valve central closure.
The safety of long‐term continuous‐flow LVAD support after aortic valve closure was studied by the Texas Heart Institute group in 16 patients and compared with those of 510 LVAD recipients without concomitant aortic valve closure. Survival was similar for LVAD‐only patients and aortic valve‐closure patients for a 2 years follow‐up. There were no deaths related to aortic valve closure. 52 The Columbia University Medical Center group in New York presented a group of 56 patients with pre‐existing mild AI who underwent continuous‐flow LVAD implantation and compared outcomes between 41 patients who underwent aortic valve repair and those 15 patients who did not. Freedom from progression of AI to moderate or severe at 2 years was significantly more prevalent in the group of patients who underwent aortic valve repair compared with those who did not. 53
Nowadays, most surgeons implanting LVADs prefer replacing an insufficient aortic valve during LVAD surgery with a bioprosthesis rather than closing the orifice. The approach to AI that develops late after LVAD implantation is different, as any repeat surgical intervention in the aortic valve may involve increased surgical risk. A non‐surgical technique for closing the regurgitant aortic valve was first described by the group from Freiburg, by using a trans‐catheter closure of the native aortic valve with an Amplatzer occluder with self‐expanding double discs and its connecting waist. 54 This technique has been reported so far in more than a dozen of patients by several groups with a significant reduction of AI, improvement of cardiac haemodynamics and reduction of the pulmonary capillary wedge pressure. 55
The first reports on successful treatment of severe AI in LVAD implanted patients using the transcatheter aortic valve replacement (TAVR) technique, came out in 2012 resulting in an immediate and long standing reduction of the AI. 56
The largest group of patients with continuous‐flow LVADs who underwent TAVR implantation was reported by the group from the Piedmont Heart Institute in Atlanta. They reported nine patients with resolution of AI, a significant improvement of the New York Heart Association classification from the time of implant to 6 months, and 89% survival at 6 months.
These last studies favour the TAVR as the current treatment of choice for LVAD patients who develop symptomatic AI. 57
Patients with significant AI going into LVAD surgery are treated by most surgeons by replacing the insufficient aortic valve with a biologic prosthesis during LVAD surgery rather than closing it
For LVAD patients with severe AR a TAVR is a considerable option.
In the surgical ward
Anaesthesia for non‐cardiac surgery of the LVAD‐supported patient
Approximately 30% of all LVAD‐supported patients will require non‐cardiac surgery, most commonly endoscopies or invasive cardiology interventions but could also be from any other discipline and either elective or urgent. 58 Successful treatment of these patients requires a multidisciplinary approach, which includes the anaesthesiologist, cardiologist, LVAD support nurse, and perfusionist. 59 In recent years, more and more procedures requiring anaesthesiologist support in LVAD patients are carried out in non‐LVAD centres, often out of the operating room, and often by a general non‐cardiac anesthesiologist. 59 , 60
Basic LVAD physiology
It is vital to understand that the LVAD patient is preload dependent, and afterload sensitive. Factors critical to preload in these patients include volemia, positioning, surgical technique (laparoscopic vs. open), arrhythmias, and right ventricular function. Afterload sensitivity means that hypertension can decrease flow causing end organ damage. Recommended MAP is 70–90 mmHg. 58 , 59 , 61 , 62
Pre‐operative evaluation
Prior to surgery it should be determined which LVAD the patient has (HM II™, HW™, HM3™) so that the appropriate console can be arranged. Medications and end organ function must be determined. Review the most recent blood tests including coagulation, renal and hepatic function, and LDH as a marker of pump thrombosis. A recent echo will provide vital information on the cannula position, valvular function, and the right ventricular function. A decision on pre‐operative stopping of the anticoagulation must be taken on a case by case basis with the risk of bleeding being weighed against the risk of a pump thrombus. 58 , 61 , 62 When possible, it is preferred to perform the surgical procedures in an LVAD centre with an experienced cardiac anaesthesiologist. Low cardiac risk surgical interventions (such as superficial dermatological surgeries, dental, minor gynaecological interventions, eye surgeries, etc.) in patients with no major comorbidities can be performed in a non‐LVAD centre preferably with an access (even remotely) to LVAD specialists. LVAD specialized centres are reserved for major procedures in patients with significant comorbidities, and where post‐operative pharmacological support is likely. 59 , 60 ICD's need to be de‐activated (with a magnet on the ICD) to allow operation with no inappropriate shocks.
Intraoperative monitoring
During the surgical procedure, the LVAD must be connected to an adequate power source (preferable mains), and parameter monitoring. 61 Standard monitors can be used. Non‐invasive BP monitoring is reliant on pulsatility and is only successful in approximately 50% of LVAD patients. Doppler ultrasound on the radial artery is successful in 94% of patients. 61 Invasive (intra‐arterial) BP monitoring is the gold standard, but its insertion may be challenging and require ultrasound or Doppler guidance. The use of arterial lines has decreased over the years but is always indicated for major surgery especially with expected fluid shifts and vasopressor/inotrope use. 58 , 61 , 62
Pulse oximetry is dependent on pulsatility and therefore may not record. Serial arterial blood gases can be taken to monitor oxygenation, and cerebral oximetry can be used to ensure brain oxygenation. 58 , 62 A central venous line or pulmonary artery catheter may be indicated for high risk procedures or for unstable patients. A trans‐oesophageal echo has to be available for the diagnoses and treatment of crisis situations. 58 , 61 , 62
Anaesthetic choice
Regional or neuroaxial anaesthesia is rarely used due to coagulopathies and anticoagulation and the subsequent risk of bleeding and complications. Monitored anaesthesia care (MAC) is commonly used especially in gastro and cardiology cases. Care must be taken with sedation and spontaneous ventilation to avoid hypoxia and hypercarbia. General anaesthesia is indicated for surgical procedures that require intubation. Drug choice is limited to drugs, which will maintain contractility, preload, and afterload. Care must be taken with positive‐pressure ventilation to avoid high tidal volumes and high positive end‐expiratory pressure (PEEP) to maintain preload. 58 , 61 , 62 , 63
Patient positioning
Trendelenburg (head down tilt) can augment venous return and cause RV dysfunction. Anti‐Trendelenburg (head up) and lateral decubitus (side) can reduce preload and LV filling, causing hypotension. Positioning has to be performed gradually and may require a fluid bolus. Prone position may also reduce preload and can also cause LVAD inflow obstruction. 58 , 62
Surgical technique
Laparoscopy can decrease preload which can decrease pump flow and cause hypotension. If the patient becomes unstable the insufflation pressures must be decreased. One‐lung ventilation is to be avoided where possible due to the likelihood of hypoxia and hypercarbia which increase pulmonary vascular resistance (PVR) and may cause RV failure. 62
Haemodynamic management
The key targets in the management of the LVAD patient are to maintain MAP 70–90 mmHg, to protect the right ventricle, to maintain adequate preload, and to maintain afterload. Pump parameters such as pulsatility index (PI), pump power, pump flow are used to help diagnose problems. 58 , 59 , 61 , 62
Intraoperative challenges
‘Suction event’ refers to when the interventricular septum shifts towards the inflow cannula of the LVAD. It results in refractory hypotension, low pump flow, low PI, ventricular arrhythmias, and haemodynamic collapse. Causes can include hypovolemia, high pump speeds, RV dysfunction, high PVR, and vasoplegia. Treatment includes fluids, vasopressors, a reduction in pump speed, and RV optimization (nitric oxide, milrinone, dobutamine, and adrenaline). 58 , 62
Bleeding is not uncommon with 40% of patients requiring blood products. This is due to a combination of acquired type 2a Von Willebrand Deficiency, AVMs due to continuous flow, and anticoagulation. 58 , 62
Arrhythmias occur in 30–50% of patients and can be atrial or ventricular. They can cause decompensated HF and instability. Suction event must be ruled out. A stable patient with an arrhythmia can be treated conservatively with amiodarone or lidocaine. If the patient is unstable cardioversion is indicated, and pump speed is adjusted if a suction event is suspected. 58 , 62
Cardiac arrest can be difficult to detect without electrocardiogram monitoring and will often result in PETCO2 being under 20 mmHg. Resuscitate as non‐LVAD patients (see relevant section in this manuscript). 58 , 62
Post‐operative care
Post‐operative extubation and recovery care are applied according to the standard procedures whilst avoiding abrupt changes in preload, afterload, and contractility. A multidisciplinary team approach is adopted. Minor cases can safely be monitored in the regular Post‐Anesthesia Care Unit whereas major cases would likely benefit from intensive care unit (ICU) or intensive cardiac care unit (ICCU). The ICD should be reset. 58 , 63
The LVAD patient is preload dependent and afterload sensitive.
Critical factors influencing preload in these patients include blood volume, positioning, surgical technique (laparoscopic vs. open), arrhythmias, and right ventricular function.
Afterload sensitivity means that hypertension can decrease flow causing end organ damage.
Special LVAD centres are reserved for the major surgical procedures especially for patients with significant comorbidities, and where pharmacological support is likely to be needed.
For post‐operative care, a multidisciplinary team approach is preferable.
Minor cases can safely be monitored in the regular Post‐Anesthesia Care Unit whereas major cases would likely benefit from ICU or ICCU.
Non‐cardiac surgery in LVAD patients
Patients with LVADs who are in need for non‐cardiac surgery demonstrate unique concerns that require specifically addressed peri‐surgical assessment to minimize the risk for complications. This include the need for adequate intra‐operative BP monitoring, peri‐operative anticoagulation management, modifications to the surgical access site due to anatomic considerations, and management of intraoperative device malfunction and postoperative complications including cardiovascular and cerebrovascular events. 59
Most common minor procedures performed in LVAD patients are gastrointestinal endoscopic examinations. 59 , 64 , 65 Other common procedures are related to implantable cardioverter devices (e.g. device extraction) and other electrophysiological procedures such as ablations. 59
Abdominal surgeries in LVAD patients are increasingly performed due the increased prevalence of LVAD‐supported patients and their improved longevity. Abdominal surgery in LVAD patients can be challenging due to both the use of anti‐thrombotic treatment and to access‐site restrictions. Pre‐operative computed tomography (CT) scan are useful to locate pump pocket and driveline location.
A study which included seven LVAD patients who underwent emergency abdominal laparotomy found 28% peri‐operative mortality rate. All surgeries were performed using a midline incision without xiphoidal extension. 66 Elective laparoscopic procedures are also feasible in LVAD patients. A study comprising of 17 elective laparoscopic procedures in LVAD patients described the periumbilical open Hasson technique as the most common abdominal entry technique used. No cases were converted to open surgery. Peri‐operative thrombotic events or LVAD complications secondary to holding anticoagulation were not reported. 67
Retrospective data from a study comprising 246 patients and 702 procedures demonstrated increased risk of acute kidney injury (18%), intra‐operative hypotension (27%, hypotension defined as MAP <70 mmHg over 20 min), elevated LDH levels (2.6%), and bleeding complications (6.4%). 59 Peri‐operative mortality was 5.3%.
Intra‐operative blood‐pressure monitoring frequently requires the use of invasive intra‐arterial catheter. However, this need is dependent upon the LVAD type, patient and surgical characteristics, and anaesthetic management and was more frequently reported in lower versus higher volume institutions. 68 Moreover, the use of a Doppler probe with a manual sphygmomanometer or a slow‐deflation cuff has been shown to increase non‐invasive monitoring success rates. 69 , 70 Target values defining hypotension in LVAD patients differ from the ones used in the general non‐LVAD population. A cut‐off of <70 mmHg to define hypotension is mostly used. 59 Moreover, the aetiology of hypotension in LVAD patients commonly differs from the general surgical population and may include the development of device thrombus, suction events, and right ventricular failure. 59 Alterations to previously stable device settings are rarely (if ever) needed intraoperatively. 65
Anticoagulation management in LVAD patients during the peri‐operative period remains challenging and is most often determined on a case‐by‐case basis. Management of active bleeding in LVAD patients does not differ from the protocols used in non‐LVAD patients) Table 2 ).
Table 2.
Suggested anticoagulation management
| Consequence of bleeding | Non‐life threatening | Life threatening |
|---|---|---|
| Elective surgery |
|
|
| Urgent/emergent surgery | If required intra‐operative:
|
Pre‐emptive:
|
FFP, fresh frozen plasma.
Acute kidney injury (AKI) that developed in the first week of surgery was observed in 17% of LVAD patients post non‐cardiac surgeries (NCSs). 59 Peri‐operative characteristics independently associated with AKI included major procedures, invasive arterial BP monitoring, and pre‐operative fresh frozen plasma transfusion; the latter most probably serve as surrogates for the complexity of the patient.
In conclusion, key decisions in LVAD patients undergoing major NCS include the presence of LVAD coordinator/anaesthesiologist during surgery, the optimal anticoagulation regimen, and the need for invasive haemodynamic monitoring. (Table 3 ).
Record pump parameter 24 h before elective NCS and monitor trends.
Consider IV hydration if ‘nil by mouth’ is prolonged.
Establish BP monitoring BEFORE induction of general anaesthesia
Maintain MAP 70–90 mmHg.
De‐activate ICD.
For abdominal conditions, laparoscopic surgery if feasible may be safer.
Recommence IV heparin 24–48 h post‐NCS until therapeutic INR levels.
Table 3.
Suggested invasive monitoring
| Likelihood of instability | Non‐general anaesthesia | General anaesthesia | ||
|---|---|---|---|---|
| Low probability | High probability | Low probability | High probability | |
| CVC | Yes | Yes | Yes | |
| AL | Yes | Yes | Yes | |
| PAC | Insert sheath | |||
| TEE | Make available | |||
AL, arterial line; CVP, ventral venous catheter; PAC, pulmonary artery catheter; TEE, trans‐oesophageal echo.
During hospitalization and at discharge
Driveline issues
The driveline (DL) is a cable that exits the body at the lower abdominal wall connecting the intra‐corporeal pump to the external controller and the energy supply delivering power and data. The DL consists of a central strength cable surrounded by six electrical conductors, three primary (two for energy supply and one for data) and three backups. Handling of the DL is usually performed by the patient himself and his close caregivers. If the close caregiver accompanies the patient, it is preferred to allow him to continue handling the DL and dressing as he is experienced and has been qualified for that. In case of an emergency when there is no close caregiver, other healthcare providers will be required to take care of the DL. There are various protocols for handling the DL, and mainly, among them all, the common denominator is that the principle of sterility must be kept.
When approaching an LVAD patient by a non‐LVAD specialist, the following issues related to the DL need attention:
(1) Verify connection of the cable to the controller.
(2) The cable is complete and not damaged.
(3) The cable is straight and not twisted or kinked.
(4)The cable is secured to the controller.
(5) The dressing is clean and undamaged.
(6) The cable is fixed to the body.
The routine care includes keeping the DL from external damage, cleaning, dressing, fixation, and shower issues.
Self‐care of the driveline
Keeping the DL from external damage: keep the DL fixed to the abdominal wall and avoid kinking or pulling of the DL.
Cleaning: wipe the skin around the DL away from the exit site and not vice versa with a sterile pad rinsed in Alcohosept (chlorhexidine 0.5% + alcohol 70%) or by ChloraPrep (chlorhexidine 2% + alcohol 70%).
Dressing—There is no one specific recommended protocol, and the dressing manner is affected mainly by equipment availability and by local experience. Before applying a dressing, ask for any known allergic‐hypersensitivity reaction to the dressing employed. There is no benefit in daily dressing change, and the recommendation is to change the dressing twice a week in a sealed dressing or once a week in a transparent dressing. This is applicable only to intact and uninfected exit sites, as the presence of infection or secretion will require more frequent dressing changes. Surrounding the DL exit site, around the DL, a simple sterile pad, or an antimicrobial or a silver dressing 71 should be used. A transparent chlorhexidine dressing, allowing daily control over the wound and requiring only once a week replacement, is widely used. The only disadvantage in that kind of dressing is that it cannot absorb any secretion from an unhealed or infected DL exit site. The replacement of the DL dressing must be made in sterile technique and can be performed when needed by the nursing team similar to the way a dressing of a pick Line is performed.
Fixation —Movement of the DL may cause injury or bleeding and infection at the DL exit site. There are many types of fixators with no advantage of one over the other. Without any relevance to the fixator used, the cable must always be attached to the abdominal wall. The fixation is replaced as needed approximately between weekly and monthly.
Shower—Showering requires preparations. The dressing must be covered with any method (large adhesive, nylon wrap) in order to prevent water penetration. The accessory equipment (batteries and controller) is inserted into a dedicated waterproof bag. Showering with the cable plugged in is prohibited. Optimal timing for the DL dressing change is after showering, and the patient is advised to plan that ahead. 72 Some LVAD programmes allow showering every day; some twice a week; and others, once a week. In any case, it is advisable to avoid showering until the exit site wound has healed. Aburjania et al. showed a significant reduction in DL infections if weekly showering. 73
Infections —Infections are the Achilles heel of the LVAD system, among which DL infection is the most common, reported at a range of 15–40% in the 3 years post‐LVAD implantation. These infections cause significant morbidity, progress to sepsis, and even be life threatening.
Preventing infection —Preventing infection in hospitalized patients is crucial. Eradication of DL infection or other LVAD‐related infection is problematic in the presence of a foreign body that cannot be extracted. The importance of hand hygiene must not be underestimated. Firm fixation of the DL to the abdominal wall is very important for the prevention of DL infection by reducing damage caused to DL exit site. A fall or pull of the bag containing the batteries and controller are the primary cause of DL infection. 27 In case of bag fall and pulling of the DL, the attending healthcare provider must perform an assessment of the wound. If signs of damage or bleeding at the DL exit site are observed, empiric antibiotic therapy is indicated for at least 1 week. First generation Cephalosporin is the preferred antibiotic class covering most of the agents causing skin infection. Replacement of the dressing by an experienced caregiver or a skilled staff member in the proper sterile technique is important for the prevention of DL infection.
In every hospital admission, the patient's methicillin‐susceptible S. aureus and methicillin‐resistance S. aureus status must be reviewed and treated accordingly. The use of secondary prophylaxis for non‐cardiac procedures such as dental, gastrointestinal respiratory, genitourinary, or cutaneous in LVAD‐supported patients has not been studied yet. Although in the American Heart Association Scientific Statement 27 the routine use of antibiotic prophylaxis in patients with indwelling devices is not recommended, the 2013 ISHLT ‘Guidelines in Mechanical Circulatory Support’, consider such secondary prophylaxis a reasonable option, aiming at reducing the risk of bacteraemia and potential seeding of the LVAD. 6
Vaccines are administrated like for every other HF patient, and personal and household hygiene are encouraged. 27
A good method for tracking the DL exit site wound is by photography. In any suspected DL issues, a picture of the DL exit site is encouraged to be shared with the LVAD team. Reviewing the ‘photo album’ of previous pictures may help in decision making. In places where due to medical confidentiality the use photographs are not permitted, comparing the patient's DL exit site wound with a poster that shows different levels of infection is advisable.
DL infections: DL infections are classified into Superficial DLI (SDLI) and Deep DLI (DDLI).
The SDLI is characterized by redness and tenderness of the soft tissue surrounding the DL exit site occasionally with some effusion. A swab culture even from the depths of the wound should be taken and empiric (until the microbiological results arrive) antibiotic therapy started. The preferred antibiotic therapy is combination of 1st generation cephalosporin (for staphylococcal infection) and quinolone (for Pseudomonas). Every centre is welcomed to change the antibiotic protocol according to the common pathogenic flora in that region. The antibiotic therapy needs modification if the causative microbial agent has been identified. During DL or any other infection, INR levels can be altered mandating frequent INR monitoring. Following blood count and CRP levels is helpful. Frequent replacements of the DL dressings might also be beneficial.
DDLI involves deeper tissues with purulent, sometimes foul‐smelling effusion from the DL exit site wound. The skin above and along the subcutaneous cable tract is painful, oedematous, and erythematous. Proximal progression of the erythema above the DL indicates worsening uncontrolled DDLI.
In DDLI, hospitalization is indicated for prompt empirical IV antibiotic therapy (according to infectious diseases consult) immediately after swab culture from the DL exit site effusion and four blood culture samples obtained.
A peripherally inserted central catheter (PICC) facilitates discharge home for long term antibiotic therapy ranging from weeks, months and even longer.
Evaluation and follow‐up of the DDLI includes ultrasonographic and CT scanning to diagnose the depth and extent of the DDLI and abscess formations along the subcutaneous DL tract. PET CT is the most reliable imaging modality enabling accurate assessment of the therapeutic response. Daily change of the dressing is mandated for assessment of the DL exit site wound. When DDLI progresses despite optimal medical therapy, surgical debridement of the wound is indicated. Surgical opening of the cable origin to point where clean non‐infected tissue is identified the exit site is shifted to a new exit site. The incision is mostly treated by vacuum‐assisted closure. When this surgery is unsuccessful and the infectious process approaches or reaches the device itself, pump replacement or emergent heart transplantation are indicated.
In any suspected external damage to the DL, contact the LVAD team or the manufacturer.
A fall or pull of the bag are the primary causes of infection.
A good fixation of the DL can reduce the incidence of DL infection.
In any case of a bag fall, the healthcare provider must perform an assessment of the wound, consider starting an empiric antibiotic, and consult the LVAD specialist.
When empiric start of antibiotic therapy, treat the common pathogens as recommended by the nearest LVAD centre.
Exercise training in patients with ventricular assist devices
Exercise training (ET) is highly recommended in HF. 74 , 75 , 76 , 77 More recently, it has been proposed also in LVAD recipients, 78 , 79 but with a non‐homogeneous implementation, as shown by the European Exercise Training Survey. 80 The limited implementation is attributed to lack of knowledge, prioritization, official recommendations, or heterogeneity of the surgical intervention (simple or shared device implantation, combined valve surgery, linked with ventricular ablation), indication, or simply because of too severely frail individuals (such as very elderly HF patients).
Based on the current consistent but still limited evidence supporting the safety and the benefit of early mobilization (EM) and ET in the LVAD population, the Heart Failure Association (HFA) of the European Society of Cardiology has developed practical advice on the modality of exercise implementation in LVAD patients. 81
Early mobilization
In every patient, as well as in LVAD recipients, EM, defined as initiating physical exercise within the early illness phase, is the first step for initiation of exercise therapy, and it constitutes the basic standard modality for ET implementation, during the post‐acute phase. EM has to be considered 82 , 83 , 84 when patient's haemodynamic state is stable (including surgical wound, skin integrity maintenance, and pulmonary hygiene), and LVAD functioning and troubleshooting have been correctly addressed. Regarding the timing of starting ET, the limited data available support the safety of 6 weeks interval after the LVAD implantation. 85 , 86 An algorithm for EM for LVAD‐supported patients and the transition to ET is here proposed, based on expert opinion, and patient's attitudes/clinical state (Figure 1 ).
Figure 1.
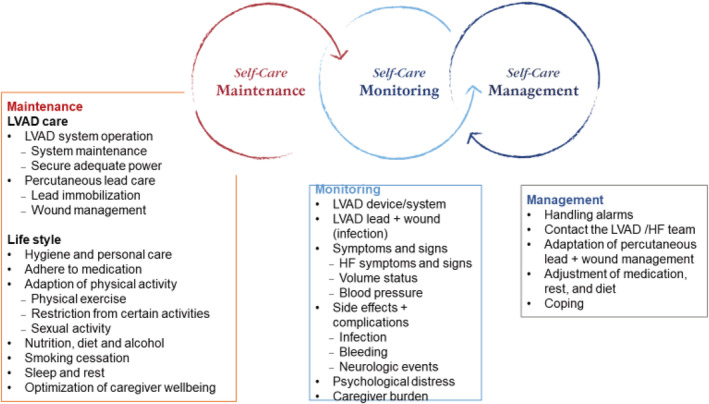
Self care if LVAD supported patient: Maintenance, Monitoring and Management.
Exercise training
No guidelines describing the specific ET setting, modality, and duration for LVAD‐supported patients are available, but, only limited evidence on implementation of light exercise intensities. Monitoring of exercise sessions is crucial, at least initially, which include the supervision of the patient, the clinical adaptation, and the VAD functioning. To optimize the exercise or the work‐load prescription, a symptom‐limited cardiopulmonary exercise testing or 6 min walking test (6MWT) according to local availability, is advisable, in order to aim at a peak workload below the predetermined ventilatory anaerobic threshold. If peak VO2 is >14 mL/kg/min or 6MWT > 300 m, a more intensive ET can be applied.
Early mobilization post‐VAD implantation, with safety of 6 weeks interval after the implantation is beneficial.
6 weeks period of changing intervals is recommended.
To optimize the exercise or the work‐load prescription, a symptom‐limited cardiopulmonary exercise testing (or 6MWT according to local availability) is advisable.
Pre‐discharge recommendations: Education and social support
Education and support of the LVAD‐supported patient and their close caregiver is vital during the different phases of the LVAD‐journey/trajectory. Communication between the healthcare provider and the LVAD‐supported patients, their family, and caregivers need to be open and address the practical, psychological, and social consequences of the LVAD implantation.
Education about living with a chronic disease has already started during the HF trajectory and should continue and shift focus during the process of evaluation for LVAD, decision making and consent, preparation, and post‐device implantation. Successful long‐term LVAD support includes a high degree of self‐care by the patient and their caregiver requiring long‐term support from a multi‐disciplinary team. 87 Self‐care is defined as process of maintaining health through health promoting practices and illness management. 88 All three components of self‐care deserve special attention once an HF patient has been supported with an LVAD, including activities regarding self‐care maintenance (related both to the device and lifestyle), self‐care monitoring (e.g. monitoring for complications or distress), and self‐care management (e.g. handling alarms or coping with living with the device).
Clinicians need to be aware of available materials in their countries that can be used for education, decision making, and support. In some countries, a broad spectrum of resources exists for patients considered for LVAD implantation or living with an LVAD, such as brochures, apps, and websites. Some centres have their own education material, while others use materials produced by the manufacturer. A recent review described that information material might be suboptimal with strong emphasis on benefits of living with the LVAD and lack of information on the potential risks, alternative options, and caregiver considerations. Most materials use outdated statistics, are above the reading level of average patients, and are biased towards favouring the LVAD therapy. It is therefore advised to scrutinize material that is used in the education and care for LVAD patients.
Finally, optimal education of the LVAD patients and their caregiver which focuses on knowledge and skills through a collaborative, adult learning approach is needed. Not only factual knowledge needs to be addressed (e.g. what is the DL) but also separate learning strategies should be used to develop skills and to apply knowledge in new situations (e.g., what to do in case of new symptoms).
The LVAD‐supported patients have to be approached by a multi‐disciplinary team that will address the practical, psychological, and social consequences of the LVAD implantation.
The patient and his caregiver need to have knowledge about self‐care maintenance, monitoring, and management for best outcomes.
Palliative care and end‐of‐life issues in LVAD‐supported patients
The integration of a palliative approach into advanced HF management has been recommended both within international guidelines 74 , 89 and expert position papers. 90 , 91 , 92 , 93 Through improved therapeutic communication and discussion of goals and preferences, informed decisions can be made by the HF team, patient, and family regarding future treatment options. However, most of the evidence regarding palliative interventions for patients with LVADs remains directed at those whereby the device was implanted for destination therapy (DT).
In a retrospective case‐note review of all patients (n = 51) awaiting LVAD implantation 94 over a 20 month period, 28 patients received a palliative care consultation prior to implantation. Results showed that the consultation did not affect LVAD placement, with seven patients (25%) requiring palliative care follow‐up. Controversially, authors concluded that although this consultation was beneficial in the management of multiple symptoms, ongoing follow‐up was not always necessary.
Clinical trials such as REMATCH have demonstrated the positive benefits of the LVAD 95 ; however, for some patients, expectations of the life post‐LVAD do not equate to reality. Furthermore, a recent study by the Mayo Clinic found that the median time from implantation to death for patients with a DT was 14 months 96 with most patients dying in hospital and only 46% receiving palliative care 1 month before death. This is not surprising given the use of hospice provision by HF patients in general, as well as considering post‐implantation complications and that many non‐LVAD specialists remain unfamiliar with the advanced technology.
The RCTs have shown a collaborative palliative and HF approach to be beneficial. 97 , 98 In the SWAP‐HF study, 99 patients with LVAD who received the palliative intervention were more likely to have a documented advance plan, indicating they had an opportunity to discuss and have choice at the end of life. Interestingly the study also found the intervention did not affect anxiety, depression, or quality of life scores.
Similar to previous ICD studies on deactivation, 100 many professionals are reluctant to engage in discussions regarding LVAD deactivation, perceiving the device as ‘life‐saving’. There was consensus that only when the patient was imminently dying would the device be deactivated, 101 with 13% of cardiologists believing LVAD deactivation was a form of euthanasia or physician‐assisted suicide.
Improved collaborative approach between palliative and HF both prior to and following LVAD implantation, irrelevant of the indication, will inform realistic goals and expectations by the patient and family. More research is required into the provision of quality end of life care for this cohort of patients, exploring the needs of the patient and family members. Finally, practical resources need to be considered that can be tailored to meet the LVAD‐supported patient's needs, as well as raising awareness of the need to educate professionals in the management of LVAD‐supported patients.
Palliative care is beneficial if started prior to the LVAD implantation.
Discuss realistic goals and expectations about LVAD implantation as well as end of life decisions.
A documented advance plan of deactivation of the LVAD might prove helpful when needed.
End of part 3.
Statement of previous publication
The authors warrant that the article is original, does not infringe upon any copyright or other proprietary right of any third party.
The article is not under consideration by another publication; and has not been previously published.
Conflict of interest
Authors have nothing to disclose.
Funding
None.
Author contributions
The authors confirm that the final manuscript has been read and each author's contribution has been approved by the appropriate author.
Gustafsson, F. , Ben Avraham, B. , Chioncel, O. , Hasin, T. , Grupper, A. , Shaul, A. , Nalbantgil, S. , Hammer, Y. , Mullens, W. , Tops, L. F. , Elliston, J. , Tsui, S. , Milicic, D. , Altenberger, J. , Abuhazira, M. , Winnik, S. , Lavee, J. , Piepoli, M. F. , Hill, L. , Hamdan, R. , Ruhparwar, A. , Anker, S. , Crespo‐Leiro, M. G. , Coats, A. J. S. , Filippatos, G. , Metra, M. , Rosano, G. , Seferovic, P. , Ruschitzka, F. , Adamopoulos, S. , Barac, Y. , De Jonge, N. , Frigerio, M. , Goncalvesova, E. , Gotsman, I. , Itzhaki Ben Zadok, O. , Ponikowski, P. , Potena, L. , Ristic, A. , Jaarsma, T. , and Ben Gal, T. (2021) HFA of the ESC position paper on the management of LVAD‐supported patients for the non‐LVAD specialist healthcare provider Part 3: at the hospital and discharge. ESC Heart Failure, 8: 4425–4443. 10.1002/ehf2.13590.
References
- 1. Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol 2015; 66: 2663–2674. [DOI] [PubMed] [Google Scholar]
- 2. Rangasamy S, Madan S, Saeed O, Goldstein DJ, Jorde UP, Negassa A, Patel SR. Noninvasive measures of pulsatility and blood pressure during continuous‐flow left ventricular assist device support. ASAIO J 2019; 65: 241–246. [DOI] [PubMed] [Google Scholar]
- 3. Nassif ME, Tibrewala A, Raymer DS, Andruska A, Novak E, Vader JM, Itoh A, Silvestry SC, Ewald GA, LaRue SJ. Systolic blood pressure on discharge after left ventricular assist device insertion is associated with subsequent stroke. J Heart Lung Transplant 2015; 34: 503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saeed O, Jermyn R, Kargoli F, Madan S, Mannem S, Gunda S, Nucci C, Farooqui S, Hassan S, Mclarty A, Bloom M, Zolty R, Shin J, D'Alessandro D, Goldstein DJ, Patel SR. Blood pressure and adverse events during continuous flow left ventricular assist device support. Circ Heart Fail 2015; 8: 551–556. [DOI] [PubMed] [Google Scholar]
- 5. Wasson LT, Yuzefpolskaya M, Wakabayashi M, Takayama H, Naka Y, Uriel N, Jorde UP, Demmer RT, Colombo PC. Hypertension: an unstudied potential risk factor for adverse outcomes during continuous flow ventricular assist device support. Heart Fail Rev 2015; 20: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, el‐Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J, International Society for Heart and Lung Transplantation . The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013; 32: 157–187. [DOI] [PubMed] [Google Scholar]
- 7. Potapov EV, Antonides C, Crespo‐Leiro MG, Combes A, Färber G, Hannan MM, Kukucka M, de Jonge N, Loforte A, Lund LH, Mohacsi P, Morshuis M, Netuka I, Özbaran M, Pappalardo F, Scandroglio AM, Schweiger M, Tsui S, Zimpfer D, Gustafsson F. 2019 EACTS Expert consensus on long‐term mechanical circulatory support. Eur J Cardiothorac Surg 2019; 56: 230–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khazanie P, Hammill BG, Patel CB, Kiernan MS, Cooper LB, Arnold SV, Fendler TJ, Spertus JA, Curtis LH, Hernandez AF. Use of heart failure medical therapies among patients with left ventricular assist devices: insights from INTERMACS. J Card Fail 2016; 22: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Randhawa VK, West L, Luthman J, Estep JD, Soltesz EG, Starling RC. Sacubitril/valsartan in patients post‐left ventricular assist device implant: a single‐centre case series. Eur J Heart Fail 2020; 22: 1490–1492. [DOI] [PubMed] [Google Scholar]
- 10. Adatya S, Uriel N, Yarmohammadi H, Holley CT, Feng A, Roy SS, Reding MT, John R, Eckman P, Zantek ND. Anti‐factor Xa and activated partial thromboplastin time measurements for heparin monitoring in mechanical circulatory support. JACC Heart Fail 2015; 3: 314–322. [DOI] [PubMed] [Google Scholar]
- 11. Sandner SE, Riebandt J, Haberl T, Mahr S, Rajek A, Schima H, Wieselthaler GM, Laufer G, Zimpfer D. Low‐molecular‐weight heparin for anti‐coagulation after left ventricular assist device implantation. J Heart Lung Transplant 2014; 33: 88–93. [DOI] [PubMed] [Google Scholar]
- 12. Imamura T, Adatya S, Chung B, Nguyen A, Rodgers D, Sayer G, Sarswat N, Kim G, Raikhelkar J, Ota T, Song T, Juricek C, Medvedofsky D, Jeevanandam V, Lang R, Estep JD, Burkhoff D, Uriel N. Cannula and pump positions are associated with left ventricular unloading and clinical outcome in patients with HeartWare left ventricular assist device. J Card Fail 2018; 24: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeVore AD, Patel PA, Patel CB. Medical management of patients with a left ventricular assist device for the non‐left ventricular assist device specialist. JACC Heart Fail 2017; 5: 621–631. [DOI] [PubMed] [Google Scholar]
- 14. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez‐Fernandez S, Miani D, Filippatos G, Maggioni AP, ESC Heart Failure Long‐Term Registry Investigators . Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 15. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel T, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP, ADHERE Scientific Advisory Committee and Investigators . Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216. [DOI] [PubMed] [Google Scholar]
- 16. Mullens W, Verbrugge FH, Nijst P, Tang WHW. Renal sodium avidity in heart failure: from pathophysiology to treatment strategies. Eur Heart J 2017; 38: 1872–1882. [DOI] [PubMed] [Google Scholar]
- 17. Roehm B, Vest AR, Weiner DE. Left ventricular assist devices, kidney disease, and dialysis. Am J Kidney Dis 2018; 71: 257–266. [DOI] [PubMed] [Google Scholar]
- 18. Brisco MA, Testani JM, Cook JL. Renal dysfunction and chronic mechanical circulatory support: from patient selection to long‐term management and prognosis. Curr Opin Cardiol 2016; 31: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ross DW, Stevens GR, Wanchoo R, Majure DT, Jauhar S, Fernandez HA, Merzkani M, Jhaveri KD. Left ventricular assist devices and the kidney. Clin J Am Soc Nephrol 2018; 13: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, Hernandez AF. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol 2013; 61: 2397–2405. [DOI] [PubMed] [Google Scholar]
- 21. Matthews JC, Pagani FD, Haft JW, Koelling TM, Naftel DC, Aaronson KD. Model for end‐stage liver disease score predicts left ventricular assist device operative transfusion requirements, morbidity, and mortality. Circulation 2010; 121: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang JA, Kato TS, Shulman BP, Takayama H, Farr M, Jorde UP, Mancini DM, Naka Y, Schulze PC. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: use of the Model of End‐stage Liver Disease (MELD) and MELD eXcluding INR (MELD‐XI) scoring system. J Heart Lung Transplant 2012; 31: 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Potthoff A, Schettler A, Attia D, Schlue J, Schmitto JD, Fegbeutel C, Strüber M, Haverich A, Manns MP, Wedemeyer H, Gebel M, Schneider A. Liver stiffness measurements and short‐term survival after left ventricular assist device implantation: a pilot study. J Heart Lung Transplant 2015; 34: 1586–1594. [DOI] [PubMed] [Google Scholar]
- 24. Russell SD, Rogers JG, Milano CA, Dyke DB, Pagani FD, Aranda JM, Klodell CT Jr, Boyle AJ, John R, Chen L, Massey HT, Farrar DJ, Conte JV, HeartMate II Clinical Investigators . Renal and hepatic function improve in advanced heart failure patients during continuous‐flow support with the HeartMate II left ventricular assist device. Circulation 2009; 120: 2352–2357. [DOI] [PubMed] [Google Scholar]
- 25. Yoshioka D, Takayama H, Colombo PC, Yuzefpolskaya M, Garan AR, Topkara VK, Han J, Kurlansky P, Naka Y, Takeda K. Changes in end‐organ function in patients with prolonged continuous‐flow left ventricular assist device support. Ann Thorac Surg 2017; 103: 717–724. [DOI] [PubMed] [Google Scholar]
- 26. Majumder K, Spratt JR, Holley CT, Roy SS, Cogswell RJ, Liao K, John R. Impact of postoperative liver dysfunction on survival after left ventricular assist device implantation. Ann Thorac Surg 2017; 104: 1556–1562. [DOI] [PubMed] [Google Scholar]
- 27. Kusne S, Mooney M, Danziger‐Isakov L, Kaan A, Lund LH, Lyster H, Wieselthaler G, Aslam S, Cagliostro B, Chen J, Combs P, Cochrane A, Conway J, Cowger J, Frigerio M, Gellatly R, Grossi P, Gustafsson F, Hannan M, Lorts A, Martin S, Pinney S, Silveira FP, Schubert S, Schueler S, Strueber M, Uriel N, Wrightson N, Zabner R, Huprikar S. An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J Heart Lung Transplant 2017; 36: 1137–1153. [DOI] [PubMed] [Google Scholar]
- 28. Cardoso JN, del Carlo C, Oliveira Junior MT, Ochiai ME, Kalil Filho R, Barretto ACP. Infection in patients with decompensated heart failure: in‐hospital mortality and outcome. Arq Bras Cardiol 2018; 110: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mondal NK, Sobieski MA, Pham SM, Griffith BP, Koenig SC, Slaughter MS, Wu ZJ. Infection, oxidative stress, and changes in circulating regulatory T cells of heart failure patients supported by continuous‐flow ventricular assist devices. ASAIO J 2017; 63: 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moro‐García MA, Echeverría A, Galán‐Artímez MC, Suárez‐García FM, Solano‐Jaurrieta JJ, Avanzas‐Fernández P, Díaz‐Molina B, Lambert JL, López‐Larrea C, Morís de la Tassa C, Alonso‐Arias R. Immunosenescence and inflammation characterize chronic heart failure patients with more advanced disease. Int J Cardiol 2014; 174: 590–599. [DOI] [PubMed] [Google Scholar]
- 31. Xydonas S, Parissis J, Lioni L, Kapsimali V, Psarra E, Farmakis D, Kremastinos D, Lekakis J, Sideris A, Tsirogianni A, Filippatos G. Immunosenescence in patients with chronic systolic heart failure. J Cardiovasc Med (Hagerstown) 2016; 17: 624–630. [DOI] [PubMed] [Google Scholar]
- 32. Hannan MM, Xie R, Cowger J, Schueler S, de By T, Dipchand AI, Chu VH, Cantor RS, Koval CE, Krabatsch T, Hayward CS, Nakatani T, Kirklin JK. Epidemiology of infection in mechanical circulatory support: a global analysis from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant 2019; 38: 364–373. [DOI] [PubMed] [Google Scholar]
- 33. Qu Y, McGiffin D, Kure C, Ozcelik B, Fraser J, Thissen H, Peleg AY. Biofilm formation and migration on ventricular assist device drivelines. J Thorac Cardiovasc Surg 2019; 491–502. [DOI] [PubMed] [Google Scholar]
- 34. Toba FA, Akashi H, Arrecubieta C, Lowy FD. Role of biofilm in Staphylococcus aureus and Staphylococcus epidermidis ventricular assist device driveline infections. J Thorac Cardiovasc Surg 2011; 141: 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaplan JB. Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J Dent Res 2010; 89: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vest AR, Mistak SM, Hachamovitch R, Mountis MM, Moazami N, Young JB. Outcomes for patients with diabetes after continuous‐flow left ventricular assist device implantation. J Card Fail 2016; 22: 789–796. [DOI] [PubMed] [Google Scholar]
- 37. Asleh R, Briasoulis A, Schettle SD, Tchantchaleishvili V, Pereira NL, Edwards BS, Clavell AL, Maltais S, Joyce DL, Joyce LD, Daly RC. Impact of diabetes mellitus on outcomes in patients supported with left ventricular assist devices: a single institutional 9‐year experience. Circ Heart Fail 2017; 10. [DOI] [PubMed] [Google Scholar]
- 38. Xia Y, Forest S, Friedmann P, Chou LC, Patel S, Jorde U, Goldstein D. Factors associated with prolonged survival in left ventricular assist device recipients. Ann Thorac Surg 2019; 107: 519–526. [DOI] [PubMed] [Google Scholar]
- 39. Yassin AS, Subahi A, Adegbala O, Abubakar H, Akintoye E, Ahmed A, Ismail A, Elhag A, Kambal A, Alade A, Shokr M. Clinical impact of diabetes mellitus on short‐term outcomes and in‐hospital mortality of cardiac mechanical support with left ventricular assist device (LVAD): a retrospective study from a national database. Cardiovasc Revasc Med 2018; 883–886. [DOI] [PubMed] [Google Scholar]
- 40. Goetz ME, Charnigo R, Guglin M. Implantation of left ventricular assist device results in immediate improvement of glucose metabolism in patients with and without diabetes mellitus. Heart Lung Circ 2020; 29: 931–935. [DOI] [PubMed] [Google Scholar]
- 41. Patel N, Gluck JA, Radojevic J, Coleman CI, Baker WL. Left ventricular assist device implantation improves glycaemic control: a systematic review and meta‐analysis. ESC Heart Fail 2018; 5: 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yen DC, Watson MH, Burgess LD, Kuchibhatla M, Patel CB, Campbell KB, Vora AK. Positive impact of continuous‐flow left ventricular assist device implantation on glycemic control in patients with type 2 diabetes mellitus and advanced chronic systolic heart failure. Pharmacotherapy 2016; 36: 1210–1216. [DOI] [PubMed] [Google Scholar]
- 43. Hawkins RB, Go K, Raymond SL, Ayzengart A, Friedman J. Laparoscopic sleeve gastrectomy in patients with heart failure and left ventricular assist devices as a bridge to transplant. Surg Obes Relat Dis 2018; 14: 1269–1273. [DOI] [PubMed] [Google Scholar]
- 44. Leviner DB, Keidar A, Ben‐Gal T, Medalion B. Cardiac function recovery following LVAD implantation and bariatric surgery in a morbidly obese patient. J Card Surg 2014; 29: 740–742. [DOI] [PubMed] [Google Scholar]
- 45. Sajgalik P, Kim CH, Stulak JM, Kushwaha SS, Maltais S, Joyce DL, Joyce LD, Johnson BD, Schirger JA. Pulmonary function assessment post‐left ventricular assist device implantation. ESC Heart Fail 2019; 6: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fang JC, Wever‐Pinzon O. Dealing with unintended consequences: continuous‐flow LVADs and aortic insufficiency. JACC Cardiovasc Imaging 2016; 9: 652–654. [DOI] [PubMed] [Google Scholar]
- 47. Rajagopal K, Daneshmand MA, Patel CB, Ganapathi AM, Schechter MA, Rogers JG, Milano CA. Natural history and clinical effect of aortic valve regurgitation after left ventricular assist device implantation. J Thorac Cardiovasc Surg 2013; 145: 1373–1379. [DOI] [PubMed] [Google Scholar]
- 48. Holley CT, Fitzpatrick M, Roy SS, Alraies MC, Cogswell R, Souslian L, Eckman P, John R. Aortic insufficiency in continuous‐flow left ventricular assist device support patients is common but does not impact long‐term mortality. J Heart Lung Transplant 2017; 36: 91–96. [DOI] [PubMed] [Google Scholar]
- 49. Gasparovic H, Kopjar T, Saeed D, Cikes M, Svetina L, Petricevic M, Lovric D, Milicic D, Biocina B. De novo aortic regurgitation after continuous‐flow left ventricular assist device implantation. Ann Thorac Surg 2017; 104: 704–711. [DOI] [PubMed] [Google Scholar]
- 50. McKellar SH, Deo S, Daly RC, Durham LA III, Joyce LD, Stulak JM, Park SJ. Durability of central aortic valve closure in patients with continuous flow left ventricular assist devices. J Thorac Cardiovasc Surg 2014; 147: 344–348. [DOI] [PubMed] [Google Scholar]
- 51. Morgan JA, Brewer RJ, Nemeh HW, Henry SE, Neha N, Williams CT, Lanfear DE, Tita C, Paone G. Management of aortic valve insufficiency in patients supported by long‐term continuous flow left ventricular assist devices. Ann Thorac Surg 2012; 94: 1710–1712. [DOI] [PubMed] [Google Scholar]
- 52. Kurihara C, Cohn WE, Kawabori M, Sugiura T, Civitello AB, Morgan JA. Long‐term continuous‐flow left ventricular assist device support after left ventricular outflow tract closure. ASAIO J 2019; 65: 558–564. [DOI] [PubMed] [Google Scholar]
- 53. Fukuhara S, Ikegami H, Polanco AR, Song JJ, Han J, Takeda K, Kurlansky PA, Takayama H, Naka Y. Concomitant repair for mild aortic insufficiency and continuous‐flow left ventricular assist devices. Eur J Cardiothorac Surg 2017; 52: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 54. Grohmann J, Blanke P, Benk C, Schlensak C. Trans‐catheter closure of the native aortic valve with an Amplatzer Occluder to treat progressive aortic regurgitation after implantation of a left‐ventricular assist device. Eur J Cardiothorac Surg 2011; 39: e181–e183. [DOI] [PubMed] [Google Scholar]
- 55. Parikh KS, Mehrotra AK, Russo MJ, Lang RM, Anderson A, Jeevanandam V, Freed BH, Paul JD, Karol J, Nathan S, Shah AP. Percutaneous transcatheter aortic valve closure successfully treats left ventricular assist device‐associated aortic insufficiency and improves cardiac hemodynamics. JACC Cardiovasc Interv 2013; 6: 84–89. [DOI] [PubMed] [Google Scholar]
- 56. Santini F, Forni A, Dandale R, Ribicini F, Franchi G, Onorati F, Vassanelli C, Mazzucco, A, Faggian G. First successful management of aortic valve insufficiency associated with HeartMate II left ventricular assist device support by transfemoral CoreValve implantation: the Columbus's egg? JACC Cardiovasc Interv 2012; 5: 114–115. [DOI] [PubMed] [Google Scholar]
- 57. Yehya A, Rajagopal V, Meduri C, Kauten J, Brown M, Dean L, Webster J, Krishnamoorthy A, Hrobowski T, Dean D. Short‐term results with transcatheter aortic valve replacement for treatment of left ventricular assist device patients with symptomatic aortic insufficiency. J Heart Lung Transplant 2019; 38: 920–926. [DOI] [PubMed] [Google Scholar]
- 58. Dalia AA, Cronin B, Stone ME, Turner K, Hargrave J, Vidal Melo MF, Essandoh M. Anesthetic management of patients with continuous‐flow left ventricular assist devices undergoing noncardiac surgery: an update for anesthesiologists. J Cardiothorac Vasc Anesth 2018; 32: 1001–1012. [DOI] [PubMed] [Google Scholar]
- 59. Mathis MR, Sathishkumar S, Kheterpal S, Caldwell MD, Pagani FD, Jewell ES, Engoren MC. Complications, risk factors, and staffing patterns for noncardiac surgery in patients with left ventricular assist devices. Anesthesiology 2017; 126: 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brown TA, Kerpelman J, Wolf BJ, McSwain JR. Comparison of clinical outcomes between general anesthesiologists and cardiac anesthesiologists in the management of left ventricular assist device patients in noncardiac surgeries and procedures. J Cardiothorac Vasc Anesth 2018; 32: 2104–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chung M. Perioperative management of the patient with a left ventricular assist device for noncardiac surgery. Anesth Analg 2018; 126: 1839–1850. [DOI] [PubMed] [Google Scholar]
- 62. Rao VK, Tsai A. Management of patients on mechanical circulatory assist devices during noncardiac surgery. Int Anesthesiol Clin 2018; 56: e1–e27. [DOI] [PubMed] [Google Scholar]
- 63. Nelson EW, Heinke T, Finley A, Guldan GJ, Gaddy P, Matthew Toole J, Mims R, Abernathy JH III. Management of LVAD patients for noncardiac surgery: a single‐institution study. J Cardiothorac Vasc Anesth 2015; 29: 898–900. [DOI] [PubMed] [Google Scholar]
- 64. Degnan M, Brodt J, Rodriguez‐Blanco Y. Perioperative management of patients with left ventricular assist devices undergoing noncardiac surgery. Ann Card Anaesth 2016; 19: 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stone M, Hinchey J, Sattler C, Evans A. Trends in the management of patients with left ventricular assist devices presenting for noncardiac surgery: a 10‐year institutional experience. Semin Cardiothorac Vasc Anesth 2016; 20: 197–204. [DOI] [PubMed] [Google Scholar]
- 66. Gündeş E, Uzun O, Çiyiltepe H, Aday U, Çetin DA, Gülmez S, Senger AS, Kırali K. Emergency abdominal surgery in patients with left ventricular assist device: short‐ and long‐term results. Postepy Kardiol Interwencyjnej 2017; 13: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vigneswaran Y, Wang V, Krezalek M, Prachand V, Wyers S, Juricek C, Uriel N, Jeevanandam V, Hussain M. Laparoscopic procedures in patients with cardiac ventricular assist devices. Surg Endosc 2019; 33: 2181–2186. [DOI] [PubMed] [Google Scholar]
- 68. Sheu R, Joshi B, High K, Thinh Pham D, Ferreira R, Cobey F. Perioperative management of patients with left ventricular assist devices undergoing noncardiac procedures: a survey of current practices. J Cardiothorac Vasc Anesth 2015; 29: 17–26. [DOI] [PubMed] [Google Scholar]
- 69. Woldendorp K, Gupta S, Lai J, Dhital K, Hayward CS. A novel method of blood pressure measurement in patients with continuous‐flow left ventricular assist devices. J Heart Lung Transplant 2014; 33: 1183–1186. [DOI] [PubMed] [Google Scholar]
- 70. Lanier GM, Orlanes K, Hayashi Y, Murphy J, Flannery M, Te‐Frey R, Uriel N, Yuzefpolskaya M, Mancini DM, Naka Y, Takayama H, Jorde UP, Demmer RT, Colombo PC. Validity and reliability of a novel slow cuff‐deflation system for noninvasive blood pressure monitoring in patients with continuous‐flow left ventricular assist device. Circ Heart Fail 2013; 6: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 71. Cagliostro B, Levin AP, Fried J, Stewart S, Parkis G, Mody KP, Garan AR, Topkara V, Takayama H, Naka Y, Jorde UP, Uriel N. Continuous‐flow left ventricular assist devices and usefulness of a standardized strategy to reduce drive‐line infections. J Heart Lung Transplant 2016; 35: 108–114. [DOI] [PubMed] [Google Scholar]
- 72. John R, Aaronson KD, Pae WE, Acker MA, Hathaway DR, Najarian KB, Slaughter MS, HeartWare Bridge to Transplant ADVANCE Trial Investigators . Drive‐line infections and sepsis in patients receiving the HVAD system as a left ventricular assist device. J Heart Lung Transplant 2014; 33: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 73. Aburjania N, Sherazi S, Tchantchaleishvili V, Alexis JD, Hay CM. Stopping conventional showering decreases Pseudomonas infections in left ventricular assist device patients. Int J Artif Organs 2017; 40: 282–285. [DOI] [PubMed] [Google Scholar]
- 74. Baddour LM, Bettmann MA, Bolger AF, Epstein AE, Ferrieri P, Gerber MA, Gewitz MH, Jacobs AK, Levison ME, Newburger JW, Pallasch TJ. Nonvalvular cardiovascular device‐related infections. Circulation 2003; 108: 2015–2031. [DOI] [PubMed] [Google Scholar]
- 75. Ponikowski P, Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Mee P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 76. Piepoli MF, Binno S, Corrà U, Seferovic P, Conraads V, Jaarsma T, Schmid JP, Filippatos G, Ponikowski PP, Committee on Exercise Physiology & Training of the Heart Failure Association of the ESC . ExtraHF survey: the first European survey on implementation of exercise training in heart failure patients. Eur J Heart Fail 2015; 17: 631–638. [DOI] [PubMed] [Google Scholar]
- 77. Piepoli MF, Davos C, Francis DP, Coats AJ, ExTraMATCH Collaborative . Exercise training meta‐analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004; 328: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL, HF‐ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA 2009; 301: 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Corrà U, Pistono M, Mezzani A, Gnemmi M, Tarro Genta F, Caruso R, Giannuzzi P. Cardiovascular prevention and rehabilitation for patients with ventricular assist device from exercise therapy to long‐term therapy. Part I: exercise therapy. Monaldi Arch Chest Dis 2011; 76: 27–32. [DOI] [PubMed] [Google Scholar]
- 80. Corrà U, Pistono M, Piepoli MF, Giannuzzi P. Ventricular assist device patients on the horizon of cardiovascular prevention and rehabilitation. Can we convert challenges into opportunities? Eur J Prev Cardiol 2012; 19: 490–493. [DOI] [PubMed] [Google Scholar]
- 81. Ben Gal T, Piepoli MF, Corrà U, Conraads V, Adamopoulos S, Agostoni P, Piotrowicz E, Schmid JP, Seferovic PM, Ponikowski P, Filippatos G, Jaarsma T, Committee on Exercise Physiology & Training of Heart Failure Association and endorsed by Cardiac Rehabilitation Section of European Association for Cardiovascular Rehabilitation and Prevention of ESC . Exercise programs for LVAD supported patients: a snapshot from the ESC affiliated countries. Int J Cardiol 2015; 201: 215–219. [DOI] [PubMed] [Google Scholar]
- 82. Adamopoulos S, Corrà U, Laoutaris ID, Pistono M, Agostoni PG, Coats AJS, Crespo Leiro MG, Cornelis J, Davos CH, Filippatos G, Lund LH, Jaarsma T, Ruschitzka F, Seferovic PM, Schmid JP, Volterrani M, Piepoli MF. Exercise training in patients with ventricular assist devices: a review of the evidence and practical advice. A position paper from the Committee on Exercise Physiology and Training and the Committee of Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 3–13. [DOI] [PubMed] [Google Scholar]
- 83. Wells CL. Physical therapist management of patients with ventricular assist devices: key considerations for the acute care physical therapist. Phys Ther 2013; 93: 266–278. [DOI] [PubMed] [Google Scholar]
- 84. Muthiah K, Gupta S, Otton J, Robson D, Walker R, Tay A, Macdonald P, Keogh A, Kotlyar E, Granger E, Dhital K, Spratt P, Jansz P, Hayward CS. Body position and activity, but not heart rate, affect pump flows in patients with continuous‐flow left ventricular assist devices. JACC Heart Fail 2014; 2: 323–330. [DOI] [PubMed] [Google Scholar]
- 85. Perme CS, Southard RE, Joyce DL, Noon GP, Loebe M. Early mobilization of LVAD recipients who require prolonged mechanical ventilation. Tex Heart Inst J 2006; 33: 130–133. [PMC free article] [PubMed] [Google Scholar]
- 86. Ganga HV, Leung A, Jantz J, Choudhary G, Stabile L, Levine DJ, Sharma SC, Wu WC. Supervised exercise training versus usual care in ambulatory patients with left ventricular assist devices: a systematic review. PLoS One 2017; 12: e0174323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Morrone TM, Buck LA, Catanese KA, Goldsmith RL, Cahalin LP, Oz MC, Levin HR. Early progressive mobilization of patients with left ventricular assist devices is safe and optimizes recovery before heart transplantation. J Heart Lung Transplant 1996; 15: 423–429. [PubMed] [Google Scholar]
- 88. Kato N, Jaarsma T, Ben Gal T. Learning self‐care after left ventricular assist device implantation. Curr Heart Fail Rep 2014; 11: 290–298. [DOI] [PubMed] [Google Scholar]
- 89. Riegel B, Jaarsma T, Stromberg A. A middle‐range theory of self‐care of chronic illness. ANS Adv Nurs Sci 2012; 35: 194–204. [DOI] [PubMed] [Google Scholar]
- 90. Fang JC, Ewald GA, Allen LA, Butler J, Westlake Canary CA, Colvin‐Adams M, Dickinson MG, Levy P, Stough WG, Sweitzer NK, Teerlink JR, Whellan DJ, Albert NM, Krishnamani R, Rich MW, Walsh MN, Bonnell MR, Carson PE, Chan MC, Dries DL, Hernandez AF, Hershberger RE, Katz SD, Moore S, Rodgers JE, Rogers JG, Vest AR, Givertz MM, Heart Failure Society of America Guidelines Committee . Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail 2015; 21: 519–534. [DOI] [PubMed] [Google Scholar]
- 91. Jaarsma T, Beattie JM, Ryder M, Rutten FH, McDonagh T, Mohacsi P, Murray SA, Grodzicki T, Bergh I, Metra M, Ekman I, Angermann C, Leventhal M, Pitsis A, Anker SD, Gavazzi A, Ponikowski P, Dickstein K, Delacretaz E, Blue L, Strasser F, McMurray J, Advanced Heart Failure Study Group of the HFA of the ESC . Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2009; 11: 433–443. [DOI] [PubMed] [Google Scholar]
- 92. Hill L, Prager Geller T, Baruah R, Beattie JM, Boyne J, Stoutz N, di Stolfo G, Lambrinou E, Skibelund AK, Uchmanowicz I, Rutten FH, Čelutkienė J, Piepoli MF, Jankowska EA, Chioncel O, Ben Gal T, Seferovic PM, Ruschitzka F, Coats AJS, Strömberg A, Jaarsma T. Integration of a palliative approach into heart failure care: a European Society of Cardiology Heart Failure Association position paper. Eur J Heart Fail 2020; 22: 2327–2339. [DOI] [PubMed] [Google Scholar]
- 93. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge‐Caballero E, de Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska‐Migaj E, McDonagh T, Seferovic P, Ruschitzka F. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 1505–1535. [DOI] [PubMed] [Google Scholar]
- 94. Salomon S, Frankel H, Chuang E, Eti S, Selwyn P. Implementing routine palliative care consultation before LVAD implantation: a single center experience. J Pain Symptom Manage 2018; 55: 1350–1355. [DOI] [PubMed] [Google Scholar]
- 95. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne‐Nickens P, Oz MC, Poirier VL, Meier P. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med 2001; 345: 1435–1443. [DOI] [PubMed] [Google Scholar]
- 96. Dunlay SM, Strand JJ, Wordingham SE, Stulak JM, Luckhardt AJ, Swetz KM. Dying with a left ventricular assist device as destination therapy. Circ Heart Fail 2016; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, Adams PA, Speck A, Johnson KS, Krishnamoorthy A, Yang H, Anstrom KJ, Dodson GC, Taylor DH Jr, Kirchner JL, Mark DB, O'Connor CM, Tulsky JA. Palliative care in heart failure: the PAL‐HF randomized, controlled clinical trial. J Am Coll Cardiol 2017; 70: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brännström M, Boman K. Effects of person‐centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail 2014; 16: 1142–1151. [DOI] [PubMed] [Google Scholar]
- 99. O'Donnell AE, Schaefer KG, Stevenson LW, DeVoe K, Walsh K, Mehra MR, Desai AS. Social Worker‐Aided Palliative Care Intervention in High‐risk Patients With Heart Failure (SWAP‐HF): a pilot randomized clinical trial. JAMA Cardiol 2018; 3: 516–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hill L, McIlfatrick S, Taylor BJ, Dixon L, Cole BR, Moser DK, Fitzsimons D. Implantable cardioverter defibrillator (ICD) deactivation discussions: Reality versus recommendations. Eur J Cardiovasc Nurs 2016; 15: 20–29. [DOI] [PubMed] [Google Scholar]
- 101. McIlvennan CK, Wordingham SE, Allen LA, Matlock DD, Jones J, Dunlay SM, Swetz KM. Deactivation of left ventricular assist devices: differing perspectives of cardiology and hospice/palliative medicine clinicians. J Card Fail 2017; 23: 708–712. [DOI] [PubMed] [Google Scholar]


