Abstract
Aims
Recent large randomized controlled trials (RCTs) have demonstrated efficacy of sodium‐glucose cotransporter‐2 inhibitors (SGLT2i) in both preventing and treating heart failure (HF). SGLT2i‐induced reversal of left ventricular remodelling has been proposed as a mechanism contributing to this effect.
Methods and results
We performed a systematic review and meta‐analysis of RCTs to compare SGLT2i versus placebo (treatment duration >3 months) on cardiac remodelling parameters as measured by cardiac magnetic resonance imaging (cMRI) in patients with HF and/or diabetes. The PubMed and ClinicalTrials.gov databases were searched until 15 June 2021. Our primary outcome was change in absolute left ventricular mass (LVM) from baseline to study endpoint. Secondary outcomes included changes in LVM indexed to body surface area, left ventricular end‐systolic volume (LVESV), left ventricular end‐diastolic volume (LVEDV), and left ventricular ejection fraction (LVEF) from baseline to study endpoint. The Cochrane Collaboration's tool was used to assess risk of bias. Five studies representing 408 patients were included. SGLT2i was associated with greater LVM regression compared to placebo (MD, −5.76 g; 95% CI, −10.87 g to −0.64 g, I 2 = 73%; overall effect, P < 0.03; four RCTs). Statistical subgroup differences were not observed in our sensitivity analysis focusing on HF with reduced ejection fraction (P = 0.37) and were observed in our sensitivity analysis focusing on diabetes (P < 0.001). SGLT2i was not associated with statistical changes in LV mass indexed to body surface area (I 2 = 75%; P = 0.16; five RCTs), LVESV (I 2 = 87%; P = 0.07; five RCTs), LVEDV (I 2 = 81%; P = 0.20; five RCTs), nor LVEF (I 2 = 85%; P = 0.19; five RCTs) versus placebo. Sixty per cent of RCTs had low risk of bias.
Conclusions
Sodium‐glucose cotransporter‐2 inhibitors treatment was associated with a reduction in left ventricular mass as assessed by cMRI.
Keywords: SGLT2i, Cardiac magnetic resonance imaging, Cardiac remodelling, Diabetes, HFrEF
Background
Sodium‐glucose cotransporter‐2 inhibitors (SGLT2i) have been shown to prevent incident heart failure in patients with type 2 diabetes and treat heart failure with a reduced ejection fraction (HFrEF) in patients with and without diabetes. 1 , 2 , 3 , 4 , 5 , 6 While several mechanisms have been suggested to mediate these benefits, 7 , 8 , 9 there has been increasing interest in the effects of these therapies on ventricular reverse remodelling.
Aims
We performed a meta‐analysis of randomized controlled trials (RCTs) comparing SGLT2i versus placebo that evaluated changes in left ventricular mass, volumes, and ejection fraction as assessed by cardiac magnetic resonance imaging (cMRI).
Methods
Search strategy and selection criteria
We searched the PubMed and ClinicalTrials.gov databases from inception to 15 June 2021 using groups of keywords for SGLT2i, diabetes mellitus, heart failure, and cardiac morphology and function. The search strategies are provided in Supporting Information, Appendix S1 . A manual search of the reference lists of all included studies and relevant reviews was also conducted. Our search was limited to publications in the English language. The inclusion criteria were: 1) study design, randomized controlled trial; 2) population, patients with diabetes or heart failure; 3) intervention, SGTL2i therapy vs. placebo; 4) outcomes, reporting any of our primary or secondary outcomes; 5) length of treatment, intervention duration of at least 3 months. A flowchart outlining the study selection process is provided in Supporting Information, Figure S1 .
Outcomes
The primary outcome was change in left ventricular mass (LVM) from baseline to study endpoint as measured by cMRI. Secondary outcomes included changes in LVM indexed to body surface area (LVMi), left ventricular end systolic volume (LVESV), left ventricular end diastolic volume (LVEDV), and left ventricular ejection fraction (LVEF) from baseline to study endpoint as measured by cMRI.
Data extraction and quality assessment
Citations were independently screened by two reviewers (N. K. D. and N. M.) to select studies that met eligibility criteria and abstract data using a structured form which included study design, population characteristics, duration and dose of treatment, and outcomes. Discrepancies were resolved by a third author (C. D. M.). Two reviewers (N. M. and R. V.) assessed quality and risk of bias across the domains of sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting as per the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions. Risk of bias was graded as either being low, high, or unclear for each respective domain within each study.
Data synthesis
Data from all studies were combined to estimate the mean difference (MD) and 95% confidence interval (CI) for each outcome using an inverse variance approach and DerSimonian and Laird's random effects‐model. Missing data were not imputed. Statistical heterogeneity was tested using an inverse weighted χ 2 test and was quantified by I 2, with values >50% being considered substantial heterogeneity. P < 0.05 was considered statistically significant. Publication bias was intended to be assessed by inspection of the funnel plot of the primary outcome; however, this was unable to be done due to too few studies meeting eligibility criteria. We planned a priori sensitivity analyses to evaluate potential differences in treatment effect amongst trials exclusively recruiting patients with HFrEF and trials exclusively recruiting patients with diabetes or prediabetes. All analyses were performed with Review Manager software (version 5.3). The protocol for this systematic review was not registered. This systematic review and meta‐analysis adheres to PRISMA guidelines.
Results
Study characteristics and study population
A total of five studies, representing 408 patients, met the eligibility criteria and were included in the meta‐analysis (Supporting Information, Figure S1 ). 10 , 11 , 12 , 13 , 14 Table 1 summarizes the characteristics of included studies. The included studies assessed a dose of 10 mg of dapagliflozin or empagliflozin daily, and treatment durations ranged from 36 weeks to 1 year. Three RCTs exclusively enrolled patients with HFrEF, and four RCTs exclusively enrolled patients with diabetes or prediabetes. Sixty per cent of the studies had a low risk of bias in at least five out of the six domains (Supporting Information, Figure S2 ; justifications are summarized in Supporting Information, Table S1 ). An overview of relevant baseline patient characteristics and cMRI parameters according to treatment group is provided for each included study in Table 2 .
Table 1.
Characteristics of included studies
| Author | Participants | Intervention and comparator | Cardiovascular magnetic resonance measurements |
|---|---|---|---|
| Brown 2020 |
Normotensive adults 18–80 years with no clinical heart failure nor LV systolic dysfunction (LVEF < 45%) with Type 2 diabetes (HbA1c 48–85 mmol/mol) and evidence of echocardiographic LV hypertrophy (LV mass indexed to BSA > 115 g/m2 [M] or >95 g/m2 [F], or LV mass indexed to height2.7 > 48 g/m2.7 [M] or >44 g/m2.7 [F]). (Randomized: N = 66) |
Dapagliflozin (10 mg) or matching placebo once daily for 12 months | Changes to LV mass (raw value and indexed to BSA, height, height1.7, and height2.7), LVEF, LVEDV (raw value), and LVESV (raw value), stroke volume, and left atrial area from baseline to 12 months |
| Lee 2021 |
Adults ≥18 with Type 2 diabetes (HbA1c 48–97 mmol/mol, diet‐controlled or stable therapy for 6 weeks prior) or prediabetes (HbA1c 39–47 mmol/mol) and HF (NYHA II‐IV) with LVEF ≤40% and stable medical therapy for 4 weeks prior. (Randomized: N = 105) |
Empagliflozin (10 mg) or matching placebo once daily for 36 weeks | Changes to LVESV (raw value and indexed to BSA), LV global longitudinal strain, LVEDV (raw value and indexed to BSA), LVEF, LV mass (raw value and indexed to BSA), LV global function index, LA volume (raw value and indexed to BSA), myocardial blood flow, and extracellular volume fraction from baseline to 36 weeks |
| Santos‐Gallego 2021 |
Adults with HF (NYHA II‐III) with LVEF <50% and stable HF symptoms as well as medical therapy for 3 months prior, with no history of diabetes. (Randomized: N = 84) |
Empagliflozin (10 mg) or matching placebo daily for 6 months | Changes to LVEDV (raw value and indexed to BSA), LVESV (raw value and indexed to BSA), LVEF, LV mass (raw value and indexed to BSA), and sphericity index from baseline to 6 months |
| Singh 2020 |
Adults 18–75 years with Type 2 diabetes and HF (NYHA I‐III) with LVEF <45% or subjective LV systolic dysfunction that was mild or worse along with stable HF symptoms, medical therapy, and no history of hospitalization for HF for ≥3 months prior. Patients were required to be on furosemide 80 mg daily (or less), or on an equivalent loop diuretic. (Randomized: N = 56) |
Dapagliflozin (10 mg) or matching placebo once daily for 1 year | Changes to LVESV (raw value and indexed to BSA), LVEDV (raw value and indexed to BSA), LV mass (indexed to BSA), LVEF, LA volume (indexed to BSA) and LV stroke volume from baseline to 1 year |
| Verma 2019 |
Adults 40–80 years with type 2 diabetes (HbA1c 6.5–10%) and established cardiovascular disease (previous MI ≥ 6 months ago or coronary revascularization ≥2 months ago), with any background antihyperglycaemic therapy that had been stable ≥2 months without recent hospitalization for HF, severe HF symptoms (NYHA‐IV) nor LVEF <30%. (Randomized: N = 97) |
Empagliflozin (10 mg) once daily or matching placebo for 6 months | Changes to LV mass (raw value and indexed to BSA, height, height1.7, and height2.7), LVEF, LVEDV (raw value and indexed to BSA), and LVESV (raw value and indexed to BSA) from baseline to 6 months |
BSA, body surface area; M, male; F, female; HF, heart failure; LV, left ventricular; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; NYHA, New York Heart Association.
Table 2.
Baseline patient characteristics and cMRI parameters
| Brown 2020 | Lee 2021 | Santos‐Gallego 2021 | Singh 2020 | Verma 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SGLT2i | Placebo | SGLT2i | Placebo | SGLT2i | Placebo | SGLT2i | Placebo | SGLT2i | Placebo | |
| Baseline characteristics | ||||||||||
| Age (years) | 64.25 ± 7.01 | 66.74 ± 6.62 | 68.2 ± 11.7 | 69.2 ± 10.6 | 64.2 ± 10.9 | 59.9 ± 13.1 | 66.9 ± 7.0 | 67.4 ± 6.8 | 64 (57, 69) b | 64 (56, 72) b |
| Male sex | 20 (62.5) | 18 (52.9) | 34 (65.4) | 43 (81.1) | 27 (64) | 27 (64) | 18 (64.3) | 19 (67.9) | 44 (90) | 46 (96) |
| BMI (kg/m2) | 32.30 ± 4.66 | 32.59 ± 4.22 | 30.9 ± 5.9 | 30.4 ± 5.1 | 29.3 ± 6 | 30 ± 6 | 33.0 ± 5.5 | 32 ± 5.2 | 27.7 ± 4.7 | 27.4 ± 5.4 |
| HbA1c | 61.75 ± 11.19 mmol/mol | 60.18 ± 10.15 mmol/mol | 7.5 ± 1.6% | 7.0 ± 1.4% | 5.8 ± 0.3% | 5.8 ± 0.5% | 63.0 ± 17.8 mmol/mol | 58.6 ± 16.4 mmol/mol | 7.9 ± 0.8% | 8.0 ± 0.9% |
| SBP (mmHg) | 130.41 ± 9.62 | 127.67 ± 10.65 | 125.8 ± 18.2 | 130.3 ± 21.6 | NR | NR | 135 ± 15.4 | 132.8 ± 18.8 | 139 ± 15 | 138 ± 15 |
| NYHA class of HF | ||||||||||
| Class I | NR | NR | 0 (0.0) | 0 (0.0) | NR | NR | 12 (42.9) | 13 (46.4) | NR | NR |
| Class II | NR | NR | 37 (71.2) | 44 (83.0) | NR | NR | 13 (46.4) | 11 (39.3) | NR | NR |
| Class III | NR | NR | 15 (28.8) | 9 (17.0) | NR | NR | 3 (10.7) | 4 (14.3) | NR | NR |
| Class IV | NR | NR | 0 (0.0) | 0 (0.0) | NR | NR | 0 (0.0) | 0 (0.0) | NR | NR |
| Baseline CMR parameters | ||||||||||
| Baseline LVM (g) | 126.47 ± 20.54 | 121.61 ± 24.20 | 121.2 ± 36.5 | 131.9 ± 44.9 | 135.2 ± 45.2 | 131.8 ± 54.4 | NR | NR | 116.5 ± 26.3 | 120.9 ± 33.0 |
| Baseline LVMi (g/m2) a | 60.92 ± 7.76 | 59.04 ± 8.73 | 61.2 ± 16.1 | 65.4 ± 19.6 | 67.9 ± 17.8 | 65.9 ± 19.8 | 69.5 ± 16.3 | 73.7 ± 19.3 | 59.3 ± 10.9 | 62.2 ± 12.8 |
| Baseline LVESV (mL) | 37.17 ± 9.92 | 33.63 ± 11.13 | 157.5 ± 68.1 | 152.9 ± 58.4 | 143.6 ± 66.3 | 135.1 ± 54.8 | 99.2 ± 40.7 | 106.4 ± 59.6 | 53.0 ± 20.8 | 62.5 ± 26.0 |
| Baseline LVEDV (mL) | 127.63 ± 22.54 | 120.66 ± 25.29 | 224.8 ± 72.2 | 222.7 ± 60.1 | 219.8 ± 75.8 | 210.4 ± 68.9 | 172.4 ± 47.7 | 188.3 ± 72.4 | 124.1 ± 33.0 | 138.4 ± 39.1 |
| Baseline LVEF (%) | 71.31 ± 5.42 | 72.54 ± 6.27 | 31.7 ± 9.9 | 33.0 ± 9.5 | 36.2 ± 8.2 | 36.5 ± 8 | 44.5 ± 12.4 | 46.5 ± 11.7 | 58.0 ± 7.5 | 55.5 ± 8.7 |
Data are mean ± SD, n (%) except where otherwise specified.
BMI, body mass index; cMRI, cardiac magnetic resonance imaging; HbA1c, haemoglobin A1c; HF, heart failure; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; LVMi, indexed left ventricular mass; LVESV, left ventricular end systolic volume; NYHA, New York Heart Association; SBP, systolic blood pressure; SGLT2i, sodium‐glucose cotransporter‐2 inhibitors.
Indexed to body surface area.
Age provided as median (IQR) for this study.
Primary outcome
SGLT2i was associated with a greater regression in LVM relative to placebo (MD, −5.76 g; 95% CI, −10.87 g to −0.64 g, I 2 = 73%; overall effect, P < 0.03; four trials; Figure 1A ). The test for subgroup differences in our sensitivity analysis focusing on HFrEF did not reveal any differences (P = 0.37). We observed subgroup differences in our sensitivity analysis focusing on diabetes, where LVM regression by SGLT2i was larger in magnitude amongst patients without diabetes (P < 0.001; Supporting Information, Figure S3A ).
Figure 1.
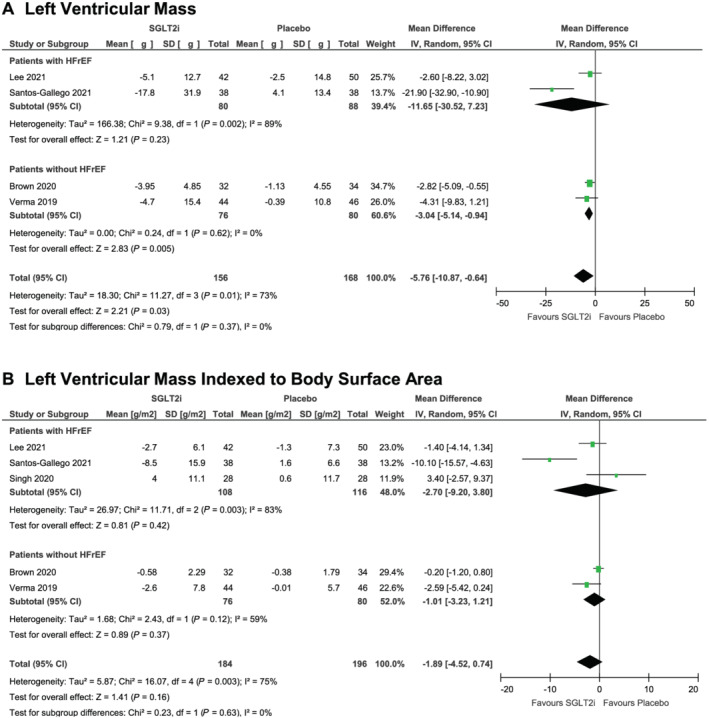
Cardiac magnetic resonance imaging‐assessed changes in left ventricular mass (A) and left ventricular mass indexed to body surface area (B) from baseline to study endpoint in randomized controlled trials of patients treated with sodium glucose transporter‐2 inhibitor therapy versus placebo.
Secondary outcomes
There were no significant differences between groups for all secondary outcomes of LVMi (MD, −1.89 g/m2; 95% CI, −4.52 to 0.74 g/m2, I 2 = 75%; overall effect, P = 0.16; five trials; Figure 1B ), LVESV (MD, −7.56 mL; 95% CI, −15.66 to 0.54 mL, I 2 = 87%; overall effect, P = 0.07; five trials; Figure 2A ), LVEDV (MD, −6.66 mL; 95% CI, −16.82 to 3.49 mL, I 2 = 81%; overall effect, P = 0.20; five trials; Figure 2B ), or LVEF (MD, 1.76%; 95% CI, −0.86% to 4.37%, I 2 = 85%; overall effect, P = 0.19; five trials; Figure 2C ). We observed no subgroup differences for each respective secondary outcome in our sensitivity analyses focusing on HFrEF. The results of our sensitivity analysis focusing on diabetes are presented in Supporting Information, Figures S3 and S4 .
Figure 2.
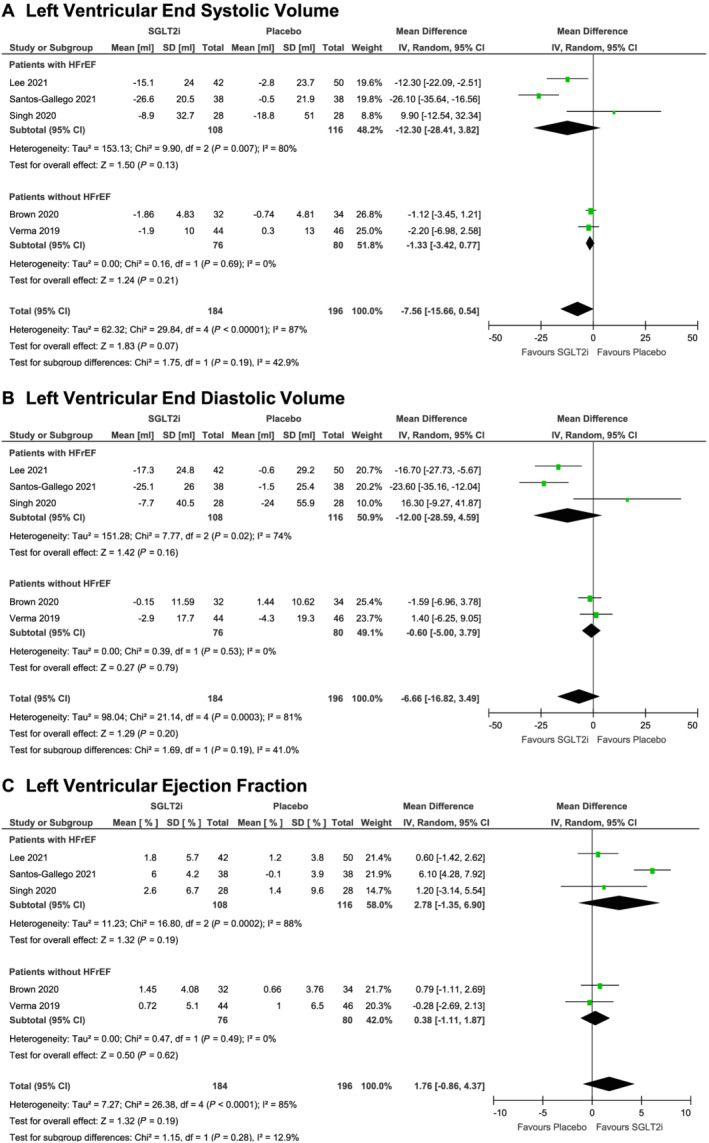
Cardiac magnetic resonance imaging‐assessed changes in left ventricular end systolic volume (A), left ventricular end diastolic volume (B), and left ventricular ejection fraction (C) from baseline to study endpoint in randomized controlled trials of patients treated with sodium glucose transporter‐2 inhibitor therapy versus placebo.
Conclusions
In this meta‐analysis of double‐blind placebo controlled RCTs evaluating left ventricular remodelling by cMRI, we observed that SGLT2i were associated with a significant reduction in left ventricular mass with a consistent benefit observed in people with and without diabetes or HFrEF. Other indices of left ventricular remodelling were not statistically significant, but there was a trend towards reduction in LVESV. The analyses are to be interpreted in the context of limitations including (i) substantial heterogeneity between studies, (ii) relatively small sample sizes amongst included studies, (iii) differing treatment durations across studies, and (iv) inconsistencies in the exact calculations for LVM indexed to body surface area. Notwithstanding these caveats, the data point towards an early effect of SGLT2i on ventricular remodelling which may help explain the clinical benefits observed in patients with diabetes and heart failure. Several potential direct and indirect mechanisms have been proposed to explain the effects of SGLT2i on myocardial remodelling. These include effects on myocardial ion channels, alterations in mitophagy/autophagy, increased cardiac bioenergetics, erythropoietin production, and changes in reno‐cardiac signalling. 7 , 8 , 9 , 15 Further studies evaluating remodelling in patients with heart failure with a preserved ejection fraction (HFpEF) would be of interest, particularly in the context of the ongoing clinical studies in these patients. 16 , 17
Conflict of interest
Nitish K. Dhingra: none declared. Nikhil Mistry: none declared. Pankaj Puar: none declared. Raj Verma: none declared. Stefan Anker: Dr Anker reports grants and personal fees from Vifor International and Abbott Vascular and personal fees from Astra‐Zeneca, Bayer, Brahms, Boehringer Ingelheim, Cardiac Dimensions, Novartis, Occlutech, Servier, and Vifor International. C. David Mazer: Advisory board honoraria from Amgen, AstraZeneca, and Boehringer Ingelheim. Subodh Verma: S.V. holds a Tier 1 Canada Research Chair in Cardiovascular Surgery. S.V. has also received grants and personal fees for speaker honoraria and advisory board participation from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Amgen, HLS, Merck, Novartis, Sun Pharmaceuticals, Toronto Knowledge Translation Working Group, Phase Bio. He also serves as President of the Canadian Medical and Surgical Knowledge Translation Research Group.
Supporting information
Table S1. Justification for Risk of Bias Assessment.
Figure S1. Study Selection.
Figure S2. Risk of Bias Assessment.
Figure S3. Changes in Left Ventricular Mass (Panel A) and Left Ventricular Mass indexed to Body Surface Area (Panel B) from Baseline to Study Endpoint in Randomized Controlled Trials of Patients Treated with Sodium Glucose Transporter‐2 Inhibitor Therapy versus Placebo – Sensitivity Analysis Focusing on Diabetes.
Figure S4. Changes in Left Ventricular End Systolic Volume (Panel A), Left Ventricular End Diastolic Volume (Panel B), and Left Ventricular Ejection Fraction (Panel C) from Baseline to Study Endpoint in Randomized Controlled Trials of Patients Treated with Sodium Glucose Transporter‐2 Inhibitor Therapy versus Placebo – Sensitivity Analysis Focusing on Diabetes.
Dhingra, N. K. , Mistry, N. , Puar, P. , Verma, R. , Anker, S. , Mazer, C. D. , and Verma, S. (2021) SGLT2 inhibitors and cardiac remodelling: a systematic review and meta‐analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Failure, 8: 4693–4700. 10.1002/ehf2.13645.
References
- 1. McMurray J, Solomon S, Inzucchi S, Køber L, Kosiborod M, Martinez F, Ponikowski P, Sabatine M, Anand I, Bělohlávek J, Böhm M, Chiang C, Chopra V, de Boer R, Desai A, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, Katova T, Kitakaze M, Ljungman C, Merkely B, Nicolau J, O'Meara E, Petrie M, Vinh P, Schou M, Tereshchenko S, Verma S, Held C, DeMets D, Docherty K, Jhund P, Bengtsson O, Sjöstrand M, Langkilde A. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 2. Petrie M, Verma S, Docherty K, Inzucchi S, Anand I, Belohlávek J, Böhm M, Chiang C, Chopra V, de Boer R, Desai A, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, Katova T, Kitakaze M, Ljungman C, Merkely B, Nicolau J, O'Meara E, Vinh P, Schou M, Tereshchenko S, Køber L, Kosiborod M, Langkilde A, Martinez F, Ponikowski P, Sabatine M, Sjöstrand M, Solomon S, Johanson P, Greasley P, Boulton D, Bengtsson O, Jhund P, McMurray J. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020; 323: 1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Packer M, Anker S, Butler J, Filippatos G, Pocock S, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi D, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey J, Kaul S, Brunner‐La Rocca H, Merkely B, Nicholls S, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde M, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 4. Anker S, Butler J, Filippatos G, Khan M, Marx N, Lam C, Schnaidt S, Ofstad A, Brueckmann M, Jamal W, Bocchi E, Ponikowski P, Perrone S, Januzzi J, Verma S, Böhm M, Ferreira J, Pocock S, Zannad F, Packer M. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status. Circulation 2021; 143: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zannad F, Ferreira J, Pocock S, Anker S, Butler J, Filippatos G, Brueckmann M, Ofstad A, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐reduced and DAPA‐HF trials. Lancet 2020; 396: 819–829. [DOI] [PubMed] [Google Scholar]
- 6. McGuire D, Shih W, Cosentino F, Charbonnel B, Cherney D, Dagogo‐Jack S, Pratley R, Greenberg M, Wang S, Huyck S, Gantz I, Terra S, Masiukiewicz U, Cannon C. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes. JAMA Cardiol 2021; 6: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verma S, McMurray J. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia 2018; 61: 2108–2117. [DOI] [PubMed] [Google Scholar]
- 8. Lopaschuk G, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co‐transporter 2 (SGLT2) inhibitors. JACC Basic Transl Sci 2020; 5: 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol 2021; 83: 503–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown AJ, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA‐LVH trial. Eur J Heart Fail 2020; 41: 3421–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee M, Brooksbank K, Wetherall K, Mangion K, Roditi G, Campbell R, Berry C, Chong V, Coyle L, Docherty K, Dreisbach J, Labinjoh C, Lang N, Lennie V, McConnachie A, Murphy C, Petrie C, Petrie J, Speirits I, Sourbron S, Welsh P, Woodward R, Radjenovic A, Mark P, McMurray J, Jhund P, Petrie M, Sattar N. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR‐DM‐HF). Circulation 2021; 143: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santos‐Gallego C, Vargas‐Delgado A, Requena‐Ibanez J, Garcia‐Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel‐Perez F, Rodriguez‐Cordero A, Zafar M, Fergus I, Atallah‐Lajam F, Contreras J, Varley C, Moreno P, Abascal V, Lala A, Tamler R, Sanz J, Fuster V, Badimon J. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2021; 77: 243–255. [DOI] [PubMed] [Google Scholar]
- 13. Singh J, Mordi I, Vickneson K, Fathi A, Donnan P, Mohan M, Choy A, Gandy S, George J, Khan F, Pearson E, Houston J, Struthers A, Lang C. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care 2020; 43: 1356–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verma S, Mazer C, Yan A, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh M, Fitchett D, Goodman S, Goldenberg R, Al‐Omran M, Gilbert R, Bhatt D, Leiter L, Jüni P, Zinman B, Connelly K. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease. Circulation 2019; 140: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 15. Mazer C, Hare G, Connelly P, Gilbert R, Shehata N, Quan A, Teoh H, Leiter L, Zinman B, Jüni P, Zuo F, Mistry N, Thorpe K, Goldenberg R, Yan A, Connelly K, Verma S. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation 2020; 141: 704–707. [DOI] [PubMed] [Google Scholar]
- 16. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M. Evaluation of the effects of sodium–glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR‐preserved trial. Eur J Heart Fail 2019; 21: 1279–1287. [DOI] [PubMed] [Google Scholar]
- 17. Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Lindholm D, Wilderäng U, Öhrn F, Claggett B, Langkilde AM, Petersson M, McMurray JJV. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail; 2021; 23: 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Justification for Risk of Bias Assessment.
Figure S1. Study Selection.
Figure S2. Risk of Bias Assessment.
Figure S3. Changes in Left Ventricular Mass (Panel A) and Left Ventricular Mass indexed to Body Surface Area (Panel B) from Baseline to Study Endpoint in Randomized Controlled Trials of Patients Treated with Sodium Glucose Transporter‐2 Inhibitor Therapy versus Placebo – Sensitivity Analysis Focusing on Diabetes.
Figure S4. Changes in Left Ventricular End Systolic Volume (Panel A), Left Ventricular End Diastolic Volume (Panel B), and Left Ventricular Ejection Fraction (Panel C) from Baseline to Study Endpoint in Randomized Controlled Trials of Patients Treated with Sodium Glucose Transporter‐2 Inhibitor Therapy versus Placebo – Sensitivity Analysis Focusing on Diabetes.


