Abstract
Aims
Weight loss (WL) is a poor prognostic factor for patients with heart failure (HF) with reduced ejection fraction. However, its prognostic impact on patients with HF with preserved ejection fraction (HFpEF) remains unestablished. The evidence regarding the effects of obesity on the prognosis of WL is also unclear. We aimed to identify the risk factors for WL and examine the association between WL and prognosis of HFpEF in obese and non‐obese patients.
Methods and results
In this multicentre cohort study, the data of 573 patients hospitalized with HFpEF [median age: 78 years (interquartile range, 71–84 years); 49.2% female] were identified from hospital databases. WL was defined as ≥5% weight reduction within 6 months after discharge. Obesity was defined according to Japanese criteria as body mass index ≥25 kg/m2. The main study outcomes were all‐cause mortality and HF rehospitalization between 6 and 24 months after hospital discharge. Logistic regression analysis and Cox proportional hazards regression analysis were performed to identify independent the risk factors associated with WL and to calculate the hazard ratios (HRs) associated with adverse outcomes. The prevalence of obesity at discharge was 21.1%. At 6 month follow‐up, WL occurred in 17.4% and 10.8% of the obese and non‐obese patients, respectively. Onset of WL in non‐obese patients was associated with prior hospitalization for HF [odds ratio (OR) 2.39, 95% confidence interval (CI) 1.22–4.68, P = 0.011] and high levels of brain natriuretic peptide (OR 2.32, CI 1.17–4.60, P = 0.015). In obese patients, WL was associated with the use of mineralocorticoid receptor antagonists (OR 3.26, CI 1.08–9.76, P = 0.03) and vasopressin receptor antagonists (OR 6.61, CI 2.03–21.2, P = 0.001). During 1021.3 person‐years of follow‐up, 31 patients died, and upon 1081.0 person‐years follow‐up, 84 patients required rehospitalization for HF. In proportional hazards analysis, WL was associated with all‐cause mortality (HR 5.12, CI 2.08–12.5, P < 0.001) and HF rehospitalization (HR 2.63, CI 1.38–5.01, P = 0.003) after adjustment for confounders in non‐obese patients, but not in obese patients.
Conclusions
Weight loss should be considered as an indicator for monitoring worsening of HF condition in non‐obese patients with HFpEF. WL was not associated with adverse events in obese patients with HFpEF, possibly due to appropriate fluid management during follow‐up.
Keywords: Heart failure with preserved ejection fraction, Weight loss, Cachexia, Obesity, Prognosis, Cohort study
Introduction
Heart failure (HF) is a chronic progressive disorder that leads to weight loss (WL) due to metabolic imbalance. 1 WL in HF is associated with oedema and elevated B‐type natriuretic peptide (BNP) levels 2 , 3 , 4 and is a powerful, poor prognostic factor given that it is suggestive of cardiac cachexia. 5 Cardiac cachexia is a systemic metabolic disturbance 6 ; therefore, WL has been interpreted as a simple and sensitive marker of disease progression. 7 However, reports on the prognostic impact of WL have focused mainly on patients with HF with reduced ejection fraction (HFrEF). 2 , 5 , 8
In elderly patients with HF, the proportion of patients with HF with preserved ejection fraction (HFpEF) is higher than that of patients with HFrEF. 9 , 10 Patients with HFpEF are older, predominantly female, and usually present multiple comorbidities such as chronic obstructive pulmonary disease, anaemia, and hypertension. 9 , 11 , 12 , 13 While factors derived from the pathogenesis of HF are associated with WL in patients with HFrEF, ageing and comorbidities have also been reported to be associated with WL. 2 , 3 , 4 This means that the underlying mechanisms of WL in patients with HFpEF who are older and have more comorbidities may be different. Although a recent study has described the prognostic impact of WL in patients with HFpEF, 14 risk factors for its development have not been sufficiently examined.
The association between WL and adverse outcomes in individuals with HF and an elevated body mass index (BMI) has been documented previously, 2 , 3 , 5 , 8 regardless of the ejection fraction. 15 , 16 , 17 However, evidence regarding the impact of WL among non‐obese patients is limited. It is likely that the clinical significance of WL varies according to the initial BMI. However, this relation remains to be examined in patients with HFpEF.
Therefore, this study aimed to examine the association between WL and outcomes in patients with HFpEF and identify the possible risk factors for this complication.
Methods
Study design and population
This study was based on the ‘multicenter prospective cohort study to develop frailty‐based prognostic criteria in HF patients (FLAGSHIP)’ study. 18 The FLAGSHIP study enrolled ambulatory patients hospitalized due to acute or exacerbated HF and those aged ≥70 years and hospitalized due to acute myocardial infarction (AMI). ‘Ambulatory’ was defined as the ability to walk 20 m at hospital discharge, with or without the assistance of a walking aid. Exclusion criteria were as follows: severe cognitive impairment defined as a Mini‐Mental State Examination score <17 points 19 ; severe mental disorder; difficulty answering questionnaires; and assumed short‐term mortality (e.g., severe aortic valve stenosis without surgical indication, terminal stage cancer). Patients were followed up for 2 years to assess frailty status and clinical events.
The FLAGSHIP study protocol was organized according to the Guidelines for the Epidemiological Research proposed by the Japanese Ministry of Health, Labour, and Welfare. Additionally, the study protocol was approved by the Ethics Committee of Nagoya University School of Medicine (approval no. 2014‐0421). This investigation conforms with the principles outlined in the Declaration of Helsinki. Ethical approval was obtained from each participating hospital. All patients provided written informed consent prior to study enrolment.
From the patients enrolled into the FLAGSHIP study, we excluded patients with AMI and selected patients with HFpEF for this study (n =1178). HFpEF was defined as a left ventricular ejection fraction (LVEF) ≥50% at discharge, assessed by transthoracic echocardiography, and calculated using Simpson's method, in accordance with recent guidelines. 20 We excluded patients who were re‐hospitalized due to HF or died within 6 months after discharge (regardless of the cause of death) (n = 223) and those with missing body weight data at 6 months after discharge (n = 382). Finally, 573 patients were eligible for analysis (Figure 1 ).
Figure 1.
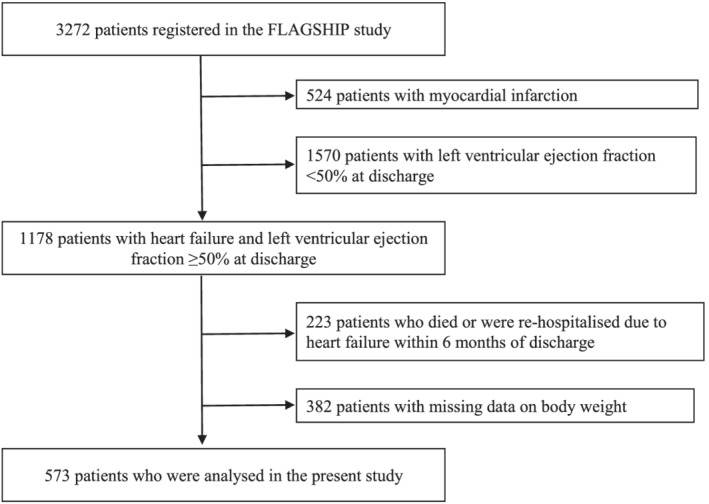
Patient selection flowchart.
Measurement of body weight and definition of weight loss
Body weight was measured using scales (Digital Health Meter, HD‐661, TANITA, Japan) distributed to patients by mail or at routine clinical visits after discharge. Body weight data at discharge and at 6 months after discharge were considered for analysis. WL was defined as a ≥5% weight loss within 6 months from discharge. According to their BMI and based on Japanese criteria, 21 patients were categorized into an obese (BMI ≥ 25 kg/m2) or non‐obese (BMI < 25 kg/m2) group.
Assessment of post‐discharge outcomes
The outcomes of this study were HF rehospitalisations and death due to any cause between 6 and 24 months after hospital discharge. Follow‐up data were retrieved from the medical records of the respective hospitals and from a mail survey conducted every 4 months. The diagnosis of HF as a cause of rehospitalization was made by cardiologists at each enrolling institution.
Data collection
Patient characteristics, including age, sex, clinical data (HF aetiology, comorbidities, previous admissions due to HF, New York Heart Association class at discharge, and medication use), were collected from medical records. Biochemical data included the levels of BNP, N‐terminal‐proBNP (NT‐proBNP), serum albumin and haemoglobin, and the estimated glomerular filtration rate (eGFR). BNP and NT‐proBNP levels ≥200 and ≥900 pg/mL, respectively, were defined as elevated. Anaemia was defined as a haemoglobin level <13 g/dL in men and <12 g/dL in women. Albumin levels <3.4 mg/dL were defined as low. Depression was defined as a score ≥2 in the Geriatric Depression Scale‐5 (GDS5). 22 Grip strength (GS) was evaluated using a Jamar dynamometer (Digital Hand Dynamometer, DHD‐1, SAEHAN Corporation, South Korea) set at the second handle position at discharge. Two attempts were made with each hand, and the maximum value (in 0.1 kg) of each hand was recorded. Weakness was defined as a GS < 30 kg for men and <17 kg for women. Furthermore, 10 m usual walking speed was performed twice, and the faster result was used as the index of the usual walking speed at discharge. Exercise capacity was assessed using the Performance Measure of Activity in Daily Living‐8 (PMADL‐8), which is strongly and negatively correlated to the peak VO2. 23 PMADL‐8 is scored from 8 to 32, with higher scores indicating more severe functional limitations than lower scores. Functional limitation was defined as a PMADL‐8 score ≥20 24 at discharge and at 6 months after discharge. Appetite was assessed using the Simplified Nutritional Appetite Questionnaire (SNAQ), which includes four items and produces a total score ranging from 4 to 20. Anorexia was defined as a SNAQ score <14 25 at discharge and at 6 months after discharge.
Statistical methods
Continuous variables are reported using medians and interquartile ranges, and categorical variables are reported using counts and percentages. Parameters following a normal distribution were compared using unpaired t tests. When data did not follow a normal distribution, the Mann–Whitney test was used for comparison. Categorical variables were analysed using the χ 2 test. Logistic regression analysis was performed to identify independent risk factors associated with WL. Variables with a P value <0.1 in univariate analysis were included in multivariable analysis. Kaplan–Meier cumulative survival curves were constructed to evaluate the effect of WL on outcomes and for illustrative purposes and compared using the Mantel–Haenszel log‐rank test. A Cox proportional hazards regression analysis was performed to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) associated with adverse outcomes. Additionally, adjustments were made for age and sex in Model 1, and for age, sex, and variables with a P value <0.05 in univariate analysis in model 2. A P value <0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS version 21 (IBM Corp., Armonk, NY, USA).
Results
Weight loss at 6 months after discharge
Patient characteristics at discharge are shown in Table 1 . The prevalence of obesity was 21.1%. At 6 months after discharge, WL had occurred in 17.4% and 10.8% of obese and non‐obese patients, respectively (P = 0.05). In the non‐obese group, patients who experienced WL had a higher prevalence of previous HF exacerbations, anaemia, low albumin levels, and high BNP levels than those who did not present WL. In the obese group, WL was associated with diuretic therapy. In the non‐obesity group at 6 months of post‐discharge, functional limitations (WL; 59.6%, non‐WL; 43.7%), and anorexia (31.9% and 17.6%) in the WL group were significantly more frequent than in the non‐WL (Figure 2 ).
Table 1.
Baseline characteristics
| Non‐obese | Obese | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 452, 78.9%) | (n = 121, 21.1%) | |||||||||
| Total | WL | non‐WL | WL | non‐WL | ||||||
| (n = 573) | (n = 49, 10.8%) | (n = 403, 89.2%) | (n = 21, 17.4%) | (n = 100, 82.6%) | ||||||
| Age, years | 78.0 [71.0–84.0] | 81.0 | [72.5–86.0] | 81.0 | [75.0–86.0] | 73.0 | [68.5–81.5] | 75.0 | [68.0–81.7] | |
| Female | 282 | (49.2) | 18 | (36.7) | 205 | (50.9) | 12 | (57.1) | 47 | (47.0) |
| BMI, kg/m2 | 22.1 | [19.6–24.2] | 21.6 | [19.6–22.9] | 21.1 | [18.9–22.7] | 26.5 | [25.7–28.0] | 27.1 | [25.7–29.0] |
| Past HF hospitalization | 116 | (20.0) | 18 | (36.7)* | 74 | (18.4) | 3 | (14.3) | 21 | (21.0) |
| Aetiology | ||||||||||
| Arrhythmia | 150 | (26.2) | 16 | (32.7) | 87 | (21.5) | 8 | (36.4) | 39 | (39.0) |
| Ischaemic heart disease | 142 | (24.8) | 11 | (22.4) | 105 | (26.1) | 3 | (14.3) | 23 | (23.0) |
| Valve heart disease | 131 | (22.9) | 11 | (22.4) | 108 | (26.7) | 2 | (9.1) | 10 | (10.0) |
| Hypertension | 71 | (12.4) | 6 | (19.0) | 48 | (11.9) | 2 | (9.1) | 15 | (15.0) |
| DCM/HCM | 52 | (9.1) | 1 | (2.0) | 40 | (9.9) | 2 | (9.1) | 9 | (9.0) |
| Pulmonary hypertension | 7 | (1.2) | 0 | (0) | 6 | (1.5) | 1 | (4.5) | 0 | (0) |
| Infiltrative cardiomyopathies | 3 | (0.5) | 0 | (0) | 3 | (0.7) | 0 | (0) | 0 | (0) |
| NYHA Class III–IV | 40 | (6.0) | 4 | (8.2) | 28 | (6.9) | 1 | (4.8) | 7 | (7.0) |
| Comorbidity | ||||||||||
| Diabetes mellitus | 174 | (30.4) | 13 | (26.5) | 111 | (27.5) | 10 | (47.7) | 40 | (40.0) |
| COPD | 28 | (4.9) | 1 | (2.0) | 22 | (5.5) | 1 | (4.8) | 4 | (4.0) |
| Af (on admission) | 234 | (40.9) | 18 | (36.7) | 165 | (40.8) | 8 | (36.4) | 43 | (43.0) |
| Cancer | 36 | (6.2) | 1 | (2.0) | 24 | (6.0) | 2 | (9.5) | 9 | (9.0) |
| Medications | ||||||||||
| β‐blocker | 373 | (65.1) | 27 | (55.1) | 264 | (65.5) | 14 | (66.7) | 68 | (68.0) |
| ACEi/ARB | 338 | (59.1) | 29 | (59.2) | 221 | (55.0) | 13 | (61.9) | 75 | (75.0) |
| MRA | 205 | (35.8) | 21 | (42.9) | 142 | (35.3) | 11 | (52.4)** | 31 | (31.0) |
| Loop diuretic | 428 | (74.6) | 37 | (75.5) | 305 | (75.7) | 19 | (90.5)** | 67 | (67.0) |
| Thiazide diuretic | 49 | (8.5) | 4 | (8.2) | 38 | (9.4) | 1 | (4.8) | 6 | (6.0) |
| Vasopressin receptor antagonist | 91 | (15.8) | 9 | (18.4) | 63 | (15.6) | 9 | (42.9)** | 10 | (10.0) |
| Statin | 203 | (35.5) | 14 | (28.6) | 136 | (33.8) | 9 | (42.9) | 44 | (44.0) |
| Oral inotropic agent | 44 | (7.7) | 3 | (6.1) | 33 | (8.2) | 2 | (9.5) | 6 | (6.0) |
| Anticoagulant | 298 | (52.1) | 24 | (49.0) | 215 | (53.8) | 11 | (52.4) | 48 | (48.0) |
| Aspirin | 226 | (39.5) | 19 | (38.8) | 164 | (40.8) | 6 | (28.6) | 37 | (37.0) |
| Calcium‐channel blocker | 202 | (35.3) | 20 | (40.8) | 122 | (30.2) | 12 | (54.5) | 49 | (49.0) |
| PCI during hospitalization | 63 | (11.0) | 4 | (8.2) | 45 | (11.1) | 3 | (13.6) | 15 | (15.0) |
| PM implantation/CRT during hospitalization | 39 | (6.8) | 3 | (6.1) | 23 | (5.7) | 0 | (0) | 13 | (13.0) |
| eGFR, mL/min/1.73 m2 | 47.0 | [36.0–61.6] | 44.2 | [31.9–61.5] | 48.1 | [36.8–62.2] | 44.0 | [34.9–59.0] | 46.0 | [35.0–58.3] |
| Anaemia a | 350 | (61.3) | 39 | (79.6)* | 252 | (62.8) | 13 | (61.9) | 46 | (46.0) |
| Low albumin b | 165 | (28.9) | 23 | (47.9)* | 115 | (28.5) | 6 | (28.6) | 21 | (21.0) |
| Elevated BNP c | 277 | (48.7) | 34 | (69.4)* | 190 | (47.5) | 13 | (61.9) | 40 | (40.4) |
| Depression d | 200 | (34.9) | 13 | (26.5) | 149 | (37.0) | 7 | (33.3) | 31 | (31.0) |
| Low GS e | 228 | (39.8) | 24 | (49) | 167 | (41.4) | 7 | (33.3) | 30 | (30.0) |
| Walking speed, m/s | 0.96 | [0.77–1.11] | 0.94 | [0.80–1.05] | 0.94 | [0.78–1.11] | 1.01 | [0.90–1.08] | 0.99 | [0.75–1.14] |
| PMADL‐8 | 15 | [15–24] | 19 | [16–23] | 20 | [15–24] | 20 | [16–23] | 19 | [14–25] |
| Anorexia f | 120 | (22.9) | 14 | (29.8) | 90 | (24.5) | 3 | (15.8) | 13 | (13.0) |
| CR after discharge | 147 | (25.7) | 9 | (18.8) | 111 | (27.6) | 5 | (23.8) | 22 | (22.0) |
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitor; Af, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; CR, cardiac rehabilitation; CRT, cardiac resynchronization therapy; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; GDS5, the Geriatric Depression Scale‐5; GS, grip strength; HCM, hypertrophic cardiomyopathy; HF, heart failure; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PM, pacemaker; PMADL, performance measure of activity in daily living; SNAQ, Simplified Nutritional Appetite Questionnaire.
Data are presented as median (interquartile range), or n (%).
Haemoglobin levels <13 g/dL in men and <12 g/dL in women.
Albumin levels <3.4 g/dL.
BNP levels ≥200 pg/mL or NT‐proBNP ≥ 900 pg/mL.
GDS5 ≥ 2 points.
GS < 30 kg for men and <17 kg for women.
SNAQ <14 points.
P < 0.05 vs. non‐weight loss in non‐obese patients.
P < 0.05 vs. non‐weight loss in obese patients.
Figure 2.
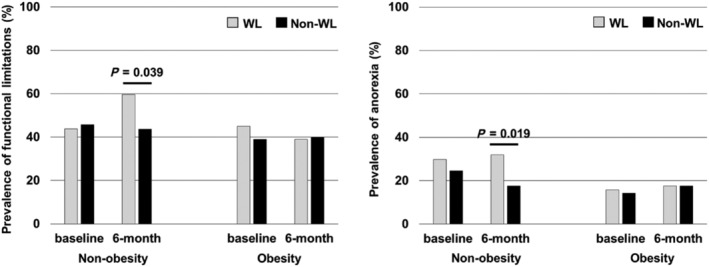
Functional limitations and anorexia at discharge and after 6 months of hospital discharge. (A) Functional limitations. (B) Anorexia. In non‐obesity group (at base line, 6 months): weight loss group; n = 49, n = 47, non‐weight loss group; n = 403, n = 375. In obesity group (at base line, 6 months): weight loss group; n = 21, n = 18, non‐weight loss group; n = 100, n = 91
A comparison of characteristics between included and excluded patients is shown in the supporting information, Table S1 . The excluded patients showed no difference in the patient background compared with our study population.
The results of univariate and multivariate analyses to identify risk factors for WL are shown in Table 2 . After adjusting for age and sex in multivariate analysis, past HF hospitalizations and high BNP levels were significantly associated with WL in the non‐obese group. Additionally, mineralocorticoid receptor antagonists and vasopressin receptor antagonists were significantly associated with WL in the obese group.
Table 2.
Logistic regression analysis for variables associated with weight loss at 6 months after discharge
| Non‐obese | Obese | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate* | Univariate | Multivariate* | |||||||||
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.01 | (0.98–1.04) | 0.44 | 0.99 | (0.96–1.03) | 0.78 | 0.99 | (0.95–1.04) | 0.86 | 0.98 | (0.93–1.03) | 0.51 |
| Female | 0.56 | (0.30–1.03) | 0.06 | 0.59 | (0.31–1.12) | 0.11 | 1.50 | (0.58–3.88) | 0.40 | 1.11 | (0.38–3.32) | 0.84 |
| BMI | 1.06 | (0.94–1.20) | 0.32 | 1.03 | (0.87–1.21) | 0.70 | ||||||
| Past HF hospitalization | 2.58 | (1.37–4.86) | <0.01 | 2.39 | (1.22–4.68) | 0.01 | 0.62 | (0.16–2.33) | 0.48 | |||
| Aetiology | ||||||||||||
| Arrhythmia | 1.75 | (0.92–3.33) | 0.08 | 1.75 | (0.88–3.47) | 0.10 | 0.96 | (0.36–2.53) | 0.93 | |||
| Ischaemic heart disease | 0.82 | (0.40–1.66) | 0.58 | 0.55 | (0.15–2.04) | 0.38 | ||||||
| Valve heart disease | 0.78 | (0.38–1.59) | 0.50 | 0.94 | (0.19–4.67) | 0.94 | ||||||
| Hypertension | 1.02 | (0.41–2.54) | 0.95 | 0.59 | (0.12–2.85) | 0.51 | ||||||
| DCM/HCM | 0.18 | (0.02–1.40) | 0.10 | 1.06 | (0.21–5.32) | 0.94 | ||||||
| Pulmonary hypertension | N/A** | N/A** | ||||||||||
| Infiltrative cardiomyopathies | N/A** | N/A** | ||||||||||
| NYHA Class III–IV | 1.19 | (0.39–3.54) | 0.75 | 0.66 | (0.07–5.70) | 0.70 | ||||||
| Comorbidity | ||||||||||||
| Diabetes mellitus | 0.95 | (0.48–1.85) | 0.88 | 1.36 | (0.53–3.50) | 0.52 | ||||||
| COPD | 0.36 | (0.04–2.73) | 0.32 | 1.20 | (0.12–11.3) | 0.87 | ||||||
| Cancer | 0.32 | (0.04–2.48) | 0.28 | 1.06 | (0.21–5.32) | 0.94 | ||||||
| Af (on admission) | 0.83 | (0.45–1.54) | 0.56 | 0.81 | (0.31–2.14) | 0.67 | ||||||
| Medications | ||||||||||||
| β‐blocker | 0.64 | (0.35–1.17) | 0.15 | 0.94 | (0.34–2.55) | 0.90 | ||||||
| ACEi/ARB | 1.18 | (0.65–2.17) | 0.57 | 0.54 | (0.20–1.45) | 0.22 | ||||||
| MRA | 1.37 | (0.73–2.54) | 0.30 | 2.44 | (0.97–2.54) | 0.06 | 3.26 | (1.08–9.76) | 0.03 | |||
| Loop diuretics | 0.26 | (0.54–1.17) | 0.80 | 1.17 | (0.75–1.81) | 0.48 | ||||||
| Thiazide diuretic | 0.77 | (0.46–1.28) | 0.32 | 0.75 | (0.19–2.95) | 0.69 | ||||||
| Vasopressin receptor antagonist | 1.21 | (0.56–2.62) | 0.62 | 6.75 | (2.28–19.9) | <0.01 | 6.61 | (2.03–21.2) | <0.01 | |||
| Statin | 0.78 | (0.40–1.50) | 0.46 | 0.95 | (0.36–2.46) | 0.92 | ||||||
| Oral inotropic agent | 0.73 | (0.21–2.47) | 0.61 | 1.64 | (0.30–8.80) | 0.55 | ||||||
| Anticoagulant | 0.83 | (0.46–1.51) | 0.56 | 1.19 | (0.46–3.05 | 0.71 | ||||||
| Aspirin | 0.91 | (0.50–1.68) | 0.78 | 0.68 | (0.24–1.90) | 0.46 | ||||||
| Calcium‐channel blocker | 1.60 | (0.87–2.94) | 0.12 | 1.38 | (0.53–3.58) | 0.49 | ||||||
| PCI during hospitalization | 0.74 | (0.25–2.16) | 0.58 | 0.64 | (0.13–3.08) | 0.58 | ||||||
| PM implantation/CRT during hospitalization | 1.07 | (0.31–3.71) | 0.90 | N/A** | ||||||||
| eGFR | 0.99 | (0.97–1.00) | 0.24 | 0.99 | (0.97–1.02) | 0.85 | ||||||
| Anaemia a | 2.30 | (1.11–4.75) | 0.02 | 1.68 | (0.77–3.65) | 0.18 | 1.90 | (0.72–5.00) | 0.18 | |||
| Low albumin b | 2.30 | (1.25–4.22) | <0.01 | 1.87 | (0.96–3.63) | 0.06 | 1.48 | (0.51–4.29) | 0.46 | |||
| Elevated BNP c | 2.50 | (1.32–4.74) | <0.01 | 2.32 | (1.17–4.60) | 0.01 | 2.39 | (0.91–6.31) | 0.07 | 2.13 | (0.72–6.31) | 0.17 |
| Depression d | 0.61 | (0.31–1.19) | 1.53 | 1.11 | (0.40–3.02) | 0.83 | ||||||
| Low GS e | 1.35 | (0.74–2.45) | 0.31 | 1.16 | (0.42–3.18) | 0.76 | ||||||
| Walking speed | 0.96 | (0.31–2.95) | 0.94 | 1.35 | (0.23–7.96) | 0.73 | ||||||
| PMADL‐8 | 0.97 | (0.92–1.03) | 0.39 | 0.99 | (0.92–1.07) | 0.84 | ||||||
| Anorexia f | 1.30 | (0.66–2.54) | 0.43 | 1.12 | (0.28–4.40) | 0.86 | ||||||
| CR after discharge | 0.60 | (0.28–1.29) | 0.19 | 1.10 | (0.36–3.36) | 0.85 | ||||||
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitor; Af, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; CR, cardiac rehabilitation; CRT, cardiac resynchronization therapy; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; GDS5, the Geriatric Depression Scale‐5; GS, grip strength; HCM, hypertrophic cardiomyopathy; HF, heart failure; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PM, pacemaker; PMADL, performance measure of activity in daily living; SNAQ, Simplified Nutritional Appetite Questionnaire.
Data are presented as odds ratio (OR) and 95% confidence interval (95% CI) as assessed by logistic regression analysis.
Data are presented as median (interquartile range), or n (%).
Haemoglobin levels <13 g/dL in men and <12 g/dL in women.
Albumin levels <3.4 g/dL.
BNP levels ≥200 pg/mL or NT‐proBNP ≥ 900 pg/mL.
GDS5 ≥ 2 points.
GS < 30 kg for men and <17 kg for women.
SNAQ <14 points.
Only variables with a P‐value <0.1 in univariate analysis were included in multivariate analysis.
Not applicable.
Weight loss and subsequent outcomes
During 1021.3 person‐years of follow‐up, 31 patients died (26 non‐obese and 5 obese). Regarding readmissions due to HF, during 1081.0 person‐years of follow‐up, 84 patients (63 non‐obese and 21 obese) experienced this complication. Obese patients had a lower risk of death of any cause (HR: 1.40; 95% CI: 0.54–3.61) and readmission due to HF (HR: 0.80; 95% CI: 0.49–1.32) than non‐obese patients.
According to the presence or absence of WL in non‐obese and obese groups, event‐free survival curves are shown in Figure 3. In the non‐obese group, WL was a risk factor for all‐cause death and readmission due to HF. However, this was not the case in the obese group. This association remained after adjusting for confounders in Cox proportional hazards analysis (Table 3 ).
Figure 3.
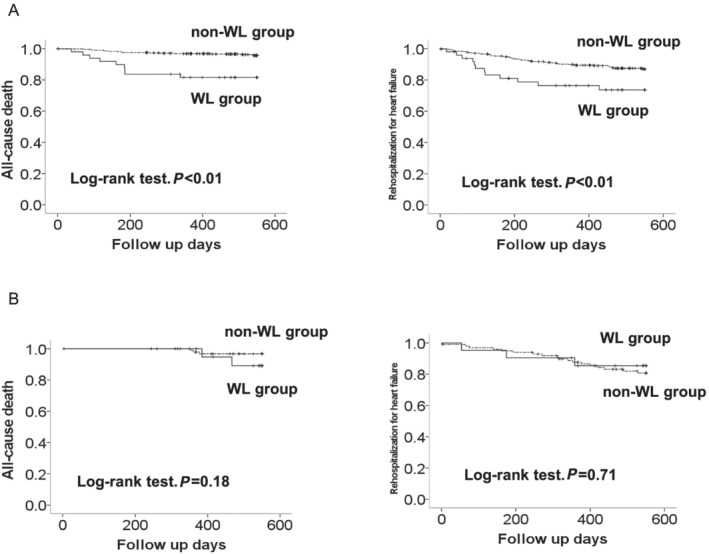
Kaplan–Meier curves of all‐cause death and rehospitalization due to heart failure. (A) Non‐obesity group. (B) Obesity group. WL, weight loss
Table 3.
Hazard ratios for all‐cause death and rehospitalization due to heart failure
| All patients | Non‐obese patients | |||||
|---|---|---|---|---|---|---|
| Non‐WL | WL | Non‐WL | WL | |||
| All‐cause death | ||||||
| No. of patients | 503 | 70 | 403 | 49 | ||
| Person‐years of follow‐up | 957.9 | 124.0 | 768.0 | 84.2 | ||
| No. of all‐cause death | 20 | 11 | 17 | 9 | ||
| Incidence rate/1000 person‐years | 20.9 | 88.7 | 22.1 | 106.9 | ||
| HR in the crude model | 1 | 4.30 | (2.06–8.99) | 1 | 4.85 | (2.15–10.81) |
| HR in the adjusted model a | 1 | 4.07 | (1.94–8.54) | 1 | 4.35 | (1.89–9.95) |
| HR in the adjusted model b , c | 1 | 3.44 | (1.61–7.35) | 1 | 5.12 | (2.08–12.5) |
| Rehospitalization due to heart failure | ||||||
| No. of patients | 503 | 70 | 403 | 49 | ||
| Person‐years of follow‐up | 909.4 | 112.3 | 730.5 | 75.0 | ||
| No. of re‐hospitalizations due to heart failure | 69 | 15 | 51 | 12 | ||
| Incidence rate/1000 person‐years | 75.9 | 133.5 | 69.8 | 159.9 | ||
| HR in the crude model | 1 | 1.82 | (1.03–3.17) | 1 | 2.37 | (1.26–4.44) |
| HR in the adjusted model a | 1 | 1.80 | (1.02–3.15) | 1 | 2.36 | (1.24–4.45) |
| HR in the adjusted model d , e | 1 | 1.90 | (1.05–3.43) | 1 | 2.63 | (1.38–5.01) |
Abbreviations: CI, confidence interval; HR, hazard ratio; WL, weight loss.
Adjusted for age and sex.
Adjusted for age, sex, past‐heart failure hospitalization, elevated levels of brain natriuretic peptide, low albumin, use of mineralocorticoid receptor antagonists, and use of vasopressin receptor antagonists.
Adjusted for age, sex, past heart failure hospitalisation, arrhythmia as an etiology, low grip strength in non‐obese patients.
Adjusted for age, sex, infiltrative cardiomyopathies as an etiology, New York Heart Association class III‐IV, use of loop diuretics, use of oral inotropic agent, low albumin, low grip stregth, walking speed in all pateints.
Adjusted for age, sex, infiltrative cardiomyopathies as an etiology, use of oral inotropic agent, low grip strength, walking speed in non‐obese patients.
The HRs of all‐cause death and readmission due to HF for variables are shown in Table S2. WL could not be evaluated as a risk factor in the obese group because the occurrence of the primary outcome was low in this group (5 and 21 cases for the WL and non‐WL groups, respectively).
Discussion
This study is the first to report the incidence and prognostic impact of WL exclusively in patients with HFpEF. We found that WL was associated with a poor prognosis in non‐obese patients, but not in obese patients. Furthermore, WL was associated with past hospitalizations due to HF and high BNP levels in the former and diuretic therapy in the latter. Therefore, our results reveal that WL in non‐obese individuals with HFpEF has a negative prognostic significance.
The reported incidence of WL in patients with HF is approximately 10–15% and increases in New York Heart Association class III and IV patients. 2 , 3 , 5 , 8 Based on our results, its incidence in patients with HFpEF seems to be in line with these figures (10.8% and 17.4% in the non‐obese and obese groups, respectively). It is worth noting that most patients included in previous studies presented with HFrEF, were younger (sixth decade of age), and had a higher mean BMI (over 25 kg/m2) than our patients (whose mean age and BMI were 78 years and 22.1 kg/m2, respectively). A previous study including lean patients (mean BMI 23 kg/m2, mean age 73 years) showed a similar incidence of WL (11%). 4 In summary, the incidence of WL in patients with HFpEF and HFrEF seems to be similar.
In the present study, non‐obese patients with WL had higher all‐cause mortality and re‐hospitalization rates than their pairs without WL. The Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity study, which included both patients with HFpEF and HFrEF, had already shown that WL impacts prognosis negatively in HF, independently of the LVEF. 2 However, most of the patients in the previous studies were obese and included patients with HFrEF. 2 , 4 The present study is novel in that it examined the prognostic impact of WL in patients with HFpEF with and without obesity and clarified the negative impact in non‐obesity patients.
Progressive WL in HF is often a hallmark of cachexia, which is associated with significantly high morbidity and mortality. 26 Cachexia is driven by various pathophysiological mechanisms, including neuroendocrine abnormalities, inflammatory system activation, increased lipolysis, and muscle wasting. 26 , 27 This complication affects 10–15% of patients with HF, becomes apparent as disease severity progresses, and is associated with a poor prognosis. 26 , 27 In our study, WL in the non‐obesity group was associated with elevated BNP and a history of HF re‐hospitalizations. In general, elevated BNP reflects the severity of HF, and patients with HF deteriorate to cardiac cachexia with repeated readmissions. Furthermore, at 6 months of hospital discharge, a high proportion of patients in the WL group in the non‐obesity group presented with functional limitations and anorexia, suggesting that their physical function and nutritional status were deteriorating. Therefore, cardiac cachexia may be associated with the clinical background of WL in the non‐obesity group. WL may indicate that the degree of neurohormonal activation has reached a clinically relevant degree; therefore, increasing the mortality risk. The non‐obesity group was a population of elderly patients presenting with cachexia with multiple comorbidities. It is reported that an exercise programme for patients with HFpEF with reduced physical function can improve exercise tolerance. 28 Therefore, we speculate that in the non‐obesity group, it is necessary to maintain appropriate body weight by monitoring weight loss as a pathological indicator, maintain appropriate nutritional status, and improve physical function through exercise programmes.
Given the small number of obese patients in our study, we could not examine the association between WL and prognosis in this subgroup. Therefore, the results in this group represent preliminary data. While the risk of death associated with WL remained unchanged when obesity was excluded from analysis, re‐hospitalizations increased. This may suggest that WL is not associated with an increased risk of re‐hospitalizations in obese patients. However, WL in the obesity group was associated with the use of diuretics at discharge in the present study. As patients with HF often present with fluid retention, elimination of oedema by diuretic therapy may be misdiagnosed as WL due to cachexia. 7 In other words, we speculate that the obesity group in this study included patients whose WL was caused by diuretics, and this may have led to an improved prognosis. Hence, WL may be particularly critical among non‐obese patients.
Obesity (defined as a BMI ≥ 25 kg/m2) was not a risk factor for adverse outcomes in the present study. In a previous study including patients with HFpEF, the incidence of the composite outcomes (all‐cause death and HF re‐hospitalizations) was higher in patients with BMIs <23.5 kg/m2 and >35 kg/m2 than in those with a BMI of 26–29 kg/m2. 16 Although approximately 75% of patients in the obese group had a BMI of 29 kg/m2 in the present study, the small number of outcomes in this group may have precluded reaching statistical significance. Furthermore, WL in the obesity group was not associated with worsening of symptoms, suggesting WL in obesity group may result from fluid management. In obese patients with HFpEF with diabetes mellitus or metabolic syndrome, hemodynamic monitoring allows for an increase in diuretic dosage, resulting in an improved prognosis. 29 Given that a high proportion of diabetes mellitus and that diuretic prescription was associated with WL in the obesity group in this study, the WL in the obesity group could have reflected the results of proper fluid management. Moreover, it has been suggested that the complication of sarcopenia in obese patients with HFpEF patients may be associated with clinical outcomes. 30 Because about 30% of the obese group in our study included a group with reduced GS, we speculate that the presence of sarcopenic obese cases may have affected the outcome. Future studies should consider the evaluation of different body compartments when assessing BMI.
Limitations
The present study had several limitations. First, only ambulatory patients at the time of discharge and with complete weight data were included in the analysis. Those who died or were readmitted within 6 months of discharge were excluded. Patients who died or early readmitted patients after discharge had a higher HF severity than our study population. Thus, the results of this study cannot be applied to them. Second, confounding factors such as medications, including SGLT2 inhibitors and intentional or unintentional WL, may have varied within 6 months after discharge and thus affected our results. Third, in the FLAGSHIP study, the scales were aligned to the same model, but other measurement conditions were not necessarily controlled, which may have led to the misidentification of weight. Fourth, we may have misclassified patients with HF with recovered EF as patients with HFpEF because the LVEF data were adopted at the time of hospital discharge. Finally, because of the small number of events in the obesity group, we refrained from performing multivariate analysis and adjusting for confounding factors to investigate the association between WL and outcomes in this subpopulation.
Conclusions
In non‐obese patients with HFpEF, WL within 6 months of hospital discharge was an independent risk factor for all‐cause mortality and rehospitalization after 6 months post‐discharge. Additionally, WL was associated with previous admissions due to HF and elevated BNP or NT‐proBNP levels. Thus, WL is an important prognostic indicator after the discharge of patients with HFpEF. Screening and management for this complication after hospitalizations may be necessary to prevent adverse outcomes in patients with HF.
Conflict of interest
None declared.
Funding
This work was supported by a Grant‐in‐Aid for Scientific Research (A) from the Japan Society for the Promotion of Science (grant number 16H01862). We did not receive any funding from commercial organizations for the conduction of this study.
Supporting information
Table S1. Comparison between the study cohort and excluded patients.
Table S2. A Cox proportional hazards regression analysis for all‐cause death and rehospitalisation due to heart failure
Acknowledgements
We wish to thank the participants of this study for their contribution, without which these analyses would not have been possible. In addition, we would like to thank all FLAGSHIP collaborating investigators.
Kamisaka, K. , Kamiya, K. , Iwatsu, K. , Iritani, N. , Imoto, S. , Adachi, T. , Iida, Y. , Yamada, S. , and FLAGSHIP collaborators (2021) Impact of weight loss in patients with heart failure with preserved ejection fraction: results from the FLAGSHIP study. ESC Heart Failure, 8: 5293–5303. 10.1002/ehf2.13619.
References
- 1. Springer J, Filippatos G, Akashi YJ, Anker SD. Prognosis and therapy approaches of cardiac cachexia. Curr Opin Cardiol 2006; 21: 229–233. [DOI] [PubMed] [Google Scholar]
- 2. Pocock SJ, McMurray JJ, Dobson J, Yusuf S, Granger CB, Michelson EL, Ostergren J, Pfeffer MA, Solomon SD, Anker SD, Swedberg KB. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J 2008; 29: 2641–2650. [DOI] [PubMed] [Google Scholar]
- 3. Rossignol P, Masson S, Barlera S, Girerd N, Castelnovo A, Zannad F, Clemenza F, Tognoni G, Anand IS, Cohn JN, Anker SD, Tavazzi L, Latini R, GISSI‐HF and Val‐HeFT Investigators . Loss in body weight is an independent prognostic factor for mortality in chronic heart failure: insights from the GISSI‐HF and Val‐HeFT trials. Eur J Heart Fail 2015; 17: 424–433. [DOI] [PubMed] [Google Scholar]
- 4. Okuhara Y, Asakura M, Orihara Y, Naito Y, Tsujino T, Ishihara M, Masuyama T, J‐MELODIC Study Investigators . Effects of weight loss in outpatients with mild chronic heart failure: findings from the J‐MELODIC study. J Card Fail 2019; 25: 44–50. [DOI] [PubMed] [Google Scholar]
- 5. Anker SD, Negassa A, Coats AJ, Afzal R, Poole‐Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet 2003; 361: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 6. Sente T, Van Berendoncks AM, Hoymans VY, Vrints CJ. Adiponectin resistance in skeletal muscle: pathophysiological implications in chronic heart failure. J Cachexia Sarcopenia Muscle 2016; 7: 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valentova M, Anker SD, von Haehling S. Cardiac cachexia revisited: the role of wasting in heart failure. Heart Fail Clin 2020; 16: 61–69. [DOI] [PubMed] [Google Scholar]
- 8. Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, Dunlap ME, Ezekowitz JA, Felker GM, Fudim M, Greene SJ, Hernandez AF, O'Connor CM, Schulte P, Starling RC, Teerlink JR, Voors AA, Mentz RJ. Body weight change during and after hospitalization for acute heart failure: patient characteristics, markers of congestion, and outcomes: findings from the ASCEND‐HF trial. JACC Heart Fail 2017; 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 10. Tsutsui H, Tsuchihashi‐Makaya M, Kinugawa S, Goto D, Takeshita A, JCARE‐CARD Investigators . Clinical characteristics and outcome of hospitalized patients with heart failure in Japan. Circ J 2006; 70: 1617–1623. [DOI] [PubMed] [Google Scholar]
- 11. Kajimoto K, Sato N, Keida T, Sakata Y, Takano T, Acute Decompensated Heart Failure Syndromes (ATTEND) Investigators . Associations of anemia and renal dysfunction with outcomes among patients hospitalized for acute decompensated heart failure with preserved or reduced ejection fraction. Clin J Am Soc Nephrol 2014; 9: 1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, ADHERE Scientific Advisory Committee and Investigators . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol 2006; 47: 76–84. [DOI] [PubMed] [Google Scholar]
- 13. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB, OPTIMIZE‐HF Investigators and Hospitals . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF registry. J Am Coll Cardiol 2007; 50: 768–777. [DOI] [PubMed] [Google Scholar]
- 14. Nishikido T, Oyama JI, Nagatomo D, Node K. A reduction of BMI predicts the risk of rehospitalization and cardiac death in non‐obese patients with heart failure. Int J Cardiol 2019; 276: 166–170. [DOI] [PubMed] [Google Scholar]
- 15. Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LA, Skali H, Pfeffer MA, Yusuf S, Swedberg K, Michelson EL, Granger CB, McMurray JJ, Solomon SD, CHARM Investigators . Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007; 116: 627–636. [DOI] [PubMed] [Google Scholar]
- 16. Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I‐PRESERVE) trial. Circ Heart Fail 2011; 4: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J, Begley A, Jackson R, Harrison M, Pellicori P, Clark AL, Cleland JGF. Body mass index and all‐cause mortality in heart failure patients with normal and reduced ventricular ejection fraction: a dose‐response meta‐analysis. Clin Res Cardiol 2019; 108: 119–132. [DOI] [PubMed] [Google Scholar]
- 18. Yamada S, Adachi T, Izawa H, Murohara T, Kondo T, FLAGSHIP collaborators . A multicenter prospective cohort study to develop frailty‐based prognostic criteria in heart failure patients (FLAGSHIP): rationale and design. BMC Cardiovasc Disord 2018; 18: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population‐based norms for the Mini‐Mental State Examination by age and educational level. JAMA 1993; 269: 2386–2391. [PubMed] [Google Scholar]
- 20. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. Wytyczne ESC dotyczace diagnostyki i leczenia ostrej i przewleklej niewydolnosci serca w 2016 roku [2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure]. Kardiol Pol 2016; 74: 1037–1147. [DOI] [PubMed] [Google Scholar]
- 21. Miyazaki S. Himansyou shinryou guideline 2016. Nihon Naika Gakkai Zasshi 2018; 107: 262–268. [Google Scholar]
- 22. Hoyl MT, Alessi CA, Harker JO, Josephson KR, Pietruszka FM, Koelfgen M, Mervis JR, Fitten LJ, Rubenstein LZ. Development and testing of a five‐item version of the Geriatric Depression Scale. J Am Geriatr Soc 1999; 47: 873–878. [DOI] [PubMed] [Google Scholar]
- 23. Kono Y, Yamada S, Iwatsu K, Nitobe S, Tanaka Y, Shimizu Y, Shinoda N, Okumura T, Hirashiki A, Murohara T. Predictive value of functional limitation for disease severity in patients with mild chronic heart failure. J Cardiol 2012; 60: 411–415. [DOI] [PubMed] [Google Scholar]
- 24. Shimizu Y, Yamada S, Suzuki M, Miyoshi H, Kono Y, Izawa H, Kato R, Murohara T. Development of the performance measure for activities of daily living‐8 for patients with congestive heart failure: a preliminary study. Gerontology 2010; 56: 459–466. [DOI] [PubMed] [Google Scholar]
- 25. Rolland Y, Perrin A, Gardette V, Filhol N, Vellas B. Screening older people at risk of malnutrition or malnourished using the Simplified Nutritional Appetite Questionnaire (SNAQ): a comparison with the Mini‐Nutritional Assessment (MNA) tool. J Am Med Dir Assoc 2012; 13: 31–34. [DOI] [PubMed] [Google Scholar]
- 26. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, Harrington D, Kox WJ, Poole‐Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997; 349: 1050–1053. [DOI] [PubMed] [Google Scholar]
- 27. von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle 2016; 7: 507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016; 5: 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB, CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet 2016; 30: 453–461. [DOI] [PubMed] [Google Scholar]
- 30. Kirkman DL, Bohmke N, Billingsley HE, Carbone S. Sarcopenic obesity in heart failure with preserved ejection fraction. Front Endocrinol (Lausanne) 2020; 11: 558271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison between the study cohort and excluded patients.
Table S2. A Cox proportional hazards regression analysis for all‐cause death and rehospitalisation due to heart failure


