Abstract
Background
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer and a highly heterogeneous disease with a diversity of phenotypes and genotypes in different populations. The purpose of this study is to investigate oncogenic alterations of lung adenocarcinoma (LUAD) in eastern China and their significance in targeted therapies.
Methods
This study enrolled 101 LUAD patients and used a customized DNA panel to detect molecular alterations. Comprehensive analysis of mutations and clinical application of genomic profiling was carried out.
Results
The most commonly mutated genes were epidermal growth factor receptor (EGFR) (53%) and tumor protein p53 (TP53) (32%). The less frequently mutated genes were erb-b2 receptor tyrosine kinase 2 (ERBB2) (25%), ATR serine/threonine kinase (ATR) (20%), CCAAT enhancer binding protein alpha (CEBPA) (16%), RB transcriptional corepressor 1 (RB1) (16%), transcription factor 7 like 2 (TCF7L2) (14%), ROS proto-oncogene 1, receptor tyrosine kinase (ROS1) (12%) and spectrin alpha, erythrocytic 1 (SPTA1) (12%). Among them, the frequency of ERBB2, ATR, CEBPA, RB1 and TCF7L2 mutations was much higher than that in the databases. Seventy percent of the patients harbored at least one actionable alteration according to the OncoKB evidence. CEBPA mutations affected the efficacy of EGFR-tyrosine kinase inhibitors. ERBB2, CEBPA and TCF7L2 mutated tumors tend to have higher tumor mutation burden (TMB).
Conclusions
LUAD patients from eastern China have a unique profile of mutations. The targeted DNA panel is helpful for personalized treatment decision of LUAD patients, and specific mutations may affect the efficacy of targeted therapies.
Keywords: lung adenocarcinoma, next-generation sequencing (NGS), genetic alteration, tumor mutation burden (TMB), targeted therapy
Introduction
Lung cancer is the most common cancer in China (an incidence rate of 35.10/100000, 774323 new cases) (1). NSCLC accounts for the most (80–85%) of all lung cancers and is mainly divided into LUAD, squamous cell carcinoma (SCC) and large cell carcinoma (LCC) (2). Targeted therapies have greatly prolonged the survival time of NSCLC patients harboring actionable driver alterations (3, 4). Besides, the application of immune checkpoint inhibitors (ICIs), has also significantly improved the overall survival (OS) of some lung cancers (5, 6). Over the last decade, next-generation sequencing (NGS) testing is increasingly used for clinical diagnosis and therapies (7–11). An NGS cancer gene panel (CGP) was applied to detect actionable driver alterations and measure TMB. Understanding the characteristics of molecular alterations in lung cancer can help patients choose personalized targeted therapy or immunotherapy.
Population reports focusing on Europe, America, South Korea, and China showed differences in the frequency of driver genes among lung cancer patients, which affect the efficacy of targeted drugs (12–15). Thus, it is necessary to study the genomic profiles of LUAD in Chinese patients. A total of 101 LUAD samples were analyzed by a targeted panel of 639 genes (Singlera OncoAims® Panoramic Detection Panel). This panel refers to cancer databases, clinical guidelines and references to detect genetic alterations including targeted drug related actionable alterations and uncommon mutations that have not been well studied in LUAD. Second, we demonstrated the utility of DNA sequencing results to guide clinical treatment and explored the impact of mutations on the efficacy of EGFR-targeted therapy to identify the predictive biomarkers.
Materials and Methods
Patients and Samples Collection
We enrolled 101 patients with diagnosed LUAD in Zhongshan Hospital Affiliated to Fudan University from January 2018 to March 2021. Two pathologists determined the histological type and degree of differentiation according to the 2015 WHO classification of lung tumors (16), and the disease staging according to the Union for International Cancer Control (UICC) classification of the tumor-node-metastasis (TNM) (17). Patients with other malignancies and multiple malignancies were not included in this study. The basic clinical information included age, gender, histological diagnosis, TNM stage, degree of differentiation, metastasis, and smoking history. Patients were divided into 3 groups according to the smoking status: never-smokers, ever-smokers (ceased >1 year) and current smokers (ongoing or ceased ≤ 1 year). This study was approved by the Ethics Committee of Zhongshan Hospital Affiliated to Fudan University. All participants signed informed consent. Formalin fixed paraffin embedded (FFPE) tumor tissues and paired blood samples were collected.
Genomic DNA Preparation
According to the manufacturer’s instructions, FFPE DNA was isolated by using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany), and gDNA from blood was extracted by using the QIAamp DNA Blood Mini Kit. The quality of DNA was detected by 1% agarose gel electrophoresis. The Qubit dsDNA HS Assay kit and Qubit 3.0 fluorimeter (Life Technologies, Eugene, Oregon, USA) were used to quantify DNA.
NGS Analysis and Somatic Alteration Detection
Singlera OncoAim® Panoramic Detection Panel (Singlera Genomics (Shanghai) Ltd., China) was conducted for mutation analysis. According to the Illumina standard library construction instructions (Illumina, Inc., California, USA), 20 ng DNA was prepared to generate libraries by using a KAPA Library Quantification Kit (Kapa, KK4824). The library products were sequenced using 75 bp paired-end runs on the Illumina MiSeq. The sequencing data was processed by bioinformatics software provided by the manufacturer. The minimum sequencing depth and allele frequency (MAF) of single nucleotide polymorphisms (SNPs) was set to ≥200x and ≥ 5%, respectively. Raw data containing sequence information and quality information was aligned to the reference human genome (UCSC hg19). Single nucleotide variants (SNVs), small insertions and deletions (InDels), mixed variants, structural variants and amino acid changes were identified using SnpEff3.0 which is the genetic variant annotation and functional effects prediction tool. SnpEff can annotate and predict the effects of variants on genes and amino acids programmatically (18, 19). Germline and somatic alterations were all detected. Variations exist in tumors but not in matched blood are viewed as somatic mutations. The list of 639 genes was shown in Supplementary Table 1 .
Actionable Alterations Analysis
OncokB (Precision Oncology Knowledge Base) website is developed and maintained by the Memorial Sloan Kettering Cancer Center (20). It contains the information of 125 cancer types related 682 genes, 5670 alterations and corresponding 103 drugs. The effects of targeted inhibitors vary with tumor lineages, even in cancers with the same mutant. Potentially actionable changes in specific cancer types are divided into four levels. When the tumor type of non-small cell lung cancer was selected, the “actionable genes” showed the genes and mutant sites/alleles targeted by evidence-based drugs. At present, a total of 8 genes have recommended drugs. When selecting the tumor type of all solid tumor, 10 additional genes have recommended drugs, and most of these mutations are level 4 alterations. These 18 genes are included in the DNA panel.
Statistical Analysis
Mutational profiling was conducted by using MAF Visualization tools (maftools) in R (version 3.6.1) (http://www.r-project.org) (21, 22). 3-D structures of protein were performed by using I-TASSER server (http://zhanglab.ccmb.med.umich.edu/I-TASSER) (23). Fisher exact test was used to test for significance between two groups. P-value < 0.05 was considered statistically significant. For all figures: asterisk (*) means P-value <0.05, (**) means P-value <0.01, (***) means P-value <0.001.
Results
Patient Characteristics and Testing Flowchart
In total, 101 patients with LUAD comprising 42 males and 59 females were enrolled in this study and patients were aged 34–88 years, with a median age of 61 years. Overall, 46.5% of the patients were diagnosed with stage I, 16.8% with stage II, 4.0% with stage III, and 32.7% with stage IV LUAD. In total, 4.0% of cases was well differentiated, 55.4% was moderate differentiated, and 40.6% was poor differentiated. Most patients (79.2%) were non-smokers ( Supplementary Table 2 ). NGS results were used to analyze common and uncommon mutations. A total of 71 patients had actionable mutations detected in tissues, of which 35 received targeted therapies. There were 32 patients with follow-up information who were evaluated for the efficacy of targeted drugs ( Figure 1 ).
Figure 1.
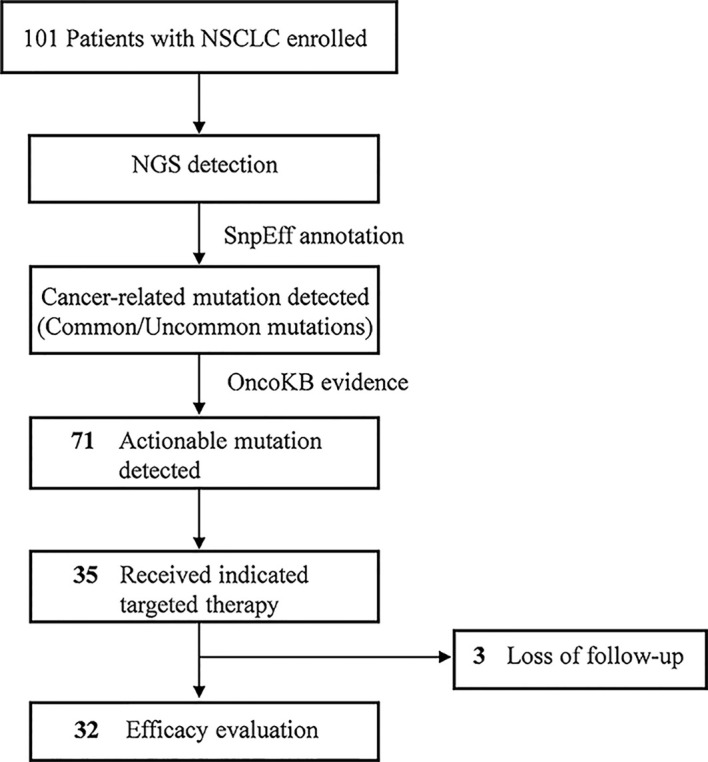
Testing Flowchart of patients. Flowchart indicates patient enrollment, DNA panel conducted, cancer-related mutations detected and targeted therapies available.
Genomic Landscape of LUAD Patients
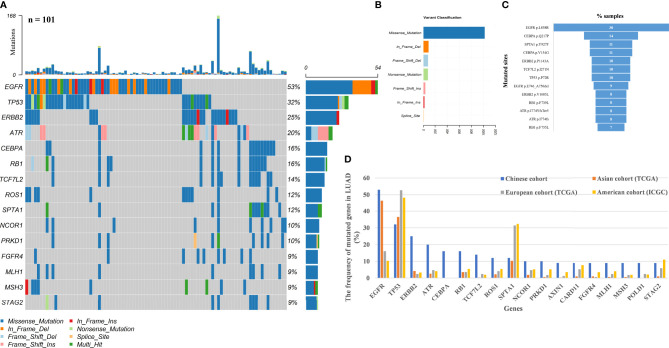
A total of 314 mutated genes were detected by using the DNA panel in LUAD patients, with a mean of 8 alterations per sample. The most commonly mutated genes were EGFR (53%, 54/101) and TP53 (32%, 32/101). Other most frequently affected genes were ERBB2 (25%, 25/101), ATR (20%), CEBPA (16%), RB1 (16%), TCF7L2 (14%), ROS1 (12%) and SPTA1 (12%) ( Figure 2A ). Among these mutations, missense mutation was the most common mutation, followed by in frame deletion and frame shift deletion ( Figure 2B ). Besides, 1.98% (2/101) was EML4-ALK fusion and 1.98% (2/101) was KIF5B-RET fusion. The most common alteration was EGFR p. L858R, followed by CEBPA p.Q217P. Multiple mutations including CEBPA p.Q217P, SPTA1 p.Y927F, CEBPA p.V154G, ERBB2 p.P1140A, TCF7L2 p.I271N, ERBB2 p.V1085L, RB1 p.F739L, ATR p.I774YfsTer5 and ATR p.I774fs have never been reported, but the possibility that they are genetic polymorphisms has been ruled out ( Figure 2C ). The mutation frequency of ERBB2, ATR, CEBPA, RB1, and TCF7L2 genes in the panel was significantly higher than that reported in the the Cancer Genome Atlas (TCGA) (Asian and European cohorts) and International Cancer Genome Consortium (ICGC) (American cohort) databases ( Figure 2D , Supplementary Table 3 ). Algorithm predicted that fibroblast growth factor receptor 1 (FGFR1), KRAS proto-oncogene, GTPase (KRAS), NRAS proto-oncogene, GTPase (NRAS), EGFR, CEBPA and RB1 were prominent driver genes. In addition, ATR and TCF7L2 were also the driver genes ( Supplementary Figure 1 ).
Figure 2.
Mutational landscape of 101 Chinese LUAD patients. (A) 15 top frequently mutated genes are shown. The X-axis represents the sample of each patient, and the Y-axis represents the mutated genes and the mutation frequency of each mutated gene. (B) Display of variation classification. (C) High frequency hot spot mutated sites in LUAD patients. (D) Comparison of the frequency of 18 significantly mutated genes identified in Chinese LUAD patients with that in the TCGA and ICGC cohorts. TCGA, The Cancer Genome Atlas. ICGC, International Cancer Genome Consortium.
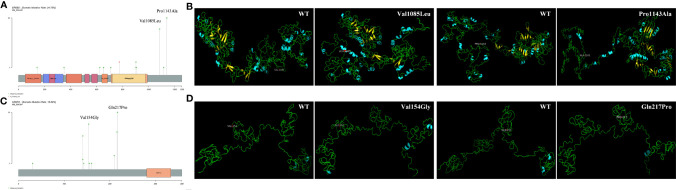
The profile of common driver genes in the current Chinese cohort was shown in Supplementary Figure 2 . We focus on novel carcinogenic mutations found in the sequencing. ERBB2 (also called HER2) is recognized as an oncogene (24, 25). More recently, ERBB2 gene amplifications and mutations have been reported in 2% to 4% of LUAD (26). In a Chinese study of 98 LUAD patients, the mutation frequency of ERBB2 was 24%, similar to that reported in this Chinese cohort (27). The two sites, ERBB2 p.P1140A and ERBB2 p.V1085L with the highest mutation frequency (10% and 8%, respectively) occurred outside the functional domain, but they changed the protein structure, which may affect ERBB2 receptor activation ( Figures 3A, B ). The role of CEBPA was first established in acute myeloid leukemia (AML) (28). CEBPA acts as a tumor suppressor and was found to be down regulated in 50% of II and IIIA LUAD (29). But the pathologic mutations of CEBPA were uncommon in lung cancer. In this study, missense mutation was the most common variant type, and it could change the structure of protein and contribute to lung malignancies ( Figures 3C, D ). Other genes with relatively high mutation frequency were ATR, RB1 and TCF7L2 genes. Although they are not the commonly mutated genes in NSCLC, their mutation frequency in this cohort was higher than that in the previously reported cohorts. Among patients, frame shift insertion/deletion of ATR and the major mutation of RB1 (RB1 p.F739L) and TCF7L2 (TCF7L2 p.I271N) changed the protein structure during simulation ( Supplementary Figure 3 ), suggesting that they might play an important role in the development of lung cancer. The mutated sites of other 15 mutated genes with high frequency were displayed in Supplementary Figure 4 (30, 31).
Figure 3.
Mutant points and 3-D protein structures of normal and mutant protein of ERBB2 (A, B) and CEBPA (C, D). Blue means helix, green means sheet. The mutant points were shown in red in the structure diagram.
Targeted Therapy Based on NGS Testing Results
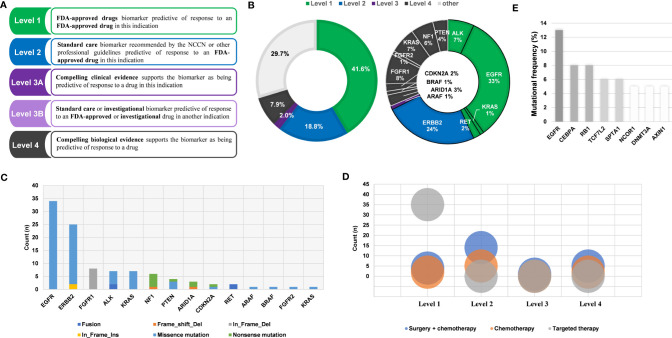
OncoKB (http://oncokb.org/) evidence was applied to evaluate whether molecular changes can guide treatment ( Figure 4A and Table 1 ). Of 101 patients, 71 (70.3%) harbored sensitizing mutations and were recommended for targeted therapies according to the OncoKB evidence. Besides, 41.6% of those had matched FDA-recognized level_1 actionable alterations including ALK fusion and oncogenic mutations, EGFR and KRAS mutations and RET fusion, 18.8% had level_2 ERBB2 alterations, and 2% had level_3 ARAF alteration. Level_4 alterations accounted for 7.9% including oncogenic mutations in ARID1A, BRAF, CDKN2A, FGFR1, FGFR2, KRAS, NF1 and PTEN. ( Figures 4B, C ). We found that targeted therapy has become the first choice for patients with level_1 alterations ( Figure 4D ). Among the 36 patients being followed up, 32 patients who used matching targeted drugs had a median remission period > 14 months, and patients No. 33, 34, 35 had mutations of the recommended targeted drug, but they received chemotherapy, and the disease progressed after an average of 7 months. Patient NO. 36 had no clinically available mutation, and his disease progressed 5 months after chemotherapy ( Table 2 ). In addition, almost 29.7% of the patients had no clinically applicable mutations of NSCLC, among which CEBPA, RB1, TCF7L2 were the most common mutated genes ( Figure 4E ), thus providing potential targets for the research and development of new targeted drugs.
Figure 4.
Clinical actionable mutations and their clinical evidence. (A) OncoKB levels of evidence. (B) Samples were divided into different grades according to mutations (left). Mutated genes in different grades (right). (C) Mutation types in actionable alterations. (D) Different treatment means in different grades. (E) The most frequently mutated genes in patients without actionable alterations.
Table 1.
Mutated genes and actionable targeted therapies.
| Level a | Gene | Alterations | Tumor type | Targeted therapy guidelines | Actionable mutational frequency, n (%) | Actual targeted therapy according to guidelines, n |
|---|---|---|---|---|---|---|
| 1 | ALK | Fusions, Oncogenic Mutations | Non-Small Cell Lung Cancer | Alectinib, Ceritinib, Crizotinib, Brigatinib, Lorlatinib | 7 (6.9) | 2 |
| 1 | BRAF | V600E | Non-Small Cell Lung Cancer | Dabrafenib+Trametinib | 0 | / |
| 1 | EGFR | 19 Del, L858R, Exon20 insertion, G719, L861Q, S768I, T790M | Non-Small Cell Lung Cancer | Afatinib, Dacomitinib, Erlotinib, Gefitinib, Osimertinib, Amivantamab, Mobocertinib | 34 (33.7) | 33 |
| 1 | KRAS | G12C | Non-Small Cell Lung Cancer | Sotorasib | 1 (1.0) | / |
| 1 | NTRK1/2/3 | Fusions | All Solid Tunors | Entrectinib, Larotrectinib | 0 | / |
| 1 | RET | Fusions | Non-Small Cell Lung Cancer | Pralsetinib, Selpercatinib | 2 (2.0) | / |
| 1 | ROS1 | Fusions | Non-Small Cell Lung Cancer | Crizotinib, Entrectinib | 0 | / |
| 2 | EGFR | A763_Y764insFQEA | Non-Small Cell Lung Cancer | Erlotinib | 0 | / |
| 2 | ERBB2 | Oncogenic Mutations | Non-Small Cell Lung Cancer | Ado-Trastuzumab Emtansine, Trastuzumab Deruxtecan | 25 (24.8) | / |
| 3 | ARAF | Oncogenic Mutations | Non-Small Cell Lung Cancer | Sorafenib | 1 (1.0) | / |
| 3 | EGFR | Exon19 insertion, Kinase Domain Duplication | Non-Small Cell Lung Cancer | Erlotinib, Gefitinib, Poziotinib, Afatinib | 0 | / |
| 4 | ARID1A | Truncating Mutations | All Solid Tunors | PLX2853, Tazemetostat | 3 (3.0) | / |
| 4 | BRAF | G464, G469A, G469R, G469V, K601, L597 | All Solid Tunors | PLX8394 | 1 (1.0) | / |
| 4 | CDKN2A | Oncogenic Mutations | All Solid Tunors | Palbociclib, Abemaciclib, Ribociclib | 1 (1.0) | / |
| 4 | EGFR | D761Y, L718V, L747P | Non-Small Cell Lung Cancer | Afatinib, Osimertinib | 0 | / |
| 4 | FGFR1/2/3 | Oncogenic Mutations | All Solid Tunors | Erdafitinib, Infigratinib, Debio1347, AZD4547 | 9 (9.0) | / |
| 4 | KRAS | Oncogenic Mutations | All Solid Tunors | Cobimetinib, Trametinib, Binimetinib | 7 (7.0) | / |
| 4 | NF1 | Oncogenic Mutations | All Solid Tunors | Trametinib, Cobimetinib | 6 (6.0) | / |
| 4 | PTEN | Oncogenic Mutations | All Solid Tunors | AZD8186, GSK2636771 | 4 (4.0) | / |
OncoKB levels of evidence.
Table 2.
Treatment follow-up records.
| Sample No. | Gender | Age | TNM stage | Actionable alteration | OncoKB level | Treatment | Drug evidence | Time to remission (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 70 | T4N3M1a | EGFR L858R | 1 | Gefitinib | FDA-approved | 25 |
| 2 | Female | 59 | T2aN2M1b | EGFR 19del | 1 | Gefitinib | FDA-approved | 18 |
| 3 | Female | 57 | T1bNxM1 | EGFR L858R | 1 | Gefitinib | FDA-approved | 21 |
| 4 | Male | 56 | T4N2M0 | EGFR 19del | 1 | Gefitinib | FDA-approved | 12 |
| 5 | Male | 76 | T4N2M1b | EGFR L858R | 1 | Erlotinib | FDA-approved | 48 |
| 6 | Male | 88 | T4N3M0 | EGFR L858R | 1 | Gefitinib | FDA-approved | 13 |
| 7 | Female | 66 | T3N3M1c | EGFR L858R | 1 | Gefitinib | FDA-approved | 29 |
| 8 | Male | 63 | T4N2M1 | EGFR 19del | 1 | Gefitinib | FDA-approved | 32 |
| 9 | Female | 78 | T4N3M1c | EGFR 19del | 1 | Gefitinib | FDA-approved | 9 |
| 10 | Male | 62 | T3N2M1c | EGFR L858R | 1 | Erlotinib | FDA-approved | 7 |
| 11 | Male | 73 | T1cN2M1c | EGFR 19del | 1 | Gefitinib | FDA-approved | 35 |
| 12 | Male | 62 | T2aN3M1a | EGFR T790M | 1 | Osimertinib | FDA-approved | 17 |
| 13 | Male | 71 | T3N3M1c | EGFR L858R | 1 | Gefitinib | FDA-approved | 7 |
| 14 | Female | 71 | T2N2M1c | EGFR L858R | 1 | Pembrolizumab + Gefitinib | FDA-approved | 15 |
| 15 | Male | 73 | T2bN0M1c | EGFR T790M | 1 | Osimertinib | FDA-approved | 29 |
| 16 | Female | 75 | T4N2M1a | EGFR L858R | 1 | Gefitinib | FDA-approved | 12 |
| 17 | Female | 72 | T4N2M1c | EGFR 19del | 1 | Gefitinib | FDA-approved | 10 |
| 18 | Female | 49 | T2N2M1c | ALK fusion | 1 | Alectinib | FDA-approved | 8 |
| 19 | Male | 68 | T3N3M1a | EGFR 19del | 1 | Osimertinib | FDA-approved | 28 |
| 20 | Female | 52 | T2N1M1c | EGFR 19del | 1 | icotinib | NMPA-approved | 4 |
| 21 | Female | 47 | T1bN2M1c | EGFR T790M | 1 | Osimertinib | FDA-approved | 14 |
| 22 | Male | 72 | cT3N2M1a | EGFR 19del | 1 | Erlotinib | FDA-approved | 5 |
| 23 | Female | 44 | cT4N3M1c | EGFR L858R | 1 | Gefitinib | FDA-approved | 6 |
| 24 | Female | 58 | cT4N3M1c | EGFR L858R | 1 | HS-10296 | NMPA-approved | 12 |
| 25 | Female | 73 | cT1cN3M1c | EGFR 19del | 1 | Gefitinib | FDA-approved | 5 |
| 26 | Female | 72 | cT4N3M1 | EGFR 19del | 1 | Gefitinib | FDA-approved | 7 |
| 27 | Female | 65 | T1bN2M0 | EGFR L858R | 1 | Gefitinib | FDA-approved | 20 |
| 28 | Female | 55 | T2aN2M0 | EGFR L858R | 1 | Gefitinib | FDA-approved | 24 |
| 29 | Female | 52 | T2aN0M0 | EGFR 19del | 1 | Gefitinib | FDA-approved | 22 |
| 30 | Female | 50 | T2aN0M0 | EGFR T790M | 1 | Osimertinib | FDA-approved | 25 |
| 31 | Female | 65 | T2aN0M0 | EGFR L861Q | 1 | Afatinib | FDA-approved | 18 |
| 32 | Male | 55 | T2aN0M0 | EGFR G719A | 1 | Erlotinib | FDA-approved | 16 |
| 33 | Male | 62 | T3N2M1c | EGFR L858R | 1 | Pemetrexed + carboplatin | / | 6 |
| 34 | Male | 73 | T1cN2M1c | EGFR 19del | 1 | Pemetrexed + carboplatin | / | 8 |
| 35 | Female | 41 | T1cN3M1c | ERBB2 p.A775_G776insYVMA | 2 | Pemetrexed + carboplatin | / | 7 |
| 36 | Male | 55 | T2aN2M0 | / | / | Pemetrexed + carboplatin | / | 5 |
FDA, U.S. Food and Drug Administration; NMPA, China National Medical Products Administration.
Response to Indicated Targeted Therapy
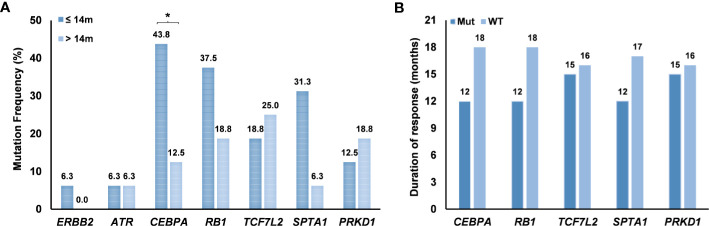
We investigated the relationship between the driver genes and the duration of EGFR-TKIs response. The results showed that CEBPA mutations were more common in patients with short duration of response to TKIs (< 14 months) than that in patients with long duration of response (> 14 months) (43.8% vs. 12.5%, P =0.031) ( Figure 5A ). The duration of response to TKIs in patients with CEBPA mutations was shorter than that in patients without CEBPA mutations (12 months vs. 18 months) regardless of other driver gene mutations ( Figure 5B ). However, RB1 and SPTA1 mutations were common in patients with short duration of response to TKIs. Patients with RB1 and SPTA1 mutations had shorter TKI remission time than those without mutations, but there was no significant difference ( Figure 5B ). TCF7L2 and PRKD1 mutations were common in patients with longer duration of response, although there was no statistical difference ( Figure 5A ).
Figure 5.
Effect of mutated genes on the efficacy of EGFR-TKIs. (A) Differences in mutation frequency of mutated genes between duration of response ≤ 14 months and duration of response > 14 months. (B) Duration of response to TKIs according to gene mutations. *P < 0.05. Mut, mutation; WT, wild type.
Tumor Mutation Burden of LUAD
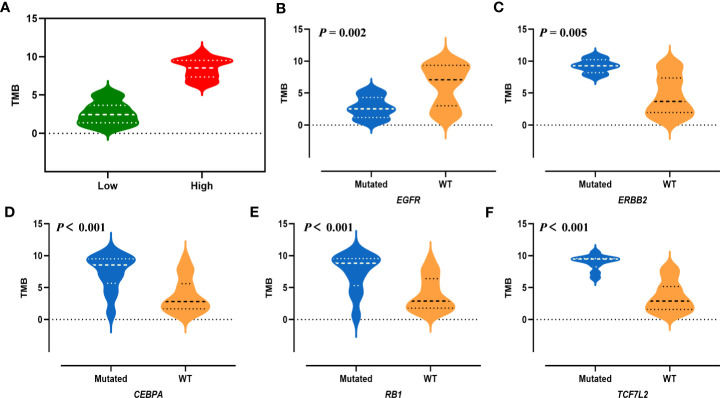
TMB is a biomarker of sensitivity to immune checkpoint inhibitors including PD-1 and PD-L1 blockade immunotherapy (32). In the current study, the median TMB of LUAD was 4.82 (range 0.65–10.50) mutations/Mb. According to the TCGA (the Cancer Genome Atlas) database, the average TMB value of lung adenocarcinoma was set as follows: low (<6.3 mutations/Mb) and high (≥ 6.3 mutations/Mb) ( Figure 6A ). EGFR mutated tumors had lower median TMB than EGFR wild-type tumors ( Figure 6B ). LUAD with somatic mutations in ERBB2, CEBPA, RB1 and TCF7L2 genes got higher TMB than tumors without those mutations ( Figures 6C–F ). But we found no difference on TMB of TP53 and ATR mutations. No significant association between TMB and lymph node metastasis (P = 0.922), peritoneal metastasis (P = 0.220) and smoking status (P = 0.873) was found (data not shown).
Figure 6.
Tumor mutation burden of LUAD. (A) The distribution of TMB across all patients, using a threshold of 6.3 mutations/Mb. Low (<6.3 mutations/Mb), and high (≥ 6.3 mutations/Mb). Comparison of TMB according to EGFR mutations (B), ERBB2 mutations (C), CEBPA mutations (D), RB1 mutations (E), TCF7L2 mutations (F).
Discussion
Nowadays, targeted therapy has greatly improved the outcomes of patients with specific molecular changes. Therapeutic decisions should be guided by the molecular changes of tumor with standardized and easily interpretable annotation. Such detailed, evidence-based information about individual somatic mutations could be found on OncoKB (33, 34). In this study, we performed genomic analysis of 101 patients with LUAD in eastern China using a customized 639 genes DNA panel, which contributed to the selection of matched targeted therapies. Nearly 70% of patients harbored mutations that matched the targeted drugs, and 41.6% of patients with actionable alterations were recommended FDA-approved drugs. About 18.8% of patients had gene mutations recommended by NCCN guidelines or other professional guidelines. Almost 29.7% of the patients had no clinically applicable mutations of NSCLC, among which, 8% had mutations in CEBPA and RB1, and 6% had TCF7L2 mutations, providing targets for clinical drug development for patients who did not have evidence of drugs. We were surprised to find that targeted therapy has become the first choice for patients with level_1 mutations. However, for patients with level_2 and below mutations, surgery and chemotherapy are still the first choice. Among the 71 patients with clinically actionable alterations, patients used matching targeted drugs, resulting in a better outcome, and 3 patients who had mutations of the recommended targeted drug, but they received chemotherapy and 1 patient without actionable alteration who was treated with chemotherapy showed worse prognosis than those received targeted agent, suggesting the significance of incorporation of NGS analysis into standard clinical practice. Though not all patients with mutations meet the indications for the use of targeted drugs, we consider and hope that patients with mutations who meet the indications can choose targeted drugs and benefit from them.
Furthermore, we found significant relationship between CEBPA mutations and the efficacy of EGFR-TKIs. Tumor heterogeneity is an important reason for the efficacy and resistance to EGFR targeted therapy in patients with NSCLC (35). Interestingly, we found that CEBPA mutations may be a biomarker for EGFR-TKIs, as the high frequency of CEBPA mutations indicated a poor prognosis of EGFR-TKIs treatment.
DNA panel can analyze the value of TMB which has become a predictive biomarker for ICI treatment. High TMB is known to be associated with DNA mismatch repair pathway genes and TP53 (36, 37), but we did not detect a correlation of them. However, statistical analysis showed the significant association between TMB and mutations in ERBB2, CEBPA, RB1 and TCF7L2, the top frequently mutated genes. If EGFR-TKI treatment is used, the efficacy of these patients may be poor, but patients may have higher TMB, which may point to the use of immunotherapeutic drugs, suggesting potential predictive biomarkers for response to PD-1 blockade immunotherapy.
The limitation of this study is that the sample size of patients with complete follow-up records is too small. In the future, more samples will be collected to elucidate the clinical value of targeted therapies based on NGS detection and to explore the clinical operability of using OncoKB database to guide patients’ treatment. Additionally, several novel mutated genes in the panel were found and they were closely associated with age, TNM stage and lymph node metastasis. Further study should be focused on the relationship between these mutations and clinical characteristics and signaling pathway to explore the mechanism of these genes leading to LUAD.
Conclusion
In conclusion, genomic profile of 101 Chinese LUAD patients was identified. Novel genes with high mutation frequency such as ERBB2, ATR, CEBPA, RB1 and TCF7L2 were closely related to the efficacy of targeted therapies and TMB. NGS is conducive to guide patients to choose targeted drugs and provide basis for the development and application of precise treatment strategies for Chinese lung cancer patients.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI-Sequence Read Archive (SRA), PRJNA774908.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Zhongshan Hospital Affiliated to Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JL and CL designed the manuscript. W-YX, MY, and ZL analyzed the data and wrote the manuscript. All the authors contributed to the article and approved the submitted version.
Funding
Shanghai Municipal Key Clinical Specialty(shslczdzk02201), Shanghai Top-Priority Clinical Key Disciplines Construction Project (2017ZZ02013), Young Medical Professionals Training Program from Shanghai Medical and Health Development Foundation (Q2017-056).
Conflict of Interest
W-YX was employed by company Singlera Genomics (Shanghai) Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the medical workers and researchers from the Department of Pulmonary and Critical Care Medicine of Zhongshan Hospital, Fudan University and Singlera Genomics (Shanghai) Ltd. for their excellent work in data collection, sequencing and analysis for this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.726547/full#supplementary-material
References
- 1. Feng RM, Zong YN, Cao SM, Xu RH. Current Cancer Situation in China: Good or Bad News From the 2018 Global Cancer Statistics? Cancer Commun (Lond) (2019) 39(1):22. doi: 10.1186/s40880-019-0368-6. Cited in: Pubmed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin (2014) 64(1):9–29. doi: 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 3. Clinical Lung Cancer Genome P. Network Genomic M . A Genomics-Based Classification of Human Lung Tumors. Sci Transl Med (2013) 5(209):209ra153. doi: 10.1126/scitranslmed.3006802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO Guidelines and the Critical Role of Immunohistochemical Markers in the Subclassification of Non-Small Cell Lung Carcinoma (NSCLC): Moving From Targeted Therapy to Immunotherapy. Semin Cancer Biol (2018) 52(Pt 1):103–9. doi: 10.1016/j.semcancer.2017.11.019.Cited in: Pubmed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and Pemetrexed With or Without Pembrolizumab for Advanced, Non-Squamous Non-Small-Cell Lung Cancer: A Randomised, Phase 2 Cohort of the Open-Label KEYNOTE-021 Study. Lancet Oncol (2016) 17(11):1497–508. doi: 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 7. Nie K, Jiang H, Zhang C, Geng C, Xu X, Zhang L, et al. Mutational Profiling of Non-Small-Cell Lung Cancer Resistant to Osimertinib Using Next-Generation Sequencing in Chinese Patients. BioMed Res Int (2018) 2018:9010353. doi: 10.1155/2018/9010353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wen S, Dai L, Wang L, Wang W, Wu D, Wang K, et al. Genomic Signature of Driver Genes Identified by Target Next-Generation Sequencing in Chinese Non-Small Cell Lung Cancer. Oncologist (2019) 24(11):e1070–81. doi: 10.1634/theoncologist.2018-0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, et al. Phylogenetic ctDNA Analysis Depicts Early-Stage Lung Cancer Evolution. Nat (2017) 545(7655):446–51. doi: 10.1038/nature22364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anagnostou V, Forde PM, White JR, Niknafs N, Hruban C, Naidoo J, et al. Dynamics of Tumor and Immune Responses During Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Res (2019) 79(6):1214–25. doi: 10.1158/0008-5472.CAN-18-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu C, Zhuang W, Chen L, Yang W, Ou WB. Frontiers of ctDNA, Targeted Therapies, and Immunotherapy in Non-Small-Cell Lung Cancer. Transl Lung Cancer Res (2020) 9(1):111–38. doi: 10.21037/tlcr.2020.01.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blons H, Garinet S, Laurent-Puig P, Oudart JB. Molecular Markers and Prediction of Response to Immunotherapy in Non-Small Cell Lung Cancer, an Update. J Thorac Dis (2019) 11(Suppl 1):S25–36. doi: 10.21037/jtd.2018.12.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao J, Chen L, Li H, Chen H, Yao J, Mu S, et al. An Accurate and Comprehensive Clinical Sequencing Assay for Cancer Targeted and Immunotherapies. Oncologist (2019) 24(12):e1294–302. doi: 10.1634/theoncologist.2019-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seo JS, Ju YS, Lee WC, Shin JY, Lee JK, Bleazard T, et al. The Transcriptional Landscape and Mutational Profile of Lung Adenocarcinoma. Genome Res (2012) 22(11):2109–19. doi: 10.1101/gr.145144.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teixeira VH, Pipinikas CP, Pennycuick A, Lee-Six H, Chandrasekharan D, Beane J, et al. Deciphering the Genomic, Epigenomic, and Transcriptomic Landscapes of Pre-Invasive Lung Cancer Lesions. Nat Med (2019) 25(3):517–25. doi: 10.1038/s41591-018-0323-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol (2015) 10(9):1243–60. doi: 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 17. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol (2016) 11(1):39–51. doi: 10.1016/j.jtho.2015.09.009. International Association for the Study of Lung Cancer S, Prognostic Factors Committee Advisory B, Participating I. [DOI] [PubMed] [Google Scholar]
- 18. Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, et al. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff: SNPs in the Genome of Drosophila Melanogaster Strain W1118; Iso-2; Iso-3. Fly (Austin) (2012) 6(2):80–92. doi: 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ulintz PJ, Wu W, Gates CM. Bioinformatics Analysis of Whole Exome Sequencing Data. Methods Mol Biol (2019) 1881:277–318. doi: 10.1007/978-1-4939-8876-1_21 [DOI] [PubMed] [Google Scholar]
- 20. Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol (2017), 1-16. doi: 10.1200/PO.17.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: Efficient and Comprehensive Analysis of Somatic Variants in Cancer. Genome Res (2018) 28(11):1747–56. doi: 10.1101/gr.239244.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu G, Wang LG, Han Y, He QY. Clusterprofiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS (2012) 16(5):284–7. doi: 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang J, Zhang Y. I-TASSER Server: New Development for Protein Structure and Function Predictions. Nucleic Acids Res (2015) 43(W1):W174–81. doi: 10.1093/nar/gkv342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in Combination With Chemotherapy Versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet (2010) 376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 25. Gajria D, Chandarlapaty S. HER2-Amplified Breast Cancer: Mechanisms of Trastuzumab Resistance and Novel Targeted Therapies. Expert Rev Anticancer Ther (2011) 11(2):263–75. doi: 10.1586/era.10.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ninomiya K, Hata T, Yoshioka H, Ohashi K, Bessho A, Hosokawa S, et al. A Prospective Cohort Study to Define the Clinical Features and Outcome of Lung Cancers Harboring HER2 Aberration in Japan (HER2-CS STUDY). Chest (2019) 156(2):357–66. doi: 10.1016/j.chest.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 27. Zhou X, Xu X, Tian Z, Xu WY, Cui Y. Mutational Profiling of Lung Adenocarcinoma in China Detected by Next-Generation Sequencing. J Cancer Res Clin Oncol (2020) 146(9):2277–87. doi: 10.1007/s00432-020-03284-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konstandin NP, Pastore F, Herold T, Dufour A, Rothenberg-Thurley M, Hinrichsen T, et al. Genetic Heterogeneity of Cytogenetically Normal AML With Mutations of CEBPA. Blood Adv (2018) 2(20):2724–31. doi: 10.1182/bloodadvances.2018016840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Halmos B, Huettner CS, Kocher O, Ferenczi K, Karp DD, Tenen DG. Down-Regulation and Antiproliferative Role of C/EBPalpha in Lung Cancer. Cancer Res (2002) 62(2):528–34. [PubMed] [Google Scholar]
- 30. Fang W, Huang Y, Gu W, Gan J, Wang W, Zhang S, et al. PI3K-AKT-mTOR Pathway Alterations in Advanced NSCLC Patients After Progression on EGFR-TKI and Clinical Response to EGFR-TKI Plus Everolimus Combination Therapy. Transl Lung Cancer Res (2020) 9(4):1258–67. doi: 10.21037/tlcr-20-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qu GP, Shi M, Wang D, Wu JH, Wang P, Gong ML, et al. Dual Targeting of MEK and PI3K Effectively Controls the Proliferation of Human EGFR-TKI Resistant Non-Small Cell Lung Carcinoma Cell Lines With Different Genetic Backgrounds. BMC Pulm Med (2021) 21(1):208. doi: 10.1186/s12890-021-01571-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Picard E, Verschoor CP, Ma GW, Pawelec G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front Immunol (2020) 11:369. doi: 10.3389/fimmu.2020.00369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saadeh C, Bright D, Rustem D. Precision Medicine in Oncology Pharmacy Practice. Acta Med Acad (2019) 48(1):90–104. doi: 10.5644/ama2006-124.246 [DOI] [PubMed] [Google Scholar]
- 34. Katsoulakis E, Duffy JE, Hintze B, Spector NL, Kelley MJ. Comparison of Annotation Services for Next-Generation Sequencing in a Large-Scale Precision Oncology Program. JCO Precis Oncol (2020) 4:212–21. doi: 10.1200/PO.19.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of Resistance to EGFR-Targeted Drugs: Lung Cancer. ESMO Open (2016) 1(3):e000060. doi: 10.1136/esmoopen-2016-000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park S, Lee H, Lee B, Lee SH, Sun JM, Park WY, et al. DNA Damage Response and Repair Pathway Alteration and Its Association With Tumor Mutation Burden and Platinum-Based Chemotherapy in SCLC. J Thorac Oncol (2019) 14(9):1640–50. doi: 10.1016/j.jtho.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 37. Xu F, Lin H, He P, He L, Chen J, Lin L, et al. A TP53-Associated Gene Signature for Prediction of Prognosis and Therapeutic Responses in Lung Squamous Cell Carcinoma. Oncoimmunology (2020) 9(1):1731943. doi: 10.1080/2162402X.2020.1731943 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI-Sequence Read Archive (SRA), PRJNA774908.