Abstract
Repeated antigen testing of 12 severe acute respiratory coronavirus virus 2 (SARS-CoV-2)–positive nursing home residents using Abbott BinaxNOW identified 9 of 9 (100%) culture-positive specimens up to 6 days after initial positive test. Antigen positivity lasted 2–24 days. Antigen positivity might last beyond the infectious period, but it was reliable in residents with evidence of early infection.
To address the disproportionate coronavirus disease (COVID-19) burden in nursing homes, in July 2020, the US Department of Health and Human Services began allocating point-of-care antigen tests to >15,000 nursing homes. 1,2 Although the results of such tests can facilitate earlier identification of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infections and implementation of prevention efforts compared with laboratory-based tests like real-time reverse transcription-polymerase chain reaction (RT-PCR), 3 published data characterizing antigen testing in infected nursing home residents are limited. Such data could better inform infection prevention and control practices, particularly during SARS-CoV-2 outbreaks. 4
The Abbott BinaxNOW COVID-19 Ag Cards (BinaxNOW, Abbott Diagnostics Scarborough, Scarborough, ME) have received emergency-use authorization (EUA) for qualitative detection of the SARS-CoV-2 nucleocapsid protein antigen. 5 McKay et al 6 evaluated the performance of BinaxNOW used for facility-wide point-prevalence surveys (PPSs) during a nursing home outbreak. In this report, we further characterize BinaxNOW using repeated testing in a sample of antigen-positive nursing home residents identified in McKay et al by comparing antigen results to RT-PCR and virus culture results at frequent intervals and describing the duration of antigen positivity.
Methods
Participant recruitment
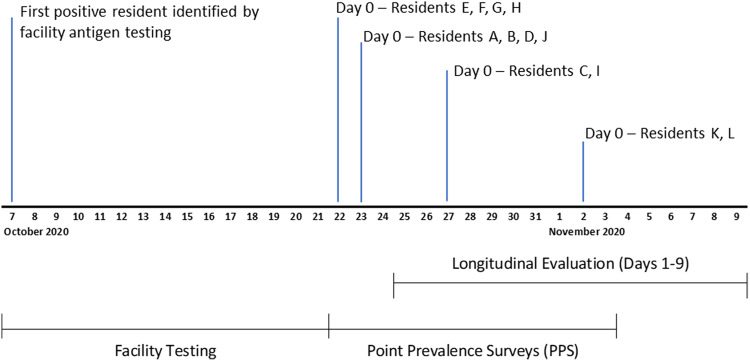
Following the identification of a SARS-CoV-2 outbreak through facility testing, the Centers for Disease Control and Prevention (CDC) conducted 3 PPSs of all residents according to CDC guidance (Fig. 1); 4 the PPS, including materials and methods is described in McKay et al. 6 Residents who tested antigen positive during a PPS and provided informed consent were enrolled in a longitudinal evaluation that included 4 additional specimen collections every 1–3 days. All antigen testing presented here was conducted using BinaxNOW. 5
Fig. 1.
Timeline of BinaxNOW antigen testing at a nursing home experiencing an outbreak of SARS-CoV-2 infections. The facility conducted antigen testing of residents using BinaxNOW from October 7 through October 21, 2020. From October 22, 2020, through November 3, 2020, the CDC conducted 3 facility-wide point-prevalence surveys (PPSs) among 127 residents using BinaxNOW (results published in McKay et al 6 ). Day 0 denotes the dates participants of the longitudinal evaluation were first identified as antigen positive during a PPS. Specimen collection for the longitudinal evaluation began 1–3 days after Day 0.
Specimen collection and testing
At each visit, paired bilateral anterior nasal swabs were collected: one was tested using BinaxNOW 5 and the other was tested for presence of SARS-CoV-2 RNA by RT-PCR using the CDC Influenza SARS-CoV-2 (Flu SC2) Multiplex Assay. 6 Cycle threshold (Ct) values <40 for the SARS-CoV-2 nucleocapsid protein gene target were defined as RT-PCR positive. To assess the presence of replication-competent virus, cultures were performed using Vero-CCL-81 cells on specimens with an RT-PCR Ct value <35 or antigen-positive/RT-PCR–negative results. 6
Statistical analysis
For each participant, results from facility testing, PPSs, and longitudinal specimens were analyzed using Microsoft Excel software (Microsoft, Redmond, WA) and SAS version 9.4 software (SAS Institute, Cary, NC). Percent positive agreement was determined by comparing antigen test results with RT-PCR and virus culture results. Percent negative agreement between antigen test and virus culture was not calculated because antigen-negative/RT-PCR–negative specimens were not sent for culture. Antigen duration was defined as the number of days between first and last antigen-positive results, inclusive. This activity was reviewed by the CDC and was conducted consistent with applicable federal law and CDC policy. 7–11
Results
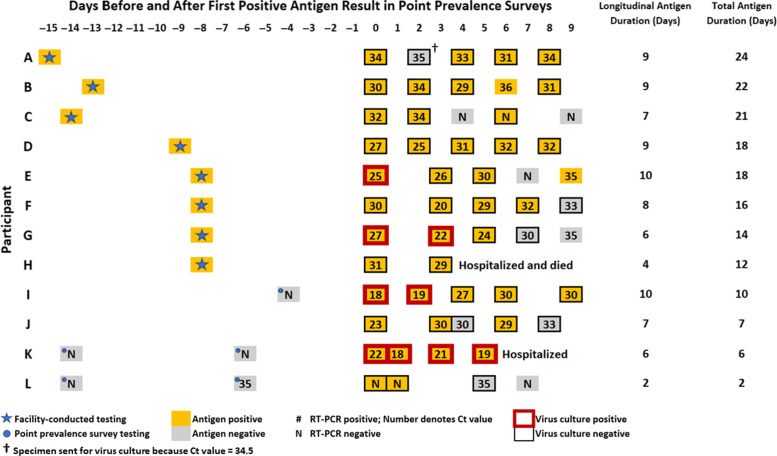
Among 127 residents tested during the PPSs, 47 (37%) tested antigen positive, and 12 (26%) enrolled in the longitudinal evaluation. The median age of the 12 participants was 74 years (range, 37–89) and 8 (67%) were male. Of 55 paired specimens, 44 (80%) were antigen positive and 48 (87%) were RT-PCR positive (Fig. 2). Between antigen tests and RT-PCR assays, the percent positive agreement was 85% (41 of 48; exact binomial 95% confidence interval [95% CI], 72%–94%) and percent negative agreement was 57% (4 of 7; 95% CI, 18%–90%). Also, 3 antigen-positive/RT-PCR–negative (ie, false positive) specimens, collected from 2 participants (C and L), had preceding and subsequent antigen-negative specimens that were either RT-PCR negative or had a Ct value of 35 (Fig. 2).
Fig. 2.
SARS-CoV-2 antigen, RT-PCR, and virus culture results from 12 nursing home residents participating in a longitudinal evaluation to characterize Abbott BinaxNOW COVID-19 Ag Cards. Specimens collected on days −15 through −1 were tested by the facility. Day 0 is the day participants first tested positive during a point-prevalence survey (PPS) conducted by the CDC. Specimens collected on days 1–9 were for the longitudinal evaluation. Cycle threshold (Ct) values were rounded to the nearest whole value; specimens with Ct values <40 were considered positive.
Among 48 RT-PCR–positive specimens, the median Ct value was 29 (range, 18–35) for antigen-positive specimens and 33 (range, 30–35) for antigen-negative specimens (Wilcoxon, P = .007). Virus culture was attempted for 48 specimens (87%). In total, 39 specimens (81%), including 3 antigen-positive/RT-PCR–negative specimens, were culture negative for SARS-CoV-2; however, 9 specimens (19%) from 4 participants (E, G, I, and K) were culture positive for 1 to at least 6 days (Fig. 2). The percent positive agreement between antigen test and virus culture among these 9 specimens was 100% (95% CI, 66%–100%). The median Ct value among antigen-positive specimens was 21 (range, 18–27) when the culture was positive and 30 (range, 20–34) when the culture was negative (Wilcoxon P < .001). Also, 5 participants (42%) tested antigen positive at all longitudinal visits. Moreover, 4 (33%) participants with intermittent antigen-negative tests (A, C, E, and J) had specimens with Ct values ≥30 preceding their first antigen-negative result (Fig. 2).
The duration between the first PPS antigen-positive result and last longitudinal antigen-positive result ranged from 2 to 10 days, when specimen collection stopped. Facility-conducted testing identified 8 participants (A–H) as antigen positive before the PPS, extending their antigen positivity duration to 12 to 24 days (Fig. 2). The maximum days observed for antigen-positive/RT-PCR–positive agreement and antigen-positive/culture-positive agreement were 10 and 6, respectively.
Discussion
In this small convenience sample of SARS-CoV-2 antigen-positive nursing home residents, BinaxNOW identified all specimens harboring replication-competent virus during repeated, frequent testing. Ct values among antigen-positive specimens were significantly lower than antigen-negative specimens, suggesting that antigen-positive residents had higher viral loads. These results suggest that BinaxNOW identified potentially infectious nursing home residents during this outbreak. Antigen was detected up to 24 days after the initial positive result, well beyond the maximum observed duration of culture positivity (6 days), indicating that antigen positivity might last beyond the infectious period in some nursing home residents. For comparison, Surie et al 12 found that PCR positivity can last as long as 48 days in a nursing home cohort.
Antigen results were consistently positive for many participants. Some false-positive and intermittently positive specimens occurred among specimens preceded or followed by antigen-negative specimens, RT-PCR–negative specimens, or specimens with high Ct values, possibly reflecting later stages of infection when viral loads are waning. McKay et al 6 found that percent positive agreement and percent negative agreement between BinaxNOW and RT-PCR were lower among specimens collected during late SARS-CoV-2 infection. 6 These findings support guidance that antigen testing not be used to determine duration of transmission-based precautions. 4
Data on SARS-CoV-2 antigen positivity duration are limited. 5 In an assessment of a different antigen test, Hirotsu et al 13 identified SARS-CoV-2 antigen-positive specimens up to 25 days after the first antigen-positive result in at least 1 of 7 patients. Although our data suggest that culture-positive specimens will also be positive when tested with BinaxNOW, antigen-positive specimens can be culture negative during late SARS-CoV-2 infection. 6 Additional studies are needed to assess the role of antigen tests in differentiating between people who are infectious and those who are not, particularly since performance might be affected by new variants and vaccination status.
This study has several limitations. The data are from a small sample of nursing home residents who tested positive with BinaxNOW during an outbreak; these findings might not be generalizable to other populations, antigen tests, or nonoutbreak settings. Antigen-negative/RT-PCR–positive residents were not enrolled; including such residents would have contributed additional antigen test performance and duration data. The longitudinal evaluation followed participants for up to 10 days, and despite including additional facility testing results, how long antigen positivity can last and what factors contribute to prolonged positivity remain unknown. The duration of infectiousness was likely underestimated because a negative culture result does not necessarily mean replication-competent virus was not present.
In conclusion, this report provides data on repeated SARS-CoV-2 antigen testing in nursing home residents and suggests that BinaxNOW can identify infectious people at risk of transmitting SARS-CoV-2 during nursing home outbreaks. Antigen positivity might last beyond the infectious period in some residents, but reliably identified residents with evidence of early infection.
Acknowledgments
The authors thank the residents and staff at the nursing home for participating. They also appreciate the support provided by Pascale Wortley (Georgia Department of Public Health), Bettina Bankamp, Leslie Barclay, Theresa Bessey, Michael D. Bowen, Hannah Browne, Davina Campbell, Preeti Chhabra, Mathew D. Esona, Brandi Freeman, Lalitha Gade, Renee Galloway, Rashi Gautam, Claire Hartloge, Amy L. Hopkins, Shilpi Jain, Brent Jenkins, Baoming Jiang, Kahaliah Joseph, Eric M. Katz, Gimin Kim, Magdalena Medrzycki, Slavica Mijatovic-Rustempasic, Sung-Sil Moon, Kenny Nguyen, Kay W. Radford, Sujan C. Reddy, Kashif Sahibzada, Patricia Shewmaker, S. Leeann Smart, Alexandra Tejada, Srinivasan Velusamy, Jan Vinjé, Phili Wong, Nhien Wynn, and HaoQiang Zheng (CDC COVID-19 Response Team). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). CDC does not endorse specific products or manufacturers.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
Authors report no conflicts of interest relevant to this article.
References
- 1.COVID-19 in nursing homes. US Government Accountability Office website. https://www.gao.gov/assets/gao-21-367.pdf. Published 2021. Accessed May 27, 2021.
- 2. Nursing home data—point of care device allocation. Centers for Medicare and Medicaid Services website. https://data.cms.gov/Special-Programs-Initiatives-COVID-19-Nursing-Home/Nursing-Home-Data-Point-of-Care-Device-Allocation/jbvf-tb74. Published 2020. Accessed January 27, 2021.
- 3.Interim guidance for antigen testing for SARS-CoV-2. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html. Published 2021. Accessed May 27, 2021.
- 4.SARS-CoV-2 antigen testing in long-term care facilities. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homes-antigen-testing.html. Published 2021. Accessed May 27, 2021.
- 5.BinaxNOW COVID-19 Ag Card—instructions for use. US Food and Drug Administration website. https://www.fda.gov/media/141570/download. Published 2020. Accessed January 27, 2021.
- 6. McKay SL, Tobolowsky FA, Moritz ED, et al. Performance evaluation of serial SARS-CoV-2 rapid antigen testing during a nursing home outbreak. Ann Intern Med 2021. doi: 10.7326/M21-0422. [DOI] [PMC free article] [PubMed]
- 7. Protection of human subjects. 45 C.F.R. part 46.102(l)(2). Code of Federal Regulations. 2018. [PubMed]
- 8. Institutional review boards. 21 C.F.R. part 56. Code of Federal Regulations. 1981.
- 9. Records maintained on individuals. 5 U.S.C. §552a. United States Code. 2018.
- 10.Purposes. 44 U.S.C. §3501. United States Code. 2018.
- 11. Research and investigations generally. 42 U.S.C. §241(d). United States Code. 2011.
- 12. Surie D, Huang JY, Brown AC, et al. Infectious period of severe acute respiratory syndrome coronavirus 2 in 17 nursing home residents—Arkansas, June–August 2020. Open Forum Infect Dis 2021. doi: 10.1093/ofid/ofab048. [DOI] [PMC free article] [PubMed]
- 13. Hirotsu Y, Maejima M, Shibusawa M, et al. Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs, including from seven serially followed patients. Int J Infect Dis 2020;99:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]