Graphical abstract
Keywords: Amniotic fluid embolism, Obstetric medicine, Transthoracic echocardiogram, Shock
Highlights
-
•
McConnell’s sign may be an early sign of acute RV strain in amniotic fluid embolus.
-
•
TTE could be considered to facilitate timely diagnosis of amniotic fluid embolus.
-
•
The presence of RV dysfunction on TTE may help guide hemodynamic therapies.
Introduction
Amniotic fluid embolism (AFE) is a rare obstetrical emergency. It is characterized by acute cardiopulmonary distress and disseminated intravascular coagulopathy (DIC), occurring during delivery or immediately in the postpartum period.1 The pathophysiology of AFE is not well understood. There is emerging evidence suggesting that the amniotic fluid embolization to the pulmonary vasculature triggers an acute inflammatory response, ultimately leading to severe pulmonary vasospasm.2,3 This subsequently results in acute pulmonary hypertension and right ventricular (RV) dysfunction. Amniotic fluid embolism is associated with significant adverse maternal and fetal outcomes; hence early recognition is key. We present the value of transthoracic echocardiography (TTE) in establishing timely diagnosis of AFE. In addition, we propose that McConnell’s sign may also be an early indicator of acute RV strain in patients with AFE.
Case Presentation
A 27-year-old G2P1 healthy female at 40 weeks of pregnancy underwent an emergency cesarean section due to an arrest of labor. Her pregnancy course was uncomplicated, with no gestational hypertension or diabetes. During the cesarean section, the patient experienced an episode of bradycardia and hypotension that spontaneously resolved. Shortly after the cesarean section, she was noted to have a postpartum hemorrhage with an estimated blood loss of ∼2 L. Despite adequate volume resuscitation, the patient developed progressive hypoxemia and refractory hypotension. Urgent surgical exploration did not reveal significant ongoing bleeding contributing to her instability, and the patient further decompensated hemodynamically. Her heart rate was 150 bpm, blood pressure was 70/50 mm Hg, and oxygen saturation was 90% on 100% nonrebreather, and she was noted to be oliguric despite resuscitation. The patient was transferred urgently to a tertiary care intensive care unit for further management.
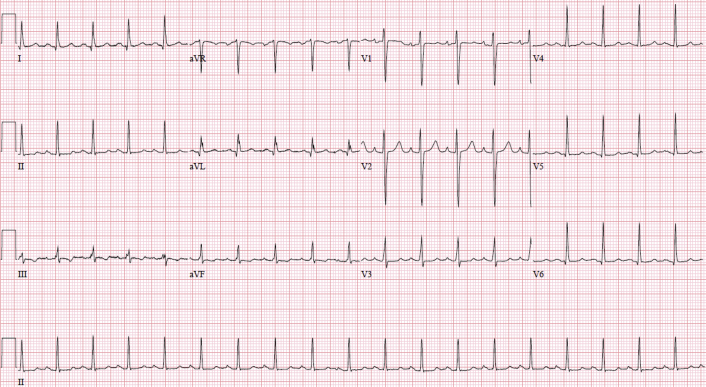
Laboratory investigations showed evidence of DIC with a hemoglobin of 115 g/L, platelet count of 81 × 10E9/L, fibrinogen of 0.4 g/L, and international normalized ratio of 1.8. The patient also had an acute kidney injury with a creatinine of 130 μmol/L. An arterial blood gas revealed a pH of 7.38, pCO2 of 26 mm Hg, pO2 of 91 mm Hg, HCO3 of 15 mmol/L, and lactate of 1.7 mmol/L. Her baseline electrocardiogram showed sinus tachycardia with nonspecific T-wave changes (Figure 1), with subsequent electrocardiograms consistent with sinus tachycardia. Her initial troponin level was elevated at 492 ng/L and subsequently trended down.
Figure 1.
Baseline electrocardiogram showing sinus tachycardia with non-specific ST- and T-wave changes in leads II, III, and aVF.
The differential diagnosis included AFE pulmonary embolism, peripartum cardiomyopathy, acute respiratory distress syndrome, anaphylaxis, and septic shock. Obstetrical causes such as uterine rupture and placental abruption were excluded based on surgical exploration. Based on the constellation of findings, AFE was strongly suspected.
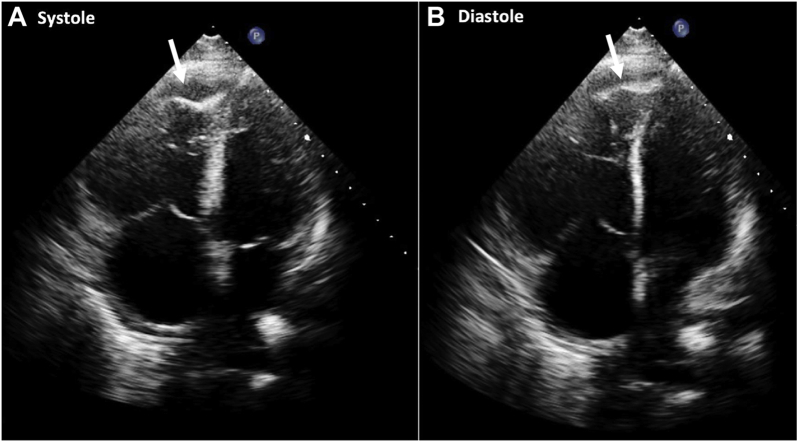
Transthoracic echocardiogram was performed to efficiently narrow down the differential diagnosis by excluding peripartum cardiomyopathy and to evaluate RV function in the setting of ongoing shock. The left ventricle (LV) was normal in size with a preserved systolic function. However, there was significant RV dilation with severely reduced systolic function based on visual assessment. Additionally, a distinct pattern of akinesia of the mid RV wall with hypercontractility of the apex was noted, consistent with McConnell’s sign (Video 1 and Figure 2). Moderate tricuspid regurgitation (TR) was also seen (TR max velocity = 310.8 cm/sec; Figure 3). Lastly, a linear structure was seen within the inferior vena cava (IVC) on several views that was most consistent with thrombus (Figure 4).
Figure 2.
Apical four-chamber view in systole (A) and diastole (B). There was akinesis of the mid RV wall with hypercontractility of the RV apex (white arrow) consistent with McConnell’s sign. Trace pericardial effusion was also noted.
Figure 3.
Continuous-wave Doppler through the tricuspid valve demonstrating moderate TR. The maximum velocity was 311 cm/sec with a calculated peak gradient of 39 mm Hg. Right atrial pressure was estimated at 15 mm Hg.
Figure 4.
Subcostal view demonstrates a linear echo-dense mass within the IVC consistent with the two-dimensional echocardiographic appearance of thrombus.
Computed tomographic pulmonary angiography confirmed a low-burden pulmonary embolism involving the lateral basal segmental pulmonary artery of the right lower lobe (Figure 5). Abdominal and pelvic computed tomography showed thrombosis within the right gonadal vein with a small amount of thrombus extending into the IVC. Additionally, there was extensive bilateral renal cortical necrosis.
Figure 5.
Computed tomographic pulmonary angiography showing low-burden pulmonary embolism (PE) involving the lateral basal segmental pulmonary artery (A) of the right lower lobe (white arrow). There was no evidence of pulmonary embolism in the right, left, or main pulmonary arteries (B).
The degree of RV dysfunction was out of proportion with the low-burden pulmonary embolism noted, and therefore it was felt that the most likely underlying diagnosis was AFE. The patient was started on norepinephrine to maintain a mean arterial pressure of at least 60 mm Hg in the setting of acute right heart failure secondary to AFE. Initially epinephrine was administered, followed by dobutamine given the presence of significant RV dysfunction. Invasive pulmonary artery pressure monitoring was not used given the presence of DIC and the high likelihood based on echocardiogram that the hemodynamic instability was primarily related to RV, rather than biventricular, dysfunction. Therapeutic anticoagulation with unfractionated heparin was also initiated at the time. Her hemodynamics improved over the next 48 hours, with successful weaning off pressor support. The patient was started on continuous renal replacement therapy in the setting of anuric renal failure with refractory volume overload and was transitioned to intermittent hemodialysis prior to discharge. At 3-month follow-up, the patient was doing well clinically and was no longer dialysis dependent. Repeat echocardiogram demonstrated normal biventricular function, but repeat computed tomography imaging has not been performed.
Discussion
Amniotic fluid embolism is a rare obstetrical emergency. It is characterized by acute cardiopulmonary collapse and DIC occurring during delivery or in the immediate postpartum period.1 The pathophysiology of AFE is not well understood.4 It has been hypothesized that direct embolization of amniotic fluid to the maternal pulmonary circulation leads to acute cardiorespiratory distress.2,3 However, Funk et al.5 recently demonstrated that mechanical obstruction of the pulmonary vasculature by fetal squamous cells alone would impact <0.1% of alveolar units. There is growing evidence suggesting that fetal antigens may trigger a profound inflammatory response.2 More specifically, the amniotic fluid microembolization to the maternal pulmonary vasculature triggers an inflammatory cascade, ultimately leading to pulmonary vasospasm.3,6 Thrombosis related to the procoagulant properties of amniotic fluid and inflammation likely contributes and can result in microemboli that are not even readily apparent pathologically.3 These processes lead to acute pulmonary hypertension, which results in intrapulmonary shunting, severe hypoxemia,3,6 and RV dysfunction.3
Cardiorespiratory collapse secondary to acute RV dysfunction and DIC are the main hallmarks of AFE. However, there are also thrombotic manifestations associated with AFE, which have been previously reported in literature. There are multiple case reports describing intracardiac thrombi detected on echocardiography in patients with AFE.7,8 As a result, it was hypothesized that massive intravascular thrombosis in the initial phase of AFE could potentially be the missing link to this syndrome.7,8 Our patient did have an IVC thrombus extending into the gonadal vein as well as a low-burden pulmonary embolus detected on imaging. It is difficult to elucidate the exact mechanism underlying these findings, but it could potentially be related to the procoagulant nature of the amniotic fluid.
Most cases of AFE are associated with significant adverse maternal and fetal outcomes. The mortality associated with AFE ranges from 20% to 60%,2 and hence early recognition is key. We present the potential value of TTE in identifying severe RV dysfunction that may be contributing to cardiovascular collapse. McConnell’s sign is a distinct echocardiographic finding that is historically associated with a high specificity for diagnosing acute pulmonary embolisms.9 However, there is emerging evidence describing McConnell’s sign in nonthromboembolic causes of pulmonary hypertension.10 McConnell’s sign is characterized by RV mid-free wall hypokinesis with apical sparing.9 Three mechanisms have been proposed to explain the physiology of this pattern.9,11 The right ventricle acutely adapts to a spherical shape, which is more physiologically effective to reduce ventricular wall stress.9 The characteristic RV apical sparing is caused by tethering of the RV apex to a compensating LV.9 It is also hypothesized that increased wall stress leads to localized ischemia of the RV free wall.9,11
Amniotic fluid embolism is primarily a clinical diagnosis, and there are no established criteria or imaging findings to diagnose it. Tomographic chest imaging may not be conclusive for diagnosing AFE unless there is a high-burden embolic event. The role of echocardiography for diagnosing AFE is not well established. Pilecky et al.12 described transesophageal echocardiography findings in a patient with AFE and paradoxical embolism. Imaging revealed multiple heterogenous masses in the right atrium and ventricle, as well as evidence of severe RV dilatation and dysfunction. There was no evidence of intracardiac thrombi in our case, but the patient did have a low-burden PE as well as an IVC thrombus extending to the gonadal vein, which support the hypothesis of concurrent thrombosis in AFE. Shechtman et al.13 reported a case of AFE in which transesophageal echocardiography findings were consistent with acute RV failure with interventricular septum bowing and severe TR. We demonstrate similar observations using TTE, which is less invasive and more readily available. We also propose that in addition to septal flattening and RV dilation—which can occur in both acute and chronic RV strain—McConnell’s sign may be helpful in leading physicians to more strongly suspect AFE given the absence of a gold standard diagnostic tool.
Conclusion
Amniotic fluid embolism is a rare obstetrical emergency that requires rapid identification and correction of abnormalities. Echocardiography can identify acute RV failure in these patients, and McConnell’s sign may be seen in these patients as a marker of acute RV dysfunction. Transthoracic echocardiography could be considered in patients with suspected AFE to facilitate timely diagnosis and subsequent initiation of guided hemodynamic therapies.
Footnotes
Conflicts of Interest: None.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2021.09.006.
Supplementary Data
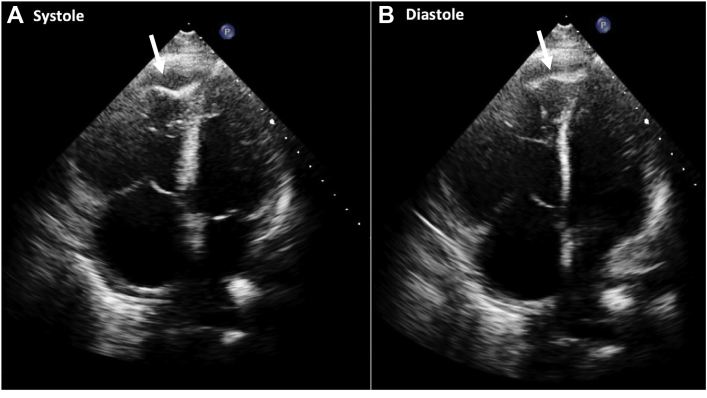
Apical four-chamber view: akinesis of the mid RV wall with hypercontractility of the RV apex consistent with McConnell's sign. Trace pericardial effusion is also noted.
References
- 1.Tamura N., Farhana M., Oda T., Itoh H., Kanayama N. Amniotic fluid embolism: pathophysiology from the perspective of pathology. J Obstet Gynaecol Res. 2017;43:627–632. doi: 10.1111/jog.13284. [DOI] [PubMed] [Google Scholar]
- 2.Clark S.L. Amniotic fluid embolism. Obstet Gynecol. 2014;123:337–348. doi: 10.1097/AOG.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 3.Kaur K., Bhardwaj M., Kumar P., Singhal S., Singh T., Hooda S. Amniotic fluid embolism. J Anaesthesiol Clin Pharmacol. 2016;32:153–159. doi: 10.4103/0970-9185.173356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locksmith G.J. Amniotic fluid embolism. Obstet Gynecol Clin N Am. 1999;26:435-vii. doi: 10.1016/s0889-8545(05)70088-7. [DOI] [PubMed] [Google Scholar]
- 5.Funk M., Damron A., Bandi V., Aagaard K., Szigeti R., Clark S. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460–461. doi: 10.1016/j.ajog.2017.12.225. [DOI] [PubMed] [Google Scholar]
- 6.Clark S.L. New concepts of amniotic fluid embolism: a review. Obstet Gynecol Surv. 1990;45:360–368. doi: 10.1097/00006254-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Porat S., Leibowitz D., Milwidsky A., Valsky D.V., Yagel S., Anteby E.Y. Transient intracardiac thrombi in amniotic fluid embolism. Br J Obstet Gynaecol. 2004;111:506–510. doi: 10.1111/j.1471-0528.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 8.Collett L.W., Sheehan P.V., Gatward J.J. Amniotic fluid embolism with right heart masses presenting as cardiac arrest during labour. Anaesth Intensive Care. 2019;47:193–196. doi: 10.1177/0310057X19838726. [DOI] [PubMed] [Google Scholar]
- 9.McConnell M.V., Solomon S.D., Rayan M.E., Come P.C., Goldhaber S.Z., Lee R.T. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469–473. doi: 10.1016/s0002-9149(96)00339-6. [DOI] [PubMed] [Google Scholar]
- 10.Walsh B.M., Moore C.L. McConnell’s sign is not specific for pulmonary embolism: case report and review of the literature. J Emerg Med. 2015;49:301–304. doi: 10.1016/j.jemermed.2014.12.089. [DOI] [PubMed] [Google Scholar]
- 11.Sosland R.P., Gupta K. Images in cardiovascular medicine: McConnell’s sign. Circulation. 2008;118:e517–e518. doi: 10.1161/CIRCULATIONAHA.107.746602. [DOI] [PubMed] [Google Scholar]
- 12.Pilecky D., Sollfrank R., Wiesinger T., Balogh E., Elsner D. Echocardiographic diagnosis of amniotic fluid embolism with paradoxical embolism. Eur Heart J Cardiovasc Imaging. 2021;22:e150. doi: 10.1093/ehjci/jeab084. [DOI] [PubMed] [Google Scholar]
- 13.Shechtman M., Ziser A., Markovits R., Rozenberg B. Amniotic fluid embolism: early findings of transesophageal echocardiography. Anesthes Analges. 1999;89:1456–1458. doi: 10.1097/00000539-199912000-00025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apical four-chamber view: akinesis of the mid RV wall with hypercontractility of the RV apex consistent with McConnell's sign. Trace pericardial effusion is also noted.