Abstract
Purpose of Review
The purpose of this article is to critically evaluate the available literature on telehealth and antimicrobial stewardship.
Recent Findings
There is limited evidence regarding the role of telehealth within the discipline of antimicrobial stewardship. A review of the available literature suggests remote antimicrobial stewardship programs conducted via telehealth can decrease antimicrobial consumption. A direct comparison between traditional antimicrobial stewardship programs and remote antimicrobial stewardship programs is lacking.
Summary
Telehealth is a promising tool for expanding antimicrobial stewardship, especially in small rural or community hospitals. Trust, team, and technology, the three Ts of remote antimicrobial stewardship derived from themes within the available literature, can serve as a framework for developing a remote antimicrobial stewardship program.
Keywords: Antimicrobial stewardship, Telehealth, Telestewardship, Antimicrobial resistance
Introduction
Telehealth is an emerging field that has a potential role in expanding antimicrobial stewardship to small and critical access hospitals. This review seeks to summarize the available literature on the utilization of telehealth for providing antimicrobial stewardship.
The 2019 United States Centers for Disease Control and Prevention (CDC) core elements for hospital antibiotic stewardship suggest the use of off-site or remote Infectious Diseases (ID)-trained providers when local resources are not available [1••]. A recent report published in the Annals of Internal Medicine found that in 2017 roughly 80% of counties in the USA did not have an ID physician [2]. A 2012 survey showed that 40% of Veteran’s Affairs Medical Centers with inpatient care did not have full-time ID physicians on staff [3]. Also in 2019, the Centers for Medicare and Medicaid Services (CMS) set a requirement for all acute-care hospitals that utilize Medicare or Medicaid to include antimicrobial stewardship by March 30, 2020 [4••].
The COVID-19 pandemic has highlighted the disparity in access to ID physicians, and novel strategies are needed to meet the demand for ID expertise. Telehealth is a potential solution to increasing ID physician access.
Additionally, the pandemic has brought telemedicine to the forefront for medical providers around the world. As of quarter three of 2020, venture capital funding in telehealth had reached $9.4 billion USD—already surpassing the previous annual record of $8.2 billion in 2018 [5].
The Infectious Diseases Society of America (IDSA) published a position statement in 2019 on the role of telehealth in the field of Infectious Diseases [6•]. They note the Health Resources Services Administration defines telehealth as “the use of electronic information and telecommunications technologies to support long-distance clinical healthcare, patient and professional health-related education, public health and health administration.” According to definitions put forth by the IDSA, telehealth with antimicrobial stewardship would primarily involve asynchronous telemedicine without direct patient communication. The IDSA supports the use of telehealth in antimicrobial stewardship to expand access to antimicrobial stewardship to community hospitals [6•].
A recent systematic review examined the effectiveness of telehealth with ID consultation demonstrating comparable efficacy to in-person consultation based on limited available evidence [7]. A separate review focused more broadly on the application of telehealth in ID practice. They demonstrated similar outcomes in the outpatient setting for patients with HCV, HIV, and tuberculosis, whether clinic visits were conducted in-person or through telehealth services [8]. In 2018 Pottinger et al. provided an overview of the literature and potential value of antimicrobial stewardship conducted via telehealth. They highlighted the need for telehealth in antimicrobial stewardship given the vast disparity between available ID specialists and the number of hospitals. They purported that the fundamentals of a successful stewardship program include building trust with a remote institution through periodic face-to-face communication [9]. To our knowledge, there has been no comprehensive systematic review on the role of telehealth in antimicrobial stewardship.
Methods
A systematic literature review was performed using PubMed on September 14, 2020. Search terms utilized were “Antimicrobial Stewardship” and “Telehealth” along with “Antibiotic Stewardship” and “Telehealth” and finally “Telestewardship.” Only English language articles were included if no translation was available. There was no date range limitation, but all articles were published between 2012 and 2020. Articles were included if they described an antimicrobial stewardship intervention performed by an off-site team utilizing telehealth capabilities such as phone calls, video conferencing, email, electronic medical record documentation, or other methods of remote communication. All references of included articles were reviewed for the relevant search terms. Conference abstracts were excluded if outcome data could not be determined. See Fig. 1 for the search process. Primary outcomes were included as specified in the article or if not specified (or if multiple primary outcomes were listed) the first listed measurable outcome was labeled as the primary outcome,1 and all other outcomes were labeled as secondary. Study quality was determined utilizing previously published methodology [10].
Fig. 1.
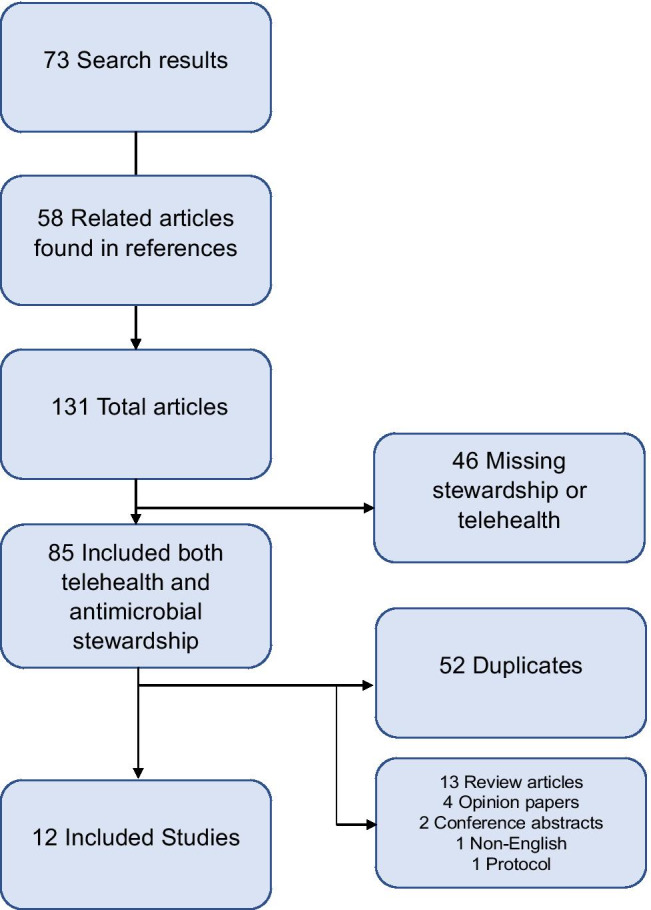
Literature search results. Seventy-three initial search results were found and an additional 58 related articles were located within the references of these initial references. Studies with horizontal arrows indicate excluded studies. Twelve studies were included in the final analysis
Outcomes were sorted based on antimicrobial stewardship intervention. Examples included prospective audit and feedback (PAF), antimicrobial restriction, IV to PO conversion, etc.
Results
A total of 131 articles were reviewed, and 12 met inclusion criteria (Table 1). The quality of included studies was generally low consisting, primarily of observational and quasi-experimental studies with only one randomized controlled trial.
Table 1.
Summary of studies reviewed
| Author | Year | Study design | Primary outcome | Secondary outcomes | Population | Intervention | Statistical significance (primary) | Statistical significance (secondary) | N | Quality | Acceptance rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| dos Santos[11] | 2019 | Quasi-experimental | Antimicrobial consumption | “Appropriateness,” antimicrobial resistance hand rub use, cost | 55-bed community hospital in Brazil | PAF1 | Significant reductions in the consumption of fluoroquinolones, first-generation cephalosporins, and ceftriaxone | “Appropriateness” and cost. Decrease in the rate of carbapenem-resistant Acinetobacter spp. isolation | 11,088 prescriptions for 6163 patients | *** | Not reported |
| Stevenson[12] | 2018 | Prospective quasi-experimental pilot study | Provider perceptions | Recommendation acceptance rate | Two rural VAMCs2 342 total acute-care and long-term care beds | Weekly meetings to discuss cases | N/A | N/A | 259 cases over 78 sessions between both sites | * | 73% |
| Shively[13] | 2020 | Prospective observational, semi-structured interviews | Antimicrobial consumption | ID consultation, cost | Two community hospitals with 461 total beds | PAF, Restriction, guideline development, provider education | Broad spectrum antimicrobial utilization decreased by 24.2% (341.1 vs. 258.7 DOT/1000 PDs – p < 0.001 | ID consultation increased from 15.4 consults per 1000 PDs compared to 21.5 consults per 1000 PDs (p = 0.001). Estimated annualized savings $142,629.83 | 78,339 PDs3 prior to intervention; 37,639 PDs after intervention | *** | 88.9% |
| Beaulac[14] | 2016 | Uninterrupted time series analysis | Antimicrobial consumption | HA-CDI4, antimicrobial consumption by group | 212 bed LTACH5 | Daily audits of EMR6 with PAF via e-mail | Non-significant decrease in absolute consumption 32.8 DDD7/1000 PD (95% CI, − 77.0 to 11.4) (p = 0.14); significant change in slope from before to after the program (− 6.58 DDD/1000 PD per month [95% CI, − 11.48 to − 1.67]; p = 0.01) | Decrease HA-CDI (incidence rate ratio, 0.57 [95% CI, 0.35–0.92]; p = 0.02). Decrease anti-MRSA antibiotic use, decrease CDI therapy, increased antipseudomonal agents | 885 recommendations about 734 patients | ** | 48% |
| dos Santos[15] | 2013 | Prospective observational | Appropriateness of empiric therapy | Appropriateness of empiric pneumonia therapy | 50-bed community hospital in Brazil | Web-based consultation portal for providers | Significant increase in local guideline appropriate empiric therapy. 60% of prescriptions after interventions compared to 40% before. (p < 0.01) | Guideline appropriate empiric pneumonia therapy increased from 41 to 63% after intervention (p = 0.01) | 81 consult requests | ** | 100% |
| Wilson[16] | 2019 | Prospective observational | Antimicrobial consumption | Duration of therapy, Mean ASI8, DOT9 per antibiotic class, ratio of DOT to antibiotic exposure, mean LOS, mortality | Two rural VAMCs 342 total acute care and long-term care beds | Weekly meetings to discuss cases | Significant decrease, in overall antibiotic DOT in acute and long-term care at both sites ranging from 8 to 22% all p < 0.001 | ASI decreased in acute/long-term care at site A (p < 0.001), but unchanged to increase at site B. Only site A acute care had decreased length of therapy (p < 0.001). At site A fluoroquinolone and broad-spectrum beta-lactams usage decreased in acute + LTC. No change in LOS10 or mortality | 259 cases over 78 sites between both sites | ** | not reported |
| Yam[17] | 2012 | Observational | Number of ASP interventions | Rate of narrowing therapy to culture results, agreement between local ASP and remote ID physician, cost, CDI | 141-bed rural community hospital | Develop local ASP with remote ID physician support. PAF, Cascade reporting | N/A | N/A | 311 cases | * | 86–100% |
| Ceradini[18] | 2017 | Observational “before and after” | Incidence of multidrug-resistant Enterobacteriaceae | HAI infections, LOS, cost, satisfaction, consumption | 220-bed pediatric hospital “suburban” | Case discussion | Rate of multidrug-resistant organism isolation decreased from 104/1000 PDs to 79/1000 PDs. (p < 0.01) | No for LOS, HAI. N/A for others | 683 patients to establish baseline: 531 patients post-intervention | ** | not reported |
| Yan[19] | 2020 | Randomized control trial | Antimicrobial prescribing rate | Diagnostic shifting to mask inappropriate prescribing | Physicians practicing primary care via telemedicine. (outpatient) | Education compared to individualized feedback on prescribing patterns | For URI11 and bronchitis, there was a greater decrease in antibiotic prescription in the intervention group compared with the control group (decrease from 15 to 7.8% of prescriptions for URI diagnosis and 64 to 32.1% for bronchitis compared to control groups p < 0.001) | OR of visits with sinusitis or pharyngitis increased in the post-period compared with that in the pre-intervention period (aOR 1.36, 95% CI [1.29, 1.44], p < 0.001). However, a larger diagnostic shift was seen in the control group | 31,473 visits in education arm and 25,519 visits in intervention arm met enrollment criteria | **** | N/A |
| Wood[20] | 2015 | Observational | Antimicrobial consumption | Cost, consumption by class, antimicrobial resistance | 6 community hospitals within one health system -413 total beds | PAF | No significant decrease in total antimicrobial consumption | Quinolone use decreased 57.4% in hospital B (p = 0.001), 65.9% in hospital D (p < 0.001), and 67.3% in hospital E (p < 0.001). Hospitals B, D, and E also had statistically significant decreases in antipseudomonal prescribing Average cost savings of $20,860.25 over 18 months | 12,904 charts reviewed between the 6 sites | ** | 81–95% |
| Morquin[21] | 2018 | Prospective observational | Adherence to recommendations | Provider perceptions of program | 2000-bed academic hospital system | PAF | N/A; 79% of diagnostic and 87% of therapeutic recommendations were accepted | N/A; most approved of the program | 6994 chart reviews for 4173 inpatients | * | 79–87% |
| Howell[22] | 2019 | Prospective observational | Time commitment | Barriers, cost | 110-bed small community hospital | PAF | N/A, 3039 min over 3.5 months. 218 min per week or 3.6 h/week. 41.1% time spent on data analysis, 20.5% reporting, 18.4% preparing for meetings, 14.6% on education, and 5.4% spent on regulatory protocols and policies | N/A, barriers—workflow, communication, consistency. Average cost savings of $17,411.02 for patients with accepted ASP interventions compared to those rejected | 724 alerts on 553 patients | * | 11% |
1PAF, prospective audit and feedback
2VAMC, Veterans Affairs Medical Center
3PD, patient days
4HA-CDI, hospital-acquired Clostridioides difficile infection
5LTACH, long-term acute care hospital
6EMR, electronic medical record
7DDD, defined daily doses
8ASI, antibiotic spectrum index
9DOT, days of therapy
10LOS, length of stay
11URI, upper respiratory tract infection
Eight studies were conducted in the USA, two in Brazil, and two in Europe. Primary study sites were community hospitals, three included long-term acute care hospitals, and one study was in the outpatient primary care setting. One study examined a pediatric inpatient population [18], the remainder focused on adults.
The most common ASP intervention was prospective audit and feedback (PAF) included in 58% (7/12) studies.
The most common primary outcome was antimicrobial consumption reported in 5 of the included studies [11, 13, 14, 16, 20]. Four of the five studies demonstrated a statistically significant decrease in antimicrobial consumption with the implementation of telehealth antimicrobial stewardship [11, 13, 14, 16].
One study demonstrated a statistically significant decrease in antimicrobial prescriptions for upper respiratory tract infections in the outpatient setting [17]. A separate study examined effects on compliance with local guidelines with a statistically significant increase in the rate of adherence [15]. One study demonstrated a significant decrease in isolation of multi-drug resistant organisms [18]. One study found a statistically significant decrease in hospital-acquired Clostridioides difficile infection as a secondary outcome [14].
A single study expressed concern the local private practice ID physician would experience decreased financial compensation from lower consult volume with the implementation of telestewardship and evaluated the effect on ID consultations. They found a 40% increase in consultation following telehealth antimicrobial stewardship implementation [13]. Another study examined the time commitment required to perform telestewardship and found an average of 3.6 h per week of time from a remote ID pharmacy resident [22].
Personnel
Of the examined studies, 83% (10/12) of telehealth antimicrobial stewardship programs consisted of a remote ID physician (one study utilized an internal medicine trained associated medical director [19], and another consisted of pharmacists [22]) and 25% (3/12) included a remote pharmacist [14, 20, 22]. One-third (4/12) of local sites created a local ASP team consisting of local providers, infection prevention nurses, and pharmacists [12, 16, 17, 23]. The other two-thirds only utilized local providers communicating with the remote ASP team.
Studies included between 1 and 4 remote ID physicians, 2 studies employed ID-trained pharmacists [14, 22], with one study included pharmacists with unspecified levels of training [20].
The size of hospitals examined ranged from 50 beds up to 2000. The average number of remote ASP providers to hospital beds was one provider for every 234 beds. The number of full-time-equivalents dedicated to remote antimicrobial stewardship was not routinely reported. The exact ratio of remote ASP providers to hospital bed size is unknown although it should be noted that only three of the eleven (27%) inpatient studies met the goal for traditional ASP programs of 1.0–1.5 FTEs per 100 hospital beds that has been described [24, 25]. CMS proposes a minimum of 0.10 physician FTEs along with 0.25 pharmacy FTEs for a “moderate size hospital” (defined as 124 beds), although acknowledges that 1.0 physician FTEs and 0.5 pharmacies FTEs may be more effective [4••].
On average, 64.6 interventions were performed per remote provider per month for the 9 studies where this could be determined. It should be noted that the reporting of interventions was not standardized among publications.
Equipment
The primary modalities of communication were telephone or videoconferencing along with electronic medical record documentation. Two studies from the same author developed web-based platforms [11, 15], three communicated via secure email [11, 14, 15], and one via secure SMS messaging [15].
Acceptance Rate of Recommendations
There was a wide variance in the provider acceptance rate of remote ASP recommendations ranging from 11 to 100%. The studies with the lowest reported compliance rates lacked face-to-face communication with the local facility—one study relied on e-mail only communication providing a 48% acceptance rate [14]. An 11% acceptance rate was seen when the remote ASP personnel communicated via TheraDoc with a local pharmacist and had no direct communication with frontline providers [22]. The remaining studies that reported on acceptance rates included some form of face-to-face communication with local providers and had acceptance rates ranging from 73 to 100%. Among the three studies that developed a local antimicrobial stewardship team and provided acceptance rates, the average acceptance rate was 86.6%. Among the 5 studies that did not create a local ASP, the average was 66.5%. It is notable that the studies not employing local ASP representatives had significantly more variance of acceptance rates and included the two lowest acceptance rates of 11 and 48%.
Financial Impact
A potential concern regarding telehealth intervention would be decreased financial compensation related to a decrease in local ID consultation. The one study that examined this demonstrated an increase in local consultation. No study reported a financial loss. Three studies reported on annual antimicrobial purchasing cost savings ranging from $13,907 to $142,630. One study reported a $17,411 savings in total hospital charges including, $10,073 in pharmacy charges over 4 months. No study directly addressed the cost of establishing or maintaining a telehealth ASP.
Discussion
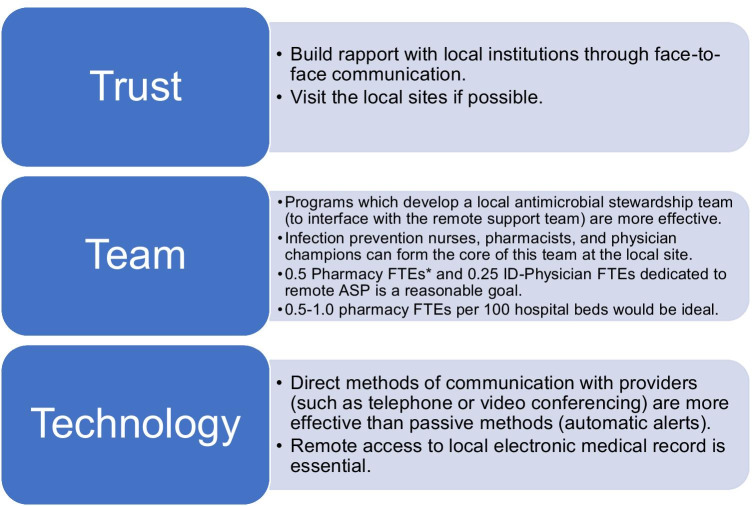
Three primary themes which emerged from the review of the available literature are described in Fig. 2. Establishing relationships and building trust with the local institution are frequently cited as vital to success in the examined articles. Examples included visiting with the local facility on a periodic basis, hosting grand rounds on topics regarding antimicrobial stewardship, or monthly teleconference meetings with local stewardship champions.
Fig. 2.
The three Ts of remote antimicrobial stewardship: trust, team, and technology. *FTE, fulltime equivalent
Another key element in the formation of remote antimicrobial stewardship is the components of the team. Infectious diseases expertise is critical to successful antimicrobial stewardship. Recommending infectious diseases consultation is a common ASP intervention. The one study article that mentioned the presence of a local ID provider prior to the implementation noted an increase in consultation [13], while in the remaining studies the remote ASP served as the primary medium of ID expertise.
According to data from the National Residency Matching Program for 2021, there were 365 applicants for 416 open positions in infectious diseases. Although this was an increase from the year prior, a quarter of programs reported an unfilled position [26]. This suggests an increase in the ID workforce is not on the immediate horizon and novel strategies are needed to meet ID expertise for stewardship staffing requirements. Telestewardship is well positioned to meet these needs by extending available ID expertise. When on-site ID consultation is not available, access to remote ID-trained physicians in conjunction with local antimicrobial stewardship champions would greatly facilitate the work of a telestewardship program.
Although additional data are required to establish with certainly, there is a signal suggesting the formation of a local ASP team is associated with a greater acceptance rate of remote ASP team recommendations. According to the National Antimicrobial Stewardship Task Force, a study of the time required to complete various ASP activities within 12 VAMCs suggested a minimum or 0.25 ID physician full-time equivalents (FTEs) and 0.5 pharmacy FTEs dedicated to an ASP regardless of hospital size with a goal of 1.0 ID-trained pharmacy FTEs per 100 patient beds for a robust ASP. It should be noted that only 21% of their facilities had more than one pharmacy FTE dedicated to ASP despite 8 of the facilities having 200 or more beds [24]. A separate study examining a survey of 244 US institutions found that institutions employing less than 0.5 dedicated FTEs to antimicrobial stewardship were less likely to be able to perform the most effective ASP activities such as prospective audit and feedback. They also demonstrated a 1.5-fold increase in achieving primary outcomes measures such as cost savings, decreased antimicrobial consumption, or decreased multi-drug resistant organisms per each 0.5 pharmacies FTEs dedicated to ASP. They proposed a minimum of 1.0 pharmacy FTEs with 0.5 physician FTEs with roughly 1 additional pharmacy FTE per 500 beds [25]. Although there is no specific guidance for telestewardship, these FTE targets are reasonable goals for a remote ASP.
Certain technological capabilities are required to conduct antimicrobial stewardship via telehealth. According to publicly released statements from the CMS, telehealth encounters have increased by 2600% in 2020 compared to 2019 [27]. This suggests a potential increase in telehealth capabilities developed during the COVID-19 pandemic that could be utilized for telestewardship activities post-pandemic.
Several limitations were noted in the included studies. For the study with the lowest acceptance rate [22], it is conceivable that this may have been related to the reluctance of the local clinical pharmacist to accept recommendations from a pharmacy resident. Robust telestewardship models ideally would include services provided by ID-trained pharmacists and physicians; any learners involved ideally would be precepted by staff clinicians. Although several studies commented on cost savings, the direct cost of establishing the remote antimicrobial stewardship programs was not discussed.
The lack of direct comparison between remote antimicrobial stewardship and traditional antimicrobial stewardship programs is notable and is a key target for future research.
Conclusion
Telehealth is a rapidly expanding field and antimicrobial stewardship programs are well-positioned to take advantage of this growing technology. Data in the existing literature are not robust but suggest facilitators include local antimicrobial stewardship champions as well as access to infectious diseases consultants (either locally or via telehealth services). More research is needed to fully characterize the role of telehealth in antimicrobial stewardship, but existing data suggest this may be a promising mechanism to support hospitals that cannot create robust local programs.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
In one study, “main topic discussed” was inferred to mean primary outcome [18].
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jacob Pierce, Email: Jacob.Pierce@vcuhealth.org.
Michael P. Stevens, Email: Michael.Stevens@vcuhealth.org
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.••CDC. Implementation of antibiotic stewardship core elements at small and critical access hospitals [Internet]. Cent. Dis. Control Prev. Natl. Cent. Emerg. Zoonotic Infect. Dis. 2020 [cited 2020 Nov 9]. Available from: https://www.cdc.gov/antibiotic-use/core-elements/small-critical.html. Guidance on critical components of an antimicrobial stewardship program.
- 2.Walensky RP, McQuillen DP, Shahbazi S, Goodson JD. Where is the ID in COVID-19? Ann Intern Med [Internet]. 2020;173:587–9. Available from: https://www.acpjournals.org/doi/10.7326/M20-2684 [DOI] [PMC free article] [PubMed]
- 3.Chou AF, Graber CJ, Jones M, Zhang Y, Goetz MB, Madaras-Kelly K, et al. Characteristics of antimicrobial stewardship programs at veterans affairs hospitals: results of a nationwide survey. infect control hosp epidemiol. 2016;2016:1–8. [DOI] [PubMed]
- 4.••CMS. Medicare and medicaid programs; regulatory provisions to promote program efficiency, transparency, and burden reduction; fire safety requirements for certain dialysis facilities; hospital and critical access hospital (CAH) changes to promote innovation, flexibility, and improvement in patient care [Internet]. 2019. Available from: https://www.federalregister.gov/documents/2019/09/30/2019-20736/medicare-and-medicaid-programs-regulatory-provisions-to-promote-program-efficiency-transparency-and. Regulatory guidance for ensuring compliance when establishing an antimicrobial stewardship program.
- 5.Rock Health. Q3 2020: a new annual record for digital health (already) [Internet]. 2020 [cited 2020 Nov 11]. Available from: https://rockhealth.com/reports/q3-2020-digital-health-funding-already-sets-a-new-annual-record/
- 6.•Young JD, Abdel-Massih R, Herchline T, McCurdy L, Moyer KJ, Scott JD, et al. Infectious diseases society of America position statement on telehealth and telemedicine as applied to the practice of infectious diseases. Clin Infect Dis. 2019;68:1437–43. IDSA perspective on role for telehealth within infectious disease including antimicrobial stewardship. [DOI] [PubMed]
- 7.Burnham JP, Fritz SA, Yaeger LH, Colditz GA. Telemedicine infectious diseases consultations and clinical outcomes: a systematic review. Open Forum Infect Dis. 2019;6:1–6. doi: 10.1093/ofid/ofz517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parmar P, Mackie D, Varghese S, Cooper C. Use of telemedicine technologies in the management of infectious diseases: a review. Clin Infect Dis. 2015;60:1084–1094. doi: 10.1093/cid/ciu1143. [DOI] [PubMed] [Google Scholar]
- 9.Pottinger PS, Kassamali Z, Wright TC, Scott JD, Martinez-Paz N, Lynch JB. Tele-antimicrobial stewardship in action. Curr Treat Options Infect Dis. Current Treatment Options in Infectious Diseases; 2018;10:229–39.
- 10.Rittmann B, Stevens MP. Clinical decision support systems and their role in antibiotic stewardship: a systematic review. Curr Infect Dis Rep. Current Infectious Disease Reports; 2019;21. [DOI] [PubMed]
- 11.dos Santos RP, Dalmora CH, Lukasewicz SA, Carvalho O, Deutschendorf C, Lima R, et al. Antimicrobial stewardship through telemedicine and its impact on multi-drug resistance. J Telemed Telecare. 2019;25:294–300. doi: 10.1177/1357633X18767702. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson LD, Banks RE, Stryczek KC, Crnich CJ, Ide EM, Wilson BM, et al. A pilot study using telehealth to implement antimicrobial stewardship at two rural veterans affairs medical centers. Infect Control Hosp Epidemiol. 2018;39:1163–1169. doi: 10.1017/ice.2018.197. [DOI] [PubMed] [Google Scholar]
- 13.Shively NR, Moffa MA, Paul KT, Wodusky EJ, Schipani BA, Cuccaro SL, et al. Impact of a telehealth-based antimicrobial stewardship program in a community hospital health system. Clin Infect Dis. 2020;71:539–545. doi: 10.1093/cid/ciz878. [DOI] [PubMed] [Google Scholar]
- 14.Beaulac K, Corcione S, Epstein L, Davidson LE, Doron S. Antimicrobial stewardship in a long-term acute care hospital using offsite electronic medical record audit. Infect Control Hosp Epidemiol. 2016;37:433–439. doi: 10.1017/ice.2015.319. [DOI] [PubMed] [Google Scholar]
- 15.dos Santos RP, Deutschendorf C, Carvalho OF, Timm R, Sparenberg A. Antimicrobial stewardship through telemedicine in a community hospital in southern Brazil. J Telemed Telecare. 2013;19:1–4. doi: 10.1177/1357633X12473901. [DOI] [PubMed] [Google Scholar]
- 16.Wilson BM, Banks RE, Crnich CJ, Ide E, Viau RA, El Chakhtoura NG, et al. Changes in antibiotic use following implementation of a telehealth stewardship pilot program. Infect Control Hosp Epidemiol. 2019;40:810–814. doi: 10.1017/ice.2019.128. [DOI] [PubMed] [Google Scholar]
- 17.Yam P, Fales D, Jemison J, Gillum M, Bernstein M. Implementation of an antimicrobial stewardship program in a rural hospital. Am J Heal Pharm. 2012;69:1142–1148. doi: 10.2146/ajhp110512. [DOI] [PubMed] [Google Scholar]
- 18.Ceradini J, Tozzi AE, D’Argenio P, Bernaschi P, Manuri L, Brusco C, et al. Telemedicine as an effective intervention to improve antibiotic appropriateness prescription and to reduce costs in pediatrics. Ital J Pediatr. Italian Journal of Pediatrics; 2017;43:10–3. [DOI] [PMC free article] [PubMed]
- 19.Du Yan L, Dean K, Park D, Thompson J, Tong I, Liu C, et al. Education vs clinician feedback on antibiotic prescriptions for acute respiratory infections in telemedicine: a randomized controlled trial. J Gen Intern Med. Journal of General Internal Medicine; 2020; [DOI] [PMC free article] [PubMed]
- 20.Wood ZH, Nicolsen NC, Allen N, Cook PP. Remote antimicrobial stewardship in community hospitals. Antibiotics. 2015;4:605–16. [DOI] [PMC free article] [PubMed]
- 21.Morquin D, Ologeanu-Taddei R, Koumar Y, Reynes J. Tele-expertise system based on the use of the electronic patient record to support real-time antimicrobial use. Int J Technol Assess Health Care. 2018;34:156–162. doi: 10.1017/S0266462318000089. [DOI] [PubMed] [Google Scholar]
- 22.Howell CK, Jacob J, Mok S. Remote antimicrobial stewardship: a solution for meeting the joint commission stewardship standard? Hosp Pharm. 2019;54:51–56. doi: 10.1177/0018578718769240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shively NR, Moffa MA, Paul KT, Wodusky EJ, Schipani BA, Cuccaro SL, et al. Impact of a telehealth-based antimicrobial stewardship program in a community hospital health system. Clin Infect Dis. 2019;1–7. doi:10.1093/cid/ciz878 [DOI] [PubMed]
- 24.Echevarria K, Groppi J, Kelly AA, Morreale AP, Neuhauser MM, Roselle GA. Development and application of an objective staffing calculator for antimicrobial stewardship programs in the Veterans Health Administration. Am J Heal Pharm. 2017;74:1785–1790. doi: 10.2146/ajhp160825. [DOI] [PubMed] [Google Scholar]
- 25.Doernberg SB, Abbo LM, Burdette SD, Fishman NO, Goodman EL, Kravitz GR, et al. Essential resources and strategies for antibiotic stewardship programs in the acute care setting. Clin Infect Dis. 2018;67:1168–1174. doi: 10.1093/cid/ciy255. [DOI] [PubMed] [Google Scholar]
- 26.NRMP. NRMP Results and data specialties matching service, 2020 appointment year [Internet]. 2020. Available from: https://mk0nrmp3oyqui6wqfm.kinstacdn.com/wp-content/uploads/2020/02/Results-and-Data-SMS-2020.pdf
- 27.CMS. Trump administration drives telehealth services in medicaid and medicare [Internet]. 2020 [cited 2020 Nov 9]. Available from: https://www.cms.gov/newsroom/press-releases/trump-administration-drives-telehealth-services-medicaid-and-medicare
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.