Abstract
Objectives
To evaluate a method of quantitative X-ray (QXR) for obtaining bone health information from standard radiographs aimed at identifying early signs of osteoporosis to enable improved referral and treatment. This QXR measurement is performed by postexposure analysis of standard radiographs, meaning bone health data can be acquired opportunistically, alongside routine imaging.
Design
The relationship between QXR and dual energy X-ray absorptiometry (DEXA) was demonstrated with a phantom study. A prospective clinical study was conducted to establish areal bone mineral density (aBMD) prediction model and a risk prediction model of a non-normal DEXA outcome. This was then extrapolated to a larger patient group with DEXA referral data.
Setting
Secondary care National Health Service Hospital.
Participants
126 consenting adult patients from a DEXA clinic.
Interventions
All participants underwent a DEXA scan to determine BMD at the lumbar spine (L2–L4) and both hips. An additional Antero-Posterior pelvis X-ray on a Siemens Ysio, fixed digital radiograph system was performed for the study.
Outcome
Performance of QXR as a risk predictor for non-normal (osteoporotic) BMD.
Results
Interim clinical study data from 78 patients confirmed a receiver operator curve (area under the ROC curve) of 0.893 (95% CI 0.843 to 0.942) for a risk prediction model of non-normal DEXA outcome. Extrapolation of these results to a larger patient group of 11 029 patients indicated a positive predictive value of 0.98 (sensitivity of 0.8) for a population of patients referred to DEXA under current clinical referral criteria.
Conclusions
This study confirms that the novel QXR method provides accurate prediction of a DEXA outcome.
Trial registration number
ISRCTN98160454; Pre-results.
Keywords: rheumatology, radiology & imaging, orthopaedic & trauma surgery, geriatric medicine, preventive medicine
Strengths and limitations of the study.
No modifications were made to the X-ray equipment, demonstrating that this quantitative X-ray (QXR) technique is compatible with current clinical workflow.
Patients consented to having a pelvis X-ray on the same day following their dual energy X-ray absorptiometry (DEXA) scan, which means that the DEXA and QXR results are directly comparable.
The radiographs were taken at as low as 15% of standard exposure due to constraints imposed by the automatic exposure control. Acquisitions therefore do not match a standard diagnostic examination. This increases random noise, implying that the reported results represent a lower bound to performance.
Exclusions were higher than expected due to issues with data collection. This was due to the natural learning curve for the new technique and are expected to reduce significantly in the second part of the study.
The study cohort only included patients preselected for a DEXA scan so were already considered at higher risk of osteoporosis. Therefore, the efficacy of QXR along with clinical risk factors as predictor of DEXA could not be assessed in the general population.
Introduction
With an ageing global population and associated increase in the risk of osteoporotic fractures, there are imminent challenges to healthcare systems and the wider economy from what has been described as the approaching fracture tsunami.1 Approximately 34% of women and 17% of men are affected by osteoporosis worldwide.2 In Europe, 3.5 million new osteoporotic fragility fractures occur annually, the most common of which are in the hip (610 000 fractures), distal forearm (560 000 fractures) and spine (520 000 fractures).3 Previous and incident fractures also accounted for 1 180 000 quality-adjusted life years lost within Europe during 2010 and the costs of treating fragility fractures are expected to increase by 25% by 2025.4
In the UK, hip fractures alone account for £1 billion of healthcare expenditure5 and have a mortality rate of between 15% and 36% in the first year post fracture.6 Once osteoporosis is diagnosed however, fracture risk can be substantially mitigated through lifestyle changes and use of pharmacological therapies.7 Pharmacological prevention is relatively inexpensive and made up just 5% of the €37 billion spent on fracture care in Europe during 2013,3 with the remaining 95% spent on treatment and long-term care.
There is a clear case for improving the early detection and treatment of osteoporosis, and wider access to screening. To be economically viable, any screening must be targeted towards those at greatest risk of osteoporosis and ideally work alongside existing clinical pathways to avoid additional risks and costs. Improved diagnosis and primary or secondary prevention through opportunistic use of the standard digital radiograph (DR) image provides the best opportunity to mitigate against the ballooning costs of fragility fractures.
We present here the interim results of a clinical study of a novel method for osteoporosis prescreening; X-ray (QXR). We show in this paper that this software-based method produces a high positive predictive value (PPV) of a non-normal dual-energy X-ray absorptiometry (DEXA) outcome for patients referred to DEXA under the current clinical referral criteria (CRC). The measurement is performed by postexposure analysis of the standard plain radiograph. This means that bone health data can be acquired opportunistically, alongside routine fracture and non-fracture imaging, with no increase to patient dose and negligible additional point of care costs for the healthcare provider.
Technical background
The QXR technique uses a novel approach to extract composition and thickness information from a standard plain radiograph (X-ray image). This information is not usually accessible from a standard DR image and typically requires a multienergy approach like DEXA.
The approach taken within the QXR algorithm is to simulate the X-ray transport through a volume of hard and soft tissue which approximates the real-world body part. The volume is then iteratively refined until a simulated radiograph can be produced which matches that observed from the real scan, to within some acceptable margin of error. By measuring the amount of bone which is present in the model, it is possible to infer the bone content within the real-world radiograph.
The advantage of the technique is that images can be acquired at the standard imaging protocol, with no changes to the X-ray hardware. This opens up the possibility to perform the measurement opportunistically, from the same scan which is taken for standard diagnostic purposes. The QXR software typical processes at lower than native image resolution for computational efficiency. In this study, a digital down sampling of the image was performed where 23×23 standard pixels were summed together to create a single super-pixel.
Assessing bone density is not conventionally achievable using a standard single energy exposure. This is because a single intensity value is not unique for a given object. The QXR approach overcomes this problem. In the presence of scatter, there exists only one anatomically plausible solution for soft and hard tissue distribution, where agreement can be found between the physics model and the ground truth. To return such information by other means typically requires some additional hardware complexity, such as quantitative CT (QCT) which is able to directly measure bone density,8 or DEXA which uses energy information from the X-ray beam to derive an areal bone mineral density (aBMD) measure.
The QXR approach returns data that is analogous to QCT, but from a single scan at a single kilovoltage peak (kVp). The standard outputs from the model are as follows: total thickness (cm), hard tissue fraction, direct beam fraction, and scatter fraction. From this, the bone thickness can be derived, where we define average bone as a compound of hydroxyapatite (HA).
From a practical implementation standpoint, the software requires the input of the raw radiograph, prior to any non-linear processing and the X-ray exposure setting. A onetime fingerprint of the X-ray system is carried out which connects the physics model to the real-world system. Calibration intervals are dependent on the stability of the X-ray hardware but based on this study are likely to be monthly or longer. The software can be provided as an offline application, taking data from a picture archiving and communications system, or installed directly onto the X-ray hardware.
Materials and methods
Study design
A 126-patient prospective clinical study was conducted, where QXR results were compared with DEXA, which is the most common clinical method of assessing osteoporosis.9 The intended sample size of 130 was calculated assuming a dropout rate of 3% and a significance level of 1%, providing a 90% power to detect equivalency between Trueview and the reference standard. Further details can found in the published protocol.10
Potential study participants were identified from appointment lists for the DEXA clinic at James Cook University Hospital between November 2019 and March 2020 and were found eligible if they met the criteria listed in table 1. Those eligible were approached to seek informed consent for participation in the study. Owing to the method of recruitment, participants formed a random series and a higher prevalence of osteoporosis and osteopenia was expected to be observed in this group than the prevalence in the general population.
Table 1.
Eligibility criteria for study participation
| Inclusion criteria | Exclusion criteria |
| 1. Caucasian male or female, at least 50 years of age. Attending for a DEXA scan of Neck of Femur (for measurement of bone mineral density); | 1. Women who are pregnant or are breastfeeding |
| 2. Patient able to comprehend and sign the Informed Consent prior to enrolment in the study | 2. Concurrent participation in another experimental intervention or drug study |
| 3. Has an implant or other radio-opaque foreign body in the location of the assessment | |
| 4. Unwilling or unable to provide informed consent |
DEXA, dual-energy X-ray absorptiometry.
All participants underwent a DEXA scan to determine BMD at the lumbar spine (L2–L4) and both hips (femoral neck and total hip sites). All BMD measurements were performed on the same DEXA scanner throughout (GE Lunar Prodigy Advanced, V.13.6). To reduce the impact of operator bias, the same independent radiographer carried out all DEXA measurements; and stability and accuracy were monitored daily using a manufacturer-supplied phantom. All quality assurance checks were well within the manufacturer’s tolerances throughout the study. T-scores were derived using the manufacturer’s reference ranges at the femoral neck. In light of the recent recommendations to use femoral neck BMD to calculate fracture risk and determine T-scores, T-scores at the hip sites were further developed using female reference databases from the Third National Health And Nutrition Examination Survey.11 For women, osteoporosis was defined as per WHO definition12 as a T-score≤−2.5 SD below the mean for a young person. For men and women, osteopenia was defined as being between >−2.5 and <−1.0 SD and normal being ≥−1. Z-scores were also determined.
Participants were then sent for an additional AP pelvis X-ray on a Siemens Ysio, fixed DR system on the same day. This X-ray was only used for the purposes of the study and had no bearing on the diagnosis or treatment of the patient. No adverse events were recorded for the index or reference tests. The only change to the clinical protocol was the removal of the anti-scatter grid (ASG). This resulted in a shortened exposure, due to automatic exposure control triggering at around 15% of the standard clinical dose. Due to trial restrictions, a manual exposure was not permitted and as such, the results presented here represent the lower bound to performance. Additionally, errors due to patient positioning and unknowns around precise operating characteristics of the X-ray system all led to suboptimal starting data. Integration with the acquisition station itself would allow much greater control to be exercised over the data capture stage.
Extractions
We report here interim results from 78 patients (after extractions) recruited to the study. Acquiring quantitative data from a DR system puts certain additional demands on patient positioning and hardware set up. Calibration measurements are required in order to relate the physics model to the X-ray hardware and X- ray protocol. Due to COVID-19 restrictions, calibrations could only be obtained for the following beam energies: 75, 79 and 81 kVp and a source to imager distance of 104.9 cm. Eighteen subjects were excluded as they were not taken at a calibrated kVp and a further 16 subjects were excluded as the source to imager distance was not 104.9 cm.
In addition, 1 subject was extracted as the ASG was not removed. Two images were extracted as the DEXA Region Of Interest was drawn incorrectly. Two were extracted as the DEXA scan had an abnormally large difference between the left and right femoral necks indicating a positioning error. Two were excluded for poor patient positioning. Seven patients were excluded as the algorithm failed to converge. Investigation of this non-convergence showed that (1) excessive abdominal fat obscured the femoral neck in four of these patients and (2) three images showed abnormally high grey level values relative to the recorded dose, suggesting a data error. After extractions for data collection errors, 94% of the remaining data were usable.
This interim analysis has helped us identify the data collection errors that account for the majority of the extractions. For subsequent conduct of the study, errors in data collection are expected to be reduced substantially by integrating the software with the image acquisition software and through improved user training.
The radiographs were exported in linear dose response form and anonymised of all patient data. Each image was assigned a key which was used to link the DR scan to the equivalent anonymised DEXA report. Images were then processed offline and the following outputs returned: total thickness, hard tissue fraction, bone thickness and direct beam intensity. The radiograph on the top of the figure is shown in raw, linear form and was acquired at an estimated 15% of the standard clinical dose.
An ROI was manually defined on the radiograph by an operator who matched to the ROI placement in the accompanying DEXA image. An example is shown in figure 1. This ROI was then applied to the HA-equivalent bone thickness map to extract a femoral neck mean HA-equivalent bone thickness. This method was applied to both the left and right femoral necks of all 78 patients, resulting in 156 measurements. Of the 156 femoral neck measurements, 87 had osteopenia, 11 had osteoporosis and 58 were normal as defined by the DEXA reference standard. In all cases, the DEXA result was not known until the index test result had been computed.
Figure 1.
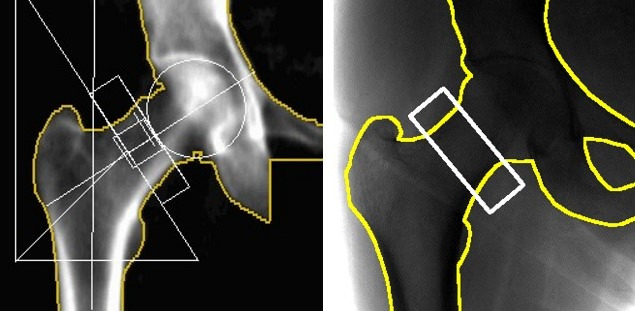
(Left) Dual-energy X-ray absorptiometry image with the standard hip geometrical markings overlaid (white) and the bone-soft tissue boundary (yellow). (Right) Digital radiograph scan with as close as achievable match to hip placement. The ROI (white) shows the region used for femoral neck bone health analysis.
Univariate analysis was first performed to assess the usefulness of the various QXR outputs (total thickness, hard tissue fraction, bone thickness and direct beam intensity) as univariate predictors of a non-normal (osteopenia or osteoporosis) DEXA outcome.
For continuous variables, the mean and SD were calculated for the normal and non-normal groups. A p value was also calculated under the null hypothesis that the population means of both groups are equal. For gender, the percentage of women is reported. A p value was also calculated, under the null hypothesis that the proportion of women in both groups are equal. To assess risk prediction performance, a logistic regression model was used,13 with both the variable being assessed and gender included as predictors. Gender was added so that the difference in thresholds for man and woman is considered in the model. The receiver operating characteristic (ROC) curve was calculated and the area under the ROC curve (AUC) is reported along with the associated 95% CI using de Long’s method.14 Following the univariate analysis of the various QXR outputs, an assessment was performed of the usefulness of QXR in two potential use cases.
Scenario 1: performance of QXR as a predictor of aBMD
Multivariate regression analysis was performed, where all outputs from the QXR method—total thickness (cm), hard tissue fraction, direct beam fraction and scatter fraction were analysed together to predict aBMD. This is a more demanding application of the data than a normal/non-normal classifier.
Forward-backward stepwise model selection with the Akaike information criterion was used to select the models presented.15 The base model had linear and quadratic terms for every variable. We report the resultant model, the value of the parameters, their SE and the p value (under the null hypothesis that the parameter associated with that variable is zero). To assess overfitting, the R-squared value was bootstrapped with 5000 samples. The bias and SD are reported.
Scenario 2: performance of QXR as a risk predictor of an osteoporosis diagnosis from DEXA
Multivariate risk prediction analysis (using multivariate logistic regression) was performed, where all variables were analysed together to assess the performance of QXR as a risk predictor for non-normal (osteoporotic) BMD. Again forward–backward stepwise model selection with the Akaike information criterion for model selection was used. We report the resultant model, the value of the parameters, their SE and the p value (under the null hypothesis that the log-odds for that variable is one). To assess the quality of the risk prediction model, ROC analysis was performed. The ROC plot and the AUC with associated 95% CI are reported. To assess overfitting, the AUC value was bootstrapped with 5000 samples. The bias and SD are reported.
The performance of the risk predictor was also extrapolated to a wider population using Bayes’ theorem. The prior probabilities were extracted from DEXA data provided by the Freeman Hospital, Newcastle. Meta data relating to age, gender and DEXA outcome were provided for 11 029 patients and the PPV was assessed under various scenarios.
The statistical analysis for the clinical study was carried out using the software package, R.16
Patient and public involvement
This study was designed based on initial input from patients and the public to understand willingness to participate and to establish understanding of aims and proposed conduct of the study. This was done using a patient focus group prior to securing funding for the study. Patients have not been involved in the conduct of the study, other than as participants. The results of the study will be made available in open access journals and publicised more widely through press channels. Patient advisors were approached during the early design stages of the study when we were applying for National Institute for Health Research funding before H2020.
Results
Table 2 contains the results of univariate analysis of the QXR outputs as predictors of DEXA normal/non-normal outcome. This shows a significant difference between the normal and non-normal groups for all continuous variables. The largest difference appears to be bone thickness, which is supported by the phantom data in the previous section and the AUC value of 0.810. This is as expected, as bone thickness is the closest QXR analogue to aBMD.
Table 2.
Results of univariate analysis of the quantitative X-ray outputs as predictors of dual-energy X-ray absorptiometry normal/non-normal outcome
| Mean normal (SD) | Mean non-normal (SD) | P value | AUC (CI) | |
| Bone thickness | 1.75 (0.403) | 2.25 (0.503) | <0.001 | 0.810 (0.741 to 0.878) |
| Intensity | 111.9 (28.3) | 87.7 (26.5) | <0.001 | 0.753 (0.673 to 0.833) |
| Thickness | 18.3 (2.15) | 19.8 (2.40) | <0.001 | 0.674 (0.587 to 0.761) |
| Alloy | 0.0969 (0.0231) | 0.115 (0.0275) | <0.001 | 0.689 (0.602 to 0.775) |
| Gender | 78.6% female | 77.6% female | 1 | 0.505 (0.437 to 0.573) |
| aBMD | 0.770 (0.0738) | 1.00 (0.120) | <0.001 | 1 (1 to 1) |
aBMD, areal bone mineral density; AUC, area under the ROC curve.
The fact that all QXR outputs show a significant difference between the normal and non-normal populations, with AUC significantly larger than 0.5, justifies their inclusion in the subsequent multivariate analysis. Gender was justified as it directly impacts the thresholds for the classification.
Scenario 1: performance of QXR as a predictor of aBMD
Table 3 contains the analysis for the selected model for aBMD prediction. Intensity was removed by forward–backward model selection as including intensity did not result in an improved model by the Akaike information criterion. The p values for the other variables do not indicate that their inclusion is unjustified. The R2 value of 0.662 (0.643 adjusted) shows that a clinically relevant amount of variation is being resolved by this model. The bootstrapping showed a bias of 0.0114 and an SE of 0.516.
Table 3.
Analysis of the significance of the various quantitative X-ray outputs as predictors of areal bone mineral density for the multivariate model selected using forward–backward stepwise model selection
| Estimate | SE | t-value | P value | |
| (Intercept) | 7.39 | 1.72 | 4.30 | <0.001 |
| Gender | 0.0481 | 0.02 | 2.40 | 0.0175 |
| Bone thickness | 2.63 | 0.913 | 2.88 | 0.0046 |
| Thickness | −0.566 | 0.132 | −4.28 | <0.001 |
| Hard tissue fraction | −46.1 | 17.5 | −2.63 | 0.0095 |
| Bone thickness2 | −0.329 | 0.111 | −2.96 | 0.0036 |
| Intensity2 | 0.00 | 0.00 | −5.57 | <0.001 |
| Thickness2 | 0.011 | 0.0024 | 4.61 | <0.001 |
| Hard tissue fraction2 | 117.5 | 40.9 | 2.87 | 0.0047 |
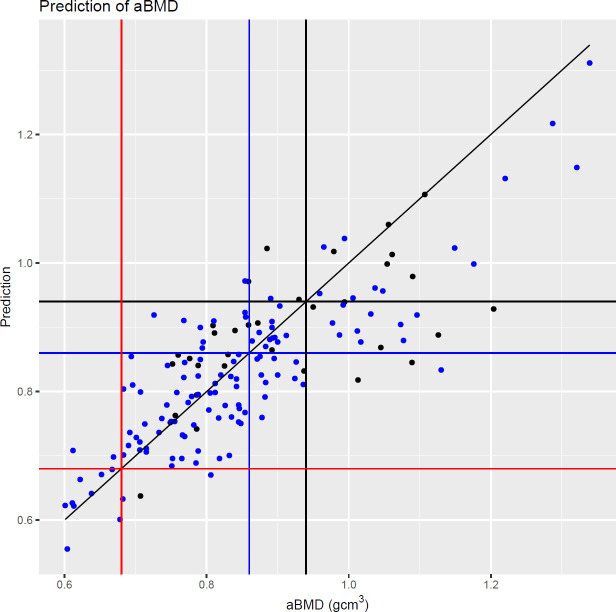
Figure 2 shows the fitted model plotted against DEXA aBMD. The vertical and horizontal lines show the male and female classification thresholds as defined by DEXA. The model predicts 81.8% (9/11) of osteoporotic femoral necks correctly. For an operating point of sensitivity=0.8 and specificity=0.81, QXR identified 67 femoral necks as being normal and 89 as non-normal.
Figure 2.
Fitted values from the model presented in table 3 against areal bone mineral density (aBMD). Black points: male patients. Blue points: female patients. Black vertical and horizontal lines: male normal bone density threshold. Blue vertical and horizontal lines: female normal bone density threshold. Red vertical and horizontal lines: female low bone density threshold (osteoporosis). Diagonal black line: y = x.
Scenario 2: performance of QXR as a risk predictor of an osteoporosis diagnosis from DEXA
Table 4 contains the analysis for the selected model for non-normal bone health prediction.
Table 4.
Analysis of the significance of the various quantitative X-ray outputs as a risk prediction model of a non-normal dual-energy X-ray absorptiometry outcome for the multivariate model selected using forward-backward stepwise model selection
| Estimate | SE | t-value | P value | |
| (Intercept) | −167.7 | 102.7 | −1.63 | 0.103 |
| Gender | −2.76 | 0.746 | −3.70 | <0.001 |
| Bone thickness | −86.7 | 54.8 | −1.58 | 0.114 |
| Intensity | 0.0876 | 0.0252 | 3.48 | <0.001 |
| Thickness | 13.8 | 7.60 | 1.82 | 0.0689 |
| Hard tissue fraction | 1494.3 | 1071.4 | 1.39 | 0.163 |
| Bone thickness2 | 11.2 | 6.47 | 1.73 | 0.0836 |
| Thickness2 | −0.246 | 0.124 | −1.99 | 0.0471 |
| Hard tissue fraction2 | −3686.6 | 2491.1 | −1.48 | 0.139 |
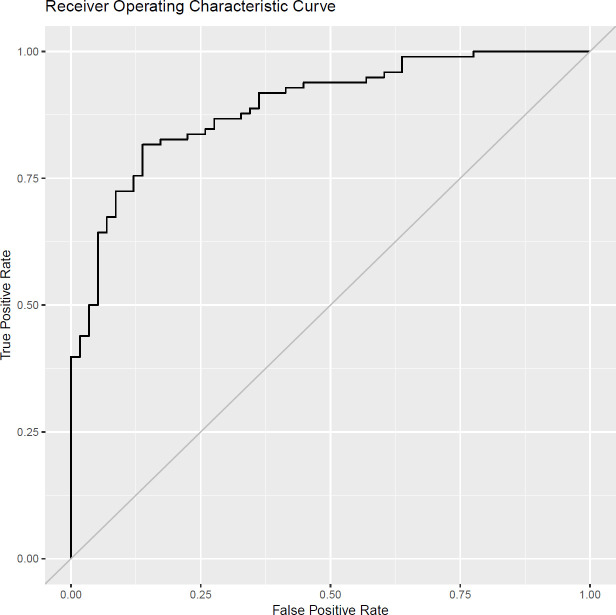
Figure 3 shows the ROC curve which is clearly far from the y=x line, highlighting that the model has value. This is further supported by the AUC, which is significantly larger than 0.5 (AUC 0.893 with 95% CI 0.843 to 0.942). The bootstrapping of AUC gave a bias of 0.013 with an SE of 0.025.
Figure 3.
Receiver operating characteristic curve for risk predictor model of non-normal dual-energy X-ray absorptiometry outcome.
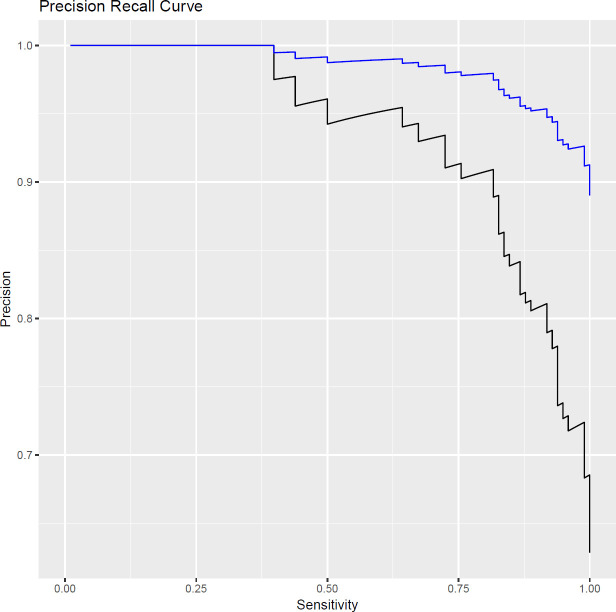
A precision recall curve is plotted in figure 4 for this model, based on the 78 patients (156 femoral necks) within the clinical study and extrapolated to a larger group of 11 029 patients, where the model performance was mapped onto the DEXA outcomes of this larger population.
Figure 4.
Precision recall curve for the risk of a non-normal dual-energy X-ray absorptiometry (DEXA) outcome, as determined by the quantitative X-ray multivariate risk model. The black line shows data from the 78-patient clinical study and the blue line shows the predicted performance on a wider population based on DEXA outcome reports.
The PPV of a non-normal DEXA outcome is 0.82, given that a patient has received a non-normal QXR outcome. Extrapolating to a wider 11 029 patient group and taking a sensitivity of 0.8 as an example operating point, the PPV is 0.98. The lower PPV for the clinical study is due to this cohort of patients being truncated more towards the normal/non-normal boundary. In the larger population, the PPV of QXR is higher than that for either age or gender when used to predict a non-normal DEXA outcome. This further highlights the potential for this technique in the wider population. The PPVs for various patient demographics are summarised in table 5
Table 5.
Positive predictive value (PPV) of a non-normal dual-energy X-ray absorptiometry outcome, based on various patient demographics, including a prior non-normal quantitative X-ray (QXR) scan for the Newcastle population
| Patient demographic | PPV |
| Referred by current CRC | 0.88 |
| CRC+female | 0.91 |
| CRC+over 65 | 0.94 |
| CRC+patient has non-normal QXR result | 0.98 |
CRC, clinical referral criteria.
Discussion
The ability of QXR to provide an accurate prediction of the likely outcome of a DEXA scan provides enormous potential for prescreening and early intervention at the point of first fracture and before. This offers clinicians powerful decision support, supplementing the current CRC.
The technique is compatible with a standard radiograph acquired for diagnostic purposes and can therefore be deployed as an opportunistic tool for measuring bone health from the same diagnostic radiograph. As a result, we expected the clinical scenario where this technique can most appropriately be deployed is within radiology departments, where the QXR result will be reported out alongside the diagnostic image, most likely as an onscreen prompt and a ‘red dotting’ on the radiograph itself. This can then be included in the radiologist’s reporting.
All QXR outputs were found to be useful inputs to a multivariate DEXA outcome predictor, with bone thickness being the most significant, with an AUC value of 0.810. This is expected, as bone thickness is the closest QXR analogue to aBMD. Two scenarios for use of QXR were tested. The first was as a predictor of DEXA aBMD and the second method was as an indicator of the risk of obtaining a non-normal DEXA result. Results suggest that QXR may provide an effective proxy for DEXA in situations where DEXA access is limited, with concordant identification of osteoporosis in 81.8% (9/11) of cases. In the second scenario, QXR was found to have high true positive and low false positive rates when used to predict a non-normal DEXA outcome, with an AUC of 0.893 (95% CI 0.843 to 0.942).
A useful point of comparison is quantitative ultrasound (QUS). With a reported sensitivity of 0.93 and a specificity of 0.84,17 QUS can also be used to provide DEXA prescreening capability. However, it cannot be provided at an economically viable cost point.17 Furthermore, uncertainties remain around the reproducibility,18 interpretation of QUS measurements19 and the heterogeneity of QUS scanners.20 In contrast, the QXR method requires no additional hardware and can be integrated into current radiographer workflow, making it possible to deliver a net benefit from a healthcare provider cost perspective.
QCT offers an alternative method of bone health measurement and has been shown to be effective as an opportunistic screening tool.21 QXR provides a complementary method for DR and is likely to benefit from significantly higher throughput of patients. QXR also avoids the primary disadvantages of QCT: slow,22 expensive,23 and most importantly, high dose radiation8 (1–3 mSv for QCT compared with 0.0001 mSv for DEXA),24 meaning that there are advantages with opportunistic DR-based bone health screening, using low radiosensitive sites such as the ultradistal radius, or the calcaneus.
The study was limited by the low permitted patient dose and as this is a new technique, there was a natural learning curve for the radiographers, resulting in suboptimal image capture and higher than anticipated exclusions. One further limitation of this study is that the patients were all preselected for DEXA prior to receiving a QXR test. As a result, we cannot conclude the performance of this technique on the wider population without preselection. The authors aim to extend this work to assess performance on forearm fractures within the general population and the utility of QXR as part of a fracture risk prediction model.
Sensitivity analysis has highlighted the importance of consistent ROI selection between the DEXA and QXR scans, with errors of up to 10% observed. This is the one area where we may expect to see reduced accuracy in the subsequent within-trial analysis if adequate operator training is not provided. This is related to practical use, rather than fundamental accuracy, so does not influence the conclusions of this study and can be addressed longer term in a similar way to DEXA, with a combination of automation and operator training. In a clinical application, ROI selection would be semiautomated to reduce ROI selection bias.
Being an X-ray technique, QXR is also expected to suffer from similar inherent limitations as DEXA, such as being affected by obesity, degenerative changes in bone morphology, joint space narrowing, calcifications and osteophytes. These factors were excluded from the study but should be investigated as part of an extended study.
In practice, fracture risk prediction with QXR should be used alongside other clinical risk factors as part of a complete risk prediction model, for example, as part of FRAX25 or QFracture.26 This can then be used to improve the accuracy of the 10-year fracture risk prediction for patients, where DEXA aBMD data are not available, and thus improve CRC.
Research agenda
QXR is likely to lead to improved detection of the early onset of osteoporosis. We anticipate the resulting impact to be a reduction in the socioeconomic cost of long-term fracture care, by bringing forward clinical intervention by up to 15 years, but rigorous health economic modelling is required to estimate the benefits of a QXR system to healthcare providers. The next stage is to begin feasibility studies with the QXR software integrated onto a DR system. If successful, this method has a valuable role to play as an early decision support tool for clinicians where DEXA is available; and as an accessible alternative where access to DEXA is limited.
Additionally, the aim is to expand the accuracy of the risk model by adding further predictors of bone health, such as volumetric BMD, trabecular bone score and finite element stress analysis modelling. These will all be used, along with the current QXR outputs to generate a more accurate 10-year fracture prediction. We also aim to expand the general utility of the technique by extending compatibility out to radiographs of the lumbar spine, wrist and calcaneus.
Conclusions
This study demonstrates that use of a novel QXR method provides accurate and early prediction of the likely DEXA outcome for a patient. Integrated into the current DR workflow, QXR can provide opportunistic screening and decision support for clinicians, at greater accuracy and on a much larger scale than is achievable with current methods. This may be used to support current CRC to improve the early detection of osteoporosis.
Acknowledgments
The authors would like to acknowledge and thank the following contributors to the study. The European Union Horizon 2020 programme for the funding to conduct the clinical study, without which this work would not have been possible. Siemens Healthineers for their ongoing support in the clinical study (funding not applicable). Our patient advisors Elizabeth Robson and Adrian Chadwick for their valuable input to the study design. Natalie Clark, Marc Atkinson and other staff at James Cook University Hospital, and patients for their participation in the clinical study.
Footnotes
Contributors: AR was Chief Investigator for the clinical study and drafted the manuscript along with PDS and BL. SPT conducted and analysed the DEXA scans. LK managed the clinical study in the hospital. MJ advised on processes in radiology. BL, TW, BC, TW and ARatcliffe advised on study design, analysis and interpretation of data. All authors reviewed and approved the manuscript. AR is the guarantor.
Funding: This work was funded by Horizon 2020 (grant number 777835).
Competing interests: The coauthors on this paper from IBEX Innovations hold the intellectual property rights over the QXR technology and have a vested interest in the adoption of this technology into clinical practice. The lead author (AR) holds grants from NIHR, ORUK and DePuy J&J Ltd unrelated to this study.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The study data are available upon reasonable request from Factory CRO or via the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was conducted in line with prevailing regulations and written informed consent to use anonymised data was obtained from all participants. This study was designed and conducted as per the Helsinki II Declaration and was approved by the Health Research Authority (HRA) and Health and Care Research Wales (HCRW) (ISRCTN98160454; Protocol number: IBX/SP1701; IRAS project ID: 255317; North-East Tyne & Wear South REC reference: 18/NE/0368).
References
- 1.Harvey N, McCloskey EV. Gaps and solutions in bone health: a global framework for improvement. International Osteoporosis Foundation (IOF), 2016. [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726–33. 10.1007/s00198-006-0172-4 [DOI] [PubMed] [Google Scholar]
- 3.Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos 2013;8:136. 10.1007/s11657-013-0136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavone V, Testa G, Giardina SMC, et al. Pharmacological therapy of osteoporosis: a systematic current review of literature. Front Pharmacol 2017;8:803. 10.3389/fphar.2017.00803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunning T, Dickinson R, Fagan E. National hip fracture database (NHFD) annual report 2018. London, UK: Royal College of Physicians, 2017. [Google Scholar]
- 6.Morri M, Ambrosi E, Chiari P, et al. One-Year mortality after hip fracture surgery and prognostic factors: a prospective cohort study. Sci Rep 2019;9:1–7. 10.1038/s41598-019-55196-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanley DA, McClung MR, Davison KS, et al. Western osteoporosis alliance clinical practice series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med 2017;130:862.e1–862.e7. 10.1016/j.amjmed.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Brett AD, Brown JK. Quantitative computed tomography and opportunistic bone density screening by dual use of computed tomography scans. J Orthop Translat 2015;3:178–84. 10.1016/j.jot.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson J, Bonner TJ, Head M, et al. Variation in bone mineral density by anatomical site in patients with proximal humeral fractures. J Bone Joint Surg Br 2009;91:772–5. 10.1302/0301-620X.91B6.22346 [DOI] [PubMed] [Google Scholar]
- 10.Ibex Trueview® study v1.0, SRCTN98160454 2021.
- 11.Schousboe JT, Shepherd JA, Bilezikian JP, et al. Executive summary of the 2013 International Society for clinical densitometry position development conference on bone densitometry. J Clin Densitom 2013;16:455–66. 10.1016/j.jocd.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a who Study Group [meeting held in Rome from 22 to 25 June 1992]. World Health Organization, 1994. [PubMed] [Google Scholar]
- 13.Glonek GFV, McCullagh P. Multivariate logistic models. J R Stat Soc Series B Stat Methodol 1995;57:533–46. 10.1111/j.2517-6161.1995.tb02046.x [DOI] [Google Scholar]
- 14.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med 2013;4:627–35. [PMC free article] [PubMed] [Google Scholar]
- 15.Bendel RB, Afifi AA. Comparison of stopping rules in forward “stepwise” regression. J Am Stat Assoc 1977;72:46–53. [Google Scholar]
- 16.R Core Team . R: a language and environment for statistical computing. Vienna, Austria, 2018. Available: https://www.R-project.org/
- 17.Sim MF, Stone M, Johansen A, et al. Cost effectiveness analysis of BMD referral for DXA using ultrasound as a selective pre-screen in a group of women with low trauma Colles' fractures. Technol Health Care 2000;8:277–84. 10.3233/THC-2000-8503 [DOI] [PubMed] [Google Scholar]
- 18.Krieg M-A, Barkmann R, Gonnelli S, et al. Quantitative ultrasound in the management of osteoporosis: the 2007 ISCD official positions. J Clin Densitom 2008;11:163–87. 10.1016/j.jocd.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 19.Baroncelli GI. Quantitative ultrasound methods to assess bone mineral status in children: technical characteristics, performance, and clinical application. Pediatr Res 2008;63:220–8. 10.1203/PDR.0b013e318163a286 [DOI] [PubMed] [Google Scholar]
- 20.Knapp KM, Blake GM, Spector TD, et al. Can the WHO definition of osteoporosis be applied to multi-site axial transmission quantitative ultrasound? Osteoporos Int 2004;15:367–74. 10.1007/s00198-003-1555-4 [DOI] [PubMed] [Google Scholar]
- 21.Dagan N, Elnekave E, Barda N, et al. Automated opportunistic osteoporotic fracture risk assessment using computed tomography scans to aid in FRAX underutilization. Nat Med 2020;26:77–82. 10.1038/s41591-019-0720-z [DOI] [PubMed] [Google Scholar]
- 22.Stagi S, Cavalli L, Cavalli T, et al. Peripheral quantitative computed tomography (pQCT) for the assessment of bone strength in most of bone affecting conditions in developmental age: a review. Ital J Pediatr 2016;42:88. 10.1186/s13052-016-0297-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Löffler MT, Jacob A, Valentinitsch A, et al. Improved prediction of incident vertebral fractures using opportunistic QCT compared to DXA. Eur Radiol 2019;29:4980–9. 10.1007/s00330-019-06018-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damilakis J, Adams JE, Guglielmi G, et al. Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol 2010;20:2707–14. 10.1007/s00330-010-1845-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanis JA, Oden A, Johansson H, et al. FRAX and its applications to clinical practice. Bone 2009;44:734–43. 10.1016/j.bone.2009.01.373 [DOI] [PubMed] [Google Scholar]
- 26.Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. BMJ 2012;344:e3427. 10.1136/bmj.e3427 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. The study data are available upon reasonable request from Factory CRO or via the corresponding author.