Abstract
We present pathology of the peripheral nerves of a patient with Adult-onset Charcot-Marie-Tooth disease 4F caused by periaxin gene mutation p.D651N. The patient was a 72-year-old woman. She had hoarseness and underwent continuous positive airway pressure therapy at night due to sleep apnea. The patient died abruptly. Remarkable demyelination with tomacula formation was found in the phrenic nerve, vagal nerve, recurrent laryngeal nerve, and oculomotor nerves. The cause of death could have been insufficient reactivity to the aspiration or sudden onset of bilateral vocal cord palsy. We must pay attention to respiratory function and cranial nerve palsies in hereditary demyelinating neuropathies.
Keywords: Charcot-Marie-Tooth disease 4F, Periaxin, Vocal cord paresis, Recurrent laryngeal nerve, Phrenic nerve, Oculomotor nerve
Highlights
-
•
Patients with hereditary demyelinating neuropathy could die suddenly.
-
•
Demyelination of the phrenic and recurrent laryngeal nerves might be relevant.
-
•
Pathology of these nerves has been rarely reported.
1. Introduction
Weakness and sensory disturbance of the limbs are well recognized in Charcot-Marie-Tooth disease (CMT). However, its complications affecting the respiratory function such as vocal cord paralysis are not so emphasized despite that impairment of these nerves is sometimes life-threatening. One reason for that is that electrophysiological assessment and histological examination of cranial nerves is difficult or impossible until death. Here, we describe a case of a female elderly patient with CMT4F accompanied by periaxin gene mutation who died suddenly. The pathological findings of the peripheral nerves including the oculomotor, recurrent laryngeal, vagal, and phrenic nerves are discussed.
2. Case report
The patient was a 72-year-old woman. She was transferred from a nursing home to our hospital due to cardiopulmonary arrest. Approximately 2 h after she took a lunch, she was discovered unconscious. A small amount of food was observed in the trachea, indicating that she vomited, but the airway was not obstructed. Intracranial bleeding and rupture of the aorta were ruled out after computed tomography. Consolidation was found in her lower lung fields. Despite cardiopulmonary resuscitation, she died.
Her clinical symptoms before death had been described previously [1]. Briefly, she was the third child of healthy non-consanguineous parents. She had contracted infantile paralysis at the age of 18 months. She noticed a distal lower limbs weakness and sensory impairment at the age of 30. She was diagnosed as having Charcot-Marie-Tooth disease when she was 40 years old. At that time, she underwent a sural nerve biopsy. Although remarkable fiber loss of myelinated and unmyelinated nerve fibers was observed, typical onion bulb formation was not found. She came to our hospital at the age of 65. She was intellectually normal. She suffered from hoarseness with vocal cord paralysis. A wheelchair was needed to move. Although she underwent continuous positive airway pressure therapy at night due to sleep apnea, she never complained of dyspnea, dysarthria, or dysphagia during daytime. There was no medical history of aspiration pneumonia or diplopia. No compound muscle action potential or sensory nerve action potential was derived in nerve conduction study of the median, ulnar, tibial, and sural nerves. Electromyography and neuroimaging study for the peripheral nerve or nerve roots were not done. Under informed consent, we performed genetic analysis. It revealed homozygous mis-sense mutation in periaxin gene (p.D651N, CMT4F).
Autopsy was performed according to her living will 44 h after her death. Macroscopically, congestion of the lungs, liver, kidney, and spleen was found. Coronary arteries were patent. The brain weight was 1290 g. The spinal roots were enlarged remarkably. In the peripheral nervous system, the oculomotor nerve adjacent to the midbrain, the phrenic and vagal nerves at the thoracic level, and the recurrent laryngeal nerve were dissected out with other somatic nerves including the anterior and posterior spinal nerve roots and the sciatic nerve. After fixation with 2.5% glutaraldehyde and 2% paraformaldehyde, osmication, and embedding in epoxy resin, these tissues were stained in toluidine blue. Teased fiber technique was also performed.
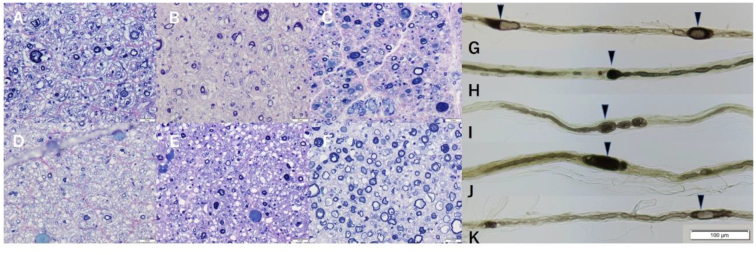
In all nerves, myelinated nerve fiber loss and thinning of the myelin were remarkable (Fig. 1A-F). Onion-bulb formation was observed in the sciatic, phrenic, vagal nerves, and the anterior and posterior spinal roots. Teasing of the myelinated fibers was technically difficult, but the remnant fibers showed demyelination pattern (Fig. 1G-K). In some nerves, tomacula formation (focal myelin thickening) was observed (Fig. 1G-K arrowheads), which were compatible with the pathological report of patients having periaxin mutations [2]. These nerves showed no cellular infiltration or thickening of the perineurium in these nerves.
Fig. 1.
Toluidine blue staining of the anterior spinal nerve root (A), the posterior spinal nerve root (B), the recurrent laryngeal nerve (C), the vagal nerve (D), the phrenic nerve (E), and the oculomotor nerve (F). Bars in the bottom right corners represent 20 μm. Teased fibers of the anterior spinal nerve root (G), the posterior spinal nerve root (H), the recurrent pharyngeal nerve (I), the vagal nerve (J), and the phrenic nerve (K). The bar in the bottom represents 100 μm. Tomacula formation (focal myelin thickening) is observed in these nerves (arrowhead). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Generalized neuronal loss was not found in the anterior horn of the spinal cord, the dorsal root ganglion, sympathetic ganglion, or oculomotor nucleus. Grouped atrophy was observed in the diaphragm muscle on HE stains [3].
3. Discussion
The likely cause of her death included asphyxia by food aspiration or acute respiratory failure by the bilateral recurrent laryngeal nerve palsy. Although she had no dyspnea in daytime, there was evidence of aspiration pneumonia. The strength of cough might have been insufficient due to the latent demyelination of the phrenic nerve. Decreased pharyngeal reflex might have been associated with her aspiration.
Periaxin is a protein expressed by Schwann cells. Homozygous mutations of this gene cause demyelinating polyneuropathy. Nonsense or frameshift mutation of this gene results in early onset CMT4F or Dejerine-Sottas neuropathy [2]. The pathological evidence of phrenic nerve involvement in Dejerine-Sottas disease has been reported [4]. Electrophysiological report on the phrenic nerve has been also published in other type CMT [5]. Vocal cord paresis due to dysfunction of the recurrent laryngeal nerve has been reported in CMT2 with mitofusin-2 mutation, CMT2C with transient receptor potential cation channel subfamily V member 4 mutation, CMT4A with ganglioside-induced differentiation-associated protein 1 mutation, and CMT4B1 with myotubularin-related protein 2 mutation [6]. Although the recurrent laryngeal nerves would undergo pathological change in CMT as well as the nerves innervating limbs, there has been no pathological evidence of this nerve to the best of our knowledge. Dysfunction of these nerves causes hoarseness or dyspnea.
Oculomotor nerve palsy has been reported in a patient with CMT1A [7]. It is difficult to evaluate oculomotor nerves electrophysiologically, and pathological changes of oculomotor nerves in CMT is rarely reported. Demyelination of the oculomotor nerve in our patient was obvious compared to those of patients without CMT [8].
4. Conclusion
In conclusion, we must pay attention to respiratory function and cranial nerve palsies in hereditary demyelinating neuropathies.
Grant support
This report received no specific grant from any funding agency in public, commercial, or not-for-profit sector.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgement
The authors would like to thank Editage (www.editage.jp) for the English language review.
References
- 1.Tokunaga S., Hashiguchi A., Yoshimura A., Maeda K., Suzuki T., Haruki H., Nakamura T., Okamoto Y., Takashima H. Late-onset Charcot-Marie-Tooth disease 4F caused by periaxin gene mutation. Neurogenetics. 2012;13:359–365. doi: 10.1007/s10048-012-0338-5. [DOI] [PubMed] [Google Scholar]
- 2.Takashima H., Boerkoel C.F., De Jonghe P., Ceuterick C., Martin J.J., Voit T., Schröder J.M., Williams A., Brophy P.J., Timmerman V., Lupski J.R. Periaxin mutations cause a broad spectrum of demyelinating neuropathies. Ann. Neurol. 2002;51:709–715. doi: 10.1002/ann.10213. [DOI] [PubMed] [Google Scholar]
- 3.Shintaku M., Maeda K., Shiohara M., Namura T., Kushima R. Neuropathology of the spinal nerve roots, spinal cord, and brain in the first autopsied case of Charcot-Marie-Tooth disease 4F with a mutation (D651N) of the periaxin gene. Neuropathology. 2021 doi: 10.1111/neup.12731. (in press) [DOI] [PubMed] [Google Scholar]
- 4.Felice K.J., Fratkin J.D., Feldman E.L., Sima A.A. Phrenic nerve involvement in Dejerine-Sottas disease: a clinicopathological case study. Pediatr. Pathol. 1994;14:905–911. doi: 10.3109/15513819409037686. [DOI] [PubMed] [Google Scholar]
- 5.Sagliocco L., Orlandi G., Calabrese R., Pellengrinetti A., Baglini O., Castelli F., Baldinotti F., Sartucci F. Electrodiagnostic evidence of phrenic nerve demyelination in Charcot-Marie-Tooth disease 1A. Am. J. Phys. Med. Rehabil. 2003;82:754–759. doi: 10.1097/01.PHM.0000087453.94529.0D. [DOI] [PubMed] [Google Scholar]
- 6.Zambon A.A., Sora M.G.N., Cantarella G., Cerri F., Quattrini A., Comi G., Previtali S.C., Bolino A. Vocal cord paralysis in Charcot-Marie-Tooth type 4b1 disease associated with a novel mutation in the myotubularin-related protein 2 gene: a case report and review of the literature. Neuromuscul. Disord. 2017;27:487–491. doi: 10.1016/j.nmd.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posa A., Emmer A., Kornhuber M.E. Unilateral oculomotor palsy in Charcot-Marie-Tooth disease 1A (CMT1A) Clin. Neurol. Neurosurg. 2017;155:20–21. doi: 10.1016/j.clineuro.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Maeda K., Yasuda H. Histological background of susceptibility of oculomotor nerve to ischemia. J. Neurol. Disord. Stroke. 2014;3:1092. [Google Scholar]