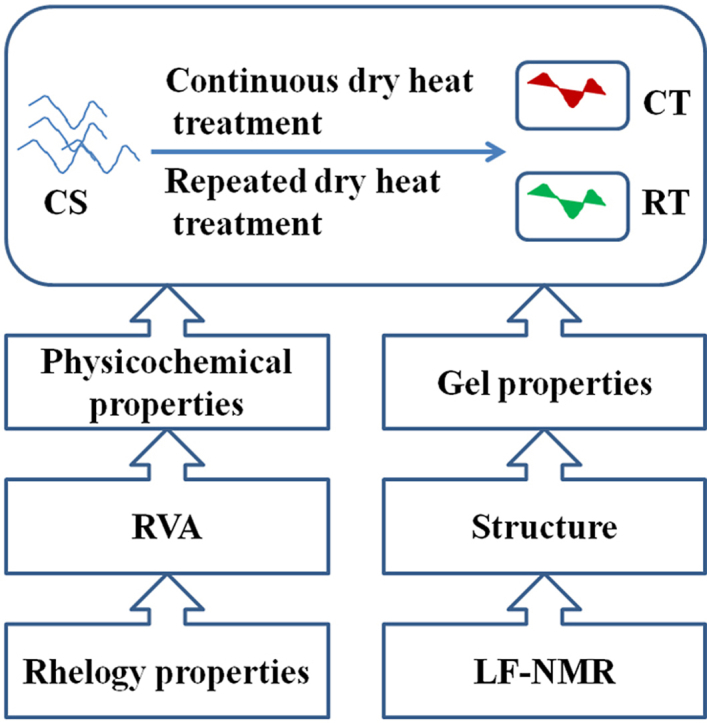
Abstract
The effects of continuous dry heat treatment (CT) and repeated dry heat treatment (RT) on gel and structural properties of chestnut starch (CS) were investigated. CT and RT both reduced the swelling degree of starch and showed significant variations in pasting viscosity, viscoelasticity, gel strength and hardness varying from high to low after dry heat treatment, and CT was lower than that of RT. Neither dry heat treatment nor gelatinization produced new functional groups, and both reduced short-range ordered degree. There were significant decrease in spin-spin relaxation time (T2) with dry heat treatment (CT and RT), which made the starch in the samples closely combine with water. These results are helpful to better understand the changes of physicochemical properties of starch gel products during dry heat treatment and provide some theoretical references for the application of CS in food industry.
Keywords: Continuous dry heat treatment, Repeated dry heat treatment, Chestnut starch
Graphical abstract
Highlights
-
•
The solubility of treated starch increased, while the swelling degree decreased.
-
•
Continuous and repeated dry heat treatment reduced the pasting viscosity of CS.
-
•
The short-range ordered degree was also reduced with dry heat treatment.
-
•
CT can change the physicochemical properties of starch more effectively than RT.
1. Introduction
Chestnut is rich in nutrition and unique in flavor which has attracted wide attention of many researchers (Zhu, 2016). As the highest content component in chestnut, chestnut starch (CS) is gluten-free and can be used as energy material for celiac disease (Mir et al., 2019). Starch is the main energy source of human diet, and its rapid digestion can induce many diseases, such as obesity, diabetes and cardiovascular diseases, so new slow digestion starch resources are being gradually developed to meet market demand. CS might serve as a novel starch source with high resistance content and applied in both food and non-food industries, which were beneficial to prevent the incidence of type-II diabetes (Cruz et al., 2013).
Native starch with the thermal instability and easy retrogradation and other undesirable characteristics during processing, starch modification has been widely concerned. During the traditional processing methods, such as baking or boiling, starch would undergo irreversible changes, and its crystal structure would be lost (Silva et al., 2016). Therefore, other starch modification methods to improve the gelatinization and structural characteristics of CS were warranted.
Compared with other modification methods, dry heat treatment has the advantages of simplicity, safety and no chemical residue (Sun et al., 2014). Dry heat treatment is continuously treat samples (moisture content less than 10%) at 140 °C for a certain time (Gou et al., 2019). Dry heat treatment usually include two types, continuous dry heat treatment (CT) and repeated dry heat treatment (RT). Sample treated by CT was that the sample was continuously heated for a period of time, while the RT meant heating for a period of time, then cooling for a period of time, which was regarded as a cycle, and then the operation of this cycle was repeated. Zhou et al. (2021) also found CT and RT had a significant effect on the pasting properties of quinoa starch, but they only focused on the properties of native starch and the effect of dry heat treatment on CS gel and structure properties has not been studied previously.
The study of starch gel properties after dry heat treatment, such as rheological properties, gel strength and hardness, can provide some theoretical reference for whether dry heat treatment starch is beneficial to the formation of gel products. And it is also interesting to understand the migration changes of internal water molecules when starch is modified. Therefore, the purpose of the present study are 1) to explore the effects of CT and RT on the pasting, gel and structural properties of CS and 2) to compare the differences between CT and RT, which will also help us to understand the characteristics of CS, thus providing some theoretical basis for the development of CS and expand the application range of CS.
2. Materials and methods
2.1. Materials and reagents
Chestnuts were obtained from Tianhong supermarket (Jiangxi, China). The starch content kit (A148-1-1) was bought from Jiancheng Bioengineering Co. (Nanjing, China). Other chemicals were all analytical grade.
2.2. Sample preparation
CS was extracted by water extraction method, and was slightly modified according to the method of Liu et al. (2021). The total starch content was 98.09% by the means of content kit. The apparent amylose content in CS was 16.23%.
Dry heat treated samples were prepared using the method of Gou et al. (2019). Native CS (water content less than 10%) was continuously heat treated in an oven at 140 °C for 3, 6, 9 and 12 h and then cooled at room temperature to obtain the CT-3, CT-6, CT-9 and CT-12 starch respectively.
Repeated treatment of starch was obtained following the steps: firstly, native CS were treated at 140 °C for 3 h, and then cooled for 1 h, which was recorded as one cycle, then obtain a sample namely RT-1, which was CT-3. Then, this cycle was repeated twice, three and four times, and then marked them as RT-2, RT-3 and RT-4, respectively. Native CS without dry heat treatment was recorded as control.
2.3. Determination of solubility and swelling power
The result of solubility and swelling power were determined with modified method (Gou et al., 2019, Li et al., 2021). 30 mL of starch suspension (2%, w/v) were shaken in a water-bath at 50 °C, 60 °C, 70 °C, 80 °C, 90 °C and heated at 120 rpm for 30 min with continuous stirring. Then cooled to room temperature and centrifuged at 4000 g for 15 min. The supernatant was then dried at 105 °C for 2 h and weighed. The value of solubility (S) and swelling power (SP) were calculated according to the following formula:
| S (%) = (weight of dried supernatant/weight of dry starch) × 100 |
| SP (g/g) = weight of sediment/ [weight of dry starch × (100−S)] |
2.4. Pasting properties
The pasting properties of starch were determined with a Rapid-Visco Analyzer (RVA, Newport Scientific, NSW, Australia) following the method of Chen et al. (2018). Dry starch (7%, w/v) and 25 mL distilled water were mixed evenly in an aluminum tank with continuous stirring at 960 rpm, the slurry was balanced at 50 °C for 1 min and heated to 95 °C at a speed of 13 °C min−1 for 3 min. Then cooled to 50 °C at the same speed, stirred at 160 rpm for 4 min, and the starch pastes were collected for later use.
2.5. Rheological properties
The rheometer (DHR-2, American Technical Consulting Company) equipped with a plate (diameter 40 mm, gap 0.5 mm), and dynamic rheological scanning was carried out in the angular frequency range of 1–10 rad s−1 based on the method of Li et al. (2020).
2.6. Gel strength and hardness
A texture analyzer (TA-XTplus, Stable Co., UK; 0.5R probe) was used for measurement. Test speed including before, during and after testing was 2 mm s−1, test distance is 10 mm, trigger force was 5 gf, and trigger type was set as automatic.
2.7. Fourier-transform infrared spectra (FT-IR)
The samples obtained from RVA were frozen in a freeze dryer at −80 °C for 3 d to obtain freeze-dried samples. The freeze-dried samples of control, CT-3, CT-6, CT-9, CT-12, RT-2, RT-3 and RT-4 were respectively designated as D-Control,da1 DCT-3, DCT-6, DCT-9, DCT-12, DRT-2, DRT-3 and DRT-4.
Samples were scanned based on the following procedures: freeze-dried samples and KBr powder in a 1:30 ratio in the mortar full grinding, pressing machine would grind the powder into a transparent path of a thin sheet and be used to determine. FT-IR (Nicolet 5700, Thermo, USA) had a scanning wavelength of 4000–400 cm−1, with a scanning resolution of 8 cm−1 over 64 scans. The spectra of CT and RT samples (freeze-dried and ungelatinized starch) were obtained, and following previously described procedures (Correia, Cruz-Lopes, & Beirão-da-Costa, 2012; Li et al., 2021; Lyu et al., 2021).
2.8. Low-field nuclear magnetic resonance (LF-NMR)
A 23 MHz NMR analyzer (EDUUMR20-015V-I, Niumag Co., Ltd., Suzhou, China) was used to obtain the T2 according to the previous method (Ma et al., 2019). Transfer 1 mL of gelatinized sample prepared by RVA to NMR glass tube, and shake thoroughly to eliminate bubbles. Set CPMG sequence for determination.
2.9. Statistical analysis
SPSS 21.0 software (IBM software, Chicago, Illinois, USA) and Origin 8.0 software (Stat-Ease Company, Minnesota, USA) were used to evaluate the significance and obtain the graph.
3. Results and discussion
3.1. Solubility and swelling power
Solubility and swelling power were used to reflect the amylose leaching degree and water absorption capacity during starch swelling. The solubility of native starch was increased by CT and RT (Table 1A), which indicated that dry heat treatment promoted the leaching of amylose. And with the increasing of temperature, the solubility of starch also increased, which was consistent with the previous research result that dry heat treatment increased the solubility of starch from Dioscorea (Vashisht et al., 2017). It was also found that the solubility was usually related to amylose, while the swelling power was related to amylopectin and also reported that this increase in solubility caused by the leaching of amylose during dry heat treatment (Gou et al., 2019). In addition, the solubility of CT was greater than that of RT, which might be related to the differences in water absorption capacities and swelling power.
Table 1A.
Solubility results of native chestnut starch and dry heat treated starch.
| Samples | Solubility (%) |
||||
|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | |
| Control | 0.15 ± 0.03a | 0.26 ± 0.02a | 1.01 ± 0.05a | 5.61 ± 0.02a | 7.79 ± 0.09a |
| CT-3 | 0.23 ± 0.02b | 0.28 ± 0.00a | 1.06 ± 0.03b | 8.02 ± 0.09b | 11.45 ± 0.42b |
| CT-6 | 0.43 ± 0.02d | 0.79 ± 0.02d | 1.56 ± 0.02d | 13.06 ± 0.19d | 17.72 ± 0.11c |
| CT-9 | 0.66 ± 0.03f | 0.94 ± 0.03e | 1.92 ± 0.03f | 18.41 ± 0.53f | 23.75 ± 0.51f |
| CT-12 | 1.01 ± 0.04h | 1.26 ± 0.02f | 2.81 ± 0.02h | 21.59 ± 0.58g | 27.66 ± 0.30g |
| RT-2 | 0.32 ± 0.02c | 0.62 ± 0.01b | 1.26 ± 0.03c | 10.52 ± 0.32c | 19.37 ± 0.33d |
| RT-3 | 0.50 ± 0.02e | 0.72 ± 0.02c | 1.73 ± 0.02e | 13.38 ± 0.45d | 21.49 ± 0.37e |
| RT-4 | 0.75 ± 0.05g | 0.92 ± 0.01e | 2.17 ± 0.06g | 16.30 ± 0.37e | 23.69 ± 0.16f |
Note: CS, native chestnut starch; CT-3, CT-6, CT-9, CT-12, preparation of chestnut starch by continuous dry heat treatment for 3 h, 6 h, 9 h, 12 h, respectively; RT-2, RT-3, RT-4, preparation of chestnut starch by repeated dry heat treatment for two cycles, three cycles, four cycles, respectively. Results are showed as mean values ± SD in triplicate. Different letters (a-g) in the same column show a significant difference (p < 0.05).
Both CT and RT decreased the swelling power of CS (Table 1B), which indicated that dry heat treatment could inhibit the swelling power of starch granules. Especially, CT-12 (50 °C) reached the lowest swelling power value of all samples. This might also be due to the aggregation of starch granules during the heat treatment of starch. However, with the increasing of temperature, the swelling power increased slightly, which was attributed to the starch reaching gelatinization temperature during heat treatment, which caused partial gelatinization.
Table 1B.
Swelling power of native chestnut starch and dry heat treated starch.
| Samples | Swelling power (g/100g) |
||||
|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | |
| Control | 1.95 ± 0.04e | 2.22 ± 0.01f | 3.85 ± 0.08f | 12.84 ± 0.13f | 22.59 ± 0.22g |
| CT-3 | 1.68 ± 0.05d | 2.08 ± 0.05e | 3.55 ± 0.09e | 10.96 ± 0.12e | 18.63 ± 0.39f |
| CT-6 | 1.55 ± 0.02c | 1.84 ± 0.10cd | 3.15 ± 0.03cd | 8.81 ± 0.13d | 15.80 ± 0.14d |
| CT-9 | 1.42 ± 0.02b | 1.62 ± 0.02b | 2.94 ± 0.05ab | 7.93 ± 0.15b | 12.69 ± 0.20b |
| CT-12 | 1.27 ± 0.02a | 1.46 ± 0.04a | 2.84 ± 0.04a | 6.67 ± 0.15a | 10.76 ± 0.20a |
| RT-2 | 1.69 ± 0.00d | 1.93 ± 0.01d | 3.22 ± 0.02d | 9.11 ± 0.08d | 15.60 ± 0.29e |
| RT-3 | 1.50 ± 0.01c | 1.83 ± 0.03c | 3.05 ± 0.03c | 8.35 ± 0.29c | 13.57 ± 0.34c |
| RT-4 | 1.34 ± 0.03a | 1.61 ± 0.01b | 2.96 ± 0.02b | 7.61 ± 0.19b | 11.28 ± 0.34a |
Note: CS, native chestnut starch; CT-3, CT-6, CT-9, CT-12, preparation of chestnut starch by continuous dry heat treatment for 3 h, 6 h, 9 h, 12 h, respectively; RT-2, RT-3, RT-4, preparation of chestnut starch by repeated dry heat treatment for two cycles, three cycles, four cycles, respectively. Results are showed as mean values ± SD in triplicate. Different letters (a-g) in the same column show a significant difference at p < 0.05.
3.2. Pasting properties
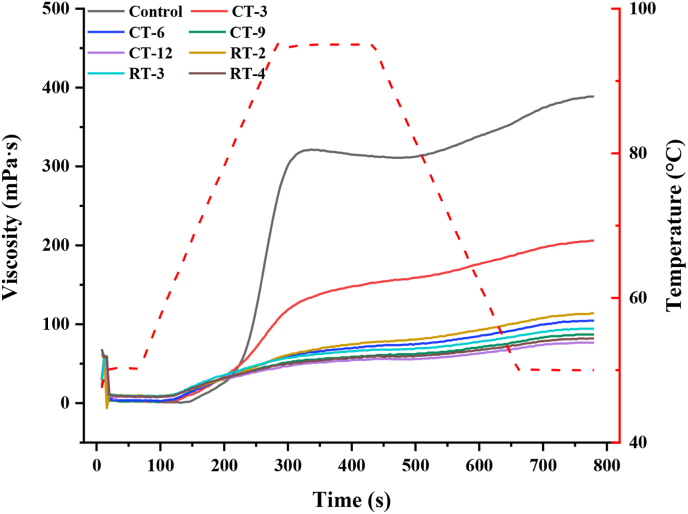
Compared with the control group, the gelatinization viscosity (PV, TV, FV, SB, BD) of CS decreased after dry heat treatment (CT and RT), and all parameters were negatively correlated with CT time or RT cycle except for BD (Fig. 1; Table 2). Especially, CT-12 reached the lowest viscosity value of all samples, and attributed the result of PV reduction to the expansion of starch granules limited by dry heat treatment (Sun et al., 2013). PV represents the swelling power of starch or its ability to combine with free water (Nawab et al., 2016). This reduction of PV induced by dry heat treatment was confirmed by the decrease of swelling power. FV was generally related to the ability of starch paste to form sticky paste during cooling, which was largely related to the leaching of amylose (Luo et al., 2020). The reduction in SB indicated that dry heat treatment made CS gel stable and not easy to deteriorate. TV was the lowest viscosity value during the whole gelatinization process, while BD, as the difference between PV and TV, was used to evaluate the short-term stability of the starch paste. The BD of CS decreased with CT and RT, showing that the treated starch was more resistant to shearing and high temperature. Compared with RT, the viscosity parameter change of CT treated starch was lower, and the reducing trend was more significant. Similar trend had been demonstrated that CT could reduce the PV, FV, TV, and SB of sweet potato starch and this might be due to the partial structural change of the starch particles during the RT, so a small amount of energy was required to form the paste, thus resulting in higher viscosity (Gou et al., 2019).
Fig. 1.
Viscosity changes of CT and RT samples.
Table 2.
The pasting and gel properties parameters of native chestnut starch and dry heat treated starch.
| Samples | PV (mPa·s) | TV (mPa·s) | BD (mPa·s) | FV (mPa·s) | SB (mPa·s) | Gel Strength (N) | Hardness (N) |
|---|---|---|---|---|---|---|---|
| Control | 321.33 ± 8.58e | 311.00 ± 7.35e | 10.33 ± 1.25d | 388.33 ± 11.8e | 77.33 ± 4.50e | 0.37 ± 0.02d | 0.47 ± 0.02f |
| CT-3 | 150.33 ± 2.05d | 141.33 ± 2.05d | 9.00 ± 0.00d | 206.00 ± 3.27d | 64.33 ± 1.25d | 0.19 ± 0.02c | 0.36 ± 0.02e |
| CT-6 | 76.33 ± 2.87c | 71.00 ± 3.27c | 5.67 ± 0.47c | 114.00 ± 4.08c | 43.00 ± 0.82c | 0.11 ± 0.01b | 0.17 ± 0.00c |
| CT-9 | 61.00 ± 0.82ab | 57.33 ± 0.47ab | 3.33 ± 0.47ab | 87.33 ± 0.47ab | 30.00 ± 0.00b | 0.07 ± 0.00a | 0.11 ± 0.00a |
| CT-12 | 55.00 ± 5.72a | 52.67 ± 5.25a | 2.33 ± 0.47a | 76.67 ± 7.59a | 24.00 ± 2.45a | 0.06 ± 0.00a | 0.10 ± 0.00a |
| RT-2 | 81.00 ± 0.82c | 74.67 ± 1.25c | 4.67 ± 0.47bc | 121.67 ± 0.94c | 45.67 ± 0.94c | 0.12 ± 0.01b | 0.20 ± 0.01d |
| RT-3 | 67.00 ± 0.00b | 63.33 ± 0.47b | 3.67 ± 0.47ab | 94.33 ± 1.70b | 31.00 ± 2.16b | 0.08 ± 0.00a | 0.14 ± 0.02b |
| RT-4 | 59.00 ± 2.45ab | 55.00 ± 1.63a | 4.00 ± 0.82b | 82.00 ± 4.90ab | 27.00 ± 3.27ab | 0.06 ± 0.01a | 0.10 ± 0.00a |
Note: CS, native chestnut starch; CT-3, CT-6, CT-9, CT-12, preparation of chestnut starch by continuous dry heat treatment for 3 h, 6 h, 9 h, 12 h, respectively; RT-2, RT-3, RT-4, preparation of chestnut starch by repeated dry heat treatment for two cycles, three cycles, four cycles, respectively. Results are showed as mean values ± SD in triplicate. The different letter showed the significant difference (p < 0.05). PV is peak viscosity; TV represents trough viscosity; FV is final viscosity; BD is breakdown viscosity; SB is setback viscosity.
3.3. Dynamic rheological measurements
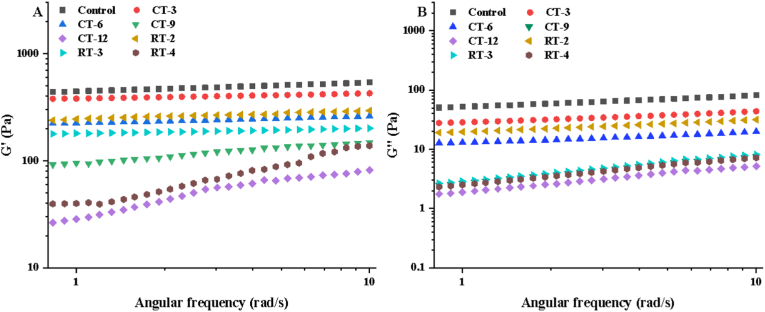
The dynamic rheological test showed the same increasing trend of storage modulus (G′) and loss modulus (G″) with angular frequency (See Fig. 2), and the G′ of all gels was larger than G″, which signified that all gels were typical weak gels (Lin et al., 2021). Meanwhile, CT and RT reduced the G′ and G″ of CS gel, and which was negatively correlated with the treatment time and treatment cycle. The decrease of G′ might be due to the following reasons: first, dry heat treatment would reduce the effective water content of starch, and reduced the effective concentration of starch in unit volume, thereby affecting the formation of gel network structure; second, the dry heat treatment would affect the swelling and water absorption performance of starch granules, thus resulting in the difference of gel viscoelasticity of starch granules (Liu et al., 2020). Additionally, G′ was related to the extra connection of starch molecular chains and the enhancement of connection tightness, which showed that dry heat treatment would weaken the entanglement points between starch gel molecules, and led to the decrease of G' (Tabarsa et al., 2017).
Fig. 2.
(A) Storage modulus (G′) and (B) loss modulus (G″) curves of CT and RT gels.
3.4. Gel properties
Dry heat treatment decreased the gel strength and hardness of CS gel, and the effect of CT was more obvious (Table 2). Compared with RT, the gel strength and gel hardness of CT treated samples were relatively small, the main difference was the existence of cooling cycle would influence the thermal degradation of starch hence influence the gel strength and hardness (Colussi et al., 2014). Especially, when the dry heat treatment with 12 h, the gel strength decreased from 0.37 N to 0.06 N and the gel hardness decreased significantly from 0.47 N to 0.10 N. This might be attributed that dry heat treatment would prevent starch retrogradation, which could be explained by the decrease of SB. The changes in gel strength and hardness after CT and RT could be due to dry heat treatment destroyed partially ordered structure of the starches thus leading to changes in the amorphous region (Luo et al., 2020). Amylose was easier to diffuse and gelatinize under dry heat treatment (CT and RT), resulted in lower gelatinization conditions than the native starch, thus leading to lower gel strength and hardness.
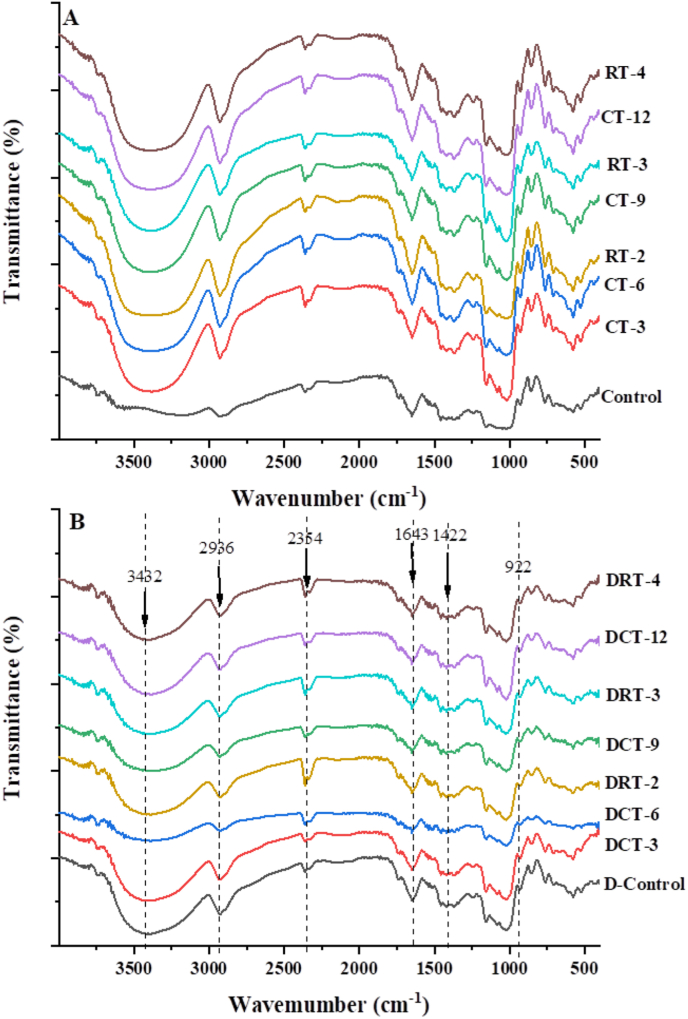
3.5. FT-IR
Compared with the control group, both CT and RT showed the similar FT-IR patterns, and there were no new absorption peaks appeared (Fig. 3A and B), which indicated that CT and RT did not cause changes in functional groups and did not destroy the original functional groups of native CS. Similar results had been found that dry heat treatment and gelatinization did not cause the generation of new chemical bonds (Zhou et al., 2021). The absorption peak at 1643 cm−1 was attributed to the O–H stretching vibration of starch amorphous region (Wang et al., 2018). It also be noticed that there was a broad peak at 3250-3500 cm−1 and at 3432 cm−1, which was mainly related to the stretching vibration of free, intramolecular and intermolecular free hydroxyl groups. The hydrophilicity of starch was the reason to explain the existence of a small absorption peak near the wavelength of 922 cm−1 (Jiang et al., 2011). The peak of 1422 cm−1 was associated with angular twisting of C–H (Jan et al., 2017).
Fig. 3.
The FTIR spectrum of ungelatinized (A) and gelatinized gel samples (B) of CT and RT.
This fact was found that the value of R1047/1022 after dry heat treatment or gelatinization was lower than that of native starch (Table 3), which suggested that dry heat treatment reduced the short-range order degree of CS. The values of R1047/1022 increased slightly and then decreased with the extension of dry heat treatment time and treatment cycle, whether CT or RT, which might be related to the arrangement and recrystallization of starch granule after dry heat treatment, thus influenced on the short-range order of starch (Luo et al., 2020). However, there was no significant difference among CT samples, RT samples and gelatinized samples, which indicated that the time and cycle of dry heat treatment had no significant effect on short-range order.
Table 3.
The absorbance value of 1047/1022 results of samples.
| Samples | 1047/1022 cm−1 |
|---|---|
| Control | 1.277 ± 0.228b |
| CT-3 | 0.886 ± 0.003a |
| CT-6 | 0.949 ± 0.038a |
| CT-9 | 0.929 ± 0.034a |
| CT-12 | 0.915 ± 0.014a |
| RT-2 | 0.946 ± 0.030a |
| RT-3 | 0.889 ± 0.004a |
| RT-4 | 0.893 ± 0.000a |
| D-Control | 0.948 ± 0.003a |
| DCT-3 | 0.940 ± 0.003a |
| DCT-6 | 0.934 ± 0.001a |
| DCT-9 | 0.934 ± 0.005a |
| DCT-12 | 0.905 ± 0.001a |
| DRT-2 | 0.944 ± 0.004a |
| DRT-3 | 0.945 ± 0.008a |
| DRT-4 | 0.931 ± 0.000a |
Note: Control, native chestnut starch; CT-3, CT-6, CT-9, CT-12, preparation of chestnut starch by continuous dry heat treatment for 3 h, 6 h, 9 h, 12 h, respectively; RT-2, RT-3, RT-4, preparation of chestnut starch by repeated dry heat treatment for two cycles, three cycles, four cycles, respectively. D-Control, DCT-3, DCT-6, DCT-9, DCT-12, DRT-2, DRT-3, DRT-4 represented the obtained freeze-dried starch samples, respectively. Results are showed as mean values ± SD in triplicate. Different letters (a-g) in the same column show a significant difference at p < 0.05.
3.6. LF-NMR
LF-NMR was used to detect the water distribution in gel samples, and T2, as an important index, mainly reflected the tightness between starch and water (Din et al., 2018; Ma et al., 2019). In T2 spectra, there are three kinds of water components in starch gel, which were tightly bound water (T21), immobile water (T22) and free water (T23), respectively. T21, T22 and T23 of CS gel treated with dry heat treatment were lower than control group, which was consistent with the result of T2 (Table 4). Moreover, there were significant differences between starch with different treatment time and cycle samples and dry heat treatment (CT and RT) decreased the T2 of CS gel. The reduction in T2 might be due to the leached amylose aggregated or rearranged, thus limiting the movement of water molecules. The T2 value of CT-12 sample was the smallest, which indicated that starch and water were most closely combined in the system. The T2 value of CT was slightly lower than RT, which was due to the existence of the cooling cycle stage of RT.
Table 4.
Water mobility results of native chestnut starch and dry heat treated starch.
| Samples | T2 (ms) | T21 (ms) | T22 (ms) | T23 (ms) |
|---|---|---|---|---|
| Control | 272.48 ± 1.96f | 299.94 ± 17.45d | 853.06 ± 18.41d | 8343.11 ± 278.80b |
| CT-3 | 250.12 ± 1.16e | 280.78 ± 21.69bcd | 754.05 ± 50.74bc | 7941.76 ± 707.53ab |
| CT-6 | 244.91 ± 0.70cd | 204.01 ± 21.94ab | 722.00 ± 28.08abc | 7572.18 ± 136.40ab |
| CT-9 | 241.68 ± 2.35abc | 214.19 ± 18.09a | 704.71 ± 21.75abc | 7141.34 ± 139.71a |
| CT-12 | 238.94 ± 0.50a | 216.53 ± 36.67abc | 678.24 ± 11.32ab | 6985.39 ± 477.83a |
| RT-2 | 245.65 ± 2.26d | 250.47 ± 13.39abcd | 776.64 ± 82.01cd | 7586.55 ± 706.59ab |
| RT-3 | 243.24 ± 0.30bcd | 280.35 ± 13.84bcd | 712.66 ± 1.65abc | 7208.77 ± 213.13a |
| RT-4 | 241.24 ± 1.84ab | 290.67 ± 70.14cd | 647.84 ± 20.05a | 7019.88 ± 156.07a |
Note: CS, native chestnut starch; CT-3, CT-6, CT-9, CT-12, preparation of chestnut starch by continuous dry heat treatment for 3 h, 6 h, 9 h, 12 h, respectively; RT-2, RT-3, RT-4, preparation of chestnut starch by repeated dry heat treatment for two cycles, three cycles, four cycles, respectively. Results are showed as mean values ± SD in triplicate. Different letters in the same column show a significant difference at p < 0.05.
4. Conclusion
Dry heat treatment, as an important physical modification method, had an influence on gelatinization, rheology and structure of starch. Dry heat treatment increased the solubility of native CS, which would expand the application of CS. The viscosity (PV, TV, FV, SB, BD), dynamic viscoelasticity, gel strength and hardness of CS after CT and RT was lower than that of native starch, which showed that the treated starch by dry heat treatment was not easy to form gel. Dry heat treatment also decreased the degree of short ordered structure of CS. LF-NMR result showed that dry heat treatment (CT and RT) also made starch molecules closely combined with water. This study also indicated that the two methods of dry heat treatment, CT and RT were different for improving the gelatinization, rheology and other properties of starch, and we find that continuous dry heat treatment was more effective in reducing pasting viscosity. Therefore, modifying starch by dry heat treatment will improve the properties of starch and expand its application range.
CRediT authorship contribution statement
Wenmeng Liu: Writing – original draft, Methodology, Conceptualization, Visualization, Data curation. Wentao Pan: Writing – review & editing, Methodology. Jinwang Li: Writing – review & editing. Yi Chen: Writing – review & editing. Qiang Yu: Writing – review & editing. Liyuan Rong: Writing – review & editing. Wenhao Xiao: Writing – review & editing. Huiliang Wen: Writing – review & editing. Jianhua Xie: Writing – original draft, Resources, Conceptualization, Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was financial supported by the National Natural Science Foundation of China (31972034), the Natural Science Foundation of Jiangxi Province, China (20181ACB20013), and the National Youth Top-notch TalentSupport Program of China.
References
- Chen L., Tian Y., Bai Y., Wang J., Jiao A., Jin Z. Effect of frying on the pasting and rheological properties of normal maize starch. Food Hydrocolloids. 2018;77:85–95. [Google Scholar]
- Colussi R., Pinto V.Z., El Halal S.L., Vanier N.L., Villanova F.A., Marques E.S.R., Zavareze E.d.R., Dias A.R.G. Structural, morphological, and physicochemical properties of acetylated high-, medium-, and low-amylose rice starches. Carbohydr. Polym. 2014;103:405–413. doi: 10.1016/j.carbpol.2013.12.070. [DOI] [PubMed] [Google Scholar]
- Correia P., Cruz-Lopes L., Beirão-da-Costa L. Morphology and structure of chestnut starch isolated by alkali and enzymatic methods. Food Hydrocolloids. 2012;28(2):313–319. [Google Scholar]
- Cruz B.R., Abraao A.S., Lemos A.M., Nunes F.M. Chemical composition and functional properties of native chestnut starch (Castanea sativa Mill) Carbohydr. Polym. 2013;94:594–602. doi: 10.1016/j.carbpol.2012.12.060. 2013. [DOI] [PubMed] [Google Scholar]
- Din Z.-u., Xiong H., Wang Z., Chen L., Ullah I., Fei P., Ahmad N. Effects of different emulsifiers on the bonding performance, freeze-thaw stability and retrogradation behavior of the resulting high amylose starch-based wood adhesive. Colloids Surf. A Physicochem. Eng. Asp. 2018;538:192–201. [Google Scholar]
- Gou M., Wu H., Saleh A.S.M., Jing L., Liu Y., Zhao K., Su C., Zhang B., Jiang H., Li W. Effects of repeated and continuous dry heat treatments on properties of sweet potato starch. Int. J. Biol. Macromol. 2019;129:869–877. doi: 10.1016/j.ijbiomac.2019.01.225. [DOI] [PubMed] [Google Scholar]
- Jan K.N., Panesar P.S., Rana J.C., Singh S. Structural, thermal and rheological properties of starches isolated from Indian quinoa varieties. Int. J. Biol. Macromol. 2017;102:315–322. doi: 10.1016/j.ijbiomac.2017.04.027. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Gao W., Li X., Zhang J. Characteristics of native and enzymatically hydrolyzed Zea mays L., Fritillaria ussuriensis Maxim. and Dioscorea opposita Thunb. starches. Food Hydrocolloids. 2011;25(3):521–528. [Google Scholar]
- Li F., Guan X., Li C. Effects of degree of milling on the starch digestibility of cooked rice during (in vitro) small intestine digestion. Int. J. Biol. Macromol. 2021;188:774–782. doi: 10.1016/j.ijbiomac.2021.08.079. [DOI] [PubMed] [Google Scholar]
- Li S., Huang L., Zhang B., Chen C., Fu X., Huang Q. Fabrication and characterization of starch/zein nanocomposites with pH-responsive emulsion behavior. Food Hydrocolloids. 2021;112:106341. [Google Scholar]
- Li S., Zhang B., Li C., Fu X., Huang Q. Pickering emulsion gel stabilized by octenylsuccinate quinoa starch granule as lutein carrier: role of the gel network. Food Chem. 2020;305:125476. doi: 10.1016/j.foodchem.2019.125476. [DOI] [PubMed] [Google Scholar]
- Lin S., Liu X., Cao Y., Liu S., Deng D., Zhang J., Huang G. Effects of xanthan and konjac gums on pasting, rheology, microstructure, crystallinity and in vitro digestibility of mung bean resistant starch. Food Chem. 2021;339:128001. doi: 10.1016/j.foodchem.2020.128001. [DOI] [PubMed] [Google Scholar]
- Liu S., Shen M., Xiao Y., Luo Y., Xie J. Effect of maize, potato, and pea starches with Mesona chinensis polysaccharide on pasting, gelatinization properties, granular morphology and digestion. Food Hydrocolloids. 2020;108:106047. [Google Scholar]
- Liu W., Wang R., Li J., Xiao W., Rong L., Yang J., Wen H., Xie J. Effects of different hydrocolloids on gelatinization and gels structure of chestnut starch. Food Hydrocolloids. 2021;120:106925. [Google Scholar]
- Luo Y., Shen M., Li E., Xiao Y., Wen H., Ren Y., Xie J. Effect of Mesona chinensis polysaccharide on pasting, rheological and structural properties of corn starches varying in amylose contents. Carbohydr. Polym. 2020;230:115713. doi: 10.1016/j.carbpol.2019.115713. [DOI] [PubMed] [Google Scholar]
- Lyu B., Wang H., Swallah M.S., Fu H., Shen Y., Guo Z., Tong X., Li Y., Yu H., Jiang L. Structure, properties and potential bioactivities of high-purity insoluble fibre from soybean dregs (Okara) Food Chem. 2021;364:130402. doi: 10.1016/j.foodchem.2021.130402. [DOI] [PubMed] [Google Scholar]
- Ma S., Zhu P., Wang M. Effects of konjac glucomannan on pasting and rheological properties of corn starch. Food Hydrocolloids. 2019;89:234–240. [Google Scholar]
- Mir S.A., Bosco S.J.D., Shah M.A. Technological and nutritional properties of gluten-free snacks based on brown rice and chestnut flour. J. Saudi Soc. Agric. Sci. 2019;18(1):89–94. [Google Scholar]
- Nawab A., Alam F., Haq M.A., Hasnain A. Effect of guar and xanthan gums on functional properties of mango (Mangifera indica) kernel starch. Int. J. Biol. Macromol. 2016;93(Pt A):630–635. doi: 10.1016/j.ijbiomac.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Silva A.P., Oliveira I., Silva M.E., Guedes C.M., Borges O., Magalhaes B., Goncalves B. Starch characterization in seven raw, boiled and roasted chestnuts (Castanea sativa Mill.) cultivars from Portugal. J. Food Sci. Technol. 2016;53(1):348–358. doi: 10.1007/s13197-015-2047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Gong M., Li Y., Xiong L. Effect of dry heat treatment on the physicochemical properties and structure of proso millet flour and starch. Carbohydr. Polym. 2014;110:128–134. doi: 10.1016/j.carbpol.2014.03.090. [DOI] [PubMed] [Google Scholar]
- Sun Q., Si F., Xiong L., Chu L. Effect of dry heating with ionic gums on physicochemical properties of starch. Food Chem. 2013;136(3–4):1421–1425. doi: 10.1016/j.foodchem.2012.09.061. [DOI] [PubMed] [Google Scholar]
- Tabarsa M., Anvari M., Joyner H.S., Behnam S., Tabarsa A. Rheological behavior and antioxidant activity of a highly acidic gum from Althaea officinalis flower. Food Hydrocolloids. 2017;69:432–439. [Google Scholar]
- Vashisht D., Pandey A., Hermenean A., Yanez-Gascon M.J., Perez-Sanchez H., Kumar K.J. Effect of dry heating and ionic gum on the physicochemical and release properties of starch from Dioscorea. Int. J. Biol. Macromol. 2017;95:557–563. doi: 10.1016/j.ijbiomac.2016.11.064. [DOI] [PubMed] [Google Scholar]
- Wang X., Reddy C.K., Xu B. A systematic comparative study on morphological, crystallinity, pasting, thermal and functional characteristics of starches resources utilized in China. Food Chem. 2018;259:81–88. doi: 10.1016/j.foodchem.2018.03.121. [DOI] [PubMed] [Google Scholar]
- Zhou Y.-l., Cui L.-h., You X.-y., Jiang Z.-h., Qu W.-h., Liu P.-d., Ma D.-y., Cui Y.-y. Effects of repeated and continuous dry heat treatments on the physicochemical and structural properties of quinoa starch. Food Hydrocolloids. 2021;113:106532. [Google Scholar]
- Zhu F. Effect of processing on quality attributes of chestnut. Food Bioprocess Technol. 2016;9(9):1429–1443. [Google Scholar]