Abstract
Background
Inhaled budesonide (BUD) is available in a range of doses for treating chronic asthma.
Objectives
To assess the efficacy and safety of budesonide at different doses in order to establish whether a clinically significant dose response profile exists.
Search methods
A search was carried out for Controlled Clinical Trials using the Cochrane Airways Group trial register.
Selection criteria
Randomised trials in children and adults comparing one dose of budesonide to a second dose in the treatment of chronic asthma. Two reviewers independently assessed articles for inclusion and methodological quality.
Data collection and analysis
One reviewer extracted data; authors were contacted to clarify missing information. Quantitative analyses where undertaken using Review Manager 4.0.4 with MetaView 3.1.
Main results
24 studies met the inclusion for the review (3907 participants). In non‐oral steroid treated, mild to moderately severe asthma, no clinically worthwhile differences in FEV1, morning PEFR, symptom scores or rescue beta2 agonist use were apparent across a dose range of 200‐1600 mcg/d. However, in moderate to severe asthma a significant reduction in the likelihood of trial withdrawal due to asthma exacerbation was apparent when treating patients with BUD 800 mcg/d compared to 200 mcg/d: Relative Risk 3.93 (95% confidence interval, 1.4 to 10.9). This result was weighted largely by a single, large, high quality RCT. In severe asthma significant improvements favouring high dose BUD (1600 mcg/d) over low dose (200 mcg/d) were apparent for FEV1 but not morning PEFR. This finding was based on two large RCTs of good quality. In oral steroid treated asthmatics no dose dependent oral steroid sparing effect was apparent for BUD 1600 mcg/d v 800 or 400 mcg/d. Statistically significant, dose dependent suppression of 24 hour urinary free cortisol excretion and serum cortisol post synthetic ACTH infusion over the dose range 800‐3200 mcg/d was apparent but the clinical significance of these findings is unclear.
Authors' conclusions
Budesonide exhibits a significant dose response effect between low and high dose for improvement in FEV1 in severe asthma and reduction of exacerbations in moderate to severe asthma. No significant dose dependent improvements in FEV1, PEFR or symptoms are evident in non‐oral steroid treated asthmatics with mild to moderate disease. Dose dependent alterations in sensitive measures of hypothalamic‐pituitary‐adrenal function were evident but the clinical significance of these changes is unclear.
Keywords: Adult, Child, Humans, Anti-Inflammatory Agents, Anti-Inflammatory Agents/therapeutic use, Asthma, Asthma/drug therapy, Bronchodilator Agents, Bronchodilator Agents/therapeutic use, Budesonide, Budesonide/therapeutic use, Chronic Disease, Randomized Controlled Trials as Topic
Plain language summary
Budesonide at different doses for asthma
Budesonide is an inhaled corticosteroid used to treat the inflammation of airways (passages to the lungs) that occurs in asthma. This review presents the effects of budesonide at different doses for people with varying degrees of asthma. In patients with mild‐moderate asthma no important differences were apparent between the lowest dose (200 mcg/d) and the highest dose (1600 mcg/d) for measures of airway opening and symptoms. However, patients with more severe asthma are less likely to experience an acute worsening of their asthma control when a higher dose (1600 mcg/d) is used regularly compared to a lower dose (200 mcg/d). Future research should report results more comprehensively, and should use quality of life questionnaires.
Background
Asthma is common disorder affecting children and adults, is increasing in incidence and constitutes a significant worldwide health problem. It is generally acknowledged that whatever the genetic factors determining the likelihood of developing disease or specific allergic/non‐allergic precipitants in particular individuals, a phenotype characterised by persistent inflammation of the airway mucosa and allied structures is common to all asthmatic individuals. Resulting airway hyper‐responsiveness with variable airway calibre probably account for the majority of symptoms experienced by asthmatic patients. Avoidance of the specific exacerbating factors (where they can be identified) may help some patients but is often difficult to achieve; altering the genetic pre‐disposition to asthma development is not currently a therapeutic option. Pharmacological treatment to reduce airway inflammation is therefore the cornerstone of therapy for most asthmatic patients. Inhaled corticosteroids (ICS) are anti‐inflammatory agents that have been available for the treatment of asthma for over 30 years; budesonide (BUD) was introduced in the early 1980's and had been widely used since.
It would seem intuitive that greater efficacy would result from using higher doses of ICS, in other words that a dose‐response relationship exists for ICS. A previous systematic review has shown that budesonide leads to significant improvements in measures of airway calibre, symptoms and bronchial hyper‐responsiveness compared to placebo (Adams 2001). Having established the basic efficacy of budesonide we were interested in assessing how this response varies with dose. This question is best addressed by a randomised controlled trial (RCT) that compares one nominal daily dose of budesonide to a second nominal daily dose using the same delivery system. This review systematically evaluates the evidence from these RCTs in a qualitative and quantitative manner.
Objectives
1. To assess the efficacy and safety outcomes in studies that have compared one nominal daily dose of budesonide to a second nominal daily dose of budesonide in treating chronic asthma.
2. To establish whether a dose response relationship exists for budesonide in the treatment of chronic asthma.
Methods
Criteria for considering studies for this review
Types of studies
Only prospective randomised trials were included. Double, single and unblinded studies were considered.
Types of participants
Studies assessing children and adults with chronic asthma were included. Studies concerned with acute asthma and/or exclusively concerned with infants (two years of age or less) were excluded. Studies enrolling both asthmatic patients and COPD sufferers were included, provided the data for asthma subjects was separately available. Studies carried out in primary care, institutional care and the hospital outpatient setting were eligible for inclusion.
Types of interventions
Budesonide at one nominal daily dose delivered by mouth inhalation versus a different nominal daily dose. Treatment periods needed to be at least one week. Studies using pressurised aerosol metered dose inhalers (MDI) with or without spacer or dry powder inhalers (DPI) were eligible for inclusion; studies using any type of nebuliser were excluded.
Types of outcome measures
All outcome measures were considered. Outcomes reported as both absolute values and change compared to baseline were considered. Important outcomes identified a priori were as follows:
1. Outcomes reflecting airway calibre: FEV1, morning and evening PEFR, diurnal variability in diary card PEFR. 2. Asthma symptoms 3. Rescue short‐acting beta2 agonist use 4. Bronchial hyper‐responsiveness (BHR) to histamine and methacholine 5. Quality of life 6. Asthma exacerbations: hospital admission rates, days off work or school, unscheduled doctor visits due to exacerbation 7. Safety outcomes: hypothalamic‐pituitary‐adrenal (HPA) axis function reflected in serum and urinary cortisol measures 8. Oropharyngeal side effects: hoarseness, sore throat, oropharyngeal Candidiasis
Search methods for identification of studies
Electronic searches
Stage 1: A search was carried out of the Cochrane Airways Group Trial Register. This consists of randomised controlled trials (RCTs) and has been compiled from searches of MEDLINE (1966‐1999), EMBASE (1980‐1999) and CINAHL (1982‐1999) and the Cochrane Controlled Trials Register (CCTR). It is also augmented by RCTs found as a result of hand searching 20 of the leading respiratory care journals. The following search terms were applied:
steroid* OR glucocorticoid* OR corticosteroid* OR beclomethasone
OR budesonide OR fluticasone OR triamcinolone OR flunisolide OR
Becotide OR Becloforte OR Pulmicort OR Flixotide
The electronic abstracts of citations resulting from this search were then imported into a bibliographic database and termed the inhaled steroid register. This was hand‐searched by two reviewers (NPA and JB) for duplicate publications, which were removed.
Stage 2: The inhaled steroid register was searched using the following terms:
budesonide OR Pulmicort
Electronic abstracts were exported to a new database and termed the budesonide register. Citations were initially excluded if it was clear that the study:
a) Was not concerned with treatment of chronic asthma in humans b) Was not an RCT c) Did not include a treatment arm with inhaled budesonide
Where uncertainty existed, the publication was retrieved in full text version
Searching other resources
The bibliographies of all papers retrieved in full text form and relevant narrative reviews were searched for additional publications. The British Journal of Clinical Research and the European Journal of Clinical Research were hand searched for relevant studies. Authors of included studies were contacted and asked if they were aware of further studies. The European headquarters of Astra Zeneca manufacturers of Pulmicort were contacted to find details of studies sponsored that may have been missed. Finally, the proceedings of meetings of the European Respiratory Society (1997/1998), British Thoracic Society (1997/1998) and American Thoracic Society (1997 to 1999) were searched for relevant trials.
Data collection and analysis
Selection of studies
Decision to exclude studies prior to full paper retrieval was made by one reviewer (NPA). Papers retrieved in full text form were assessed independently by two reviewers (NPA and JB); disagreement as to which papers to include was resolved by consensus.
Data extraction and management
One reviewer (NPA) extracted data for each outcome from the published results of included trials. In the case of continuous outcomes such as spirometry:
1. Where outcomes were evaluated at a number of time points, only data from the last evaluable time point was used. 2. Data were extracted from graphical plots when presented in this form; and an attempt was made to verify such data by contacting authors. 3. If an intention‐to‐treat analysis was not used by the investigators, and it was not explicit in the presentation of results how many subjects were in each group at the time of last evaluation of that outcome, the appropriate N value for each intervention group was calculated by subtracting the number of patients who withdrew in each intervention group from those randomised to each intervention group. Authors were written to (by mail, fax and/or electronic mail) on at least two occasions to clarify details of randomisation and/or request missing outcome data. Attempt was made to send requests to correct current addresses by searching MEDLINE, EMBASE and hospital World Wide Web (WWW) sites for up‐ to‐date contact details. Astra Zeneca were approached for data for those trials in which contact authors did not initially reply or when authors suggested doing so and which the company had sponsored.
Assessment of risk of bias in included studies
Two reviewers (NPA and JB) who were blinded to the author's names, institution and funding sources independently assessed each study for methodological quality. The trials were scored using the Cochrane approach:
Grade A: adequate allocation concealment Grade B: unclear allocation concealment Grade C: clearly inadequate concealment
The methodological quality of included studies was also assessed using a 5‐point scoring instrument (Jadad 1996):
a) Was the study described as randomised? (yes=1 no=0) b) Was the study described as double blind? (yes=1 no=0) c) Was there a description of withdrawals and dropouts? (yes=1 no=0) d) Was the method of randomisation well described and appropriate? (yes=1 no=0) e) Was the method of double blinding well described and appropriate? (yes=1 no=0) f) Deduct 1 point if method of randomisation or blinding inappropriate
Inter‐rater agreement was measured using the kappa statistic. Disagreement was resolved by consensus.
Data synthesis
A weighted treatment effect across trials was calculated using the Cochrane statistical package RevMan 4.0.4 with MetaView 3.1. For continuous outcomes, a weighted mean difference (WMD) or standardised mean difference (SMD) was calculated as appropriate. For dichotomous outcomes a relative risk (RR) was calculated. Pooled treatments effects are expressed with their 95% confidence intervals (95% CI). Heterogeneity of effect size across studies pooled was calculated, with p< 0.05 used as the cut‐off level for significance. A number of a priori conditions were established regarding the comparisons made:
1. Studies were categorised into those in which patients were a) not treated with regular oral steroids b) dependent upon regular oral steroid treatment prior to study. It was recognised, a priori, that trials in which the efficacy of inhaled corticosteroids was being assessed in patients dependent upon oral steroids may have an 'oral steroid down‐titration' design using reduction in the use of oral steroids as an outcome measure, whilst maintaining a given level of asthma control. However, studies in which patients were not treated with regular oral steroids are more likely to have a design aimed at detecting improvements in asthma control. It would be inappropriate to combine trials with such different designs and aims.
2. The results of parallel and crossover trials were not pooled because there is no agreement on the methods for this.
3. It was anticipated that measures of bronchial hyper‐responsiveness (PD20 FEV1, PC20 FEV1) would often be reported as geometric means. Presentation of results in this way indicates that data has been logarithmically transformed prior to analysis by investigators to take account of skewed distribution. Data for such outcomes were only pooled across studies where the mean and standard deviation of logged values (from which geometric means are derived) could be calculated.
Sensitivity analysis
Sensitivity analyses were performed on the basis of methodological quality. Results were re‐analysed using studies of only the highest quality scores (Jadad 3 to 5). Subgroup analyses based upon patient age, delivery device, study duration and asthma severity were planned.
Results
Description of studies
Results of the search
Stage 1 electronic search: 6494 citations retrieved, 2162 unique citations
Stage 2 electronic search: 1036 unique citations 331 not RCT (on basis of abstract) 195 not chronic asthma in humans 129 not concerned with inhaled steroid treatment 40 not concerned with BUD treatment
341 papers retrieved in full text form:
46 not RCT 239 not involving a comparison of two or more doses of BUD 12 infants 1 treatment period of less than 1 week 4 nebuliser delivery device 6 comparison of dosing regimens 10 comparison of delivery devices 22 included studies
Additional sources: one study (Rees 1993) was identified as a result of hand searching the European Journal of Clinical Research.
Agreement between the two independent assessments of study quality were as follows:
Randomisation: kappa=1 Double‐blind: kappa=0.9 Withdrawal/dropout: kappa=0.5 Method of randomisation: kappa=0.5 Method of double‐blinding: kappa=0.8
Included studies
28 publications (representing 24 studies) met the inclusion criteria for the review. All were published in English, although none were excluded on the basis of language.
We would like to draw the reader's attention to the 17 citations listed under Studies awaiting assessment. Due to unforeseen delays in the publication of this review, a number of potentially relevant trials have been identified after an updated search of the electronic sources from April 1999 ‐ August 2001. These studies will be fully evaluated and included as appropriate in a future update at the earliest possible opportunity.
Populations The studies were conducted in North America (USA, Canada) Europe (Denmark, Finland, Norway, Sweden, The Netherlands, Turkey, UK) and Israel. 7 (30%) studies assessed children, 16 (70%) studies were in adult asthmatics.
Diagnosis of asthma Country specific or international asthma guideline criteria were used in six studies (Boe 1989, Busse 1998, Johansson 1988, Jonasson 1998, Pedersen 1996, van der Molen 1998). In five (21%) studies it was stated that patients had episodic respiratory symptoms consistent with a diagnosis of asthma; in eight (33%) studies 15% or greater FEV1/PEFR variability over time or reversibility in response to short acting beta2 agonist was an inclusion criterion. In three studies (Juniper 1990, Kraan 1988, Swystun 1998) the diagnosis of asthma was supported by demonstrable methacholine BHR (PC20 FEV1 < 8 mg/ml). In seven studies (Aaronson 1998, Agertoft 1997, Bisgaard 1991, Laursen 1986, Toogood 1984, Tukiainen 1987, Wolthers 1991, Wolthers 1992) the criteria upon which a diagnosis of asthma was made was not clear.
Asthma severity Attempt was made to classify asthma severity for patients' enrolled to each study using current GINA 1995/NHLBI 1997 criteria. This classification of severity is based upon FEV1 (% predicted), frequency of symptoms and exacerbations and diurnal PEFR variability. Four grades of severity are defined: severe, moderate, mild persistent and mild intermittent. The classification applies to untreated patients. These guidelines also recommend a range of daily doses of ICS for disease at each severity grade. For example, BUD 200‐400 mcg/d for mild persistent disease, 400‐600 mcg/d for moderate disease, > 600 mcg/d for severe disease in adult patients. For studies in which patients were using an ICS at the time of enrolment, an attempt was made to classify severity according to baseline ICS use using these ranges.
Severe asthma In two parallel group design studies (Laursen 1986, Nelson 1998) and one crossover design study (Tukiainen 1987), dependence on oral prednisolone for asthma control was an inclusion criterion. The parallel group studies were conducted with the primary objective of determining if inhaled BUD demonstrated a dose dependent oral corticosteroid sparing effect. In two further parallel group studies (Busse 1998, Shapiro 1998a) and two crossover studies (Johansson 1988, Toogood 1984) a proportion of enrolled patients were treated with oral corticosteroids for asthma control. In these trials no attempt was made to taper the oral corticosteroid dose. In the studies recruiting oral corticosteroid treated asthmatics, baseline symptom frequency was not reported at all; however in those studies which reported baseline FEV1 (% predicted) this was significantly reduced in all cases (see Included study characteristics). It appears that asthma control was sub optimal in most of these studies. Demonstration of a requirement in most/all recruited patients for oral steroid treatment would class the asthmatic in these studies as having severe disease.
Moderate to severe asthma: the FACET study In one study use of a regular ICS was an inclusion criterion. This is was the multicentre trial undertaken by the Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group (Pauwels 1997a/Pauwels 1997b). This was a large study (852 adults) in which the relative efficacy of budesonide at either 200 mcg/d or 800 mcg/d were evaluated, either with or without the addition of formoterol 24 mcg/d in a four arm parallel group design. The mean daily dose of beclomethasone/budesonide used by patients randomised to treatment was between 823 and 856 mcg/d. This is an influential study and is discussed in detail below (see Results: 200 v 800 mcg/d).
Mild to moderate asthma In the four studies that enrolled non‐ICS treated asthmatics, there was an indication that symptoms were not adequately controlled at the time of enrolment (Campbell 1998b, Pedersen 1995, Pedersen 1996, van der Molen 1998). In these studies asthma severity was classed as mild to moderate. In five studies (Bisgaard 1991, Boe 1989, Juniper 1990, Kraan 1988, Rees 1993) some/all patients were receiving a regular ICS at the time of enrolment.
Mild asthma Three studies (Agertoft 1997, Wolthers 1991, Wolthers 1992) gave no indication of symptom frequency or baseline FEV1 (% predicted) hence the degree of severity and level of control was unclear although investigators stated that disease was mild. In two studies (Aaronson 1998, Jonasson 1998) no indication of symptom frequency at baseline was provided, but baseline FEV1 (% predicted) of enrolled patients approached 100 %. In one study (Swystun 1998) symptom control was clearly good, and baseline FEV1 > 74 % predicted. In these studies asthma severity was classed as mild.
Study design 15 (63%) studies were of a parallel group design; nine (37%) were crossover studies. Only four crossover design studies employed a washout period between treatments (Agertoft 1997, Swystun 1998, Toogood 1984, Wolthers 1991). 10 (42%) studies employed a treatment period of between one and four weeks, 11 (46%) had a treatment period of one to five months. Three studies (Juniper 1990, Pauwels 1997a/Pauwels 1997b, Pedersen 1996) employed a treatment period of between nine and 12 months.
Interventions Ranges of different nominal daily doses of BUD were compared (see Included study characteristics). Identical delivery devices (either MDI, MDI+spacer or DPI) were used to deliver different nominal daily doses of BUD in all studies. In one six period crossover study (Toogood 1984) patients received BUD with both MDI and MDI+spacer during different treatment periods. One study (Pauwels 1997) employed four parallel intervention groups: BUD 200 mcg/d, BUD 800 mcg/d, BUD 200 mcg/d+formoterol 24 mcg/d, BUD 800 mcg/d+formoterol 24 mcg/d. The latter two intervention groups were included in the meta‐analysis. The rationale for this is as follows: an additive effect of formoterol and BUD was assumed, and since we were interested in the relative efficacy of the two doses of BUD, it seemed appropriate to treat formoterol given by equal doses in each group as a controlled intervention that would not influence the relative efficacy comparison between the two doses of BUD. To include these results, the Pauwels study has been listed as two studies: Pauwels 1997a and Pauwels 1997b. This was necessary to allow inclusion of data for the second comparison group in the meta‐analysis.
Outcomes A wide range of efficacy and safety outcomes was assessed (see Included study characteristics). A number of studies evaluated specific outcomes that have not been considered in this review. These included growth assessment by lower leg knemometry (Agertoft 1997, Wolthers 1991, Wolthers 1992) and biochemical markers of bone turnover (Wolthers 1991). These are the subject of separate reviews in preparation. One study (Rees 1993) included a cost effectiveness analysis. Cost effectiveness of ICS's is likely to be the subject of a future analysis and has not been considered here. Incomplete outcome data that could not be included in the meta‐analysis is listed in Table 1.
1. Outcome data not included in meta‐analysis.
| Study | Data |
| Aaronson 1998 | Plasma cortisol Data not presented in a usable form |
| Agertoft 1997 | Morning PEFR Evening PEFRDaytime and night‐time asthma symptom scoresUrinary cortisol excretion No SD values available for above outcomes |
| Bisgaard 1991 | FEV1 Urinary free cortisol No numerical data presented for above outcomes |
| Boe 1989 | FEV1 FVC Morning PEFR Daily asthma symptom score No numerical data available for above outcomes |
| Busse 1998 | Change in FEV1 compared to baseline Change in FVC compared to baseline Change in morning PEFR compared to baseline Change FEF25‐50 (L) compared to Change evening PEFR compared to Change daytime asthma symptom compared to Change nighttime asthma symptom compared to baseline daily beta2 agonist use Early morning plasma cortisol Early morning plasma cortisol post ACTH No SD values available for above outcomes |
| Jonasson 1998 | FEV1 Morning PEFR Methacholine BHR PD20 FEV1 Daytime asthma symptom scoreNight‐time asthma symptoms scoreNo numerical data available for above outcomes |
| Juniper 1990 | Methacholine BHRPC20 FEV1Log transformed data not available |
| Kraan 1988 | Clinic assessed asthma symptom score No numerical data available |
| Laursen 1986 | Morning PEFREvening PEFRDaily use of beta2 agonistsPlasma cortisol Plasma cortisol 30 mins post 250 mcg iv cosyntropinNo standard SD available for above outcomes |
| Nelson 1998 | Percentage reduction in daily dose oral prednisolone compared to baselinePercentage change in FEV1 (litres) compared to baselinePercentage change in morning PEFR (litres/min) compared to baselinePercentage change in evening PEFR (litres/min) compared to baselinePercentage change in FEF25‐50 (litres/min) compared to baseline Change in daytime asthma symptom score compared to baselineChange in night‐time symptom score compared to baseline Change in daily use of beta2 agonists compared to baseline (pfs/d)Morning plasma cortisol (nmol/litre)Plasma cortisol 60 min post 25 units cosyntropin IM (nmol/litre)No standard SD values available for above outcomes |
| Pauwels 1997 | Night‐time asthma symptom score Daytime asthma symptom score Night‐time awakening (No. per night) Night‐time use of beta2 agonists Daytime use of beta2 agonists No SD values available for above outocomes |
| Pedersen 1995 | FEV1Maximum percentage fall in FEV1 following exercise testFVCFEF 25‐75Maximum percentage fall in FEF25‐75 following exercise testClinic PEFRMorning PEFREvening PEFRDaytime asthma symptom scoreNighttime asthma symptom scoreDaily beta2 agonist useNo SD values available for any of the above outcomes |
| Shapiro 1998 | Change in % predicted FEV1 compared to baseline Change in morning PEFR (litres/min) compared to baselineChange in daytime asthma symptom scoreChange in night‐time asthma symptom scoreChange in daily use of beta2 agonist (pfs/d)Change in morning plasma cortisol (nmol/litre) compared to baselineChange in plasma cortisol 30 min post 25 units iv cosyntropin (nmol/litre) compared to baselineNo SD values available for above outcomes |
| Swystun 1998 | Methacholine BHR PC15 FEV1Allergen BHR PC15 FEV1Log transformed data not available for above outcomes |
| Wolthers 1992 | FEV1 Morning PEFR Evening PEFR Daily use of beta2 agonist No SD values available for above outcomes |
| Wolthers 1991 | FEV1 Morning PEFR Evening PEFR Urinary free cortisol excretion No SD values available for above outcomes |
Risk of bias in included studies
The overall quality of included studies was high. All studies were randomised. 21 (88%) were double blind, and in 19 (80%) the number of patients withdrawn and the reasons for withdrawal were stated. 19 (80%) of studies were graded with a Jadad score of three or greater. Only five (20%) studies were graded with a Jadad score of 2 or less. In eight (33%) studies allocation concealment was clearly employed.
Effects of interventions
When examining the graphs and results in the text, it should be recalled that a negative sign for continuous variables such as FEV1, PEF, beta2‐agonist use etc favours BUD over placebo (see Methods of Analysis).
Non oral steroid treated asthmatics
Two fold dose comparisons
100 v 200 mcg/d
A single parallel design study (Jonasson 1998) of high quality (Jadad score 4) in children with mild, controlled asthma (163 subjects) assessed the relative efficacy of BUD 100 v 200 mcg/d over a 12 week treatment period. No significant difference in the maximal percentage fall in FEV1 following a six minute exercise test was apparent between treatment groups: WMD 0.7% (95% CI ‐2.0 to 3.5 %). Similarly no differences between doses were apparent for FEV1, morning PEFR, daytime and night‐time symptom scores or methacholine BHR (PD20 FEV1).
A single crossover design study of high quality (Jadad score 4) in 19 children with moderate, uncontrolled asthma assessed BUD 100 v 200 with four week treatment periods (Pedersen 1995). No significant difference between treatments were apparent for FEV1, FVC, clinic and diary card assessed PEFR, symptom scores, rescue beta2 agonist use and maximum percentage fall in FEV1 following six minute exercise test.
200 v 400 mcg/d
Three crossover design studies assessed the relative efficacy of BUD 200 v 400 mcg/d. All recruited inhaled steroid naïve asthmatics. Two enrolled children, with either mild asthma (Agertoft 1997) or moderate, uncontrolled asthma (Pedersen 1995). One assessed adults with mild, controlled disease (Swystun 1998). They were of high methodological quality (Jadad score 4). Treatment periods ranged between one and four weeks. A number of common outcomes were assessed: FEV1, morning PEFR, evening PEFR, daytime and night‐time symptom scores, 24 hour urinary free cortisol excretion (corrected for urinary creatinine). No significant differences were apparent between the BUD doses for these outcomes in any study, although data were not presented in a form suitable for inclusion in a meta‐analysis. However, Pedersen 1995 demonstrated a significantly smaller drop in post exercise test FEV1 for patients receiving 400 mcg/d (9.9%) compared to 200 mcg/d (20.1%), p <0.01. No significant differences were found for allergen and methacholine bronchial hyper‐responsiveness expressed as PC15 FEV1 (Swystun 1998).
400 v 800 mcg/d
Three parallel group studies assessed the relative efficacy and safety of BUD 400 v 800 mcg/d. One enrolled ICS naïve adults with mild, sub‐optimally controlled asthma (Campbell 1998b) and was of high methodological quality (Jadad score 4); one study enrolled adults with moderately severe uncontrolled asthma (Rees 1993) and was graded poorly for quality (Jadad score 1). Both were large studies (>500 adult subjects) and had intervention periods of six weeks. The third study assessed children with mild asthma (Wolthers 1992) and was of high quality (Jadad score 5). Different outcomes were assessed in each study. No significant differences were apparent between doses for change in morning PEFR, rescue beta2 agonist use or symptom scores compared to baseline (Campbell 1998b); morning PEFR, evening PEFR or rescue beta2 agonist use (Rees 1993, Wolthers 1992); daytime symptom score or and sleep disturbance score (Rees 1992); FEV1 (Wolthers 1992). One three limbed crossover study allowed a comparison of 400 vs 800 mcg/d (Swystun 1998), but no significant difference between any measured outcome was apparent.
A single, high quality (Jadad score 4) parallel group study (Juniper 1990) had a unique study design. Patients already receiving treatment with inhaled beclomethasone dipropionate (BDP) had their daily dose titrated during a run‐in period to optimise their asthma control such that they were not woken at night by symptoms and did not experience exercise‐related symptoms. They were then randomised to the same or double the nominal daily dose of BUD as that of BDP required to control symptoms. Patients were therefore randomised to BUD 200‐400 mcg/d or 400‐800 mcg/d, i.e. a two fold differences in dose. No significant differences were apparent for change in FEV1 (litres), methacholine BHR, clinic assessed symptom score or rescue beta2 agonist use compared to baseline. No significant differences in the number of patients withdrawn due to asthma exacerbation or experiencing oropharyngeal side effects were apparent.
One further crossover design study (Boe 1989), of good quality (Jadad score 4) in patients with moderately severe disease (level of control unclear) also assessed BUD 400 v 800 mcg/d in adult patients. No numerical data were presented but no significant differences between doses were apparent for FEV1, morning PEFR, evening PEFR or daily symptom score. One three limbed crossover study allowed a comparison of 400 vs 800 mcg/d (Swystun 1998), but no significant difference between any measured outcome was apparent.
800 v 1600 mcg/d
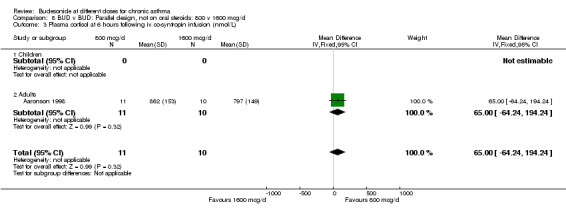
Two parallel design studies assessed the relative effects of BUD 800 v 1600 mcg/d in adult asthmatics on measures of HPA function. One study of reasonable quality (Jadad score 3) assessed the effects of treatment over a six week period in mild, well controlled asthma (Aaronson 1998). The second study (Turkas 1995) was of low quality (Jadad score 2) and used a four week treatment period. Prior ICS use and degree of control were unclear. Both studies assessed basal morning cortisol levels. Patients treated with BUD 800 mcg/d had significantly higher serum cortisol levels WMD 114 nmol/L (95% CI, 26 to 203 nmol/L). However when a sensitivity analysis was done excluding the lower quality study no significant difference between doses was apparent. Individual studies assessed further outcomes. BUD 800 mcg/d resulted in significantly higher 24 hour urinary free cortisol levels WMD 68 mcg (95% CI, 46 to 90) compared to BUD 1600 mcg/d treated patients (Turkas 1995). It should also be noted that mean post treatment 24‐hour urinary free cortisol levels were suppressed below the normal range in patients treated with BUD 1600 mcg/d. Aaronson 1998 assessed serum cortisol post co‐syntropin infusion. No difference was apparent between treatments: WMD 65 nmol/L (95% CI ‐64 to 194 nmol/L). This study also included a very high dose BUD 3200 mcg/d treatment arm. No differences between treatment groups in terms of basal or stimulated cortisol levels were apparent for the two fold dose comparison BUD 1600 mcg/d v 3200 mcg/d.
Four fold dose comparisons:
100 v 400 mcg/d
A single crossover design study of high quality (Jadad score 4) in 19 ICS naïve children assessed BUD 100 v 400 mcg/d over four week treatment periods but with no washout (Pedersen 1995). No significant differences were apparent between daily doses for FEV1, FVC, clinic and diary card assessed PEFR, symptom scores or rescue beta2 agonist use. However, a significantly smaller drop in post exercise test FEV1 was demonstrated by patients receiving 400 mcg/d (9.9%) compared to 100 mcg/d (25.7%), p <0.0001.
200 v 800 mcg/d
Four parallel design studies assessed the relative effects of BUD 200 mcg/d v 800 mcg/d. Two studies (van der Molen 1998, Wolthers 1992) were undertaken in ICS naïve asthmatics with mild disease. Two studies (Kraan 1988, Pauwels 1997a/Pauwels 1997b) were undertaken in patients with moderate to severe disease. A number of common outcomes were reported in these studies; results were pooled. No significant difference between treatment groups were apparent for FEV1, WMD 1.4% predicted (95% CI, 0.8 to 3.6 %predicted) or morning PEFR, WMD 1.5 L/min (95% CI, 13 to 16 L/min). It should be noted that in the largest study (Pauwels 1997a/Pauwels 1997b), comprising 852 subjects a more powerful ANCOVA model was used to assess group differences and small but statistically significant differences in favour of BUD 800 mcg/d were apparent for these outcomes. The mean differences between groups in this single study were of the similar magnitude to those found in the meta‐analysis.
There were significantly fewer patient withdrawals due to asthma exacerbation for BUD 800 mcg/d compared to 200 mcg/d RR 3.93 (95% CI, 1.4 to 10.9). The results are largely weighted by one large trial (Pauwels 1997a/Pauwels 1997b). No heterogeneity in effect size across studies was apparent. All studies were rated as having good methodological quality (Jadad scores 3‐4).
Individual studies examined other outcomes. No significant differences between doses were apparent for evening PEFR, daytime asthma symptom score, rescue beta2 agonist use (van der Molen 1998); methacholine BHR, blood eosinophil count or clinic assessed asthma symptom score (Kraan 1988). In the most influential study in this group (Pauwels 1997) a significantly higher number of patients experienced severe exacerbations when receiving BUD 200 mcg/d compared to 800 mcg/d RR 1.5 (95% CI, 1.2 to 1.8), although this was not reflected in differences in number of patients hospitalised due to exacerbation: RR 0.6 (95% CI, 0.2 to 2.0).
Significantly lower daytime symptom scores and night‐time rescue beta2 agonist use were apparent in the higher dose group compared to the lower dose group. The primary outcome measure in (Pauwels 1997a,Pauwels 1997b) was an annualised rate of severe and mild exacerbations (exacerbations/patient/year). A significantly lower rate was apparent in the higher dose BUD dose group corresponding to approximately one less severe exacerbation every 2‐3 years and 8‐13 less mild exacerbations every year for BUD 800 mcg/d compared to BUD 200 mcg/d.
Three crossover design studies assessed the relative effects of BUD 200 v 800 mcg/d. Two studies were conducted in children and one in adult patients. All were small (12 ‐ 33 subjects) and of variable quality (Jadad scores 2‐4). Patients recruited were ICS naïve with mild asthma (Swystun 1998, Wolthers 1991) or had moderately severe disease already receiving 200‐400 mcg/d of an ICS (Bisgaard 1991). The degree of control (good) was clear in only one study (Swystun 1998). FEV1 was assessed in all three studies, but complete data for this outcome was available from only one (Swystun 1998) so a pooled treatment effect could not be calculated. A significant difference between doses was not apparent in two studies (Swystun 1998, Wolthers 1991). In one study (Bisgaard 1991) effects on FEV1 were difficult to assess due to the way in which results were presented. Individual studies assessed further outcomes: no significant differences were apparent between daily doses for allergen and methacholine BHR PC15 FEV1 (Swystun 1998), 24 hour urinary free cortisol (Bisgaard 1991, Wolthers 1991), morning PEFR, evening PEFR or rescue beta2 agonist use (Wolthers 1991).
400 v 1600 mcg/d A single parallel design study (Pedersen 1996) in ICS naïve adult asthmatics with mild, sup‐optimally controlled disease assessed the relative efficacy of BUD 400 v 1600 mcg/d. This study was of lower methodological quality (Jadad score 2). No significant differences in FEV1 (% predicted), histamine BHR or blood eosinophil count were apparent between treatment groups.
800 v 1600 mcg/d Significantly higher serum cortisol was apparent for the lower dose: WMD 114 nmol/L (26 to 203). Two studies undertaken in adults contributed to this analysis (Aaronson 1998, Turkas 1995). No significant differences between doses were apparent for cortisol levels at 6 hours post co‐syntropin infusion.
Oral steroid treated asthmatics
Two large (> 400 subjects) parallel design studies of 12 weeks duration assessed the relative efficacy and safety of BUD over a range of different doses. A significant proportion of patients in each study were oral steroid treated but no attempt was made to taper prednisolone use. Both were of fair methodological quality (Jadad score 3) and studied either adult patients (Busse 1998) or children (Shapiro 1998b) who already required large doses of ICS and/or regular oral steroids for asthma control. Each assessed the relative efficacy of BUD 200, 400 and 800 mcg/d. Busse assessed a fourth active treatment arm of BUD 1600 mcg/d 1998. No outcome data were presented in a form that allowed pooling in the meta‐analysis. In both studies outcomes were reported as change compared to baseline.
Significant differences in certain outcomes between high and low dose BUD were apparent when comparing the highest and lowest daily doses. Differences were consistently in favour of the higher dose. In the case of Busse 1998 this was BUD 1600 v 200 mcg/d (eight fold dose difference); for Shapiro 1998 BUD 800 v 200 mcg/d (four fold dose difference). The results of these studies are summarised below:
Busse 1998 BUD 1600 v 200 mcg/d Improvement in FEV1 compared to baseline: 0.13 litres( p<0.05) Improvement in morning PEFR compared to baseline: 18 L/min (p<0.01)
Shapiro 1998b BUD 800 mcg/d v 200 mcg/d Improvement in FEV1 compared to baseline: 4 (%predicted) (p=0.015) Improvement in morning PEFR compared to baseline: 2.3 (% predicted) (p<0.001) Reduction in daily beta agonist use (puffs/day) 16% (p=0.036)
One study (Busse 1998) demonstrated a significantly increased risk of withdrawal due to asthma exacerbation for BUD 200 mcg/d v 1600 mcg/d RR (95% CI) 2.78 (1.2 to 6.3). No difference in the rate of withdrawal between BUD 800 mcg/d and 200 mcg/d was apparent (Shapiro 1998).
No significant differences were apparent between any daily dose in either study when assessing daytime and night‐time symptom scores, morning plasma cortisol and cortisol post co‐syntropin.
A single parallel group study (Laursen 1986) of lower quality (Jadad score 2) assessed the relative oral prednisolone sparing efficacy of BUD 1600 v 400 mcg/d in adult patients. The rationale by which decision was made to taper the daily dose was not clear. No significant difference in the absolute daily dose of oral prednisolone at end of treatment was apparent between the two groups WMD 2.0 mg (95% CI ‐0.6 to 4.6 mg).
A single large (159 subjects) parallel group study (Nelson 1998) of fair quality (Jadad score 3) assessed the relative oral prednisolone sparing efficacy of BUD 1600 v 800 mcg/d in adult patients. Prednisolone dose tapering was undertaken using a forced down titration approach (see: Included study characteristics). No significant differences were apparent between BUD doses for the percentage reduction in daily prednisolone dose compared to baseline or the number of patients able to discontinue prednisolone completely.
Three crossover design studies assessed the relative efficacy and safety of BUD 1600 v 400 mcg/d in oral steroid treated adults. Two studies (Johansson 1988, Tukiainen 1987) were of high quality (Jadad score 5) one of fair quality (Toogood 1984). However no studies employed an ICS free washout period. A number of common outcome measures were assessed. No significant differences in morning PEFR WMD ‐21 L/min (95% CI ‐63 to 21 L/min); evening PEFR WMD ‐15 L/min (95% CI ‐52 to 22 L/min) or daily beta2 agonist use WMD ‐0.1 puffs/day (95% CI ‐1.5 to 1.3) were apparent. No differences were apparent between BUD doses for daytime and night‐time symptom scores.
Individual studies assessed other outcomes. No differences in FEV1, FVC, or morning plasma cortisol or were apparent (Tukiainen 1987). Toogood 1984 assessed efficacy outcomes expressed as change compared to baseline. This study had a complex six period crossover design (see: Included study characteristics). Statistically significant advantages of high dose BUD over low dose BUD were apparent for change in FEV1 and change in FEF25‐75 compared to baseline. However, due to the way in which the results were presented it is difficult to quantitatively assess the difference between doses.
Discussion
Higher doses of inhaled BUD have been found to be more effective than lower doses of BUD (so demonstrating a dose response effect) in the following situations:
1. Treatment with BUD 400 mcg/d resulted in significant reductions in the fall in FEV1 following exercise when treating ICS naïve, symptomatic children compared to either 200 or 100 mcg/d (results based on a single, high quality study). The clinical value of the difference in effect between doses is difficult to assess, but it could reasonably argued that children with exercise related symptoms not controlled on low dose BUD (100‐200 mcg/d) should have their daily dose increased to 400 mcg/d.
2. Treatment of adults with BUD 800 mcg/d significantly reduced the number of exacerbations/patient/year relative to BUD 200 mcg/d in a single large study (Pauwels 1997a,Pauwels 1997b). The reductions appear to be clinically significant. An important caveat regarding these findings concerns the fact that patients enrolled to this study had demonstrated a requirement for moderately high doses of BDP prior to randomisation. It is possible therefore that these conclusions only apply to patients with moderate to severe asthma and cannot necessarily be extrapolated to asthmatics with mild disease. The study also demonstrated significant reductions in daytime asthma symptom score and night‐time rescue beta2 agonist use, but the clinical importance of these differences is harder to assess.
3. In adults and children with sub optimally controlled, severe asthma receiving oral corticosteroids and/or high dose ICS, improvements in FEV1, morning PEFR and rescue beta2 agonist use were apparent when high dose BUD 800‐1600 mcg was compared to low dose BUD 200 mcg/d. The differences in FEV1 are probably of clinical importance, the differences in PEFR less clearly so.
In non oral corticosteroid treated asthmatics there was no evidence of a clinically meaningful dose response effect for FEV1, morning PEFR, symptom scores or rescue beta2 agonist use. This seemed to be the case irrespective of the estimated severity of disease. Statistically significant but very small advantages of BUD 800 mcg/d over 200 mcg/d were found for FEV1 (2% predicted), and morning PEFR (2 L/min) in one study only, demonstrated using an ANCOVA analysis (Pauwels 1997a/Pauwels 1997b). These are probably not of clinical significance.
Treatment of non‐oral steroid treated adults with BUD 800 mcg/d resulted in significantly higher post treatment 24 hour urinary free cortisol excretion rates compared to 1600 mcg/d, suggesting dose‐dependent suppression over this range. However this was reported a single low quality study. Dose‐dependent reductions in serum cortisol level post co‐syntropin infusion were apparent over a dose range of 800 ‐ 3200 mcg/d. This again was the finding of a single (fair quality) study. The clinical relevance of perturbations in these sensitive measures of HPA function is not clear. No trials have assessed the long term risk of adrenal crisis in children or adults treated with different doses of BUD (or indeed any other ICS).
In oral steroid treated asthmatics no dose dependent oral steroid sparing effect was apparent. This concerned comparisons over the dose range 1600 v 800 mcg/d and 1600 v 400 mcg/d.
Methodological limitations:
Assessment of the relative efficacy of BUD at different doses is difficult because a wide range of doses are available, so a large number of dose combinations are possible. Baseline severity and symptom control are likely to be important determinants of relative response, depending on the particular outcome assessed. If three levels of severity are defined (mild, moderate, severe), two levels of control (well controlled, poorly controlled), six nominal daily doses (100 ‐1600 mcg/d) and two age groups (children and adults) there are 180 possible combinations. In other words 180 separate studies would need to be conducted to assess each dose combination in each given clinical situation. If a distinction between studies of different duration is made (<1‐4 weeks, 1‐5 months, > 6 months) using different delivery device (MDI, DPI, MDI+spacer) this number rises to over 1600. The total number of studies included in this analysis is only 24. Assessment of different doses under all conditions has clearly not been covered and the reader should be aware of this limitation when assessing the results of this review.
Outcome measures common to different studies were often reported in different ways. Absolute values (e.g. FEV1 in litres), standardised values (e.g. FEV1 as % predicted) or change in either of these metrics compared to baseline were used. This factor, combined with the fact that a significant proportion of results were incompletely reported (e.g. no standard deviations around means) limited the poolable data and hence the power of the meta‐analysis.
Too few studies were available to undertake meaningful subgroup analyses to assess the influence of patient age, delivery device or study duration on the results of analysis.
Authors' conclusions
Implications for practice.
There appears to be no benefit of higher doses of budesonide in mild asthma. Clinically meaningful improvements in asthma control may be achieved when patients with moderate to severe asthma are treated with doses in the range 800‐1600 mcg/d. This effect appears to be greatest in terms of the effect on exacerbations. This review supports current guideline recommendations that the dose of inhaled corticosteroids should be increased in patients who demonstrate inadequate control. There is no evidence for increased effect in doses > 1600 mcg/d. There is a lack of reliable evidence concerning the relative safety of different doses in terms of their long‐term effect on HPA function.
Implications for research.
1. Quantitative meta analyses concerning the relative efficacy and safety of inhaled corticosteroids would be improved by more complete reporting of numerical data in the published reports of primary studies. 2. Assessment of the influence of baseline severity and asthma control when assessing relative efficacy and safety measures would be improved by the adoption of a standardised scale of symptom frequency and uniform reporting of baseline FEV1 (% predicted) in primary studies. 3. Assessment of the impact of different doses of BUD on sensitive respiratory disease specific quality of life instruments is lacking and would be a useful area of future research.
What's new
| Date | Event | Description |
|---|---|---|
| 21 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 4 December 1999 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Anna Bara for running the initial electronic search, Steve Milan for assistance with statistical methods. We also like to thank Dr L Campbell, Dr S Johansson, Dr G Jonasson, Professor E Juniper, Dr H Nelson, Professor R Pauwels, Professor P Venge, Dr V Swystun, Professor J Toogood, Dr P Tukiainen, Dr I Turktas and Dr T van der Molen who were kind enough to provide additional information regarding their studies.
Data and analyses
Comparison 1. BUD v BUD: Parallel design, not on oral steroids: 100 v 200 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximal percentage fall in FEV1 post 6 minute exercise test | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐2.05, 3.45] |
| 1.1 Children | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐2.05, 3.45] |
| 1.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Withdrawal due to asthma exacerbation (No. of patients) | 1 | 81 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Children | 1 | 81 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
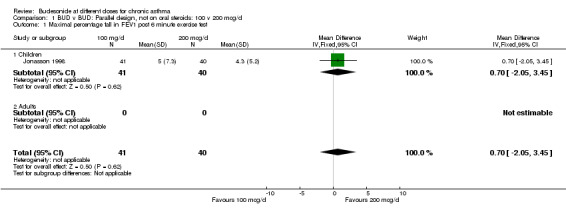
Comparison 1 BUD v BUD: Parallel design, not on oral steroids: 100 v 200 mcg/d, Outcome 1 Maximal percentage fall in FEV1 post 6 minute exercise test.
1.2. Analysis.
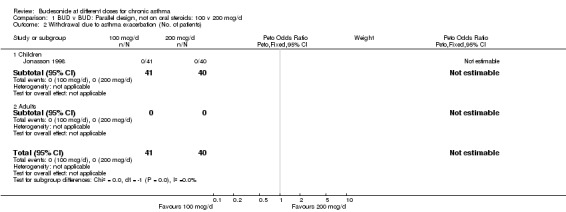
Comparison 1 BUD v BUD: Parallel design, not on oral steroids: 100 v 200 mcg/d, Outcome 2 Withdrawal due to asthma exacerbation (No. of patients).
Comparison 2. BUD v BUD: Crossover design, not on oral steroids: 200 v 400 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.57, 0.81] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.57, 0.81] |
| 2 24 hour Urinary free cortisol excretion corrected for creatinine (nmol cortisol/mmol creatinine) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.44, 2.64] |
| 2.1 Children | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.44, 2.64] |
| 2.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
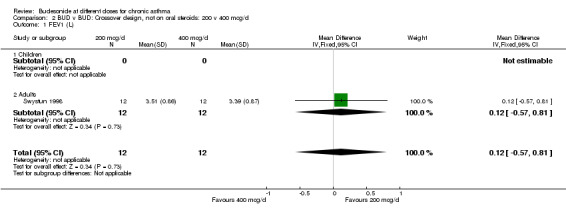
Comparison 2 BUD v BUD: Crossover design, not on oral steroids: 200 v 400 mcg/d, Outcome 1 FEV1 (L).
2.2. Analysis.
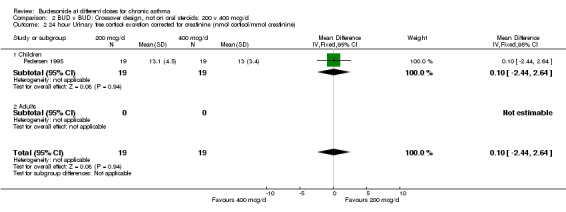
Comparison 2 BUD v BUD: Crossover design, not on oral steroids: 200 v 400 mcg/d, Outcome 2 24 hour Urinary free cortisol excretion corrected for creatinine (nmol cortisol/mmol creatinine).
Comparison 3. BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morning PEFR (L/min) | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | ‐4.00 [‐25.20, 13.20] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | ‐4.00 [‐25.20, 13.20] |
| 2 Change in morning PEFR compared to baseline (L/min) | 1 | 628 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐21.73, 1.73] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 628 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐21.73, 1.73] |
| 3 Evening PEFR (L/min) | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐30.04, 8.04] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐30.04, 8.04] |
| 4 Experience of cough, wheeze and/or breathlessness (days/week) | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.10, 0.90] |
| 4.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.10, 0.90] |
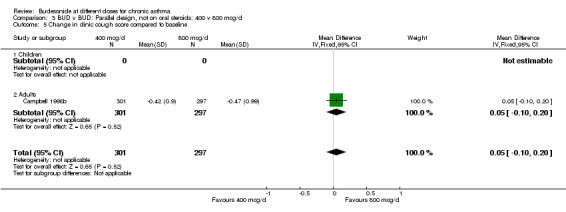
| 5 Change in clinic cough score compared to baseline | 1 | 598 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.10, 0.20] |
| 5.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 1 | 598 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.10, 0.20] |
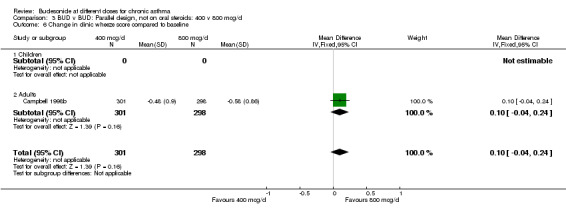
| 6 Change in clinic wheeze score compared to baseline | 1 | 599 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.04, 0.24] |
| 6.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 1 | 599 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.04, 0.24] |
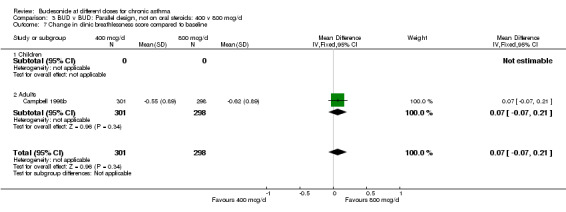
| 7 Change in clinic breathlessness score compared to baseline | 1 | 599 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| 7.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 1 | 599 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
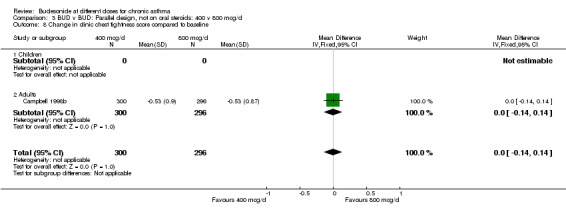
| 8 Change in clinic chest tightness score compared to baseline | 1 | 596 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.14, 0.14] |
| 8.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 1 | 596 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.14, 0.14] |
| 9 Daily use of beta2 agonists (puffs/day) | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.10, 0.50] |
| 9.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.10, 0.50] |
| 10 Change in daytime asthma symptom score compared to baseline | 1 | 625 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 10.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults | 1 | 625 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 11 Change in night‐time asthma symptom score compared to baseline | 1 | 631 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 11.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Adults | 1 | 631 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 12 Withdrawal due to asthma exacerbation (No. of patients) | 2 | 582 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 Children | 1 | 26 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Adults | 1 | 556 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
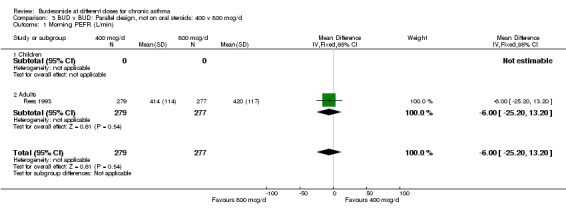
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 1 Morning PEFR (L/min).
3.2. Analysis.
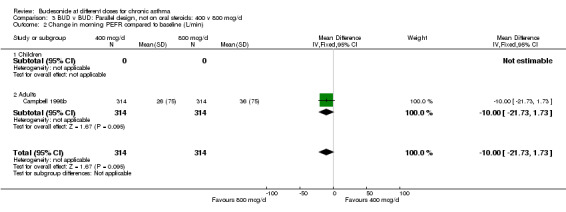
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 2 Change in morning PEFR compared to baseline (L/min).
3.3. Analysis.
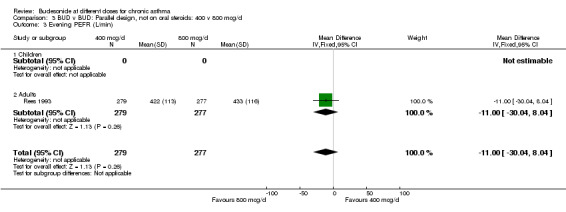
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 3 Evening PEFR (L/min).
3.4. Analysis.
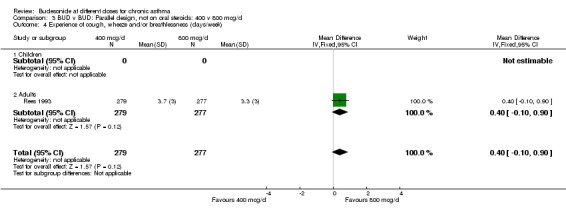
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 4 Experience of cough, wheeze and/or breathlessness (days/week).
3.5. Analysis.
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 5 Change in clinic cough score compared to baseline.
3.6. Analysis.
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 6 Change in clinic wheeze score compared to baseline.
3.7. Analysis.
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 7 Change in clinic breathlessness score compared to baseline.
3.8. Analysis.
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 8 Change in clinic chest tightness score compared to baseline.
3.9. Analysis.
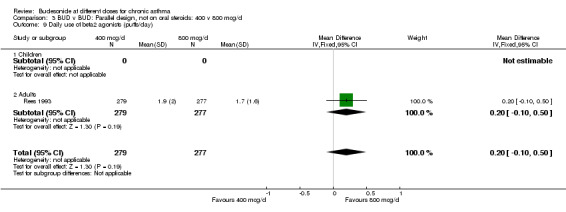
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 9 Daily use of beta2 agonists (puffs/day).
3.10. Analysis.
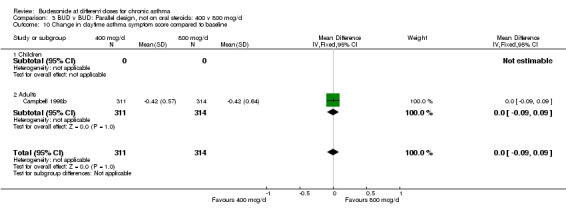
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 10 Change in daytime asthma symptom score compared to baseline.
3.11. Analysis.
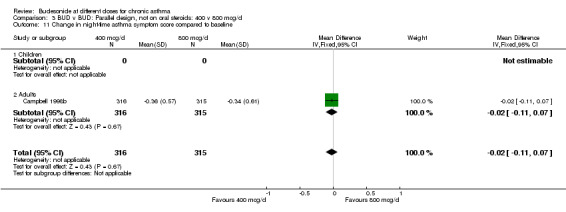
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 11 Change in night‐time asthma symptom score compared to baseline.
3.12. Analysis.
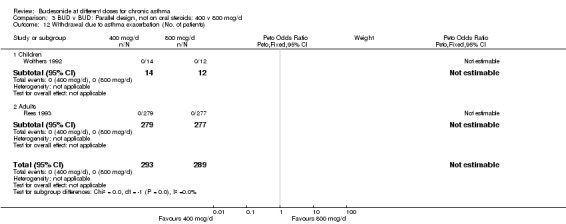
Comparison 3 BUD v BUD: Parallel design, not on oral steroids: 400 v 800 mcg/d, Outcome 12 Withdrawal due to asthma exacerbation (No. of patients).
Comparison 4. BUD v BUD: Crossover design, not on oral steroids: 400 v 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.81, 0.57] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.81, 0.57] |
4.1. Analysis.
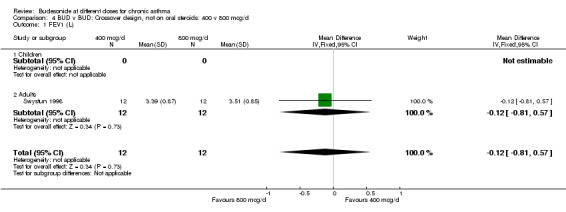
Comparison 4 BUD v BUD: Crossover design, not on oral steroids: 400 v 800 mcg/d, Outcome 1 FEV1 (L).
Comparison 5. BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 4 | 940 | Mean Difference (IV, Fixed, 95% CI) | ‐1.39 [‐3.58, 0.80] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 4 | 940 | Mean Difference (IV, Fixed, 95% CI) | ‐1.39 [‐3.58, 0.80] |
| 2 Morning PEFR (L/min) | 3 | 906 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐16.10, 13.13] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 3 | 906 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐16.10, 13.13] |
| 3 Evening PEFR (L/min) | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 7.95 [‐35.43, 51.33] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 7.95 [‐35.43, 51.33] |
| 4 Methacholine bronchial responsiveness (log 10 PC20 FEV1) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.59, 0.09] |
| 4.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.59, 0.09] |
| 5 Daytime asthma symptom score | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.32, 0.20] |
| 5.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.32, 0.20] |
| 6 Daily use of beta2 agonists (puffs/day) | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.60, 0.46] |
| 6.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.60, 0.46] |
| 7 Blood eosinophil count (x10 9/L) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.06, 0.22] |
| 7.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.06, 0.22] |
| 8 Patients with severe exacerbation | 2 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.18, 1.81] |
| 8.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 2 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.18, 1.81] |
| 9 Withdrawal due to asthma exacerbation (No. of patients) | 4 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.93 [1.41, 10.94] |
| 9.1 Children | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 3 | 882 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.93 [1.41, 10.94] |
| 10 Hospital admission due to asthma exacerbation (No. of patients) | 2 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.17, 1.95] |
| 10.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults | 2 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.17, 1.95] |
5.1. Analysis.
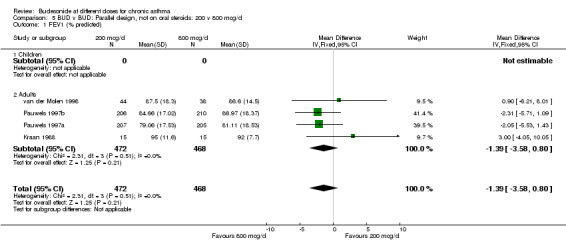
Comparison 5 BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d, Outcome 1 FEV1 (% predicted).
5.2. Analysis.
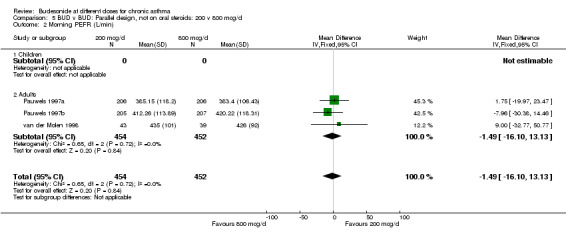
Comparison 5 BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d, Outcome 2 Morning PEFR (L/min).
5.3. Analysis.
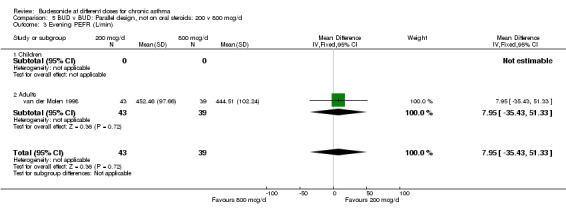
Comparison 5 BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d, Outcome 3 Evening PEFR (L/min).
5.4. Analysis.
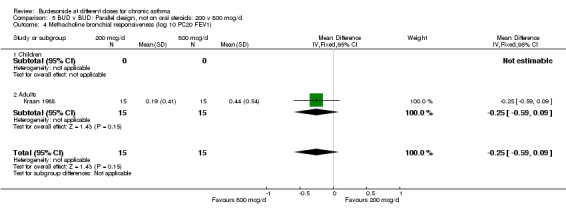
Comparison 5 BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d, Outcome 4 Methacholine bronchial responsiveness (log 10 PC20 FEV1).
5.5. Analysis.
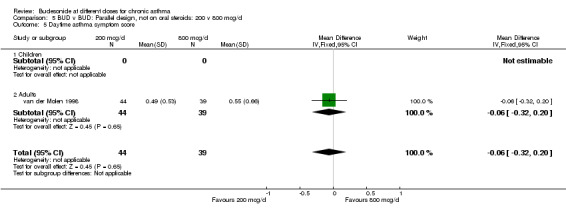
Comparison 5 BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d, Outcome 5 Daytime asthma symptom score.
5.6. Analysis.
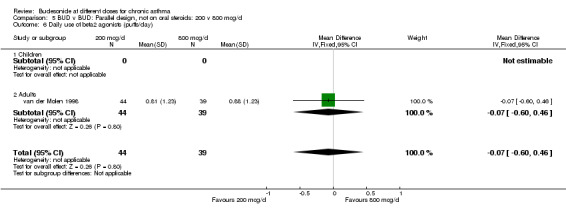
Comparison 5 BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d, Outcome 6 Daily use of beta2 agonists (puffs/day).
5.7. Analysis.
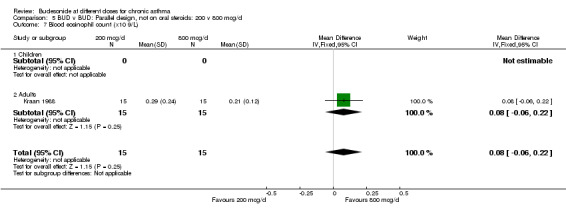
Comparison 5 BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d, Outcome 7 Blood eosinophil count (x10 9/L).
5.8. Analysis.
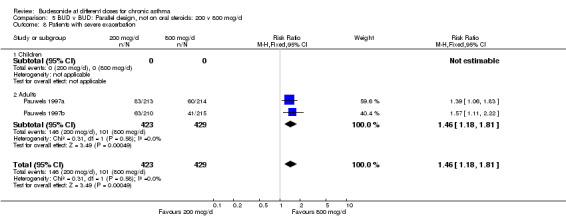
Comparison 5 BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d, Outcome 8 Patients with severe exacerbation.
5.9. Analysis.
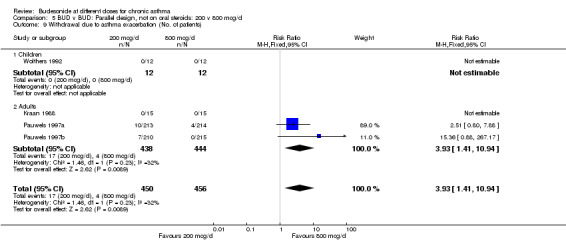
Comparison 5 BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d, Outcome 9 Withdrawal due to asthma exacerbation (No. of patients).
5.10. Analysis.
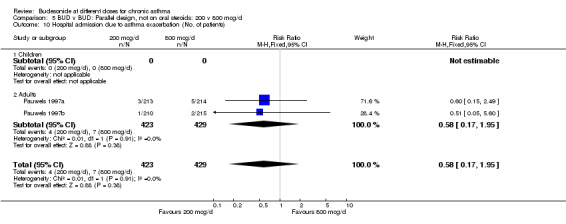
Comparison 5 BUD v BUD: Parallel design, not on oral steroids: 200 v 800 mcg/d, Outcome 10 Hospital admission due to asthma exacerbation (No. of patients).
Comparison 6. BUD v BUD: Crossover design, not on oral steroids: 200 v 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.68, 0.68] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.68, 0.68] |
6.1. Analysis.
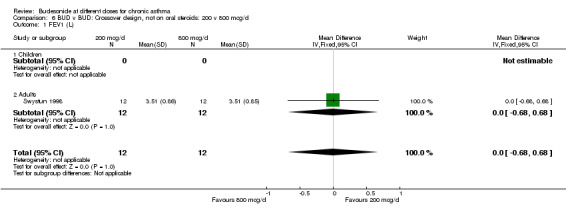
Comparison 6 BUD v BUD: Crossover design, not on oral steroids: 200 v 800 mcg/d, Outcome 1 FEV1 (L).
Comparison 7. BUD v BUD: Parallel design, not on oral steroids: 400 v 1600 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Histamine bronchial responsiveness (log 10 PC20 FEV1) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.64, 0.24] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.64, 0.24] |
| 2 FEV1 (% predicted) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐21.98, 3.98] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐21.98, 3.98] |
| 3 Blood eosinophil count (x10 9/L) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.06] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.06] |
7.1. Analysis.
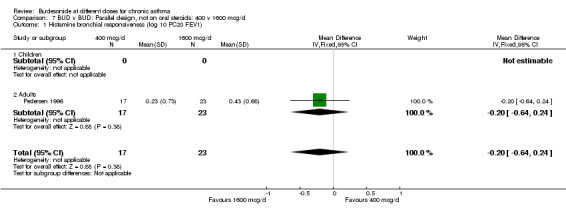
Comparison 7 BUD v BUD: Parallel design, not on oral steroids: 400 v 1600 mcg/d, Outcome 1 Histamine bronchial responsiveness (log 10 PC20 FEV1).
7.2. Analysis.
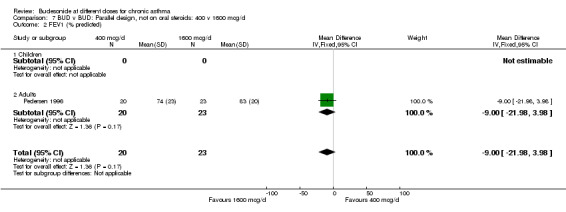
Comparison 7 BUD v BUD: Parallel design, not on oral steroids: 400 v 1600 mcg/d, Outcome 2 FEV1 (% predicted).
7.3. Analysis.
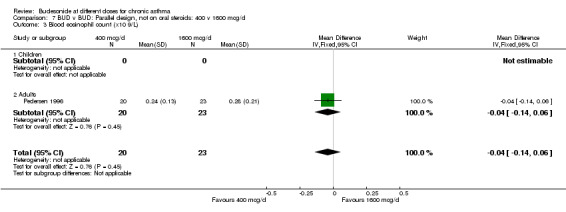
Comparison 7 BUD v BUD: Parallel design, not on oral steroids: 400 v 1600 mcg/d, Outcome 3 Blood eosinophil count (x10 9/L).
Comparison 8. BUD v BUD: Parallel design, not on oral steroids: 800 v 1600 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum cortisol (nmol/L) | 2 | 41 | Mean Difference (IV, Fixed, 95% CI) | 114.49 [26.09, 202.89] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 2 | 41 | Mean Difference (IV, Fixed, 95% CI) | 114.49 [26.09, 202.89] |
| 2 24 hour urinary free cortisol (nmol) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 68.0 [46.32, 89.68] |
| 2.1 children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 68.0 [46.32, 89.68] |
| 3 Plasma cortisol at 6 hours following iv co‐syntropin infusion (nmol/L) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 65.0 [‐64.24, 194.24] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 65.0 [‐64.24, 194.24] |
| 4 Withdrawal due to asthma exacerbation (No. of patients) | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
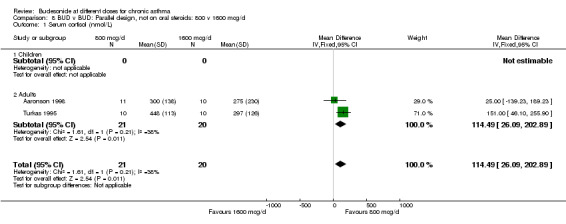
Comparison 8 BUD v BUD: Parallel design, not on oral steroids: 800 v 1600 mcg/d, Outcome 1 Serum cortisol (nmol/L).
8.2. Analysis.
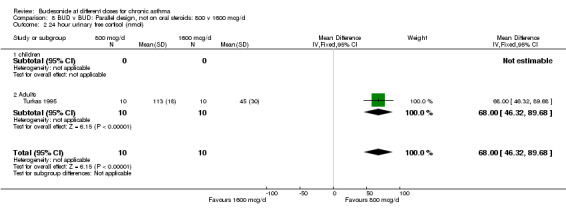
Comparison 8 BUD v BUD: Parallel design, not on oral steroids: 800 v 1600 mcg/d, Outcome 2 24 hour urinary free cortisol (nmol).
8.3. Analysis.
Comparison 8 BUD v BUD: Parallel design, not on oral steroids: 800 v 1600 mcg/d, Outcome 3 Plasma cortisol at 6 hours following iv co‐syntropin infusion (nmol/L).
8.4. Analysis.
Comparison 8 BUD v BUD: Parallel design, not on oral steroids: 800 v 1600 mcg/d, Outcome 4 Withdrawal due to asthma exacerbation (No. of patients).
Comparison 9. BUD v BUD: Parallel design, not on oral steroids: 1600 v 3200 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morning plasma cortisol (nmol/L) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 80.0 [‐107.70, 267.70] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 80.0 [‐107.70, 267.70] |
| 2 Plasma cortisol at 6 hours following iv co‐syntropin infusion (nmol/L) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 118.0 [‐16.60, 252.60] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 118.0 [‐16.60, 252.60] |
9.1. Analysis.
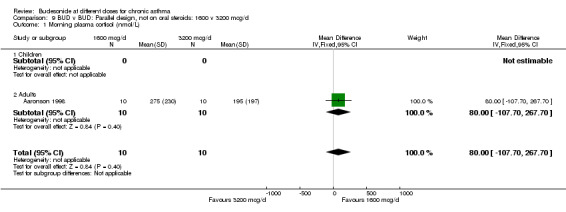
Comparison 9 BUD v BUD: Parallel design, not on oral steroids: 1600 v 3200 mcg/d, Outcome 1 Morning plasma cortisol (nmol/L).
9.2. Analysis.
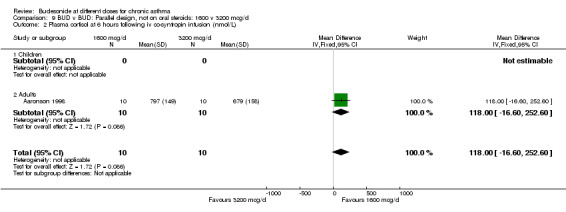
Comparison 9 BUD v BUD: Parallel design, not on oral steroids: 1600 v 3200 mcg/d, Outcome 2 Plasma cortisol at 6 hours following iv co‐syntropin infusion (nmol/L).
Comparison 10. BUD v BUD: Parallel design, not on oral steroids: 800 v 3200 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morning plasma cortisol (nmol/L) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 105.0 [‐41.83, 251.83] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 105.0 [‐41.83, 251.83] |
| 2 Plasma cortisol at 6 hours following iv co‐syntropin infusion (nmol/L) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 183.0 [49.72, 316.28] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 183.0 [49.72, 316.28] |
10.1. Analysis.
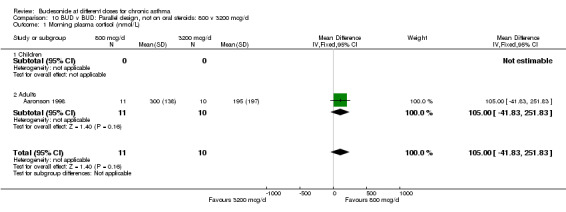
Comparison 10 BUD v BUD: Parallel design, not on oral steroids: 800 v 3200 mcg/d, Outcome 1 Morning plasma cortisol (nmol/L).
10.2. Analysis.
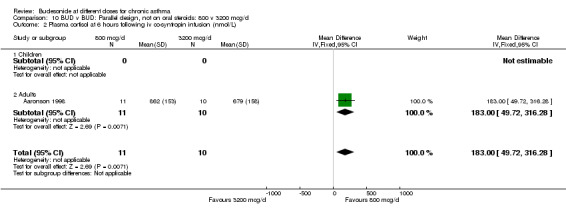
Comparison 10 BUD v BUD: Parallel design, not on oral steroids: 800 v 3200 mcg/d, Outcome 2 Plasma cortisol at 6 hours following iv co‐syntropin infusion (nmol/L).
Comparison 11. BUD v BUD: Parallel design, oral steroid treated: 200 v 400 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawal due to asthma exacerbation (No. of patients) | 2 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.76, 2.15] |
| 1.1 Children | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.80] |
| 1.2 Adults | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.90, 3.77] |
11.1. Analysis.
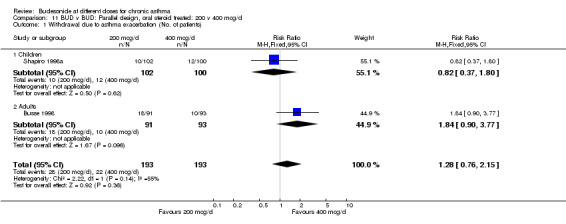
Comparison 11 BUD v BUD: Parallel design, oral steroid treated: 200 v 400 mcg/d, Outcome 1 Withdrawal due to asthma exacerbation (No. of patients).
Comparison 12. BUD v BUD: Parallel design, oral steroid treated: 400 v 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawal due to asthma exacerbation (No. of patients) | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.56, 1.69] |
| 1.1 Children | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.39, 1.61] |
| 1.2 Adults | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.55, 3.23] |
12.1. Analysis.
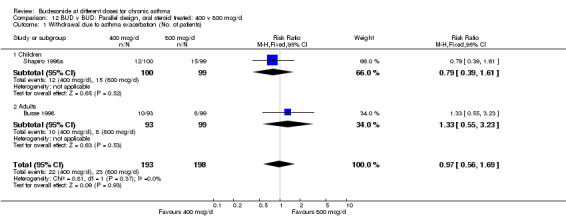
Comparison 12 BUD v BUD: Parallel design, oral steroid treated: 400 v 800 mcg/d, Outcome 1 Withdrawal due to asthma exacerbation (No. of patients).
Comparison 13. BUD v BUD: Parallel design, oral steroid treated: 200 v 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawal due to asthma exacerbation (No. of patients) | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.75, 2.09] |
| 1.1 Children | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.31, 1.37] |
| 1.2 Adults | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.12, 5.35] |
13.1. Analysis.
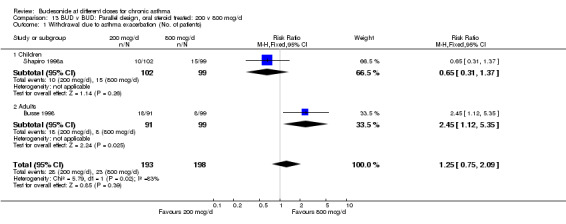
Comparison 13 BUD v BUD: Parallel design, oral steroid treated: 200 v 800 mcg/d, Outcome 1 Withdrawal due to asthma exacerbation (No. of patients).
Comparison 14. BUD v BUD: Parallel design, oral steroid treated: 400 v 1600 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Daily dose of oral prednisolone (mg) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.63, 4.63] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.63, 4.63] |
| 2 Withdrawal due to asthma exacerbation (No. of patients) | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.60, 3.79] |
| 2.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.60, 3.79] |
14.1. Analysis.
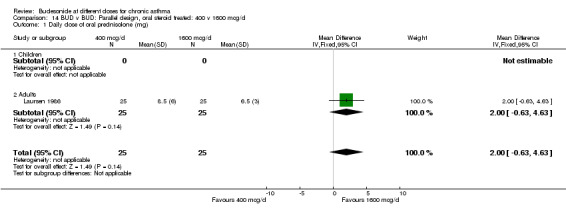
Comparison 14 BUD v BUD: Parallel design, oral steroid treated: 400 v 1600 mcg/d, Outcome 1 Daily dose of oral prednisolone (mg).
14.2. Analysis.
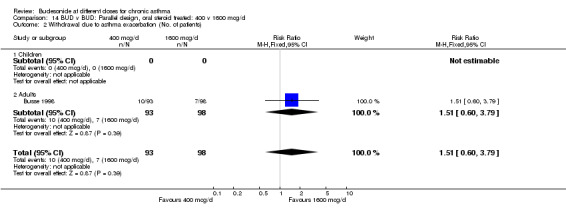
Comparison 14 BUD v BUD: Parallel design, oral steroid treated: 400 v 1600 mcg/d, Outcome 2 Withdrawal due to asthma exacerbation (No. of patients).
Comparison 15. BUD v BUD: Parallel design, oral steroid treated: 800 v 1600 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation of oral steroids (No. of patients) | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
| 1.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
15.1. Analysis.
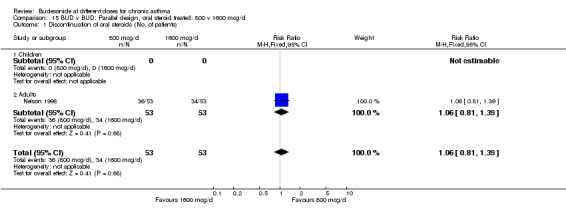
Comparison 15 BUD v BUD: Parallel design, oral steroid treated: 800 v 1600 mcg/d, Outcome 1 Discontinuation of oral steroids (No. of patients).
Comparison 16. BUD v BUD: Crossover design, oral steroid treated: 400 v 1600 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.72, 0.22] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.72, 0.22] |
| 2 FVC (litres) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.96, 0.30] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.96, 0.30] |
| 3 Morning PEFR (L/min) | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐20.62 [‐63.01, 21.78] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐20.62 [‐63.01, 21.78] |
| 4 Evening PEFR (L/min) | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐15.29 [‐52.33, 21.75] |
| 4.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐15.29 [‐52.33, 21.75] |
| 5 Daily beta2 agonist use (puffs/day) | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐1.51, 1.34] |
| 5.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐1.51, 1.34] |
| 6 Daytime breathlessness score | 2 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.43, 0.43] |
| 6.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 2 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.43, 0.43] |
| 7 Night‐time breathlessness score | 2 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.31, 0.55] |
| 7.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 2 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.31, 0.55] |
16.1. Analysis.
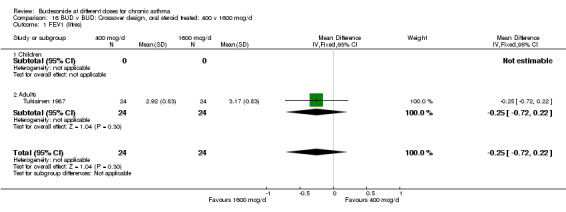
Comparison 16 BUD v BUD: Crossover design, oral steroid treated: 400 v 1600 mcg/d, Outcome 1 FEV1 (litres).
16.2. Analysis.
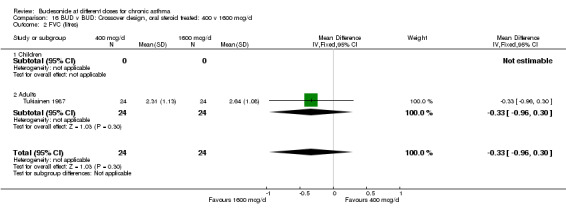
Comparison 16 BUD v BUD: Crossover design, oral steroid treated: 400 v 1600 mcg/d, Outcome 2 FVC (litres).
16.3. Analysis.
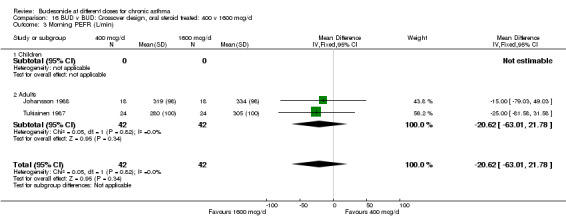
Comparison 16 BUD v BUD: Crossover design, oral steroid treated: 400 v 1600 mcg/d, Outcome 3 Morning PEFR (L/min).
16.4. Analysis.
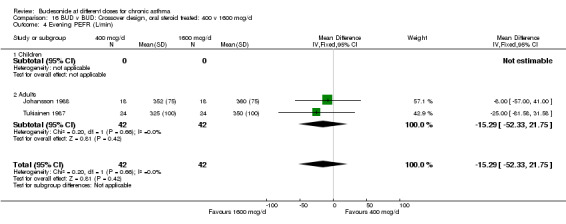
Comparison 16 BUD v BUD: Crossover design, oral steroid treated: 400 v 1600 mcg/d, Outcome 4 Evening PEFR (L/min).
16.5. Analysis.
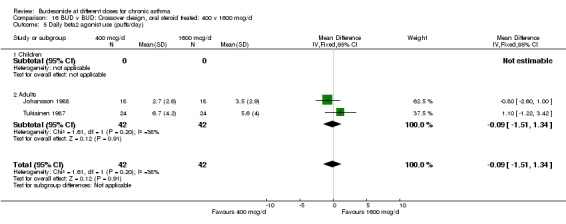
Comparison 16 BUD v BUD: Crossover design, oral steroid treated: 400 v 1600 mcg/d, Outcome 5 Daily beta2 agonist use (puffs/day).
16.6. Analysis.
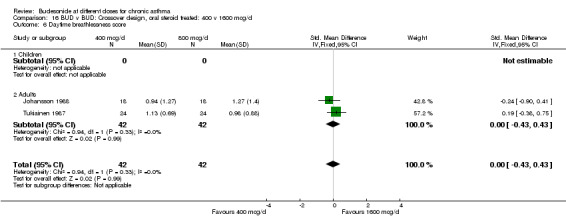
Comparison 16 BUD v BUD: Crossover design, oral steroid treated: 400 v 1600 mcg/d, Outcome 6 Daytime breathlessness score.
16.7. Analysis.
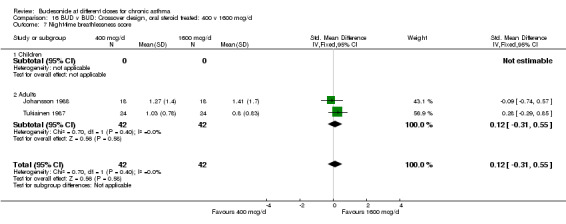
Comparison 16 BUD v BUD: Crossover design, oral steroid treated: 400 v 1600 mcg/d, Outcome 7 Night‐time breathlessness score.
Comparison 17. BUD v BUD: Crossover design, not on oral steroids: 100 v 400 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 24 hour Urinary free cortisol excretion corrected for creatinine (nmol cortisol/mmol creatinine) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐0.98, 4.38] |
| 1.1 Children | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐0.98, 4.38] |
| 1.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
17.1. Analysis.
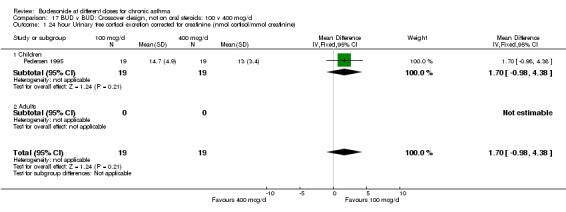
Comparison 17 BUD v BUD: Crossover design, not on oral steroids: 100 v 400 mcg/d, Outcome 1 24 hour Urinary free cortisol excretion corrected for creatinine (nmol cortisol/mmol creatinine).
Comparison 18. BUD v BUD: Parallel design, oral steroid treated: 200 v 1600 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawal due to asthma exacerbation (No. of patients) | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.21, 6.32] |
| 1.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.21, 6.32] |
18.1. Analysis.
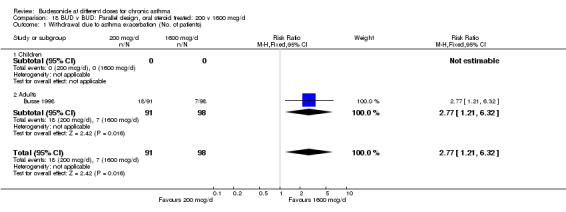
Comparison 18 BUD v BUD: Parallel design, oral steroid treated: 200 v 1600 mcg/d, Outcome 1 Withdrawal due to asthma exacerbation (No. of patients).
Comparison 19. BUD v BUD: Parallel studies, not on oral steroids: low v high dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) and FEV1 (% predicted) combined | 5 | 983 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.23, 0.02] |
| 1.1 200 v 800 mcg/d | 4 | 940 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.22, 0.03] |
| 1.2 400 v 1600 mcg/d | 1 | 43 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.02, 0.19] |
| 2 Morning PEFR (L/min) | 4 | 1462 | Mean Difference (IV, Fixed, 95% CI) | ‐3.14 [‐14.77, 8.49] |
| 2.1 400 v 800 mcg/d | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | ‐4.00 [‐25.20, 13.20] |
| 2.2 200 v 800 mcg/d | 3 | 906 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐16.10, 13.13] |
| 3 Evening PEFR (L/min) | 2 | 638 | Mean Difference (IV, Fixed, 95% CI) | ‐7.94 [‐25.37, 9.49] |
| 3.1 400 v 800 mcg/d | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐30.04, 8.04] |
| 3.2 200 v 800 mcg/d | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 7.95 [‐35.43, 51.33] |
| 4 Daily use of beta2 agonists (puffs/day) | 2 | 639 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.13, 0.40] |
| 4.1 400 v 800 mcg/d | 1 | 556 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.10, 0.50] |
| 4.2 200 v 800 mcg/d | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.60, 0.46] |
| 5 Withdrawal due to asthma exacerbation (No. of patients) | 7 | 1568 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.93 [1.41, 10.94] |
| 5.1 100 v 200 mcg/d | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 400 v 800 mcg/d | 1 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 200 v 800 mcg/d | 4 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.93 [1.41, 10.94] |
| 5.4 800 v 1600 mcg/d | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
19.1. Analysis.
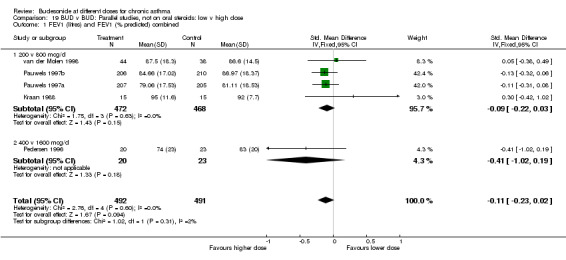
Comparison 19 BUD v BUD: Parallel studies, not on oral steroids: low v high dose, Outcome 1 FEV1 (litres) and FEV1 (% predicted) combined.
19.2. Analysis.
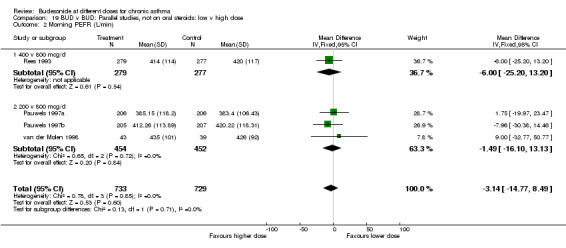
Comparison 19 BUD v BUD: Parallel studies, not on oral steroids: low v high dose, Outcome 2 Morning PEFR (L/min).
19.3. Analysis.
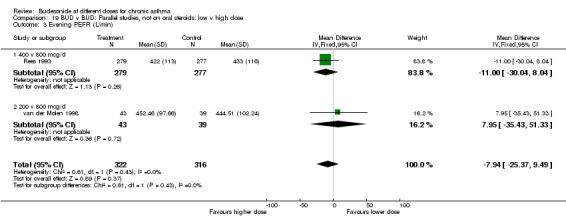
Comparison 19 BUD v BUD: Parallel studies, not on oral steroids: low v high dose, Outcome 3 Evening PEFR (L/min).
19.4. Analysis.
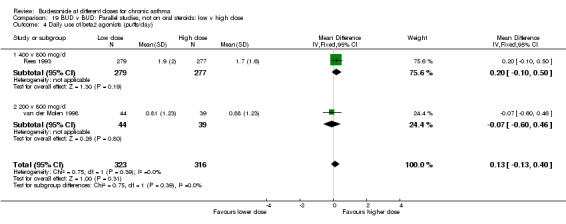
Comparison 19 BUD v BUD: Parallel studies, not on oral steroids: low v high dose, Outcome 4 Daily use of beta2 agonists (puffs/day).
19.5. Analysis.
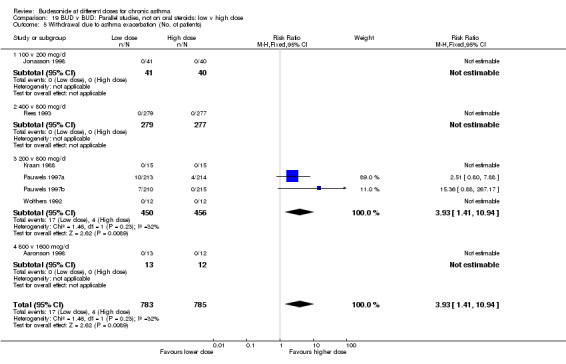
Comparison 19 BUD v BUD: Parallel studies, not on oral steroids: low v high dose, Outcome 5 Withdrawal due to asthma exacerbation (No. of patients).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aaronson 1998.
| Methods | Setting: multicentre study, USA, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Length of intervention period: 6 weeks Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 64 adults: 49M 15F
Age range: 18‐56 years
Inclusion criteria:
Adults patients with diagnosis of asthma (not otherwise defined)
FEV1 (% predicted) 65 or greater
Able to use Turbuhaler effectively and achieve inspiratory flow rate of at least 50 litres/min
Exclusion criteria:
Hospitalization due to asthma or respiratory tract infection in last 4 weeks
Serious concurrent illness
Any corticosteroid therapy in last 6 months or investigational drug in last 4 weeks
Significantly abnormal cortisol levels (basal or post cosyntropin)
Pregnancy/lactation Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): >65 |
|
| Interventions | 1. BUD 200 mcg 2 pfs 2xdaily (800 mcg/d) via Turbuhaler DPI 2. BUD 400 mcg 2pfs 2xdaily (1600 mcg/d) via Turbuhaler DPI 3. BUD 800 mcg 2 pfs 2xdaily (3200 mcg/d) via Turbuhaler DPI all with placebo tablet 1xdaily to maintain blinding |
|
| Outcomes | Basal plasma cortisol Plasma cortisol at 6 hours following continuous iv cosyntropin infusion, 0.25 mg/500 ml saline over 6 hours Withdrawal due to asthma exacerbation |
|
| Notes | No reply from author to clarify details of randomisation method. Placebo and oral prednisolone intervention groups were also studies: results not considered here. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Agertoft 1997.
| Methods | Setting: Denmark, paediatric outpatient clinic Randomisation: yes, computer generated algorithm Allocation concealment: unclear Design: crossover, 2 week washout Length of intervention period: 2 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 48 children: 27M 21F
Age range: 6 to 12 years
Inclusion criteria:
Clinical diagnosis of mild asthma requiring treatment with inhaled beta2 agonist only
Exclusion criteria:
Oral or inhaled corticosteroid use Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): not stated |
|
| Interventions | 1. BUD 200 mcg/day via Turbuhaler DPI 2. BUD 400 mcg/day via Turbuhaler DPI |
|
| Outcomes | Lower leg growth by knemometry Morning PEFR Evening PEFR Daytime asthma symptom score Night‐time asthma symptom score Urinary free cortisol excretion corrected for creatinine | |
| Notes | Reply from author but unwilling to clarify details of randomisation method or provide outcome data. Placebo and fluticasone groups were also studied: results not considered here. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bisgaard 1991.
| Methods | Setting Denmark, paediatric outpatient clinic. Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Length of intervention period: 8 weeks Masking: single blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: not described Jadad score: 2 | |
| Participants | 33 children: 14M 19F
Age range: 6‐15 years
Inclusion criteria:
Clinical diagnosis of asthma with a requirement for 200‐400 mcg/day of inhaled steroid
Exclusion criteria:
Oral steroid use in last year
Unable to use a metered dose inhaler Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): not stated |
|
| Interventions | 1. BUD 200 mcg/day via MDI 2. BUD 400 mcg/day via MDI 3. BUD 800 mcg/day via MDI All treatments delivered in divided twice daily regimen |
|
| Outcomes | FEV1 24 hour urinary free cortisol | |
| Notes | No reply from author to clarify details of randomisation method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Boe 1989.
| Methods | Setting: multicentre study Sweden, hospital outpatient clinic Randomisation: yes, using 4x4 Williams square Allocation concealment: unclear Masking: double blind Design: crossover, no washout Length of intervention period: 4 weeks Excluded: not stated Withdrawals: stated Baseline characteristics: no baseline demographic data presented Jadad score: 4 | |
| Participants | 128 adults: 67M 61F
Age range 18‐77 years
Inclusion criteria:
Asthma as defined by ATS criteria
Requirement for inhaled steroids in a dose range of 400‐800 micrograms
Exclusion criteria:
Cardiovascular disease or diabetes mellitus Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): not stated |
|
| Interventions | 1. BUD 200 mcg 2xdaily (400 mcg/d) 2.) BUD 400 mcg 2xdaily (800 mcg/d) Delivery device: MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Daily asthma symptom score | |
| Notes | No reply from author to clarify if allocation concealment had been employed. This study was designed as a four period crossover trial. Additional treatment periods with BDP 400 mcg/d and 1000 mcg/d were included: results not considered here. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Busse 1998.
| Methods | Setting: multicentre study USA, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Masking: double‐blind Design: parallel group Length of intervention period: 12 weeks Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | Subjects: 473 adults: 217M 256F
Age range: 18 ‐ 70 years
Inclusion criteria:
Diagnosis of asthma (American Thoracic Society criteria) for at least 6 months
15% reversibility of FEV1 with inhaled beta2 agonist or greater
Use of inhaled or inhaled and oral corticosteroids for at least 6 months
Use of between 300 and 1000 mcg daily inhaled BDP for 1 month prior to study
Exclusion criteria:
History of carcinoma or significant co‐existing disease
Use of injectable corticosteroids
Irregular use of oral corticosteroids Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): range 40‐75 |
|
| Interventions | 1. BUD 200 mcg daily 2. BUD 400 mcg daily 3. BUD 800 mcg daily 4. BUD 1600 mcg daily All doses delivered as twice daily regimen via Turbuhaler DPI | |
| Outcomes | Change in FEV1 compared to baseline Change morning PEFR compared to baseline Change evening PEFR compared to baseline Change daytime asthma symptom compared to baseline Change nighttime asthma symptom compared to baseline daily beta2 agonist use Early morning plasma cortisol Early morning plasma cortisol post ACTH | |
| Notes | No reply from author to clarify details of randomisation method. Placebo treatment group was also studied: results not considered here. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Campbell 1998b.
| Methods | Setting: multicentre study UK and Ireland, primary care Randomisation: yes, computer generated algorithm Allocation concealment: yes Design: parallel group Length of intervention period: 6 weeks Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 681 adults: 297M 384F
Mean (SD) age: 400 mcg/day group 33.3 (15.6) years, 800 mcg/day group 33.5 (13.8) years
Inclusion criteria:
Over 12 years of age with a diagnosis of asthma
Asthma symptoms 2/7 days prior to enrolment and symptoms and on at least 1/3 days of run‐in period
Use of inhaled beta2 agonist for at least 2 weeks and on at least 1/3 days of run‐in period
Requiring (in opinion of investigator) further treatment with an inhaled corticosteroid
15% diurnal variability in PEFR on at least 2/4 days of run‐in period
Exclusion criteria:
PEFR (% predicted) < 60
Treatment with beta2 blockers, sodium cromoglycate, nedocromil in last 3 months
Pregnancy/breast feeding Baseline asthma control Symptom frequency: symptoms on at least 1 out of 3 days during run‐in period FEV1 (% predicted): not stated |
|
| Interventions | 1. BUD 800 mcg/day for 6 weeks followed by 400 mcg/day for 12 weeks via Turbohaler DPI 2. BUD 400 mcg/day for 18 weeks via Turbohaler DPI Patients received appropriate number of actuations of additional placebo Turbohaler to maintain blinding |
|
| Outcomes | Change in morning PEFR compared to baseline Change in daytime use of beta2 agonists compared to baseline Change in clinic cough score compared to baseline Change in clinic wheeze score compared to baseline Change in clinic breathlessness score compared to baseline Change in clinic chest tightness score compared to baseline Change in daytime asthma symptom score compared to baseline (diary card) Change in night‐time asthma symptom score compared to baseline (diary card) Change in night‐time sleep disturbance compared to baseline ( nights/week) | |
| Notes | Reply from author clarifying method of random order generation and use of allocation concealment. Study comprised an initial 6 week period during which patients were randomised to either 400 mcg/d or 800 mcg/d of inhaled BUD via Turbohaler. This was followed by a further 18 week period during which all patients openly received BUD 400 mcg/d. The results from the final 18 week period not included in this analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Johansson 1988.
| Methods | Setting: Sweden, hospital outpatient clinic Randomisation: yes, computer generated random number sequence Allocation concealment: yes (pharmaceutical company sponsors provided coded envelopes) Design: crossover, no washout Length of intervention period: 2 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: no demographic data by treatment sequence Jadad score: 5 | |
| Participants | 18 adults: 8M 10F
Age range: 28‐68 years
Inclusion criteria:
Diagnosis of bronchial asthma (ATS criteria)
Requiring some form of additional therapy (inhaled BDP or oral prednisolone) in addition to beta2 agonists, methylxanthines or sodium cromoglycate
Exclusion criteria:
Chronic bronchitis or restrictive lung disease Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): not stated |
|
| Interventions | BUD 25 mcg 1 puff 4xdaily (100 mcg/d) via MDI BUD 100 mcg 1 puff 4xdaily (400 mcg/d) via MDI BUD 400 mcg 1 puff 4xdaily (1600 mcg/d) via MDI |
|
| Outcomes | Morning PEFR Evening PEFR Daily breathlessness score Daily sleep disturbance score Daily use of additional beta2 agonist (puffs/day) | |
| Notes | Reply from author clarifying method of random order generation and use of allocation concealment. Four patients enrolled were using regular oral prednisolone for asthma control. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jonasson 1998.
| Methods | Setting: Norway, paediatric outpatient clinic Randomisation: yes, computer generated sequence Allocation concealment: yes Design: parallel group Length of intervention period: 12 weeks Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 163 children: 107M 56F
Age range: 7‐16 years
Inclusion criteria:
Children with a diagnosis of asthma (based on criteria of an International Consensus Report 1992)
3 previous obstructive episodes or 1 previous obstructive episodes with atopy
Exclusion criteria:
Use of inhaled corticosteroids within last 2 months
Use of cromoglycate or nedocromil within last 4 weeks
Asthma exacerbation requiring emergency room visit or hospital admission within last 4 weeks
Lower respiratory tract infection within last 4 weeks Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): mean > 100 |
|
| Interventions | 1. BUD 100 mcg 1xdaily morning (100 mcg/day) via Turbuhaler DPI 2. 200 mcg 1xdaily (200 mcg/day) morning via Turbuhaler DPI 3. 100 mcg 2xdaily via Turbuhaler DPI (200 mcg/d) Each group received appropriate number of actuations of placebo to maintain blinding 4. Placebo: 2xdaily via Turbuhaler DPI |
|
| Outcomes | FEV1 Maximal percentage fall in FEV1 post exercise test Morning PEFR FEF 25 FEF 50 FEF 75 Methacholine bronchial responsiveness (PD20 FEV1) Daytime asthma symptom score Night‐time asthma symptom score Daytime use of beta2 agonists Night‐time use of beta2 agonist Withdrawal due to asthma exacerbation | |
| Notes | Reply from author clarifying method of random order generation, use of allocation concealment and provided numerical data for exercise challenge. Exercise test involved a six minute run on a motor driven treadmill with speed adjusted to achive a submaximal exercise load and a steady state heart rate of 170‐180 bpm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Juniper 1990.
| Methods | Setting: Canada, hospital outpatient clinic Randomised: Yes, computer generated sequence Allocation concealment: yes Design: parallel group Length of intervention period: 12 months Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | Low dose group: 16 adults (6M 10F) Mean (SD) age 42 (14.4) years
High dose group: 16 adults (7M 9F) mean (SD) age 41 (10) years
Inclusion criteria:
Methacholine bronchial responsiveness (PC20 FEV1) < 8 mg/ml
Asthma symptoms requiring regular treatment with bronchodilators and regular inhaled corticosteroid
FEV1 > 70 (% predicted), no night‐time wakening, no limitation of activities on established minimal dose of BDP during run period
Exclusion criteria:
Symptoms not stabilized using BDP 400mcg/day prior to randomisation
Use of oral prednisolone or cromoglycate within last 2 months. Baseline asthma control Symptom frequency: symptoms fully controlled FEV1 (% predicted): > 70 |
|
| Interventions | Patients stratified as either low (BDP 100 or 200 mcg/day) or high (BDP 300 or 400 mcg/day) at randomisation Patients in low dose group randomised to either 200 or 400 mcg/day Patients in the high dose group randomised to either 400 or 800 mcg/day. |
|
| Outcomes | Change in FEV1 compared to baseline Methacholine bronchial responsiveness (PC20 FEV1) Change in clinic assessed asthma symptom score compared to baseline Change in clinic assessed asthma severity score compared to baseline Change in daily beta2 agonist use compared to baseline Withdrawal due to asthma exacerbation Requirement for oral steroids due to asthma exacerbation Oropharyngeal side effects Oral Candidiasis | |
| Notes | Reply from author clarifying method of random order generation and use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kraan 1988.
| Methods | Setting: The Netherlands, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Length of intervention period: 8 weeks Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline Characteristics: significant difference between groups for FEV1 Jadad score: 3 | |
| Participants | 30 adults: 22M 8F
Age range: 18‐37 years
Inclusion criteria:
History of episodic wheezing
Strongly positive skin tests for at least two common allergens including house dust mite
Mild symptoms are controlled by bronchodilators or prophylactic drugs (cromolyn sodium or inhaled steroids)
FEV1 (% predicted) 70 or greater
Methacholine bronchial responsiveness (PC20 FEV1) less than 8 mg/ml
Exclusion criteria:
Regular use of oral steroids Baseline asthma control Symptom frequency: symptoms controlled FEV1 (% predicted): range 76‐108 |
|
| Interventions | 1. BUD 50 mcg 2 puffs 2xdaily (200mcg/day) via MDI 2. BUD 200 micrograms 2 puffs 2xdaily (800 mcg/day) via MDI |
|
| Outcomes | FEV1 (% predicted) Methacholine bronchial responsiveness (PC20 FEV1) Clinic assessed asthma symptom score Blood eosinophil count Withdrawal due to asthma exacerbation | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Laursen 1986.
| Methods | Setting: Denmark, hospital outpatient clinic Randomisation: yes, not method stated Allocation concealment: unclear Design: parallel Length of intervention period: 15 weeks Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: no demographic characteristics by intervention group Jadad score: 2 | |
| Participants | 50 adults: 29M 21F
Age range: 17‐73 years
Inclusion criteria:
Adults with severe, oral steroid‐dependent asthma
Daily oral prednisolone requirement of 10 mg or more for at least 3 months
Exclusion criteria:
Not stated Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): mean 77 |
|
| Interventions | 1. BUD 50 mcg 4 pfs 2xdaily (400 mcg/d) via MDI+spacer 2. BUD 200 mcg 4pfs 2xdaily (1600 mcg/d) via MDI+spacer | |
| Outcomes | Daily oral prednisolone dose (mg/day) Morning PEFR Evening PEFR Daily use of beta2 agonists Plasma cortisol Plasma cortisol 30min post 250 mcg iv cosyntropin | |
| Notes | Reply from author but unable to clarify details concerning randomisation method. Prior to randomisation all patients were treated with 40 mg of oral prednisolone for 1 week. This was tailed off within a week of starting the randomised part of the study. Study was divided into 2 parts: an initial 15 week randomised parallel group trial, and a second 36 week period when all patients were openly treated with BUD. The results for the latter are not included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Nelson 1998.
| Methods | Setting: multicentre study USA, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Length of intervention period: 20 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 159 adults: 80M 79F
Age range: 20‐69 years
Inclusion criteria:
Patients with chronic oral steroid‐dependent asthma
Oral steroid treatment for a minimum of 6 months (5‐30 mcg/day or equivalent)
Exclusion criteria:
Use of inhaled corticosteroids other than BDP
Sodium cromoglycate, nedocromil sodium or investigational drug within last 4 weeks
Methotrexate within last 3 months
Hospitalised due to asthma within last 30 days Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): < 60 |
|
| Interventions | 1. BUD 400 mcg x daily (800 mcg/d) via Turbuhaler 2. BUD 800 mcg 2xdaily (1600 mcg/d) via Turbuhaler 3. Placebo 2 x daily via Turbuhaler | |
| Outcomes | Number of patients discontinuing oral prednisolone Percentage reduction in daily dose oral prednisolone compared to baseline Percentage change in FEV1 (litres) compared to baseline Percentage change in morning PEFR (litres/min) compared to baseline Percentage change in evening PEFR (litres/min) compared to baseline Percentage change in FEF25‐50 (litres/min) compared to baseline Change in daytime asthma symptom score compared to baseline Change in night‐time symptom score compared to baseline Change in daily use of beta2 agonists compared to baseline (pfs/d) Morning plasma cortisol (nmol/litre) Plasma cortisol 60 min post 25 units cosyntropin IM (nmol/litre) | |
| Notes | Reply from author clarifying method of random order generation and use of allocation concealment. At baseline all subjects had their inhaled BDP converted to an 'equivalent' dose of oral prednisolone. A forced down‐titration design was used to reduce oral prednisolone: at 2 weekly intervals a) subjects receiving > 10 mg/d prednisolone decreased daily dose by 5 mg/d b) subjects receiving 10 mg/d or less of prednisolone decreased daily dose by 2.5 mg/d | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pauwels 1997a.
| Methods | Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group Setting: multicentre study (Canada, Europe, Israel) hospital outpatient clinic Randomisation: yes, computer generated sequence Allocation concealment: yes Design: parallel group Length of intervention period: 12 months Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 852 adults: 416 M 436F
Age range: 18‐70 years
Inclusion criteria:
Adult patients with asthma for at least 6 months
Treatment with an inhaled corticosteroid for at least 3 months
FEV1 (% predicted) 50 or greater
15% or greater improvement in FEV1 following inhaled beta2 agonist
Asthma stable by a priori defined criteria.
Exclusion criteria:
Requirement for > 2000 mcg BDP or 1600 mcg BUD daily via MDI or > 800 mcg BUD or 800 mcg FP via DPI daily.
3 or more courses of oral corticosteroids in past
Hospitalized due to asthma in previous 6 months Baseline asthma control Symptom frequency: symptoms well controlled FEV1 (% predicted): > 50 |
|
| Interventions | 1. BUD 100 mcg 2xdaily (200 mcg daily) via Turbuhaler DPI 2. BUD 400 mcg 2xdaily (800 mcg daily) via Turbuhaler DPI |
|
| Outcomes | Severe asthma exacerbations/patient/year Mild asthma exacerbations/patient/year Patients without severe exacerbation (%) FEV1 (% predicted) Morning PEFR Daytime asthma symptom score Nighttime asthma symptom score Daytime use of beta2 agonist Nighttime use of beta2 agonists Nighttime awakenings (No. per night) Withdrawal due to asthma exacerbation Hospital admission due to asthma exacerbation | |
| Notes | Reply from author clarifying method of random order generation, use of allocation concealment and provided data for FEV1 and PEFR. Four week run‐in period during which patients received BUD 1600 mcg daily via DPI with terbutaline as needed. Severe exacerbations defined as one requiring oral steroids, decrease on morning PEFR of 30% or more below baseline on two consecutive days. Mild exacerbations defined as morning PEFR 20% or more below baseline, requiring > 3 additional puffs short‐acting beta2 agonist or wakening at night due to asthma. Patients with three or more severe exacerbations within 3 months or a total of 5 or more exacerbations were withdrawn. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pauwels 1997b.
| Methods | See Pauwels 1997a | |
| Participants | See Pauwels 1997a | |
| Interventions | 1. BUD 100 mcg 2xdaily (200 mcg daily) via Turbuhaler+ Formoterol 12 mcg 2xdaily (24 mcg daily) via Turbuhaler 2. BUD 400 mcg 2xdaily (800 mcg daily) via Turbuhaler+ Formoterol 12 mcg 2xdaily (24 mcg daily) via Turbuhaler |
|
| Outcomes | See Pauwels 1997a | |
| Notes | See Pauwels 1997a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pedersen 1995.
| Methods | Setting: Denmark, paediatric outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Length of intervention period: 4 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: no demographic data according to treatment sequence Jadad score: 4 | |
| Participants | 19 children: 17M 2F
Age range: 6‐15 years.
Inclusion criteria:
Troubled with asthma symptoms on at least three days per week
Reversibility in FEV1 of 20% or greater after inhaled beta2 agonist or a drop of 20% or greater after exercise challenge
Exclusion criteria:
Respiratory illness other than asthma
Oral prednisolone use within last month Baseline asthma control Symptom frequency: symptoms on at least 3 days per week FEV1 (% predicted): not stated |
|
| Interventions | 1. BUD 50 mcg 1 puff 2xdaily (100 mcg/day) via MDI+spacer 2. BUD 100 mcg 1 puff 2xdaily (200mcg/day) via MDI+spacer 3. BUD 200 mcg 1 puff 2xdaily (400 mcg/day) via MDI+spacer |
|
| Outcomes | FEV1 Maximum percentage fall in FEV1 following exercise test FVC FEF 25‐75 Maximum percentage fall in FEF25‐75 following exercise test Clinic PEFR Morning PEFR Evening PEFR Daytime asthma symptom score Nighttime asthma symptom score Daily beta2 agonist use 24 hour urinary free cortisol excretion | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pedersen 1996.
| Methods | Setting: Denmark, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Length of intervention period: 9 months Masking: double blind Excluded: not stated Withdrawals: not stated Baseline Characteristics: comparable Jadad score: 2 | |
| Participants | 85 adults: 47M 38F
Mean (SD) age: 46.2 (12) years
Inclusion criteria:
Diagnosis of asthma according to American Thoracic Society criteria
Requiring regular maintenance therapy due to attacks of breathlessness, cough and wheeze
15 % or greater reversibility in FEV1 after inhaled beta2 agonist
Exclusion criteria:
Not stated Baseline asthma control Symptom frequency: unclear FEV1 (% predicted): mean 68‐71 |
|
| Interventions | 1. BUD 400mcg/d MDI 2. BUD 1600mcg/d via MDI |
|
| Outcomes | FEV1 (% predicted) Histamine bronchial responsiveness (PC20 FEV1) Blood eosinophil count Serum eosinophil cationic protein Serum eosinophil‐derived neurotoxin Serum myeloperoxidase Serum lactoferrin | |
| Notes | Reply from authors providing numerical data for histamine challenge. Study also included an oral theophylline treatment arm: results not assessed in this analysis. Investigators distinguish between smokers and non‐smokers. Only the outcome data pertaining to the non‐smokers has been included in this analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rees 1993.
| Methods | Setting: multicentre study UK, primary care Randomisation: yes, method not stated Allocation concealment: yes Design: parallel group Length of intervention period: 6 weeks Masking: unblinded, open Excluded: 65 patients failed to meet inclusion criteria after run in Withdrawals: not stated Baseline Characteristics: comparable Jadad score: 1 | |
| Participants | 556 adults: 269M 287 F
Mean (SD) age: 40 (16) years
Inclusion criteria:
At least 16 years of age with chronic asthma
Requirement for beta2 agonist use and /or symptoms of cough, wheeze of breathlessness on at least 3 out of 6 days of run‐in period
Exclusion criteria:
Pregnancy/lactation
PEFR < 60 (% predicted) Asthma exacerbation within last two months
Use of BDP > 400mcg/day Baseline asthma control Symptom frequency: symptoms on at least 3 out of 6 days during run‐in period FEV1 (% predicted): not stated |
|
| Interventions | 1. BUD 200 mcg 2xdaily (400mcg/day) via DPI for 12 weeks 2. 200 mcg mcg 2xdaily (400mcg/day) for 6 weeks increased to 400 mcg 2x daily (800mcg/day) via DPI for 6 weeks |
|
| Outcomes | Morning PEFR Evening PEFR Sleep disturbance score (nights/week) Cough, wheeze and breathlessness score (days/week) Daily use of beta2 agonists Withdrawal due to asthma exacerbation Economic analysis | |
| Notes | Reply from author but unable to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Shapiro 1998a.
| Methods | Setting: multicentre study USA, paediatric outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Length of intervention period: 12 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 404 children: 314M 90F
Age range: 6‐18 years
Inclusion criteria:
Children with asthma (not otherwise defined)
Dependent on inhaled corticosteroids for asthma control for at least 6 months
15% or greater improvement in FEV1 after inhaled beta2 agonist
At least 2 different asthma medications daily for last 6 months (at least 1 and inhaled steroid)
Exclusion criteria:
Significant coexistent disease Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): range 36‐123 |
|
| Interventions | BUD: 1. 100 mcg 1actuation 2xdaily (200 mcg/d) via Turbuhaler DPI 2. 200 mcg 1 actuation 2xdaily (400 mcg/d) via Turbuhaler DPI 3. 400 mcg 1 actuation 2xdaily (800 mcg/d) via Turbuhaler DPI 4. Placebo: 1 actuation 2xdaily | |
| Outcomes | FEV1 (% predicted) Morning PEFR Daytime asthma symptom score Night‐time asthma symptom score Daily use of beta2 agonist Morning plasma cortisol (nmol/litre) Plasma cortisol 30 min post 25 units iv cosyntropin (nmol/litre) All outcomes assessed as change compared to baseline Withdrawals due to asthma exacerbations | |
| Notes | No reply from author to clarify details of randomisation method. A proportion of patients (30/404) were receiving regular oral steroid treatment at enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Swystun 1998.
| Methods | Setting: Canada, hospital outpatient clinic Randomisation: yes, computer generated sequence Allocation concealment: yes Design: crossover, 6 day washout Length of intervention period: 7 days Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 12 adults: 5M 7F
Age range: 20‐48 years
Inclusion criteria:
Adults with mild to moderate atopic asthma
Documented response to a specific allergen in skin prick testing or inhalation challenge
% predicted FEV1 > 70
Methacholine bronchial responsiveness PC20 FEV1 8 mg/ml or less
Exclusion criteria:
Requirement for any asthma medication within last month
Respiratory tract infection within last month Baseline asthma control Symptom frequency: no requirement for any asthma medication within last 4 weeks FEV1 (% predicted): > 70 |
|
| Interventions | 1. BUD 200 mcg/day 2. BUD 400 mcg/day 3. BUD 800 mcg/day 4. Placebo: 1 actuation 2xdaily All treatments delivered by Turbuhaler DPI | |
| Outcomes | Allergen bronchial responsiveness PC15 FEV1 Methacholine bronchial responsiveness PC15 FEV1 FEV1 (litres) | |
| Notes | Reply from author clarifying method of random order generation and use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Toogood 1984.
| Methods | Setting: Canada, hospital outpatient clinic Randomisation: yes, computer generated sequence Allocation concealment: unclear Design: 6 period crossover trial Length of treatment period: 2 weeks Masking: double blind regarding dose, unblinded regarding device Excluded: not stated Withdrawals: not stated Baseline characteristics: clinical and spirometric data presented Jadad score: 3 | |
| Participants | 35 adults: 21M 14F
Mean(SD) age: 54.2 (13) years
Inclusion criteria:
Chronic asthma (no further details) requiring regular treatment with inhaled BDP
Exclusion criteria:
None stated Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): mean 60 |
|
| Interventions | Patients received each of the following treatments for 2 week periods. Patients were randomised to receive each daily dose (400 or 1600 cmg/d) and within each dose level delivery device was also provided in random order. There was no washout between devices, but a 2 week 'washout' period between doses of BDP 400 mcg/d BUD 400 mcg/d via MDI BUD 400 mcg/d via MDI+tube spacer BUD 400 mcg/d via MDI+large volume spacer BUD 1600 mcg/d via MDI BUD 1600 mcg/d via MDI+tube spacer BUD 1600 mcg/d via MDI+large volume spacer |
|
| Outcomes | Change in FEV1 compared to baseline Change in FEF25‐75 compared to baseline | |
| Notes | A proportion of patients (18/35) were receiving regular oral steroid treatment at enrolment. 2 week run in period during which time all patients received BDP 400 mcg/d. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Tukiainen 1987.
| Methods | Setting: two centre study Finland, hospital outpatient clinic Randomisation: yes, computer generated sequence Allocation concealment: unclear Design: crossover, no washout Length of intervention period: 4 weeks Masking: double blind Excluded: not stated Withdrawals stated Baseline characteristics: no demographic data by treatment sequence Jadad score: 5 | |
| Participants | 24 adults: 7M 17F
Age range: 30‐70 years
Inclusion criteria:
Patients with severe bronchial asthma dependent on oral steroid for asthma control
Exclusion criteria:
> spontaneous 20% increase in PEFR over three day run in period Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): not stated |
|
| Interventions | 1. BUD 50 mcg 4 puffs 2xdaily (400 mcg/day) via MDI+spacer 2. BUD 200 mcg 4 puffs 2xdaily (1600 mcg/day) via MDI+spacer |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Daytime breathlessness score Night‐time breathlessness score Daily rescue beta2 agonist use Morning plasma cortisol Incidence of oral Candidiasis | |
| Notes | Method of random order generation confirmed by authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Turkas 1995.
| Methods | Setting: Turkey, paediatric outpatient clinic Randomisation: yes, computer generated random sequence Allocation concealment: unclear Study design: parallel group Length of intervention period: 4 weeks Masking: no details Excluded: not stated Withdrawals: not stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 20 adults: 4M 16F
Age range: 19‐34 years
Inclusion criteria:
Patients with moderate to severe asthma
20% reversibility in airways obstruction
Allergic to seasonal or perennial allergens
Exclusion criteria:
Significant concurrent illness Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): not stated |
|
| Interventions | 1. BUD 800 mcg/day via MDI+spacer 2. BUD 1600 mcg/day via MDI+spacer |
|
| Outcomes | Serum cortisol 24 hour urinary free cortisol Serum triglyceride Serum cholesterol Serum high density lipoprotein (HDL) cholesterol | |
| Notes | Reply from author clarifying method of random order generation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
van der Molen 1998.
| Methods | Setting: multicentre study The Netherlands, primary care setting Randomisation: yes, computer generated sequence Allocation concealment: yes Design: parallel group Length of intervention period: 4 weeks Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 91 adults enrolled, 84 randomised: 40M 44F
Age range: 18‐50 years
Inclusion criteria:
Diagnosis of asthma (Dutch College of General Practice criteria 1992)
FEV1 (% predicted) 50 or greater
Requiring 3 or more doses inhaled bronchodilator/week in previous month
Exclusion criteria:
Smoking history > 20 pack years
Corticosteroid treatment for asthma exacerbation in last 2 months
Serious concurrent disease Baseline asthma control Symptom frequency: requiring < 3 puffs beta2 agonist/week FEV1 (% predicted): > 50 |
|
| Interventions | 1. BUD 100 mcg 2xdaily (200 mcg/d) via Turbuhaler DPI 2. BUD 400 mcg 2xdaily (800mcg/day) via Turbuhaler DPI |
|
| Outcomes | Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Change in daytime asthma symptom score compared to baseline Change in daily beta2 agonist use compared to baseline Time to reach optimal asthma control % predicted FEV1 | |
| Notes | Reply from author clarifying method of random order generation, use of allocation concealment and provision of numerical data for spirometry, PEFR and symptom scores. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wolthers 1991.
| Methods | Setting: Denmark, paediatric outpatient clinic Randomised: yes, computer generated sequence Allocation concealment: unclear Design: crossover, 18 day washout Length of intervention period: 18 days Masking: double blind Excluded: not stated Withdrawals: stated Baseline Characteristics: no separate demographic data by treatment sequence Jadad score: 4 | |
| Participants | 15 children: 9M 6F
Age range: 6‐13 years
Inclusion criteria:
Pre‐adoescent children with no signs of puberty
Diagnosis of mild asthma requiring treatment with beta2 agonists as required
Exclusion criteria:
Use of inhaled or oral corticosteroids within last 2 months Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): not stated |
|
| Interventions | 1. BUD 50 mcg 2 puffs 2xdaily (200mcg/day) via MDI+spacer 2. BUD 200 mcg 2 puffs 2xdaily (800 mcg/day) via MDI+spacer |
|
| Outcomes | Lower leg growth by knemometry FEV1 Morning PEFR Evening PEFR Serum insulin‐like growth factor (IGF‐1) Serum insulin‐like growth factor binding protein (IGFBP‐3) Serum carboxy terminal propeptide of type 1 collagen (ICTP) Serum amino terminal propeptide of type III collagen (PIIINP) Urinary free cortisol excretion | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wolthers 1992.
| Methods | Setting: Denmark, hospital outpatient clinic Randomisation: yes, computer generated sequence Allocation concealment: unclear Design: parallel group Length of intervention period: 8 weeks Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 5 | |
| Participants | 43 children: 30M 13F
Age range: 6‐14 years
Inclusion criteria:
Diagnosis of mild asthma
Requiring beta‐2‐agonists only for symptom control
Exclusion criteria:
Use of oral or inhaled steroids within last two months Baseline asthma control Symptom frequency: not stated FEV1 (% predicted): not stated |
|
| Interventions | 1. BUD 200 mcg/day via MDI+spacer 2. BUD 400 mcg/day via MDI+spacer 3. BUD 800 mcg/day via MDI+spacer Patients in each group received appropriate number of puffs of placebo to maintain blinding |
|
| Outcomes | Lower leg growth by knemometry FEV1 Morning PEFR Evening PEFR Daily use of beta2 agonists Withdrawal due to asthma exacerbation | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agertoft 1993 | Design aims of this study were to compare different delivery systems (MDI+Nebuhaler spacer versus Turbuhaler). Children were randomised to either BUD via Nebuhaler or half their minimal effective daily nominal dose using Nebuhaler via the alternative Turbuhaler. |
| Agertoft 1994 | Population studied exclusively infants. Concerned the comparison of different spacer devices (Nebuhaler, Aerochamber, Babyspacer) used to deliver equal single nominal doses of BUD. The single outcome assessed was the amount of budesonide deposited on a filter paper placed between the spacer outlet and the mouth of the patient. |
| Bisgaard 1995 | Concerned with assessment of the drug delivery characteristics of two types of spacer device. Evaluation of the in‐vitro kinetics of passive aerosol disappearance and estimation of the fraction of a single equal nominal dose of BUD delivered to subjects using each delivery method. |
| Bisgaard 1998 | Concerned with a comparison of different delivery methods (Nebuliser versus MDI+spacer). |
| Campbell 1995 | Concerned with the comparison of different dosing regimens. All patients received BUD 800 mcg/day, either as a regimen of 800 mcg 1xdaily at night or 400 mcg 2xdaily. |
| Campbell 1998a | Concerned with the comparison of different dosing regimens. All patients received BUD 400 mcg/day, either as a regimen of 400 mcg 1xdaily at night or 200 mcg 2xdaily. |
| Chisholm 1998 | Concerned with the comparison of different dosing regimens. All patients received BUD 200 mcg/day, either as a regimen of 200 mcg 1xdaily at night or 100 mcg 2xdaily. |
| Engel 1989 | Concerned with the comparison of different delivery devices (Turbuhaler DPI v MDI). Patients were divided into two groups and received either BUD 800 mcg/day or 1600 mcg/day; however allocation to different doses was not randomised and was determined according to each individual's previous inhaled steroid dose prior to enrolment. |
| Grimfeld 1994 | Population studied exclusively infants. Nebuliser delivery device employed. |
| Heuck 1998 | Concerned with the comparison of different dosing regimens. All patients received BUD 800 mcg/day, either as a regimen of 800 mcg 1xdaily morning or 400 mcg 2xdaily. |
| Kenyon 1998 | Concerned with comparison of different delivery devices (plastic Nebuhaler versus plastic Volumatic versus metal Aerochamber) on radiolabeled budesonide lung deposition measured by gamma scintography. Patients were randomised to receive single equal nominal doses of BUD via each device. |
| Nieminen 1995 | Concerned with the comparison of different delivery devices (Turbuhaler DPI v MDI) using the same nominal daily dose of BUD. |
| Nyholm 1984 | Concerned with the comparison of different dosing regimens. All patients received BUD 400 mcg/day, either as a regimen comprising 4 puffs 2xdaily or 2 puffs 4xdaily. |
| Pauwels 1996 | Concerned with the comparison of different delivery devices (Turbuhaler DPI v MDI). The nominal daily dose of BUD received at randomisation was the same as the nominal dose of maintainance inhaled corticosteroid used by the patient prior to the study. |
| Pedersen 1993 | Pharmokinetic study designed to evaluate the extent to which orophayngeal deposition of budesonide contributes to systemic availability of drug. Evaluation of plasma concentrations of budesonide following single inhaled nominal dose of budesonide via Turbuhaler versus single equal nominal dose of budesonide via Turbuhaler with measures to prevent oral systemic absorbtion of drug. |
| Reiser 1986 | Concerned with a comparison of different delivery devices (MDI versus MDI+spacer). All patients received an identical nominal daily dose of budesonide. |
| Rocca 1996 | Patients were not randomised to receive different nominal daily doses of budesonide. |
| Shapiro 1998b | Nebuliser delivery device used. |
| Thorsson 1998 | Concerned with comparison of different delivery devices (MDI versus MDI+spacer versus Turbuhaler DPI) on radiolabeled budesonide lung deposition measured by gamma scintography. Patients were randomised to receive single equal nominal doses of BUD via each device. |
| Toogood 1982 | Patients were randomised to different sequences of treatment ( with different dosing frequencies and dosing schedules). Patients were not however randomised to different doses within each treatment schedule. |
| Toogood 1997 | Concerned with a comparison of different delivery devices (Turbuhaler versus MDI+Nebuhaler spacer). Patients were randomised to receive escalating doses of budesonide (400mcg/day to 2400 mcg/day) from each delivery system in a parallel design study. All patients received identical dose regimens. |
| Vikre‐Jorgensen 1997 | Nebuliser delivery device used. |
| Volovitz 1998 | Nebuliser delivery device used. |
| Wennergren 1996 | Population studied exclusively infants. Nebuliser delivery device used. |
| Wilson 1998 | Randomised crossover design study comparing different nominal doses of BUD and triamcinolone. However intervention period of 3 days only for each treatment period. |
| Wolthers 1998 | Concerned with the comparison of different dosing regimens. Patients received BUD 800 mcg/day, either as a regimen of 800 mcg 1xdaily morning or 400 mcg 2xdaily. Only outcome assessed serum osteocalcin levels. The effects of inhaled corticosteroids on bone turnover is the subject of a future review. |
Contributions of authors
Nick Adams retrieved papers identified by electronic search, handsearched additional sources for relevant studies, assessed trials for methodological quality, contacted authors to clarify details of trial design and/or request missing data, extracted data from included trials and wrote text of review. Janine Bestall retrieved papers identified by search, assessed trials for methodological quality, contacted authors for clarification or trial details and/or request missing data. Paul Jones helped design the review and provided editorial support .
Sources of support
Internal sources
No sources of support supplied
External sources
NHS Research and Development, UK.
Garfield Weston Foundation, UK.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Aaronson 1998 {published data only}
- Aaronson D, Kaiser H, Dockhorn R, Findlay S, Korenblat P, Thorsson L, et al. Effects of budesonide by means of the Turbuhaler on the hypothalmic‐pituitary‐adrenal axis in asthmatic subjects: a dose‐response study. Journal of Allergy & Clinical Immunology 1998;101(3):312‐9. [DOI] [PubMed] [Google Scholar]
Agertoft 1997 {published data only}
- Agertoft L, Pedersen S. Short‐term knemometry and urine cortisol excretion in children treated with fluticasone propionate and budesonide: a dose response study. European Respiratory Journal 1997;10(7):1507‐12. [DOI] [PubMed] [Google Scholar]
Bisgaard 1991 {published data only}
- Bisgaard H, Pedersen S, Damkjaer Nielsen M, Osterballe O. Adrenal function in asthmatic children treated with inhaled budesonide. Acta Paediatrica Scandinavica 1991;80(2):213‐7. [DOI] [PubMed] [Google Scholar]
Boe 1989 {published data only}
- Boe J, Rosenhall L, Alton M, Carlsson LG, Carlsson U, Hermansson BA, et al. Comparison of dose‐response effects of inhaled beclomethasone dipropionate and budesonide in the management of asthma. Allergy: European Journal of Allergy & Clinical Immunology 1989;44(5):349‐55. [DOI] [PubMed] [Google Scholar]
Busse 1998 {published data only}
- Busse WW, Chervinsky P, Condemi J, Lumry WR, Petty TL, Rennard S, et al. Budesonide delivered by Turbuhaler is effective in a dose‐dependent fashion when used in the treatment of adult patients with chronic asthma. Journal of Allergy & Clinical Immunology 1998;101(4 Pt 1):457‐63. [DOI] [PubMed] [Google Scholar]
Campbell 1998b {published data only}
- Campbell LM, Gooding TN, Aitchison WR, Smith N, Powell JA. Initial loading (400 micrograms twice daily) versus static (400 micrograms nocte) dose budesonide for asthma management. PLAN Research Group. International Journal of Clinical Practice 1998;52(6):361‐8, 370. [PubMed] [Google Scholar]
Johansson 1988 {published data only}
- Johansson SA, Dahl R. A double‐blind dose‐response study of budesonide by inhalation in patients with bronchial asthma. Allergy: European Journal of Allergy & Clinical Immunology 1988;43(3):173‐8. [DOI] [PubMed] [Google Scholar]
Jonasson 1998 {published data only}
- Jonasson G, Carlsen KH, Blomqvist P. Clinical efficacy of low‐dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. European Respiratory Journal 1998;12(5):1099‐104. [DOI] [PubMed] [Google Scholar]
Juniper 1990 {published data only}
- Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Long‐term effects of budesonide on airway responsiveness and clinical asthma severity in inhaled steroid‐dependent asthmatics. European Respiratory Journal 1990;3(10):1122‐7. [PubMed] [Google Scholar]
Kraan 1988 {published data only}
- Kraan J, Koeter GH, Mark TW, Boorsma M, Kukler J, Sluiter HJ, et al. Dosage and time effects of inhaled budesonide on bronchial hyperreactivity. American Review of Respiratory Disease 1988;137(1):44‐8. [DOI] [PubMed] [Google Scholar]
Laursen 1986 {published data only}
- Laursen LC, Taudorf E, Weeke B. High‐dose inhaled budesonide in treatment of severe steroid‐dependent asthma. European Journal of Respiratory Diseases 1986;68(1):19‐28. [PubMed] [Google Scholar]
Nelson 1998 {published data only}
- Nelson HS, Bernstein IL, Fink J, Edwards TB, Spector SL, Storms WW, et al. Oral glucocorticosteroid‐sparing effect of budesonide administered by Turbuhaler: a double‐blind, placebo‐controlled study in adults with moderate‐to‐severe chronic asthma. Pulmicort Turbuhaler Study Group. Chest 1998;113(5):1264‐71. [DOI] [PubMed] [Google Scholar]
Pauwels 1997a {published data only}
- Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. New England Journal of Medicine 1997;337(20):1405‐11. [DOI] [PubMed] [Google Scholar]
Pauwels 1997b {published data only}
- Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. New England Journal of Medicine 1997;337(20):1405‐11. [DOI] [PubMed] [Google Scholar]
Pedersen 1995 {published data only}
- Pedersen S, Hansen OR. Budesonide treatment of moderate and severe asthma in children: a dose‐response study. Journal of Allergy & Clinical Immunology 1995;95(1 Pt 1):29‐33. [DOI] [PubMed] [Google Scholar]
Pedersen 1996 {published data only}
- Pedersen B, Dahl R, Karlstrom R, Peterson CG, Venge P. Eosinophil and neutrophil activity in asthma in a one‐year trial with inhaled budesonide. The impact of smoking. American Journal of Respiratory & Critical Care Medicine 1996;153(5):1519‐29. [DOI] [PubMed] [Google Scholar]
Rees 1993 {published data only}
- Campbell LM, Simpson RJ, Turbitt ML, Richardson PDI. .A comparison of the cost effectiveness of budesonide 400 mug/day and 800 mug/day in the management of mild‐to‐moderate asthma in general practice. British Journal of Medical Economics 1993;6:67‐74. [Google Scholar]
- Rees TP, Lennox B, Timney AP, Hossain M, Turbitt ML, Richardson PDI. Comparison on increasing the dose of budesonide to 800mg/day with a maintained dose of 400mg/day in mild‐to‐moderate asthmatic patients. European Journal of Clinical Research 1993;4:67‐77. [Google Scholar]
Shapiro 1998a {published data only}
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH, et al. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. Journal of Pediatrics 1998;132(6):976‐82. [DOI] [PubMed] [Google Scholar]
Swystun 1998 {published data only}
- Swystun VA, Bhagat R, Kalra S, Jennings B, Cockcroft DW. Comparison of 3 different doses of budesonide and placebo on the early asthmatic response to inhaled allergen. Journal of Allergy & Clinical Immunology 1998;102(3):363‐7. [DOI] [PubMed] [Google Scholar]
Toogood 1984 {published data only}
- Toogood JH, Baskerville J, Jennings B, Lefcoe NM, Johansson SA. Use of spacers to facilitate inhaled corticosteroid treatment of asthma. American Review of Respiratory Disease 1984;129(5):723‐9. [DOI] [PubMed] [Google Scholar]
- Toogood JH, Jennings B, Baskerville J, Johansson SA. Clinical use of spacer systems for corticosteroid inhalation therapy: a preliminary analysis. European Journal of Respiratory Diseases ‐ Supplement 1982;122:100‐7. [PubMed] [Google Scholar]
Tukiainen 1987 {published data only}
- Tukiainen P, Lahdensuo A. Effect of inhaled budesonide on severe steroid‐dependent asthma. European Journal of Respiratory Diseases 1987;70(4):239‐44. [PubMed] [Google Scholar]
Turkas 1995 {published data only}
- Turktas I, Gokcora N, Yavuz O, Elbek S, Cevik C, Demirsoy S. Effect of inhaled budesonid on lipid metabolism and hypothalamic‐pituitary‐adrenal axis function in patients with bronchial asthma. Turkish Journal of Medical Sciences 1995;25(3):183‐6. [Google Scholar]
van der Molen 1998 {published data only}
- Molen T, Meyboom‐de Jong B, Mulder HH, Postma DS. Starting with a higher dose of inhaled corticosteroids in primary care asthma treatment. American Journal of Respiratory & Critical Care Medicine 1998;158(1):121‐5. [DOI] [PubMed] [Google Scholar]
Wolthers 1991 {published data only}
- Wolthers OD, Juul A, Hansen M, Muller J, Pedersen S. The insulin‐like growth factor axis and collagen turnover in asthmatic children treated with inhaled budesonide. Acta Paediatrica 1995;84(4):393‐7. [DOI] [PubMed] [Google Scholar]
- Wolthers OD, Pedersen S. Growth of asthmatic children during treatment with budesonide: a double blind trial. BMJ 1991;303(6795):163‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolthers OD, Pedersen S. Measures of systemic activity of inhaled glucocorticosteroids in children: A comparison of urine cortisol excretion and knemometry. Respiratory Medicine 1995;89(5):347‐9. [DOI] [PubMed] [Google Scholar]
Wolthers 1992 {published data only}
- Wolthers OD, Pedersen S. Controlled study of linear growth in asthmatic children during treatment with inhaled glucocorticosteroids. Pediatrics 1992;89(5 Pt 1):839‐42. [PubMed] [Google Scholar]
References to studies excluded from this review
Agertoft 1993 {published data only}
- Agertoft L, Pedersen S. Importance of the inhalation device on the effect of budesonide. Archives of Disease in Childhood 1993;69(1):130‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agertoft L, Pedersen SE. [Budesonide administered via Turbuhaler and a nebulator]. Ugeskrift for Laeger 1994;156:4134‐7. [PubMed] [Google Scholar]
Agertoft 1994 {published data only}
- Agertoft L, Pedersen S. Influence of spacer device on drug delivery to young children with asthma. Archives of Disease in Childhood 1994;71(3):217‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisgaard 1995 {published data only}
- Bisgaard H. A metal aerosol holding chamber devised for young children with asthma. European Respiratory Journal 1995;8(5):856‐60. [PubMed] [Google Scholar]
Bisgaard 1998 {published data only}
- Bisgaard H, Nikander K, Munch E. Comparative study of budesonide as a nebulized suspension vs pressurized metered‐dose inhaler in adult asthmatics. Respiratory Medicine 1998;92(1):44‐9. [DOI] [PubMed] [Google Scholar]
Campbell 1995 {published data only}
- Campbell LM, Gunn SD, Sweeney D, Smithers AJ, Zurek AAA, Golightly L, et al. Once daily budesonide: effective control of moderately severe asthma with 800 mcg once daily inhaled via Turbohaler when compared with 400 mcg twice daily. European Journal of Clinical Research 1995;7:1‐14. [Google Scholar]
Campbell 1998a {published data only}
- Campbell LM, Bodalia B, Gogbashian CA, Gunn SD, Humphreys PJ, Powell JP. Once‐daily budesonide: 400 micrograms once daily is as effective as 200 micrograms twice daily in controlling childhood asthma. PETITE Research Group. International Journal of Clinical Practice 1998;52(4):213‐9. [PubMed] [Google Scholar]
Chisholm 1998 {published data only}
- Chisholm SL, Dekker FW, Knuistingh Neven A, Petri H. Once‐daily budesonide in mild asthma. Respiratory Medicine 1998;92(3):421‐5. [DOI] [PubMed] [Google Scholar]
Engel 1989 {published data only}
- Engel T, Heinig JH, Malling HJ, Scharling B, Nikander K, Madsen F. Clinical comparison of inhaled budesonide delivered either via pressurized metered dose inhaler or Turbuhaler. Allergy: European Journal of Allergy & Clinical Immunology 1989;44(3):220‐5. [DOI] [PubMed] [Google Scholar]
Grimfeld 1994 {published data only}
- Grimfeld A, Lesbros D, Ostinelli J, Caswell C. Long‐term study of nebuilsed budesonide in young children with moderate to severe asthma. European Respiratory Journal. 1994; Vol. 7, issue Suppl 18:27.
Heuck 1998 {published data only}
- Heuck C, Wolthers OD, Kollerup G, Hansen M, Teisner B. Adverse effects of inhaled budesonide (800 micrograms) on growth and collagen turnover in children with asthma: a double‐blind comparison of once‐daily versus twice‐daily administration. Journal of Pediatrics 1998;133(5):608‐12. [DOI] [PubMed] [Google Scholar]
Kenyon 1998 {published data only}
- Kenyon CJ, Thorsson L, Borgstrom L, Newman SP. The effects of static charge in spacer devices on glucocorticosteroid aerosol deposition in asthmatic patients. European Respiratory Journal 1998;11(3):606‐10. [PubMed] [Google Scholar]
Nieminen 1995 {published data only}
- Nieminen MM, Lahdensuo A. Inhalation treatment with budenoside in asthma. A comparison of Turbuhaler(TM) and metered dose inhalation with Nebuhaler(TM). Acta Therapeutica 1995;21(3‐4):179‐92. [Google Scholar]
Nyholm 1984 {published data only}
- Nyholm E, Frame MH, Cayton RM. Therapeutic advantages of twice‐daily over four‐times daily inhalation budesonide in the treatment of chronic asthma. European Journal of Respiratory Diseases 1984;65(5):339‐45. [PubMed] [Google Scholar]
Pauwels 1996 {published data only}
- Pauwels RA, Hargreave FE, Camus P, Bukoski M, Stahl E. A 1‐year comparison of turbuhaler vs pressurized metered‐dose inhaler in asthmatic patients. Chest 1996;110(1):53‐7. [DOI] [PubMed] [Google Scholar]
Pedersen 1993 {published data only}
- Pedersen S, Steffensen G, Ohlsson SV. The influence of orally deposited budesonide on the systemic availability of budesonide after inhalation from a Turbuhaler. British Journal of Clinical Pharmacology 1993;36(3):211‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reiser 1986 {published data only}
- Reiser J, Frame MH, Warner JO. The potential value of a 750‐ml spacer for the administration of inhaled corticosteroids to children. Pediatric Pulmonology 1986;2(4):237‐43. [DOI] [PubMed] [Google Scholar]
Rocca 1996 {published data only}
- Rocca Serra JP, Malka M, Caekert A, Marty M. [Influence of inhalation device on asthmatic patients quality of life: Switch MDI to Turbuhaler]. Allergie Et Immunologie 1996;28(6):202‐17. [Google Scholar]
Shapiro 1998b {published data only}
- Shapiro G, Mendelson L, Kraemer MJ, Cruz‐Rivera M, Walton‐Bowen K, Smith JA. Efficacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroid‐dependent, persistent asthma. Journal of Allergy & Clinical Immunology 1998;102(5):789‐96. [DOI] [PubMed] [Google Scholar]
Thorsson 1998 {published data only}
- Thorsson L, Kenyon C, Newman SP, Borgstrom L. Lung deposition of budesonide in asthmatics: A comparison of different formulations. International Journal of Pharmaceutics 1998;168(1):119‐27. [Google Scholar]
Toogood 1982 {published data only}
- Toogood J, Baskerville J, Jennings B, Lefcoe N, Johansson SA. Effect of reducing the dose frequency of aerosol steroid for asthma: Critical importance of the 'baseline state'. Chest. 1982; Vol. 82, issue 2:246.
- Toogood JH, Baskerville JC, Jennings B, Lefcoe NM, Johansson SA. Influence of dosing frequency and schedule on the response of chronic asthmatics to the aerosol steroid, budesonide. Journal of Allergy & Clinical Immunology 1982;70(4):288‐98. [DOI] [PubMed] [Google Scholar]
Toogood 1997 {published data only}
- Toogood JH, White FA, Baskerville JC, Fraher LJ, Jennings B. Comparison of the antiasthmatic, oropharyngeal, and systemic glucocorticoid effects of budesonide administered through a pressurized aerosol plus spacer or the Turbuhaler dry powder inhaler. Journal of Allergy & Clinical Immunology 1997;99(2):186‐93. [DOI] [PubMed] [Google Scholar]
Vikre‐Jorgensen 1997 {published data only}
- Vikre‐Jorgensen J, Agertoft L, Pedersen S. Dose titration of nebulized budesonide in young children. Pediatric Pulmonology 1997;23(4):270‐7. [DOI] [PubMed] [Google Scholar]
Volovitz 1998 {published data only}
- Volovitz B, Soferman R, Blau H, Nussinovitch M, Varsano I. Rapid induction of clinical response with a short‐term high‐dose starting schedule of budesonide nebulizing suspension in young children with recurrent wheezing episodes. Journal of Allergy & Clinical Immunology 1998;101(4 Pt 1):464‐9. [DOI] [PubMed] [Google Scholar]
Wennergren 1996 {published data only}
- Wennergren G, Nordvall SL, Hedlin G, Moller C, Wille S, Asbrink Nilsson E. Nebulized budesonide for the treatment of moderate to severe asthma in infants and toddlers. Acta Paediatrica 1996;85(2):183‐9. [DOI] [PubMed] [Google Scholar]
Wilson 1998 {published data only}
- Wilson AM, Brewster HJ, Lipworth BJ. Dose‐response comparison of systemic bioactivity with inhaled budesonide and triamcinolone acetonide in asthmatic adults. Journal of Allergy & Clinical Immunology 1998;102(5):751‐6. [DOI] [PubMed] [Google Scholar]
Wolthers 1998 {published data only}
- Wolthers OD, Heuck C. Differential effects of inhaled budesonide on serum osteocalcin in children and adolescents with asthma. Pediatric Allergy & Immunology 1998;9(3):150‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aziz 2000 {published data only}
- Aziz I, Wilson AM, Lipworth BJ. Effects of once‐daily formoterol and budesonide given alone or in combination on surrogate inflammatory markers in asthmatic adults. Chest 2000;118(4):1049‐58. [DOI] [PubMed] [Google Scholar]
Derom 1999 {published data only}
- Derom E, Schoor J, Verhaeghe W, Vincken W, Pauwels R. Systemic effects of inhaled fluticasone propionate and budesonide in adult patients with asthma. American Journal of Respiratory & Critical Care Medicine 1999;160(1):157‐61. [DOI] [PubMed] [Google Scholar]
Foresi 2000 {published data only}
- Foresi A, Morelli MC, Catena E. Low‐dose budesonide with the addition of an increased dose during exacerbations is effective in long‐term asthma control. Chest 2000;117(2):440‐6. [DOI] [PubMed] [Google Scholar]
Gauvreau 2000 {published data only}
- Gauvreau GM, Sulakvelidze I, Watson RM, Inman MD, Rerecich TJ, O'Byrne PM. Effects of once daily dosing with inhaled budesonide on airway hyperresponsiveness and airway inflammation following repeated low‐dose allergen challenge in atopic asthmatics. Clinical & Experimental Allergy 2000;30(9):1235‐43. [DOI] [PubMed] [Google Scholar]
Jatakanon 1999 {published data only}
- Jatakanon A, Kharitonov S, Lim S, Barnes PJ. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax 1999;54(2):108‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jonasson 2000 {published data only}
- Jonasson G, Carlsen KH, Hultquist C. Low‐dose budesonide improves exercise‐induced bronchospasm in schoolchildren. Pediatric Allergy & Immunology 2000;11(2):120‐5. [DOI] [PubMed] [Google Scholar]
Kaiser 1999 {published data only}
- Kaiser H, Aaronson D, Dockhorn R, Edsbacker S, Korenblat P, Kallen A. Dose‐proportional pharmacokinetics of budesonide inhaled via Turbuhaler(TM). British Journal of Clinical Pharmacology 1999;48(3):309‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemp 1999a {published data only}
- Kemp JP, Skoner DP, Szefler SJ, Walton‐Bowen K, Cruz‐Rivera M, Smith JA. Once‐daily budesonide inhalation suspension for the treatment of persistent asthma in infants and young children. Annals of Allergy 1999;83(3):231‐9. [DOI] [PubMed] [Google Scholar]
Kemp 1999b {published data only}
- Kemp J, Wanderer AA, Ramsdell J, Southern DL, Weiss S, Aaronson D, et al. Rapid onset of control with budesonide Turbuhaler in patients with mild‐ to‐moderate asthma. Annals of Allergy 1999;82(5):463‐71. [DOI] [PubMed] [Google Scholar]
McFadden 1999 {published data only}
- McFadden ER, Casale TB, Edwards TB, Kemp JP, Metzger WJ, Nelson HS, et al. Administration of budesonide once daily by means of Turbuhaler to subjects with stable asthma. Journal of Allergy & Clinical Immunology 1999;104(1):46‐52. [DOI] [PubMed] [Google Scholar]
Miyamoto 2000a {published data only}
- Miyamoto T, Takahashi T, Nakajima S, Makino S, Yamakido M, Mano K, et al. A double‐blind, placebo‐controlled dose‐response study with budesonide Turbuhaler in Japanese asthma patients. Respirology 2000;5(3):247‐56. [DOI] [PubMed] [Google Scholar]
Miyamoto 2000b {published data only}
- Miyamoto T, Takahashi T, Nakajima S, Makino S, Yamakido M, Mano K, et al. A double‐blind, placebo‐controlled steroid‐sparing study with budesonide Turbuhaler in Japanese oral steroid‐dependent asthma patients. Respirology 2000;5(3):231‐40. [DOI] [PubMed] [Google Scholar]
Nielsen 2000 {published data only}
- Nielsen LP, Dahl R. Therapeutic ratio of inhaled corticosteroids in adult asthma: A dose‐range comparison between fluticasone propionate and budesonide, measuring their effect on bronchial hyperresponsiveness and adrenal cortex function. American Journal of Respiratory & Critical Care Medicine 2000;162(6):2053‐7. [DOI] [PubMed] [Google Scholar]
Reddel 2000 {published data only}
- Reddel HK, Jenkins CR, Marks GB, Ware SI, Xuan W, Salome CM, et al. Optimal asthma control, starting with high doses of inhaled budesonide. European Respiratory Journal 2000;16(2):226‐35. [DOI] [PubMed] [Google Scholar]
Shapiro 2001 {published data only}
- Shapiro GG, Mendelson LM, Pearlman DS. Once‐Daily Budesonide Inhalation Powder (Pulmicort Turbuhaler) Maintains Pulmonary Function and Symptoms of Asthmatic Children Previously Receiving Inhaled Corticosteroids. Annals of Allergy Asthma & Immunology 2001;86(6):633‐40. [DOI] [PubMed] [Google Scholar]
Tukiainen 2000 {published data only}
- Tukiainen H, Taivainen A, Majander R, Poussa T, Svahn T, Puolijoki H, et al. Comparison of high and low dose of the inhaled steroid, budesonide, as an initial treatment in newly detected asthma. Respiratory Medicine 2000;94(7):678‐83. [DOI] [PubMed] [Google Scholar]
Wilson 2000 {published data only}
- Wilson AM, Lipworth BJ. Dose‐response evaluation of the therapeutic index for inhaled budesonide in patients with mild‐to‐moderate asthma. American Journal of Medicine 2000;108(4):269‐75. [DOI] [PubMed] [Google Scholar]
Additional references
Adams 2001
- Adams N, Bestall J, Jones PW. Budesonide for chronic asthma in children and adults (Cochrane Review). The Cochrane Library 2001, Issue 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society. The British guidelines on asthma management 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐20. [Google Scholar]
GINA 1995
- National Asthma Education and Prevention Program. Global strategy for asthma management and prevention NHBLI/WHO workshop report. National Institute of Health, Bethseda, MD 1995, issue NIH Publication No. 95‐3659.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996 Feb;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
NHLBI 1997
- National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Managment of Asthma, Expert Panel Report No. 2. Bethseda MD: NIH/National Heart, Lung and Blood Institute 1997, issue Publication No. 97‐4051.


