Abstract.
We aimed to determine the prevalence of early puberty in girls with premature pubarche and analyze the time interval between their pubarche and succeeding thelarche. This study included 60 female children with premature pubarche. We retrospectively collected clinical, laboratory, and radiological findings from all participants. We investigated the time interval between pubarche and thelarche in cases wherein premature pubarche was followed by thelarche. The mean age at onset of pubarche was 6.93 ± 0.79 yr old. Among the participants, 16.7% were preterm, 20% were small for gestational age (SGA), and 55% were overweight or obese. The mean time interval between pubarche and thelarche was 11.20 ± 7.41 mo. The mean serum DHEA-S level was higher in the preterm group (p = 0.016), and DHEA-S levels were generally higher in the SGA group (p = 0.004). This study documented the presence of being overweight or obese and having more advanced growth than their genetic potential in half of the patients who had premature pubarche. In addition to these identified risk factors, obesity-independent DHEA-S levels were observed to be higher in patients who had early puberty with the first six months of their follow-up considered to be the most critical time in predicting early puberty.
Keywords: premature pubarche, premature adrenarche, exaggerated adrenarche, precocious puberty, dehydroepiandrosterone sulfate (DHEA-S)
Highlights
● Being overweight or obese and having advanced growth were detected in half of our patients.
● The risk of early puberty had higher serum DHEA-S levels, which were independent of their weight.
● The most critical time for precocious puberty is the first 6 mo of the follow-up period.
Introduction
Observing Tanner stage 2 pubertal findings in a girl younger than 8 yr old is called “precocious puberty” (1). This condition may have central or peripheral causes. Regarding the timing of precocious puberty, pubertal findings typically occur at the age range of 7.5–8.5 yr old, which is indicated as “early puberty” or defined as the “gray zone” (1). Premature pubarche is a subtype of precocious puberty, and its risk factors are similar to those of early puberty (1).
Typically, an increase in adrenal androgen precursor levels between the ages of 5 and 8 yr old is defined as adrenarche, a biochemical process without any clinical signs (2). Normally, pubic and axillary hair growth, which are the clinical signs of the adrenal androgen effect, appear after the age of 8 yr old in girls (2). The presence of androgenic signs such as pubic and axillary hair before this age is defined as premature pubarche and is diagnosed by excluding organic causes of androgen excess, including congenital adrenal hyperplasia androgen-producing tumors and exogenous androgen exposure.
In recent years, many studies have shown that etiological factors such as intrauterine growth retardation (IUGR), small for gestational age (SGA), and rapid weight gain in infancy increase the level of dehydroepiandrosterone sulfate (DHEA-S), causing premature pubarche (2, 3). Isolated premature pubarche is regarded as a warning sign of intrauterine-programmed metabolic syndrome rather than a normal and benign variant condition. Additionally, pubarche may be a precursor sign of polycystic ovary syndrome (PCOS) especially in prepubertal girls with SGA, which may accelerate the pubertal tempo, triggering gonadarche and causing early menarche (2, 4).
In this study, we aimed to investigate whether puberty occurs at a younger age in girls who present with premature pubarche as well as the onset of thelarche after pubarche; in doing so, we also aimed to investigate any associations between premature pubarche and early puberty. Additionally, we aimed to investigate which of the risk factors of isolated premature pubarche are considered to be most important in determining early puberty. Thus, we aimed to determine the time interval between premature pubarche and the succeeding thelarche as well as early puberty risk factors.
Materials and Methods
This study was approved by the Ethics Committee of Ankara University Faculty of Medicine, Ankara, Turkey (Approval No. 12-807-18). Written consent was obtained from all children and their parents who agreed to participate in the study.
Participants in this study were girls who had premature pubarche and girls who were diagnosed with central precocious puberty (CPP) whose first pubertal sign was pubarche; these participants were referred to our outpatient clinic. The demographic, anthropometric, laboratory, and imaging findings of the participants and their pubertal findings during six monthly follow-up consults were retrospectively reviewed.
Information about gestational age, birth weight, maternal menarche age, and diagnosis of maternal PCOS were collected using patient follow-up forms. Those born before the gestational age of 37 wk were defined as preterm. Additionally, SGA was defined as having a birth weight or length more than two standard deviations (SDs) below the mean.
Age at onset of pubarche, weight, height, height standard deviation score (SDS), body mass index (BMI), relative body mass index (RBMI), target height (TH), and target height SDS were interpreted against national reference data (5).
Serum LH, FSH, and DHEA-S levels were measured using the immunochemiluminescence (ICMA) method via Access DXI 800 (Beckman Coulter®). Serum estradiol levels were measured using the electrochemiluminescence method via Modular E170 Immunological Analyzer system (Roche Diagnostics®), and 17-hydroxyprogesterone (17-OHP) levels were measured using the radioimmunoassay (RIA) method (DIAsource ImmunoAssays®).
In studies of healthy Turkish children at Tanner 2 pubarche, serum concentrations of DHEA-S measuring above 35 μg/dL and 17-OHP levels measuring below 2 ng/mL were regarded as reference values and as biochemical findings of adrenarche (6); considering these, DHEA-S levels above 124 μg/dL were accepted as exaggerated adrenarche (6). Patients with 17-OHP levels above 2 ng/mL were evaluated as non-classical congenital adrenal hyperplasia (NCCAH), and they were excluded from our study. Those with basal LH levels ≥ 0.3 IU/L by ICMA and stimulated LH levels ≥ 5 IU/L by ICMA upon gonadotropin-releasing hormone (GnRH) stimulation test were diagnosed with CPP (7).
The bone age (BA) of every patient was determined based on an x-ray of the left wrist using the method of Greulich and Pyle, and the predicted adult height (PAH) was estimated using the Bayley and Pinneau method. It is accepted that Δbone age divided by Δchronological age (ΔBA/ΔCA) measuring ≥ 1.2 is in favor of BA more than + 2 SD. The corrected height SDS for genetic potential was calculated by subtracting the target height SDS from the height SDS. A cut-off point of less than + 1 SDS below the mean is used to identify whether a child is growing appropriately at their genetic potential or not, and a cut-off point of more than + 1 SDS above the mean is used to classify whether a child is growing faster than their genetic potential or not (8).
Statistical analysis
Statistical analysis was performed using SPSS® for Windows version 11.5 (SPSS, Inc., Chicago, IL, USA). G * Power 3.1 was used to analyze the statistical power. Frequencies and percentages are presented as descriptive statistics for categorical variables. Quantitative data were checked using the Shapiro-Wilk test. Mean ± SD values and median (minimum–maximum) were used for continuous variables. Student’s t-test was used to compare the average values of numeric outcomes between the two groups. The Mann-Whitney U test was used if there were doubts regarding the suitability of using a t-test, most notably, if the distribution was non-normal. The Chi-square and Fisher’s exact tests were used for categorical modeling between the two qualitative variables. Pearson correlation coefficient was used to precisely measure the relationship between the two variables. Statistical significance was defined as p < 0.05.
Results
The mean age at onset of pubarche in our patients was 6.93 ± 0.79 (4.5–7.9) yr old. Additionally, 16.7% of them were preterm, the mean birth weight was 2,918 ± 644 g, the mean maternal menarche age was 12.98 ± 1.23 yr old, and 15% percent of the cases had a history of maternal PCOS. The mean bone age of our patients was 8.24 ± 1.36 yr old, and the mean ΔBA/ΔCA value was 1.10 ± 0.15.
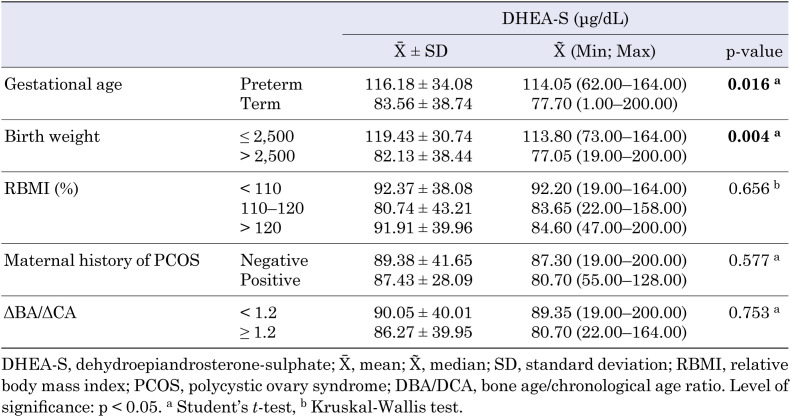
Anthropometric evaluation showed that the mean height SDS of our patients was 0.89 ± 1.01. Additionally, their mean RBMI was 112.49 ± 19.39%, mean target height was 161.60 ± 4.77 cm, mean target height SDS was –0.16 ± 0.88, and mean genetic potential was 1.05 ± 0.94. Thirty-two patients (53.3%) showed more advanced growth than their genetic potential, and bone age was greater than + 2 SD in 26.7% of our patients. Despite the advanced bone age of these patients, their target heights remained compatible with their predicted final adult heights. The mean serum DHEA-S level at admission was 89.09 ± 39.68 μg/dL, and the mean serum 17-OHP level was 1.08 ± 0.49 ng/mL.
When the term and preterm groups were compared, mean serum DHEA-S level was higher in the preterm group (p = 0.016) and DHEA-S levels were also higher in the SGA group (p = 0.004) (Table 1). A significant negative correlation was found between DHEA-S levels and birth weight (r = –0.41, p = 0.001).
Table 1. Relationship between serum DHEA-S levels and patients characteristics.
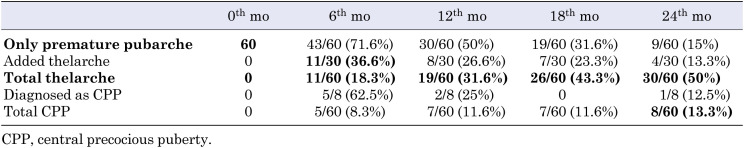
During follow-up, thelarche followed pubarche in 30 (50%) out of the 60 patients who presented with premature pubarche; in 11 (36.6%) of these 30 patients, thelarche occurred within the first six months of the follow-up period. Among the 60 patients, eight (13.3%) were diagnosed with CPP, and five (62.5%) out of eight were diagnosed within the first six months of the follow-up period. Nine out of the 60 patients (15%) who presented with premature pubarche continued to have isolated premature pubarche at the end of the 24-mo follow-up period (Table 2).
Table 2. Two-year follow-up results of patients with premature pubarche.
In 30 patients, thelarche followed pubarche; the mean decimal age of these patients was 7.75 ± 0.84 yr old. The mean time interval between their pubarche and thelarche was 11.20 ± 7.41 mo.
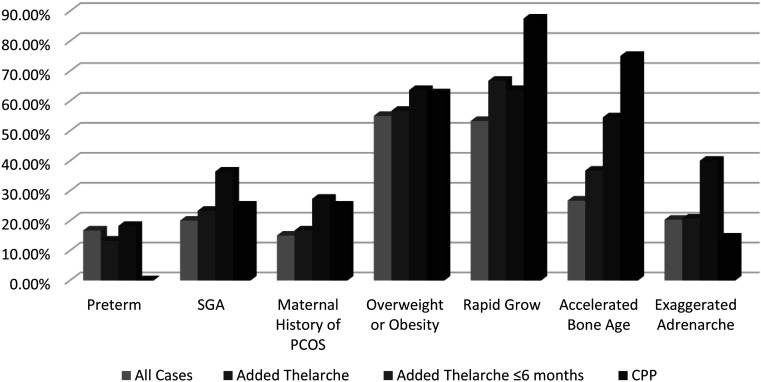
The comparison of related risk factors between the patient groups is shown graphically in Fig. 1. Maternal history of PCOS, SGA, and frequency of exaggerated adrenarche were significantly higher in the group in which thelarche followed pubarche within the first six months of the follow-up period compared to the other groups (p = 0.027). Being overweight or obese was seen in the majority of cases across all groups. As expected, more advanced growth in comparison to genetic potential and accelerated BA were observed in the majority of patients diagnosed with CPP.
Fig. 1.
Percentages of risk factors of all cases, including those that developed thelarche after pubarche, that developed thelarche after pubarche within the first six months of the follow-up period, and were diagnosed with CPP.
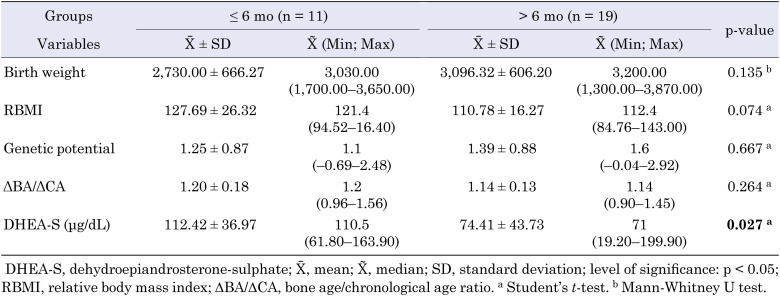
Serum DHEA-S levels of patients in which thelarche followed pubarche within the first six months were significantly higher than those who had thelarche six months after pubarche (p = 0.027) (Table 3).
Table 3. Relationship between thelarche developing after pubarche within the first six months and after six months.
Discussion
Puberty is a complex process involving developmental changes regulated by multiple genetic and environmental factors and hormonal mechanisms. In the literature, precocious puberty has been indicated as the onset of pubertal development in girls before the age of 8 yr old (9,10,11,12,13,14,15). However, there continue to be many debates about the age limit used for diagnosing precocious puberty (12). Onset of pubarche before the age of 8 yr old is considered an early finding, which in most cases requires further evaluation. In line with this, a study conducted with 820 girls aged between four and 8 yr old in Turkey emphasized a similar pattern. Guran et al. reported that the prevalence of premature pubarche was 4.3% (6).
Previous studies from various ethnicities have demonstrated that onset of pubarche was between 6.5 and 7.5 yr old in girls with premature pubarche (11, 16,17,18). Agreeing with other studies, our results showed that the mean age at onset of pubarche was 6.93 yr old in 60 girls. Williams et al. found that the mean onset of pubarche was 7.2 yr old in girls presenting with isolated premature pubarche over a two-year follow-up period (19). This observation is also in accordance with our study.
The Barker hypothesis asserts that the intrauterine environment of the fetus significantly contributes to health problems during childhood. Intrauterine exposure to nutrients, oxygen, or placental hormones has been associated with chronic diseases. Children born SGA experience higher rates of developing hypertension, glucose intolerance, diabetes mellitus, and mortality from cardiovascular disease (20). For the first time in the literature, Persson et al. reported that intrauterine exposure affected the onset of puberty. It was reported that “girls born with SGA were five months earlier than normal girls at puberty onset and menarche” (21). In the following studies, preterm birth, SGA, and IUGR were accepted as predisposing factors for premature pubarche (15, 22,23,24). Santos-Silva et al. documented that the mean birth weight was 2,945 g in Caucasian girls diagnosed with premature pubarche (16). In contrast, Oettingen et al. reported that the mean birth weight was 3.236 g in girls of various ethnicities; among these, 13% were preterm (17). Charkaluk et al. showed that 18.7% of girls presenting with premature pubarche had IUGR (24). Similar to previous studies, we showed that the mean birth weight of our participants was 2,918 g. About 16.7% of them were born at or before the 37th gestational week. In our cohort, 20% of the cases presenting with pubarche were born SGA, which is consistent with the literature. Although intrauterine limited growth has been implicated in premature adrenarche, it remains controversial. More recent studies have focused on this issue (18, 25).
Previous studies have suggested that being overweight and obese causes premature activation of the hormonal axis, initiating the onset of puberty (18, 26,27,28,29). Atay et al. reported a more significant increase in premature thelarche with an increase in BMI compared with premature pubarche (24). Similarly, Rosenfield et al. concluded that being overweight and obese were respectively about 1.5 times and about two times more common in girls with signs of earlier pubertal development (29). In contrast, the rate of being overweight or obese in Australian girls and boys who were eventually diagnosed with premature pubarche was 65%. This rate was reported to be 60% in Caucasian girls diagnosed with premature pubarche (16, 18). Charkaluk et al. documented that only 32.5% of the participants were obese in French girls with premature pubarche (23). Utriainen et al. found that the BMI of Northern Europeans who were eventually diagnosed with premature pubarche was significantly higher at 114% versus 104% for girls with normal pubertal prognosis (28). Similar to these prior studies, 55% of the patients in our study who presented with pubarche were overweight or obese. This finding further supports the finding of previous studies that obesity triggers the onset of puberty (1, 26, 28, 30,31,32,33,34,35,36).
In recent years, there has been an increase in studies showing that food types, endocrine disruptors, and chemicals may affect the onset and tempo of both premature pubarche and precocious puberty (26, 37, 38). However, due to the retrospective nature of our study, these risk factors could not be evaluated.
As it is known, precocious puberty causes accelerated growth, advanced BA, and early maturation. Ferran et al. found that 20% of girls who were eventually diagnosed with premature pubarche had advanced BA; however, Oettigen et al. calculated the mean ΔBA/ΔCA to be at 0.97 in girls followed up with pubarche (17, 32). In our study, the mean ΔBA/ΔCA was 1.10. Our data also showed that a BA of 26.7% of the patients was advanced. This rate was at 22.2% in patients who remained in isolated pubarche after two years. Seventy-five percent of patients diagnosed with CPP and thelarche following pubarche had advanced BA. Approximately 53.3% of patients who presented with pubarche deviated from their genetic potential and grew rapidly. Although some studies have pointed out that shifting the onset of puberty to an earlier time will cause a final height deficit, this situation does not affect the final height in patients with early puberty. However, their prepubertal growth tempo may increase slightly. It is important to note that this condition does not affect the onset and progression of normal puberty (1, 4, 11). In fact, in our study, the PAH of patients was compatible with their TH despite their advanced BA; Erdeve et al. followed patients with early puberty up to their final adult height and showed that there was no loss of target height (39). Goktug et al. observed that pubertal progression and final adult height were not affected in patients diagnosed with premature pubarche (37). Additionally, Santos-Silva et al. found a positive correlation between BA advancement and serum DHEA-S levels. They reported that overweight or obese girls with premature pubarche have advanced BA and higher androgen levels compared to premature pubarche girls with normal BMI (16).
Previous studies have shown an increase in the incidence of anovulation, functional ovarian hyperandrogenism, and metabolic syndrome after puberty in girls with premature pubarche. In particular, metabolic syndrome can manifest before puberty; Ibanez et al. reported that hyperinsulinism in girls decreased SHBG and IGFBP-1 levels. It can also cause PCOS in later periods by causing adrenal and ovarian hyperandrogenism (25, 30, 31, 40, 41). Recently, a diagnosis of PCOS is no longer possible by molecular genetic analysis alone, but it has been shown to be common in patients with a positive family history. Ferran et al. reported family history of PCOS in 9.6% of their patients with their mean age of menarche at 12.2 yr old (32). In our study, 15% of the mothers of those with premature pubarche had a history of PCOS, and their maternal menarche ages were generally normal with a mean age of 12.98 yr old.
In the literature, a serum DHEA-S level above 40 μg/dL is accepted as a sign of biochemical adrenarche, and a serum level above 222 μg/dL has been accepted as exaggerated adrenarche (2, 6, 9, 19). However, in recent years, it has been emphasized that adrenarche should more appropriately be interpreted based on age and pubertal stage. Based on normal reference levels in our country, DHEA-S reference values determined based on standardized age, sex, and puberty stage were used in our study. As such, we accepted DHEA-S levels of 35–124 μg/dL as normal adrenarche and levels above 124 μg/dL as exaggerated adrenarche (6). Santos-Silva et al. reported that the mean DHEA-S level was found at 102 μg/dL in girls with premature pubarche (16). Oettigen et al. reported that the mean serum DHEA-S level of girls with premature pubarche was 65.1 ± 35.9 μg/dL (26). In our study, the mean serum DHEA-S level was 89.09 ± 39.68 µg/dL. Among the participants, 69.5% had serum DHEA-S levels considered to be biochemical adrenarche in Turkish children based on pubertal stage and sex, and the level was accepted as exaggerated adrenarche in 20.3% of them.
Many case-control studies have documented that children born SGA have higher androgen levels than their peers born with appropriate sizes according to their gestational age (41, 42). Our findings are in agreement with those of previous studies; serum DHEA-S levels were found to be significantly higher in those with a history of SGA and prematurity. This finding supports the hypothesized relationship between intrauterine programming and early adrenal maturation.
Santos-Silva et al. reported obesity in 29% of girls and 49% of boys in a study that included 82 girls and 15 boys. Serum DHEA-S levels were found to be significantly higher in overweight or obese patients than in those with normal BMI (16). In our study, no significant relationship was found between being overweight or obese and DHEA-S levels. This may be due to the sample size and the inclusion of only girls in the study.
Pere et al. reported that the mean age of menarche in Finnish girls with a diagnosis of premature adrenarche was six months earlier compared to the general population (43). Similarly, Ibanez et al. showed that menarche was seen earlier in Catalan girls with a diagnosis of premature adrenarche compared to the local population. Menarche was three times more common before the age of 12 yr old among those with SGA (15). In another study, Ibanez et al. showed that puberty started earlier and was completed faster in girls born SGA and diagnosed with premature pubarche. Menarche occurred approximately 8–10 mo earlier, and adult height decreased by a mean of 6.5 cm (4).
In our study, it was observed that premature thelarche followed in 30 of 60 patients with premature pubarche after a two-year follow-up. Thelarche followed pubarche in 11 (36.6%) of these patients within the first six months. In the follow-up period, eight patients (13%) were diagnosed with CPP, and five (62%) of them were diagnosed within the first six months wherein treatment was also initiated. In this context, it is crucial to know that the first six months are the most critical time when it comes to diagnosing CPP during follow-up consults of patients diagnosed with premature pubarche.
DHEA-S levels were found to be significantly higher in patients who had thelarche after pubarche within six months compared to those whose thelarche followed after six months. Similar to previous studies, our results showed that serum DHEA-S levels were significantly higher in patients with a history of IUGR and SGA. CPP in patients with pubarche can be quickly diagnosed with well-established prenatal and natal history and high serum DHEA-S levels at admission.
Although the risk factors are well defined, the cause of premature pubarche remains unknown. Premature pubarche is considered an exaggerated variant of normal adrenarche. The fact remains that the levels of serum DHEA-S, which is the main hormone of adrenarche, was higher in obese patients, and early puberty seems to support this theoretical prediction. However, adrenarche was not exaggerated in any of our patients. This means that an elevated DHEA-S level is not the only factor responsible for early puberty. Although our study is based on retrospective data, the strength of our study relies on the fact that patients with organic hyperandrogenism were excluded.
Conclusion
In conclusion, being overweight or obese and having advanced growth compared to genetic potential were detected in half of our patients with premature pubarche. In addition to these risks, those at risk of early puberty had higher serum DHEA-S levels, which were independent of their weight. We also noted that the most critical time for detecting precocious puberty is the first six months of the follow-up period.
Conflict of Interests
The authors have no conflicts of interest to declare.
Acknowledgments
We thank all children and adolescents who participated in the study.
References
- 1.Berberoğlu M. Precocious puberty and normal variant puberty: definition, etiology, diagnosis and current management. J Clin Res Pediatr Endocrinol 2009;1: 164–74. doi: 10.4274/jcrpe.v1i4.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Utriainen P, Laakso S, Liimatta J, Jääskeläinen J, Voutilainen R. Premature adrenarche--a common condition with variable presentation. Horm Res Paediatr 2015;83: 221–31. doi: 10.1159/000369458 [DOI] [PubMed] [Google Scholar]
- 3.Farello G, Altieri C, Cutini M, Pozzobon G, Verrotti A. Review of the literature on current changes in the timing of pubertal development and the incomplete forms of early puberty. Front Pediatr 2019;7: 147. doi: 10.3389/fped.2019.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibáñez L, de Zegher F. Puberty after prenatal growth restraint. Horm Res 2006;65(Suppl 3): 112–5. [DOI] [PubMed] [Google Scholar]
- 5.Neyzi O, Bundak R, Gökçay G, Günöz H, Furman A, Darendeliler F, et al. Reference values for weight, height, head circumference, and body mass index in Turkish children. J Clin Res Pediatr Endocrinol 2015;7: 280–93. doi: 10.4274/jcrpe.2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guran T, Firat I, Yildiz F, Kaplan Bulut I, Dogru M, Bereket A. Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche. Clin Endocrinol (Oxf) 2015;82: 712–8. doi: 10.1111/cen.12612 [DOI] [PubMed] [Google Scholar]
- 7.Carel JC, Léger J. Clinical practice. Precocious puberty. N Engl J Med 2008;358: 2366–77. doi: 10.1056/NEJMcp0800459 [DOI] [PubMed] [Google Scholar]
- 8.Rogol AD, Hayden GF. Etiologies and early diagnosis of short stature and growth failure in children and adolescents. J Pediatr 2014;164(5): S1–14.e6. doi: 10.1016/j.jpeds.2014.02.027 [DOI] [PubMed] [Google Scholar]
- 9.Sultan C, Gaspari L, Maimoun L, Kalfa N, Paris F. Disorders of puberty. Best Pract Res Clin Obstet Gynaecol 2018;48: 62–89. doi: 10.1016/j.bpobgyn.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 10.Rosenfield RL, Cooke DW, Radovick S. Puberty and its disorders in the female. In: Pediatric Endocrinology: Fourth Edition: Elsevier Inc.; 2014. p. 569-663. e1. [Google Scholar]
- 11.Sancho Rodríguez ML, Bueno Lozano G, Labarta Aizpún JI, de Arriba Muñoz A. Natural progression of premature pubarche and underlying diseases. An Pediatr (Engl Ed) 2018;89: 238–45(in Spanish). [DOI] [PubMed] [Google Scholar]
- 12.Carel JC, Lahlou N, Roger M, Chaussain JL. Precocious puberty and statural growth. Hum Reprod Update 2004;10: 135–47. doi: 10.1093/humupd/dmh012 [DOI] [PubMed] [Google Scholar]
- 13.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44: 291–303. doi: 10.1136/adc.44.235.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novello L, Speiser PW. Premature adrenarche. Pediatr Ann 2018;47: e7–11. doi: 10.3928/19382359-20171214-04 [DOI] [PubMed] [Google Scholar]
- 15.Ibáñez L, Jiménez R, de Zegher F. Early puberty-menarche after precocious pubarche: relation to prenatal growth. Pediatrics 2006;117: 117–21. doi: 10.1542/peds.2005-0664 [DOI] [PubMed] [Google Scholar]
- 16.Santos-Silva R, Costa C, Castro-Correia C, Fontoura M. Clinical, biochemical and gender characteristics of 97 prepubertal children with premature adrenarche. J Pediatr Endocrinol Metab 2019;32: 1247–52. doi: 10.1515/jpem-2019-0185 [DOI] [PubMed] [Google Scholar]
- 17.von Oettingen J, Sola Pou J, Levitsky LL, Misra M. Clinical presentation of children with premature adrenarche. Clin Pediatr (Phila) 2012;51: 1140–9. doi: 10.1177/0009922812456238 [DOI] [PubMed] [Google Scholar]
- 18.Neville KA, Walker JL. Precocious pubarche is associated with SGA, prematurity, weight gain, and obesity. Arch Dis Child 2005;90: 258–61. doi: 10.1136/adc.2004.053959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams RM, Ward CE, Hughes IA. Premature adrenarche. Arch Dis Child 2012;97: 250–4. doi: 10.1136/archdischild-2011-300011 [DOI] [PubMed] [Google Scholar]
- 20.Barker DJ. Fetal growth and adult disease. Br J Obstet Gynaecol 1992;99: 275–6. doi: 10.1111/j.1471-0528.1992.tb13719.x [DOI] [PubMed] [Google Scholar]
- 21.Persson I, Ahlsson F, Ewald U, Tuvemo T, Qingyuan M, von Rosen D, et al. Influence of perinatal factors on the onset of puberty in boys and girls: implications for interpretation of link with risk of long term diseases. Am J Epidemiol 1999;150: 747–55. doi: 10.1093/oxfordjournals.aje.a010077 [DOI] [PubMed] [Google Scholar]
- 22.Voutilainen R, Jääskeläinen J. Premature adrenarche: etiology, clinical findings, and consequences. J Steroid Biochem Mol Biol 2015;145: 226–36. doi: 10.1016/j.jsbmb.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 23.Charkaluk ML, Trivin C, Brauner R. Premature pubarche as an indicator of how body weight influences the onset of adrenarche. Eur J Pediatr 2004;163: 89–93. doi: 10.1007/s00431-003-1358-9 [DOI] [PubMed] [Google Scholar]
- 24.Atay Z, Turan S, Guran T, Furman A, Bereket A. The prevalence and risk factors of premature thelarche and pubarche in 4- to 8-year-old girls. Acta Paediatr 2012;101: e71–5. doi: 10.1111/j.1651-2227.2011.02444.x [DOI] [PubMed] [Google Scholar]
- 25.Ibáñez L, Potau N, Marcos MV, De Zegher F. Adrenal hyperandrogenism in adolescent girls with a history of low birthweight and precocious pubarche. Clin Endocrinol (Oxf) 2000;53: 523–7. doi: 10.1046/j.1365-2265.2000.01133.x [DOI] [PubMed] [Google Scholar]
- 26.Adair LS, Gordon-Larsen P. Maturational timing and overweight prevalence in US adolescent girls. Am J Public Health 2001;91: 642–4. doi: 10.2105/AJPH.91.4.642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mäntyselkä A, Jääskeläinen J, Lindi V, Viitasalo A, Tompuri T, Voutilainen R, et al. The presentation of adrenarche is sexually dimorphic and modified by body adiposity. J Clin Endocrinol Metab 2014;99: 3889–94. doi: 10.1210/jc.2014-2049 [DOI] [PubMed] [Google Scholar]
- 28.Utriainen P, Jääskeläinen J, Romppanen J, Voutilainen R. Childhood metabolic syndrome and its components in premature adrenarche. J Clin Endocrinol Metab 2007;92: 4282–5. doi: 10.1210/jc.2006-2412 [DOI] [PubMed] [Google Scholar]
- 29.Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics 2009;123: 84–8. doi: 10.1542/peds.2008-0146 [DOI] [PubMed] [Google Scholar]
- 30.Ibáñez L, Potau N, Francois I, de Zegher F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab 1998;83: 3558–62. doi: 10.1210/jcem.83.10.5205 [DOI] [PubMed] [Google Scholar]
- 31.Ibáñez L, Díaz R, López-Bermejo A, Marcos MV. Clinical spectrum of premature pubarche: links to metabolic syndrome and ovarian hyperandrogenism. Rev Endocr Metab Disord 2009;10: 63–76. doi: 10.1007/s11154-008-9096-y [DOI] [PubMed] [Google Scholar]
- 32.de Ferran K, Paiva IA, Garcia LS, Gama MP, Guimarães MM. Isolated premature pubarche: report of anthropometric and metabolic profile of a Brazilian cohort of girls. Horm Res Paediatr 2011;75: 367–73. doi: 10.1159/000324107 [DOI] [PubMed] [Google Scholar]
- 33.Ong KK, Petry CJ, Emmett PM, Sandhu MS, Kiess W, Hales CN, et al. ALSPAC study team. Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia 2004;47: 1064–70. doi: 10.1007/s00125-004-1405-8 [DOI] [PubMed] [Google Scholar]
- 34.Vuguin P, Linder B, Rosenfeld RG, Saenger P, DiMartino-Nardi J. The roles of insulin sensitivity, insulin-like growth factor I (IGF-I), and IGF-binding protein-1 and -3 in the hyperandrogenism of African-American and Caribbean Hispanic girls with premature adrenarche. J Clin Endocrinol Metab 1999;84: 2037–42. [DOI] [PubMed] [Google Scholar]
- 35.Mäntyselkä A, Jääskeläinen J, Lindi V, Viitasalo A, Tompuri T, Voutilainen R, et al. The presentation of adrenarche is sexually dimorphic and modified by body adiposity. J Clin Endocrinol Metab 2014;99: 3889–94. doi: 10.1210/jc.2014-2049 [DOI] [PubMed] [Google Scholar]
- 36.Kaya G, Yavas Abali Z, Bas F, Poyrazoglu S, Darendeliler F. Body mass index at the presentation of premature adrenarche is associated with components of metabolic syndrome at puberty. Eur J Pediatr 2018;177: 1593–601. doi: 10.1007/s00431-018-3211-1 [DOI] [PubMed] [Google Scholar]
- 37.Göktuğ A, Aycan Z, Önder A, Sağsak E, Keskin M, Çetinkaya S. Prematüre Pubarş Olgularının Değerlendirilmesi: Tek Merkez Deneyimi. Turkish J Pediatr Dis 11(1): 34-9.
- 38.Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr 2012;77: 137–45. doi: 10.1159/000336325 [DOI] [PubMed] [Google Scholar]
- 39.Savaş-Erdeve Ş, Şıklar Z, Hacıhamdioğlu B, Kocaay P, Çamtosun E, Öcal G, et al. Gonadotropin-releasing hormone analogue treatment in females with moderately early puberty: no effect on final height. J Clin Res Pediatr Endocrinol 2016;8: 211–7. doi: 10.4274/jcrpe.2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, et al. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediatr 2017;88: 371–95. doi: 10.1159/000479371 [DOI] [PubMed] [Google Scholar]
- 41.Ibáñez L, Lopez-Bermejo A, Díaz M, Suárez L, de Zegher F. Low-birth weight children develop lower sex hormone binding globulin and higher dehydroepiandrosterone sulfate levels and aggravate their visceral adiposity and hypoadiponectinemia between six and eight years of age. J Clin Endocrinol Metab 2009;94: 3696–9. doi: 10.1210/jc.2009-0789 [DOI] [PubMed] [Google Scholar]
- 42.Saenger P, Dimartino-Nardi J. Premature adrenarche. J Endocrinol Invest 2001;24: 724–33. doi: 10.1007/BF03343917 [DOI] [PubMed] [Google Scholar]
- 43.Pere A, Perheentupa J, Peter M, Voutilainen R. Follow up of growth and steroids in premature adrenarche. Eur J Pediatr 1995;154: 346–52. doi: 10.1007/BF02072100 [DOI] [PubMed] [Google Scholar]