Abstract.
The novel coronavirus disease (COVID-19) has emerged as a global pandemic. This was a prospective, case-control study conducted in Izmir, Turkey. The aim of this study was to assess the relationship between COVID-19 and new-onset T1DM. We included pediatric patients (aged 6 mo–18 yr) with new-onset type-1 diabetes mellitus (T1DM) diagnosed during the COVID-19 pandemic, between April 2020 and January 2021. Polymerase chain reaction was used to diagnose COVID-19 after hospital admission. An enzyme-linked immunoassay for IgM and IgG against SARS-CoV-2 was performed after the diagnosis was confirmed. In the control group, the blood antibody test was conducted as close as possible to the time of the T1DM patient referral. A total of 118 participants were included in the study, comprising 57 (48%) patients with new-onset T1DM and 61 (52%) healthy controls. Of the 57 patients, 36 (63.2%) presented with DKA, 17 (29.7%) with diabetic ketosis, and four (7%) incidentally. The SARS-CoV-2 antibody test was positive in five (8.7%) patients with T1DM and six (10%) controls. The rate of positivity did not differ between the two groups (p = 0.901). It was not possible to demonstrate a clear association between SARS-CoV-2 infection and new-onset T1DM. Whether SARS-CoV-2 increases susceptibility to diabetes by triggering islet cell autoimmunity and affects the timing of overt diabetes in patients with existing autoimmunity should be studied in large cohorts.
Keywords: COVID-19, diabetes mellitus, type-1, children
Highlights
● The incidence of COVID-19 in newly diagnosed pediatric patients with type 1 diabetes was the same as that in healthy children.
● The incidence of diabetic ketoacidosis in newly diagnosed pediatric patients with type 1 diabetes was high during the pandemic period (63.2%).
● We found no association between COVID-19 and new-onset pediatric type 1 diabetes mellitus.
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune condition in which β-cells are destroyed by autoreactive CD4+ and CD8+ T cells. There is an associated genetic predisposition to T1DM, with more than 50 candidate genes involved (1), although other factors are thought to be involved in its pathogenesis. The relationship between viral infections and T1DM is complex. Well-established seasonal variation in new-onset T1DM has led to the suggestion of viral infections playing a role in its etiology (2, 3). Destruction of > 90% of β-cells by direct viral-mediated damage leads to non-autoimmune diabetes; limited lysis releases islet cell antigens, which in conjunction with enhanced immune response results in autoimmunity (1).
Strong evidence of the relationship between coronaviruses and diabetes is based on the 2003 SARS epidemic. In severe acute respiratory syndrome (SARS), hyperglycemia is an independent predictor of morbidity and mortality in both diabetic and non-diabetic patients (4). Hyperglycemia was found in patients with mild respiratory symptoms, strengthening the hypothesis of acute damage to β-cells due to viral replication in the pancreas (5). During the 2012 Middle East Respiratory Syndrome Coronavirus (MERS-CoV) epidemic, the prevalence of T1DM increased by approximately 50% (6). Coronavirus infection may have caused an increase in T1DM manifestations by affecting immune regulation, causing direct damage to pancreatic β-cells, or both (7).
With the emergence of the novel coronavirus disease (COVID-19) as a global pandemic requiring unprecedented countermeasures, studies on its effects on diabetes have begun (5). Pre-existing diabetes mellitus is a risk factor for developing severe COVID-19 and related complications. Indeed, there have been reports of COVID-19-induced severe metabolic decompensation of pre-existing or new-onset diabetes, such as diabetic ketoacidosis (DKA) or a hyperglycemic, hyperosmolar state (HHS); COVID-19 may also induce new-onset T1DM (8).
The aim of this study was to assess the relationship between SARS-CoV-2 and new-onset T1DM.
Materials and Methods
This was a prospective, case-control study conducted at two tertiary hospitals in Izmir, Turkey. Pediatric patients (aged 6 mo–18 yr) with new-onset T1DM, diagnosed during the COVID-19 pandemic, between April 2020 and January 2021, were included. Antibodies against glutamic acid decarboxylase (GAD-65) and insulin autoantibodies (IAA) were measured. Written informed consent was obtained from the families, and verbal or written consent was obtained from the children. Within 24 h of hospital admission, polymerase chain reaction (PCR) was conducted on a nasopharyngeal swab sample to diagnose COVID-19. The sensitivity of the PCR test for laboratory-confirmed inpatient cases was 87.8%, and specificity was 88.9% (9). None of the patients had any COVID-19-related complaints or history of COVID-19. All patients were unvaccinated since there was no vaccine in the country during the study period. An enzyme linked immuno-assay microELISA (Abbott Laboratories Inc.) test for IgM and IgG against SARS-CoV-2 was performed within one month of TIDM diagnosis to determine whether patients with newly diagnosed T1DM had asymptomatic antecedent SARS-CoV-2 infection. This test cannot discriminate between IgM or IgG titers, but it has a high sensitivity (96%) and high specificity (100%) (10). Height, weight, and BMI were measured and converted to Z-scores using standard references for Turkish children (11). The control group consisted of healthy children who were regularly followed up at the pediatric clinics of the same hospitals, similar populations. The control children were matched for age and sex with patients with newly diagnosed T1DM. In the controls, the blood antibody test was performed as close as possible to the time of the T1DM patients’ referral as the rates of infection changed during the course of the study period. In controls, the same enzyme immuno-assay microELISA (Abbott Laboratories Inc.) test for IgM and IgG against SARS-CoV-2 was performed; controls did not undergo PCR testing.
In the patient group, DKA and T1DM were diagnosed according to the International Society of Pediatric and Adolescent Diabetes/International Diabetes Federation guidelines (12). Regular follow-up was performed and medical care administered by the same pediatric endocrinology team, and all data were recorded in the patient records.
Statistical analysis
All analyses were performed using SPSS version 21.0 (IBM Inc., Chicago, IL, USA). Categorical data are described using observed frequency and percentage, and continuous variables are summarized using mean and standard deviation (SD). In cases of nonparametric distribution of data, median and interquartile range are used. Patient and control data were compared using the chi-square test. Statistical significance was set at a p-value < 0.05.
Informed consent
Informed consent was obtained from the parents of the children in the study and control groups, and verbal or written consent was obtained from the children.
Ethics committee approval was obtained from Ege University (confirmation number 21-4T-54).
Results
Between April 2020 and January 2021, during the midst of the COVID-19 pandemic, a total of 106 patients had newly diagnosed T1DM in the participating centers. When the clinical characteristics at presentation were examined, DKA was detected in 62 (58.5%) and diabetic ketosis in 36 (34%); eight (7.5%) patients were incidentally diagnosed. Of the 106 patients; 57 (53.8%) agreed to participate in the study.
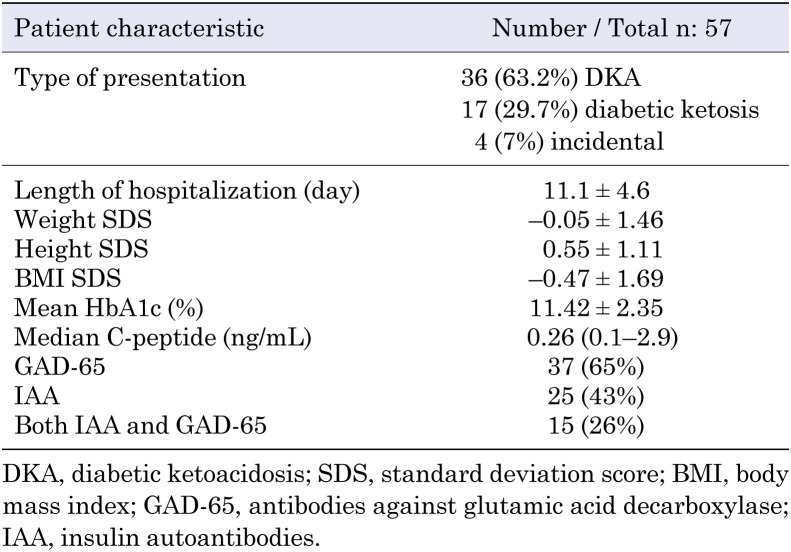
A total of 118 participants were included in the study, comprising 57 (48%) patients with new-onset T1DM and 61 (52%) healthy controls. COVID-19 PCR was performed after the patients were admitted to the hospital. The mean ages of the patients with T1DM and controls were 10.33 ± 4.5 yr and 10.35 ± 4.8 yr, respectively (p = 0.843). In the T1DM group, 32 (56%) of participants were male, and 25 (44%) were female; in the control group, 30 (49%) were male, and 31 (51%) were female (p = 0.441). The demographic features of the patients are presented in Table 1. Of the 57 patients with T1DM, 36 (63.2%) presented with DKA, 17 (29.7%) with diabetic ketosis, and four (7%) incidentally. All PCR tests were negative at the time of diagnosis in the T1DM group. The SARS-CoV-2 antibody test was positive in five (8.7%) patients with T1DM and six (10%) controls. Since patients denied experiencing COVID-19 symptoms, it was thought that this positivity was related to previous asymptomatic infection. The rate of positivity did not differ between the two groups (p = 0.901). Four (80%) of five SARS-CoV-2 antibody-positive patients were treated for DKA, three (60%) of whom had severe DKA. The median age was 12.57 (0.86–13.55) yr, and median glycated hemoglobin A1c level was 9.7%.
Table 1. Demographic, clinical, and laboratory characteristics of patients with new-onset type 1 diabetes.
Discussion
The role of viral infections in the pathogenesis of T1DM has long been suspected, as being reported in studies (13). In theory, any association with T1DM may be due to the nonspecific immunological mechanisms associated with infections. Respiratory infections in early life and T1DM have been linked, and the number of respiratory infection episodes within a nine-month period has been associated with the subsequent onset of islet autoimmunity within the following three months (14, 15).
Although the effects of the current COVID-19 pandemic on T1DM are not fully elucidated, previous sero-epidemiologic investigations have shown an association between SARS-CoV-2 and T1DM (16, 17). New-onset hyperglycemia is being increasingly described with COVID-19 in children without a history of diabetes (18). While infection-induced inflammation, cytokine activation, and resultant insulin resistance could lead to stress hyperglycemia, it is unclear to what extent islet cells are destroyed by SARS-CoV-2, leading to reduced insulin production and secretion.
SARS-CoV-2 can act as an infectious trigger that may precipitate DKA in patients with new-onset T1DM. Lawrence et al. reported a significant increase in the frequency of children and adolescents presenting with severe DKA at the onset of T1DM during the COVID-19 pandemic and attributed it to the difficulty of reaching hospitals and healthcare professionals (19). Ebekozien et al. reported 33 COVID-19-positive patients with T1DM, and the most prevalent adverse outcome was DKA (45.5%); however, only six cases were new-onset T1DM (20). Usworth et al. reported on the association between SARS-CoV-2 and new-onset T1DM from March 23 to June 4, 2020 (8). In this study, of 30 children, five were either SARS-CoV-2 PCR positive or SARS-CoV-2 serum IgG antibody positive. Twenty-one patients (70%) presented with DKA. Unsworth et al., thus, concluded that there was an apparent increase in the incidence of new-onset T1DM in children during the COVID-19 pandemic, compared to the incidence reported in previous reports. In our study, 36 (63.2%) patients presented with DKA. This value is higher than that seen in a previous report from the same city in Turkey that assessed ketosis (n = 58, 41.7%) and DKA (n = 57, 41%) at presentation in patients with newly diagnosed T1DM (21). We hypothesized that the high rate of DKA in the current cohort was due to a delay in diagnosis; it was not possible to attribute this to SARS-CoV-2 infection per se since the rate of seropositive patients was similar in both groups. Diagnosis could be delayed due to respiratory symptoms mimicking the respiratory distress caused by COVID-19; difficulties with and parental reluctance to access healthcare services; and redirection of healthcare resources to combat COVID-19, with less resources available for normal, albeit emergency, services.
Infection control measures, such as self-isolation, social distancing, and lockdown, may reduce the incidence of infections other than SARS-CoV-2, which are known triggers of diabetes. Thus, we would expect a decrease in the incidence of diabetes; although, if we found a constant or increased incidence, we may hypothesize that SARS-CoV-2 triggers diabetes. It is not possible to comment on the reason for the increase in the frequency of new-onset T1DM seen in our study as we did not compare it with the incidence in previous years.
Psychological stress is known to be a risk factor for ill health that potentially increases the risk of T1DM. Social distancing during the pandemic could be perceived as a stressful situation for children and adolescents who could not attend kindergarten or school and could not pursue normal social interactions, such as team sports or meeting friends. Perceived stress caused by feelings of isolation may have increased the risk of T1DM, and we think this must be considered when evaluating T1DM incidence. To understand whether SARS-CoV-2 is able to trigger an immune reaction against β-cells, it would be necessary to evaluate how many patients with COVID-19 developed T1DM or at least developed specific islet antibodies with long-term follow-up.
We reported on 57 pediatric patients diagnosed with T1DM and 61 healthy controls between April 2020 and December 2020. The incidence of COVID-19 immunity was similar between groups. SARS-CoV-2 infection did not seem to cause T1DM; however, our study did not include information about autoantibodies before T1DM diagnosis. Although autoimmunity develops within three months of infection, the development of overt diabetes may occur after months, and long-term observational studies are required to show a causative association. Hence, the present study was unable to conclude whether SARS-CoV-2 infections induced or accelerated autoimmunity.
Conclusion
It was not possible to demonstrate a clear association between SARS-CoV-2 infection and new-onset T1DM. Whether COVID-19 increases susceptibility to diabetes, triggers islet cell autoimmunity, and affects the time of overt diabetes in patients with existing autoimmunity should be studied in large cohorts in the long term.
Limitations
We did not perform PCR testing in the control group; as a result, we could not exclude active SARS-CoV-2 infection in the control group.
Conflict of Interests
None of the authors have any potential conflicts of interest associated with this research.
References
- 1.Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes 2008;57: 2863–71. doi: 10.2337/db07-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coppieters KT, Boettler T, von Herrath M. Virus infections in type 1 diabetes. Cold Spring Harb Perspect Med 2012;2: a007682–007682l. doi: 10.1101/cshperspect.a007682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr 2020;14: 2211–7. doi: 10.1016/j.dsx.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang JK, Feng Y, Yuan MY, Yuan SY, Fu HJ, Wu BY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med 2006;23: 623–8. doi: 10.1111/j.1464-5491.2006.01861.x [DOI] [PubMed] [Google Scholar]
- 5.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 2010;47: 193–9. doi: 10.1007/s00592-009-0109-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis 2016;49: 129–33. doi: 10.1016/j.ijid.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell 2020;27: 125–36.e7. doi: 10.1016/j.stem.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unsworth R, Wallace S, Oliver NS, Yeung S, Kshirsagar A, Naidu H, et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care 2020;43: e170–1. doi: 10.2337/dc20-1551 [DOI] [PubMed] [Google Scholar]
- 9.Jarrom D, Elston L, Washington J, Prettyjohns M, Cann K, Myles S, et al. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: a rapid systematic review. BMJ Evid-Based Med. 2020; bmjebm-2020-111511. [DOI] [PubMed]
- 10.Narasimhan M, Mahimainathan L, Araj E, Clark AE, Markantonis J, Green A, et al. Clinical Evaluation of the Abbott Alinity SARS-CoV-2 spike-specific quantitative IgG and IgM assays among infected, recovered, and vaccinated groups. J Clin Microbiol 2021;59: e0038821. doi: 10.1128/JCM.00388-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demir K, Konakçı E, Özkaya G, Kasap Demir B, Özen S, Aydın M, et al. New features for child metrics: further growth references and blood pressure calculations. J Clin Res Pediatr Endocrinol 2020;12: 125–9. doi: 10.4274/jcrpe.galenos.2019.2019.0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer-Davis EJ, Kahkoska AR, Jefferies C, Dabelea D, Balde N, Gong CX, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes 2018;19(Suppl 27): 7–19. doi: 10.1111/pedi.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lönnrot M, Lynch KF, Elding Larsson H, Lernmark Å, Rewers MJ, Törn C, et al. TEDDY Study Group. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia 2017;60: 1931–40. doi: 10.1007/s00125-017-4365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen T, Witsø E, Tapia G, Stene LC, Rønningen KS. Self-reported lower respiratory tract infections and development of islet autoimmunity in children with the type 1 diabetes high-risk HLA genotype: the MIDIA study. Diabetes Metab Res Rev 2011;27: 834–7. doi: 10.1002/dmrr.1258 [DOI] [PubMed] [Google Scholar]
- 15.Beyerlein A, Donnachie E, Jergens S, Ziegler AG. Infections in early life and development of type 1 Diabetes. JAMA 2016;315: 1899–901. doi: 10.1001/jama.2016.2181 [DOI] [PubMed] [Google Scholar]
- 16.Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 2020;8: 813–22. doi: 10.1016/S2213-8587(20)30272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klatman EL, Besançon S, Bahendeka S, Mayige M, Ogle GD. COVID-19 and type 1 diabetes: Challenges and actions. Diabetes Res Clin Pract 2020;166: 108275. doi: 10.1016/j.diabres.2020.108275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with Coronavirus disease 19 pneumonia. Gastroenterology 2020;159: 367–70. doi: 10.1053/j.gastro.2020.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence C, Seckold R, Smart C, King BR, Howley P, Feltrin R, et al. Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary centre during the COVID-19 pandemic. Diabet Med 2021;38: e14417. doi: 10.1111/dme.14417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 Diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care 2020;43: e83–5. doi: 10.2337/dc20-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demir K, Buyukinan M, Dizdarer C, Goksen D, Ozen S, Asar G, et al. The frequency and associated factors of diabetic ketoacidosis at diagnosis in children with type 1 diabetes. Güncel Pediatri 2010;8: 52–5. [Google Scholar]