Abstract
An increase in opioid-overdose deaths was evident before the COVID-19 pandemic, and has escalated since its onset. Fentanyl, a highly potent synthetic opioid, is the primary driver of these recent trends. The current study used two inbred mouse strains, C57BL/6J and A/J, to investigate the genetics of behavioral responses to fentanyl. Mice were tested for conditioned place preference and fentanyl-induced locomotor activity. C57BL/6J mice formed a conditioned place preference to fentanyl injections and fentanyl increased their activity. Neither effect was noted in A/J mice. We conducted RNA-sequencing on the nucleus accumbens of mice used for fentanyl-induced locomotor activity. Surprisingly, we noted few differentially expressed genes using treatment as the main factor. However many genes differed between strains. We validated differences in two genes: suppressor APC domain containing 1 (Sapcd1) and Glyoxalase 1 (Glo1), with quantitative PCR on RNA from the nucleus accumbens and prefrontal cortex (PFC). In both regions A/J mice had significantly higher expression of both genes than did C57BL/6J. In prefrontal cortex, fentanyl treatment decreased Glo1 mRNA. Glyoxalase 1 catalyzes the detoxification of reactive alpha-oxoaldehydes such as glyoxal and methylglyoxal, is associated with anxiety and activity levels, and its inhibition reduces alcohol intake. We suggest that future studies assess the ability of Glo1 and related metabolites to modify opioid intake.
Keywords: RNA-sequencing, opioids, metabolism, fentanyl, conditioned place preference, acute activity, conditioned activity
Introduction
Drug use disorders are the product of both genetic and environmental conditions, and are often caused by an interaction between the two. Data from twin studies estimate the heritability of opioid use disorder (OUD) as h2=0.4. The heritability of analgesic response to opioids is similar.1,2,3,4 This correlation points to a possible connection between the overall physiological response to opioids (including factors like the effectiveness of opioids to relieve pain) and the susceptibility to developing an OUD. One method that can be applied to this question is to compare behavioral and genetic differences between inbred mouse strains.
Inbred mouse lines are a powerful tool for pinpointing genetic differences that may contribute to a variety of phenotypes including drug-related behaviors. For example, intravenous (IV) self-administration (SA) studies comparing DBA/2J versus C57BL/6J (C6J) mice showed that C6J mice readily acquired morphine SA, while DBA/2J mice failed to acquire. After morphine exposure, several genes were differentially expressed in C6J but not in DBA/2J brain.5 Chromosome substitution lines using A/J chromosomes in a C6J background showed that both fentanyl- and methamphetamine-induced activity varied between the two inbred strains and that a quantitative trait locus (QTL) on Chromosome 11 was responsible.6 A/J and C6J mice are two of the eight paternal mouse strains used to produce CC and DO mice, both of which have been used for drug addiction research.7 Collaborative cross (CC) mouse lines have been assayed for acute activity, conditioned place preferences (CPP) and IVSA in response to cocaine.8 A small group of diversity outbred (DO) mice were tested on several novelty tasks, and used for cocaine acquisition studies with IVSA.9
Our goal was to use behavioral differences in response to fentanyl to discover novel genes that may facilitate vulnerability to the rewarding and behaviorally activating effects of fentanyl. As a first step we compared behavioral responses to fentanyl in two inbred mouse strains; C6J and A/J. We selected these two strains because they show significant differences in a variety of relevant motivated behaviors including exploratory locomotor activity, voluntary ethanol consumption, and acquisition of cocaine self-administration.10,11,12 The amount of activity induced by cocaine in these two strains is on opposite ends of a spectrum measured in 45 strains.13 Additionally, A/J mice were less sensitive than C6J to the depressive effects of fentanyl on respiration.14
We examined responses to fentanyl in two behavioral tests.15 One was CPP and the other was a 3-day test, which quantifies acute activity after an initial fentanyl exposure and conditioned responses to the test chamber in which fentanyl was previously experienced.15,6 Also using both strains and sexes, an initial characterization of transcriptomic differences was conducted on nucleus accumbens (NAc) tissue, with RNA-Sequencing (RNA-seq). Based on these data we selected two genes from the RNA-seq data; Glyoxalase 1 (Glo1) and Suppressor APC domain containing 1 (Sapcd1) and confirmed either strain or treatment effects with quantitative PCR. Lastly, we extended these data to another brain region involved in the dopamine mesolimbic pathway, the prefrontal cortex (PFC).
Methods and Materials
Animals
Male and female adult (60–90 days of age) mice from two inbred lines (A/J and C6J) were tested for behavior. All mice (except 4 A/Js from Jackson Labs, Bar Harbor ME) were born in the Biological Resources Facility at North Carolina State University. Mice were weaned at 21 days of age and housed in groups of 2–3 by strain, sex, and age. All mice had ad libitum access to water and food (Envigo Teklad 2020, Madison, WI, USA). Lights were on a 12:12 reverse light schedule. Tests for CPP were conducted prior to lights off. Mice in the activity study were tested (under red light) in the dark, after room lights went off. Female estrous cycles were not monitored. All animal care and procedures were approved by the NCSU animal care and use committee and in accordance with AAALAC standards.
Drugs
Mice were administered fentanyl HCL at a dose of 0.2 mg/kg via IP injection. This is a moderate dose that has been shown to be effective in C6J mice in both tasks used here15. Fentanyl HCL was dissolved in sterile physiological saline, all injections were administered at 10 mL/kg.
CPP
Mice were handled daily for at least 10 days prior to use and habituated to the testing room for 60 minutes during the two days prior to testing. The Plexiglas conditioning apparatus consisted of two square (15 cm x 15 cm) chambers with a hole between them which, when opened, allowed the mice free access to both chambers. A clear start box (4.5 × 15 cm) ran along the outside of both chambers. To begin each test session the mouse was placed in the start box with open access to both sides. When the mouse left the start box access back into it was blocked. One chamber consisted of white floors and walls, and the other consisted of black floors and walls. Additionally, the black chamber had a black plastic covered hard-wire cloth on the floor. These differences gave each side of the chamber unique texture and visual cues.16 The conditioning apparatus was cleaned with Peroxigard after each subject, and with 70% alcohol at the end of each day’s tests.
Initial side preferences were determined during a 15-minute test. For each individual the initially least preferred side (LPS) was paired with fentanyl injections (0.2 mg/kg ip), and the most preferred side (MPS) was paired with saline. After initial preference was established, 8 days of conditioning took place. Conditioning days alternated between fentanyl and saline injections, and fentanyl was always given on the first conditioning day. Conditioning sessions were 30 minutes long and were not recorded. There was a two-day break between experimental days 5 and 6. On day 10, final preferences were assessed with a 15-minute free access test. The initial and final preference tests were recorded and analyzed with Noldus EthoVision XT (Leesburg, VA), which tracked the amount of time the subject spent in each chamber. We tested 28 C6J mice (12 males and 16 females) and 22 A/J mice (12 males and 10 females).
Acute and Conditioned Activity
Mice of both strains and sexes either received fentanyl or saline before the first test in the Open Field (OF). In all subsequent tests mice received saline. This created 4 groups in each strain (n=8 per group): males + saline, males + fentanyl, females + saline, females + fentanyl. All mice were handled daily for two to four minutes in the three days prior to testing, and were habituated to the behavioral testing room each day for one hour before tests began. On day 1, mice received an injection of saline or fentanyl (0.2 mg/kg IP), and acute locomotor activity was recorded in the OF boxes. Total activity was recorded in the entire box (60 × 60 × 45 cm) and activity in the center was scored in the middle (30 × 30 cm ) square for 20 min. The next day all mice were injected with saline and immediately placed back into their home cages. On the third day, mice were injected with saline and then were placed in the OF and recorded for 20 min. Activity data (cm moved in the horizontal plane) and location (time spent in center) were analyzed on days 1 and 3 using Noldus EthoVision XT (Leesburg, VA).
Higher Doses of Fentanyl
Because A/J mice exhibited significantly less locomotor activity in response to fentanyl we conducted one more round of activity tests. We tested an additional four groups of A/J mice (n=8/group, dose x sex). Each mouse received one of two doses of fentanyl on the first day of the study (0.25 or 0.5 mg/kg). Only horizontal activity were analyzed but otherwise all other procedures were identical to those described above.
RNA-Sequencing
Mice used in the activity experiment (n=6 per group) were killed 24 hours after the final behavior test. An injection of either saline or fentanyl was given one hour prior to collecting brains. Mice in the control group (only exposed to saline during testing) received saline, and mice in the fentanyl treated group received fentanyl. This injection was given approximately 96 hours after their first and only other fentanyl treatment. Brains were rapidly removed and in a cold brain dissection mold we isolated bilateral nucleus accumbens (NAc) and the prefrontal cortex (PFC). The tissues were then rapidly frozen on dry ice. The NAc was shipped on dry ice to the University of Virginia for RNA-sequencing.
Flash frozen tissues were homogenized on ice in RLT buffer (Qiagen) containing 40mM DTT with a Tissue-Tearor (BiosSpec Products, Inc) at low speed (setting 3) for 10s. The resulting lysate was extracted with an equal volume of acid-phenol:chloroform (Thermo Fisher, cat# AM9722) and RNA was further purified from the aqueous phase with RNeasy mini kit (Qiagen) following the manufacture’s recommendations. Samples were DNased (DNA-free DNA removal kit, Thermo Fisher) and RNA concentrations were determined using a Qubit Fluorimeter (Thermo Fisher). DNA-free total RNA was sent to Psomagen (Rockville, MD) for mRNA-seq. The 32 samples were sequenced to an average read count depth of 14.89 ± 2.9 million.
RNA-seq analysis
Adaptors from raw sequence files were trimmed using Trimmomatic (https://doi.org/10.1093/bioinformatics/btu170). HISAT2 was used to align sequence reads to the GRCm38 (mm10) genome reference (https://doi.org/10.1038/nmeth.3317). StringTie was used for quantification of gene expression (PMID: 25690850). The GRCm38 – mm10 assembly was used for this analysis.
Principal Components Analysis (PCA) was performed to assess global differences due to strain and fentanyl exposure. DESeq2 was used to conduct differential gene expression analysis (doi: 10.1186/s13059–014-0550–8). Features with less than 10 reads total were excluded from analysis. DESeq2 fits a negative binomial generalized linear model for each gene along with a Wald test for significance testing. A total of three differential analyses were conducted. Significant genes were determined from each analysis using the default Benjamini-Hochberg FDR of <0.05. The three univariate models were fit using strain, sex, and treatment independently (n=32).
Quantitative PCR
We conducted qPCR using RNA from mice used for the RNA-seq study. For the NAc qPCR we had 4 mice in each group (except for the C6J control females, n=3 mice). and from the PFC we had 4–5 samples per group. We used Taqman primers for both genes (Table 1). For data evaluation, the comparative ΔΔCt method was used. Each sample was analyzed in triplicate. Expression levels of target genes were normalized to an endogenous control gene, glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Separate plates were used for each gene to eliminate plate-to-plate variation. Samples with values 2-fold above (or below) the standard deviation were excluded from the analyses.
Table 1:
TaqMan Probes
| GENE NAME | ABBREVIATION | TAQMAN ASSAY ID |
|---|---|---|
| SUPPRESSOR APC DOMAIN CONTAINING 1 | Sapcd1 | Mm004584449_m1 |
| GLYOXALASE 1 | Glo1 | Mm00844954_s1 |
| GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE | Gapdh | Mm99999915_g1 |
Statistics
To evaluate data from the CPP tests we assessed the amount of time spent on each side of the CPP apparatus on the first day (habituation) and the final test day. We examined three dependent measures. The initial difference in preference was the time spent on the black versus the white sides of the CPP box on the habituation test. Time spent on the final test day on the fentanyl-associated side of the test cage, minus the saline-associated side, was the final preference. Lastly, we scored time spent on the final test day in the fentanyl-associated side of the test cage minus the time spent on the first test day in the initially least preferred side, this is the change in preference. We employed two-way ANOVAs with sex and strain as the two independent variables. For comparisons between groups we used Bonferroni-corrected comparisons.
To analyze activity data in the OF a three-way repeated measures ANOVAs (strain x group x sex) was conducted. The data from A/J mice given higher doses of fentanyl were analyzed using a two-way repeated measure ANOVA, with dose and sex as the factors. For the qPCR analysis three-way ANOVA were used to compare the groups. Planned comparisons were made with Bonferroni-corrected t-tests.
To evaluate the qPCR data each region was analyzed with two-way ANOVAs (the factors were sex and strain). Planned comparisons were made using Bonferroni-corrected comparisons.
All the analyses were conducted using the statistical package Statistical Package for the Social Sciences (SPSS) 25 for Windows (SPSS, Chicago, IL, USA) or NCSS 2021 (Kaysville, Utah). Differences were considered significant if the probability of error was less than 5 %.
Results
C6J mice, but not A/J mice, form a conditioned place preference for fentanyl.
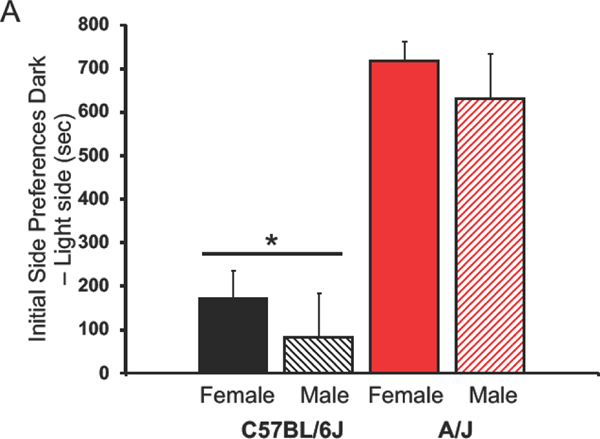
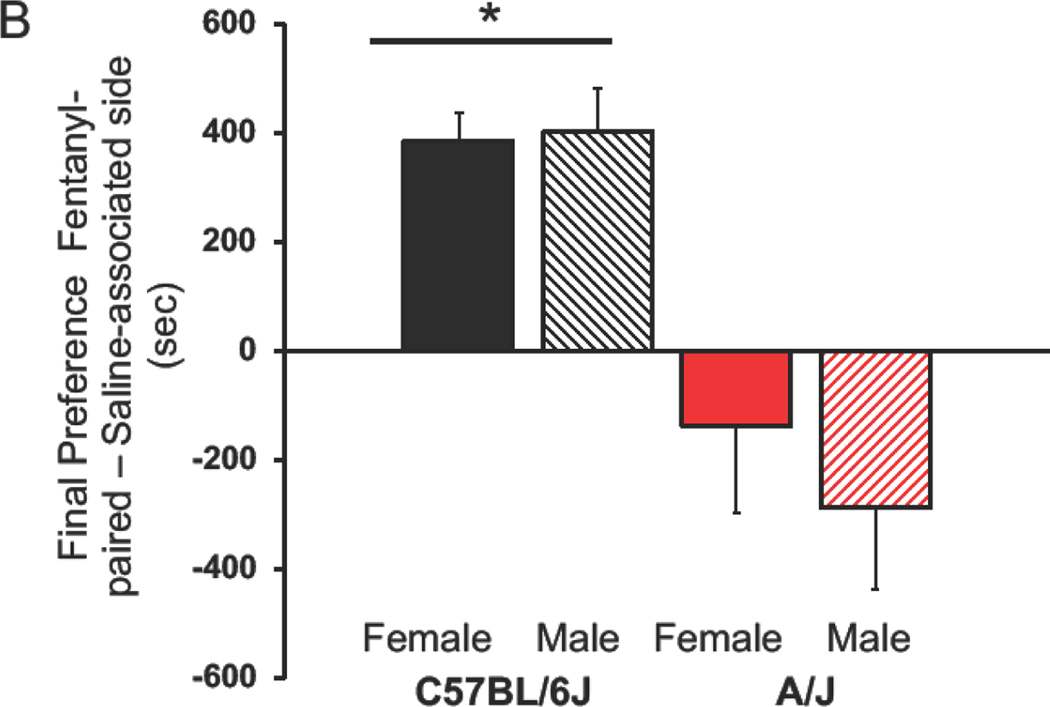
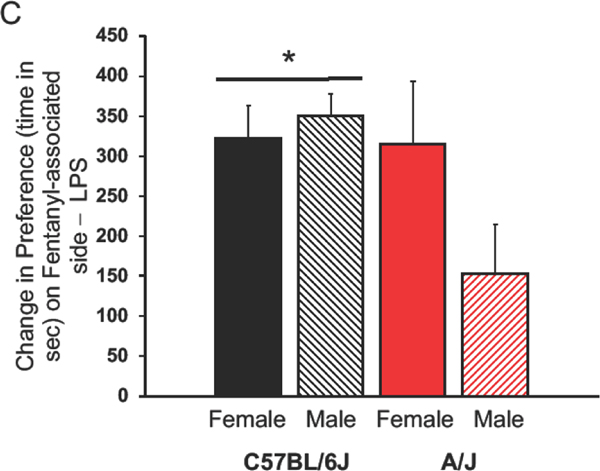
Initially, C6J mice did not have a significant side preference, but A/J mice strongly preferred the black side of the chamber (Figure 1A). This strain difference was significant (F(1,49)=43.52, p<0.00001), no effect of sex was found (F(1,49)=1.12) nor was there an interaction between the two factors (F(1,49)=0.01). Final preferences also indicated a significant strain effect (F(1,49)=31.05, p<0.00001). A/J mice did not form a preference to the fentanyl-paired side, but C6J mice did so (p<0.05, Figure 1B). No effect of sex was noted, nor was there an interaction (F(1,49)=0.39 and 0.61 respectively). Another strain effect was noted between initial time spent in the LPS and the final preference for the fentanyl-associated side (F(1,49)=4.87, p<0.035, Figure 1C). This was attributed to a larger change in preference in the C6J than the A/J mice. No significant sex difference was observed nor was there an interaction between the factors (F(1,49)=1.72, 2.59 respectively).
Figure 1:
Conditioned Place Preference (CPP) for Fentanyl.
Mean + SEM for C6J and A/J mice. A) Initial side preferences are expressed as time spent on the dark minus the time spent on the light side of the CPP chamber. B) Acquisition of a preference for the fentanyl-paired side is shown here. We calculated differences on the final test day between time spent in the fentanyl-associated minus the saline-associated sides of the CPP chamber. C) Change in preferences are calculated by subtracting time spent in the fentanyl-paired side on the final day minus initial preference for the least preferred side. Data from females are in solid colored histograms and males are denoted in stripped histograms. Data from C57BL/6J mice are in black and A/J are in red.* Significant effect of strain, p<0.00001. We tested 12 male and 16 female C6J mice along with 12 male and 10 female A/J mice. Sec=seconds, LPS=least preferred side.
C6J mice display increased activity after acute fentanyl exposure.
Activity in response to an initial acute fentanyl injection, and after re-introduction into the OF (now associated with fentanyl) were greater in C6J than A/J mice (Figure 2). We noted a highly significant effect of strain (F(1,127)= 238.29, p<0.00001), and an interaction between strain and treatment (F(1,127)=13.77, p<0.0005). The interaction was produced by the fentanyl-treated C6J mice, which were more active than all other groups (p<0.05). Activity on the first test day was significantly higher than on the third day (F(1,127)=43.87, p<0.00001). A final significant interaction, between strain and test day (F(1,127)=93.53, p<0.00001) was due to the fentanyl treated mice which traveled more on the first test day than any other group (p<0.05).
Figure 2:
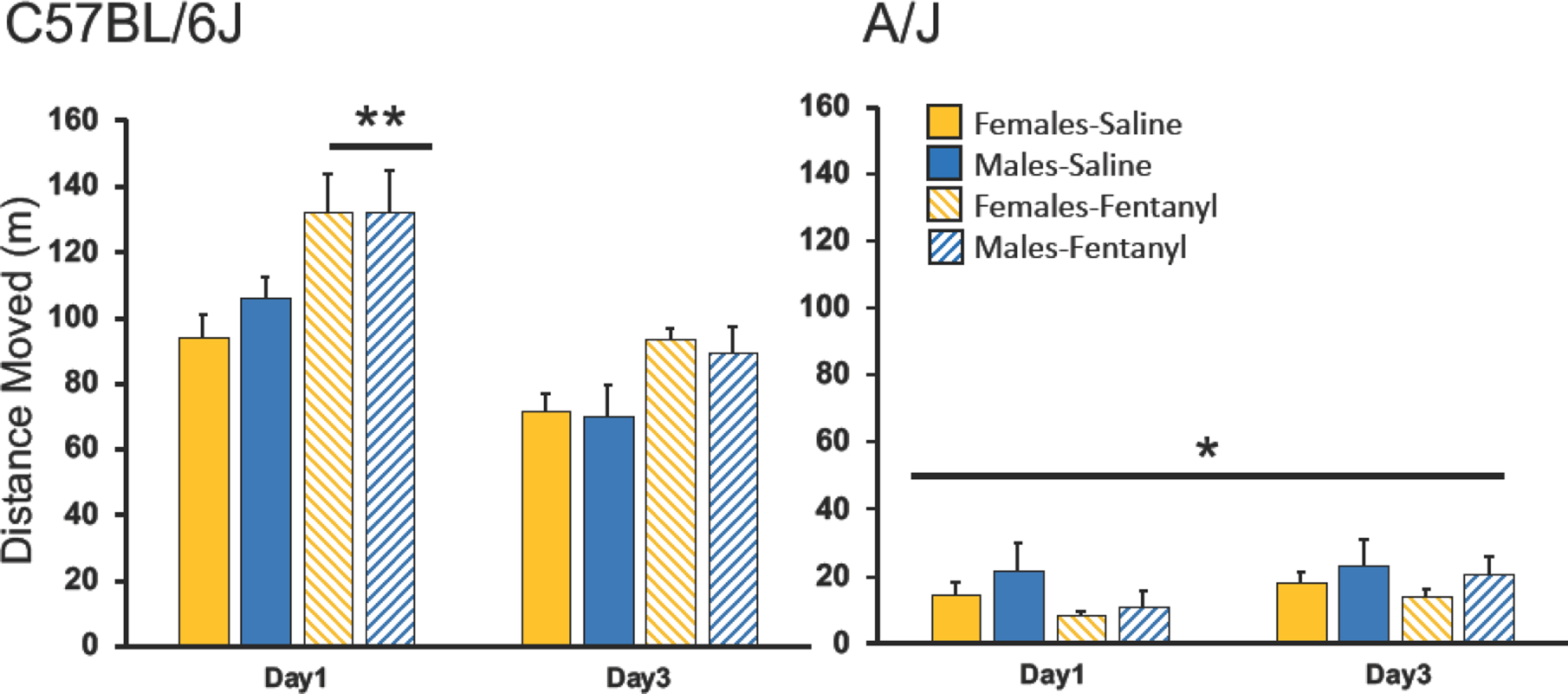
Activity in Response to Fentanyl.
Mean + SEM horizontal activity in centimeters. Activity for C6J mice is shown on the left, and A/J activity data is shown on the right. Data from females is shown in yellow histograms and males are in blue histograms. The first test day mice received either fentanyl (stripped histograms) or saline (solid histograms) and activity was recorded in the open field (OF). On the third day all mice received saline prior to the OF test. *Significant main effect of strain, p<0.00001. **Significantly more activity than any other group or test day, p<0.00001. Total test time was 20-min. cm=centimeters. n=8 per group.
To examine the conditioned response, we performed two-way ANOVAs for each strain on the data from day 3. In C6J mice we noted a significant effect of treatment (F(1,31)=9.06, p<0.005). Because the mice with prior fentanyl experience were more active on this test than the saline exposed mice we can conclude that their activity was a conditioned response to the OF, now associated with fentanyl (p<0.05). The A/J mice showed little activity, and this was not affected by prior fentanyl treatment (F(1,31)=0.48).
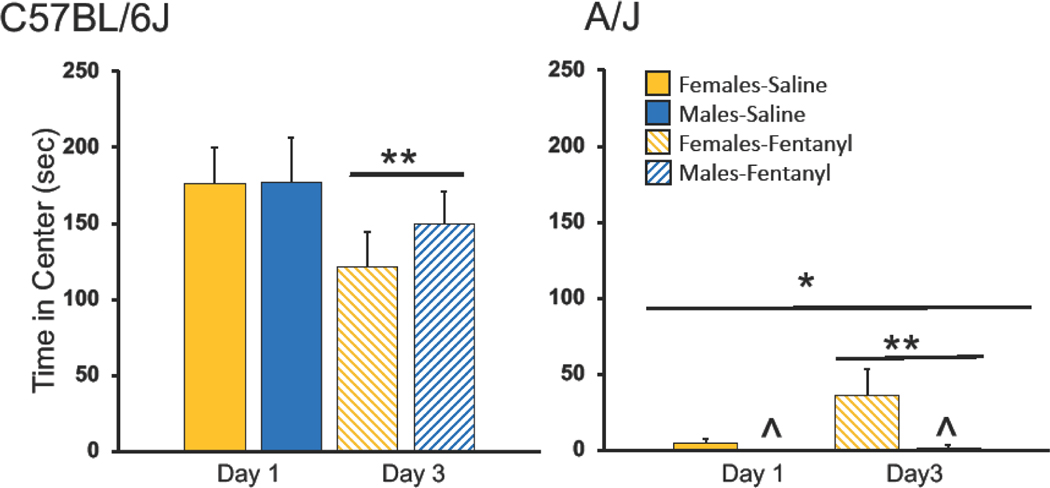
We also examined time spent in the center of the OF. A three-way repeated measures ANOVA revealed a significant strain effect (F(1,56)=129.07, p<0.00001, Figure 3) on location in the OF, with A/J mice spending far less time in the central square than did C6J mice. There was also an interaction between strain and treatment (F(1,56)=5.07, p<0.035) due to the opposite effects of fentanyl between the two strains; C6J mice treated with fentanyl spent less time in the center and A/J mice receiving fentanyl increased their time in the center. We performed two-way ANOVAs for each strain. A sex difference was noted in A/J mice (F(1,28)=4.65, p<0.05), with A/J females spending more time in the center of the arena than males. In C6J mice, the two-way ANOVA did not reveal any treatment or sex effects (F(1,28)=2.93, 0.37, respectively).
Figure 3:
Time spent in the center of the OF.
Mean + SEM time in the center of the OF. On the left activity in C6J mice is shown and on the right activity in A/J mice. Data from females is shown in yellow histograms and males are in blue histograms. The first test day mice received either fentanyl (stripped histograms) or saline (solid histograms) and activity was recorded in the open field (OF). On the third day all mice received saline prior to the OF test. *Significant effect of strain, p<0.00001. **Significant effect of treatment p<0.035. ^ Significant sex effect for A/J mice only p<0.05. Total test time was 20-min, sec=seconds. n=8 per group.
Higher doses of fentanyl did not enhance activity in A/J mice.
When A/J mice received more than double the original dose of fentanyl it had no impact on their activity. A two-way repeated measures ANOVA did not yield any dose, sex, or interaction effects in A/J mice (F(1,191)=0.16, 0.17 and 0.17 respectively, Table 2). The only significant effect was a sex by test day interaction (F(1,191)=5.42, p<0.03), but Bonferroni-corrected comparisons did not reveal any significant differences. Time spent in the center of the OF was likewise not affected by higher doses of fentanyl. (Table 2). The analysis of time spent in center also revealed a sex by day interaction (F(1,63)=5.42, p<0.03),
Table 2.
Horizontal Activity for A/J Mice. Mean +/− SEM horizontal activity (in meters) in three-day spontaneous and conditioned activity tests. No significant differences were found between groups based on sex or dose. n=8 per group.
| Fentanyl Dose | Spontaneous Activity (Day 1) 0.25 mg/kg | 0.5 mg/kg | Spontaneous Activity (Day 3) 0.25 mg/kg | 0.5 mg/kg |
|---|---|---|---|---|
| Male | 9.15 +/− 1.30 | 13.24 +/− 2.68 | 26.72 +/− 7.36 | 31.98 +/− 4.12 |
| Female | 8.26 +/− 1.51 | 23.73 +/− 14.61 | 19.73 +/− 5.69 | 21.59 +/− 2.82 |
Differences in gene expression primarily caused by strain.
We performed RNA-seq in tissue from the nucleus accumbens (NAc) to investigate transcriptomic differences due to fentanyl exposure in C6J and A/J mice. We analyzed a series of linear models testing the effect of strain, sex, and fentanyl exposure.
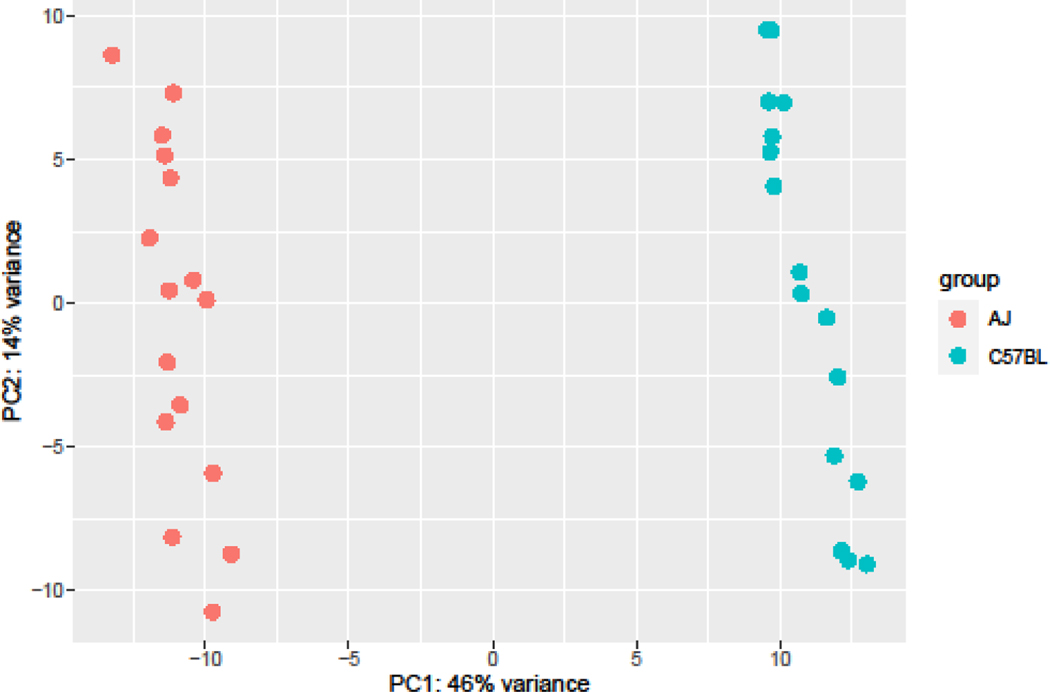
We first performed a principal components analysis (PCA) to understand major sources of variation in the data (Figure 4). As would be expected, the first principal component (PC1) separated the mice into two different groups, representing the two different strains. Mice did not separate based on sex or treatment (fentanyl or saline).
Figure 4:
Principal components analysis of nucleus accumbens RNA-seq data demonstrates clear separation of mice by strain.
Univariate models 1–3 examined the effects of strain, sex, and treatment on differential gene expression independently. Consistent with the PCA, we identified 5,769 significantly differentially expressed genes as a function of strain (Supplemental Table 2). A total of 14 genes were significantly differentially expressed as a function of sex (Supplemental Table 3). Most of these genes were located on the sex chromosomes. The final univariate model for treatment contained no significantly differentially expressed genes.
Models 4 and 5 were fit within C6J and A/J mice respectively because strain accounted for 40% of the variance. Model 4 was fit with only C6J mice (n=16) and model 5 was fit with only A/J mice (n=16). Both models controlled for treatment. No significantly differentially genes were detected.
Genes from RNA-Seq data set primarily show strain differences in two brain regions.
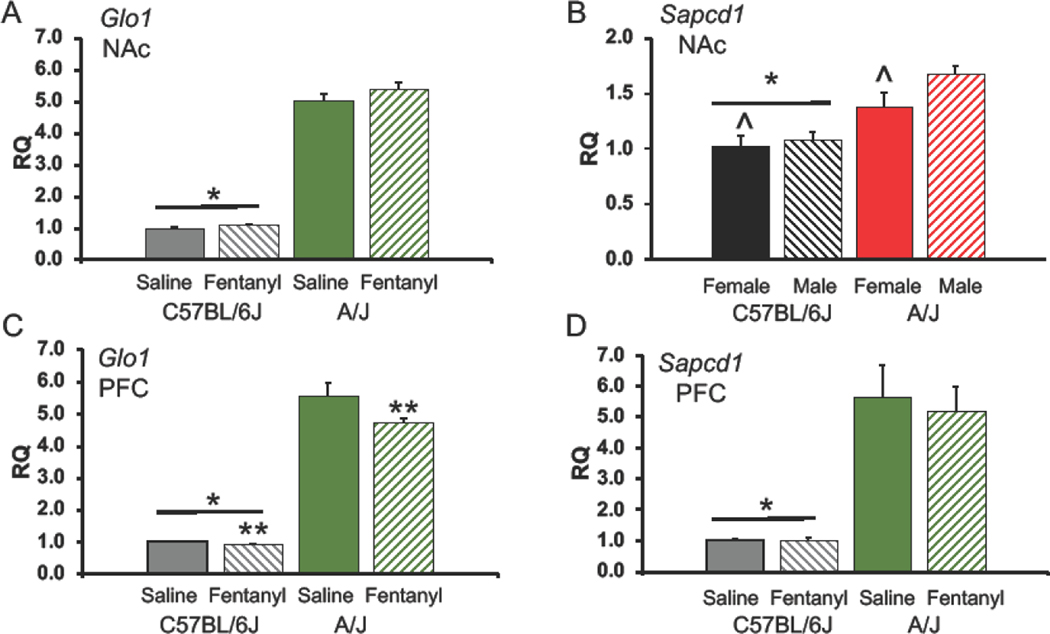
To confirm the RNA-seq data we performed qPCR for two gene candidates: Glo1 and Sapcd1. In the NAc we found a significant effect of strain (F(1,30)=1714.13, p<0.00001, Figure 5A) which indicated a greater amount of Glo1 mRNA in the NAc from A/J as compared to C6J mice. We noted a trend (F(1,30)=3.59, p=0.071) for a treatment effect and no sex differences (F(1,30)=2.47). Likewise, Sapcd1 in the NAc was significantly different between the two strains (F(1,29)=19.65, p<0.00025) with A/J mice showing higher expression than C6J mice. (Figure 5B, p<0.05). No significant differences were noted for treatment (F(1,29)=0.03) but there was a trend for a sex difference (F(1,29)=3.82, p=0.063), females had less Sapcd1 mRNA in the NAc than did males.
Figure 5:
Levels of Glo1 and Sapcd1 in nucleus accumbens and prefrontal cortex.
Data shown are (Mean + SEM). A) Levels of Glo1 mRNA in the Nucleus Accumbens (NAc). B) Sapcd1 mRNA in the NAc. C) Glo1 mRNA in the Prefrontal Cortex (PFC). D) mRNA for Sapcd1 in the PFC. In A, C, and D solid histograms represent mice of both sexes treated with saline and stripped histograms denote mice treated with fentanyl. In B, solid histograms represent female mice and stripped bars denote males. *Significant difference between strains, p<0.001, or less. **Significantly less in fentanyl versus saline treatment groups (p<0.008). ^ trend for a sex difference (p=0.063). n=4–5 per group
In the PFC we found treatment (F(1,30=8.61, p<0.008) and strain (F(1,30=1836.4, p<0.00001, Figure 5C) effects for Glo1, but no sex differences (F(1,30=2.39). Fentanyl treatment decreased mRNA for Glo1 in the PFC (p<0.05). The strain difference, as in the NAc, was produced by more Glo1 expression in A/J than C6J PFC (p<0.05). For Sapcd1 mRNA in the PFC we noted a large strain effect (F(1,30=196.62, p<0.00001), with expression also higher in A/J than C6J PFC (Figure 5D, p<0.05). No sex or treatment effects were found (F(1,30=0.02, 0.57, respectively).
Discussion
In both behavioral tasks, the inbred strains displayed striking differences. C6J mice showed robust conditioning in the CPP task, as reported previously.15 Both males and females showed strong preferences, after conditioning, for the side of the CPP chamber associated with fentanyl injections. In fact, their preferences for the fentanyl-side (previously the LPS) were as great as their initial preferences for the MPS. Data from A/J mice were more complex. The A/J mice initially displayed stronger preferences for one side of the chamber than did the C6J mice. We suspect this bias is due to their reduced activity levels and longer latencies to explore new areas.12 After conditioning A/J mice had a reduced preference for the initially MPS, but they did not have a statistical preference for the fentanyl-paired side.
Sex differences in vulnerability to rewarding effects of several drugs (mainly cocaine) have been well documented.17,18 Only a few studies have examined either sex differences or estrous cycle effects using fentanyl as the rewarding substance. In both short and extended access studies, female rats trained to self-administer (SA) fentanyl consume more drug and/or acquired fentanyl faster than males.19,20 Extinction responding is initially higher in females than in males after SA.20 Estrous cycles were not monitored during acquisition in either study, but in one study no effects of cycle day were present at during cue-induced reinstatement19 in the other females in high estradiol states has heightened response rates.20 In a recent study using more extensive spontaneous activity tests males had lower responses than females to fentanyl, but no differences in CPP were found.21In pain studies, responses to fentanyl (but not other opioids) are not sexually dimorphic.22 We regret that we did not cycle the females in the activity study, this should be done in future studies.
Inbred mouse strains are known to vary in a number of physiological and behavioral traits, including responses to a number of behavioral paradigms that assess response to rewarding stimuli. C6J mice are commonly used to study addictive behaviors in mice, presumably because of their robust responses to multiple drugs of abuse.23 There have been previous behavior studies which used the A/J strain. Kutlu et. al (2015) used eight inbred mouse strains, including C6J and A/J, to assess development of CPP after multiple conditioning sessions which paired one side of the apparatus with nicotine and the other with saline.24 After conditioning, three of the mouse strains (including C6J) showed nicotine-induced place preference, and four strains (including A/J) did not show place preferences for nicotine. Matthews et. al (2008) investigated the effects of a foot shock on consumption of an ethanol solution by multiple mouse strains, including C6J and A/J. A mild foot shock increased the consumption of ethanol in C6J, but not in A/J, mice. Acute locomotor activity in response to a cocaine injection was robust in C6J but barely detectable in A/J mice.10 Finally, in a cocaine self-administration study, all of the C6J mice acquired self-administration, while only two out of seven A/J mice acquired. Reinstatement responses of the A/J mice were significantly lower than for C6J mice.10
These two inbred strains also differ in their responses to opioids. Male C6J and A/J tested for acute activity after morphine displayed the same differences noted here, with high activity demonstrated by C6J mice and little or low activity in A/J mice, plus A/J mice did not display dose-responses.25,26 Both strains have shown physical dependence on morphine.25 Withdrawal jumping responses from morphine after naloxone were greater in C6J than in A/J mice.27 Susceptibility to common side effects of opioids (constipation and respiratory capacity), were greater in C6J than A/J mice and comparable doses yielded similar blood levels of drug.28 Genetic differences in responses of rostral ventral medial medulla neurons to the opioid mu receptor agonist DAMGO given prior to a painful stimulus showed that the drug is less effective in male A/J as compared with C6J mice.29
Some of these drug-responses may be caused by differences in activity and/or anxiety between the strains. For example our results in the activity test showed pronounced strain differences. The C6J mice were about 5 times more active than the A/J mice in the OF regardless of treatment. The A/J mice were largely inactive and administration of fentanyl did not enhance their activity. In fact when higher doses were given to A/J mice no dose-response augmentation was seen. Interestingly, the A/J mice spent less time in the center of the OF relative to total than did the C6J mice. This observation agrees with other studies that suggest higher anxiety in A/J than C6J mice.30,23 However the data are concurrent with lower levels of activity. In a previous study of multiple inbred lines, OF activity in A/J mice was on the low end of the spectrum with C6J among highest. Moreover, A/J mice spent less time in the center of the OF than C6J.31 Milner and Crabbe (2008) used the light dark (LD) transition test and found higher activity in C6J mice as compared with A/J.32 In home cage tests A/J move less than C6J mice.31 In another anxiety task, the elevated plus maze (EPM), A/J mice spent as much time in the open arms as did C6J mice.33 Whereas Solberg et al (2006) found the reverse,31 and using an elevated zero maze, A/J spent less time than C6J in open arms.32 This confound between activity and anxiety is important for the interpretation of our genetic data. Regardless of whether A/J mice are more anxious or less active they are certainly less vulnerable to fentanyl. As anxiety is typically positively correlated with vulnerability to the reinforcing actions of drugs,34 we suggest that anxiety is not the root cause of these strain differences.
Behavioral phenotyping in and of itself is useful but more useful is its application to genetic causes of behavioral differences. Here we assessed two genes from our strain and treatment RNA-seq data bases, Sapcd1 and Glo1. Suppressor APC domain containing 1 is associated with the human and mouse MHC region. In humans this is the most gene-dense region of genome.35Sapcd1 is associated with an uncharacterized protein and the gene was identified in an epidemiological study of schizophrenia where it was associated with a single nucleotide polymorphism in the major histocompatibility (MHC) region.36 While intriguing the other gene we examined has an established role in behavior.
Inbred mouse strains, several behavior tests, microarray and qPCR have shown that mRNA levels of glyoxalase 1 (Glo1) or its enzyme product correlate positively with anxiety; strains with relatively more Glo1 RNA (such as A/J) spend less time in the center of the OF, do not habituate readily to novel environments, and are less active in the light/dark task.29,37,38 There is a single copy number variation in the Glo1 gene among some strains of inbred mice.37 A/J mice have three times the gene copy number, as compared with C6J mice, and comparatively elevated Glo1. In fact, according the JAX.org mouse strain database, the two strains we used here have 11 different single nucleotide polymorphisms in Glo1. Our results confirm these strain differences and extend them as we report an effect of fentanyl on Glo1 mRNA in PFC.
When Glo1 levels have been decreased with knockout or knockdown mouse models, or by using a small molecule inhibitor, anxiety was reduced.29,39,40,41 Overexpression of Glo1 had the reverse outcome.29,42,37 Glyoxalase 1 is an enzyme in the oxoaldehyde metabolic pathway.38 The enzyme detoxifies methylgloxal (MG) a byproduct of glycolysis and the two enzymes (Gol 1 and MG) have reciprocal actions on a number of behaviors including locomotion and anxiety.38,43,42 This pathway is involved in apoptosis, metabolism, and reactive oxygen species.38
Recent studies have manipulated Glo1 to examine its effects on alcohol intake in several behavioral assays including drinking in the dark44 in mice, self-administration in rats40 and locomotor impairment in mice.45 In all three studies decreased Glo1 resulted in reduced alcohol intake. Potential mechanisms of action include MG’s action as a partial agonist on the GABAA receptor.38,39 Using higher doses MG can decrease dopamine levels in the PFC.43 In diabetic animal models46,37 and in patients47 pain is reduced when Glo1 mRNA is decreased. No other studies to our knowledge have examined the relationship between Glo1 and vulnerability to the reinforcing actions of drugs. A direct connection between Glo1 and opioids or opioid receptors has not been established but future studies on opioid-induced behaviors and these metabolic enzymes could be very worthwhile.
Supplementary Material
Highlights:
C6J and A/J mice display large behavioral differences in response to fentanyl.
C6J and A/J mice display differential gene expression in the nucleus accumbens.
No sex differences in conditioned place preferences for fentanyl in C6J mice.
Acknowledgements:
This work was funded by NIH DA048638 (EFR) and P30ES025128 to the NCSU Center for Human Health and the Environment. We thank Drs. Wendy Lynch and Abraham Palmer for useful discussions of this work.
Footnotes
Data Availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crist RC, Reiner BC, Berrettini WH; A review of opioid addiction genetics. Curr Opin Psychol. 2019;(27):31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillespie NA, Bates TC, Hickie IB, et al. ; Genetic and environmental risk factors in the non-medical use of over-the-counter or prescribed analgesics, and their relationship to major classes of licit and illicit substance use and misuse in a population-based sample of young adult twins. Addiction. 2019;114(12):2229–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angst MS, Phillips NG, Drover DR, et al. ; Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain. 2012;153(7):1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brick LA, Micalizzi L, Knopik VS, et al. ; Characterization of DSM-IV Opioid Dependence Among Individuals of European Ancestry. J Stud Alcohol Drugs. 2019;80(3):319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tapocik JD, Luu TV, Mayo CL, et al. ; Neuroplasticity, axonal guidance and micro-RNA genes are associated with morphine self-administration behavior. Addict Biol. 2013;18(3):480–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant CD, Chang HP, Zhang J, et al. ; A major QTL on chromosome 11 influences psychostimulant and opioid sensitivity in mice. Genes Brain Behav. 2009;8(8):795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saul MC, Philip VM, Reinholdt LG, et al. ; High-diversity mouse populations for complex traits. Trends in Genetics. 2019;35(7):501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenrock SA, Kumar P, Gomez AA, et al. ; Characterization of genetically complex Collaborative Cross mouse strains that model divergent locomotor activating and reinforcing properties of cocaine. Psychopharmacology (Berl). 2020;237(4):979–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson PE, Miller MM, Calton MA, et al. ; Systems genetics of intravenous cocaine self-administration in the BXD recombinant inbred mouse panel. Psychopharmacology (Berl). 2016;233(4):701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomsen M, Caine SB; False positive in the IV drug self-administration test in C57BL/6 mice. Behavioral Pharmacology. 2011;22(3):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews DB, Morrow AL, O’Buckley T, et al. ; Acute mild footshock alters ethanol drinking and plasma corticosterone levels in c57bl/6j male mice, but not dba/2j or a/j male mice. Alcohol. 2008;42(6):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodkin ES, Carlezon WA, Haile CN, et al. ; Genetic analysis of behavioral, neuroendocrine, and biochemical parameters in inbred rodents: initial studies in Lewis and Fischer 344 rats and in A/J and C57BL/6J mice. Brain Research. 1998;805(1–2):55–68. [DOI] [PubMed] [Google Scholar]
- 13.Wiltshire T, Ervin RB, Duan H, et al. ; Initial locomotor sensitivity to cocaine varies widely among inbred mouse strains. Genes Brain Behav. 2016;14(3):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fechtner L, El Ali M, Sattar A, et al. ; Fentanyl effects on breath generation in C57BL/6J and A/J mouse strains. Respir Physiol Neurobiol. 2015;215:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant CD, Roberts KW, Culbertson CS, et al. ; Pavlovian conditioning of multiple opioid-like responses in mice. Drug Alcohol Depend. 2009;103(1–2):74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudwa AE, Dominguez-Salazar E, Cabrera DM, et al. ; Dopamine d5 receptor modulates male and female sexual behavior in mice. Psychopharmacology. 2005;180(2):206–214. [DOI] [PubMed] [Google Scholar]
- 17.Lynch WJ, Roth ME, Mickelberg JL, et al. ; Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacology Biochemistry and Behavior. 2001;68(4): 641–646. [DOI] [PubMed] [Google Scholar]
- 18.Becker JB, Koob GF; Sex differences in animal models: Focus on Addiction. Pharmacological Reviews. 2016;68(2), 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malone SG, Keller PS, Hammerslag LR, et al. ; Escalation and reinstatement of fentanyl self-administration in male and female rats. Psychopharmacology. 2021;238(8), 2261–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Towers EB, Bakhti-Suroosh A, Lynch WJ; Females develop features of an addiction-like phenotype sooner during withdrawal than males. Psychopharmacology. 2021;238(8), 2213–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant CD, Healy AF, Ruan QT, et al. ; Fentanyl-induced antinociception, reward, reinforcement, and withdrawal in HNRNPH1 mutant mice. 2020 [Google Scholar]
- 22.Dahan A, Kest B, Waxman AR, et al. ; Sex-specific responses to opiates: Animal and human studies. Anesthesia & Analgesia. 2008;107(1), 83–95. [DOI] [PubMed] [Google Scholar]
- 23.Crawley JN, Paylor R; A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Hormones and Behavior. 1997;31(3):197–211. [DOI] [PubMed] [Google Scholar]
- 24.Kutlu MG, Ortega LA, Gould TJ; Strain-dependent performance in nicotine-induced conditioned place preference. Behavioral Neuroscience. 2015;129(1):37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brase DA, Loh HH, Way EL; Comparison of the effects of morphine on locomotor activity, analgesia and primary and protracted physical dependence in six mouse strains. J Pharmacol Exp Ther. 1977;201(2):368–374. [PubMed] [Google Scholar]
- 26.Gill KJ, Boyle AE; Genetic influences on drug-induced psychomotor activation in mice. Genes Brain Behav. 2008;7(8):859–868. [DOI] [PubMed] [Google Scholar]
- 27.Kest B, Smith S, Schorscher-Petcu A, et al. ; Gnao1 (GαO protein) is a likely genetic contributor to variation in physical dependence on opioids in mice. Neuroscience. 2009;162(4):1255–1264. [DOI] [PubMed] [Google Scholar]
- 28.Young A, Viswanath A, Kalladka M, et al. ; Mouse model demonstrates strain differences in susceptibility to opioid side effects. Neurosci Lett. 2018;675 :110–115. [DOI] [PubMed] [Google Scholar]
- 29.Sugino S, Namiki A, Yamakage M; Genetic differences in response properties of rostral ventromedial medulla neurons to the mu-opioid receptor agonist DAMGO in mouse inbred strains. Neurosci Lett. 2012;517(2):107–112. [DOI] [PubMed] [Google Scholar]
- 30.Hovatta I, Tennant RS, Helton R, et al. ; Glyoxalase 1 and glutathione Reductase 1 regulate anxiety in mice. Nature. 2005;438(7068):662–666. [DOI] [PubMed] [Google Scholar]
- 31.Solberg LC, Valdar W, Gauguier D, et al. ; A protocol for high-throughput phenotyping, suitable for quantitative trait analysis in mice. Mamm Genome. 2006;17(2):129–146. [DOI] [PubMed] [Google Scholar]
- 32.Milner LC, Crabbe JC; Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav. 2008;7(4):496–505. [DOI] [PubMed] [Google Scholar]
- 33.Moy S, Nadler J, Young N, et al. ; Mouse behavioral tasks relevant to Autism: Phenotypes of 10 inbred strains. Behavioural Brain Research. 2007;176(1):4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vorspan F, Mehtelli W, Dupuy G, et al. ; Anxiety and substance use disorders: co-occurrence and clinical issues. Curr Psychiatry Rep. 2015;17(2):4. [DOI] [PubMed] [Google Scholar]
- 35.Xie T; Analysis of the gene-dense major histocompatibility complex class iii region and its comparison to mouse. Genome Research. 2003;13(12):2621–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada K, Hattori E, Iwayama Y, et al. ; Population-dependent contribution of the major histocompatibility complex region to schizophrenia susceptibility. Schizophrenia Research. 2015;168(1–2):444–449. [DOI] [PubMed] [Google Scholar]
- 37.Williams R, Lim JE, Harr B, et al. ; A common and unstable copy number variant is associated with differences in glo1 expression and anxiety-like behavior. PLoS ONE. 2009;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Distler MG, Plant LD, Sokoloff G, et al. ; Glyoxalase 1 increases anxiety by reducing gabaa receptor agonist methylglyoxal. Journal of Clinical Investigation. 2012;122(6):2306–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMurray KM, Ramaker MJ, Barkley-Levenson AM, et al. ; Identification of aNovel, fast-acting Gabaergic antidepressant. Molecular Psychiatry. 2018;23(2):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Guglielmo G, Conlisk DE, Barkley-Levenson AM, et al. ; Inhibition of glyoxalase 1 reduces alcohol self-administration in dependent and nondependent rats. Pharmacology Biochemistry and Behavior. 2018;167:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang S, Kwon DM, Kwon K, et al. ; Generation and characterization of mouse Knockout For glyoxalase 1. Biochemical and Biophysical Research Communications. 2017;490(2):460–465. [DOI] [PubMed] [Google Scholar]
- 42.McMurray K, Du X, Brownlee M, et al. ; Neuronal overexpression of Glo1 or Amgdylar Microinjection of Methylglyoxal is sufficient to regulate anxiety-like behavior in mice. Behavioural Brain Research. 2016;301:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szczepanik JC, De Almeida GR, Cunha MP, et al. ; Repeated Methylglyoxal Treatment Depletes dopamine in the prefrontal cortex, and causes memory impairment and Depressive-Like behavior in mice. Neurochemical Research. 2019;45(2):354–370. [DOI] [PubMed] [Google Scholar]
- 44.McMurray KM, Sidhu PS, Cook JM, et al. ; Genetic and pharmacological manipulation Of glyoxalase 1 regulates voluntary ethanol consumption in mice. Addiction Biology. 2017;22(2):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barkley-Levenson AM, Lagarda FA, Palmer AA; Glyoxalase 1 (GLO1) inhibition or Genetic Overexpression does not alter ethanol’s Locomotor Effects: Implications for GLO1 as a therapeutic target in alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2018;42(5):869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jack MM, Ryals JM, Wright DE; Characterisation of glyoxalase I in A streptozocin-induced mouse model of diabetes with painful and insensate neuropathy. Diabetologia. 2011;54(8):2174–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peculis R, Konrade I, Skapare E, et al. l; Identification of glyoxalase 1 polymorphisms associated with enzyme activity. Gene. 2013;515(1):140–143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.