Abstract
Background
Inhaled budesonide is a widely used inhaled corticosteroid for asthma.
Objectives
The objectives of this review was to compare the efficacy of budesonide with placebo in the treatment of chronic asthma.
Search methods
The Cochrane Airways Group Trial Register and reference lists of articles was searched. We contacted trialists for additional studies and searched abstracts of major respiratory society meetings (1997‐1999).
Selection criteria
Randomised trials in children and adults comparing budesonide to placebo in the treatment of chronic asthma.
Data collection and analysis
Two reviewers independently assessed articles for inclusion and methodological quality. One reviewer extracted data.
Main results
43 studies met the inclusion criteria (2801 paticipants). In non‐oral steroid treated asthmatics, budesonide led to significant improvements in a number of measures of airway function. These included FEV1, Weighted Mean Difference (WMD) 3.7% predicted (95% CI 0.1, 7.2%); improvement in morning peak flow (PEF) from baseline WMD 29 L/min (95% CI 22, 36 L/min); improvement in evening PEF from baseline WMD 21 L/min (95% CI 13, 29 L/min). Varying methods of reporting symptoms limited the pooling of studies but all high methodological quality studies demonstrated significant improvements compared to placebo. Health status was not reported. Risk of trial withdrawal due to asthma exacerbation was lower with budesonide compared to placebo, relative risk 0.17 (95% CI 0.09, 0.33). Doses of 500‐800 mcg/d appeared to have slightly larger effect sizes than lower doses, but no advantage for high doses were apparent. A single high quality RCT reported significant reductions in daily prednisolone requirement and the number of patients able to discontinue prednisolone completely in budesonide treated subjects compared to placebo. No difference in risk of oropharyngeal soreness/hoarseness or oral Candidiasis was apparent for budesonide compared to placebo. Long‐term risk of adrenal insufficiency was not reported.
Authors' conclusions
This review strongly supports use of budesonide in chronic asthma. Consensus guidelines for chronic asthma suggest titrating inhaled steroid dose to individual requirements. Evidence from this review of trials does not present a case for routine dose titration above 800 mcg/d.
Keywords: Adult, Child, Humans, Anti-Inflammatory Agents, Anti-Inflammatory Agents/therapeutic use, Asthma, Asthma/drug therapy, Bronchodilator Agents, Bronchodilator Agents/therapeutic use, Budesonide, Budesonide/therapeutic use, Chronic Disease, Randomized Controlled Trials as Topic
Plain language summary
Budesonide versus placebo for asthma
Budesonide is highly effective preventative treatment for all patients with asthma, irrespective of age or severity of their disease. Most benefits are seen with low‐moderate doses.
Background
Inhaled corticosteroids (ICS) form the cornerstone of pharmacotherapy in the modern treatment of chronic asthma. Their importance is highlighted in all recent asthma management guidelines, and they are recommended as prophylactic therapy for all but mildest disease with intermittent symptoms (BTS 1997, NHLBI 1997). Budesonide (BUD) is an ICS licensed for use in children and adults. Since it's first introduction in the 1980's it has been widely used, and is generally acknowledged to have a favourable benefit:risk ratio. This is afforded by potent anti‐inflammatory action at the site of the mucosal inflammatory response characteristic of asthma, combined with limited systemic availability.
A large number of studies have been conducted to assess the effects of BUD when used to treat chronic asthma, and a large range outcome measures have been used to assess its efficacy and safety. However despite the undoubted value of BUD in treatment, a number of questions remain to be answered. No single study has examined all outcomes, yet it would be desirable to quantitatively assess the magnitude of response across the available outcome measures in order to gain a clearer idea of which aspects of disease control improve relative to others. It is also unclear as to how the response to treatment varies with dose, treatment duration, and patient age and asthma severity.
Prospective trials that compare BUD to placebo are likely to provide the clearest treatment effect, both in terms of efficacy and associated risk of side effects.
This review aims to synthesise the findings from studies that have compared BUD to placebo in the treatment of chronic asthma, in an attempt to provide answers to these questions.
Objectives
1. Compare the efficacy and safety of inhaled budesonide (BUD) with placebo in the treatment of chronic asthma.
2. To establish if a dose response relationship exists for budesonide in the treatment of chronic asthma.
Methods
Criteria for considering studies for this review
Types of studies
Only prospective randomised trials were included. Double, single and unblinded studies were considered.
Types of participants
Studies assessing children and adults with chronic asthma were included. Studies concerned with acute asthma and/or exclusively concerned with infants (two years of age or less) were excluded. Studies carried out in primary care, institutional care and the hospital outpatient setting were eligible for inclusion.
Types of interventions
Budesonide delivered by mouth inhalation versus placebo. Any dose of budesonide was considered, but nominal daily dose had to be stated. Nominal daily dose was calculated as the actuator dose multiplied by the number of actuations per day. Treatment needed to be for at least one week. Delivery could be by pressurised metered dose inhaler (MDI) with or without holding chamber/spacer, breath‐actuated MDI or dry powder inhaler (DPI). Any co‐intervention was acceptable, including the use of oral corticosteroids. Studies concerned with treatment delivered by nebuliser were excluded.
Types of outcome measures
Important outcomes identified a priori were as follows:
1. Outcomes reflecting airway calibre: FEV1, morning and evening PEFR, diurnal variability in diary card PEFR. 2. Asthma symptoms 3. Rescue short‐acting beta2 agonist use 4. Bronchial hyper‐responsiveness (BHR) to histamine and methacholine 5. Health related quality of life (HRQOL) 6. Asthma exacerbations: hospital admission rates, days off work or school, unscheduled doctor visits due to exacerbation 7. Safety outcomes: hypothalamic‐pituitary‐adrenal (HPA) axis function reflected in serum and urinary cortisol measures 8. Oropharyngeal side‐effects: hoarseness, sore throat, oropharyngeal Candidiasis
All outcomes were considered except those related to growth in children (height and lower leg knemometry) and biochemical markers of bone turnover and bone densitometry.
Search methods for identification of studies
Electronic searches
A search was carried out of the Cochrane Airways Group Trial Register using the terms:
steroid* OR glucocorticoid* OR corticosteroid* OR beclomethasone OR budesonide OR fluticasone OR triamcinolone OR flunisolide OR Becotide OR Becloforte OR Pulmicort OR Flixotide
The electronic abstracts of citations resulting from this search were then imported into a bibliographic database and termed the inhaled steroid register. This was hand‐searched by two reviewers (NPA and JB) for duplicate publications, which were removed.
Stage 2: The inhaled steroid register was searched using the following terms:
budesonide OR Pulmicort
Electronic abstracts were exported to a new database and termed the budesonide register. Citations were initially excluded if it was clear that the study:
a) Was not concerned with treatment of chronic asthma in humans b) Was not an RCT c) Did not include a treatment arm with an inhaled budesonide
Where uncertainty existed, the publication was retrieved in full text version
Searching other resources
The bibliographies of all papers retrieved in full text form and relevant narrative reviews were searched for additional publications. The British Journal of Clinical Research and the European Journal of Clinical Research which are not currently indexed on MEDLINE or EMBASE were hand searched for relevant studies. Authors of included studies were contacted and asked if they were aware of further studies. The European headquarters of Astra Zeneca manufacturers of Pulmicort were contacted to find details of studies sponsored which may have been missed. Finally, the proceedings of meetings of the European Respiratory Society (1997/1998), British Thoracic Society (1997/1998) and American Thoracic Society (1997/1998) were searched for relevant trials.
Data collection and analysis
Selection of studies
Decision to exclude studies prior to full paper retrieval was made by one reviewer (NPA). Papers retrieved in full text form were assessed independently by two reviewers (NPA and JB); disagreement as to which papers to include was resolved by consensus.
Data extraction and management
One reviewer (NPA) extracted data for each outcome from the published results of included trials. In the case of continuous outcomes such as spirometry:
1. Where outcomes were evaluated at a number of time points, only data from the last evaluable time point was used. 2. Data were extracted from graphical plots when presented in this form; attempt was made to verify such data by contacting authors. 3. If an intention‐to‐treat analysis was not used by the investigators, and it was not explicit in the presentation of results how many subjects were in each group at the time of last evaluation of that outcome, the appropriate N value for each intervention group was calculated by subtracting the number of patients who withdrew in each intervention group from those randomised to each intervention group.
It should be noted that continuous outcome data such as spirometry and PEFR are displayed using negative figures as the sign convention built into the RevMan software interprets smaller numbers as favourable. This ensures that results favouring a given nominal daily dose of budesonide are consistently displayed to the side of that dose in the graphical meta‐analysis display. Authors were written to (by mail, fax and/or electronic mail) to clarify details of randomisation and/or request missing outcome data. Attempt was made to send requests to correct current addresses by searching MEDLINE, EMBASE and hospital World Wide Web (WWW) sites for up‐to‐date contact details. Astra Zeneca were approached for data for those trials in which contact authors did not initially reply or when authors suggested doing so and which had been sponsored by the company.
Assessment of risk of bias in included studies
Two reviewers (NPA and JB) who were blinded to the author's names independently assessed each study for methodological quality. The trials were scored using the Cochrane approach:
Grade A: adequate allocation concealment Grade B: unclear allocation concealment Grade C: clearly inadequate concealment
The methodological quality of included studies was also assessed using a 5 point scoring instrument (Jadad 1996):
a) Was the study described as randomised? (yes=1 no=0) b) Was the study described as double blind? (yes=1 no=0) c) Was there a description of withdrawals and dropouts? (yes=1 no=0) d) Was the method of randomisation well described and appropriate? (yes=1 no=0) e) Was the method of double blinding well described and appropriate? (yes=1 no=0) f) deduct 1 point if method of randomisation or blinding inappropriate
Inter‐rater agreement was measured using the kappa statistic. Disagreement was resolved by consensus.
Data synthesis
A weighted treatment effect across trials was calculated using the Cochrane statistical package RevMan 4.0.4 with MetaView 3.1. For continuous outcomes, a weighted mean difference (WMD) or standardised mean difference (SMD) was calculated as appropriate. For dichotomous outcomes a Relative Risk (RR) was calculated. Pooled treatments effects are expressed with their 95% confidence intervals (95% CI). Heterogeneity of effect size across pooled studies was calculated, with p< 0.05 used as the cut‐off level for significance. A number of a priori conditions were established regarding the comparisons made:
1. The results of parallel and crossover trials were not pooled.
2. It was expected that most trials in patients recruited taking regular oral corticosteroids would use a 'steroid‐sparing' design in which the daily dose of oral steroid was progressively reduced. In such studies the principal outcome variable is the dose of oral steroid needed to maintain asthma control unchanged. However, studies in which patients were not treated with regular OCS are more likely to have a design aimed at detecting improvements in asthma control. Trials with these different designs and aims would be combined inappropriately.
3. It was anticipated that measures of bronchial hyper‐responsiveness (BHR) such as the provocative dose of challenge substance required to produce 20% fall in FEV1 (PD20 FEV1) or provocative concentration of challenge substance required to produce 20% fall in FEV1 (PC20 FEV1) would often be reported as geometric means. Presentation of results in this way indicate that data has been logarithmically transformed prior to analysis by investigators to take account of skewed distribution. Data for such outcomes was only pooled across studies where the mean and standard deviation of logged values (from which geometric means are derived) could be calculated.
Sensitivity analyses were performed on the basis of methodological quality. Results were re‐analysed using studies of only the highest quality scores (Jadad 3 to 5). Subgroup analyses based upon patient age, delivery device, study duration and asthma severity were planned.
Classification of asthma severity based upon baseline FEV1 (as % predicted) was planned, but we found this measurement was reported inconsistently. An attempt was made to classify severity based upon contemporary Global Initiative for Asthma (GINA) criteria. This uses a combination of FEV1 (% predicted), PEFR variability and/or symptom frequency to class asthma severity. In trials where this was not available investigators' opinion as to the severity of recruited patients was used as a last resort.
Results
Description of studies
48 publications were selected for inclusion in the review representing 43 studies. All were published in the English language. No studies were excluded on the basis of language, however four additional studies are awaiting translation. Three of these appear to be randomised controlled trials from their English language abstracts (Sekerel 1997b, Chyrek‐Borowska 1994, Damsbo 1994). One study had a placebo control group but was not described as randomised (Gorski 1993).
POPULATIONS
The studies came from all around the world including the UK, Western Europe, USA, Canada, Israel, Taiwan, Singapore and Thailand. Ten were multicentre studies (Aaronson 1998, Busse 1998, Campbell 1991, Essen‐Zandvliet 1992, Jones 1994, Lorentzson 1990, Nelson 1998, O'Byrne 1996, Osterman 1997, Shapiro 1998a). Three studies were undertaken in a primary care setting (Campbell 1991, Jones 1994, O'Byrne 1996), the remaining studies were in a secondary care setting.
Eleven studies were conducted in children (Agertoft 1997, Baki 1998, de Baets 1990, Boner 1995, Henriksen 1985, Heuck 1997b, Gleeson 1988, Jonasson 1998, Sekerel 1997a, Shapiro 1998a, Essen‐Zandvliet 1992). The remaining studies were undertaken in adults, or adolescents and adults.
One study (Prieto 1994) specifically recruited patients with seasonal asthma and was undertaken during a period of maximum allergen exposure.
ORAL CORTICOSTEROID TREATMENT
In the majority of studies patients were not treated with regular oral steroids at the time of enrolment. This was an exclusion criterion, or was clear from a description of the baseline characteristics. In only one study (Nelson 1998) was current regular treatment with oral corticosteroids an inclusion criterion. Patients were required to have needed treatment with prednisolone 5‐30 mg/d for a minimum of 6 months. This study used a forced down‐titration design to reduce prednisolone dose through the course of the study. In two further studies (Busse 1998, Shapiro 1998a) a proportion of randomised patients were receiving regular treatment with oral corticosteroids at enrolment. In both studies the daily dose of prednisolone was not altered during the trial.
DIAGNOSIS OF ASTHMA
In 9 studies (21%) there no clear indication of the criteria used to make a diagnosis of asthma. Diagnosis appears to have been at the discretion of the investigators. Criteria for the remaining studies are as follows:
a) American Thoracic Society standards: seven studies (Burke 1996, Busse 1998, Rodriguez 1991, Kuo 1994, Sekerel 1997a, Tan 1998, Yates 1996) b) NHLBI guidelines: two studies (Jonasson 1998, Wongtim 1995). c) FEV1 variability, histamine BHR and skin test positivity to house dust mite: one study (Wempe 1992a, Wempe 1992b) d) characteristic symptoms with reversibility in FEV1 after inhaled beta2 agonist: one study (Jones 1994) e) characteristic symptoms with methacholine BHR: five studies (Bel 1991, Jatakanon 1998, Juniper 1990, Kharitonov 1996, Yates 1996) f) characteristic symptoms and specific serum IgE or skin prick sensitivity to common airborne allergens: two studies (de Jong 1996, O'Connor 1992) g) histamine BHR and skin prick sensitivity to common allergen: one study (Swystun 1998) h) FEV1 reversibility to beta2 agonist in combination with BHR to histamine or methacholine: two studies (Kivity 1994, Wong 1994) i) a single one of the following: symptoms, FEV1/PEFR reversibility to beta2 agonist, histamine/methacholine BHR or skin prick sensitivity to allergen: 13 studies (Baki 1998, Boner 1995, Campbell 1991, de Baets 1990, Haahtela 1994, Nelson 1998, O'Byrne 1996, Osterman 1997, Prieto 1994, Shapiro 1998a, Toogood 1990, Essen‐Zandvliet 1992, Vathenen 1991).
SEVERITY OF ASTHMA
Mild In 24 studies (56%) asthma severity was classed as mild. In 21 studies patients had not received regular treatment with inhaled corticosteroids prior to enrolment. In 19 of these it was clear that baseline FEV1 was 70 (% predicted) or greater, either as an inclusion criterion of the study, or as judged from baseline demographic data. In two studies (Agertoft 1997, O'Byrne 1996) no information regarding baseline FEV1 of participants was available and symptom frequency was not stated. However patients had not been treated prior to study with regular ICS's and in the opinion of the investigators had mild asthma. In one study (Haahtela 1994) patients had received regular ICS therapy prior to enrolment. Baseline FEV1 ranged 84‐89 (% predicted) and had mild asthma in the opinion of the investigators.
Mild to moderate In 8 studies (19%) asthma severity was classed as mild to moderate. In four of these (Baki 1998, Jones 1994, Kivity 1994, Swystun 1998) participants had not received regular ICS prior to enrolment. Symptom frequency was not described but baseline FEV1 was > 60 (% predicted) for all studies and in the opinion of the investigators asthma severity for recruited patients ranged between mild and moderate. In a further four studies a proportion of patients had received regular ICS treatment prior to enrolment and baseline FEV1 ranged between 58 and 106 (% predicted). No information regarding symptom frequency was available. Investigators did not state an opinion regarding disease severity (Essen‐Zandvliet 1992, Wempe 1992a, Wempe 1992b), regarded asthma as mild (de Jong 1996) or mild to moderate (Heuck 1997b). Moderate In three studies asthma severity was classed as moderate. In one (Tan 1998) symptom frequency of recruited subjects was consistent with a GINA estimate of moderate severity disease. In two studies (Sekerel 1997a, Toogood 1990) baseline FEV1 was between 55 and 70 (% predicted); symptom frequency was not described. It is likely that in all studies patients had received prior treatment with inhaled corticosteroids.
Moderate to severe In three studies asthma severity of subjects recruited was classed as moderate to severe. In one study (Nelson 1998) prior regular treatment with oral steroids was an inclusion criterion for the study. In two (Busse 1998, Shapiro 1998a) a proportion of patients were oral steroid treated. Baseline FEV1 ranged from 40 to 76 (% predicted). STUDY DESIGN
29 studies (70%) were of a parallel group design, 13 (30%) of crossover design. The majority of crossover studies used a washout period between treatments. Only two studies (Cockcroft 1995, Wempe 1992a, Wempe 1992b) did not employ a washout period.
DAILY DOSE OF BUDESONIDE
19 (45%) studies assessed a daily nominal dose of BUD of 400 mcg or less, 15 studies (36%) assessed 500‐800 mcg/d, 11 studies (26%) assessed a daily dose of BUD of 1000 mcg or greater. Nine studies assessed the effects of two or more nominal daily doses of BUD versus placebo (Aaronson 1998, Agertoft 1997, Busse 1998, Jonasson 1998, Lorentzson 1990, Nelson 1998, O'Byrne 1996, Shapiro 1998a, Swystun 1998).
STUDY DURATION
19 studies (44%) had one to four week treatment periods, 19 (44%) had treatment periods of one to five months. Only four studies (Essen‐Zandvliet 1992, Juniper 1990, Osterman 1997, Haahtela 1994) were of six months duration or longer.
DELIVERY DEVICE
24 studies (60%) used a dry‐powder delivery device (DPI). 13 (31%) used aerosol metered dose inhalers (MDI) with a chamber/spacer. Only three studies used an MDI alone. In one study (Baki 1998) the type of delivery device was not stated.
OUTCOMES ASSESSED
A large number of outcomes were reported. All were considered except those listed in Table 1 since they were not considered a priori as being important efficacy measures and their role in assessing the efficacy of anti‐inflammatory therapy for asthma is unclear. Incomplete outcome data that could not be included in the meta‐analysis are listed in Table 2.
1. Outcomes reported in studies but not considered in review.
| Study ID | Outcomes |
| Burke 1996 | Bronchial wall biopsy inflammatory cell profiles (detected immunohistochemically) |
| Gauvreau 1996 | Sputum eosinophil count |
| Heuck 1997 | Serum carboxy‐terminal propetide of type 1 collagen (PICP) Serum carboxy‐terminal pyridinioline cross‐liked telopeptide of type 1 collagen (ICTP) Serum amino‐terminal propeptide of type III procollagen (PIIINP) Urinary concentration pyridinoline crosslinks(PYD) Urinary concentration deoxypyridinoline crosslinks (DPD) Serum insulin‐like growth factor I (IGF‐I) Serum insulin‐like growth factor binding protein‐3 (IGFBP‐3) |
| Jatakanon 1998 | Sputum eosinophil count Sputum eosinophil cationic protein Sputum tumour necrosis factor alpha Exhaled air nitric oxide concentration |
| Kharitonov 1996 | Exhaled air nitric oxide concentration |
| Osterman 1997 | Serum myeloperoxidase Serum neutrophil chemotactic activity Serum eosinophil chemotactic activity |
| Tan 1998 | Serum neutrophil chemotactic activity Thoracic gas volume |
2. Outcome data not included in meta‐analysis.
| Study ID | Outcomes |
| Boner 1995 | Daily use of beta2 agonist No numerical data available |
| Burke 1996 | Bronchial responsiveness to histamine (PC20 FEV1) No standard deviation values available for logged data % change FEF 25‐75 compared to baseline % change in PEFR compared to baseline Data not presented in a usable form for above outcomes |
| de Jong 1996 | Bronchial responsiveness to methacholine (PC20 FEV1) Data not presented in a usable form |
| Essen‐Zandvliet 1992 | Daily use of beta2 agonist Data reported using medians and ranges |
| Gleeson 1988 | % predicted morning PEFR % predicted evening PEFR No standard deviation values available for above outcomes Nocturnal cough and wheeze score A significant carryover effect was present for this outcome: first period data only not available |
| Henriksen 1985 | % predicted FEV1 No standard deviation values available |
| Heuck 1997 | Morning PEFR Evening PEFR No standard deviation values available for above outcomes |
| Jatakanon 1998 | Diurnal PEFR variability Daily asthma symptom score Daily use of beta 2 agonists No numerical data available for above outcomes |
| Jonasson 1998 | FEV1 FEF 25, 50, 75 Morning PEFR Daytime symptom score Night‐time symptom score Bronchial responsiveness to methacholine (PD20 FEV1) No numerical data available |
| Juniper 1990 | Bronchial responsiveness to methacholine (PC20 FEV1) Data not in form suitable for analysis |
| Kivity 1994 | Change in FEV1 compared to baseline No standard deviation values available Daily symptom score Daily use of beta2 agonist No numerical data available Bronchial responsiveness to methacholine (PD20 FEV1) Data not in a usable form for analysis |
| Kuo 1994 | Bronchial responsiveness to methacholine (PC20 FEV1) Data not in a usable form for analysis (log transformed values not available) |
| Osterman 1997 | Bronchial responsiveness to histamine (PD20 FEV1) No standard deviation values available for logged data |
| Swystun 1998 | Allergen bronchial responsiveness PC15 FEV1 Methacholine bronchial responsiveness PC15 FEV1 No standard deviation values available for above outcomes |
| Tan 1998 | Histamine bronchial responsiveness (PC20 FEV1) Data not presented in a usable form |
| Wong 1994 | Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Change in daytime asthma symptom score compared to baseline Change in rescue beta2 agonist use compared to baseline Change in early and late allergen bronchial responsiveness (PC20 FEV1) compared to baseline Above outcomes reported using medians and ranges, analysed using non‐parametric statistics |
| Wongtim 1995 | Daily asthma symptom score Weekly use of beta2 agonist No standard deviation values available for above outcomes Bronchial responsiveness to methacholine (PC20 FEV1) Data not presented in a usable form |
| Yates 1996 | Methacholine bronchial responsiveness (PC20 FEV1) Asthma symptom score Data not in a form usable for meta‐analysis |
Results of the search
Stage 1 electronic search: 6494 citations retrieved, 2162 unique citations
Stage 2 electronic search: 1036 unique citations 331 not RCT (on basis of abstract) 195 not chronic asthma in humans 129 not concerned with inhaled steroid treatment 40 not concerned with BUD treatment
341 papers retrieved in full text form:
46 not RCT 219 no placebo arm 15 infants 8 treatment period of less than 1 week 3 nebuliser delivery device 8 excluded for other reasons (see Excluded study notes) 42 included studies
One study (Campbell 1991) was identified from hand searching the British Journal of Clinical Research.
Agreement between the two independent assessments of study quality were as follows:
Randomisation: kappa=1 Double‐blind: kappa=0.9 Withdrawals/dropouts: kappa=0.8 Method of randomisation: kappa=0.9 Method of blinding: kappa=0.9
Risk of bias in included studies
The overall quality of included studies was high. All were randomised and all were described as double blind. As assessed by the Jadad method four studies (9%) achieved a score of five (Essen‐Zandvliet 1992, Gleeson 1988, Osterman 1997, Toogood 1990), 36 studies (84%) achieved a score of three or four. Only three studies (7%) scored poorly for methodological quality with a score of two (Henriksen 1985, Kharitonov 1996, Rodriguez 1991). Reporting of the use of allocation concealment was generally poor. Only 11 studies (26%) clearly employed allocation concealment (Cochrane grading of A). In the majority of cases this was only established from correspondence with the authors.
Effects of interventions
Results from the meta‐analysis are reported by outcome. All comparisons concern BUD versus placebo, and a structured approach has been used to report results. A hierarchy of result reporting has been established: A. Study design: parallel group or crossover B. Background therapy: with or without oral corticosteroids (OCS) C. Outcome: FEV1, PEFR etc. D. Unit of measurement: i.e. absolute, % predicted or change compared to baseline Within each component subgroup analyses have been undertaken according to:
A. Age group (children or adult) B. BUD daily dose (400 mcg/d or less, 500‐800 mcg/d, 1000 mcg/d or greater) C. Asthma severity (mild, mild to moderate, moderate to severe, severe) D. Delivery device (MDI, MDI+spacer, DPI) E. Length of intervention period (1‐4 weeks, 1‐5 months, 6 months or longer)
Therefore:
a) Comparisons 1‐6 consider each outcome according to dose (400 mcg/d or less, 500‐800 mcg/d, 1000 mcg/d or greater). The results for these comparisons are discussed in the text of the results first for each outcome.
b) Studies were pooled, irrespective of BUD dose (Comparisons 7 and 8). This allowed:
1. An overall effect size to be calculated for each outcome. 2. A test of whether the effect size varied by dose (i.e. whether there was heterogeneity between studies that could be explained by nominal daily dose of BUD) 3. Because studies were divided into subgroups according to daily dose, it allowed an impression to be gained of any dose response relationship by comparing the 95% confidence intervals around the mean effect size for each dose subgroup. In instances where the confidence intervals around the effect size for each dose range overlapped heavily it was concluded that no dose response effect was evident.
c) The findings of subgroup analyses based on delivery device, study duration and asthma severity are reported (Comparisons 9 to 14).
NON ORAL STEROID TREATED ASTHMATICS
SPIROMETRY
FEV1 (% predicted)
Parallel group studies No difference in FEV1 (% predicted) was apparent for BUD 400 mcg/d or less versus placebo. A significant improvement was demonstrated for BUD 500‐800 mcg/d versus placebo, WMD 4.7% (95% CI 0.4 to 9%). No studies comparing BUD 1000 mcg/d or greater versus placebo reported this outcome. When studies were pooled across all doses (eight studies, 217 subjects), a significant effect in favour of BUD was present: WMD 3.7% (95% CI 0.3 to 7.1%). The 95% confidence intervals around the treatment effect for each BUD dose subgroup overlapped widely, but BUD 500‐800 mcg/d appears to be effective whereas BUD 400 mcg/d or less did not (Comparison 07, Outcome 01).
Crossover studies No significant difference between BUD and placebo was apparent when considering FEV1 (% predicted) for any of the three daily dose ranges. No difference between BUD and placebo were apparent when studies were pooled across all doses WMD 4.5% (95% CI ‐2.2. to 11.3%) (Comparison 08, Outcome 01).
FEV1 (litres)
Parallel group studies No difference in FEV1 (litres) was apparent between BUD 500‐800 mcg/d or BUD 1000 mcg/d versus placebo. When studies were pooled across doses (four studies, 103 subjects) no difference between BUD and placebo was apparent.
Crossover studies No differences were apparent between BUD and placebo for any daily dose; no difference between treatments was apparent when studies were pooled across all doses (six studies, 138 subjects).
There was\no evidence for a different size of effect with different doses for either parallel group or crossover studies.
Change in FEV1 (litres) compared to baseline
Two parallel group studies reported FEV1 as change compared to baseline (Haahtela 1994, Osterman 1997). Both studies assessed BUD 400 mcg/d versus placebo and were conducted in adult patients. A significant difference in favour of BUD was apparent: WMD 0.20 litres (95% CI 0.07 to 0.33 litres). It should be noted that in both studies this differences resulted from a small relative improvement in FEV1 compared to baseline in the BUD treated groups combined with a relative deterioration in FEV1 compared to baseline in the placebo treated groups.
Other spirometric measures:
A number of other spirometric measures were reported by small individual studies. One parallel group study (Osterman 1997) of high methodological quality (Jadad score 5) conducted in 62 adult patients assessed BUD 400 mcg/d versus placebo and reported improvement in FEV1 (% predicted) compared to baseline. Boner 1995 (Jadad score 3) assessed the efficacy of BUD 400 mcg/d versus placebo in 20 children and reported FVC (% predicted) and FEF 25‐75 (L/second); Haahtela 1994 (Jadad score 3) assessed BUD 400 mcg/d versus placebo in 37 adults and reported change in FVC (litres) compared to baseline. One parallel group study (Rodriguez 1991) of low quality (Jadad score 2) conducted in adults assessed BUD 400‐800 mcg/d versus placebo and reported FVC (% predicted) and FEF 50 (% predicted). No differences between treatment groups were apparent for any of these outcomes.
PEAK FLOW
Diary card morning PEFR (L/min)
Parallel group studies One study (Boner 1995) assessed BUD 400 mcg/d, two studies (de Baets 1990, van Essen‐Zandvliet 1992) assessed BUD 500‐800 mcg/d versus placebo. No differences between treatments were apparent for either dose range. When studies were pooled across all doses, there were no significant differences between BUD and placebo and no evidence of a dose response effect.
Crossover studies Three crossover studies (Fuller 1991, Jatakanon 1998, Yates 1996) assessed BUD 1000 mcg/d or greater versus placebo. No difference between treatments was apparent.
Change in diary card morning PEFR (L/min) compared to baseline (Comparison 07, Outcome 05)
Five parallel group studies, all conducted in adolescents/adults, assessed BUD 400 mcg/d and reported change in morning PEFR compared to baseline (O'Byrne 1996, Lorentzson 1990, Haahtela 1994, Jones 1994, Campbell 1991). A significant effect in favour of BUD compared to placebo was apparent: WMD 29 L/min (95% CI 22 to 36 L/min). Two parallel group studies in adults (Kivity 1994, O'Byrne 1996) assessed BUD 500‐800 mcg/d or greater. A significant effect in favour of BUD was apparent compared to placebo: WMD 51 L/min (95% CI 26 to 76 L/min). A significant effect in favour of treatment with BUD was apparent when studies were pooled across all doses: WMD 29 L/min (95% CI 22 to 36 L/min). Significant heterogeneity was present when studies were pooled, this is discussed below.
Diary card evening PEFR (L/min)
Parallel group studies Only one parallel group study (Boner 1995) reported diary card evening PEFR. This was a small study (17 subjects) of fair methodological quality (Jadad score 3) conducted in children assessing BUD 400 mcg/d. No difference between BUD and placebo was apparent.
Crossover studies Two crossover studies (Fuller 1991, Yates 1996) reported evening PEFR. Both assessed BUD 1000 mcg/d or greater and were conducted in adults. No difference between BUD and placebo was apparent.
Change in diary card evening PEFR (L/min) compared to baseline (Comparison 07, Outcome 06)
Five parallel group studies, all conducted with adolescents/adults, assessed BUD 400 mcg/d and reported change in evening PEFR compared to baseline (Campbell 1991, Haahtela 1994, Jones 1994, Lorentzson 1990, O'Byrne 1996). A significant effect in favour of BUD compared to placebo was apparent: WMD 19 L/min (95% CI 10 to 19 L/min). Two parallel group studies (Kivity 1994, O'Byrne 1996) conducted in adults assessed BUD 500‐800 mcg/d. A significant effect in favour of BUD compared to placebo was apparent: WMD 40 L/min (95% CI 23 to 58 L/min). When studies were pooled across all doses (six studies, 469 subjects) there was a significant effect in favour of BUD: WMD 21 L/min (95% CI 13 to 29 L/min), however there was no evidence for a dose response effect.
Other peak flow measures
Individual studies reported other peak flow measures. Boner 1995 (Jadad score 3) assessed the efficacy of BUD 400 mcg/d versus placebo in 20 children and reported clinic measured PEFR (L/min). Kuo 1994 (Jadad score 3) assessed the efficacy of BUD 800 mcg/d in 27 adults and reported mean daily diary card PEFR (L/min) and diurnal variability in PEFR (L/min).
No differences between treatment groups were apparent for these outcomes.
ASTHMA SYMPTOMS
A range of scoring systems was used to assess the effect of treatment on symptoms.
Absolute symptom scores
Parallel group studies One study (Juniper 1990) of high methodological quality (Jadad score 4) assessed the efficacy of BUD 400 mcg/d in 29 adults over a one‐year period, and reported a monthly asthma symptom/severity score. A scoring system based on the reporting of awakening at night with symptoms, awakening in morning with symptoms, limitation of normal activities, production of sputum, rescue beta2 agonist use > 4x daily and FEV1 < 70 (% predicted) was used. One point was scored for each item that had been positive one or more days in the last week. BUD treatment resulted in a significantly lower score compared to placebo: SMD 4.1 (95% CI 2.8 to 5.5).
A single parallel group study in 47 adults (O'Byrne 1996) of high methodological quality (Jadad score 4) assessed BUD at two doses of 400 mcg/d and 800 mcg/d versus placebo. Both doses resulted in a significantly lower percentage of patients waking at night compared to placebo: 400 mcg/d RR 0.06 (95% CI 0.00 to 0.98); 800 mcg/d RR 0.22 (95% CI 0.05 to 0.9). There were also significant fewer patients with early morning wakening due to symptoms compared to placebo: 400 mcg/d RR 0.12 (95% CI 0.02 to 0.83); 800 mcg/d RR 0.30 (95% CI 0.1 to 0.93).
One parallel group study (Tan 1998) reported the number of night‐time awakenings per week due to asthma symptoms. This study was of fair methodological quality (Jadad score 3) and assessed BUD 1600 mcg/d versus placebo over a one month treatment period. BUD treated subjects experienced significantly fewer awakenings: WMD 3.7 awakenings/week (95% CI 3.1 to 4.3 awakenings/week).
One parallel group study (Rodriguez 1991) of low methodological quality (Jadad score 2) assessed the efficacy of BUD 400‐800 mcg/d over a four week period in 26 adults. Asthma symptoms were assessed at the end of the treatment period, using a three point scoring scale for each of the following elements: dyspnoea, cough, wheeze and rescue beta2 agonist use. No difference between BUD and placebo was apparent.
Crossover studies One study (Gleeson 1988) assessed the effect of BUD 400 mcg/d on daytime cough, wheeze and breathlessness scores in 56 children. This study was of high methodological quality (Jadad score 5) and incorporated a three week washout period between six week treatment periods. No difference between active treatment and placebo was apparent for any symptom‐related outcome.
Two small studies (Wempe 1992a, Wempe 1992b) of fair quality (Jadad score 3) assessed the effect of BUD 800 mcg/d on a range of day and night‐time specific symptoms in adults. BUD treatment resulted in a significantly lower scores for a number symptom measures including daytime wheeze score SMD 0.73 (95% CI 0.02 to 1.43); night‐time wheeze score SMD 1.08 (95% CI 0.01 to 2.15) and night‐time breathlessness score SMD 0.71 (95% CI 0.01 to 1.41). No difference between BUD and placebo was apparent for daytime cough, night‐time cough or daytime breathlessness scores.
Change scores compared to baseline
Parallel group studies Three studies (Haahtela 1994, Jones 1994, Lorentzson 1990) reported change in daytime symptom scores compared to baseline; two studies (Jones 1994, Lorentzson 1990) also reported change in night‐time symptom scores compared to baseline. A significantly greater favourable change in both daytime symptom score SMD 0.26 (95% CI 0.02 to 0.49) and night‐time symptom score SMD 0.48 (95% CI 0.22 to 0.74) was apparent for BUD 400 mcg/d compared to placebo.
RESCUE BETA2 AGONIST USE
A range of measures were used to assess the effect of treatment on rescue beta2 agonist use in studies of parallel group design.
One study (Juniper 1990) of high methodological quality (Jadad score 4) in 29 adults assessed the effect of BUD 400 mcg/d on daily beta2 agonist use (puffs/d). BUD resulted in significantly lower rescue beta2 agonist use compared to placebo: WMD 1.6 puffs/d (95% CI 0.3 to 3.0 puffs/d). A further small study (Kuo 1994) of fair quality (Jadad score 3) conducted in 27 adults reported weekly use of rescue beta2 agonist and found a significantly lower requirement in patients treated with BUD 800 mcg/d compared to placebo: WMD 11.4 puffs/week (95% CI 9.3 to 13.5). O'Byrne 1996 (Jadad score 4) reported the percentage of patients requiring > four puffs beta2 agonist/d for adults treated with BUD 400 mcg/d and BUD 800 mcg/d compared to placebo. Both doses led to lower beta2 agonist use compared to placebo, but these did not reach significance: BUD 400 mcg/d RR 0.17 (95% CI 0.02 to 1.23); BUD 800 mcg/d 0.07 (95% CI 0.00 to 1.09).
Five studies (Comparison 07, Outcome 07) assessed BUD 400 mcg/d and reported change in daytime use of rescue beta2 agonist compared to baseline; four assessed adults (Campbell 1991, Haahtela 1994, Lorentzson 1990, Osterman 1997) one assessed children (Jones 1994). A significantly greater favourable change was apparent for BUD compared to placebo: WMD 0.4 puffs/daytime (95% CI 0.1 to 0.8 puffs/daytime). Three studies (Lorentzson 1990, Osterman 1997, Jones 1994) also reported change in night‐time use of rescue beta2 agonist compared to baseline (Comparison 07, Outcome 08). A significant effect in favour of active treatment was apparent: WMD 0.7 puffs/night‐time (95% CI 0.3 to 1.1 puffs/night‐time).
BRONCHIAL HYPER‐RESPONSIVENESS (BHR)
A range of measures was used to assess bronchial hyper‐responsiveness.
Methacholine BHR
Parallel group studies (Comparison 07, Outcome 09) One study (Baki 1998) assessed BHR as the concentration of methacholine required to produce a 20% fall in FEV1 (PC20 FEV1). Children were treated with BUD 400 mcg/d. Two studies reported methacholine BHR following treatment with BUD 800 mcg/d or placebo. Prieto 1994 reported PC20 FEV1, Rodriguez 1991 reported the dose of methacholine required to produce a 20% fall in FEV1 (PD20 FEV1). No difference was apparent between BUD and placebo at either dose. When studies were pooled across all doses (three studies, 76 subjects) no difference between BUD and placebo was apparent: WMD 0.04 (95% CI ‐0.21 to 0.29).
One study (Boner 1995) of fair quality (Jadad score 3) conducted in 20 children reported the effects of treatment with BUD 400 mcg/d versus placebo. Methacholine BHR was reported in the form of doubling dose steps (log transformed for analysis). A significantly lower methacholine BHR was apparent in BUD treated subjects: 1.22 doubling doses steps higher in BUD treated subjects compared to placebo (95% CI 0.05 to 2.38 doubling dose steps). Data was supplied by authors and is not included in the meta‐analysis. Bel 1990 assessed the effects of BUD 400 mcg/d versus placebo in 16 adults. This study was of fair methodological quality (Jadad score 3). Treatment with BUD resulted in a significant reduction in methacholine BHR compared to placebo: 1.70 doubling dose steps higher in BUD treated subjects compared to placebo (95% CI 1.21 to 2.19 doubling dose steps).
Crossover studies (Comparison 08, Outcome 05) One small study (Gauvreau 1996) conducted in adults and of high methodological quality (Jadad score 4) assessed the effects of BUD 400 mcg/d and reported PC20 FEV1. No difference between treatment groups was apparent. Three studies (Cockcroft 1995, Jatakanon 1998, O'Connor 1992) assessed BUD 1600 mcg/d and reported PC20 FEV1. Active treatment resulted in a significantly lower methacholine BHR: WMD 0.40 log 10 PC20 FEV1 (95% CI 0.16 to 0.63 log 10 PC20 FEV1). When studies were pooled across all doses a significant effect in favour of BUD was apparent: WMD 0.4 log 10 PC20 FEV1 (95% CI 0.18 to 0.63 log 10 PC20 FEV1).
Histamine BHR
Parallel group studies One study (Haahtela 1994) in 37 adults and of fair quality (Jadad score 3) assessed BUD 400 mcg/d. Histamine BHR was reported as doubling dose steps (log transformed for analysis). BUD led to a significantly lower histamine BHR compared to placebo with a difference between treatments of 1.5 doubling dose steps favouring BUD (p=0.025, data not included in meta‐analysis). Wong 1994 assessed BUD 800 mcg/d versus placebo in 19 adult subjects and was of fair quality (Jadad score 3). Histamine BHR was reported as doubling dose steps (non log transformed). BUD led to a significantly lower histamine BHR compared to placebo with a difference between treatments of 1.7 doubling dose steps (95% CI 0.33 to 3.07 doubling dose steps).
Two studies in children (de Baets 1990, Essen‐Zandvliet 1992) assessed BUD 600 mcg/d and reported histamine PD20 FEV1 (Comparison 07, Outcome 10). A significantly lower histamine BHR was apparent for BUD treated subjects compared to placebo: WMD 0.40 log 10 PD20 FEV1 (95% CI 0.11 to 0.70 log 10 PD20 FEV1).
Crossover studies Two studies in adults (Wempe 1992a, Wempe 1992b) assessed BUD 800 mcg/d and reported histamine PC20 FEV1. Significantly lower histamine BHR was apparent for BUD treated subjects compared to placebo: WMD 1.38 log 2 PC20 FEV1 (95% CI 0.18 to 2.58 log 2 PC20 FEV1).
A single study in adults (Fuller 1991) assessed BUD 1200 mcg/d and reported BHR as the dose of histamine required to produce a 35% fall in specific airways resistance (PD35 SGaw). This was a small study (20 adults) and was of low methodological quality (Jadad score 2). No significant difference between BUD and placebo was apparent.
BHR: other measures
A number of individual studies reported the effects of treatment on bronchial hyper‐responsiveness using bronchial challenges other than methacholine and histamine.
Parallel group studies One study (Jonasson 1998) conducted in 78 children reported the effect of BUD 400 mcg/d versus placebo on the maximal percentage fall in FEV1 following exercise challenge. This study was of high methodological quality (Jadad score 4). A beneficial effect in favour of treatment was apparent: WMD 4% (95% CI 0.2 to 8.4%). A single study (de Baets 1990) in 31 children reported the effect of BUD 600 mcg/d versus placebo on house dust mite allergen bronchial responsiveness (PD20 FEV1). This study was of fair quality (Jadad score 3). No difference between treatment groups was apparent.
Crossover studies One study (Henriksen 1985) conducted in 28 children reported the effect of BUD 400 mcg/d versus placebo on the maximal percentage fall in FEV1 following exercise challenge. This study was of low methodological quality (Jadad score 2). A beneficial effect in favour of BUD was apparent: WMD 30% (95% CI 19 to 41%).
Gauvreau 1996 assessed the effect of one week's treatment with either BUD 400 mcg/d or placebo in adult patients. This study was of high methodological quality (Jadad score 4) and reported the effect of treatment on maximal percentage fall in FEV1 following inhaled allergen challenge (house dust mite, ragweed, cat dander). A significantly lower maximal percentage fall in FEV1 was evident for BUD treated subjects compared to placebo after three to seven hours: WMD 17% (95% CI 7 to 26%), and within 2 hours (p=0.01, data not included in meta‐analysis).
O'Connor 1992 assessed the effect of one month's treatment with either BUD 1600 mcg/d or placebo on adenosine monophosphate (AMP) and sodium metabisulphite bronchial responsiveness in adults. This study was of high quality (Jadad score 4). BUD resulted in a significantly lower AMP BHR compared to placebo: WMD 0.85 log 10 PC20 FEV1 (95% CI 0.24 to 1.46 log 10 PC20 FEV1). BUD also resulted in significantly lower sodium metabisulphite BHR (p< 0.01, data not included in meta‐analysis).
Fuller 1991 assessed the effects of three week's treatment with either BUD 1200 mcg/d or placebo on bradykinin bronchial responsiveness in adults. Bradykinin BHR was expressed as the dose required to produce a 35% fall in specific airways conductance. This study was of low methodological quality (Jadad score 2). BUD resulted in a significantly lower bradykinin BHR compared to placebo: WMD 0.67 log 10 PD35 SGaw (95% CI 0.02 to 1.33 log 10 PD35 Sgaw).
Cockcroft 1995 assessed the effects of one week's treatment with either BUD 1600 mcg/d or placebo on allergen (cat, horse, grass, birch or weed) BHR in adults. This study was of high methodological quality (Jadad score 4). BUD resulted in significantly lower allergen BHR compared to placebo (p<0.001, data not included in meta‐analysis).
ASTHMA EXACERBATIONS
Trial withdrawal due to exacerbation
A significantly lower risk of trial withdrawal due to asthma exacerbation was apparent for patients treated over the lower two BUD dose ranges compared to placebo:
BUD 400 mcg/d or less: RR 0.25 (95% CI 0.15 to 0.43) (Comparison 01, Outcome 26) BUD 500‐800 mcg/d: RR 0.26 (95% CI 0.16 to 0.41) (Comparison 02, Outcome 20) Only one parallel group study (Burke 1996) assessed BUD 1000 mcg/d or greater and reported withdrawal rates due to asthma exacerbation. This was a small study (18 adults) of six weeks duration and of fair quality (Jadad score 3). No significant difference in the risk of withdrawal from treatment groups was apparent.
When studies were pooled across all doses there was a significantly lower risk of withdrawal due to exacerbation: RR 0.17 (95% CI 0.09 to 0.33), with no evidence of a dose response effect.
Exacerbations requiring oral corticosteroids
Two studies (Haahtela 1994, Juniper 1990) assessing BUD 400 mcg/d reported numbers of adults requiring one or more courses of oral steroid for asthma exacerbation compared to placebo. No significant difference between treatment groups was apparent. One study (Essen‐Zandvliet 1992) assessed BUD 600 mcg/d in children. Fewer subjects treated with BUD required one or more courses of oral steroid for exacerbation compared to placebo: RR 0.29 (95% CI 0.14 to 0.57). Two studies (de Jong 1996, Vathenen 1991) assessed BUD 1000 mcg/d or greater. No significant difference between treatment groups was apparent. When studies were pooled across all doses, BUD resulted in significantly fewer patients requiring at least one course of oral prednisolone for asthma exacerbation: RR 0.34 (95% CI 0.19 to 0.59). There was no evidence of a dose response effect.
Exacerbations leading to emergency room attendance, hospital admission, school absence
Only one study (O'Byrne 1996) reported emergency room (ER) attendance due to asthma exacerbation. This was a high quality study (Jadad score 4) conducted in adults and assessed BUD at two daily doses of 400 and 800 mcg/d versus placebo over a four month treatment period. Seventeen patients received BUD 400 mcg/d, 20 received BUD 800 mcg/d and 20 received placebo. Only four patients needed to attend ER during the trial, all in the placebo group, although there was no significant difference in attendance rates for either BUD dose compared to placebo. Essen‐Zandvliet 1992 was the only study to report hospitalisations due to asthma exacerbation. This was a high quality study (Jadad score 5) conducted in children and assessed BUD 600 mcg/d versus placebo over a 22 month treatment period. Two of 58 patients were hospitalised on at least one occasion in the placebo group; none of the 58 patients randomised to BUD were hospitalised during treatment. The difference in hospitalisations was not statistically significant. This study was the only study to report school absences due to asthma symptoms/exacerbations. 26 of 58 patients in the BUD group and 35 of 58 patients in the placebo group experienced one or more school absences over the 22 month treatment period, although the difference in rates failed to reach statistical significance RR 0.74 (95%CI 0.52 to 1.06).
Time to asthma relapse
A single parallel design study (Toogood 1990) assessed time to asthma relapse. This study was of high methodological quality (Jadad score 5) and assessed the effects of BUD 400 mcg/d versus placebo in adult asthmatics over an eight week treatment period. This study had a unique design. Patients enrolled entered a four week run‐in period during which time they were treated with BDP at individually titrated doses in an attempt to achieve maximal asthma control. Following randomisation to BUD or placebo asthma relapse was defined as the time (in days) to the point at which the seven day median lower morning and evening PEFR fell below the lower 95% confidence interval for lower morning and evening PEFR at baseline. BUD treated patients had a significantly longer time to relapse (22 days) compared to placebo treated patients (9 days), p=0.02.
ORAL SIDE EFFECTS
Local oral side effects were not widely reported. Three studies (Haahtela 1994, Juniper 1990, Lorentzson 1990) assessing BUD 400 mcg/d reported the incidence of sore throat/sore mouth, hoarseness and/or 'pharyngitis'. Two studies (Essen‐Zandvliet 1992, Wong 1994) assessed BUD 500‐800 mcg/d and reported the same outcome. No difference in the number of patients experiencing these local effects were apparent when comparing BUD and placebo treated patients at either dose range. When studies were pooled across all doses, no difference in the incidence of these local effects was apparent: RR 0.81 (95% CI 0.53 to 1.23).
The incidence of oral Candidiasis was rarely reported. Only three studies (Haahtela 1994, Juniper 1990, Lorentzson 1990) reported this outcome. All assessed BUD 400 mcg/d versus placebo in adults. It was not clearly stated in any study how diagnosis was made (clinical inspection only and/or mouth swab culture etc). Studies were pooled. The overall incidence of Candidiasis was low (1 out 73 BUD treated subjects affected, 0 of 66 placebo treated subjects affected). The difference in event rate between groups was non significant: RR 3.00 (95% CI 0.13 to 68.57).
HYPOTHALAMIC‐PITUITARY‐ADRENAL FUNCTION
Hypothalamic‐pituitary‐adrenal axis (HPA) function was infrequently reported and assessed using a number of different methods.
Parallel group studies A single parallel group study (Aaronson 1998) reported plasma cortisol at six hours post co‐syntropin infusion. This was a study of fair methodological quality (Jadad score 3) undertaken in adults and assessed BUD at three doses of 800 mcg/d, 1600 mcg/d and 3200 mcg/d versus placebo over a six week treatment period. No significant difference between BUD 800 mcg/d and placebo or BUD 1600 mcg/d or placebo were apparent. Post treatment post co‐syntropin plasma cortisol following BUD 3200 mcg/d was 679 nmol/L, following placebo 879 nmol/L. The difference between highest dose BUD and placebo was statistically significant (ANCOVA analysis of original study, p=0.03). Post‐treatment basal early morning plasma cortisol levels were also reported, no difference between any BUD treatment group and placebo was apparent (data not included in meta‐analysis).
Crossover studies Gleeson 1988 reported early morning plasma cortisol. This high quality study (Jadad score 5) assessed BUD 400 mcg/d versus placebo in pre‐school children. No difference between treatment groups was apparent. Two studies in children reported 24 hour urinary free cortisol excretion. One study (Agertoft 1997) of high quality (Jadad score 4) assessed BUD 200 mcg/d and 400 mcg/d versus placebo over two week treatment periods with two week washout periods. Data was not presented in a form suitable for inclusion in the meta‐analysis. No significant difference between BUD 200 mcg/d and placebo was apparent. BUD 400 mcg/d resulted in a significantly lower 24 hour urinary free cortisol excretion compared to placebo: mean difference 2.5 nmol/mmol creatinine (95% CI 1.1 to 3.9 nmol/mmol creatinine, original ANCOVA analysis). The second study (Henriksen 1985) was of low methodological quality (Jadad score 2). In this study children received BUD 400 mcg/d or placebo over three week treatment periods with two week washout. No difference between BUD and placebo was apparent.
EOSINOPHIL RELATED OUTCOMES
Two crossover studies reported blood eosinophil counts. Both were of high methodological quality (Jadad score 4). One study (Gauvreau 1996) assessed BUD 400 mcg/d versus placebo in adults over a one week treatment period with three week washout. No difference between groups was apparent. The second study (O'Connor 1992) assessed BUD 1000 mcg/d versus placebo over a two week treatment period with four week washout. BUD treated patients had a significantly lower blood eosinophil count compared to placebo treated subjects: WMD 0.21 x10^9/L (95% CI 0.10 to 0.32x10^9/L).
A single parallel group study (Osterman 1997) of high quality (Jadad score 5) reported change in serum eosinophil cationic protein compared to baseline. This study assessed BUD 400 mcg/d versus placebo in adults over a 12 week treatment period. No difference between BUD and placebo was apparent.
Sensitivity analysis
The following table lists the comparisons in which low quality studies were eliminated in a sensitivity analysis. In each case this made no difference to the direction, size or significance of the outcome.
Study Design Outcome
Rodriguez 1991 Parallel group FEV1 (% predicted) Kharitonov 1996 Crossover group FEV1 (% predicted) Fuller 1991 Crossover group FEV1 (litres), morning PEFR, evening PEFR
Subgroup analyses
For the majority of outcomes no heterogeneity was apparent when studies were pooled across all doses. For these outcomes, subgroup analyses based on patient delivery device, asthma severity and study duration did not identify any groups who appeared to preferentially benefit from treatment. These outcomes are listed below:
Parallel group studies/Crossover studies
FEV1 (% predicted) FEV1 (% predicted) FEV1 (litres) FEV1 (litres) Change in FEV1 compared to baseline (litres) Methacholine BHR (log 10 values) Morning PEFR (L/min) Methacholine BHR (log 10 values) Histamine BHR (log 10 values) Withdrawal due to asthma exacerbation (No. patients) Requirement for one or more courses of oral steroid (No. patients) Oropharyngeal side‐effects (No. of patients)
For a number of outcome measures heterogeneity was apparent when studies were grouped according to daily dose. In the case of change in morning PEFR and change in evening PEFR compared to baseline, significant heterogeneity was present for the two studies (Kivity 1994, O'Byrne 1996) assessing BUD 500‐800 mcg/d. In the case of both outcomes, heterogeneity was reduced to a non‐significant level when studies were grouped according to delivery device or study duration. Change in night‐time use of short‐acting beta2 agonist compared to baseline was reported by three studies (Jones 1994, Lorentzson 1990, Osterman 1997). All studies recruited adults and all assessed BUD 400 mcg/d. Heterogeneity was only reduced to a non‐significant level when studies were sub grouped according to delivery device. These findings may suggest that, when considering these outcomes, delivery device and study duration are more important determinants of response than dose but this finding needs to be interpreted with caution (see Discussion).
ORAL STEROID TREATED ASTHMATICS
OCS sparing studies
Only one study (Nelson 1998) assessing BUD versus placebo and recruiting oral steroid treated subjects had the specific aim of determining the relative OCS sparing effect of treatment. This study was of fair methodological quality (Jadad score 3) and recruited 159 adults. Two daily doses of BUD were assessed, 800 mcg/d and 1600 mcg/d. Prednisolone dose tapering was undertaken using a forced down titration approach (see study notes). Mean daily prednisolone dose at baseline was between 18 and 20 mg/day for all treatment groups. Both BUD doses allowed a significantly greater reduction in daily prednisolone dose compared to placebo:
BUD 800 mcg/d v placebo:16.2 mg v 5.4 mg (p< 0.001) BUD 1600 mcg/d v placebo: 15.1 mg v 5.4 mg (p< 0.001)
In addition treatment with both daily doses of BUD allowed a significantly greater percentage of patients to discontinue prednisolone treatment completely:
BUD 800 mcg/d v placebo:68% v 8% (p<0.001) BUD 1600 mcg/d v placebo:64% v 8% (p<0.001)
A number of other outcomes were assessed in this study. These included FEV1, FEF 25‐50, diary card morning and evening PEFR, daytime and night‐time symptom scores and rescue beta2 agonist use. All outcomes were expressed as change compared to baseline. Statistically significant improvements were apparent for all outcomes for both BUD doses compared to placebo. The reader is referred to the original study for details.
Non‐OCS sparing studies
Two trials, one in adults (Busse 1998) and one in children (Shapiro 1998a) recruited oral steroid treated subjects but were not designed to assess the OCS sparing effects of treatment. Both studies were of parallel group design, fair methodological quality (Jadad score 3) and evaluated over 400 patients for 12 week treatment periods. In one study (Busse 1998) oral prednisolone dependence for asthma control was an inclusion criterion, whilst is one study (Shapiro 1998a) a proportion of subjects (30/404) were receiving prednisolone at the time of enrolment.
The effects of BUD over a range of daily doses were studied. In both trials the relative efficacy of BUD 200, 400 and 800 mcg/d was assessed versus placebo. Busse 1998 assessed a fourth active treatment arm of BUD 1600 mcg/d. A principal aim in both trials was to test for a dose response effect by comparing active treatments (i.e. different BUD doses) for a range of efficacy outcomes. These dose comparisons are considered in a separate systematic review in progress (Adams 2000). However in both studies, for all outcomes assessed, every BUD dose resulted in a significant improvement compared to placebo. In both studies plasma cortisol levels pre and post ACTH were assessed. No significant difference in the number of patients with subnormal range basal plasma cortisol levels at the end of the treatment period were apparent in either study when comparing any nominal daily dose of BUD and placebo. In (Shapiro 1998b) no difference between any dose of BUD and placebo was apparent when assessing change in post ACTH plasma cortisol compared to baseline. Busse 1998 demonstrated a statistically significant difference between highest dose BUD and placebo.
Discussion
This review has assessed the relative efficacy and safety of inhaled BUD compared to placebo when treating both non‐oral steroid dependent and oral steroid treated patients with chronic asthma. Only randomised, placebo controlled trials were included and the overall quality of studies was high. A total of 43 studies conducted worldwide between 1984 and 1998 in 2833 children and adults were assessed.
EFFICACY
When considering outcomes that relate to airway calibre, evaluation of response to treatment appears to be partly determined by the metric used to assess the outcome. Trials of parallel group design provide evidence that BUD results in significant improvement in FEV1 compared to placebo when this is expressed either as a % predicted value, WMD 3.7% (95% CI 0.1 to 7.2%), or as change compared to baseline, WMD 0.20 litres (95% CI 0.07 to 0.33 litres). No difference between treatments was apparent when FEV1 values in the treatment and control groups were compared at the end of the study. For diary card PEFR, trials of parallel design provide evidence that BUD leads to improvement in morning PEFR compared to placebo when expressed as change compared to baseline, WMD 29 L/min (95% CI 22 to 36 L/min), and evening PEFR when expressed as a change compared to baseline, WMD 21 L/min (95% CI 13 to 29 L/min). Few studies reported differences in PEFR between treatment groups at the end of the study and no difference between BUD and placebo was apparent for morning or evening end of study PEFR expressed in L/min.
Symptoms were reported using a diverse set of measures, and it was inappropriate to pool different measures used in individual studies. However, all the high quality studies (Jadad score >3) that reported any of the following measure showed a significant improvement in BUD treated patients compared to placebo:
a) absolute symptom score b) number of night‐time awakenings per week c) percentage of patients waking at night or experiencing early morning awakening due to symptoms d) reduction in daytime and night‐time symptom score compared to baseline
Rescue beta2 agonist use was consistently lower in BUD treated subjects compared to placebo. This was apparent when expressed as either reduction in daytime use compared to baseline, WMD 0.4 puffs/daytime (95% CI 0.1 to 0.8 puffs/daytime); reduction in night‐time use compared to baseline WMD 0.7 puffs/night‐time (95% CI 0.3 to 1.1 puffs/night‐time); end of study difference in daily use (puffs/d) or weekly use (puffs/week).
Bronchial hyper‐responsiveness to methacholine and histamine were reported in many studies. Differences in the metrics used to report fall in FEV1 (doubling dose steps or absolute PC20/PD 20) and variations between studies in terms of log transformation of data prior to analysis limited the possibilities for pooling studies. Nevertheless, budesonide consistently reduced BHR compared to placebo. Asthma exacerbations were consistently reduced by BUD. BUD treated subjects experienced fewer exacerbations necessitating one or more courses of rescue oral steroid, RR 0.34 (95% CI 0.19 to 0.59), and fewer trial withdrawals due to exacerbation, RR 0.17 (95% CI 0.09 to 0.33) when compared to placebo treated subjects.
Hospital admission and emergency room attendance rates due to exacerbation and days lost from school due to exacerbation were rarely reported. However the two studies which did report such outcomes (O'Byrne 1996, Essen‐Zandvliet 1992) were of high methodological quality and had treatment periods of 4 or 22 months. No significant difference between treatment groups was apparent.
Only one study assessed the role of BUD as an oral steroid sparing agent in chronic asthma (Nelson 1998). However this large trial clearly demonstrated that BUD allows a clinically significant reduction in oral prednisolone dose when used for asthma control, and allows a significantly greater number of patients to discontinue prednisolone completely compared to placebo. It should be noted that this study was conducted in adults, generalisation to children with OCS‐dependent asthma therefore needs to be made with some caution.
SAFETY
Local oral side‐effects (sore throat/hoarseness, Candidiasis) were reported infrequently but the available evidence does not demonstrate an increased risk of such occurrences with active BUD treatment compared to placebo.
The effect of BUD on hypothalamic‐pituitary‐adrenal axis function was rarely reported. Only one study (Aaronson 1998) reported the results of a dynamic stimulation test, i.e. post synthetic ACTH infusion plasma cortisol levels. This test assesses the ability of the adrenal glands to mount a cortisol secretion response. Significantly lower levels were apparent in BUD treated adults compared to placebo, however this was only the case in subjects treated with very high dose BUD (3200 mcg/d). At lower doses (800 and 1600 mcg/d) no differences between BUD and placebo were apparent. Only one fair quality study (Agertoft 1997) reported the results of a sensitive assay of basal adreno‐cortical function. 24‐hour urinary free cortisol levels corrected for creatinine were no different in children treated with either BUD 200 or 400 mcg/d compared to placebo. Translating these outcomes to clinical practice is very difficult. The probability of an acute adrenal insufficiency crisis cannot be estimated from this data.
DOSE RESPONSE
The data presented in this review does not provide clear evidence for a dose response effect for the efficacy of BUD in the treatment of non‐oral steroid treated chronic asthmatics, although in the parallel group studies, the effect sizes for FEV1 and PEFR tended to be higher with BUD 500‐800 mcg/d compared to 400 mcg/d or less. This needs to be interpreted with some caution, however. Assessment of the magnitude of response for a given outcome for those trials that compared one dose of BUD to placebo with that of trials that compared a different dose of BUD to placebo is the only way dose response could be addressed in this review, but the patients who received the different doses were not drawn from the same population of patients. The most appropriate approach to this question is to assess trials that have randomised subjects to different doses of BUD. These trials were not included in the scope of this review and are analysed in a companion Cochrane review (Adams 2001). Current guidelines (BTS 1997, GINA 1995, NHLBI 1997) assume a dose response relationship for all available ICS's by recommending larger doses for patients in whom asthma symptoms are not controlled, and titrating dose to individual requirements. The evidence presented in this review neither supports or refutes this approach.
METHODOLOGICAL LIMITATIONS
Systematic bias a) Bias related to missed trials: a large volume of literature was searched for relevant studies. Despite a comprehensive search strategy it is possible relevant studies were missed.
b) Bias related to unpublished 'negative' trials: selective publication of randomised trials with positive findings may lead to a biased over‐estimate of treatment effect in favour of BUD. However attempt to minimise such bias was made by searching unpublished respiratory society meeting abstracts and asking trialists and Astra Zeneca for further studies (who found none).
Power A significant proportion of outcome data from individual trials could not be included in the meta‐analysis because either standard deviation values for mean effect sizes were not presented in the original citation or no numerical data was presented at all (see Table 2). Authors were approached for this data but were rarely able or willing to provide it. This missing data will have diminished the power of the meta‐analysis.
Generalisability The findings of this review are broadly generalisable. Children, adolescents and adults were studied who demonstrated a spectrum of disease severity, were treated with a wide range of daily doses of BUD using both MDI, MDI+spacer and DPI devices over a wide range of treatment periods from 1 week to 22 months. However, infants were specifically excluded from this review due to special difficulties in defining and diagnosing asthma and delivering inhaled treatment in this age group. Considerable caution should therefore be used in extrapolating the findings of this review to children under two years of age.
Authors' conclusions
Implications for practice.
This systematic review strongly supports the use of inhaled BUD in the treatment of chronic asthma. This applies to children over the age of two years and adults. It applies to asthma of all degrees of severity, with medication delivered by either MDI, MDI with spacer or DPI device. Three daily dose ranges were specified in this review: 400 mcg/d or less, 500‐800 mcg/d and 1000 mcg/d or greater. When compared to placebo, patients treated within the higher dose ranges do not appear to gain greater benefit than those treated at the lower dose range compared to placebo. Current guidelines recommend titration of dose to individual response. The data from the trials comparing BUD to placebo do not support the case for dose titration above 400 mcg/d.
Implications for research.
Outcomes
Asthmatics experience varying degrees of baseline airway obstruction, hence assessment of changes in airway calibre (FEV1, PEFR) has value as an outcome measure. These 'physiological' outcomes have been reported in a consistent manner in a large number of studies, which has allowed a fairly powerful meta‐analysis. 'Patient‐centred' outcomes were less consistently reported across studies. Despite the fact that symptoms were commonly assessed, a quantitative synthesis was hampered by the fact that varying scoring systems were used. This situation is understandable because as far as we are aware no validated symptom scores for asthma have been developed for use in clinical trials for either adults or children. Development of such a scale would be a helpful advance both for asthma trialists and for meta‐analysts. Assessment of health status/health related quality of life (HRQOL) using asthma‐specific instruments was notably absent in the studies included in this review. Such outcomes would be usefully included in any future studies designed to assess the efficacy of BUD versus placebo. The safety of inhaled BUD is an important consideration in all age groups. Few studies included in this review assessed HPA function, and further studies are required to define the effects of BUD on adrenal function as assessed by the available sensitive basal and dynamic tests. A concern, particularly in children, who may require long‐term treatment with BUD for many years is the ultimate risk of adrenal insufficiency/adrenal crisis at times of stress. In order to assess such risks long term, parallel groups studies would be needed. Because such events are likely to be rare, large numbers of patients would need to be recruited in order for the study to have sufficient power to detect treatment and control differences. Such studies will be exceptionally difficult to set up and extremely expensive. In the absence of such RCT's knowledge regarding these risks will probably need to be derived from well conducted, retrospective observational studies.
Study reports
Very few studies provided details regarding the randomisation method used and whether or not allocation concealment was employed. Assessment of methodological quality and the accuracy of systematic reviews would be improved with the adoption of CONSORT 1996 recommendations for the reporting of randomised controlled trials.
What's new
| Date | Event | Description |
|---|---|---|
| 21 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 29 June 1999 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Anna Bara for running the initial electronic search, Steve Milan for assistance with statistical methods. We would like to thank the following authors who were able to provide additional information regarding their studies: Professor A.L. Boner, Dr L.M. Campbell, Dr D.W. Cockcroft, Dr G.M. Gauvreau, Dr J.G. Gleeson, Dr A. Jatakanon, Dr G. Jonasson, Professor E.F. Juniper, Dr Han‐Pin Kuo, Professor P. O'Byrne, Dr B.J. O'Conner, Dr L. Prieto, Professor J.H. Toogood and Dr D.H. Yates. We would also like to thank Marie Carlholm of Astra Zeneca who provided additional information regarding a number of Astra sponsored studies.
Data and analyses
Comparison 1. BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 3 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐2.09 [‐7.33, 3.16] |
| 1.1 Children | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐8.74, 6.70] |
| 1.2 Adults | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐10.15, 4.15] |
| 2 Change in FEV1 (% predicted) compared to baseline | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐4.3 [‐9.33, 0.73] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐4.3 [‐9.33, 0.73] |
| 3 Change in FEV1 (litres) compared to baseline | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 4 FVC (% predicted) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐5.88, 12.68] |
| 4.1 Children | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐5.88, 12.68] |
| 4.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Change in FVC (litres) compared to baseline | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.37, 0.03] |
| 5.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.37, 0.03] |
| 6 Morning PEFR (L/min) | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐29.46 [‐107.88, 48.96] |
| 6.1 Children | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐29.46 [‐107.88, 48.96] |
| 6.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Change in morning PEFR (litres/min) compared to baseline | 5 | 431 | Mean Difference (IV, Fixed, 95% CI) | ‐28.89 [‐36.19, ‐21.59] |
| 7.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 5 | 431 | Mean Difference (IV, Fixed, 95% CI) | ‐28.89 [‐36.19, ‐21.59] |
| 8 Evening PEFR (L/min) | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐28.05 [‐105.75, 49.65] |
| 8.1 Children | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐28.05 [‐105.75, 49.65] |
| 8.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in evening PEFR (litres/min) compared to baseline | 5 | 431 | Mean Difference (IV, Fixed, 95% CI) | ‐18.55 [‐26.65, ‐10.45] |
| 9.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 5 | 431 | Mean Difference (IV, Fixed, 95% CI) | ‐18.55 [‐26.65, ‐10.45] |
| 10 Clinic PEFR (L/min) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐95.20, 86.40] |
| 10.1 Children | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐95.20, 86.40] |
| 10.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 FEF 25‐75 (L/second) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.88, 0.00] |
| 11.1 Children | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.88, 0.00] |
| 11.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Monthly symptom/asthma severity score | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐4.14 [‐5.50, ‐2.78] |
| 12.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Adults | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐4.14 [‐5.50, ‐2.78] |
| 13 Change in daytime symptom score compared to baseline | 3 | 279 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.49, ‐0.02] |
| 13.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Adults | 3 | 279 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.49, ‐0.02] |
| 14 Change in night‐time symptom score compared to baseline | 2 | 240 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.74, ‐0.22] |
| 14.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Adults | 2 | 240 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.74, ‐0.22] |
| 15 Patients waking at night due to asthma symptoms (last month) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.98] |
| 15.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Adults | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.98] |
| 16 Patients with early morning wakening (in last month) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.83] |
| 16.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Adults | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.83] |
| 17 Patients requiring beta2 agonist > 4xdaily (last month) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.23] |
| 17.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Adults | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.23] |
| 18 Daily use of beta2 agonist (puffs/day) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.93, ‐0.27] |
| 18.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Adults | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.93, ‐0.27] |
| 19 Change in daytime use of short‐acting beta2 agonists compared to baseline (puffs/daytime) | 5 | 480 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.80, ‐0.06] |
| 19.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Adults | 5 | 480 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.80, ‐0.06] |
| 20 Change in night‐time use of short‐acting beta2 agonists compared to baseline (puffs/night‐time) | 3 | 304 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.09, ‐0.33] |
| 20.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Adults | 3 | 304 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.09, ‐0.33] |
| 21 Methacholine bronchial responsiveness (log 10 PC20 FEV1) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.31, 0.39] |
| 21.1 Children | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.31, 0.39] |
| 21.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Maximal percentage fall in FEV1 post exercise challenge | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐4.3 [‐8.41, ‐0.19] |
| 22.1 Children | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐4.3 [‐8.41, ‐0.19] |
| 22.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Change in serum ECP compared to baseline (mcg/L) | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐7.31, 3.71] |
| 23.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Adults | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐7.31, 3.71] |
| 24 Withdrawal due to asthma exacerbations (No. of patients) | 8 | 620 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.15, 0.43] |
| 24.1 Children | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.12, 0.43] |
| 24.2 Adults | 6 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.91] |
| 25 Requirement one or more courses of oral steroid for asthma exacerbation (No. of patients) | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.18, 1.40] |
| 25.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Adults | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.18, 1.40] |
| 26 Requirement for one or more emergency dept attendances due to asthma exacerbation (No. of patients) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.25] |
| 26.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Adults | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.25] |
| 27 Oropharyngeal side effects: sore mouth/throat, hoarseness, pharyngitis (No. of patients) | 3 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.83] |
| 27.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Adults | 3 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.83] |
| 28 Oropharyngeal Candidiasis (No. of patients) | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 28.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Adults | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
1.1. Analysis.
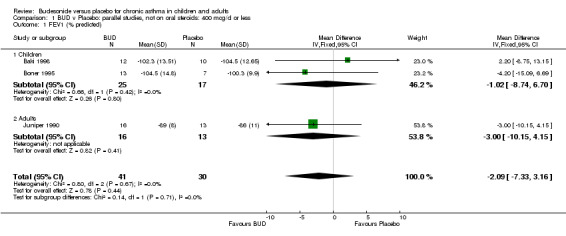
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 1 FEV1 (% predicted).
1.2. Analysis.
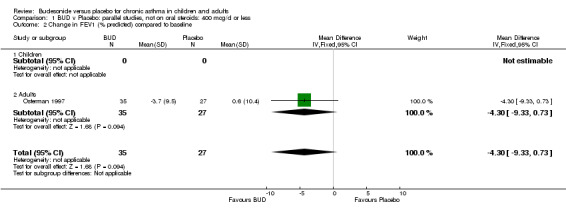
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 2 Change in FEV1 (% predicted) compared to baseline.
1.3. Analysis.
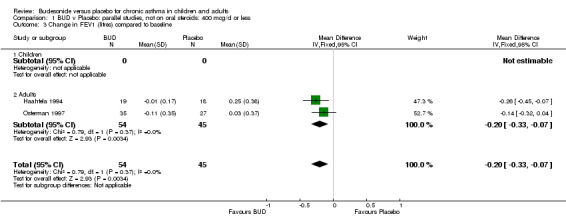
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 3 Change in FEV1 (litres) compared to baseline.
1.4. Analysis.
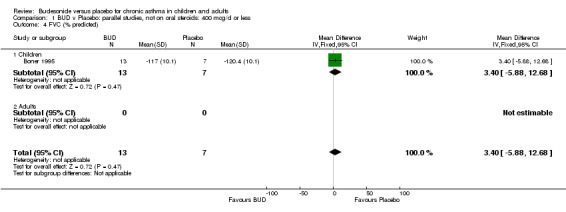
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 4 FVC (% predicted).
1.5. Analysis.
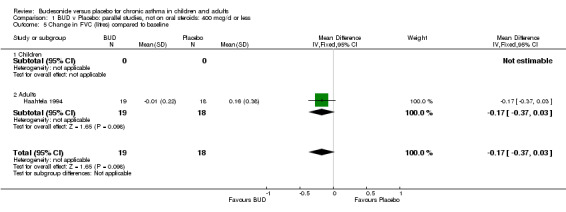
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 5 Change in FVC (litres) compared to baseline.
1.6. Analysis.
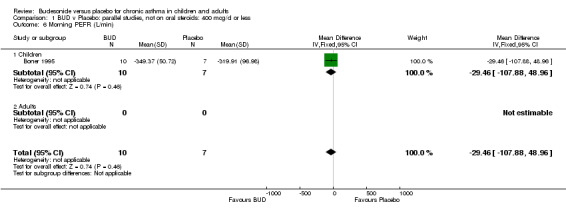
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 6 Morning PEFR (L/min).
1.7. Analysis.
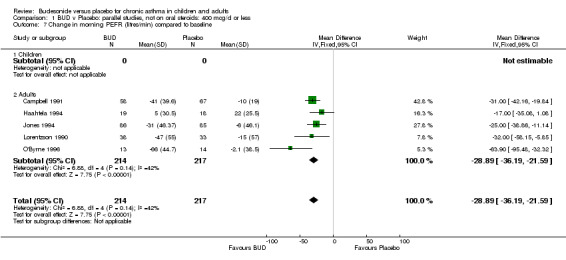
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 7 Change in morning PEFR (litres/min) compared to baseline.
1.8. Analysis.
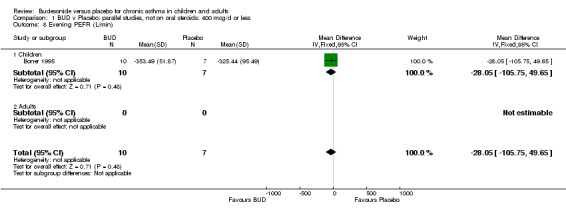
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 8 Evening PEFR (L/min).
1.9. Analysis.
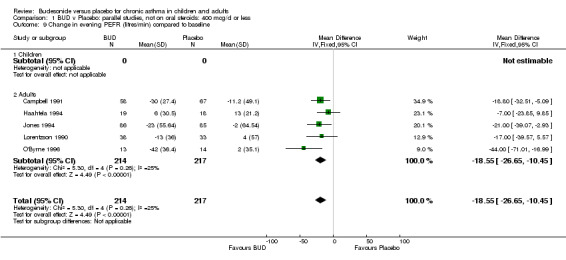
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 9 Change in evening PEFR (litres/min) compared to baseline.
1.10. Analysis.
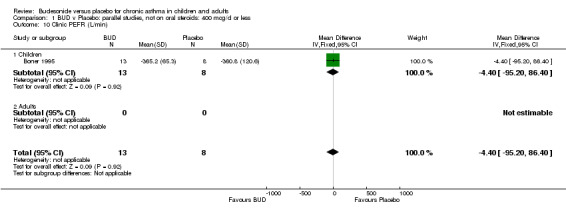
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 10 Clinic PEFR (L/min).
1.11. Analysis.
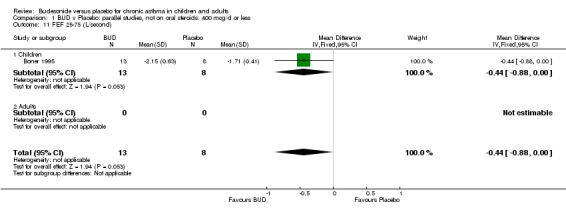
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 11 FEF 25‐75 (L/second).
1.12. Analysis.
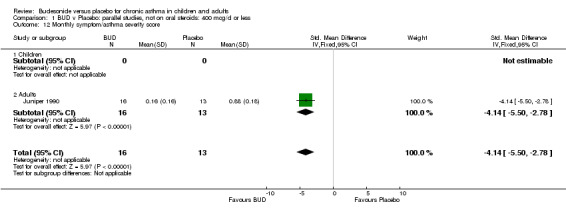
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 12 Monthly symptom/asthma severity score.
1.13. Analysis.
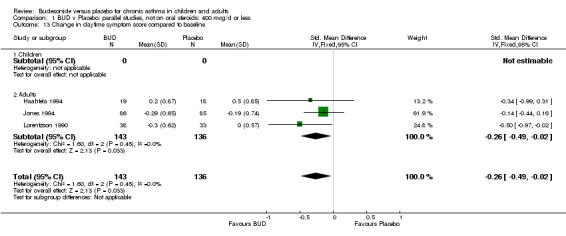
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 13 Change in daytime symptom score compared to baseline.
1.14. Analysis.
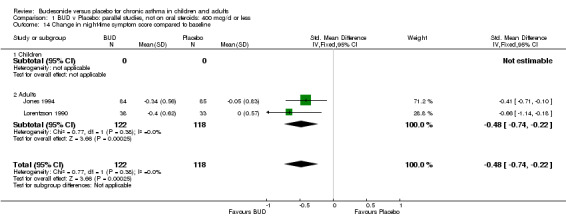
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 14 Change in night‐time symptom score compared to baseline.
1.15. Analysis.
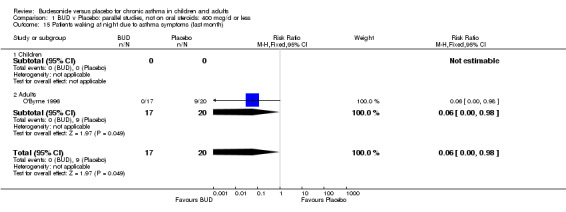
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 15 Patients waking at night due to asthma symptoms (last month).
1.16. Analysis.
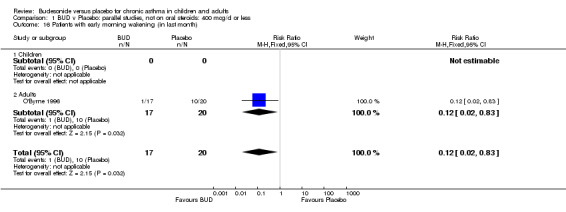
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 16 Patients with early morning wakening (in last month).
1.17. Analysis.
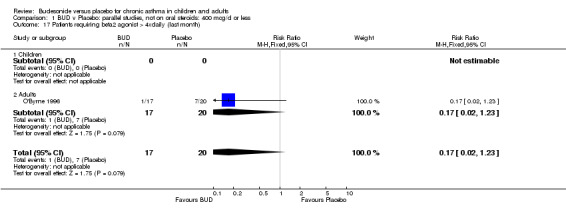
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 17 Patients requiring beta2 agonist > 4xdaily (last month).
1.18. Analysis.
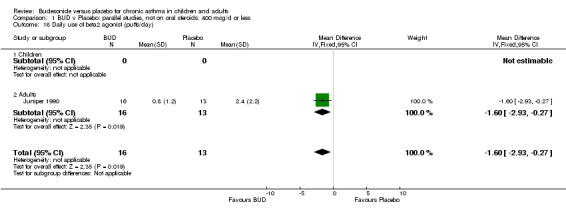
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 18 Daily use of beta2 agonist (puffs/day).
1.19. Analysis.
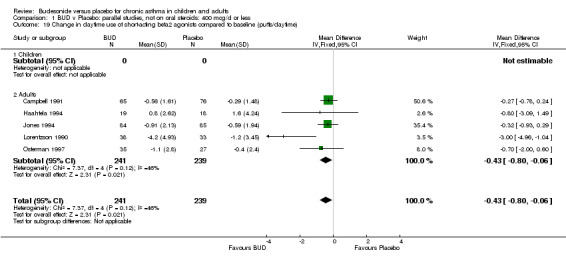
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 19 Change in daytime use of short‐acting beta2 agonists compared to baseline (puffs/daytime).
1.20. Analysis.
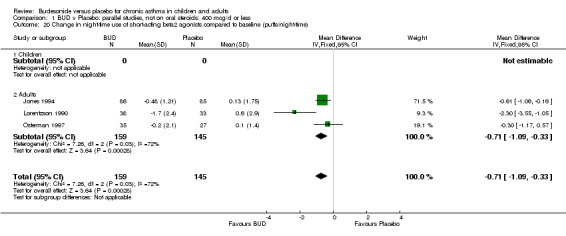
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 20 Change in night‐time use of short‐acting beta2 agonists compared to baseline (puffs/night‐time).
1.21. Analysis.
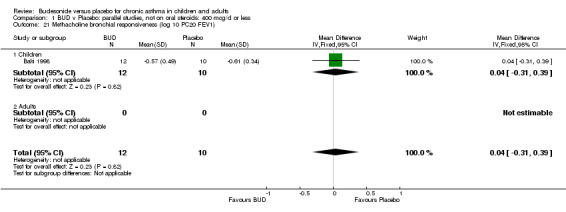
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 21 Methacholine bronchial responsiveness (log 10 PC20 FEV1).
1.22. Analysis.
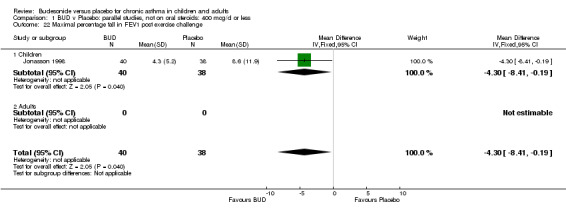
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 22 Maximal percentage fall in FEV1 post exercise challenge.
1.23. Analysis.
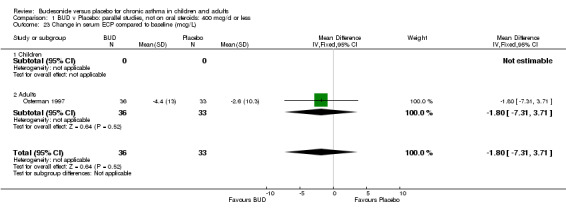
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 23 Change in serum ECP compared to baseline (mcg/L).
1.24. Analysis.
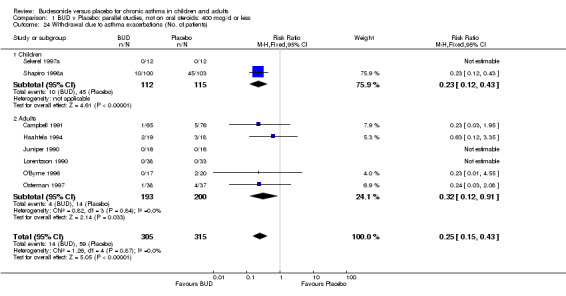
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 24 Withdrawal due to asthma exacerbations (No. of patients).
1.25. Analysis.
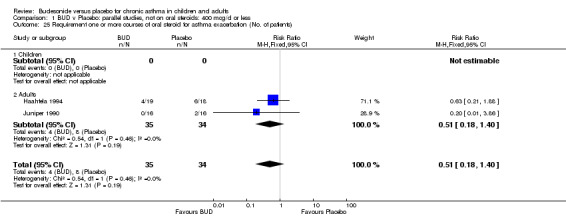
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 25 Requirement one or more courses of oral steroid for asthma exacerbation (No. of patients).
1.26. Analysis.
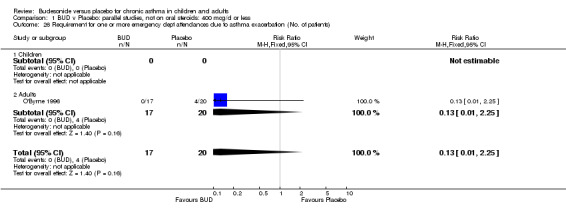
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 26 Requirement for one or more emergency dept attendances due to asthma exacerbation (No. of patients).
1.27. Analysis.
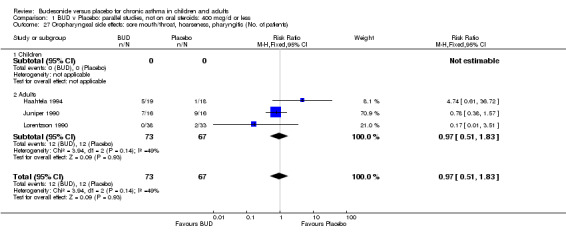
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 27 Oropharyngeal side effects: sore mouth/throat, hoarseness, pharyngitis (No. of patients).
1.28. Analysis.
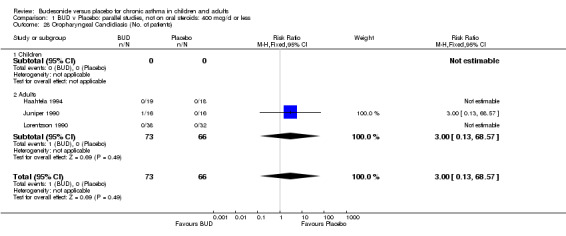
Comparison 1 BUD v Placebo: parallel studies, not on oral steroids: 400 mcg/d or less, Outcome 28 Oropharyngeal Candidiasis (No. of patients).
Comparison 2. BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 5 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐4.86 [‐9.31, ‐0.42] |
| 1.1 Children | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐6.72 [‐13.57, 0.13] |
| 1.2 Adults | 3 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐3.52 [‐9.35, 2.31] |
| 2 FEV1 (litres) | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.27, 0.55] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.27, 0.55] |
| 3 FVC (% predicted) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐11.50, 9.10] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐11.50, 9.10] |
| 4 FEF50 (% predicted) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 12.30 [‐1.90, 26.50] |
| 4.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 12.30 [‐1.90, 26.50] |
| 5 Morning PEFR (L/min) | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐31.22 [‐71.25, 8.80] |
| 5.1 Children | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐31.22 [‐71.25, 8.80] |
| 5.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Change in morning PEFR (L/min) compared to baseline | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐51.34 [‐76.38, ‐26.30] |
| 6.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐51.34 [‐76.38, ‐26.30] |
| 7 Change in evening PEFR (litres/min) compared to baseline | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐40.33 [‐57.88, ‐22.78] |
| 7.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐40.33 [‐57.88, ‐22.78] |
| 8 Daily PEFR (L/min) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐61.00 [‐133.32, 11.32] |
| 8.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐61.00 [‐133.32, 11.32] |
| 9 Diurnal variability in PEFR (L/min) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [‐3.20, 8.00] |
| 9.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [‐3.20, 8.00] |
| 10 Daily symptom score | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐2.31, 0.45] |
| 10.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐2.31, 0.45] |
| 11 Patients waking at night due to asthma symptoms in last month | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.90] |
| 11.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Adults | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.90] |
| 12 Patients with early morning wakening last month | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.10, 0.93] |
| 12.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Adults | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.10, 0.93] |
| 13 Use of short‐acting bronchodilator for symptom relief (puffs/week) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐11.4 [‐13.51, ‐9.29] |
| 13.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Adults | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐11.4 [‐13.51, ‐9.29] |
| 14 Patients requiring beta2 agonist > 4xdaily (last month) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.09] |
| 14.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Adults | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.09] |
| 15 Methacholine bronchial responsiveness (log 10 values) | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.48, 0.23] |
| 15.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Adults | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.48, 0.23] |
| 16 Methacholine bronchial responsiveness (PC20 FEV1): doubling dose steps | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.19, ‐1.21] |
| 16.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Adults | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.19, ‐1.21] |
| 17 Histamine bronchial responsiveness (log 10 PD20 FEV1) | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.70, ‐0.11] |
| 17.1 Children | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.70, ‐0.11] |
| 17.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Change in histamine bronchial responsiveness (PD20 FEV1) compared to baseline: doubling dose steps | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐1.7 [‐3.07, ‐0.33] |
| 18.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Adults | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐1.7 [‐3.07, ‐0.33] |
| 19 House dust mite allergen bronchial responsiveness (log 10 PD20 FEV1) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.37, 0.27] |
| 19.1 Children | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.37, 0.27] |
| 19.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Withdrawal due to asthma exacerbation (No. of patients) | 10 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.16, 0.41] |
| 20.1 Children | 3 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.16, 0.42] |
| 20.2 Adults | 7 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.27] |
| 21 Requirement for one or more courses of oral steroid due to asthma exacerbation (No. of patients) | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.14, 0.57] |
| 21.1 Children | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.14, 0.57] |
| 21.2 Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Requirement for one or more emergency dept attendance for exacerbation of asthma (No. of patients) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.94] |
| 22.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Adults | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.94] |
| 23 One or more hospital admission due to exacerbation of asthma (No. of patients) | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.08] |
| 23.1 Children | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.08] |
| 23.2 Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 One or more absences from school due to asthma exacerbation (No. of patients) | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.52, 1.06] |
| 24.1 Children | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.52, 1.06] |
| 24.2 Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Oropharyngeal side‐effects: sore mouth/throat, hoarseness, pharyngitis (No. of patients) | 2 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.41, 1.26] |
| 25.1 Children | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.00] |
| 25.2 Adults | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.18 [0.50, 133.66] |
| 26 Plasma cortisol 6 hour post cosyntropin infusion (nmol/L) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [‐140.66, 174.66] |
| 26.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Adults | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [‐140.66, 174.66] |
2.1. Analysis.
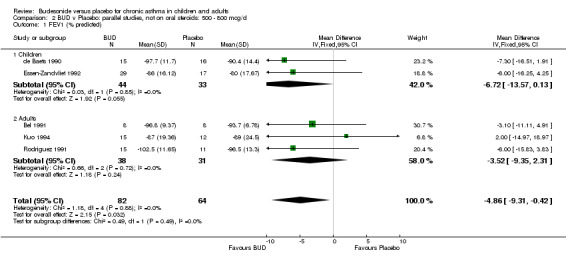
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 1 FEV1 (% predicted).
2.2. Analysis.
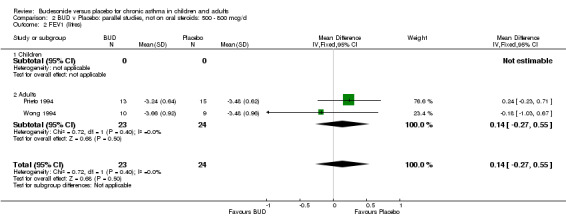
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 2 FEV1 (litres).
2.3. Analysis.
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 3 FVC (% predicted).
2.4. Analysis.
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 4 FEF50 (% predicted).
2.5. Analysis.
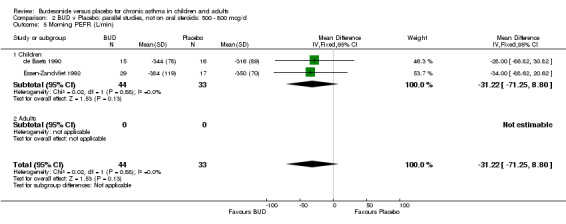
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 5 Morning PEFR (L/min).
2.6. Analysis.
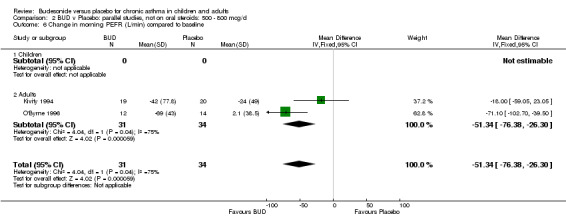
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 6 Change in morning PEFR (L/min) compared to baseline.
2.7. Analysis.
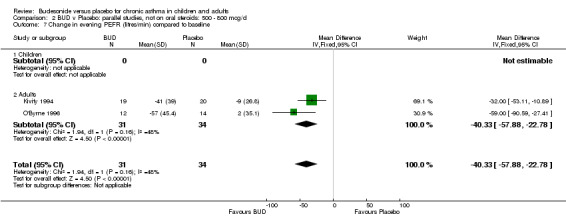
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 7 Change in evening PEFR (litres/min) compared to baseline.
2.8. Analysis.
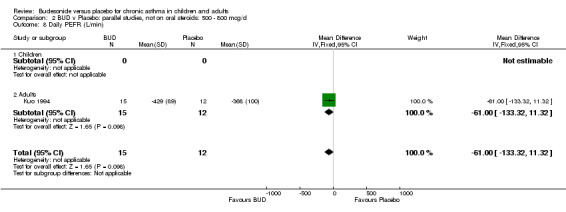
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 8 Daily PEFR (L/min).
2.9. Analysis.
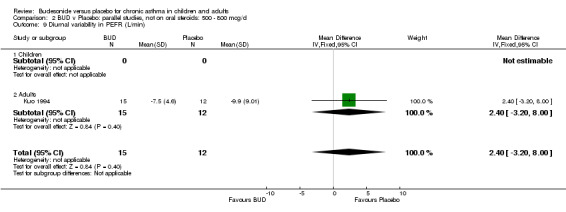
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 9 Diurnal variability in PEFR (L/min).
2.10. Analysis.
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 10 Daily symptom score.
2.11. Analysis.
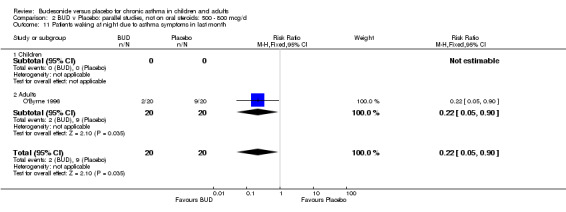
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 11 Patients waking at night due to asthma symptoms in last month.
2.12. Analysis.
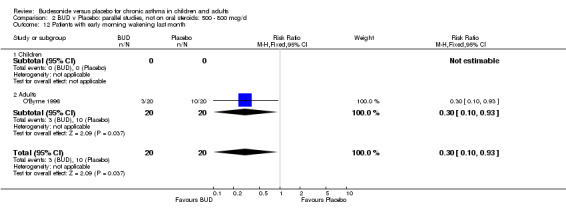
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 12 Patients with early morning wakening last month.
2.13. Analysis.
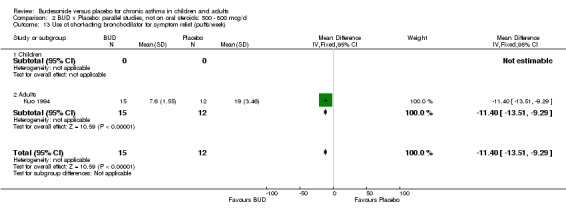
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 13 Use of short‐acting bronchodilator for symptom relief (puffs/week).
2.14. Analysis.
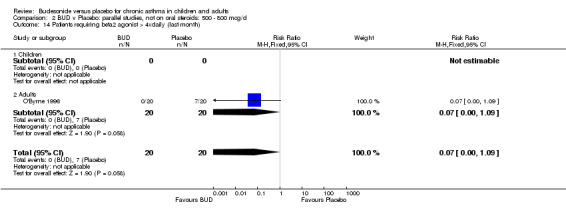
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 14 Patients requiring beta2 agonist > 4xdaily (last month).
2.15. Analysis.
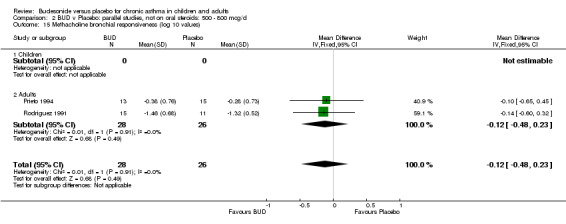
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 15 Methacholine bronchial responsiveness (log 10 values).
2.16. Analysis.
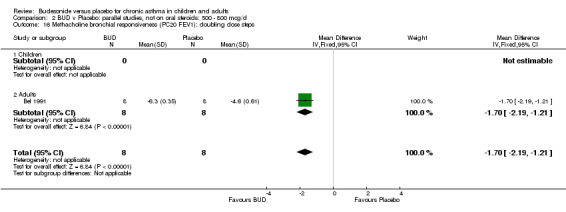
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 16 Methacholine bronchial responsiveness (PC20 FEV1): doubling dose steps.
2.17. Analysis.
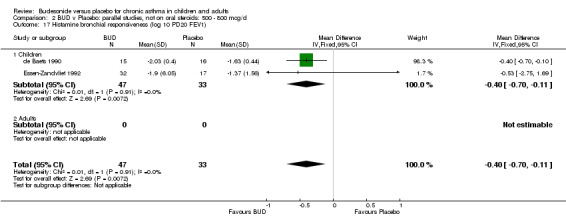
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 17 Histamine bronchial responsiveness (log 10 PD20 FEV1).
2.18. Analysis.
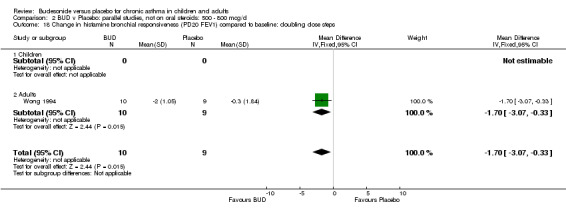
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 18 Change in histamine bronchial responsiveness (PD20 FEV1) compared to baseline: doubling dose steps.
2.19. Analysis.
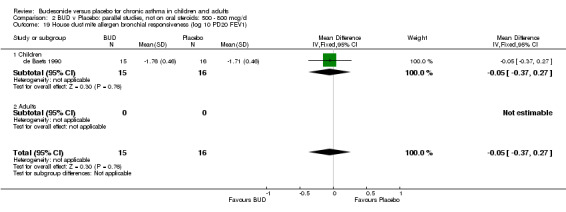
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 19 House dust mite allergen bronchial responsiveness (log 10 PD20 FEV1).
2.20. Analysis.
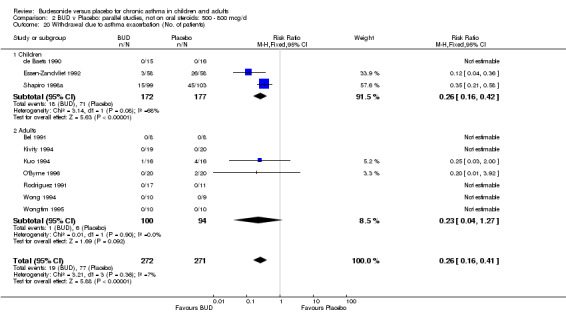
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 20 Withdrawal due to asthma exacerbation (No. of patients).
2.21. Analysis.
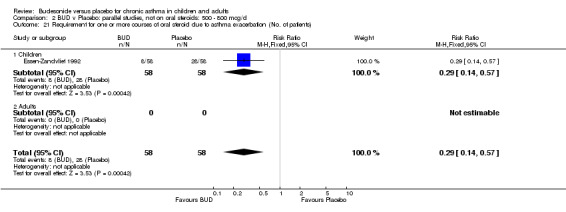
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 21 Requirement for one or more courses of oral steroid due to asthma exacerbation (No. of patients).
2.22. Analysis.
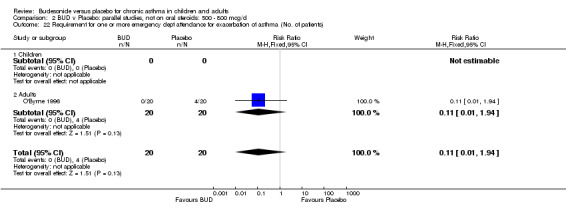
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 22 Requirement for one or more emergency dept attendance for exacerbation of asthma (No. of patients).
2.23. Analysis.
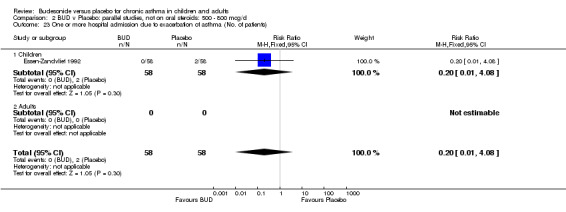
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 23 One or more hospital admission due to exacerbation of asthma (No. of patients).
2.24. Analysis.
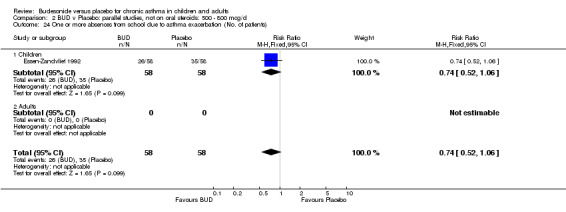
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 24 One or more absences from school due to asthma exacerbation (No. of patients).
2.25. Analysis.
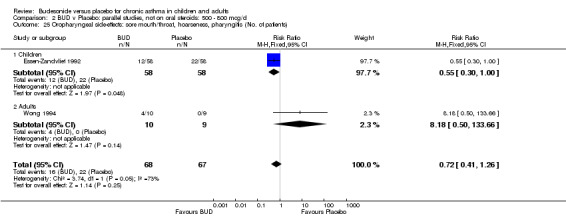
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 25 Oropharyngeal side‐effects: sore mouth/throat, hoarseness, pharyngitis (No. of patients).
2.26. Analysis.
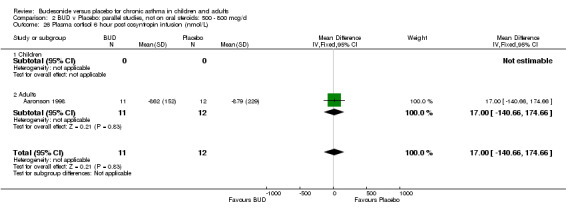
Comparison 2 BUD v Placebo: parallel studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 26 Plasma cortisol 6 hour post cosyntropin infusion (nmol/L).
Comparison 3. BUD v Placebo: parallel studies, not on oral steroids: 1000 mcg/d or greater.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.57, 0.49] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.57, 0.49] |
| 2 Number of night‐time wakenings due to asthma per week | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐4.32, ‐3.08] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐4.32, ‐3.08] |
| 3 Withdrawal due to asthma exacerbation (No. of patients) | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.24] |
| 3.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.24] |
| 4 Requirement for one or more courses or oral steroid for asthma exacerbation (No. of patients) | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.94] |
| 4.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.94] |
| 5 Plasma cortisol 6 hours post cosyntropin infusion (nmol/L) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 82.0 [‐77.11, 241.11] |
| 5.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 82.0 [‐77.11, 241.11] |
3.1. Analysis.
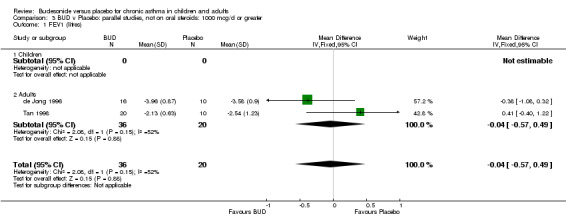
Comparison 3 BUD v Placebo: parallel studies, not on oral steroids: 1000 mcg/d or greater, Outcome 1 FEV1 (litres).
3.2. Analysis.
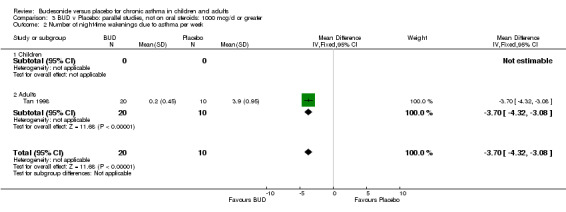
Comparison 3 BUD v Placebo: parallel studies, not on oral steroids: 1000 mcg/d or greater, Outcome 2 Number of night‐time wakenings due to asthma per week.
3.3. Analysis.
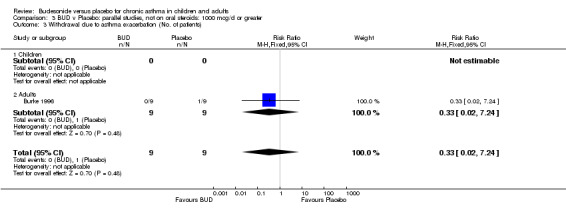
Comparison 3 BUD v Placebo: parallel studies, not on oral steroids: 1000 mcg/d or greater, Outcome 3 Withdrawal due to asthma exacerbation (No. of patients).
3.4. Analysis.
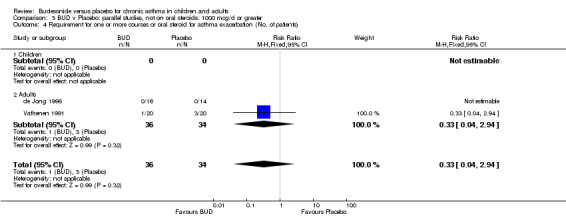
Comparison 3 BUD v Placebo: parallel studies, not on oral steroids: 1000 mcg/d or greater, Outcome 4 Requirement for one or more courses or oral steroid for asthma exacerbation (No. of patients).
3.5. Analysis.
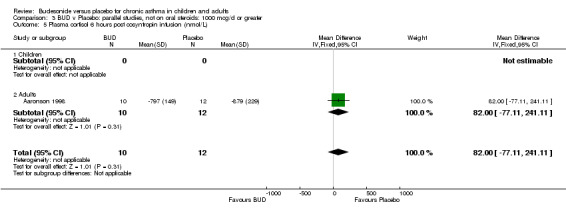
Comparison 3 BUD v Placebo: parallel studies, not on oral steroids: 1000 mcg/d or greater, Outcome 5 Plasma cortisol 6 hours post cosyntropin infusion (nmol/L).
Comparison 4. BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (litres) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.56, 0.64] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.56, 0.64] |
| 2 Daytime cough score | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.80, 0.25] |
| 2.1 Children | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.80, 0.25] |
| 2.2 Adults | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Daytime wheeze score | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.86, 0.20] |
| 3.1 Children | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.86, 0.20] |
| 3.2 Adults | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Breathlessness on exertion score | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.81, 0.25] |
| 4.1 Children | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.81, 0.25] |
| 4.2 Adults | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Methacholine bronchial responsiveness (log 10 PC20FEV1) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.12, 0.22] |
| 5.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.12, 0.22] |
| 6 Maximal % fall in FEV1 post exercise challenge compared to pre‐exercise baseline value | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐30.0 [‐41.14, ‐18.86] |
| 6.1 Children | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐30.0 [‐41.14, ‐18.86] |
| 6.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Largest % fall in FEV1 3‐7 hrs after inhaled allergen (% change compared to baseline) : late allergen response | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐17.1 [‐26.97, ‐7.23] |
| 7.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐17.1 [‐26.97, ‐7.23] |
| 8 Morning plasma cortisol (nmol/L) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐29.0 [‐81.93, 23.93] |
| 8.1 Children | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐29.0 [‐81.93, 23.93] |
| 8.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 24 hour urinary free cortisol excretion (nmol/L) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 11.00 [‐14.19, 36.19] |
| 9.1 Children | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 11.00 [‐14.19, 36.19] |
| 9.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Blood eosinophil count (x10 4 per ml) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐15.37, 27.37] |
| 10.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐15.37, 27.37] |
4.1. Analysis.
Comparison 4 BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 1 FEV1 (litres).
4.2. Analysis.
Comparison 4 BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 2 Daytime cough score.
4.3. Analysis.
Comparison 4 BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 3 Daytime wheeze score.
4.4. Analysis.
Comparison 4 BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 4 Breathlessness on exertion score.
4.5. Analysis.
Comparison 4 BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 5 Methacholine bronchial responsiveness (log 10 PC20FEV1).
4.6. Analysis.
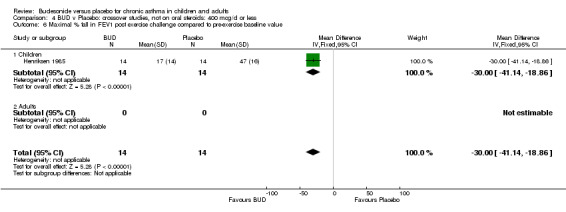
Comparison 4 BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 6 Maximal % fall in FEV1 post exercise challenge compared to pre‐exercise baseline value.
4.7. Analysis.
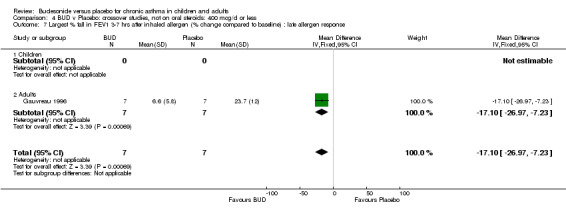
Comparison 4 BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 7 Largest % fall in FEV1 3‐7 hrs after inhaled allergen (% change compared to baseline) : late allergen response.
4.8. Analysis.
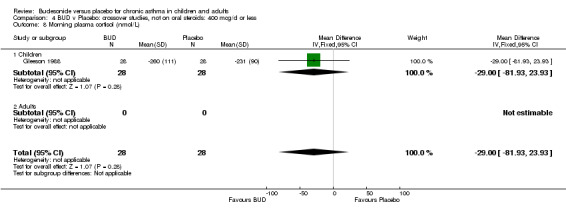
Comparison 4 BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 8 Morning plasma cortisol (nmol/L).
4.9. Analysis.
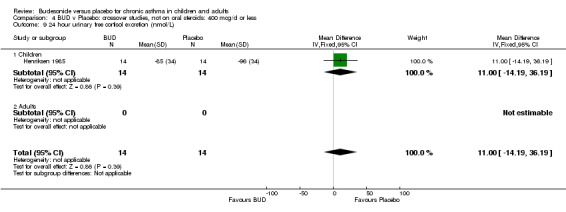
Comparison 4 BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 9 24 hour urinary free cortisol excretion (nmol/L).
4.10. Analysis.
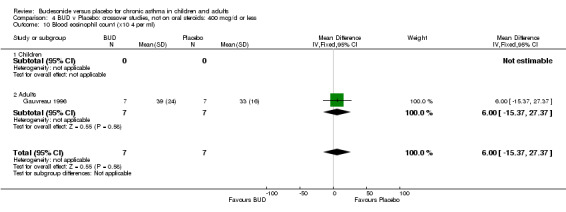
Comparison 4 BUD v Placebo: crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 10 Blood eosinophil count (x10 4 per ml).
Comparison 5. BUD v Placebo: crossover studies, not on oral steroids: 500 ‐ 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐4.74 [‐12.59, 3.11] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐4.74 [‐12.59, 3.11] |
| 2 FEV1 (litres) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.67, 0.51] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.67, 0.51] |
| 3 Daytime cough score | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.34, 0.05] |
| 3.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.34, 0.05] |
| 4 Daytime breathlessness score | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.16, 0.22] |
| 4.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.16, 0.22] |
| 5 Daytime wheeze score | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.43, ‐0.02] |
| 5.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.43, ‐0.02] |
| 6 Nighttime cough score | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Night‐time breathlessness score | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.41, ‐0.01] |
| 7.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.41, ‐0.01] |
| 8 Night‐time wheeze score | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.08 [‐2.15, ‐0.01] |
| 8.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 2 | 34 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.08 [‐2.15, ‐0.01] |
| 9 Histamine bronchial responsiveness (log2 PC20 FEV1) | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐2.58, ‐0.18] |
| 9.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐2.58, ‐0.18] |
5.1. Analysis.
Comparison 5 BUD v Placebo: crossover studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 1 FEV1 (% predicted).
5.2. Analysis.
Comparison 5 BUD v Placebo: crossover studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 2 FEV1 (litres).
5.3. Analysis.
Comparison 5 BUD v Placebo: crossover studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 3 Daytime cough score.
5.4. Analysis.
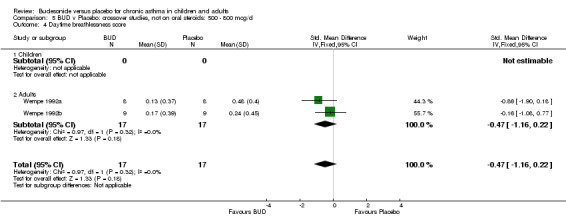
Comparison 5 BUD v Placebo: crossover studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 4 Daytime breathlessness score.
5.5. Analysis.
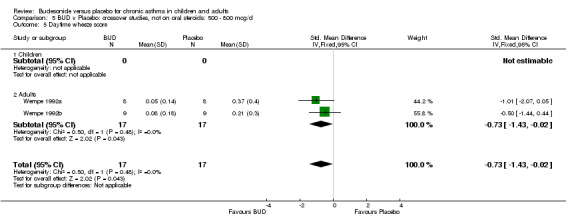
Comparison 5 BUD v Placebo: crossover studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 5 Daytime wheeze score.
5.6. Analysis.
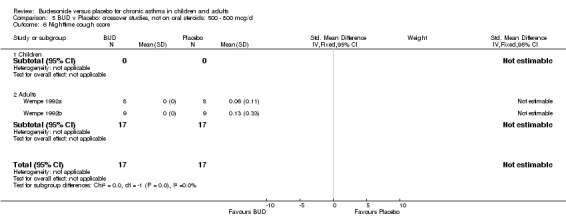
Comparison 5 BUD v Placebo: crossover studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 6 Nighttime cough score.
5.7. Analysis.
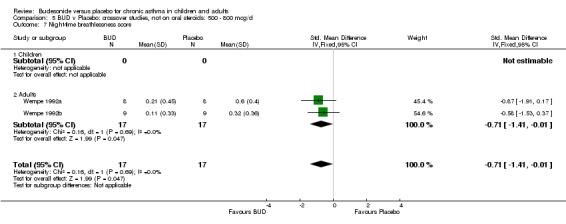
Comparison 5 BUD v Placebo: crossover studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 7 Night‐time breathlessness score.
5.8. Analysis.
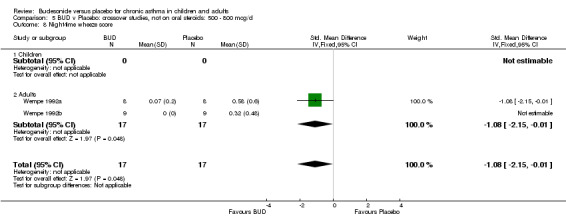
Comparison 5 BUD v Placebo: crossover studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 8 Night‐time wheeze score.
5.9. Analysis.
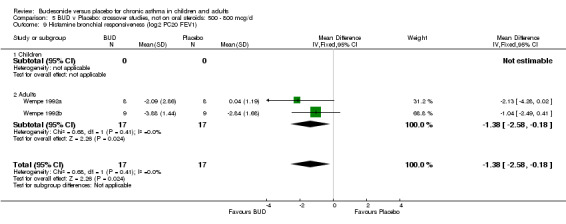
Comparison 5 BUD v Placebo: crossover studies, not on oral steroids: 500 ‐ 800 mcg/d, Outcome 9 Histamine bronchial responsiveness (log2 PC20 FEV1).
Comparison 6. BUD v Placebo: crossover studies, not on oral steroids: 1000 mcg/d or greater.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐17.10, 9.10] |
| 1.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐17.10, 9.10] |
| 2 FEV1 (litres) | 5 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.44, 0.09] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 5 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.44, 0.09] |
| 3 Morning PEFR (L/min) | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐18.90 [‐66.94, 29.14] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐18.90 [‐66.94, 29.14] |
| 4 Evening PEFR (L/min) | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐16.36 [‐45.93, 13.22] |
| 4.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐16.36 [‐45.93, 13.22] |
| 5 Methacholine bronchial responsiveness (log 10 PC20 FEV1) | 3 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.63, ‐0.16] |
| 5.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 3 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.63, ‐0.16] |
| 6 Histamine bronchial responsiveness (log 10 PD35 SGaw) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.08, 0.02] |
| 6.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.08, 0.02] |
| 7 Adenosine monophosphate bronchial responsiveness (log 10 PC20 FEV1) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.46, ‐0.24] |
| 7.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐1.46, ‐0.24] |
| 8 Bradykinin bronchial responsiveness (log 10 PD35 SGaw) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.32, ‐0.02] |
| 8.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.32, ‐0.02] |
| 9 Blood eosinophil count (x10 9/L) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.32, ‐0.10] |
| 9.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.32, ‐0.10] |
6.1. Analysis.
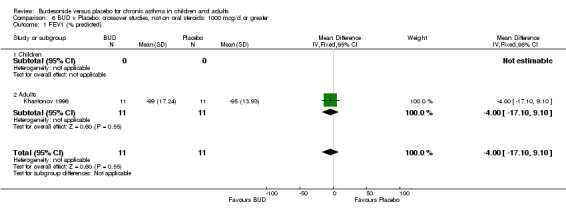
Comparison 6 BUD v Placebo: crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 1 FEV1 (% predicted).
6.2. Analysis.
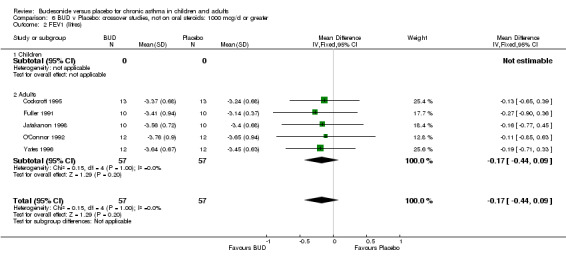
Comparison 6 BUD v Placebo: crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 2 FEV1 (litres).
6.3. Analysis.
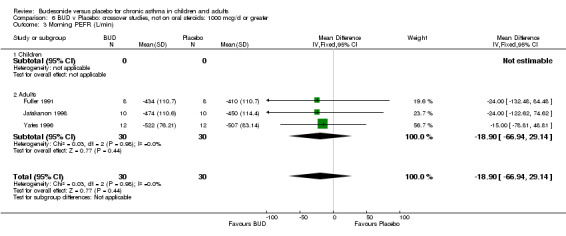
Comparison 6 BUD v Placebo: crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 3 Morning PEFR (L/min).
6.4. Analysis.
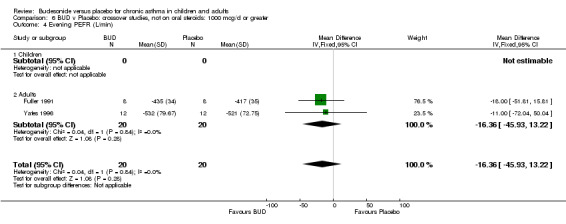
Comparison 6 BUD v Placebo: crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 4 Evening PEFR (L/min).
6.5. Analysis.
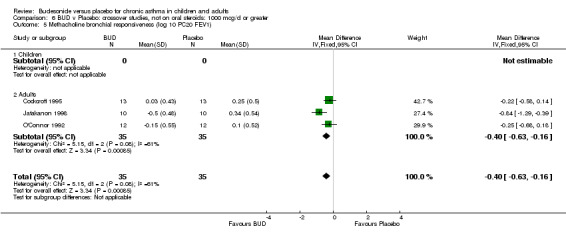
Comparison 6 BUD v Placebo: crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 5 Methacholine bronchial responsiveness (log 10 PC20 FEV1).
6.6. Analysis.
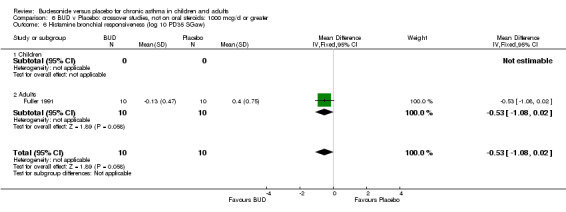
Comparison 6 BUD v Placebo: crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 6 Histamine bronchial responsiveness (log 10 PD35 SGaw).
6.7. Analysis.
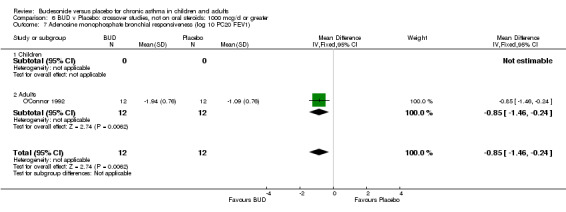
Comparison 6 BUD v Placebo: crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 7 Adenosine monophosphate bronchial responsiveness (log 10 PC20 FEV1).
6.8. Analysis.
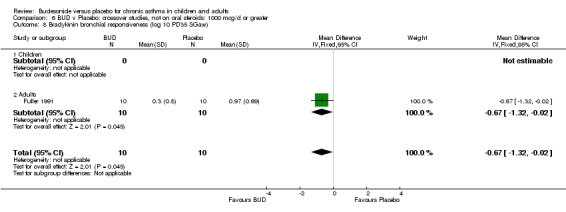
Comparison 6 BUD v Placebo: crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 8 Bradykinin bronchial responsiveness (log 10 PD35 SGaw).
6.9. Analysis.
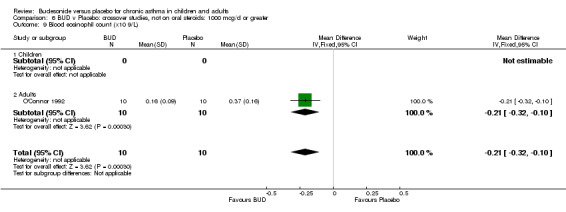
Comparison 6 BUD v Placebo: crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 9 Blood eosinophil count (x10 9/L).
Comparison 7. BUD v Placebo: parallel studies, not on oral steroids: all doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 8 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐7.09, ‐0.31] |
| 1.1 400 mcg/day or less | 3 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐2.09 [‐7.33, 3.16] |
| 1.2 500‐800 mcg/day | 5 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐4.86 [‐9.31, ‐0.42] |
| 1.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (litres) | 4 | 103 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.25, 0.40] |
| 2.1 400 mcg/day or less | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 500‐800 mcg/day | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.27, 0.55] |
| 2.3 1000 mcg/day or greater | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.57, 0.49] |
| 3 Change in FEV1 (litres) compared to baseline | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 3.1 400 mcg/day or less | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 3.2 500‐800 mcg/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Morning PEFR (L/min) | 3 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐30.86 [‐66.51, 4.79] |
| 4.1 400 mcg/day or less | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐29.46 [‐107.88, 48.96] |
| 4.2 500‐800 mcg/day | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐31.22 [‐71.25, 8.80] |
| 4.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Change in morning PEFR (L/min) compared to baseline | 6 | 469 | Mean Difference (IV, Fixed, 95% CI) | ‐28.92 [‐36.11, ‐21.74] |
| 5.1 400 mcg/day or less | 4 | 404 | Mean Difference (IV, Fixed, 95% CI) | ‐26.91 [‐34.42, ‐19.41] |
| 5.2 500‐800 mcg/day | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐51.34 [‐76.38, ‐26.30] |
| 5.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Change in evening PEFR (L/min) compared to baseline | 6 | 469 | Mean Difference (IV, Fixed, 95% CI) | ‐20.64 [‐28.29, ‐13.00] |
| 6.1 400 mcg/day or less | 4 | 404 | Mean Difference (IV, Fixed, 95% CI) | ‐16.03 [‐24.53, ‐7.54] |
| 6.2 500‐800 mcg/day | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐40.33 [‐57.88, ‐22.78] |
| 6.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Change in daytime use of short‐acting beta2 agonist (puffs/daytime) compared to baseline | 5 | 480 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.80, ‐0.06] |
| 7.1 400 mcg/d or less | 5 | 480 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.80, ‐0.06] |
| 7.2 500‐800 mcg/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Change in night‐time use of short‐acting beta2 agonist (puffs/night) compared to baseline | 3 | 304 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.09, ‐0.33] |
| 8.1 400 mcg/day or less | 3 | 304 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.09, ‐0.33] |
| 8.2 500‐800 mcg/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Methacholine bronchial responsiveness (log 10 values) | 3 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.29, 0.21] |
| 9.1 400 mcg/day or less | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.31, 0.39] |
| 9.2 500‐800 mcg/day | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.48, 0.23] |
| 9.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Histamine bronchial responsiveness (log 10 values) | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.70, ‐0.11] |
| 10.1 400 mcg/day or less | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 500‐800 mcg/day | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.70, ‐0.11] |
| 10.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Withdrawal due to asthma exacerbation (No. of patients) | 17 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.09, 0.33] |
| 11.1 400 mcg/day or less | 7 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.08, 0.57] |
| 11.2 500‐800 mcg/day | 9 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.05, 0.35] |
| 11.3 1000 mcg/day or greater | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.24] |
| 12 Requirement for one or more courses of oral steroid for asthma exacerbation (No. of patients) | 5 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.19, 0.59] |
| 12.1 400 mcg/day or less | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.18, 1.40] |
| 12.2 500‐800 mcg/day | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.14, 0.57] |
| 12.3 1000 mcg/day or greater | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.94] |
| 13 Oropharyngeal side effects (No. of patients) | 5 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.23] |
| 13.1 400 mcg/day or less | 3 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.83] |
| 13.2 500‐800 mcg/day | 2 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.41, 1.26] |
| 13.3 1000 mcg/day or greater | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
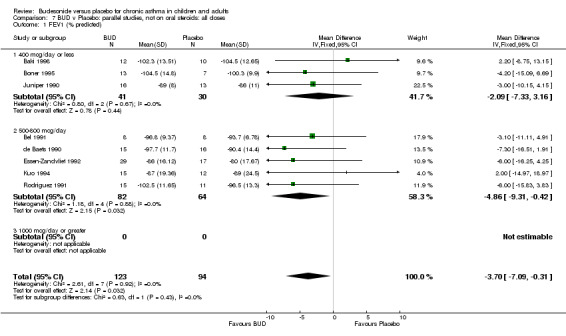
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 1 FEV1 (% predicted).
7.2. Analysis.
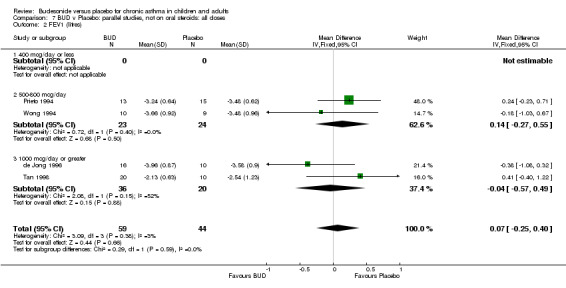
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 2 FEV1 (litres).
7.3. Analysis.
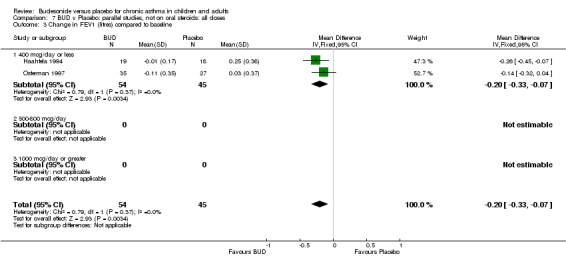
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 3 Change in FEV1 (litres) compared to baseline.
7.4. Analysis.
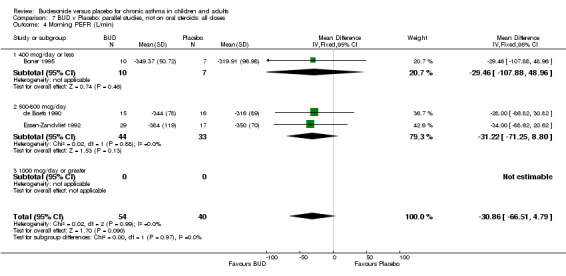
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 4 Morning PEFR (L/min).
7.5. Analysis.
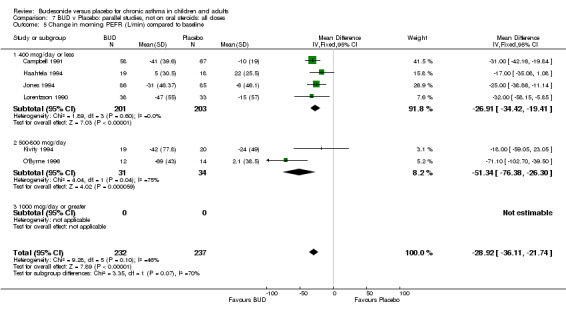
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 5 Change in morning PEFR (L/min) compared to baseline.
7.6. Analysis.
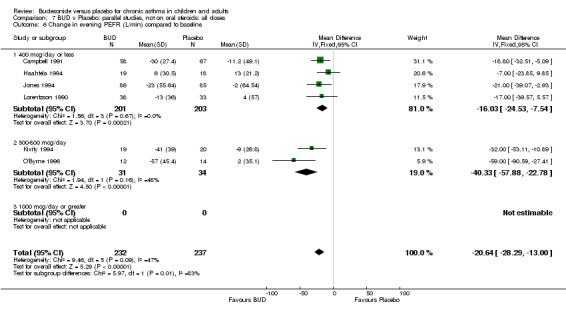
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 6 Change in evening PEFR (L/min) compared to baseline.
7.7. Analysis.
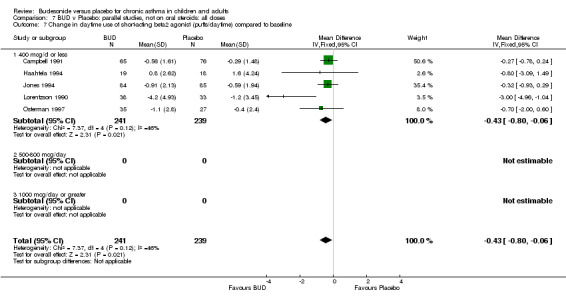
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 7 Change in daytime use of short‐acting beta2 agonist (puffs/daytime) compared to baseline.
7.8. Analysis.
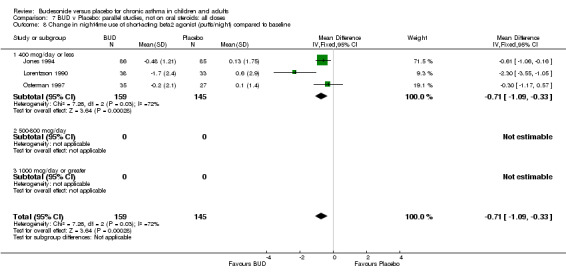
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 8 Change in night‐time use of short‐acting beta2 agonist (puffs/night) compared to baseline.
7.9. Analysis.
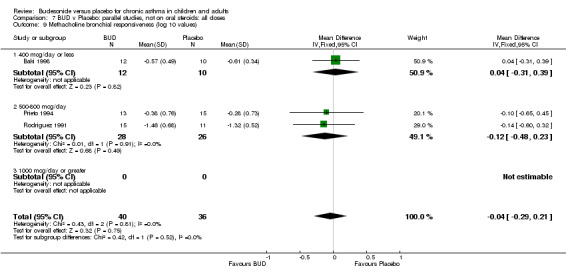
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 9 Methacholine bronchial responsiveness (log 10 values).
7.10. Analysis.
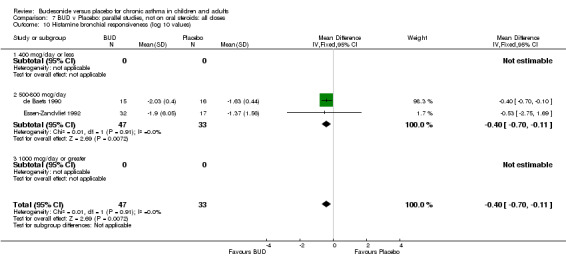
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 10 Histamine bronchial responsiveness (log 10 values).
7.11. Analysis.
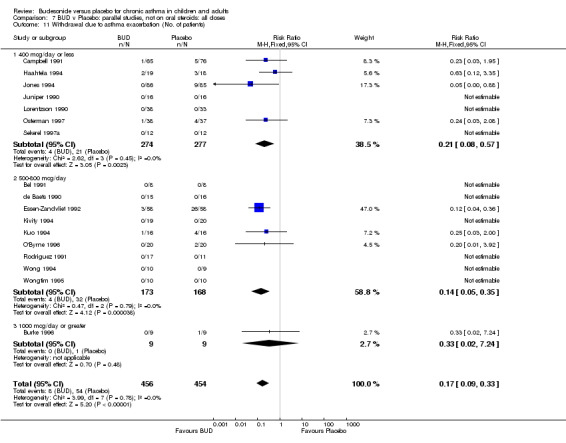
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 11 Withdrawal due to asthma exacerbation (No. of patients).
7.12. Analysis.
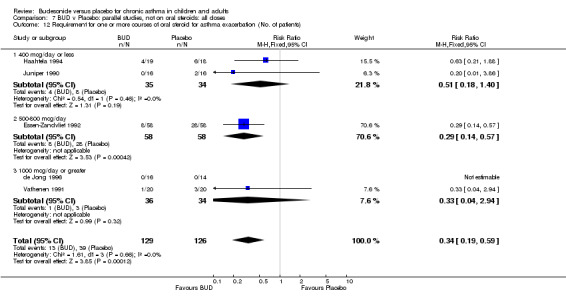
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 12 Requirement for one or more courses of oral steroid for asthma exacerbation (No. of patients).
7.13. Analysis.
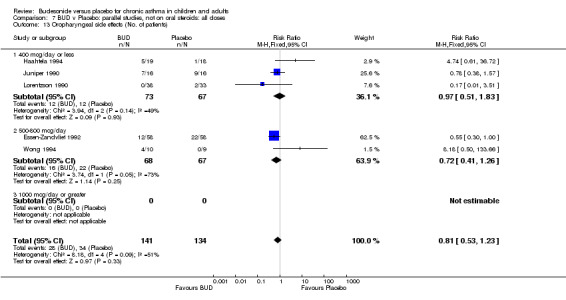
Comparison 7 BUD v Placebo: parallel studies, not on oral steroids: all doses, Outcome 13 Oropharyngeal side effects (No. of patients).
Comparison 8. BUD v Placebo: crossover studies, not on oral steroids: all doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 3 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐4.55 [‐11.28, 2.19] |
| 1.1 400 mcg/day or less | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 500‐800 mcg/day | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐4.74 [‐12.59, 3.11] |
| 1.3 1000 mcg/day or greater | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐17.10, 9.10] |
| 2 FEV1 (litres) | 6 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.40, 0.08] |
| 2.1 400 mcg/day or less | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 500‐800 mcg/day | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.67, 0.51] |
| 2.3 1000 mcg/day or greater | 5 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.44, 0.09] |
| 3 Morning PEFR (L/min) | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐18.90 [‐66.94, 29.14] |
| 3.1 400 mcg/day or less | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 500‐800 mcg/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 1000 mcg/day or greater | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐18.90 [‐66.94, 29.14] |
| 4 Evening PEFR (L/min) | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐16.36 [‐45.93, 13.22] |
| 4.1 400 mcg/day or less | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 500‐800 mcg/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 1000 mcg/day or greater | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐16.36 [‐45.93, 13.22] |
| 5 Methacholine bronchial responsiveness (log 10 values) | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.63, ‐0.18] |
| 5.1 400 mcg/day or less | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.12, 0.22] |
| 5.2 500‐800 mcg/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 1000 mcg/day or greater | 3 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.63, ‐0.16] |
8.1. Analysis.
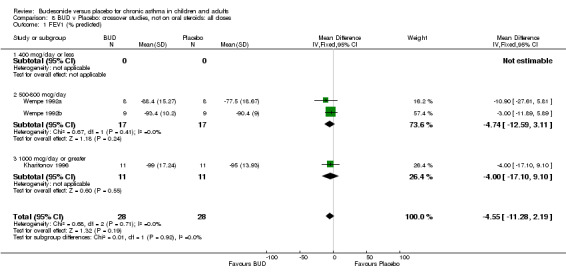
Comparison 8 BUD v Placebo: crossover studies, not on oral steroids: all doses, Outcome 1 FEV1 (% predicted).
8.2. Analysis.
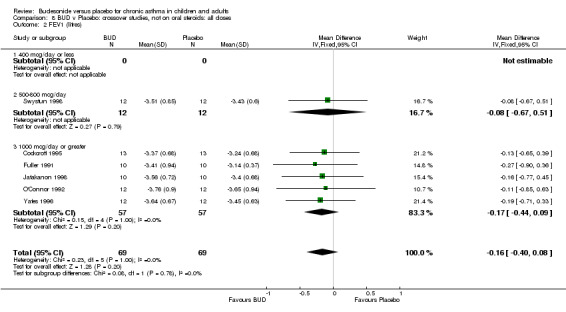
Comparison 8 BUD v Placebo: crossover studies, not on oral steroids: all doses, Outcome 2 FEV1 (litres).
8.3. Analysis.
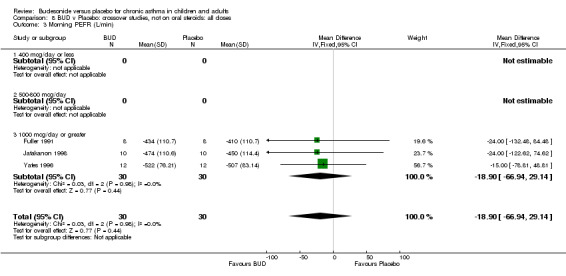
Comparison 8 BUD v Placebo: crossover studies, not on oral steroids: all doses, Outcome 3 Morning PEFR (L/min).
8.4. Analysis.
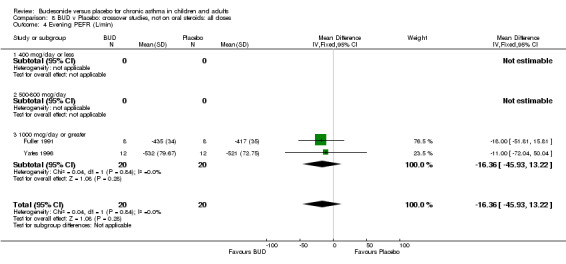
Comparison 8 BUD v Placebo: crossover studies, not on oral steroids: all doses, Outcome 4 Evening PEFR (L/min).
8.5. Analysis.
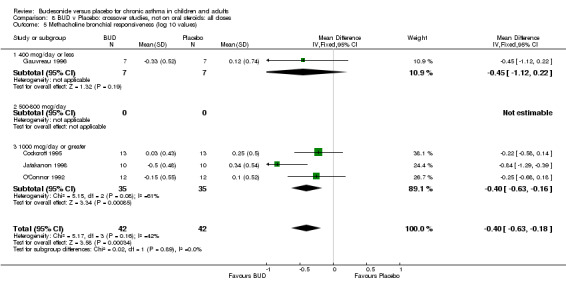
Comparison 8 BUD v Placebo: crossover studies, not on oral steroids: all doses, Outcome 5 Methacholine bronchial responsiveness (log 10 values).
Comparison 9. BUD v Placebo: parallel studies, not on oral steroids: all severities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 7 | 197 | Mean Difference (IV, Fixed, 95% CI) | ‐3.65 [‐7.22, ‐0.08] |
| 1.1 Mild | 5 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐4.09 [‐8.14, ‐0.03] |
| 1.2 Mild to moderate | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐2.17 [‐9.65, 5.31] |
| 1.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (litres) | 4 | 103 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.25, 0.40] |
| 2.1 Mild | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.27, 0.55] |
| 2.2 Mild to moderate | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐1.08, 0.32] |
| 2.3 Moderate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.40, 1.22] |
| 2.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change in FEV1 (litres) compared to baseline | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 3.1 Mild | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 3.2 Mild to moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Morning PEFR (L/min) | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐31.22 [‐71.25, 8.80] |
| 4.1 Mild | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐28.0 [‐86.82, 30.82] |
| 4.2 Mild to moderate | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐34.0 [‐88.62, 20.62] |
| 4.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Change in morning PEFR (L/min) compared to baseline | 6 | 469 | Mean Difference (IV, Fixed, 95% CI) | ‐28.92 [‐36.11, ‐21.74] |
| 5.1 Mild | 4 | 259 | Mean Difference (IV, Fixed, 95% CI) | ‐30.91 [‐39.50, ‐22.32] |
| 5.2 Mild to moderate | 2 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐24.28 [‐37.41, ‐11.15] |
| 5.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Change in evening PEFR (L/min) compared to baseline | 6 | 469 | Mean Difference (IV, Fixed, 95% CI) | ‐20.64 [‐28.29, ‐13.00] |
| 6.1 Mild | 4 | 259 | Mean Difference (IV, Fixed, 95% CI) | ‐18.39 [‐27.60, ‐9.19] |
| 6.2 Mild to moderate | 2 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐25.65 [‐39.38, ‐11.93] |
| 6.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Change in daytime use of short‐acting beta2 agonist (puffs/daytime) compared to baseline | 5 | 480 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.80, ‐0.06] |
| 7.1 Mild | 4 | 311 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.95, ‐0.04] |
| 7.2 Mild to moderate | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.93, 0.29] |
| 7.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Change in night‐time use of short‐acting beta2 agonist compared to baseline (puffs/night) | 3 | 304 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.09, ‐0.33] |
| 8.1 Mild | 2 | 133 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.67, ‐0.24] |
| 8.2 Mild to moderate | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐1.06, ‐0.16] |
| 8.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Methacholine bronchial responsiveness (log 10 values) | 3 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.29, 0.21] |
| 9.1 Mild | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.48, 0.23] |
| 9.2 Mild to moderate | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.31, 0.39] |
| 9.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Histamine bronchial responsiveness (log 10 values) | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.70, ‐0.11] |
| 10.1 Mild | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.70, ‐0.10] |
| 10.2 Mild to moderate | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐2.75, 1.69] |
| 10.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Withdrawal due to asthma exacerbation (No. of patients) | 17 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.09, 0.33] |
| 11.1 Mild | 12 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.13, 0.81] |
| 11.2 Mild to moderate | 4 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.28] |
| 11.3 Moderate | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.4 Moderate to severe | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Requirement for one or more courses of oral steroid for asthma exacerbation (No. of patients) | 5 | 255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.13, 0.48] |
| 12.1 Mild | 3 | 109 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.13, 1.13] |
| 12.2 Mild to moderate | 2 | 146 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.09, 0.44] |
| 12.3 Moderate | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 Moderate to severe | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Oropharyngeal side effects (No. of patients) | 5 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.23] |
| 13.1 Mild | 4 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.69, 2.31] |
| 13.2 Mild to moderate | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.00] |
| 13.3 Moderate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 Moderate to severe | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
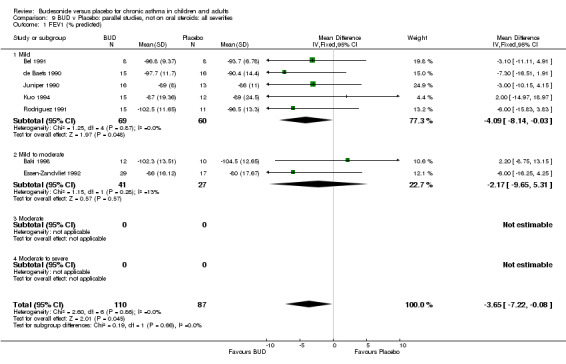
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 1 FEV1 (% predicted).
9.2. Analysis.
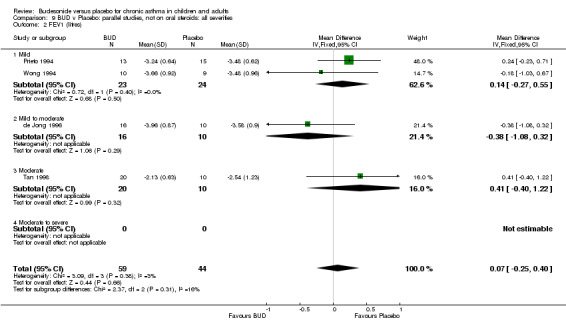
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 2 FEV1 (litres).
9.3. Analysis.
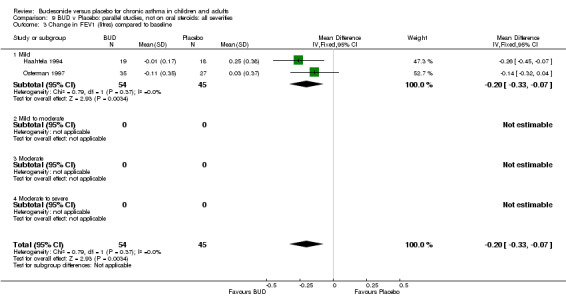
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 3 Change in FEV1 (litres) compared to baseline.
9.4. Analysis.
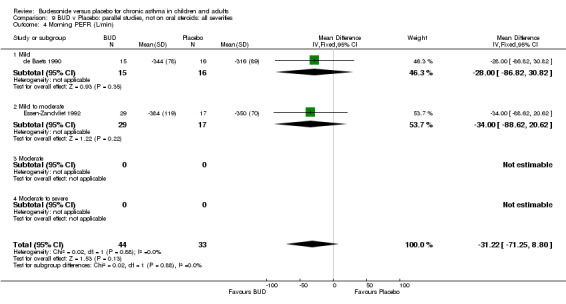
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 4 Morning PEFR (L/min).
9.5. Analysis.
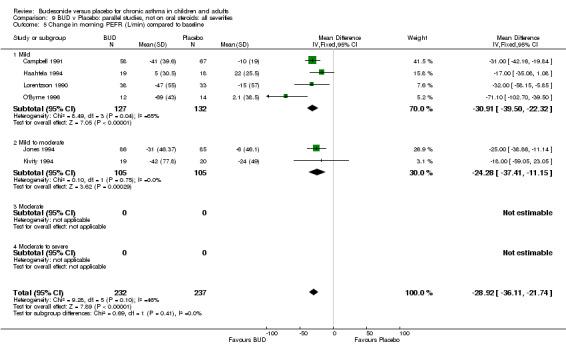
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 5 Change in morning PEFR (L/min) compared to baseline.
9.6. Analysis.
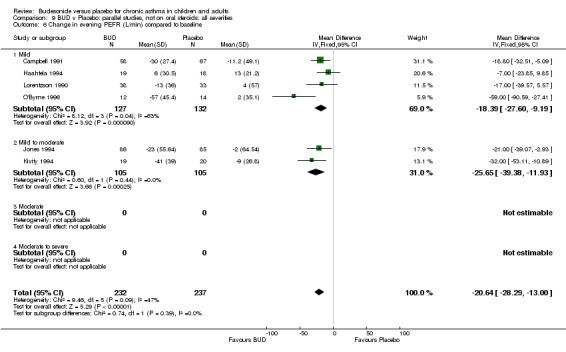
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 6 Change in evening PEFR (L/min) compared to baseline.
9.7. Analysis.
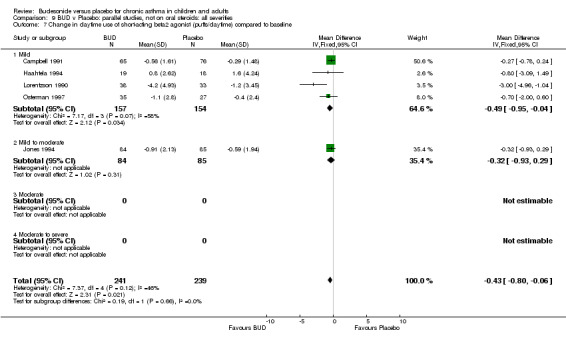
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 7 Change in daytime use of short‐acting beta2 agonist (puffs/daytime) compared to baseline.
9.8. Analysis.
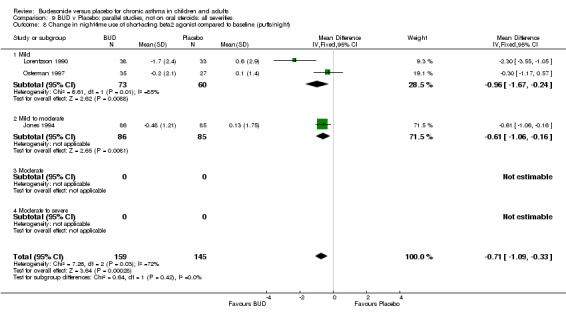
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 8 Change in night‐time use of short‐acting beta2 agonist compared to baseline (puffs/night).
9.9. Analysis.
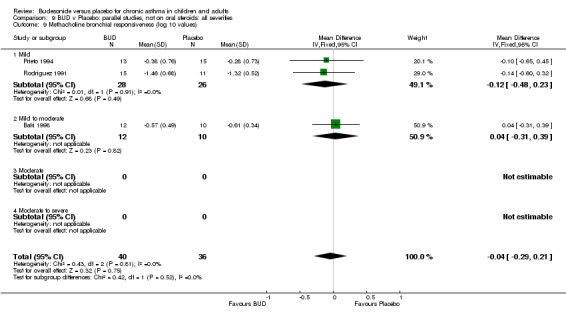
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 9 Methacholine bronchial responsiveness (log 10 values).
9.10. Analysis.
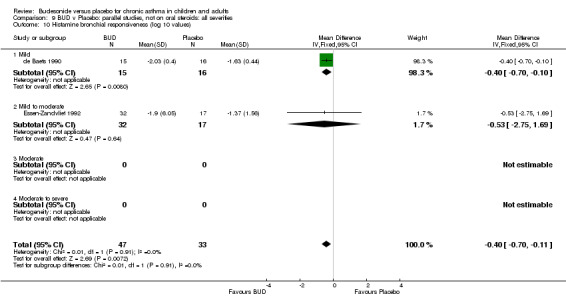
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 10 Histamine bronchial responsiveness (log 10 values).
9.11. Analysis.
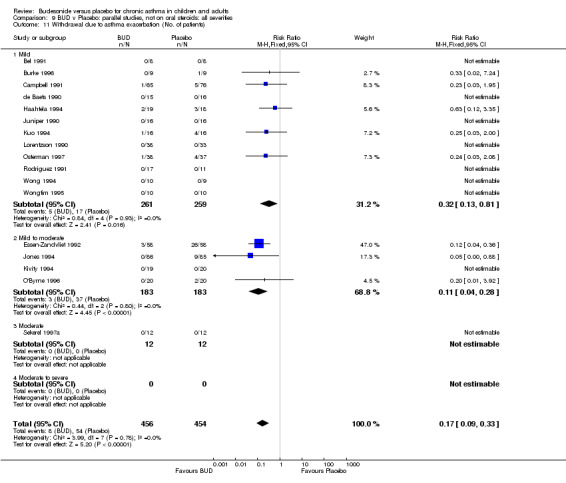
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 11 Withdrawal due to asthma exacerbation (No. of patients).
9.12. Analysis.
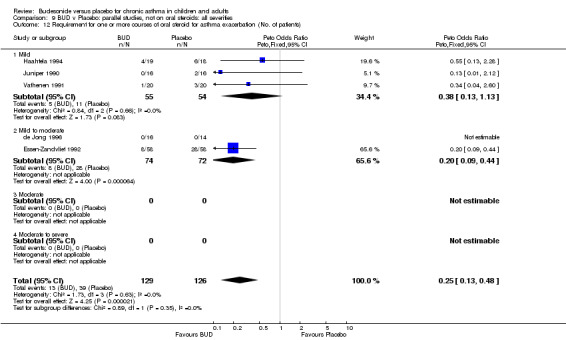
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 12 Requirement for one or more courses of oral steroid for asthma exacerbation (No. of patients).
9.13. Analysis.
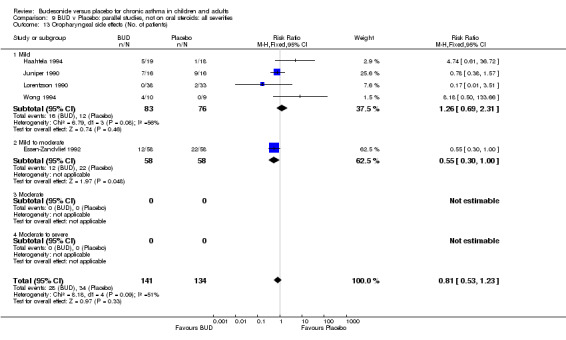
Comparison 9 BUD v Placebo: parallel studies, not on oral steroids: all severities, Outcome 13 Oropharyngeal side effects (No. of patients).
Comparison 10. BUD v Placebo: crossover studies, not on oral steroids: all severities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 3 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐4.55 [‐11.28, 2.19] |
| 1.1 Mild | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐17.10, 9.10] |
| 1.2 Mild to moderate | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐4.74 [‐12.59, 3.11] |
| 1.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (litres) | 6 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.40, 0.08] |
| 2.1 Mild | 5 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.44, 0.09] |
| 2.2 Mild to moderate | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.67, 0.51] |
| 2.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Morning PEFR (L/min) | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐18.90 [‐66.94, 29.14] |
| 3.1 Mild | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐18.90 [‐66.94, 29.14] |
| 3.2 Mild to moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Evening PEFR (L/min) | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐16.36 [‐45.93, 13.22] |
| 4.1 Mild | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐16.36 [‐45.93, 13.22] |
| 4.2 Mild to moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Methacholine bronchial responsiveness (log 10 values) | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.63, ‐0.18] |
| 5.1 Mild | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.63, ‐0.18] |
| 5.2 Mild to moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Moderate | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.
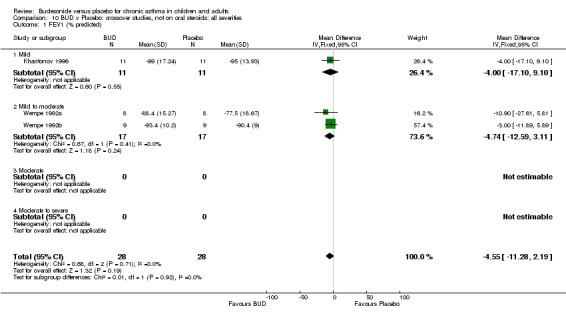
Comparison 10 BUD v Placebo: crossover studies, not on oral steroids: all severities, Outcome 1 FEV1 (% predicted).
10.2. Analysis.
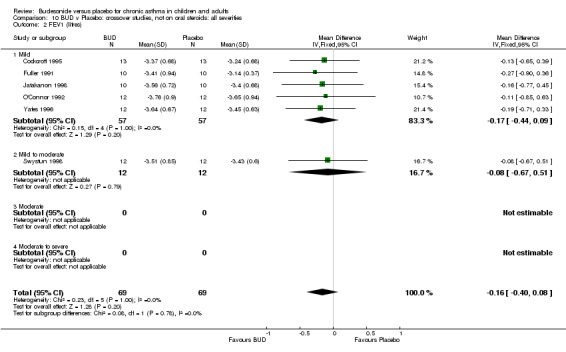
Comparison 10 BUD v Placebo: crossover studies, not on oral steroids: all severities, Outcome 2 FEV1 (litres).
10.3. Analysis.
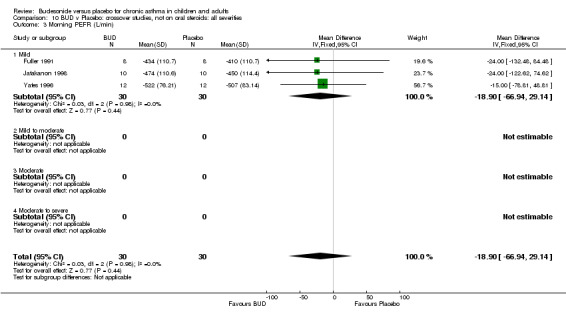
Comparison 10 BUD v Placebo: crossover studies, not on oral steroids: all severities, Outcome 3 Morning PEFR (L/min).
10.4. Analysis.
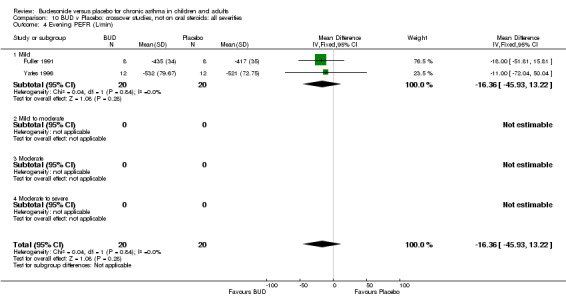
Comparison 10 BUD v Placebo: crossover studies, not on oral steroids: all severities, Outcome 4 Evening PEFR (L/min).
10.5. Analysis.
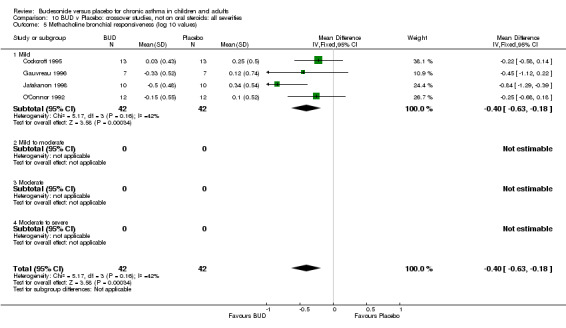
Comparison 10 BUD v Placebo: crossover studies, not on oral steroids: all severities, Outcome 5 Methacholine bronchial responsiveness (log 10 values).
Comparison 11. BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 7 | 195 | Mean Difference (IV, Fixed, 95% CI) | ‐4.33 [‐7.89, ‐0.77] |
| 1.1 MDI | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐13.09, 1.09] |
| 1.2 MDI+SPACER | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐10.26, 1.03] |
| 1.3 DPI | 3 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐8.83, 3.24] |
| 2 FEV1(litres) | 4 | 103 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.25, 0.40] |
| 2.1 MDI | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.40, 1.22] |
| 2.2 MDI+SPACER | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.23, 0.71] |
| 2.3 DPI | 2 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.84, 0.24] |
| 3 Change in FEV1 (litres) compared to baseline | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 3.1 MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 MDI+SPACER | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 DPI | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 4 Morning PEFR (L/min) | 3 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐30.86 [‐66.51, 4.79] |
| 4.1 MDI | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐34.0 [‐88.62, 20.62] |
| 4.2 MDI+SPACER | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐28.0 [‐86.82, 30.82] |
| 4.3 DPI | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐29.46 [‐107.88, 48.96] |
| 5 Change in morning PEFR (L/min) compared to baseline | 6 | 469 | Mean Difference (IV, Fixed, 95% CI) | ‐28.92 [‐36.11, ‐21.74] |
| 5.1 MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 MDI+SPACER | 3 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐42.09 [‐60.18, ‐24.00] |
| 5.3 DPI | 3 | 333 | Mean Difference (IV, Fixed, 95% CI) | ‐26.45 [‐34.29, ‐18.62] |
| 6 Change in evening PEFR (L/min) compared to baseline | 6 | 469 | Mean Difference (IV, Fixed, 95% CI) | ‐20.64 [‐28.29, ‐13.00] |
| 6.1 MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 MDI+SPACER | 3 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐31.54 [‐45.39, ‐17.69] |
| 6.3 DPI | 3 | 333 | Mean Difference (IV, Fixed, 95% CI) | ‐15.88 [‐25.04, ‐6.71] |
| 7 Change in daytime use of short‐acting beta2 agonist (puffs/daytime) compared to baseline | 5 | 480 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.80, ‐0.06] |
| 7.1 MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 MDI+SPACER | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐4.96, ‐1.04] |
| 7.3 DPI | 4 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.71, 0.03] |
| 8 Change in night‐time use of short‐acting beta2 agonist compared to baseline (puffs/night) | 3 | 304 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.09, ‐0.33] |
| 8.1 MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 MDI+SPACER | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐2.3 [‐3.55, ‐1.05] |
| 8.3 DPI | 2 | 233 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.95, ‐0.14] |
| 9 Methacholine bronchial responsiveness (log 10 values) | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.48, 0.23] |
| 9.1 MDI | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.60, 0.32] |
| 9.2 MDI+SPACER | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.65, 0.45] |
| 9.3 DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Histamine bronchial responsiveness (log 10 values) | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.70, ‐0.11] |
| 10.1 MDI | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐2.75, 1.69] |
| 10.2 MDI+SPACER | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.70, ‐0.10] |
| 10.3 DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Withdrawal due to asthma exacerbation (No. of patients) | 17 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.09, 0.33] |
| 11.1 MDI | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.04, 0.36] |
| 11.2 MDI+SPACER | 6 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 11.3 DPI | 9 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.09, 0.53] |
| 12 Requirement for one or more courses of oral steroid for asthma exacerbation (No. of patients) | 5 | 255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.13, 0.48] |
| 12.1 MDI | 1 | 116 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.09, 0.44] |
| 12.2 MDI+SPACER | 2 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.05, 1.26] |
| 12.3 DPI | 2 | 67 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.13, 2.28] |
| 13 Oropharyngeal side effects (No. of patients) | 5 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.23] |
| 13.1 MDI | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.00] |
| 13.2 MDI+SPACER | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.32, 1.29] |
| 13.3 DPI | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.90 [1.14, 30.62] |
11.1. Analysis.
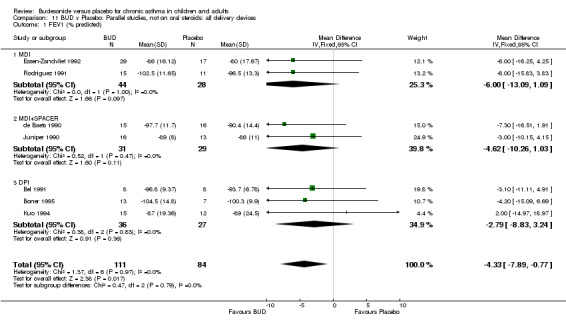
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 1 FEV1 (% predicted).
11.2. Analysis.
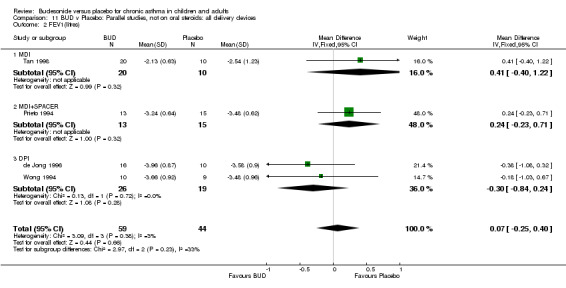
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 2 FEV1(litres).
11.3. Analysis.
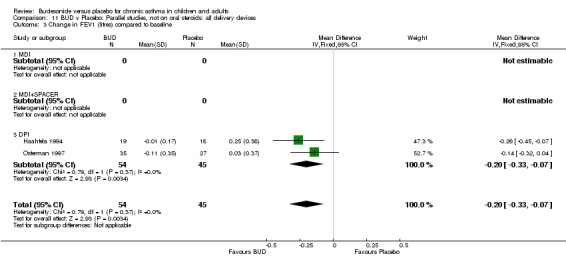
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 3 Change in FEV1 (litres) compared to baseline.
11.4. Analysis.
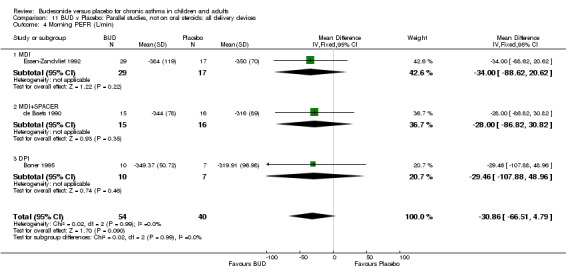
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 4 Morning PEFR (L/min).
11.5. Analysis.
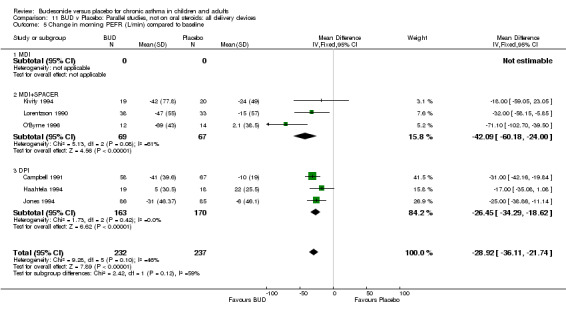
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 5 Change in morning PEFR (L/min) compared to baseline.
11.6. Analysis.
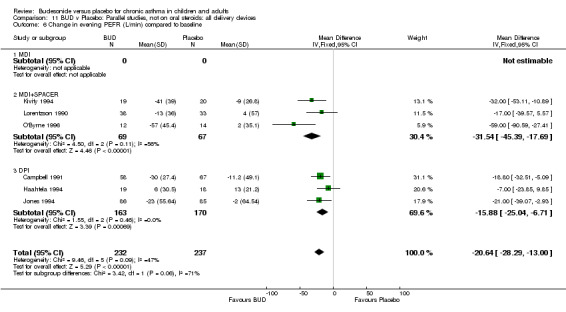
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 6 Change in evening PEFR (L/min) compared to baseline.
11.7. Analysis.
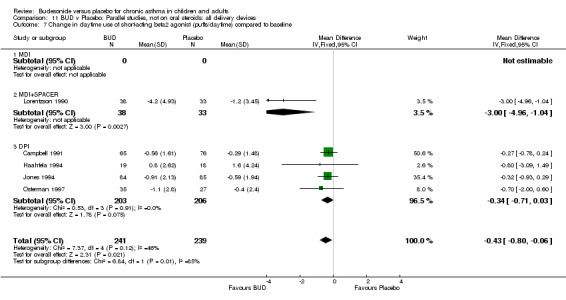
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 7 Change in daytime use of short‐acting beta2 agonist (puffs/daytime) compared to baseline.
11.8. Analysis.
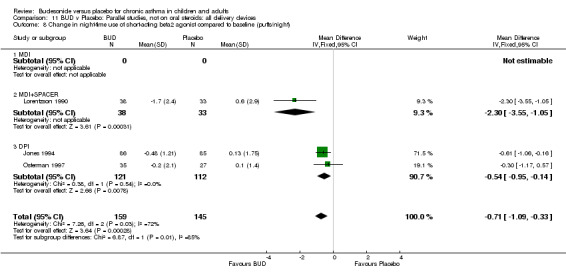
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 8 Change in night‐time use of short‐acting beta2 agonist compared to baseline (puffs/night).
11.9. Analysis.
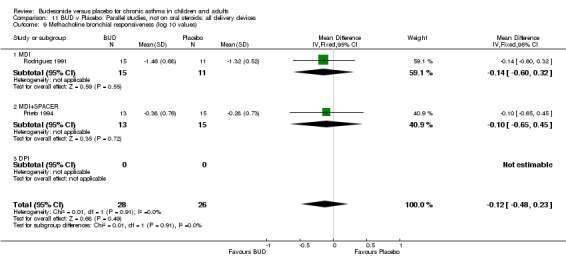
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 9 Methacholine bronchial responsiveness (log 10 values).
11.10. Analysis.
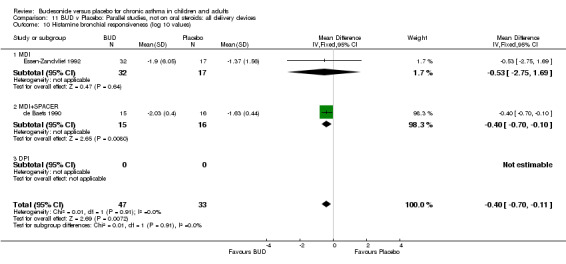
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 10 Histamine bronchial responsiveness (log 10 values).
11.11. Analysis.
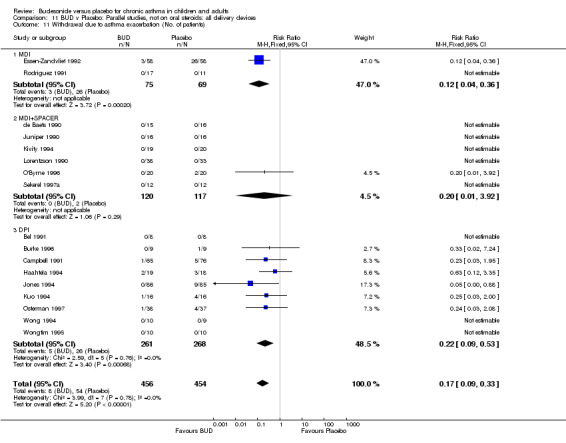
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 11 Withdrawal due to asthma exacerbation (No. of patients).
11.12. Analysis.
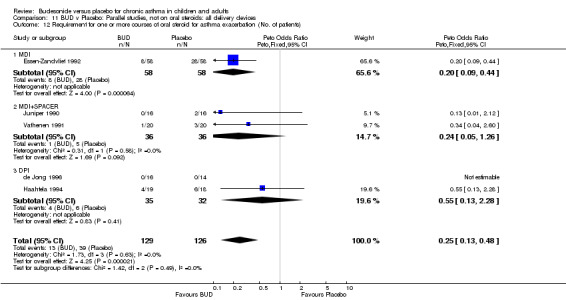
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 12 Requirement for one or more courses of oral steroid for asthma exacerbation (No. of patients).
11.13. Analysis.
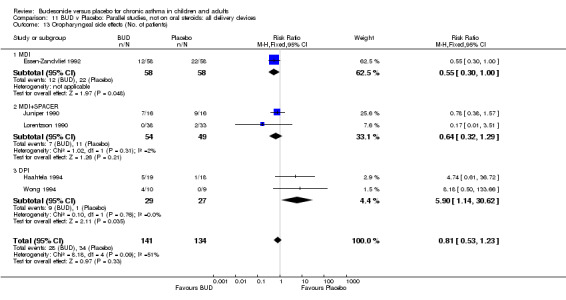
Comparison 11 BUD v Placebo: Parallel studies, not on oral steroids: all delivery devices, Outcome 13 Oropharyngeal side effects (No. of patients).
Comparison 12. BUD v Placebo: Crossover studies, not on oral steroids: all delivery devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 3 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐4.55 [‐11.28, 2.19] |
| 1.1 MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 MDI+SPACER | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 DPI | 3 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐4.55 [‐11.28, 2.19] |
| 2 FEV1 (litres) | 6 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.40, 0.08] |
| 2.1 MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 MDI+SPACER | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.90, 0.36] |
| 2.3 DPI | 5 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.40, 0.12] |
| 3 Morning PEFR (L/min) | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐18.90 [‐66.94, 29.14] |
| 3.1 MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 MDI+SPACER | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐24.0 [‐132.48, 84.48] |
| 3.3 DPI | 2 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐17.66 [‐71.23, 35.92] |
| 4 Evening PEFR (L/min) | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐16.36 [‐45.93, 13.22] |
| 4.1 MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 MDI+SPACER | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐18.0 [‐51.81, 15.81] |
| 4.3 DPI | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐72.04, 50.04] |
| 5 Methacholine bronchial responsiveness (log 10 values) | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.63, ‐0.18] |
| 5.1 MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 MDI+SPACER | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 DPI | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.63, ‐0.18] |
12.1. Analysis.
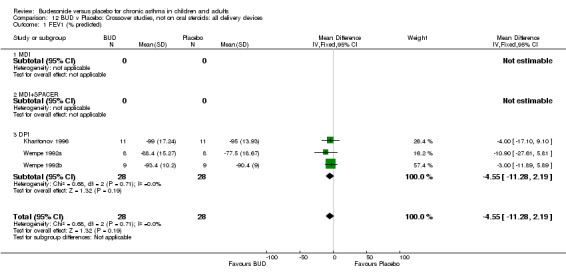
Comparison 12 BUD v Placebo: Crossover studies, not on oral steroids: all delivery devices, Outcome 1 FEV1 (% predicted).
12.2. Analysis.
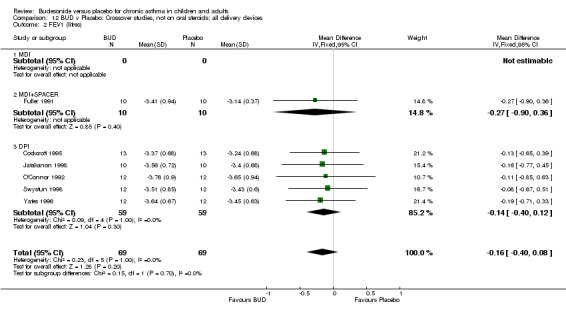
Comparison 12 BUD v Placebo: Crossover studies, not on oral steroids: all delivery devices, Outcome 2 FEV1 (litres).
12.3. Analysis.
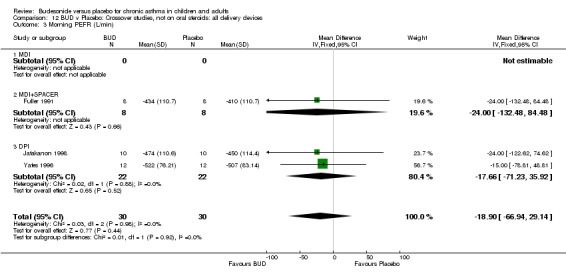
Comparison 12 BUD v Placebo: Crossover studies, not on oral steroids: all delivery devices, Outcome 3 Morning PEFR (L/min).
12.4. Analysis.
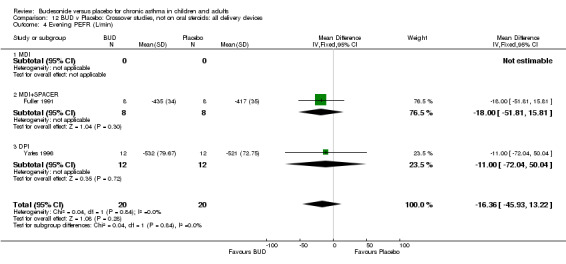
Comparison 12 BUD v Placebo: Crossover studies, not on oral steroids: all delivery devices, Outcome 4 Evening PEFR (L/min).
12.5. Analysis.
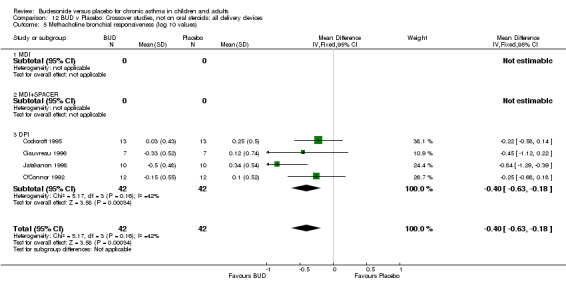
Comparison 12 BUD v Placebo: Crossover studies, not on oral steroids: all delivery devices, Outcome 5 Methacholine bronchial responsiveness (log 10 values).
Comparison 13. BUD v Placebo: Parallel studies, not on oral steroids: all study durations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 8 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐7.09, ‐0.31] |
| 1.1 1‐4 weeks | 3 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐6.88, 5.20] |
| 1.2 1‐5 months | 3 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐6.00 [‐11.72, ‐0.29] |
| 1.3 6 months or greater | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐3.98 [‐9.85, 1.88] |
| 2 FEV1 (litres) | 4 | 103 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.25, 0.40] |
| 2.1 1‐4 weeks | 3 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.53, 0.37] |
| 2.2 1‐5 months | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.23, 0.71] |
| 2.3 6 months or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change in FEV1 (litres) compared to baseline | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 3.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 6 months or greater | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 4 Morning PEFR (L/min) | 3 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐30.86 [‐66.51, 4.79] |
| 4.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 1‐5 months | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐28.53 [‐75.58, 18.53] |
| 4.3 6 months or greater | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐34.0 [‐88.62, 20.62] |
| 5 Change in morning PEFR (L/min) compared to baseline | 6 | 469 | Mean Difference (IV, Fixed, 95% CI) | ‐28.92 [‐36.11, ‐21.74] |
| 5.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 1‐5 months | 5 | 432 | Mean Difference (IV, Fixed, 95% CI) | ‐31.16 [‐37.00, ‐23.33] |
| 5.3 6 months or greater | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐17.0 [‐35.08, 1.08] |
| 6 Change in evening PEFR (L/min) compared to baseline | 6 | 469 | Mean Difference (IV, Fixed, 95% CI) | ‐20.64 [‐28.29, ‐13.00] |
| 6.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 1‐5 months | 5 | 432 | Mean Difference (IV, Fixed, 95% CI) | ‐24.18 [‐32.76, ‐15.60] |
| 6.3 6 months or greater | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐23.85, 9.85] |
| 7 Change in daytime use of short‐acting beta2 agonist (puffs/daytime) compared to baseline | 5 | 480 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.80, ‐0.06] |
| 7.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 1‐5 months | 3 | 381 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.78, ‐0.01] |
| 7.3 6 months or greater | 2 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.85, 0.40] |
| 8 Change in night‐time use of short‐acting beta2 agonist (puffs/night) compared to baseline | 3 | 304 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.09, ‐0.33] |
| 8.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 1‐5 months | 2 | 242 | Mean Difference (IV, Fixed, 95% CI) | ‐0.81 [‐1.23, ‐0.38] |
| 8.3 6 months or greater | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.17, 0.57] |
| 9 Methacholine bronchial responsiveness (log 10 values) | 3 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.29, 0.21] |
| 9.1 1‐4 weeks | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.30, 0.25] |
| 9.2 1‐5 months | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.65, 0.45] |
| 9.3 6 months or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Histamine bronchial responsiveness (log 10 values) | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.70, ‐0.11] |
| 10.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 1‐5 months | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.70, ‐0.10] |
| 10.3 6 months or greater | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐2.75, 1.69] |
| 11 Withdrawal due to asthma exacerbation (No. of patients) | 17 | 910 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.09, 0.33] |
| 11.1 1‐4 weeks | 3 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.00] |
| 11.2 1‐5 months | 10 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.52] |
| 11.3 6 months or greater | 4 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.08, 0.41] |
| 12 Requirement for one or more courses of oral steroid for asthma exacerbation (No. of patients) | 5 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.19, 0.59] |
| 12.1 1‐4 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 1‐5 months | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.94] |
| 12.3 6 months or greater | 3 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.19, 0.60] |
| 13 Oropharyngeal side effects (No. of patients) | 5 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.23] |
| 13.1 1‐4 weeks | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.18 [0.50, 133.66] |
| 13.2 1‐5 months | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.51] |
| 13.3 6 months or greater | 3 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.48, 1.16] |
13.1. Analysis.
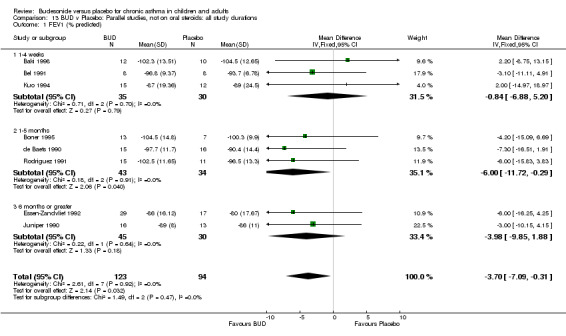
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 1 FEV1 (% predicted).
13.2. Analysis.
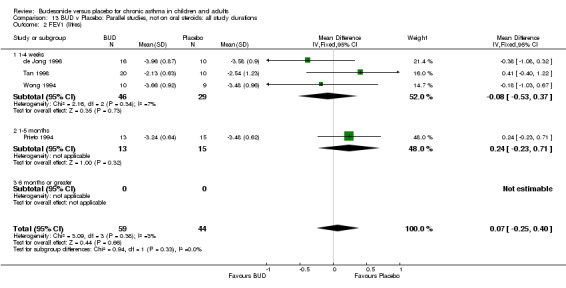
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 2 FEV1 (litres).
13.3. Analysis.
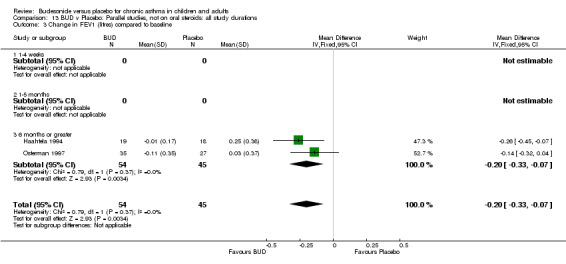
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 3 Change in FEV1 (litres) compared to baseline.
13.4. Analysis.
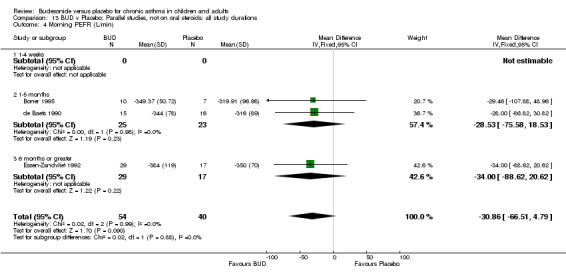
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 4 Morning PEFR (L/min).
13.5. Analysis.
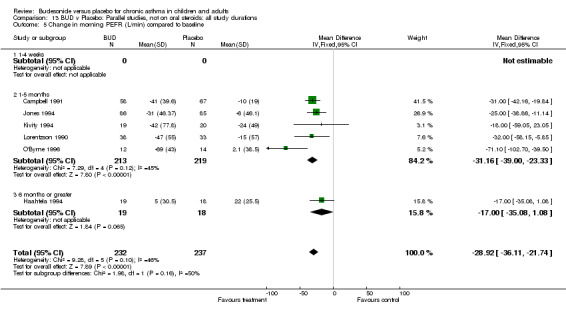
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 5 Change in morning PEFR (L/min) compared to baseline.
13.6. Analysis.
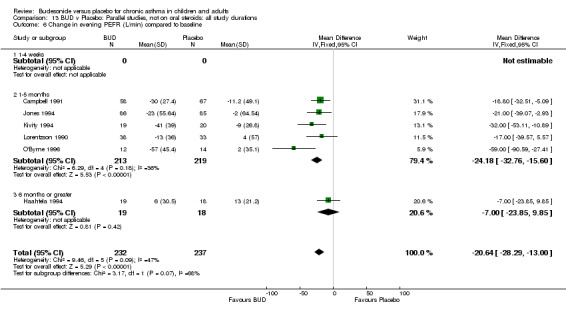
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 6 Change in evening PEFR (L/min) compared to baseline.
13.7. Analysis.
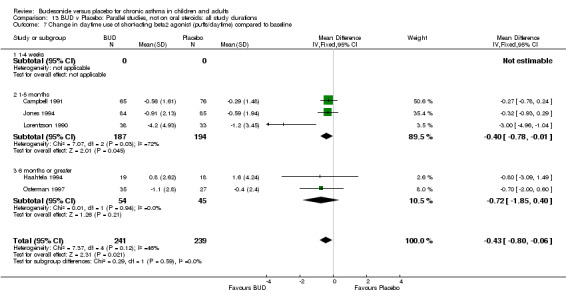
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 7 Change in daytime use of short‐acting beta2 agonist (puffs/daytime) compared to baseline.
13.8. Analysis.
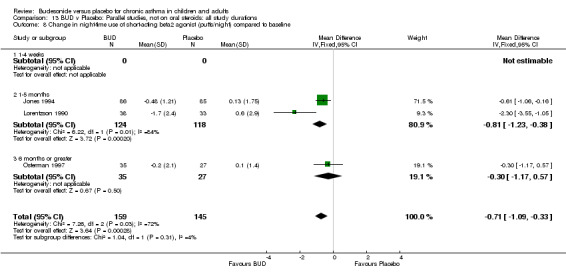
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 8 Change in night‐time use of short‐acting beta2 agonist (puffs/night) compared to baseline.
13.9. Analysis.
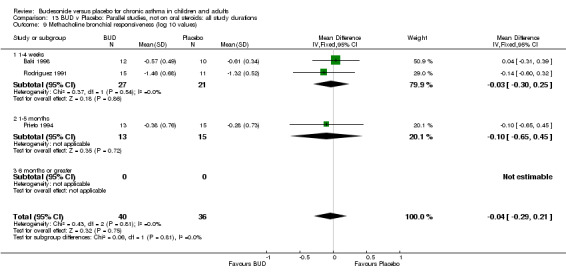
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 9 Methacholine bronchial responsiveness (log 10 values).
13.10. Analysis.
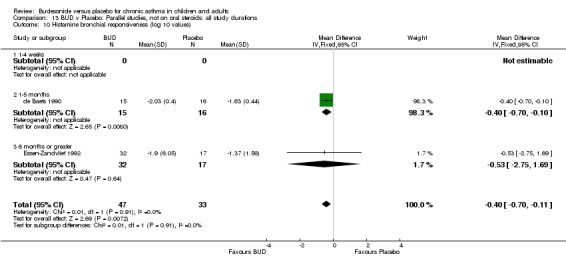
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 10 Histamine bronchial responsiveness (log 10 values).
13.11. Analysis.
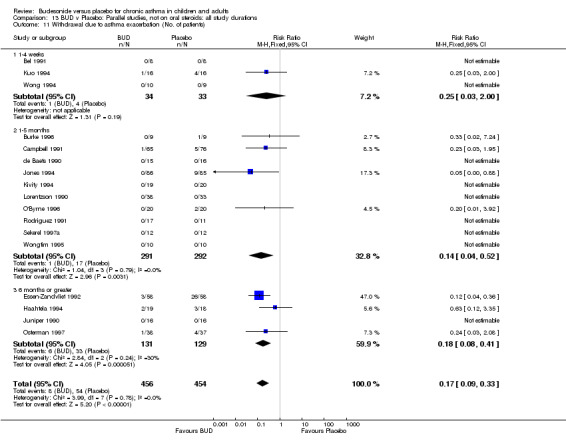
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 11 Withdrawal due to asthma exacerbation (No. of patients).
13.12. Analysis.
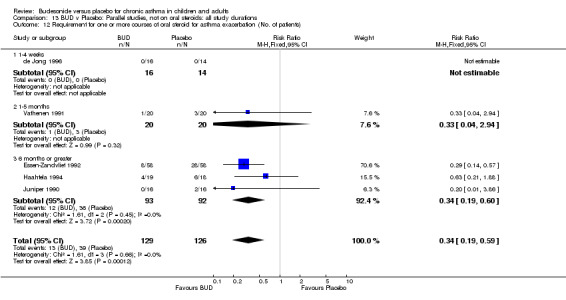
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 12 Requirement for one or more courses of oral steroid for asthma exacerbation (No. of patients).
13.13. Analysis.
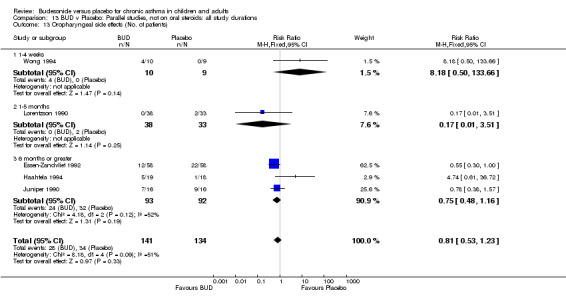
Comparison 13 BUD v Placebo: Parallel studies, not on oral steroids: all study durations, Outcome 13 Oropharyngeal side effects (No. of patients).
Comparison 14. BUD v Placebo: Crossover studies, not on oral steroids: all study durations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 3 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐4.55 [‐11.28, 2.19] |
| 1.1 1‐4 weeks | 3 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐4.55 [‐11.28, 2.19] |
| 1.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 6 months or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (litres) | 6 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.40, 0.08] |
| 2.1 1‐4 weeks | 6 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.40, 0.08] |
| 2.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 6 months or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Morning PEFR (L/min) | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐18.90 [‐66.94, 29.14] |
| 3.1 1‐4 weeks | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐18.90 [‐66.94, 29.14] |
| 3.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 6 months or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Evening PEFR (L/min) | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐16.36 [‐45.93, 13.22] |
| 4.1 1‐4 weeks | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐16.36 [‐45.93, 13.22] |
| 4.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 6 months or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Methacholine bronchial responsiveness (log 10 values) | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.63, ‐0.18] |
| 5.1 1‐4 weeks | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.63, ‐0.18] |
| 5.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 6 months or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.1. Analysis.
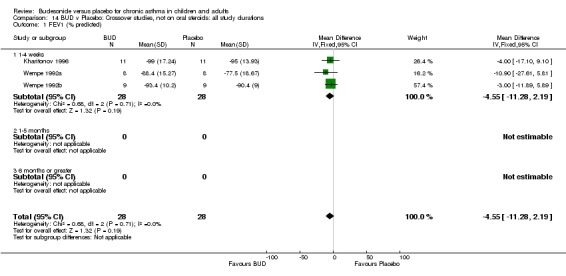
Comparison 14 BUD v Placebo: Crossover studies, not on oral steroids: all study durations, Outcome 1 FEV1 (% predicted).
14.2. Analysis.
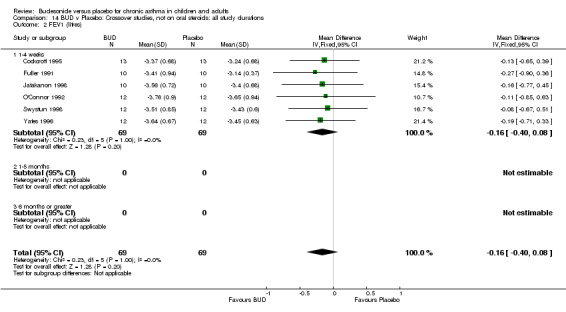
Comparison 14 BUD v Placebo: Crossover studies, not on oral steroids: all study durations, Outcome 2 FEV1 (litres).
14.3. Analysis.
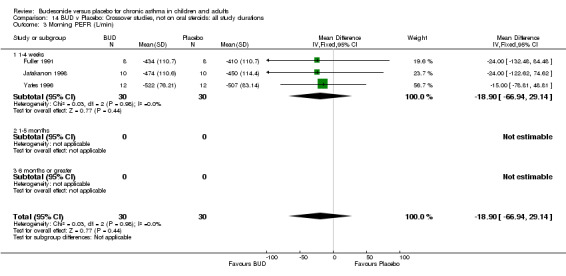
Comparison 14 BUD v Placebo: Crossover studies, not on oral steroids: all study durations, Outcome 3 Morning PEFR (L/min).
14.4. Analysis.
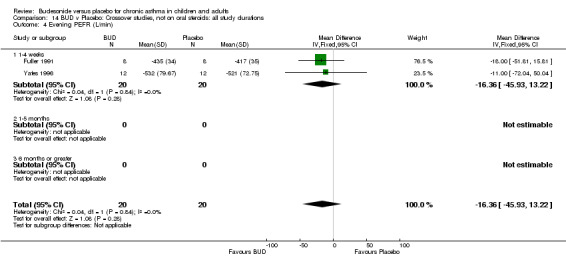
Comparison 14 BUD v Placebo: Crossover studies, not on oral steroids: all study durations, Outcome 4 Evening PEFR (L/min).
14.5. Analysis.
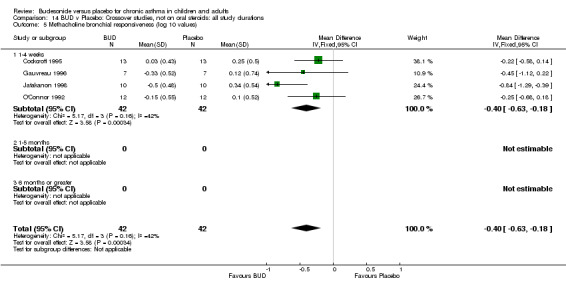
Comparison 14 BUD v Placebo: Crossover studies, not on oral steroids: all study durations, Outcome 5 Methacholine bronchial responsiveness (log 10 values).
Comparison 15. PAPER Figure 2b.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 8 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐7.09, ‐0.31] |
| 1.1 Children | 4 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐4.21 [‐9.33, 0.92] |
| 1.2 Adults | 4 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐3.31 [‐7.83, 1.21] |
15.1. Analysis.
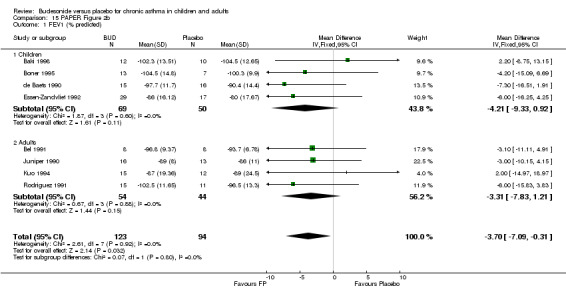
Comparison 15 PAPER Figure 2b, Outcome 1 FEV1 (% predicted).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aaronson 1998.
| Methods | Setting: multicentre study, USA, hospital outpatient clinics Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 64 adults: 49M 15F Age range: 18 ‐ 56 years Inclusion criteria: Adults patients with diagnosis of asthma (not otherwise defined) Aged 18‐65 years FEV1 (% predicted) 65 or greater Able to use Turbuhaler effectively and achieve inspiratory flow rate of at least 50 L/min Exclusion criteria: Hospitalisation due to asthma or respiratory tract infection in last 4 weeks Other significant disease Any corticosteroid therapy in last 6 months Significantly abnormal cortisol levels (basal or post cosyntropin) | |
| Interventions | BUD:
200 mcg 2 pfs 2xdaily (800 mcg/d)
400 mcg 2pfs 2xdaily (1600 mcg/d)
800 mcg 2 pfs 2xdaily (3200 mcg/d) Placebo: 2 pfs 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | Plasma cortisol at 6 hours following continuous iv cosyntropin infusion, 0.25 mg/500 ml saline over 6 hours (nmol/L) Basal plasma cortisol (nmol/L) | |
| Notes | No reply from author to clarify details of randomisation method. Prednisolone treatment arm included in study: results not considered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Agertoft 1997.
| Methods | Setting: Denmark, paediatric outpatient clinic Randomisation: yes, computerised scheme prepared in balanced blocks Allocation concealment: unclear Design: crossover, 2 week washout Intervention period: 2 weeks Masking: double blind Excluded: none Withdrawals: stated Baseline characteristics: comparable Jadad score=4 | |
| Participants | 48 children: 27M 21F Age range: 6 to 12 years Inclusion criteria: Clinical diagnosis of mild asthma requiring treatment with beta2 agonists only Exclusion criteria: Use of oral or inhaled steroids | |
| Interventions | BUD:
1. 200 mcg/d
2. 400 mcg/d Placebo Delivery device: Turbohaler DPI |
|
| Outcomes | Knemometry 24 urinary free cortisol excretion | |
| Notes | This study also included treatment arms with fluticasone 200 mcg/d and 400 mcg/d: results not considered in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Baki 1998.
| Methods | Setting: Turkey, paediatric outpatient clinic Length of intervention period: 3 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable FEV1 and methacholine bronchial responsiveness but no other demographic data presented Jadad score=3 | |
| Participants | 43 children: 22M 21F Age range: 7‐17 years Inclusion criteria: Diagnosis of mild to moderate asthma Age 7‐17 years FEV (% predicted) > 70 Methacholine BHR (PC20 FEV1) < 12 mg/ml Exclusion criteria: Respiratory tract infection within last 4 weeks | |
| Interventions | BUD: 200 mcg 2xdaily (400 mcg/d) Placebo: 2xdaily, device not stated Delivery device: not stated |
|
| Outcomes | FEV1(% predicted) Methacholine BHR (PC20 FEV1) | |
| Notes | No reply from author to clarify details of randomisation method or delivery device. Individual patient methacholine challenge data was estimated from graph and log transformed. Nedocromil and salmeterol treatment arms were also used: results not considered in this meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bel 1991.
| Methods | Setting: The Netherlands, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 4 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 16 patients: 8M 8F. Age range: 19‐34 years Inclusion criteria: History of episodic wheezing and dyspnoea, positive skin prick test to one or more airborne allergens. FEV1 (% predicted) > 80 and methacholine BHR (PC20 FEV1) 1‐7 mg/ml Symptoms controlled with bronchodilators only Exclusion criteria: Prior use of oral or inhaled corticosteroids | |
| Interventions | BUD: 200 mcg 2pfs 2xdaily (800 mcg/d) Placebo: 2 pfs 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | FEV1 (% predicted) Methacholine BHR (PC20 FEV1) Withdrawal due to asthma exacerbation | |
| Notes | Reply from author but unable to clarify details of randomisation method. Unclear whether methacholine challenge data log transformed prior to analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Boner 1995.
| Methods | Setting: Italy, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 3 months at low altitude, 2 weeks at high altitude Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 20 children completed the study: 13M 7F Age range 6‐14 years Inclusion criteria: Clinical history of asthma Positive skin allergen test to house dust mite or grass pollen FEV1 (% predicted) > 75 Methacholine BHR (PC20 FEV1) < 10mg/ml Exclusion criteria: Respiratory infection during the previous 4 weeks Significant disease other than asthma or related allergic diseases Use of oral, inhaled or injectable steroids during previous 3 months | |
| Interventions | BUD: 200 mcg 1pf 2xdaily (400mcg/d) Placebo: 1 pf 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | FEV1 (% predicted) FVC (% predicted) FEF 25‐75 (% predicted) Change in morning PEFR (% predicted) compared to baseline Change in evening PEFR (% predicted) compared to baseline Methacholine BHR (PC20 FEV1) Daily asthma symptom score | |
| Notes | Reply form authors who provided numerical data for clinic spirometry, methacholine challenge and diary card PEFR. Authors did not clarify whether allocation concealment was employed in randomisation procedure. Study comprised two parts: an initial period at high altitude avoiding allergen exposure where patients were treated for 2 weeks and a second period at low altitude when patients were treated for a further 3 months. The data included in the meta‐analysis is for the second period at low altitude. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Burke 1996.
| Methods | Setting: Ireland, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 6 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: wide range in FEV1 and PEFR (%predicted) Jadad score=3 | |
| Participants | 18 subjects: 16M 2F Age range: 19‐45 years Inclusion criteria: Clinical diagnosis of asthma (ATS criteria) Lifelong non‐smokers Clinically stable for at least 2 months prior to study No symptoms or signs of respiratory tract infection Beta2 agonist treatment only Only patients who 'reported symptoms of asthma' and required extra terbutaline in addition to 500 mcg twice daily during run‐in period Exclusion criteria: Other significant illness | |
| Interventions | BUD: 800 mcg 1 pf 2xdaily (1600 mcg/d) Placebo: 1 pf 2xdaily Delivery device: Turbohaler DPI |
|
| Outcomes | % change morning PEFR compared to baseline (clinic reading) % change FEF25‐50 compared to baseline (clinic reading) Histamine BHR (PC20 FEV1) Immunohistochemical examination of inflammatory cells on bronchial biopsy Withdrawal due to asthma exacerbation | |
| Notes | No reply from author to clarify details of randomisation method or provide numerical data for clinic spirometry, histamine challenge or diary card PEFR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Busse 1998.
| Methods | Setting: multicentre study USA, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 12 weeks Masking: double‐blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | Subjects: 473 adults: 217M 256F Age range: 18 ‐ 70 years Inclusion criteria: Diagnosis of asthma (ATS criteria) for at least 6 months 15% reversibility of FEV1 with inhaled beta2 agonist or greater Use of inhaled or inhaled and oral corticosteroids for at least 6 months Use of BDP 300 ‐1000 mcg/d for one month prior to study Exclusion criteria: History of carcinoma or other significant disease Use of injectable corticosteroids Irregular use of oral corticosteroids | |
| Interventions | BUD:
1. 200 mcg/d
2. 400 mcg/d
3. 800 mcg/d
4. 1600 mcg/d
All treatments given twice daily in divided doses Placebo: twice daily lactulose Delivery device: Turbuhaler DPI |
|
| Outcomes | Change in FEV1 compared to baseline Change FVC compared to baseline Change FEF25‐50 compared to baseline Change morning PEFR compared to baseline Change evening PEFR compared to baseline Change daytime asthma symptom compared to baseline Change nighttime asthma symptom compared to baseline daily beta2 agonist use Early morning plasma cortisol Early morning plasma cortisol post ACTH | |
| Notes | No reply from author to clarify details concerning randomisation method or provide standard deviation values for clinic spirometry, diary card PEFR, symptom scores or plasma cortisol levels. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Campbell 1991.
| Methods | Setting: multicentre study UK, primary care. Randomisation: yes, computer‐generated randomisation schedule Allocation concealment: yes Design: parallel Intervention period: 8 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=4 | |
| Participants | 141 subjects: 74M 67F Inclusion criteria: Age > 12 years Reversibility of airway obstruction in response to inhaled beta2 agonists Inhaled bronchodilator at least once per day or experiencing asthma symptoms despite current treatment Exclusion criteria: Oral steroid use within previous month Current respiratory tract infection Pregnancy | |
| Interventions | BUD: 200mcg 2 pfs 1xdaily (400 mcg/d) Placebo: 2 pfs 1xdaily Delivery device: Turbohaler DPI |
|
| Outcomes | Change in morning PEFR compared to baseline
Change in evening PEFR compared to baseline
Change in clinic PEFR compared to baseline
Change in beta2 agonist use (puffs/d) compared to baseline % patients with improved: wheeze at rest and on activity; difficulty in breathing; cough; disturbed sleep; interference with usual routine (clinic assessment) % patients with improved: sleep disturbance; ease of falling asleep; no wheeze on waking; rested on waking; wheeze at rest; limitation of activity; hours active per day; satisfaction with lifestyle (diary card assessment) Withdrawal due to asthma exacerbation |
|
| Notes | Reply from author confirming details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Cockcroft 1995.
| Methods | Setting: Canada, hospital outpatient clinic and recruitment from advertisement. Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Intervention period: 1 week Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: no data according to treatment sequence Jadad score=4 | |
| Participants | 13 adults: 5M 8F Age range: 19‐47 years Inclusion criteria: Mildly atopic asthmatic patients with well controlled asthma, Not having needed beta2 agonists for a minimum of 4 weeks prior to the study Exclusion criteria: No exposure to allergens or chest infection in previous month | |
| Interventions | BUD: 200 mcg 2 pfs 4xdaily (1600 mcg/d) Placebo: 2pfs 4xdaily Delivery device: Turbohaler DPI |
|
| Outcomes | FEV1 Methacholine BHR (PC20 FEV1) Allergen (cat, horse, grass, birch or weed) BHR (PC15 FEV1) | |
| Notes | Reply from author but details of randomisation method unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
de Baets 1990.
| Methods | Setting: The Netherlands, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 8 weeks Masking: double blind. Excluded: stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score=3 | |
| Participants | 31 children: 20M 11F Age : mean (SD) budesonide 10 (2.1) years, placebo 10.3 (1.9) years Inclusion criteria: Children with mild asthma and atopy to house dust mite Baseline spirometry measures > 75% predicted Clinically stable for 3 weeks prior to study Exclusion criteria: Use of oral or inhaled corticosteroids | |
| Interventions | BUD: 200 mcg 1 pf 3xdaily (600 mcg/d) Placebo: 1 pf 3xdaily Delivery device: MDI+spacer |
|
| Outcomes | FEV1 (% predicted) Morning PEFR Histamine BHR (PD20 FEV1) House dust mite BHR (PD20 FEV1) Withdrawal due to asthma exacerbation | |
| Notes | No reply from author to clarify details of randomisation method . | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
de Jong 1996.
| Methods | Setting: The Netherlands, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 2 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 30 adults: 17M 13F Age range: 21‐46 years Inclusion criteria: Mild asthma and a history of episodic wheezing, dyspnoea Symptoms well controlled by inhaled bronchodilator FEV (% predicted) 55 or greater Methacholine BHR (PC20 FEV1) < 4mg/ml Exclusion criteria: Respiratory tract infection or asthma exacerbation in last 6 weeks | |
| Interventions | BUD: 400mcg 1 pf 3xdaily (1200mcg/d) Placebo: 1 pf 3xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | FEV1 Methacholine BHR (PC20 FEV1) Requiring one or more courses or oral steroids for asthma exacerbation (number of patients) | |
| Notes | No reply from author to clarify details of randomisation or provide numerical data for methacholine challenge. A principal aim of this study was to assess the effects of inhaled terbutaline on rebound airway obstruction and bronchial responsiveness during treatment with either budesonide or placebo. The data used here concerns the 2 week period prior to treatment with terbutaline when patients received only budesonide or placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Essen‐Zandvliet 1992.
| Methods | Setting: multicentre study, The Netherlands, paediatric outpatient clinic Randomisation: yes, computer‐generated randomisation schedule Allocation concealment: yes Design: parallel Intervention period: 22 months Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=5 | |
| Participants | 116 children: 86M 30F Age range: 7 to 16 years Inclusion criteria: All of the following 1. Clinical diagnosis of asthma by paediatric respiratory physician defined as a history of chronic or episodic breathlessness, wheeze and/or cough 2. FEV1 (% predicted) 55‐90 and or FEV1/FVC ratio 50 to 75 3. 15% or greater Increase in FEV1 following inhaled beta2 agonist 4. Histamine BHR (PD20 FEV1) 150mcg or less Exclusion criteria: Previous use of oral corticosteroids | |
| Interventions | BUD: 200 mcg 1 pf 3xdaily (600mcg/d) with salbutamol 200mcg 1 pf 3xdaily Placebo: 1pf 3xdaily with salbutamol 200mcg 1 pf 3xdaily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) Morning PEFR Diurnal variability PEFR Histamine BHR (PD20 FEV1) No. of patients with symptom free days No. of patients with no additional beta2 agonist use No. of patients requiring a course of oral prednisolone due to asthma exacerbation No. of patients requiring hospital admission due to asthma No. of patients with no school absences due to asthma Withdrawal due to asthma exacerbation Incidence of oropharyngeal side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fuller 1991.
| Methods | Setting: UK, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, 3 week washout Intervention period: 3 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 10 subjects: 6M 4F Age range: 18‐45 years Inclusion criteria: Mild asthma requiring only irregular therapy with inhaled beta2 agonists Exclusion criteria: Not stated | |
| Interventions | BUD: 600 mcg 2xdaily (1200 mcg/d) Placebo: twice daily Delivery device: MDI+spacer |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR Bradykinin BHR (PD 35 Sgaw) Histamine BHR (PD 35 Sgaw) | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gauvreau 1996.
| Methods | Setting: Canada, hospital outpatient clinic Randomisation: yes, computer‐generated randomisation schedule Allocation concealment: yes, randomisation sequence concealed in envelopes. Design: crossover, 3 week washout Intervention period: 1 week Masking: double blind Excluded: not stated Withdrawals: stated Baseline data: no data according to treatment sequence Jadad score=4 | |
| Participants | 9 adults: 4M 5F Age range: 19‐24 years Inclusion criteria: Mild asthma FEV1 (% predicted) > 70 Non‐smokers Exclusion criteria: Respiratory tract infection or exacerbation of asthma in previous 6 weeks | |
| Interventions | BUD: 400 mcg/d Placebo Delivery device: DPI |
|
| Outcomes | Methacholine BHR (PC20 FEV1) pre and 24 hr post allergen challenge Largest % fall in FEV1 within 2 hrs of inhaled allergen (house dust mite, rag weed or cat): early allergen response Largest % fall in FEV1 3‐7 hrs after inhaled allergen: late allergen response Sputum eosinophil count Blood eosinophil count |
|
| Notes | Reply from author confirming use of allocation concealment, delivery device used. Author also provided log transformed data for pre‐allergen challenge methacholine bronchial responsiveness (log 10 PC20 FEV1). Only the pre‐allergen challenge data for methacholine bronchial challenge is included in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gleeson 1988.
| Methods | Setting: UK, hospital outpatient clinic Randomisation: yes, computer‐generated randomisation schedule Allocation concealment: yes, codes held in pharmacy Design: crossover, 3 week washout Intervention period: 6 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=5 | |
| Participants | 39 children Age range: 26‐71 months Inclusion criteria: Poorly controlled asthma as defined by at least 1 attack in each of the previous 3 months or asthma symptoms on most days Able to use Nebuhaler Parents able to reliably fill‐in diary card Exclusion criteria: Regular use of oral or inhaled corticosteroids | |
| Interventions | BUD: 200 mcg 1pf 2xdaily (400mcg/d) Placebo: I pf 2xdaily Delivery device: MDI+spacer |
|
| Outcomes | Morning PEFR (% predicted) Evening PEFR (% predicted) Daytime cough score Daytime wheeze score Breathlessness on exertion a score Nocturnal cough and wheeze score Morning plasma cortisol | |
| Notes | Reply from authors confirming use of allocation concealment. 2 week run‐in period during which patients and their parents were assessed to ensure they could use Nebuhaler and keep diary card records respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Haahtela 1994.
| Methods | Setting: Finland, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 12 months Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score=3 | |
| Participants | 37 adults: 11M 26F Mean (SD) age: budesonide group 35.9 (2.4) years, placebo 41.0 (2.8) years Inclusion criteria: Adults with asthma 15% or greater increase in FEV1 after inhaled beta2 agonist or 15% or greater decrease in FEV1 following exercise test FEV1 (% predicted) > 80 Histamine BHR (PC15 FEV1): < 32 mg/ml Exclusion criteria: Regular corticosteroid or cromolyn treatment Smokers | |
| Interventions | BUD: 200 mcg 1 pf 2xdaily (400 mcg/d) Placebo: 1 pf 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | Change in FEV1 compared to baseline Change in FVC compared to baseline Change in morning PEFR a compared to baseline Change in evening PEFR compared to baseline Histamine BHR (PC15 FEV1): change in doubling doses compared to baseline Change in daily beta2 agonist use compared to baseline Change in daytime symptom score compared to baseline Withdrawal due to asthma exacerbation Requiring one or more courses of oral prednisolone for asthma exacerbation (number of patients) Incidence of oropharyngeal side effects Incidence of oropharyngeal Candidasis | |
| Notes | No reply form author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Henriksen 1985.
| Methods | Setting: Denmark, paediatric outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, 2 week washout Intervention period: 3 weeks Masking: double blind Excluded: not stated Withdrawals: numbers given but reasons not stated Baseline characteristics: no demographic data presented Jadad score=2 | |
| Participants | 16 children Age range: 8 to 15 years Inclusion criteria: Children with perennial asthma who demonstrated 20% or greater decrease in FEV1 following exercise challenge Exclusion criteria: Treatment with corticosteroids of any sort in previous 3 months | |
| Interventions | BUD: 200mcg 1 pf 2xdaily (400 mcg/d) Placebo: 1 pf 2xdaily Delivery device: MDI+spacer |
|
| Outcomes | FEV1 (% predicted) Fall in FEV1 post exercise challenge as percentage of pre‐exercise baseline value 24 hour urinary free cortisol excretion Incidence of hoarseness | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Heuck 1997b.
| Methods | Setting: Denmark, paediatric outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, computer‐generated randomisation schedule Allocation concealment: unclear Design: crossover, 1 week washout period Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=4 | |
| Participants | 16 adolescents: 12M 4F Age range: 12.9‐16.6 years Inclusion criteria: Adolescents with mild to moderate asthma Requiring treatment with only beta2 agonists or low dose inhaled corticosteroids Exclusion criteria: Inhaled or oral steroids in month prior to study | |
| Interventions | BUD: 200 mcg 2 actuations 2xdaily (800 mcg/d) Placebo: 2actuations 2xdaily Delivery device: MDI+Nebuhaler spacer |
|
| Outcomes | Lower leg growth velocity by knemometry Serum carboxy‐terminal propetide of type 1 collagen (PICP) Serum carboxy‐terminal pyridinioline cross‐liked telopeptide of type 1 collagen (ICTP) Amino‐terminal propeptide of type III procollagen (PIIINP) Urinary concentration pyridinoline crosslinks (PYD) Urinary concentration deoxypyridinoline crosslinks (DPD) Insulin‐like growth factor I (IGF‐I) Insulin‐like growth factor binding protein‐3 (IGFBP‐3) Morning PEFR Evening PEFR Daily use of beta2 agonists | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jatakanon 1998.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: yes Design: crossover with 4 week washout period Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score=4 | |
| Participants | 10 adults: 2M 8F Age range: 24‐36 years Inclusion criteria: Diagnosis of asthma by chest physician with a history of wheezing and chest tightness FEV1 (% predicted) > 80 Methacholine BHR (PC20 FEV1) 4 mg/ml or less Exclusion criteria: Previous inhaled or oral corticosteroid use Asthma exacerbation within the last 12 months | |
| Interventions | BUD: 800 mcg 2xdaily (1600 mcg/d) Placebo: 2xdaily Delivery device: Turbohaler DPI |
|
| Outcomes | FEV1 Methacholine BHR (PC20 FEV1) Morning PEFR Daytime PEFR variability Daily asthma symptom score Daily beta2 agonist use Sputum eosinophil count Sputum eosinophil cationic protein Sputum tumour necrosis facto‐alpha Exhaled nitric oxide | |
| Notes | Reply from author confirming use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jonasson 1998.
| Methods | Setting: Norway, paediatric outpatient clinic Length of intervention period: 12 weeks Randomisation: yes, computer‐generated randomisation schedule Allocation concealment: yes Design: parallel Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score=4 | |
| Participants | 163 children: 107M 56F Age range: 7‐16 years Inclusion criteria: Children with a diagnosis of asthma (based on criteria of an International Consensus Report 1992) 3 previous obstructive episodes or 1 previous obstructive episodes with atopy Exclusion criteria: Use of inhaled corticosteroids within last 2 months Use of cromoglycate or nedocromil within last 4 weeks Asthma exacerbation requiring emergency room visit or hospital admission within last 4 weeks Lower respiratory tract infection within last weeks | |
| Interventions | BUD:
1. 100 mcg 1xdaily morning (100 mcg/d)
2. 200 mcg 1xdaily morning (200 mcg/d)
3. 100 mcg 2xdaily (200 mcg/d) Placebo: 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | Maximal percentage fall in FEV1 post exercise test FEV1 FEF 25, 50, 75 Morning PEFR Methacholine BHR (PD20 FEV1) Daytime asthma symptom score Night‐time asthma symptom score Daytime beta2 agonist use Night‐time beta2 agonist use | |
| Notes | Reply from author confirming use of allocation concealment and provided standard deviation values for mean fall is FEV1 post exercise challenge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jones 1994.
| Methods | Setting: multicentre study UK, primary care Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 12 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=4 | |
| Participants | 340 subjects: 178M 166F Age range: 12‐70 years Inclusion criteria: Mild to moderate asthma and a 'documented response' to inhaled beta2 agonists PEFR (% predicted) 60 or greater Symptom score of 1 or greater during run‐in Exclusion criteria: Regular use of corticosteroids within last 6 months Short courses of corticosteroid within last 2 months Use of nedocromil or sodium cromoglycate within last 2 months Asthma exacerbation within last 2 months | |
| Interventions | BUD:
1. 400 mcg morning (400mcg/d)
2. 400 mcg evening (400mcg/d)
3. 200 mcg 2xdaily (400mcg/d) Placebo: 2 pfs 2xdaily Delivery device: Turbohaler DPI |
|
| Outcomes | Change morning PEFR compared to baseline Change evening PEFR compared to baseline Change in daytime and night‐time symptom scores compared to baseline Change in daytime and night‐time beta2 agonist use compared to baseline Withdrawal due to asthma exacerbation | |
| Notes | No reply from author to clarify details of randomisation method. Three daily dose schedules were evaluated, all assessing 400 mcg/d. Only the data for outcomes for those patients assigned to BUD 200 mcg twice daily were used in this meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Juniper 1990.
| Methods | Setting: Canada, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: yes Design: parallel Intervention period: 12 months Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=4 | |
| Participants | 32 adults: 13M 19F Mean (SD) age: budesonide 42.4(11.6) years, placebo 35.1(15.8) years Inclusion criteria: Clinical diagnosis of asthma Methacholine BHR (PC20 FEV1) <8mg/ml FEV1 (% predicted) 70 or greater Exclusion criteria: Use of inhaled or oral steroids or cromoglycate within last 6 weeks Pregnancy | |
| Interventions | BUD: 200 mcg 1 pf 2xdaily (400mcg/d) Placebo: 1 pf 2xdaily Dlievery device: MDI+spacer |
|
| Outcomes | FEV1 (% predicted) Methacholine BHR (PC20 FEV1) Asthma severity score Daily beta2 agonist use No. of asthma exacerbations Withdrawal due to asthma exacerbation Requiring one or more courses of oral prednisolone for asthma exacerbation (number of patients) Incidence of oropharyngeal side effects Incidence of oropharyngeal Candidasis | |
| Notes | Reply from author confirming use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kharitonov 1996.
| Methods | Setting: UK, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, 3 week washout Intervention period: 3 weeks Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: comparable Jadad score=2 | |
| Participants | 11 adults: 8M 3F Age range: 26‐36 years Inclusion criteria: Atopic asthma Positive skin prick test to at least 2 allergens FEV1 (% predicted) >70 Methacholine BHR (PC20 FEV1) < 8 mg/ml Exclusion criteria: Regular oral or inhaled steroid use Respiratory tract infection or asthma exacerbation in last month | |
| Interventions | BUD: 800 mcg 2xdaily (1600 mcg/d) Placebo: 2xdaily Delivery device: Turbohaler DPI |
|
| Outcomes | FEV1 (% predicted) Methacholine BHR (PC20 FEV1) Exhaled air nitric oxide concentration | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kivity 1994.
| Methods | Setting: Israel, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 8 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 39 adults: 23 M 16F Age range: 17‐49 years Inclusion criteria: Mild to moderate asthma FEV1 (% predicted) > 60 15% or greater increase in FEV1 after inhaled beta2 agonist, or 15% fluctuation in daily PEFR during run‐in Methacholine BHR (PC20 FEV1) < 8mg/ml Exclusion criteria: Co‐existent serious illness or other pulmonary disease | |
| Interventions | BUD: 400 mcg 1 pf 2xdaily (800mcg/d) Placebo: 1 pf 2xdaily Delivery device: Turbohaler DPI |
|
| Outcomes | Change in FEV1 compared to baseline Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Methacholine BHR (PD20 FEV1) Daily asthma symptom score Daily use of beta2 agonists Withdrawal due to asthma exacerbation | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kuo 1994.
| Methods | Setting: Taiwan, hospital outpatient clinic Randomisation: yes, computer‐generated random number sequence Allocation concealment: unclear Design: parallel Intervention period: 4 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable (those completing study) Jadad score=3 | |
| Participants | 32 adults: 19M 13F Age range: 18‐64 years Inclusion criteria: Diagnosis of asthma (ATS criteria 1962) 15% or greater improvement in FEV1 after inhaled beta2 agonist Normal chest radiograph Exclusion criteria: Use of inhaled or oral steroids previous 6 weeks Current smokers or greater than 5 pack year smoking history Acute respiratory infection in last 2 weeks | |
| Interventions | BUD: 800 mcg/d ) Placebo Delivery device: Turbuhaler DPI |
|
| Outcomes | FEV1 (% predicted) Methacholine BHR (PC20 FEV1) Daily PEFR Diurnal variability PEFR Blood hypodense eosinophil count Withdrawal due to asthma exacerbation | |
| Notes | Reply from author: clarification of method of random order generation but unclear regarding allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lorentzson 1990.
| Methods | Setting: multicentre study Sweden & Norway, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 6 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 103 adults: 58M 45F Age range: 16‐59 years Inclusion criteria: Mild asthma Use of 35 puffs rescue beta2‐agonist during run in week and mean variation of 10‐30% in PEFR PEFR (% predicted) 75 or greater Exclusion criteria: Use of oral or inhaled steroids Serious co‐existent disease Respiratory infection or acute asthma exacerbation in last month | |
| Interventions | BUD:
1. 100 mcg 1pf 2xdaily (200 mcg/d)
2. 200 mcg 1pf 2xdaily (400 mcg/d) Delivery device: MDI+spacer Placebo: 1 pf 2xdaily via MDI+spacer |
|
| Outcomes | Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Change in daily use beta2 agonists compared to baseline Daily asthma symptom score Withdrawal due to asthma exacerbation Incidence of oropharyngeal side effects Incidence of oropharyngeal Candidasis | |
| Notes | No reply from author to clarify details of randomisation method. Two dosage schedules are used in this trial. Only data for those patients assigned to 400 mcg/d has been used in the meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Nelson 1998.
| Methods | Setting: multicentre study USA, hospital outpatient clinic Length of intervention period: 20 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 159 adults: 80M 79F Age range: 20‐69 years Inclusion criteria: Patients with chronic oral steroid‐dependent asthma Oral steroid treatment for a minimum of 6 months (5‐30 mcg/d or equivalent) Exclusion criteria: Use of inhaled corticosteroids other than BDP Sodium cromoglycate, nedocromil sodium or investigational drug within last 4 weeks Methotrexate within last 3 months Hospitalised due to asthma within last 30 days | |
| Interventions | BUD:
1. 400 mcg 2 x daily (800 mcg/d)
2. 800 mcg 2x daily (1600 mcg/d) Placebo; 2 x daily Delivery device: Turbuhaler DPI |
|
| Outcomes | Reduction in daily dose oral prednisolone compared to baseline Number of patients discontinuing oral prednisolone Percentage change in FEV1 compared to baseline Percentage change in morning PEFR compared to baseline Percentage change in evening PEFR compared to baseline Percentage change in FEF25‐50 compared to baseline Change in daytime asthma symptom score compared to baseline Change in night‐time symptom score compared to baseline Change in daily use of beta2 agonists compared to baseline Morning plasma cortisol Plasma cortisol 60 min post 25 units cosyntropin IM | |
| Notes | No reply from author to clarify details of randomisation method. At baseline all subjects had their daily dose of inhaled beclomethasone replaced with an 'equivalent' dose of oral prednisolone. A forced dose down titration design was used to reduce oral prednisolone: at 2 weekly intervals a) subjects receiving > 10mg/d prednisolone decreased daily dose by 5mg/d b) subjects receiving 10mg/d or less of prednisolone decreased daily dose by 2.5 mg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
O'Byrne 1996.
| Methods | Setting: Canada, multicentre study, primary care Randomisation: yes, by computer‐generated randomisation sequence Allocation concealment: yes (coding undertaken centrally by pharmaceutical company sponsors) Design: parallel Intervention period: 4 months Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=4 | |
| Participants | 47 adults: 25M 32F Mean (SD) age: BUD 400 32(9.0) years, BUD 800 37(17.8) years, placebo 36(12.7) years Inclusion criteria: Adults over the age of 18 with asthma requiring the use of inhaled bronchodilators Diurnal PEFR variability 10% or greater for at least 3 out of 7 days of the screening period Exclusion criteria: Inhaled or oral steroids in last 3 months Felt to require inhaled corticosteroids by primary care physician at the time of enrolment | |
| Interventions | BUD:
1. 100mcg 2pfs 2xdaily (400 mcg/d)
2. BUD 200mcg 2pfs 2xdaily (800 mcg/d) Placebo: 2 pfs 2xdaily Delivery device: MDI+spacer |
|
| Outcomes | Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline % of patients reporting: any symptoms; waking at night; waking early due to asthma; requiring bronchodilators > 4xdaily during previous month No. of patients attending hospital emergency dept due to acute asthma exacerbation Withdrawal due to asthma exacerbation Economic analysis | |
| Notes | Reply from author confirming use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
O'Connor 1992.
| Methods | Setting: UK, hospital outpatient clinic Randomisation: yes, computer‐generated randomisation sequence Allocation concealment: yes Design: crossover, 4 week washout Intervention period: 2 weeks Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable FEV1 (% predicted) Jadad score=4 | |
| Participants | 12 adults: 6M 6F Age range: 20 to 46 years Inclusion criteria: Non smoking adult patients with mild asthma Positive allergen skin prick test to a common airborne allergen (Dermatophagoides pteronyssinus, mixed grass pollen, cat or dog fur) FEV1 (% predicted) > 80 Exclusion criteria: Exacerbation of asthma or respiratory infection in previous 6 weeks | |
| Interventions | BUD: 400 mcg 2pfs 2xdaily (1600 mcg/d) Placebo: 2 pfs 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | FEV1 Methacholine BHR (PC20 FEV1) Sodium metabisulphite BHR (PC20 FEV1) Adenosine monophosphate BHR (PC20 FEV1) Blood eosinophil count | |
| Notes | Reply from author clarifying method of random order generation and confirming use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Osterman 1997.
| Methods | Setting: multicentre study Sweden, hospital outpatient clinic Randomisation: yes, computer‐generated randomisation sequence Allocation concealment: yes (codes held by pharmaceutical company sponsors) Design: parallel Intervention period: 12 months Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=5 | |
| Participants | 75 subjects: 33M 42F Age range: 18‐68 years Inclusion criteria: Clinical diagnosis of asthma, made not more than 1 year previously Histamine BHR (PD20 FEV1) < 0.66 mg Exclusion criteria: Teatment with anti‐inflammatory drugs within previous 3 months | |
| Interventions | BUD: 200 mcg 1pf 2xdaily (400 mcg/d) Placebo: 1pf 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | Change in FEV1 and % predicted FEV1 compared to baseline Histamine BHR (PD20 FEV1) Change in daytime and night‐time beta2 agonist use Change in serum ECP, MPO, ECA and NCA compared to baseline Withdrawal due to asthma exacerbation | |
| Notes | Reply from pharmaceutical company sponsors confirming use of allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Prieto 1994.
| Methods | Setting: Spain, hospital outpatient clinic Randomised: yes, computer‐generated randomisation schedule. Allocation concealment: not employed Design: parallel Intervention period: 4 months Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 30 adults, 28 completed: 8M 20F Mean (SD) age: budesonide 33(3) years, placebo 35 (2) years Inclusion criteria: Patients with seasonal asthma for at least 2 years FEV1 (% predicted) > 80 Positive skin prick test to grass or other pollens but not to other allergens such as house dust mite, cat or dog Exclusion criteria: Inhaled corticosteroid use Serious coexistent disease Chest infection within last 4 weeks | |
| Interventions | BUD: 400 mcg 1 pf 2xdaily (800mcg/d) Placebo: 1 pf 2xdaily Delivery device: MDI+spacer |
|
| Outcomes | FEV1 Methacholine BHR (PC20 FEV1) | |
| Notes | Reply from author clarifying method of random order generation and that allocation concealment was not employed. Study was undertaken during the period of maximum allergen exposure (March to June). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Rodriguez 1991.
| Methods | Setting: Spain, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 8 weeks Masking: not stated Excluded: not stated Withdrawals: none Baseline characteristics: no demographic data presented Jadad score=2 | |
| Participants | 28 subjects: 13M 15F Age range: 14‐30 years Inclusion criteria: Diagnosis of asthma (ATS criteria 1962) FEV1 (%predicted) > 80 Positive methacholine bronchial challenge Normal paranasal sinus X‐ray | |
| Interventions | BUD: 800 mcg/d for 4 weeks then 400 mcg/d for 4 weeks Placebo: 2 inhalations 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) FVC (% predicted) FEF50 Methacholine BHR (PD20 FEV1) Asthma symptom score Withdrawal due to asthma exacerbation | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sekerel 1997a.
| Methods | Setting: Turkey, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 8 weeks Masking: double blind. Excluded: stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score=4 | |
| Participants | 24 children: 12M 12F Mean (SD) age: BUD 11.9 (1.1) years, placebo 10.6 (1.2) years Inclusion criteria: Diagnosis of asthma (ATS criteria) 15% or greater improvement in FEV1 following inhaled beta2 agonist No increase in bronchodilator dose or receipt of oral steroids 1 month prior to study Exclusion criteria: Seasonal allergic asthma FEV1 (% predicted) < 55 | |
| Interventions | BUD: 200 mcg 1 pf 2xdaily (400 mcg/d) Placebo: 1 pf 2xdaily Delivery device: MDI+spacer |
|
| Outcomes | FEV1 (% predicted) Morning PEFR (% predicted) Evening PEFR (% predicted) Specific airways conductance (sGaw) TLC (% predicted) RV (% predicted) Methacholine BHR (PC20 FEV1) Day and night‐time asthma symptom score Daily beta2 agonist use Withdrawal due to asthma exacerbation | |
| Notes | No reply from author to clarify details of randomisation method. All continuous outcomes are reported using medians and ranges and have not been included in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Shapiro 1998a.
| Methods | Setting: multicentre study USA, paediatric outpatient clinic Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 404 children: 314M 90F Age range: 6‐18 years Inclusion criteria: Children with asthma (not otherwise defined) Dependent on inhaled corticosteroids for asthma control for at least 6 months 15% or greater improvement in FEV1 after inhaled beta2 agonist At least 2 different asthma medications daily for last 6 months (at least 1 and inhaled steroid) Exclusion criteria: Significant co‐existent disease | |
| Interventions | BUD:
1. 100 mcg 1actuation 2xdaily (200mcg/d)
2. 200 mcg 1 actuation 2xdaily (400mcg/d)
3. 400 mcg 1 actuation 2xdaily (800 mcg/d) Placebo: 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | Change in FEV1 (% predicted) compared to baseline Change in morning PEFR compared to baseline Change in daytime asthma symptom score compared to baseline Change in night‐time asthma symptom score compared to baseline Change in daily use of beta2 agonist compared to baseline Change in morning plasma cortisol compared to baseline Change in plasma cortisol 30 min post 25 units iv cosyntropin compared to baseline Withdrawal due to asthma exacerbation | |
| Notes | No reply from author to clarify details of randomisation method. A small proportion of patients were receiving treatment with oral corticosteroids at the time of enrolment (30/404). These patients continued with oral steroids at constant dose throughout study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Swystun 1998.
| Methods | Setting: Canada, hospital outpatient clinic Length of intervention period: 7 days Randomisation: yes, computer‐generated randomisation sequence Allocation concealment: yes Design: 4 way crossover with 6 day washout periods Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=4 | |
| Participants | 12 adults: 5M 7F Age range: 20‐48 years Inclusion criteria: Adults with mild to moderate atopic asthma Documented response to a specific allergen in skin prick testing or inhalation challenge FEV1 (% predicted FEV1) > 70 Methacholine BHR (PC20 FEV1) 8 mg/ml or less Exclusion criteria: Requirement for any asthma medication in last 4 weeks Respiratory tract infection within last 4 weeks | |
| Interventions | BUD:
1. 100 mcg 1 actuation 2xdaily (200 mcg/d)
2. 200 mcg 1 actuation 2xdaily (400 mcg/d)
3. 400 mcg 1 actuation 2xdaily (800 mcg/d) Placebo: 1 actuation 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | Allergen BHR (PC15 FEV1) Methacholine BHR (PC15 FEV1) FEV1 | |
| Notes | Reply from author to clarifying method of random order generation and that allocation concealment was employed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Tan 1998.
| Methods | Setting: Singapore, hospital outpatient clinic Length of intervention period: 1 month Randomisation: yes, method not described Allocation concealment: unclear Design: parallel (with crossover in one arm) Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score=3 | |
| Participants | 30 adults: 20M 10F Age range: 16‐65 years Inclusion criteria: Diagnosis of asthma for at least 1 year (ATS criteria) Nocturnal wakening by wheeze or cough more than once per week in preceding month 20% or greater fall in FEV1 or PEFR from evening to morning on 2 consecutive days before study 15 % or greater Improvement in FEV1 after inhaled beta2 agonist Histamine BHR (PC20FEV1) 8mg/ml or less Exclusion criteria: History of smoking Respiratory tract infection in last 6 weeks | |
| Interventions | BUD: 1600 mcg/d Placebo Delivery device: MDI with built in extension piece (Pulmicort Misthaler) |
|
| Outcomes | 4 pm, 10 pm, 4 am FEV1 4 pm, 10 pm, 4 am Thoracic Gas Volume 4 pm histamine BHR (PD20FEV1) 4 am histamine BHR (PD20FEV1) 4pm, 10pm, 4 am specific airway conductance (SGaw) Serum neutrophil chemotactic activity Number of night‐time wakenings per week due to asthma | |
| Notes | No reply from author to clarify details of randomisation method. Patients were randomised to either a) 8 weeks of treatment with inhaled BUD or b) 4 weeks of treatment with placebo followed by 4 weeks of treatment with inhaled BUD. In this analysis results from the first 4 weeks only have been considered. This study was specifically concerned with assessing the effects of each intervention on diurnal variability of the outcomes assessed. However in this analysis only the 4pm measurements have been considered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Toogood 1990.
| Methods | Setting: Canada, hospital outpatient clinic Length of intervention period: 8 weeks Randomation: yes, computer generated random number sequence Allocation concealment: unclear Design: parallel Masking: double blind Excluded: stated (3 patients whose asthma control could not be stabilised) Withdrawals: stated Baseline characteristics: comparable Jadad score=5 | |
| Participants | 32 adults: 19M 13F Mean (SD) age: BUD 52.4(13.6) years, placebo 53.6(16.4) years Inclusion criteria: Adults with asthma as defined by history of variable wheezing and dyspnoea relieved by beta2 agonists, diffuse wheezing on ausculation Exclusion criteria: Regular oral steroid treatment Patients not well controlled at the end of baseline | |
| Interventions | BUD: 100 mcg 1 puff 4xdaily (400 mcg/d) Placebo: 1 puff 4xdaily Delivery device: MDI+spacer (extendible tube, 110ml) |
|
| Outcomes | Time to relapse (days) | |
| Notes | Reply from author to clarify method of random order generation and blinding. During the 4 week run in period , patients were aggressively treated with BDP to achieve maximum control of asthma. A priori defined criteria for asthma relapse: time to point at which 7‐day mean lower morning and evening PEFR (LPFD) fell below 95% CI for LPFD at baseline. This study also included an oral budesonide treatment arm: results not considered in this systematic review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vathenen 1991.
| Methods | Setting; UK, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 6 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 40 adults: 28M 12F Age range: 18‐45 years Inclusion criteria: Asthma for at least 2 years FEV1 (% predicted) > 50 Histamine BHR (PD20 FEV1) 4micromols or less Exclusion criteria: Chest infection within 6 weeks of study Current smokers | |
| Interventions | BUD: 800 mcg 2xdaily (1600 mcg daily) via aerosol MDI+Nebuhaler spacer Placebo: 2xdaily via identical delivery system |
|
| Outcomes | FEV1 and FEV1 (% predicted) Morning PEFR Evening PEFR Histamine BHR (PD20 FEV1) BHR to eucapnic hyperventilation PV 20 FEV1 BHR to exercise by six minute walk challenge (% fall in FEV1 after exercise) Daily asthma symptom score Daily beta2 agonist use Requiring one or more courses or oral steroids for asthma exacerbation (number of patients) | |
| Notes | No reply from author to clarify details of randomisation method. All outcome data reported using medians and ranges and analysed using non‐parametric methods | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wempe 1992a.
| Methods | Setting: The Netherlands, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Intervention period: 4 weeks Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score=3 | |
| Participants | 8 adults: 5M 3F Age range: 18 ‐40 years Inclusion criteria: Adult patients with asthma Positive skin prick test and elevated specific IgE to house dust mite FEV1 (% predicted) > 60 Histamine BHR (PC20 FEV1) < 8 mg/ml Diurnal PEFR variability > 15% Exclusion criteria: Asthma exacerbation within last 2 months | |
| Interventions | BUD: 200mcg 2 pfs 2 x daily (800mcg/d) Placebo: 2 pfs 2 x daily Delivery device: Turbuhaler DPI Bambuterol: 20mg tablet 1xdaily Each group received appropriate additional placebo tablet or inhaler to maintain blinding |
|
| Outcomes | 24 hour mean FEV1 (% predicted) Diurnal change in FEV1 (% predicted) Histamine BHR (PC20 FEV1) Diurnal change in histamine BHR (PC20 FEV1) Daytime cough, breathlessness and wheeze scores Night‐time cough, breathlessness and wheeze scores | |
| Notes | No reply from author to clarify details of randomisation method. Patient group with daily PEFR variability > 15% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wempe 1992b.
| Methods | Setting: The Netherlands, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Intervention period: 4 weeks Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score=3 | |
| Participants | 9 adults: 6M 3F Age range: 18 ‐39 years Inclusion criteria: Adult patients with asthma Positive skin prick test and elevated specific IgE to house dust mite FEV1 (% predicted) > 60 Histamine BHR (PC20 FEV1) < 8 mg/ml Diurnal PEFR variability < 15% Exclusion criteria: Asthma exacerbation within last 2 months | |
| Interventions | BUD: 200mcg 2 pfs 2 xdaily (800mcg/d) Placebo: 2 pfs 2xdaily Delivery device: Turbuhaler DPI Bambuterol: 20mg tablet 1xdaily Each group received appropriate addtional placebo tablet or inhaler to maintain blinding |
|
| Outcomes | 24 hour mean FEV1 (% predicted) Diurnal change in FEV1 (% predicted) Histamine BHR (PC20 FEV1) Diurnal change in histamine BHR (PC20 FEV1) Daytime cough, breathlessness and wheeze scores Night‐time cough, breathlessness and wheeze scores | |
| Notes | Patient group with daily PEFR variability < 15% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wong 1994.
| Methods | Setting: UK, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 2 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 19 adults Mean age: budesonide 30 years, placebo 31years Inclusion criteria: Clinical diagnosis of asthma FEV1 (% predicted) >65 15% or greater increase in FEV1 after inhaled beta2 agonist Histamine BHR (PC20 FEV1) < 4 micromols Positive skin prick test to cat fur, grass pollen or house dust mite Exclusion criteria: Treatment other than beta2 agonist Smokers within last 5 years | |
| Interventions | 1. BUD 400mcg 2xdaily (800mcg/d)
2. Placebo 2xdaily 3. BUD 400 mcg2xdaily (800 mcg/d)+ terbutaline 1000 mcg/d 4. Terbutaline 1000 mcg/d Delivery device: Turbohaler DPI |
|
| Outcomes | Change in FEV1 compared to baseline Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Change in allergen BHR (PC20 FEV1) compared to baseline Change in histamine BHR (PD20 FEV1) compared to baseline Change in daytime asthma symptom score compared to baseline Change in daily beta2 agonist use compared to baseline Withdrawal due to asthma exacerbation Incidence of oropharyngeal side effects | |
| Notes | No reply from author to clarify details of randomisation method. 2 intervention groups received either BUD+terbutaline or terbutaline alone. The results for these groups have not been considered in this meta‐analysis. Allergen challenge, diary card PEFR, symptom scores and beta2 agonist use were reported using medians and ranges and analysed using non‐parametric methods. The data has therefore not been used in the meta‐analysis. Histamine challenge data not log transformed prior to analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wongtim 1995.
| Methods | Setting: Thailand, hospital outpatient clinic Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Intervention period: 8 weeks Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score=3 | |
| Participants | 20 adults: 10M 10F Mean (SD) age: budesonide 33.2(7.5) years, placebo 32.8(8.6) years Inclusion criteria: Mild asthma with presence of coughing or wheezing within the last 3 years 15% or greater improvement in FEV1after inhaled beta2 agonist Exclusion criteria: Use of systemic steroids, ketotifen or disodium cromoglycate within last 3 months | |
| Interventions | BUD: 200 mcg 2 pfs 2xdaily (800 mcg/d) Placebo: 2 pfs 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | Methacholine BHR (PC20 FEV1) Daily asthma symptom score Weekly beta2 agonist use Withdrawal due to asthma exacerbation | |
| Notes | No reply from author to clarify details of randomisation method. Methacholine challenge data not log transformed prior to analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yates 1996.
| Methods | Setting: UK, hospital outpatient clinic Randomisation: yes, computer‐generated random number sequence Allocation concealment: yes Design: crossover, 3 week washout Intervention period: 3 weeks Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score=3 | |
| Participants | 14 adults: 9M 5F Age range: 25‐47 years Inclusion criteria: Occasional symptoms of variable wheeze or dyspnoea controlled by beta2 agonists alone FEV1 (% predicted) >70 Methacholine BHR (PC20 FEV1) < 8mg/ml Exclusion criteria: Use of inhaled or oral corticosteroids in last 2 months Asthma exacerbation or respiratory infection within 6 weeks | |
| Interventions | BUD: 800 mcg 2xdaily (1600 mcg/d) Placebo: 2 pfs 2xdaily Delivery device: Turbuhaler DPI |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR Methacholine BHR (PC20 FEV1) Morning chest tightness score Daytime asthma symptom score Night‐time asthma symptom score | |
| Notes | Reply from author clarifying method or random order generation and that allocation concealment was employed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ATS: American Thoracic Society BHR: bronchial hyper‐responsiveness BUD: budesonide DPI: dry powder inhaler ECA: serum eosinophil chemotactic activity ECP: eosinophil cationic protein FEF25, 50, 75: maximal expiratory flow at 25%, 50% and 75% of FVC FEF25‐75: forced expiratory flow at 25 to 75% of FVC FEV1: forced expired volume in one second FVC: forced vital capacity MDI: metered dose inhaler MPO: serum myeloperoxidase NCA: serum neutrophil chemotactic activity PC15 FEV1: provocative concentration of inhalant required to produce a 15% fall in FEV1 PC20 FEV1: provocative concentration of inhalant required to produce a 20% fall in FEV1 PD20 FEV1: provocative dose of inhalant required to produce a 20% fall in FEV1 PD35 Sgaw: provocative dose of inhalant required to produce a 35% fall in SGaw PEFR: peak expiratory flow rate RV: residual volume Sgaw: specific airway conductance TLC: total lung capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bisgaard 1990 | Age range of children recruited 11 to 36 months |
| Bisgaard 1993 | Age range of children recruited 10 to 36 months |
| Booms 1997 | This study was designed to test the hypothesis that inhaled budesonide can alter the capacity of unlimited airway narrowing in those patients with asthma who have previously been demonstrated to exhibit this type of response to inhaled methacholine. A key inclusion criterion was the presence of unlimited airways narrowing in response to methacholine. All outcomes assessed were related to the shape of the methacholine dose response curve. It was felt that such outcomes were of uncertain clinical significance at the current time and therefore the study was excluded for this reason. |
| Clark 1997 | Treatment period of less than one week |
| Connett 1993 | Age range of children 1 to 3 years |
| Dahl 1982 | Single dose study |
| de Blic 1996 | Age of children under 2 years (infants), nebuliser delivery device. |
| Engel 1991 | Single dose study |
| Essen Zandvliet 1993 | Single dose study |
| Fuglsang 1995 | This randomised trial was designed to assess the influence on the shape of the dose response curve after cumulative doses of inhaled terbutaline. |
| Heuck 1997a | This study examined knemometric growth rates in children treated with budesonide. No other outcomes were assessed. |
| Ilangovan 1993 | Nebulizer delivery device employed |
| Kidney 1997 | Single dose study |
| Kozak‐Szkopek 1997 | Study concerned with patients with chronic bronchitis, not asthma |
| Noble 1992 | Age range of children recruited 4 to 17 months |
| Paggiaro 1994 | Single dose study |
| Rocca 1996 | All patients recieved budesonide via Pulmicort Turbohaler DPI throughout study. Patients were randomised to recieve either terbutaline via either an MDI or the Turbohaler DPI. |
| Shapiro 1998b | Nebuliser delivery device used |
| Sutochnikova 1996 | The study is described as randomised, however it is not clear from the English language translation of the paper if the control group received placebo or no intervention at all. Awaiting confirmation from author. |
| Van Bever 1990 | Age range of children 3 to 17 months, nebuliser delivery device used. |
| Waalkens 1990 | Not an RCT |
| Wilson 1995 | This study examined the effects of continuous prophylactic inhaled budesonide in preventing recurrent acute episodes of viral induced episodic wheeze in pre‐school children. It is not using a population of children with defined 'asthma' |
| Wilson 1998 | Placebo arm not randomised |
| Wolthers 1998 | This randomised controlled trial assessed the effects of budesonide versus placebo on serum osteocalcin levels in asthmatic children. The effects of inhaled corticosteroids on bone metabolism are being addressed by another review. No other outcome measures were assessed. |
| Yates 1998 | Single dose study |
Contributions of authors
NPA: Protocol initiation; study assessment; data extraction; analysis & write‐up
JB: Study assessment; data extraction
PWJ: Write‐up
Sources of support
Internal sources
No sources of support supplied
External sources
NHS Research and Development, UK.
Garfield Weston Foundation, UK.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Aaronson 1998 {published data only}
- Aaronson D, Kaiser H, Dockhorn R, Findlay S, Korenblat P, Thorsson L, et al. Effects of budesonide by means of the Turbuhaler on the hypothalmic‐pituitary‐adrenal axis in asthmatic subjects: a dose‐response study. Journal of Allergy & Clinical Immunology 1998 Mar;101(3):312‐9. [DOI] [PubMed] [Google Scholar]
Agertoft 1997 {published data only}
- Agertoft L, Pedersen S. Short‐term knemometry and urine cortisol excretion in children treated with fluticasone propionate and budesonide: a dose response study. European Respiratory Journal 1997;10(7):1507‐12. [DOI] [PubMed] [Google Scholar]
Baki 1998 {published data only}
- Baki A, Karaguzel G. Short‐term effects of budesonide, nedocromil sodium and salmeterol on bronchial hyperresponsiveness in childhood asthma. Acta Paediatrica Japonica 1998;40(3):247‐51. [DOI] [PubMed] [Google Scholar]
Bel 1991 {published data only}
- Bel EH, Timmers MC, Zwinderman AH, Dijkman JH, Sterk PJ. The effect of inhaled corticosteroids on the maximal degree of airway narrowing to methacholine in asthmatic subjects. American Review of Respiratory Disease 1991;143(1):109‐13. [DOI] [PubMed] [Google Scholar]
Boner 1995 {published and unpublished data}
- Boner AL, Comis A, Schiassi M, Venge P, Piacentini GL. Bronchial reactivity in asthmatic children at high and low altitude. Effect of budesonide. American Journal of Respiratory and Critical Care Medicine 1995;151(4):1194‐200. [DOI] [PubMed] [Google Scholar]
Burke 1996 {published data only}
- Burke CM, Sreenan S, Pathmakanthan S, Patterson J, Schmekel B, Poulter LW. Relative effects of inhaled corticosteroids on immunopathology and physiology in asthma: a controlled study. Thorax 1996;51(10):993‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Busse 1998 {published data only}
- Busse WW, Chervinsky P, Condemi J, Lumry WR, Petty TL, Rennard S, et al. Budesonide delivered by Turbuhaler is effective in a dose‐dependent fashion when used in the treatment of adult patients with chronic asthma. Journal of Allergy and Clinical Immunology 1998;101(4 Pt 1):457‐63. [DOI] [PubMed] [Google Scholar]
Campbell 1991 {published data only}
- Campbell LM, Watson DG, Venables TL, Taylor MD, Richardson PDI. Once daily budesonide turbohaler compared to placebo as initial prophylactic therapy for asthma. British Journal of Clinical Research 1991;2:111‐22. [Google Scholar]
Cockcroft 1995 {published data only}
- Cockcroft DW, Swystun VA, Bhagat R. Interaction of inhaled beta 2 agonist and inhaled corticosteroid on airway responsiveness to allergen and methacholine. American Journal of Respiratory and Critical Care Medicine 1995;152(5 Pt 1):1485‐9. [DOI] [PubMed] [Google Scholar]
de Baets 1990 {published data only}
- Baets FM, Goeteyn M, Kerrebijn KF. The effect of two months of treatment with inhaled budesonide on bronchial responsiveness to histamine and house‐dust mite antigen in asthmatic children. American Review of Respiratory Disease 1990;142(3):581‐6. [DOI] [PubMed] [Google Scholar]
de Jong 1996 {published data only}
- Jong JW, Mark TW, Koeter GH, Postma DS. Rebound airway obstruction and responsiveness after cessation of terbutaline: effects of budesonide. American Journal of Respiratory and Critical Care Medicine 1996;153(1):70‐5. [DOI] [PubMed] [Google Scholar]
Essen‐Zandvliet 1992 {published data only}
- Merkus PJ, Essen‐Zandvliet EE, Duiverman EJ, Houwelingen HC, Kerrebijn KF, Quanjer PH. Long‐term effect of inhaled corticosteroids on growth rate in adolescents with asthma. Pediatrics 1993;91(6):1121‐6. [PubMed] [Google Scholar]
- Essen‐Zandvliet EE. Long‐term intervention in childhood asthma: the Dutch study results. Dutch Chronic Nonspecific Lung Disease Study Group. Monaldi Archives of Chest Disease 1995;50(3):201‐7. [PubMed] [Google Scholar]
- Essen‐Zandvliet EEM, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. Effects of 22 months of treatment with inhaled corticosteroids and/or beta‐2‐agonists on lung function, airway responsiveness, and symptoms in children with asthma. American Review of Respiratory Disease 1992;146(3):547‐554. [DOI] [PubMed] [Google Scholar]
Fuller 1991 {published data only}
- Fuller RW, Choudry NB, Eriksson G. Action of budesonide on asthmatic bronchial hyperresponsiveness. Effects on directly and indirectly acting bronchoconstrictors. Chest 1991;100(3):670‐4. [DOI] [PubMed] [Google Scholar]
Gauvreau 1996 {published and unpublished data}
- Gauvreau GM, Doctor J, Watson RM, Jordana M, O'Byrne PM. Effects of inhaled budesonide on allergen‐induced airway responses and airway inflammation. American Journal of Respiratory and Critical Care Medicine 1996;154(5):1267‐71. [DOI] [PubMed] [Google Scholar]
Gleeson 1988 {published data only}
- Gleeson JG, Price JF. Controlled trial of budesonide given by the nebuhaler in preschool children with asthma. BMJ 1988;297(6642):163‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A, Pool J, Gleeson JG, Price JF. Effect of budesonide on pulmonary hyperinflation in young asthmatic children. Thorax 1988;43(11):937‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haahtela 1994 {published data only}
- Haahtela T, Jarvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. New England Journal of Medicine 1994;331(11):700‐5. [DOI] [PubMed] [Google Scholar]
Henriksen 1985 {published data only}
- Henriksen JM. Effect of inhalation of corticosteroids on exercise induced asthma: randomised double blind crossover study of budesonide in asthmatic children. British Medical Journal Clinical Research Ed 1985;291(6490):248‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heuck 1997b {published data only}
- Heuck C, Wolthers OD, Hansen M, Kollerup G. Short‐term growth and collagen turnover in asthmatic adolescents treated with the inhaled glucocorticoid budesonide. Steroids 1997;62(10):659‐64. [DOI] [PubMed] [Google Scholar]
Jatakanon 1998 {published data only}
- Jatakanon A, Lim S, Chung KF, Barnes PJ. An inhaled steroid improves markers of airway inflammation in patients with mild asthma. European Respiratory Journal 1998 Nov;12(5):1084‐8. [DOI] [PubMed] [Google Scholar]
Jonasson 1998 {published data only}
- Jonasson G, Carlsen KH, Blomqvist P. Clinical efficacy of low‐dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. European Respiratory Journal 1998;12(5):1099‐104. [DOI] [PubMed] [Google Scholar]
Jones 1994 {published data only}
- Jones AH, Langdon CG, Lee PS, Lingham SA, Nankani JP, Follows RM, et al. Pulmicort Turbohaler once daily as initial prophylactic therapy for asthma. Respiratory Medicine 1994;88(4):293‐9. [DOI] [PubMed] [Google Scholar]
Juniper 1990 {published data only}
- Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Effect of long‐term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid‐dependent asthmatics. American Review of Respiratory Disease 1990;142(4):832‐6. [DOI] [PubMed] [Google Scholar]
Kharitonov 1996 {published data only}
- Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. American Journal of Respiratory and Critical Care Medicine 1996;153(1):454‐7. [DOI] [PubMed] [Google Scholar]
Kivity 1994 {published data only}
- Kivity S, Fireman E, Greif J, Schwarz Y, Topilsky M. Effect of budesonide on bronchial hyperresponsiveness and pulmonary function in patients with mild to moderate asthma. Annals of Allergy 1994;72(4):333‐6. [PubMed] [Google Scholar]
Kuo 1994 {published data only}
- Kuo HP, Yu TR, Yu CT. Hypodense eosinophil number relates to clinical severity, airway hyperresponsiveness and response to inhaled corticosteroids in asthmatic subjects. European Respiratory Journal 1994;7(8):1452‐9. [DOI] [PubMed] [Google Scholar]
Lorentzson 1990 {published data only}
- Lorentzson S, Boe J, Eriksson G, Persson G. Use of inhaled corticosteroids in patients with mild asthma. Thorax 1990;45(10):733‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 1998 {published data only}
- Nelson HS, Bernstein IL, Fink J, Edwards TB, Spector SL, Storms WW, et al. Oral glucocorticosteroid‐sparing effect of budesonide administered by Turbuhaler: a double‐blind, placebo‐controlled study in adults with moderate‐to‐severe chronic asthma. Pulmicort Turbuhaler Study Group. Chest 1998;113(5):1264‐71. [DOI] [PubMed] [Google Scholar]
O'Byrne 1996 {published data only}
- O'Byrne P, Cuddy L, Taylor DW, Birch S, Morris J, Syrotuik J. Efficacy and cost benefit of inhaled corticosteroids in patients considered to have mild asthma in primary care practice. Canadian Respiratory Journal 1996;3(3):169‐75. [Google Scholar]
O'Connor 1992 {published data only}
- Evans PM, O'Connor BJ, Fuller RW, Barnes PJ, Chung KF. Effect of inhaled corticosteroids on peripheral blood eosinophil counts and density profiles in asthma. Journal of Allergy & Clinical Immunology 1993;91(2):643‐50. [DOI] [PubMed] [Google Scholar]
- O'Connor BJ, Ridge SM, Barnes PJ, Fuller RW. Greater effect of inhaled budesonide on adenosine 5'‐monophosphate‐induced than on sodium‐metabisulfite‐induced bronchoconstriction in asthma. American Review of Respiratory Disease 1992;146(3):560‐4. [DOI] [PubMed] [Google Scholar]
Osterman 1997 {published data only}
- Osterman K, Carlholm M, Ekelund J, Kiviloog J, Nikander K, Nilholm L, et al. Effect of 1 year daily treatment with 400 microg budesonide (Pulmicort Turbuhaler) in newly diagnosed asthmatics. European Respiratory Journal 1997;10(10):2210‐5. [DOI] [PubMed] [Google Scholar]
Prieto 1994 {published data only}
- Prieto L, Berto JM, Gutierrez V, Tornero C. Effect of inhaled budesonide on seasonal changes in sensitivity and maximal response to methacholine in pollen‐sensitive asthmatic subjects. European Respiratory Journal 1994;7(10):1845‐51. [DOI] [PubMed] [Google Scholar]
Rodriguez 1991 {published data only}
- Estrada Rodriguez JL, Belchi Hernandez J, Florido Lopez JF, Lopez Serrano C, Martinez Alzamora F, Ojeda Casas JA. Short‐term treatment of asthma with budesonide versus placebo. Journal of Investigational Allergology & Clinical Immunology 1991;1(4):266‐70. [PubMed] [Google Scholar]
Sekerel 1997a {published data only}
- Sekerel BE, Tuncer A, Saraclar Y, Adalioglu G. Inhaled budesonide reduces lung hyperinflation in children with asthma. Acta Paediatrica 1997;86(9):932‐6. [DOI] [PubMed] [Google Scholar]
Shapiro 1998a {published data only}
- Shapiro G, Bronsky EA, LaForce CF, Mendelson L, Pearlman D, Schwartz RH, et al. Dose‐related efficacy of budesonide administered via a dry powder inhaler in the treatment of children with moderate to severe persistent asthma. Journal of Pediatrics 1998;132(6):976‐82. [DOI] [PubMed] [Google Scholar]
Swystun 1998 {published data only}
- Swystun VA, Bhagat R, Kalra S, Jennings B, Cockcroft DW. Comparison of 3 different doses of budesonide and placebo on the early asthmatic response to inhaled allergen. Journal of Allergy & Clinical Immunology 1998;102(3):363‐7. [DOI] [PubMed] [Google Scholar]
Tan 1998 {published data only}
- Tan WC, Koh TH, Hay CS, Taylor E. The effect of inhaled budesonide on the diurnal variation in airway mechanics, airway responsiveness and serum neutrophil chemotactic activity in Asian patients with predominant nocturnal asthma. Respirology 1998;3(1):13‐20. [DOI] [PubMed] [Google Scholar]
Toogood 1990 {published and unpublished data}
- Toogood JH, Frankish CW, Jennings BH, Baskerville JC, Borga O, Lefcoe NM, et al. A study of the mechanism of the antiasthmatic action of inhaled budesonide. Journal of Allergy & Clinical Immunology 1990;85(5):872‐80. [DOI] [PubMed] [Google Scholar]
Vathenen 1991 {published data only}
- Vathenen AS, Knox AJ, Wisniewski A, Tattersfield AE. Effect of inhaled budesonide on bronchial reactivity to histamine, exercise, and eucapnic dry air hyperventilation in patients with asthma. Thorax 1991;46(11):811‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vathenen AS, Knox AJ, Wisniewski A, Tattersfield AE. Time course of change in bronchial reactivity with an inhaled corticosteroid in asthma. American Review of Respiratory Disease 1991;143(6):1317‐21. [DOI] [PubMed] [Google Scholar]
Wempe 1992a {published data only}
- Wempe JB, Tammeling EP, Koeter GH, Hakansson L, Venge P, Postma DS. Blood eosinophil numbers and activity during 24 hours: Effects of treatment with budesonide and bambuterol. Journal of Allergy & Clinical Immunology 1992;90(5):757‐65. [DOI] [PubMed] [Google Scholar]
- Wempe JB, Tammeling EP, Postma DS, Auffarth B, Teengs JP, Koeter GH. Effects of budesonide and bambuterol on circadian variation of airway responsiveness and nocturnal symptoms of asthma. Journal of Allergy & Clinical Immunology 1992;90(3 I):349‐57. [DOI] [PubMed] [Google Scholar]
Wempe 1992b {published data only}
- Wempe JB, Tammeling EP, Koeter GH, Hakansson L, Venge P, Postma DS. Blood eosinophil numbers and activity during 24 hours: Effects of treatment with budesonide and bambuterol. Journal of Allergy & Clinical Immunology 1992;90(5):757‐65. [DOI] [PubMed] [Google Scholar]
- Wempe JB, Tammeling EP, Postma DS, Auffarth B, Teengs JP, Koeter GH. Effects of budesonide and bambuterol on circadian variation of airway responsiveness and nocturnal symptoms of asthma. Journal of Allergy & Clinical Immunology 1992;90(3 I):349‐57. [DOI] [PubMed] [Google Scholar]
Wong 1994 {published data only}
- Wong CS, Wahedna I, Pavord ID, Tattersfield AE. Effect of regular terbutaline and budesonide on bronchial reactivity to allergen challenge. American Journal of Respiratory & Critical Care Medicine 1994;150(5 Pt 1):1268‐73. [DOI] [PubMed] [Google Scholar]
Wongtim 1995 {published data only}
- Wongtim S, Mogmued S, Chareonlap P, Limthongkul S. Effect of inhaled corticosteroids on bronchial hyperresponsiveness in patients with mild asthma. Asian Pacific Journal of Allergy & Immunology 1995;13(2):81‐5. [PubMed] [Google Scholar]
Yates 1996 {published data only}
- Yates DH, Kharitonov SA, Barnes PJ. An inhaled glucocorticoid does not prevent tolerance to the bronchoprotective effect of a long‐acting inhaled beta 2‐agonist [published erratum appears in Am J Respir Crit Care Med 1997 Apr;155(4):1491]. American Journal of Respiratory & Critical Care Medicine 1996;154(6 Pt 1):1603‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bisgaard 1990 {published data only}
- Bisgaard H, Munck SL, Nielsen JP, Petersen W, Ohlsson SV. Inhaled budesonide for treatment of recurrent wheezing in early childhood. Lancet 1990;336(8716):649‐51. [DOI] [PubMed] [Google Scholar]
Bisgaard 1993 {published data only}
- Bisgaard H. Systemic activity of inhaled topical steroid in toddlers studied by knemometry. Acta Paediatrica 1993;82(12):1066‐71. [DOI] [PubMed] [Google Scholar]
Booms 1997 {published data only}
- Booms P, Cheung D, Timmers MC, Zwinderman AH, Sterk PJ. Protective effect of inhaled budesonide against unlimited airway narrowing to methacholine in atopic patients with asthma. Journal of Allergy & Clinical Immunology 1997;99(3):330‐7. [DOI] [PubMed] [Google Scholar]
Clark 1997 {published data only}
- Clark DJ, Lipworth BJ. Evaluation of corticotropin releasing factor stimulation and basal markers of hypothalamic‐pituitary‐adrenal axis suppression in asthmatic patients. Chest 1997;112(5):1248‐52. [DOI] [PubMed] [Google Scholar]
Connett 1993 {published data only}
- Connett GJ, Lenney W, McConchie SM. The cost effectiveness of budesonide in severe asthmatics aged one to three years. British Journal of Medical Economics 1993;6:127‐34. [Google Scholar]
- Connett GJ, Warde C, Wooler E, Lenney W. Use of budesonide in severe asthmatics aged 1‐3 years. Archives of Disease in Childhood 1993;69(3):351‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahl 1982 {published data only}
- Dahl R, Johansson SA. Effect on lung function of budesonide by inhalation, terbutaline s.c. and placebo given simultaneously or as single treatments. European Journal of Respiratory Diseases ‐ Supplement 1982;122:132‐7. [PubMed] [Google Scholar]
de Blic 1996 {published data only}
- Blic J, Delacourt C, Bourgeois M, et al. Efficacy of nebulized budesonide in treatment of severe infantile asthma: a double‐blind study. Journal of Allergy & Clinical Immunology 1996;98(1):14‐20. [DOI] [PubMed] [Google Scholar]
Engel 1991 {published data only}
- Engel T, Dirksen A, Heinig JH, Nielsen NH, Weeke B, Johansson SA. Single‐dose inhaled budesonide in subjects with chronic asthma. Allergy: European Journal of Allergy & Clinical Immunology 1991;46(7):547‐53. [DOI] [PubMed] [Google Scholar]
Essen Zandvliet 1993 {published data only}
- Essen‐Zandvliet EE, Hop WC, Jong H, Ferwerda A, Kerrebijn KF. Minor acute effect of an inhaled corticosteroid (budesonide) on bronchial hyperresponsiveness to methacholine in children with asthma. European Respiratory Journal 1993;6(3):383‐6. [PubMed] [Google Scholar]
Fuglsang 1995 {published data only}
- Fuglsang G, Agertoft L, Vikre‐Jorgensen J, Pedersen S. Influence of budesonide on the response to inhaled terbutaline in children with mild asthma. Pediatric Allergy & Immunology 1995;6(2):103‐8. [DOI] [PubMed] [Google Scholar]
Heuck 1997a {published data only}
- Heuck C, Wolthers OD. Calculation of knemometric growth rates in group studies of children treated with exogenous glucocorticoids. Annals of Human Biology 1997;24(5):411‐8. [DOI] [PubMed] [Google Scholar]
Ilangovan 1993 {published data only}
- Ilangovan P, Pedersen S, Godfrey S, Nikander K, Noviski N, Warner JO. Treatment of severe steroid dependent preschool asthma with nebulised budesonide suspension. Archives of Disease in Childhood 1993;68(3):356‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kidney 1997 {published data only}
- Kidney JC, Boulet LP, Hargreave FE, et al. Evaluation of single‐dose inhaled corticosteroid activity with an allergen challenge model. Journal of Allergy & Clinical Immunology 1997;100(1):65‐70. [DOI] [PubMed] [Google Scholar]
Kozak‐Szkopek 1997 {published data only}
- Kozak‐Szkopek E, Ulmer WT. Inhalative glucocorticosteroids in chronic bronchitis [Inhalative budesonid‐therapie bei chronischer bronchitis]. Atemwegs Und Lungenkrankheiten 1997;23(9):542‐6. [MEDLINE: ; CN‐00230575 ‐ CCTR] [Google Scholar]
Noble 1992 {published data only}
- Noble V, Ruggins NR, Everard ML, Milner AD. Inhaled budesonide for chronic wheezing under 18 months of age. Archives of Disease in Childhood 1992;67(3):285‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paggiaro 1994 {published and unpublished data}
- Paggiaro PL, Dente FL, Morelli MC, Bancalari L, Franco A, Giannini D, et al. Postallergen inhaled budesonide reduces late asthmatic response and inhibits the associated increase of airway responsiveness to methacholine in asthmatics. American Journal of Respiratory & Critical Care Medicine 1994;149(6):1447‐51. [DOI] [PubMed] [Google Scholar]
Rocca 1996 {published data only}
- Rocca S, Malka M, Caekert A, Marty M. Influence of inhalation device on asthmatic patients quality of life: Switch MDI to Turbuhaler. Allergie Et Immunologie 1996;28(6):202‐17. [Google Scholar]
Shapiro 1998b {published data only}
- Shapiro G, Mendelson L, Kraemer MJ, Cruz‐Rivera M, Walton‐Bowen K, Smith JA. Efficacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroid‐dependent, persistent asthma. Journal of Allergy & Clinical Immunology 1998;102(5):789‐96. [DOI] [PubMed] [Google Scholar]
Sutochnikova 1996 {published data only}
- Sutochnikova OA, Samsonova MV, Cherniak AV, Cherniaev AL, Sokolov AS, Chuchalin AG. [The effect of the Russian inhalant glucocorticosteroid budesonide on bronchial inflammation and hyperreactivity during the long‐term treatment of bronchial asthma patients]. Ter Arkh 1996;68(3):48‐50. [PubMed] [Google Scholar]
Van Bever 1990 {published data only}
- Bever HP, Schuddinck L, Wojciechowski M, Stevens WJ. Aerosolized budesonide in asthmatic infants: a double blind study. Pediatric Pulmonology 1990;9(3):177‐80. [DOI] [PubMed] [Google Scholar]
Waalkens 1990 {published data only}
- Waalkens HJ, Essen‐Zandvliet EE, Gerritsen J, Duiverman EJ, Kerrebijn KF, Knol K. The effect of an inhaled corticosteroid (budesonide) on exercise‐induced asthma in children. Dutch CNSLD Study Group. European Respiratory Journal 1993;6(5):652‐6. [PubMed] [Google Scholar]
Wilson 1995 {published data only}
- Wilson N, Sloper K, Silverman M. Effect of continuous treatment with topical corticosteroid on episodic viral wheeze in preschool children. Archives of Disease in Childhood 1995;72(4):317‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1998 {published data only}
- Wilson AM, Brewster HJ, Lipworth BJ. Dose‐response comparison of systemic bioactivity with inhaled budesonide and triamcinolone acetonide in asthmatic adults. Journal of Allergy & Clinical Immunology 1998;102(5):751‐6. [DOI] [PubMed] [Google Scholar]
Wolthers 1998 {published data only}
- Wolthers OD, Heuck C. Differential effects of inhaled budesonide on serum osteocalcin in children and adolescents with asthma. Pediatric Allergy & Immunology 1998;9(3):150‐5. [DOI] [PubMed] [Google Scholar]
Yates 1998 {published data only}
- Yates DH, Worsdell M, Barnes PJ. Effect of an inhaled glucocorticosteroid on mast cell and smooth muscle beta 2 adrenergic tolerance in mild asthma. Thorax 1998;53(2):110‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chyrek‐Borowska 1994 {published data only}
- Chyrek‐Borowska S, Siergiejko Z, Poludniewska B. [Horacort (new steroid aerosol preparation) for treatment of bronchial asthma and allergic rhinitis]. Pneumonologia i Alergologia Polska 1994;62(1‐2):56‐63. [PubMed] [Google Scholar]
Damsbo 1994 {published data only}
- Damsbo N. [Budesonide in the treatment of mild asthma. A placebo‐controlled multicenter study in general practice]. Ugeskr Laeger 1994;156(43):6384‐8. [PubMed] [Google Scholar]
Gorski 1993 {published data only}
- Gorski P, Palczynsk C. [Clinical evaluation of budesonide forte action in patients with atopic bronchial asthma]. Polski Tygodnik Lekarski 1993;48:190‐2. [PubMed] [Google Scholar]
Sekerel 1997b {published data only}
- Sekerel BE, Tuncer A, Saraclar Y, Adalioglu G. The effect of inhaled budesonide on bronchial hyperreactivity in children with bronchial asthma. Cocuk Sagligi Ve Hastaliklari Dergisi 1997;40(3):351‐360. [Google Scholar]
Additional references
BTS 1997
- British Thoracic Society. The British guidelines on asthma management 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐20. [Google Scholar]
CONSORT 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637‐9. [DOI] [PubMed] [Google Scholar]
GINA 1995
- National Asthma Education and Prevention Program. Global initiative for asthma management and prevention NHBLI/WHO workshop report. Bethseda, MD: National Institute of Health, 1995. [NIH Publication No. 95‐3659] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996 Feb;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
NHLBI 1997
- National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Managment of Asthma, Expert Panel Report No. 2. Bethesda MD: NIH/National Heart, Lung and Blood Institute 1997, issue NIH Publication No. 97‐4051.


