ABSTRACT
Chronic kidney disease (CKD) perturbs the crosstalk with others organs, with the interaction between the kidneys and the heart having been studied most intensively. However, a growing body of data indicates that there is an association between kidney dysfunction and disorders of the central nervous system. In epidemiological studies, CKD is associated with a high prevalence of neurological complications, such as cerebrovascular disorders, movement disorders, cognitive impairment and depression. Along with traditional cardiovascular risk factors (such as diabetes, inflammation, hypertension and dyslipidaemia), non-traditional risk factors related to kidney damage (such as uraemic toxins) may predispose patients with CKD to neurological disorders. There is increasing evidence to show that uraemic toxins, for example indoxyl sulphate, have a neurotoxic effect. A better understanding of factors responsible for the elevated prevalence of neurological disorders among patients with CKD might facilitate the development of novel treatments. Here, we review (i) the potential clinical impact of CKD on cerebrovascular and neurological complications, (ii) the mechanisms underlying the uraemic toxins’ putative action (based on pre-clinical and clinical research) and (iii) the potential impact of these findings on patient care.
Keywords: cardiovascular, CKD, indoxyl sulphate, stroke, uraemic toxins
Graphical Abstract
Graphical Abstract.
INTRODUCTION
In 2017, the estimated worldwide prevalence of chronic kidney disease (CKD) was 9%, which corresponds to almost 850 million individuals [1]. Kidney disease has a major effect on overall health, both as a direct cause of morbidity and mortality, and as an important risk factor for cardiovascular disease [1]. CKD perturbs the crosstalk with other organs, with the interaction between the kidneys and the heart having been studied most intensively. However, a growing body of data indicates that diseases of the kidney are associated with diseases of the central nervous system. Mild CKD is highly prevalent in the general population and is already known to be associated with cognitive dysfunction [2]. Indeed, the epidemiologic data suggest that individuals at all stages of CKD have a higher risk of developing cognitive disorders and dementia. Furthermore, patients with CKD are more likely to present with clinically evident stroke and subclinical cerebrovascular disease (e.g. white matter hyperintensities), relative to the general population [3]. Given the high prevalence of kidney disease in the general population, the identification of mechanisms associated with cerebrovascular and neurological complications is critically important in the CKD population.
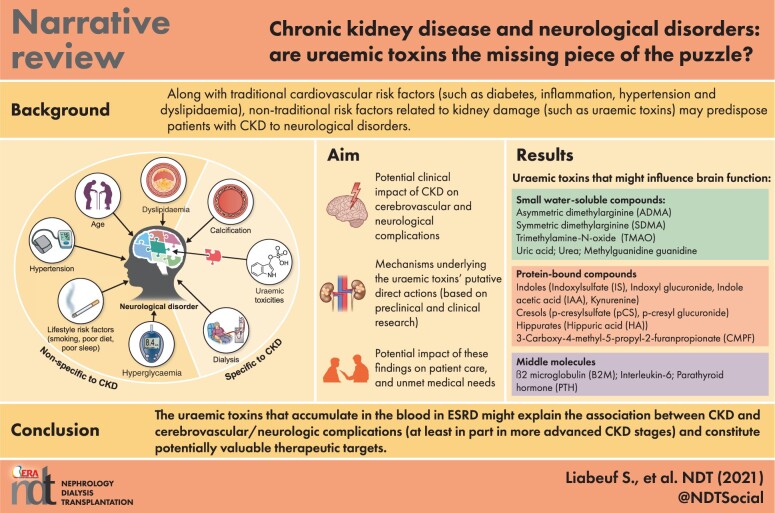
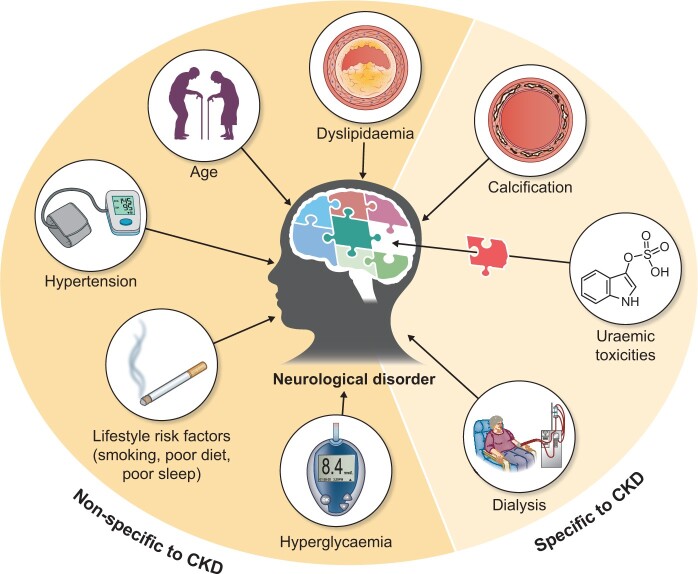
Along with traditional cardiovascular risk factors (such as diabetes mellitus, inflammation, hypertension and dyslipidaemia), non-traditional risk factors (such as uraemic toxins) related to kidney damage may predispose patients with CKD to neurological disorders (Figure 1). According to the European Uraemic Toxins Work Group (EUTox), uraemic toxins are harmful compounds that accumulate in the body during periods of renal function decline [4]. They are classified into three groups on the basis of their protein-binding properties and molecular weight: protein-bound solutes, middle molecules and small water-soluble solutes [4] (Table 1). Protein-bound toxins and certain large middle molecules are cleared poorly by dialysis. Uraemic toxins may have direct neurotoxic actions (such as astrocyte activation and neuronal death) and/or indirect actions via vascular effects (such as cerebral endothelial dysfunction, calcification and inflammation) [5]. Indeed, various uraemic toxins such as indoxyl sulphate (IS) have been implicated in the pathogenesis of neurological disorders in patients with CKD [6–8].
FIGURE 1.
The complicated puzzle of risk factors associated with neurological disorders in patients with CKD. Along with traditional cardiovascular risk factors (such as diabetes, hypertension and dyslipidaemia), non-traditional risk factors related to kidney damage (such as uraemic toxicities) may predispose patients with CKD to neurological disorders.
Table 1.
Uraemic toxins that might influence brain function
| Small water-soluble compounds | Protein-bound compounds | Middle molecules |
|---|---|---|
|
ADMA SDMA TMAO Uric acid Urea Methylguanidine guanidine |
Indoles
Cresols
Hippurates
|
B2M IL-6 PTH |
ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; TMAO, trimethylamine N-oxide; CMPF, 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid.
Here, we review (i) the potential clinical impact of CKD on cerebrovascular and neurological complications, (ii) the mechanisms underlying the uraemic toxins’ putative direct actions (based on pre-clinical and clinical research), (iii) the potential impact of these findings on patient care and (iv) unmet medical needs.
THE CLINICAL IMPACT OF CKD ON CENTRAL NERVOUS SYSTEM COMPLICATIONS
Cerebrovascular disease
A growing body of epidemiologic data suggests that impaired renal function is strongly associated with an increased risk of cerebrovascular disease. Indeed, atrial fibrillation and its major complication—ischaemic stroke—are very common in CKD patients [9]. Individuals in the general population with a slight decrease in their estimated glomerular filtration rate (eGFR) (≤60 mL/min/1.73 m2) have a greater risk of fatal or non-fatal ischaemic stroke, and a low eGFR (≤30 mL/min/1.73 m2) increases the risk of haemorrhagic stroke [3, 10]. In a meta-analysis of 63 cohort studies (encompassing 2 085 225 participants), 20 randomized controlled trials (encompassing 168 516 participants) and 30 392 reported strokes, the stroke risk increased linearly and additively with declining eGFR and increasing albuminuria. The risk of stroke increased by 7% [risk ratio (RR) (95% confidence interval, CI): 1.07 (1.04–1.09)] for every 10 mL/min/1.73 m2 decrement in the eGFR. Furthermore, a 25 mg/mmol increment in the albumin–creatinine ratio was associated with a 10% increase in the risk of stroke [RR (95% CI): 1.10 (1.01–1.20)] [11]. Moreover, stroke was the third most common cardiovascular cause of death in patients with CKD [11]. Importantly, CKD and its components (proteinuria and low eGFR) also affect the clinical outcome after ischaemic stroke. Indeed, patients with CKD had significantly higher risks of neurologic deterioration, in-hospital mortality and a poor functional outcome [12]. End-stage renal disease (ESRD) is also associated with other types of cerebrovascular damage, such as cerebral microbleeds and silent cerebral infarcts. In particular, patients on haemodialysis (HD) have an exceptionally high incidence of cerebral microbleeds [13].
Cognitive disorders
Various research results suggest that patients at all CKD stages may have a higher risk of cognitive disorders than patients without CKD. The eGFR is inversely correlated with overall cognitive function in patients with CKD [14]. The estimated prevalence of cognitive impairment in this population ranges from 30% to 60%, depending on the definition of cognitive impairment that varies between studies [15].
As in the general population, quantifying neurological impairment is particularly difficult to evaluate, indeed the method used to assess cognitive impairment in patients with CKD has a major influence on the outcome. The various screening tests do not have the same degree of accuracy—especially in patients on HD [16]. Neuropsychological assessments must be standardized and comprehensive, since impairments can develop early in CKD and the various skills do not decline at the same rate [17]. However, comprehensive neurological assessments can be long and tiring, and cognitive performance fluctuates in patients on HD over the course of a dialysis session [15, 16]. In a study of a small number of patients on HD (n = 28), cognitive performance was worst during the dialysis session itself (probably due to haemodynamic effects) and best shortly before the session or on the day after dialysis [18]. Some data observed an improvement of cognitive performance after an HD session compared with before [19]. Neuropsychological and electrophysiological performance seems to be more stable (and close to normal values) in patients on continuous ambulatory peritoneal dialysis (PD) than in patients on HD [20]. However, Drew et al.’s [21] study of 40 patients did not find a difference in cognitive performance between the hour before the HD session and the first hour of the session, suggesting that cognitive assessment during an HD session is still relevant to screen cognitive disorders in those patients. Executive functions are most frequently impaired in patients with CKD [16]. Sleep disorders and depression frequently affect patients with CKD and are able to interfere with cognitive assessments [22]. The interpretation of cognitive performance in patients with CKD remains tricky and requires multidisciplinary expertise (for a review, see [23]).
The use of certain drugs may have an (indirect) adverse effect on cognitive function of patients with CKD. Some drugs frequently used in patients with CKD such as opiates, benzodiazepine, antidepressants and drugs with anticholinergic properties are known to aggravate cognitive disorders or induce delirium [24, 25]. In patients with CKD, poor drug clearance and greater drug penetration through the blood–brain barrier (BBB) might lower the threshold for neurotoxicity and contribute to cognitive dysfunction. Indeed, most patients with CKD take several medications on a daily basis [26–28].
Movement disorders: Parkinson’s disease
Movement disorders are among the central nervous system complications in uraemic patients. Asterixis, multifocal myoclonus and restless leg syndrome are well documented in this population and so will not be reviewed here [29, 30].
A few large epidemiologic studies have found an increased risk of Parkinson’s disease risk in patients with CKD [31–34]. All the studies were performed in Asia (in Taiwan and South Korea). In 3 580 435 individuals aged ≥65 years who had received health check-ups from the South Korea National Health Insurance Service, 30 813 developed Parkinson’s disease; renal dysfunction and proteinuria were independent risk factors for the development of Parkinson’s disease, with a hazard ratio (95% CI) of 1.36 (1.31–1.42) for an eGFR of 30–60 mL/min/1.73 m2 and 1.74 (1.32–1.63) for an eGFR <30 mL/min/1.73 m2 [31]. The progressive association between decreasing of eGFR and increased risk of Parkinson’s disease incidence was confirmed in another study of data from the South Korea National Health Insurance Service [32]. In a Taiwanese study of patients with newly diagnosed ESRD versus controls, the incidence of Parkinson’s disease was 1.55-fold higher in the ESRD cohort than in the control cohort (48.8 versus 31.7/10 000 person-years, respectively), giving an adjusted hazard ratio (95% CI) of 1.73 (1.39–2.15) [33]. Hence, patients with CKD are more prone to develop Parkinsonism, independent of their diabetic status [34]. CKD and Parkinson’s disease share some pathophysiologic mechanisms, such as oxidative stress, hypertension, vitamin D deficiency and anaemia (for a review, see [35]). Nonetheless, the mechanisms that underlie this relationship have not been fully elucidated.
Anxiety and depression
Anxiety is associated with poor health outcomes among patients with CKD. A recent meta-analysis reported that the pooled prevalence of anxiety disorders (nine studies, n = 11 071) among patients with CKD was 19%, while that of elevated anxiety symptoms (52 studies, n = 110 739) was 43%, with a higher prevalence in dialysis patients than in pre-dialysis patients [36]. Depression is highly prevalent among patients with CKD and healthcare professionals must be aware of this important issue since it has a major impact on quality of life and is often not recognized. A systematic review and meta-analysis of 55 982 patients with CKD found a prevalence of 26.5% for depressive symptoms and 21.4% for clinically significant depression [37]. Accordingly, the antidepressant prescription rate in patients with CKD is nearly 1.5 times higher than in general population [38]. Anxiety and depression can further reduce a CKD patient’s quality of life, which is already lower than in general population [39].
Dialysis and transplantation affect central nervous system complications
Cerebrovascular disease is a common cause of death in patients on long-term dialysis [40]. Indeed, the risk of hospitalization for ischaemic and haemorrhagic stroke is 4- to 10-fold greater in patients on HD than in the general population [11]. It has been suggested that patients on HD have a higher prevalence of silent cerebral infarction (relative to control) [9] and an even higher prevalence of subclinical cerebral lacunar infarctions, microbleeds and loss of white matter integrity (relative to patients with CKD) [3, 41, 42]. In elderly patients, the incidence of stroke rose within a month of starting HD and remained high thereafter, when compared with the period before HD [43]. Although HD is a life-saving renal replacement therapy, a growing body of data shows that the side effects of the HD procedure contribute to cerebrovascular damage. For example, Eldehni et al. [44] showed that patients starting HD treatment had an impressive progression of white matter lesions during the first year of HD. Interestingly, lowering the dialysate temperature was associated with greater intrasession haemodynamic stability and slower progression of white matter lesions [44]. Using intradialytic positron emission tomography [45, 46], near-infrared spectroscopy [46, 47] or ultrasound measurement of the mean flow velocity in the cerebral arteries [48], it has been shown that HD induces an acute reduction in brain perfusion [46–49]. These reductions in brain perfusion were accompanied by an intrasession decline in cognitive functions, including global function, executive function and verbal fluency [49]. Repeated acute reductions in brain perfusion may have harmful long-term consequences. The percentage decline in mean blood flow velocity in the cerebral arteries was significantly correlated with the progression of the white matter hyperintensity burden over a 12-month period [49]. These findings strongly suggest that HD-induced haemodynamic changes have a pathophysiological role in the accelerated progression of brain damage in patients on HD. Accordingly, Polinder-Bos et al. [45] found a significant association between a higher ultrafiltration volume and lower intradialytic cerebral blood flow. However, the researchers did not find a significant association between changes in intradialytic blood pressure course and brain perfusion or brain tissue oxygenation [45, 46]. Likewise, a study of a larger cohort of patients by MacEwen et al. [47] (n = 58) showed that changes in blood pressure were poor predictors of downstream brain ischaemia. It is therefore possible that HD-related factors other than haemodynamic changes are involved, for example an interaction between blood and the extracorporeal circuit, which might lead to HD-induced systemic inflammation and thus endothelial dysfunction. Furthermore, an intradialytic rise in the plasma bicarbonate concentration and blood pH can affect brain perfusion. Indeed, a higher blood pH was associated with a lower cerebral blood flow in most brain regions shortly after the start of HD, relative to the pre-dialysis values [45]. It was also recently reported that even with synthetic dialysers, intradialytic hypoxia occurs in a large proportion of patients and is associated with higher all-cause hospitalization and mortality rates [49]; these observations have renewed interest in studying repeated intradialytic hypoxia as a cause of accumulating brain damage.
Although patients with CKD share many of the same risk factors for cerebrovascular disease as the general population, there are a number of uraemia-related risk factors (such as uraemic toxins), that have an additional role in promoting neurological disorders. Indeed, the fact that cognitive function improves after kidney transplantation [50, 51] suggests that uraemic toxins have a role in this impairment. In fact, a marked reduction in uraemic toxin levels was noted 1 month after kidney transplantation [52, 53]. Interestingly, van Sandwijk et al. [50] recently highlighted an improvement in white matter integrity after kidney transplantation.
IMPACTS OF URAEMIC TOXINS ON CEREBROVASCULAR DISEASES AND COGNITION
The progressive loss of kidney function typically observed in CKD is accompanied by the retention of a range of metabolites; this is due to decreased renal clearance and (in some cases) an increase in generation. Many of these solutes (uraemic toxins) have been shown to exert biological activity, hence affecting the functioning of cells and organs, resulting in the uraemic syndrome [4, 54]. As mentioned in the Introduction section, EUTox classifies uraemic toxins as small water-soluble compounds, protein-bound compounds or middle molecules [4, 54].
We systematically searched the literature (the MEDLINE database, up until 31 March 2021) for publications on the relationship between uraemic toxins and neurological complications by combining the following key words (‘uremic toxins and brain’ + ‘uremic toxins and stroke’ + ‘uremic toxins and cognitive’ + ‘uremic toxins and depression’ + ‘uremic toxins and anxiety’ + ‘uremic toxins and neurological’ + ‘uremic toxins and neurodegeneration’ + ‘uremic toxins and Parkinson’). A total of 164 articles were analysed; after the exclusion of 56 reviews, 15 redundant articles and 71 irrelevant articles, a total of 22 original articles were selected. The uraemic toxins cited in these publications are listed in Table 1. Most of these studies focused on the direct impact of protein-bound compounds on the brain. Below, we describe the origin of the incriminated uraemic toxins and their putative direct effects on the brain.
THE ORIGIN OF URAEMIC TOXINS
The origin of uraemic toxins is presented in the Supplementary data.
DIRECT EFFECTS OF URAEMIC TOXINS ON THE BRAIN
Protein-bound uraemic toxins can have direct and/or indirect effects on the brain. Most of the recent literature has focused on direct effects (Table 2).
Table 2.
Summary of pre-clinical and clinical studies of direct associations between uraemic toxins and cerebrovascular/neurologic complications
| First author | Model | Uraemic toxin(s) studied | Main findings |
|---|---|---|---|
| In vitro studies | |||
| Oshima et al. [55] | Bulbospinal neurons in the RVLM |
Uric acid IS Methylguanidine |
Uric acid, IS and methylguanidine directly stimulate bulbospinal RVLM neurons via specific transporters, such as OAT1, OAT3 OCT3 and URAT1 |
| Adesso et al. [8] |
Glioma cell line (C6) Sera from 18 participants: 4 healthy people, 8 patients with CKD and 6 patients on HD |
IS | The sera of patients with CKD induced significant inflammation in astrocyte cells, in proportion to the serum IS concentration. The IS adsorbent AST-120 reduced this inflammatory response. |
| Lin et al. [56] | Human primary astrocytes | IS | IS stimulated the release of reactive oxygen species, increased levels of NRF-2 and reduced the mitochondrial membrane potential. IS also triggered astrocyte apoptosis by inhibiting the mitogen-activated protein kinase pathway |
| Watanabe et al. [57] | Mouse hippocampal neuronal HT-22 cells |
Indole IS IAA pCS Hippurate |
Indole, IS, pCS and IAA significantly decreased the viability of HT-22 cells, which was associated with a significant decrease in glutathione levels. |
| Animal studies | |||
| Ohtsuki et al. [58] |
Adult male Wistar rats, mature female Xenopus laevis frogs Determination of brain efflux index and OAT3 expression |
IS | OAT3 mediates the brain-to-blood transport of IS and is also involved in the efflux of neurotransmitter metabolites and drugs |
| Sato et al. [59] | 4 groups of mice: (i) control (n = 16), (ii) AST-120 (n = 16), (iii) RF (n = 17) and (iv) RF + AST-120 (n = 16); evaluation of uraemic toxin accumulation in different organs, including the brain |
IS pCS |
IS and pCS accumulated in the brain of mice with RF. The oral adsorbent AST-120 prevented (to some extent) the tissue accumulation of IS and pCS |
| Mair et al. [60] | CSF and plasma ultrafiltrate were obtained from rats 48 h after a sham operation (control; n = 10) or bilateral nephrectomy (n = 10). The samples were analysed using an established metabolomic protocol | 248 solutes including IS, TMAO, hippurate and urea | The CSF levels of the great majority of uraemic solutes were elevated in rats with RF but typically increased less than in the plasma ultrafiltrate |
| Karbowska et al. [7] |
3 groups of rats: (i) control group (tap water without IS), (ii) experimental group with 100 mg/kg of body weight of IS/day and (iii) experimental group with 200 mg/kg of body weight of IS/day IS concentrations were measured in the cerebellum, brainstem, cortex, hypothalamus and striatum with hippocampus. Behavioural tests were performed and brain levels of monoamines (norepinephrine, epinephrine, dopamine and serotonin) and their metabolites were assayed |
IS |
IS accumulation was greatest in the brainstem IS led to behavioural alterations involving apathetic behaviour, increased stress sensitivity and reduced locomotor and exploratory activity. IS might contribute to the impairment of spatial memory and motor coordination. These results could not be explained completely by changes in cerebral monoamine concentrations and turnovers |
| Bobot et al. [61] | 3 rat models of CKD: (i) an adenine-rich diet, (ii) 5/6 nephrectomy and (iii) AhR−/− knockout mice overloaded with IS in the drinking water. Evaluations of BBB disruption using SPECT/CT, BBB permeability using imaging markers and neurologic impairments using neurobehavioural tests | IS | IS led to BBB disruption. Cognitive impairment in the three models was correlated with serum levels of IS and with BBB disruption. Non-CKD AhR−/− knockout mice were protected against IS-induced BBB disruption and cognitive impairment |
| Sun et al. [62] |
2 groups of mice: (i) kidney-intact controls (n = 20) and (ii) unilaterally nephrectomized (n = 120) mice divided into four groups (n = 15 per group) receiving various doses of pCS (0, 1, 10 and 100 mg/kg/day) intraperitoneally For the intervention, two groups of unilaterally nephrectomized mice were treated daily with pCS (100 mg/kg): (i) control (normal saline administration) (n = 16) and (ii) AST-120 (400 mg/kg) via oral gavage Behavioural evaluation |
pCS | Apparent deposition of pCS in the prefrontal cortical tissues was associated with several abnormal behaviours, such as depression, anxiety and cognitive impairment. However, pCS accumulation and behavioural changes were not observed at lower doses of 1 and 10 mg/kg/day in the unilateral nephrectomy group or at doses of 1, 10 and 100 mg/kg/day in the kidney-intact controls. These changes were alleviated by the uraemic toxin adsorbent AST-120 |
| Watanabe et al. [57] |
2 groups of rats: (i) controls (n = 14) and (ii) CKD (n = 15) induced by an adenine-rich diet Histological examination of rat brain. Behavioural assessments of spatial learning and memory |
No uraemic toxin measurements | The CKD group had larger numbers of pyknotic neuronal cells. The two groups did not differ in spatial learning and memory abilities |
| Clinical studies: observational studies | |||
| Bossola [63] |
80 patients on HD Assessment for depression and anxiety with the BDI and the HARS |
IL-6 PTH |
In a multivariate analysis, there was a direct, inverse correlation between BDI and IL-6 and creatinine. The HARS score was significantly correlated with the PTH levels in a univariate analysis only |
| Hsu et al. [64] | A cross-sectional study of 209 patients with CKD and a history of depression |
IS pCS Urea B2M |
Depressive patients had lower IS levels. The levels of urea, B2M and pCS were not significantly associated with depression |
| Yeh et al. [65] |
199 patients with CKD and 84 matched non-CKD participants Cognitive function was evaluated using comprehensive neuropsychological tests (executive function, memory, information processing speed, language, visuospatial function and attention): WAIS-similarity, trail-making B Frontal Assessment Battery, controlled oral word association, selective reminding tests, WAIS-digit symbol test, the trail-making A test, WAIS-vocabulary test, visual, discrimination test and WAIS-digit span test |
IS pCS |
In early-stage CKD, IS was associated with poorer executive function but pCS was not significantly associated with cognitive function |
| Ye et al. [66] |
271 healthy subjects, 596 patients with mild cognitive impairment and 197 patients with Alzheimer’s disease The MMSE and the AD Assessment Scale—cognitive subscale (ADAS-cog) were assessed serially |
Uric acid | Higher levels of uric acid were associated with slower cognitive decline |
| Sleeman et al. [67] |
154 patients with newly diagnosed Parkinson’s disease and 99 age-matched controls The Movement Disorders Society Unified Parkinson’s Disease Scale Part III was used to assess motor severity and the Montreal Cognitive Assessment was used to assess global cognition |
Urate Homocysteine |
A lower serum urate concentration was associated with worsening motor function, while a higher homocysteine concentration was associated with cognitive decline and worse motor function |
| Efstathiadou et al. [68] |
Mendelian randomization approaches Clinical outcomes: cognitive function, Alzheimer’s disease, coronary heart disease, myocardial infarction, systolic blood pressure and stroke |
28 genetic variants related to the serum uric acid | A Mendelian randomization study did not evidence a clinically relevant causal effect of genetically determined serum urate on a range of cardiovascular and neurovascular outcomes |
| Lin et al. [69] |
260 patients on HD Cognitive functions were evaluated with the MMSE and the CASI |
IS pCS |
Both free IS and free pCS were negatively associated with the CASI and MMSE scores. After controlling for confounders, circulating free IS levels were still negatively associated with MMSE and CASI scores but there was no correlation between free pCS and the total MMSE score or the total CASI score |
| Lin et al. [70] |
230 patients on HD Cognitive functions evaluated with the MMSE and the CASI |
IAA HA |
Serum IAA was associated with cognitive impairment, based on the MMSE and CASI scores. There was no correlation between the serum HA level and cognitive status |
| Li et al. [71] |
222 patients on PD Global cognition (the modified MMSE) and executive function tests |
Urea B2M |
Higher middle molecule clearance was independently associated with better performance in general cognition and executive function tests in patients with PD |
| Linde et al. [72] |
10 kidney transplants recipients and 18 controls [9 patients on HD, and 9 patients with CKD Stages 4 or 5 (eGFR <30 mL/min/1.73 m2) who were not on dialysis] Extensive neuropsychological assessment |
IS, pCS, IAA, TMAO, indoxyl glucuronide, p-cresyl glucuronide, phenylglucuronide, CMPF, HA, phenyl sulphate, kynurenine, tryptophan, kynuraenic acid, tyrosine, phenylalanine and phenylacetylglutamine | The serum concentration of most uraemic toxins decreased significantly within 1 week of kidney transplantation. There were no significant improvements in cognitive function that could be specifically related to kidney transplantation in the first 3 months after the procedure |
|
Sankowski et al. [73] |
(i) patients with Parkinson’s disease (n = 18) and (ii) controls (n = 9) Plasma and CSF samples |
IS pCS TMAO ADMA SDMA |
In patients with Parkinson’s disease, the CSF: plasma ratios for IS and pCS were higher than in controls. Patients with motor fluctuations had higher CSF levels of pCS (P = 0.0043), IS (P = 0.0361), ADMA (P = 0.0017), SDMA (P = 0.02614) and TMAO (P = 0.0179) than other Parkinson's patients did |
WAIS, Wechsler Adult Intelligence Scale; OCT, organic cation transporter; TMAO, trimethylamine N-oxide; ADMA, asymmetric dimethylarginine; CMPF, 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid; SDMA, symmetric dimethylarginine.
In vitro studies
Some recent data suggest that uraemic toxins can have a direct impact on neurons. For example, Oshima et al. [55] reported that uric acid, IS and methylguanidine directly stimulate neurons in the bulbospinal rostral ventrolateral medulla (RVLM) by increasing the oxidative stress. Watanabe et al. [57] recently examined the effects of uraemic toxins on the mouse hippocampal neuronal cell line HT-22. Indole, IS and indole acetic acid (IAA) significantly decreased the viability of the HT-22 cells, with a parallel decrease in glutathione levels [57]. Similarly, sera from patients with CKD induced significant inflammation in astrocytes; the inflammation was proportional to the serum IS concentration, and treatment with the activated charcoal adsorbent AST-120 reduced this inflammatory response. IS further increased inflammation and oxidative stress in primary central nervous system cells (via nuclear factor-κB and AhR activation) and induced neuron death [6, 8]. In an in vitro study of human astrocytes, IS activated the production of reactive oxygen species and downregulated the production of cell-protective factors like nuclear factor (erythroid-derived 2)-like 2. Moreover, IS triggered astrocyte apoptosis by inhibiting the mitogen-activated protein kinase pathway [56].
Animal studies
Hénaut et al. [74] investigated the cellular and molecular mechanisms by which uraemia worsened the severity of ischaemic stroke after transient middle cerebral artery occlusion (tMCAO) in mice. In the acute phase of stroke recovery, CKD aggravated tMCAO-induced ischaemic brain damage by increasing neuronal loss and apoptosis both in the ischaemic penumbra and the ischaemic core. The increased ischaemic damage observed in CKD mice was associated with impaired post-stroke recovery (relative to non-CKD control mice), as evidenced by greater weight loss, decreased muscle strength, impaired motor coordination and reduced physical resistance to tiredness. An early increase in local inflammation and an impairment in tissue repair in CKD mice were associated with greater ischaemic damage during the acute phase of stroke recovery [74].
It has been suggested that the negative impact of uraemia on cerebrovascular disease and cognition might be due (at least in part) to uraemic toxins in general and their accumulation in the cerebrospinal fluid (CSF) in particular. Indeed, pre-clinical studies have suggested that levels of many uraemic solutes are normally kept in low concentration in the CSF (relative to the plasma ultrafiltrate) by the action of the BBB and blood–CSF barriers. These barriers remain functional when the kidney function fails but cannot prevent the accumulation of uraemic solutes in the CSF [60]. Under normal conditions, the concentration of IS in the brain is reportedly 3.4 times lower than in the serum [60]. Although the source of IS in the brain has not been identified, this limited distribution might be due to the brain-to-blood transfer of IS by the organic anion transporter (OAT) at the BBB [58]. Ohtsuki et al. [58] used the brain efflux index method to examine this transport at the BBB and the involvement of OAT3 in IS transport. These results suggested that OAT3 mediates the brain-to-blood transport of IS and is also involved in the efflux of neurotransmitter metabolites and drugs like acyclovir, cefazolin, baclofen, 6-mercaptopurine, benzoic acid and ketoprofen [58]. Therefore, inhibition of OAT3-mediated brain-to-blood transport might occur in CKD; this would lead to the accumulation of neurotransmitter metabolites and drugs in the brain.
In a mouse model of renal failure (RF), Sato et al. [59] used liquid chromatography–tandem mass spectrometry to determine the levels of IS and p-cresyl sulphate (pCS) in various organs, including the brain. Brain levels of IS and pCS were significantly higher in mice with RF than in control mice. Furthermore, treatment with AST-120—an orally administered polymer that adsorbs low-molecular-weight compounds like indole and p-cresol (precursors of IS and pCS)—was associated with a decrease in brain IS and pCS levels in mice with RF [59].
In a single-photon emission computed tomography (SPECT) imaging study of three rodent models of CKD, Bobot et al. [61] showed that AhR activation by IS leads to disruption of the BBB. Cognitive impairment and BBB disruption were correlated with the serum IS levels [61].
A recent study in the rat confirmed that IS accumulates in the brain (in the brainstem, cerebellum, striatum and hippocampus) and influences the animals’ behavioural profile and cerebral monoamine levels, and thus provided evidence of IS’s neurotoxic activity [7]. The researchers also showed that chronic exposure to IS leads to behavioural alterations, with apathetic behaviour, increased stress sensitivity and reduced locomotor and exploratory activity. Furthermore, IS can contribute to impairments in spatial memory and motor coordination [7]. Watanabe et al. [57] recently reported that rats with adenine-induced CKD presented elevated levels of oxidative stress markers and (in some cases) had pyknotic neuronal cells in the hippocampus.
In unilaterally nephrectomized mice, the serum pCS concentration increased progressively during the administration of pCS at a dose of 100 mg/kg/day. Furthermore, pCS deposition in the prefrontal cortical tissues appeared to be associated with several abnormal behaviours, such as depression, anxiety and cognitive impairment. However, pCS accumulation and behavioural changes were not observed at doses of 1 and 10 mg/kg/day in unilaterally nephrectomized mice or at doses of 1, 10 and 100 mg/kg/day in healthy mice. These changes were alleviated by administration of the adsorbent AST-120 [62].
Clinical studies
Clinical studies have confirmed the above-described pre-clinical findings in which uraemic toxins are present in the CSF. A recent study measured the concentrations of uraemic toxins (including IS and pCS) in plasma and CSF from control patients and patients with Parkinson’s disease; the CSF–plasma ratio was higher in the latter group. Patients with motor fluctuations had higher levels of uraemic toxins in the CSF but not in the plasma [73].
Only a few clinical studies have evaluated the correlation between neurologic symptoms and uraemic toxin levels. In a cross-sectional study of 204 patients with CKD, individuals with symptoms of depression had surprisingly lower plasma IS levels [64]. The levels of urea, β2 microglobulin (B2M) and pCS were not significantly associated with depression [64]. In 80 patients on HD, there was a direct, inverse correlation between the Beck Depression Inventory (BDI) score and the levels of interleukin-6 (IL-6) [P = 0.042, odds ratio (95% CI): 1.31 (1.01–1.71)] and creatinine [P = 0.050, odds ratio (95% CI): 0.73 (0.54–1.00)], and the Hamilton Anxiety Rating Scale (HARS) correlated significantly with parathyroid hormone (PTH) levels only in univariate analysis [63]. Indeed, IL-6 has been consistently found to be elevated in stress reactions and depression patients, highlighting the important role of inflammatory processes in depression pathogenesis [75, 76].
Similarly, clinical data on the impact of uraemic toxins on cognitive functions are scarce. A cohort of 199 patients with CKD had poorer cognitive function and higher serum pCS and IS levels than 84 patients with normal renal function. In the patients with CKD, a higher serum IS level was independently associated with poor executive function but the serum pCS level was not associated with cognitive function [65]. A recent study that included patients on HD sought to investigate the association between circulating levels of free IS, pCS, IAA and hippuric acid (HA) on cognitive function, as assessed with the Mini-Mental State Examination (MMSE) and the Cognitive Abilities Screening Instrument (CASI). Both free IS and free pCS levels were negatively associated with the MMSE and CASI scores. After controlling for confounders, the MMSE and CASI scores were still associated with the circulating free IS level but not with the free pCS level [69]. Serum IAA was associated with cognitive impairment, according to the MMSE and CASI scores. There was no correlation between serum HA levels and cognitive function [70]. Cognitive function improves after kidney transplantation [50, 51] suggests that uraemic toxins have a role in this impairment. A recent exploratory study of 10 kidney transplant recipients and 18 non-transplanted patients with CKD sought to evaluate five major cognitive domains (memory, attention and concentration, information processing speed, abstract reasoning and executive function) via an extensive neuropsychological assessment before kidney transplantation and then 1 week and 3 months after transplantation. The researchers did not find any evidence of cognitive changes after kidney transplantation, relative to the control groups. The small number of patients might explain the lack of a significant change. However, the researchers observed clear changes in the serum levels of uraemic toxins after transplantation—even within 3 days [72], when previously reported a marked reduction after 1 month after kidney transplantation [52, 53].
The findings on the relationship between uric acid and cognitive functions are contradictory. Low serum levels of uric acid are associated with dementia and Parkinson’s disease [67]. Therefore, one would expect high levels to be neuroprotective—perhaps because uric acid is a major antioxidant in the plasma [66, 77]. In a clinical trial in non-CKD patients with acute ischaemic stroke, the addition of uric acid to thrombolytic therapy did not increase the proportion of patients who achieved excellent outcome after stroke compared with placebo [RR (95% CI): 1.23 (0.96–1.56)] [78]. A reanalysis of the trial concluded that in women, who usually have lower serum urate levels, the administration of uric acid reduced infarct growth and was better than placebo to reach excellent outcome [RR (95% CI): 2.09 (1.05–4.15)] [79]. However, a high serum uric acid level is a hallmark of vascular disease. Hence, any neuroprotective benefit of hyperuricaemia is probably counterbalanced by the effects of neurovascular disease [68]. Accordingly, gout (in which hyperuricaemia is usually unrelated to CKD) has never been linked to cognitive deficits. Therefore, an association between CKD-related hyperuricaemia and cognitive dysfunction is surprising [80] and should be interpreted with caution, given the large number of other toxins that inevitably accompany CKD.
INDIRECT EFFECTS OF URAEMIC TOXINS ON THE BRAIN
Uraemic toxins might also have harmful indirect effects on the brain via oxidative stress [6, 81], vascular dysfunction [82, 83], vascular calcification [84–86], coagulation disorders [87], and cardiovascular diseases like atrial fibrillation and hypertension [88, 89]. These indirect effects have been reviewed in detail elsewhere and will not be described here [5, 83].
POTENTIAL IMPACTS ON PATIENT CARE
Uraemic toxins appear to be factors that (in addition to others) specifically influence the increased prevalence of cerebrovascular and neurological signs and symptoms in patients with CKD. Drug treatments that modulate uraemic toxin concentrations (e.g. the administration of intestinal chelators) might prevent the development of harmful effects in patients with CKD. However, there are few therapeutic options. Although phosphate binders can effectively reduce phosphate levels, repositioned products from this drug class do not appear to greatly decrease circulating levels of other uraemic toxins [90]. The orally administered activated charcoal adsorbent AST-120 is widely used in Asian countries to specifically decrease uraemic toxin levels. However, its putative effect on neurological outcomes has yet to be assessed in clinical trials.
It is unlikely that uric acid has a role in brain dysfunction, and so the value of widely used urate-lowering drugs in the treatment of the cerebrovascular and neurological signs and symptoms of CKD can be questioned. Indeed, allopurinol does not modify cognitive impairment in a rare genetic form of hyperuricaemia (Lesch–Nyhan syndrome) [91]. However, high doses of allopurinol and febuxostat reportedly reduce the risk of dementia [92]. An ongoing trial expected to be completed in 2021 is seeking to determine allopurinol’s potential value in ischaemic stroke in patients with eGFR ≥30 mL/min/1.73 m2 [93]. Future clinical studies of CKD and cognitive impairment should also consider the use of urate-lowering drugs.
Patients with CKD are frequently affected by gut dysbiosis, which might increase the production of gut-derived uraemic toxins [94]. Probiotics and other nutritional therapies might reduce gut dysbiosis and decrease circulating uraemic toxin levels, oxidative stress and inflammation [95]. Since protein-bound uraemic toxins are difficult to remove by HD, the gut microbiota might be an alternative target for reducing circulating levels of these compounds and thus their neurologic and other toxic effects in patients with CKD.
New dialysis techniques or methods for better removing protein-bound uraemic toxins might help to reduce the neurologic signs and symptoms that frequently affect patients on HD [96, 97].
Lastly, and as mentioned above, kidney transplantation is followed by a rapid decrease in uraemic toxin levels [56, 74], an improvement in cognitive function [55, 57] and greater white matter integrity [55].
CONCLUSION
It is important to identify factors that explain the association between CKD and cerebrovascular/neurologic complications. The uraemic toxins that accumulate in the blood in ESRD might explain this association (at least in part in more advanced CKD stages) and constitute potentially valuable therapeutic targets.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Professor Giovambattista Capasso, acting chair of CONNECT Action and members of COST Action, for their support.
APPENDIX
CONNECT collaborators are
Giovambattista Capasso; Alexandre Andrade; Maie Bachmann; Inga Bumblyte; Adrian Constantin Covic; Pilar Delgado; Nicole Endlich; Andreas Engvig; Denis Fouque; Casper Franssen; Sebastian Frische; Liliana Garneata; Loreto Gesualdo; Konstantinos Giannakou; Dimitrios Goumenos; Ayşe Tuğba Kartal; Laila-Yasmin Mani; Hans-Peter Marti; Christopher Mayer; Rikke Nielsen; Vesna Pešić; Merita Rroji (Molla); Giorgos Sakkas; Goce Spasovski; Kate I. Stevens; Evgueniy Vazelov; Davide Viggiano; Lefteris Zacharia; Ana Carina Ferreira; Jolanta Malyszko; Ewout Hoorn; Andreja Figurek; Robert Unwin; Carsten A. Wagner; Christoph Wanner; Annette Bruchfeld; Marion Pepin; Andrzej Więcek; Dorothea Nitsch; Ivo Fridolin; Gaye Hafez; Maria José Soler; Michelangela Barbieri; Bojan Batinić; Laura Carrasco; Sol Carriazo; Ron Gansevoort; Gianvito Martino; Francesco Mattace Raso; Ionut Nistor; Alberto Ortiz; Giuseppe Paolisso; Daiva Rastenytė; Gabriel Stefan; Gioacchino Tedeschi; Ziad A. Massy; Boris Bikbov; Karl Hans Endlich; Olivier Godefroy; Jean-Marc Chillon; Anastassia Kossioni; Justina Kurganaite; Norberto Perico; Giuseppe Remuzzi; Tomasz Grodzicki; Francesco Trepiccione; Carmine Zoccali; Mustafa Arici; Peter Blankestijn; Kai-Uwe Eckardt; Danilo Fliser; Eugenio Gutiérrez Jiménez; Maximilian König; Ivan Rychlik; Michela Deleidi; George Reusz.
Contributor Information
Sophie Liabeuf, Department of Pharmacology, Amiens University Medical Center, Amiens, France; MP3CV Laboratory, EA7517, University of Picardie Jules Verne, Amiens, France.
Marion Pepin, Université Paris-Saclay, UVSQ, Inserm, Clinical Epidemiology Team, CESP (Centre de Recherche en Epidémiologie et Santé des Populations), Villejuif, France; Department of Geriatrics, Ambroise Paré University Medical Center, APHP, Boulogne-Billancourt, France.
Casper F M Franssen, Department of Nephrology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Davide Viggiano, Department of Nephrology, University of Campania “Luigi Vanvitelli”, Naples, Italy.
Sol Carriazo, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain.
Ron T Gansevoort, Department of Nephrology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Loreto Gesualdo, Department of Emergency and Organ Transplantation, University of Bari “Aldo Moro”, Bari, Italy.
Gaye Hafez, Department of Pharmacology, Faculty of Pharmacy, Altinbas University, Istanbul, Turkey.
Jolanta Malyszko, Department of Nephrology, Dialysis and Internal Medicine, Medical University of Warsaw, Warsaw, Poland.
Christopher Mayer, Center for Health and Bioresources, Biomedical Systems, AIT Austrian Institute of Technology, Vienna, Austria.
Dorothea Nitsch, Faculty of Epidemiology and Population Health, London School of Hygiene & Tropical Medicine, London, UK.
Alberto Ortiz, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain.
Vesna Pešić, Faculty of Pharmacy, University of Belgrade, Belgrade, Serbia.
Andrzej Wiecek, Department of Nephrology, Transplantation and Internal Medicine, Medical University of Silesia, in Katowice, Katowice, Poland.
Ziad A Massy, Université Paris-Saclay, UVSQ, Inserm, Clinical Epidemiology Team, CESP (Centre de Recherche en Epidémiologie et Santé des Populations), Villejuif, France; Department of Nephrology, Ambroise Paré University Medical Center, APHP, Boulogne-Billancourt/Paris, France.
CONNECT Action (Cognitive Decline in Nephro-Neurology European Cooperative Target):
Giovambattista Capasso, Alexandre Andrade, Maie Bachmann, Inga Bumblyte, Adrian Constantin Covic, Pilar Delgado, Nicole Endlich, Andreas Engvig, Denis Fouque, Casper Franssen, Sebastian Frische, Liliana Garneata, Loreto Gesualdo, Konstantinos Giannakou, Dimitrios Goumenos, Ayşe Tuğba Kartal, Laila-Yasmin Mani, Hans-Peter Marti, Christopher Mayer, Rikke Nielsen, Vesna Pešić, Merita Rroji, Giorgos Sakkas, Goce Spasovski, Kate I Stevens, Evgueniy Vazelov, Davide Viggiano, Lefteris Zacharia, Ana Carina Ferreira, Jolanta Malyszko, Ewout Hoorn, Andreja Figurek, Robert Unwin, Carsten Wagner, Christoph Wanner, Annette Bruchfeld, Marion Pepin, Andrzej Wiecek, Dorothea Nitsch, Ivo Fridolin, Gaye Hafez, Maria José Soler Romeo, Michelangela Barbieri, Bojan Batinić, Laura Carrasco, Sol Carriazo, Ron Gansevoort, Gianvito Martino, Francesco Mattace Raso, Ionut Nistor, Alberto Ortiz, Giuseppe Paolisso, Daiva Rastenytė, Gabriel Stefan, Gioacchino Tedeschi, Ziad Massy, Boris Bikbov, Karl Hans Endlich, Olivier Godefroy, Jean-Marc Chillon, Anastassia Kossioni, Justina Kurganaite, Norberto Perico, Giuseppe Remuzzi, Tomasz Grodzicki, Francesco Trepiccione, Carmine Zoccali, Mustafa Arici, Peter Blankestijn, Kai-Uwe Eckardt, Danilo Fliser, Eugenio Gutiérrez Jiménez, Maximilian Konig, Ivan Rychlik, Michela Deleidi, and George Reusz
FUNDING
This article is published as part of a supplement financially supported by the COST Action CA19127-Cognitive Decline in Nephro-Neurology: European Cooperative Target (CONNECT).
CONFLICT OF INTEREST STATEMENT
The authors do not have any conflict of interest relating with this manuscript. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFRENCES
- 1. Bikbov B, Purcell CA, Levey AS. et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joosten H, van Eersel MEA, Gansevoort RT. et al. Cardiovascular risk profile and cognitive function in young, middle-aged, and elderly subjects. Stroke 2013; 44: 1543–1549 [DOI] [PubMed] [Google Scholar]
- 3. Chillon J-M, Massy ZA, Stengel B.. Neurological complications in chronic kidney disease patients. Nephrol Dial Transplant 2016; 31: 1606–1614 [DOI] [PubMed] [Google Scholar]
- 4. Vanholder R, De Smet R, Glorieux G. et al. ; for the European Uremic Toxin Work Group (EUTox). Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int 2003; 63: 1934–1943 [DOI] [PubMed] [Google Scholar]
- 5. Assem M, Lando M, Grissi M. et al. The impact of uremic toxins on cerebrovascular and cognitive disorders. Toxins 2018; 10: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adesso S, Magnus T, Cuzzocrea S. et al. Indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: interaction between astrocytes and microglia. Front Pharmacol 2017; 8: 370. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5466960/ (15 February 2021, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karbowska M, Hermanowicz JM, Tankiewicz-Kwedlo A. et al. Neurobehavioral effects of uremic toxin-indoxyl sulfate in the rat model. Sci Rep 2020; 10: 9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adesso S, Paterniti I, Cuzzocrea S. et al. AST-120 reduces neuroinflammation induced by indoxyl sulfate in glial cells. J Clin Med 2018; 7: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olesen JB, Lip GYH, Kamper AL. et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012; 367: 625–635 [DOI] [PubMed] [Google Scholar]
- 10. Holzmann MJ, Aastveit A, Hammar N. et al. Renal dysfunction increases the risk of ischemic and hemorrhagic stroke in the general population. Ann Med 2012; 44: 607–615 [DOI] [PubMed] [Google Scholar]
- 11. Masson P, Webster AC, Hong M. et al. Chronic kidney disease and the risk of stroke: A systematic review and meta-analysis. Nephrol Dial Transplant 2015; 30: 1162–1169 [DOI] [PubMed] [Google Scholar]
- 12. Kumai Y, Kamouchi M, Hata J. et al. ; for the FSR Investigators. Proteinuria and clinical outcomes after ischemic stroke. Neurology 2012; 78: 1909–1915 [DOI] [PubMed] [Google Scholar]
- 13. Naganuma T, Takemoto Y, Shoji T. et al. Cerebral microbleeds predict intracerebral hemorrhage in hemodialysis patients. Stroke 2015; 46: 2107–2112 [DOI] [PubMed] [Google Scholar]
- 14. Kurella M, Chertow GM, Fried LF. et al. Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 2005; 16: 2127–2133 [DOI] [PubMed] [Google Scholar]
- 15. Bugnicourt JM, Godefroy O, Chillon JM. et al. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J Am Soc Nephrol 2013; 24: 353–363 [DOI] [PubMed] [Google Scholar]
- 16. Puy L, Bugnicourt JM, Liabeuf S. et al. Cognitive impairments and dysexecutive behavioral disorders in chronic kidney disease. J Neuropsychiatry Clin Neurosci 2018; 30: 310–317 [DOI] [PubMed] [Google Scholar]
- 17. Berger I, Wu S, Masson P. et al. Cognition in chronic kidney disease: A systematic review and meta-analysis. BMC Med 2016; 14: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murray AM, Pederson SL, Tupper DE. et al. Acute variation in cognitive function in hemodialysis patients: A cohort study with repeated measures. Am J Kidney Dis 2007; 50: 270–278 [DOI] [PubMed] [Google Scholar]
- 19. Schneider SM, Malecki AK, Müller K. et al. Effect of a single dialysis session on cognitive function in CKD5D patients: a prospective clinical study. Nephrol Dial Transplant 2015; 30: 1551–1559 [DOI] [PubMed] [Google Scholar]
- 20. Tilki HE, Akpolat T, Tunali G. et al. Effects of haemodialysis and continuous ambulatory peritoneal dialysis on P300 cognitive potentials in uraemic patients Upsala J Med Sci 2004; 109: 43–48 [DOI] [PubMed] [Google Scholar]
- 21. Drew DA, Tighiouart H, Scott TM. et al. Cognitive performance before and during hemodialysis: A randomized cross-over trial. Nephron Clin Pract 2013; 124: 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viggiano D, Wagner CA, Blankestijn PJ. et al. Mild cognitive impairment and kidney disease: Clinical aspects. Nephrol Dial Transplant 2020; 35: 10–17 [DOI] [PubMed] [Google Scholar]
- 23. Pepin M, Ferreira AC, Arici M. et al. Cognitive disorders in patients with chronic kidney disease: specificities of clinical assessment. Nephrol Dial Transplant [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naja M, Zmudka J, Hannat S. et al. In geriatric patients, delirium symptoms are related to the anticholinergic burden. Geriatr Gerontol Int 2016; 16: 424–431 [DOI] [PubMed] [Google Scholar]
- 25. Liabeuf S, Gras-Champel V, Moragny J. et al. Trospium chloride for overactive bladder may induce central nervous system adverse events. Eur Geriatr Med 2014; 5: 220–224 [Google Scholar]
- 26. Laville SM, Metzger M, Stengel B. et al. ; on behalf of the Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) Study Collaborators. Evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD-REIN cohort. Br J Clin Pharmacol 2018; 84: 2811–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marienne J, Laville SM, Caillard P. et al. Evaluation of changes over time in the drug burden and medication regimen complexity in ESRD patients before and after renal transplantation. Kidney Int Rep 2020; 6: 128–137. Available from: https://www.kireports.org/article/S2468-0249(20)31649-1/abstract (18 December 2020, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liabeuf S, Laville M.. Drug prescription in patients with chronic kidney disease: A true challenge. Nephrol Dial Transplant 2021; 36: 385–386 [DOI] [PubMed] [Google Scholar]
- 29. Brouns R, De Deyn PP.. Neurological complications in renal failure: a review. Clin Neurol Neurosurg 2004; 107: 1–16 [DOI] [PubMed] [Google Scholar]
- 30. Trenkwalder C, Paulus W, Walters AS.. The restless legs syndrome. Lancet Neurol 2005; 4: 465–475 [DOI] [PubMed] [Google Scholar]
- 31. Nam GE, Kim NH, Han K. et al. Chronic renal dysfunction, proteinuria, and risk of Parkinson’s disease in the elderly. Mov Disord 2019; 34: 1184–1191 [DOI] [PubMed] [Google Scholar]
- 32. Wang S, Yun JM, Shin D. et al. Chronic kidney disease: A risk factor for Parkinson’s disease. Korean J Clin Geriatr 2017; 18: 95–101 [Google Scholar]
- 33. Wang IK, Lin CL, Wu YY. et al. Increased risk of Parkinson’s disease in patients with end-stage renal disease: A retrospective cohort study. Neuroepidemiology 2014; 42: 204–210 [DOI] [PubMed] [Google Scholar]
- 34. Lin HL, Lin HC, Chen YH.. Increased risks of parkinsonism in the 3 years after chronic renal failure. Int J Clin Pract 2012; 66: 499–503 [DOI] [PubMed] [Google Scholar]
- 35. Meléndez-Flores JD, Estrada-Bellmann I.. Linking chronic kidney disease and Parkinson’s disease: a literature review. Metab Brain Dis 2021; 36: 1–12 [DOI] [PubMed] [Google Scholar]
- 36. Huang CW, Wee PH, Low LL. et al. Prevalence and risk factors for elevated anxiety symptoms and anxiety disorders in chronic kidney disease: A systematic review and meta-analysis. Gen Hosp Psychiatry 2021; 69: 27–40 [DOI] [PubMed] [Google Scholar]
- 37. Palmer S, Vecchio M, Craig JC. et al. Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int 2013; 84: 179–191 [DOI] [PubMed] [Google Scholar]
- 38. Iwagami M, Tomlinson LA, Mansfield KE. et al. Prevalence, incidence, indication, and choice of antidepressants in patients with and without chronic kidney disease: A matched cohort study in UK Clinical Practice Research Datalink. Pharmacoepidemiol Drug Saf 2017; 26: 792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Legrand K, Speyer E, Stengel B. et al. Perceived health and quality of life in patients with CKD, including those with kidney failure: Findings from national surveys in France. Am J Kidney Dis 2020; 75: 868–878 [DOI] [PubMed] [Google Scholar]
- 40. Foley RN, Parfrey PS, Sarnak MJ.. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32: S112–S119 [DOI] [PubMed] [Google Scholar]
- 41. Yao H, Takashima Y, Hashimoto M. et al. Subclinical cerebral abnormalities in chronic kidney disease. Contribut Nephrol 2013; 179: 24–34 [DOI] [PubMed] [Google Scholar]
- 42. Chai C, Wang Z, Fan L. et al. Increased number and distribution of cerebral microbleeds is a risk factor for cognitive dysfunction in hemodialysis patients: A longitudinal study. Medicine 2016; 95: e2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murray AM, Seliger S, Lakshminarayan K. et al. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol 2013; 24: 1166–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eldehni MT, Odudu A, McIntyre CW.. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 2015; 26: 957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Polinder-Bos HA, García DV, Kuipers J. et al. Hemodialysis induces an acute decline in cerebral blood flow in elderly patients. J Am Soc Nephrol 2018; 29: 1317–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Polinder-Bos HA, Elting JWJ, Aries MJ. et al. Changes in cerebral oxygenation and cerebral blood flow during hemodialysis—A simultaneous near-infrared spectroscopy and positron emission tomography study. J Cereb Blood Flow Metab 2020; 40: 328–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. MacEwen C, Sutherland S, Daly J. et al. Relationship between hypotension and cerebral ischemia during hemodialysis. J Am Soc Nephrol 2017; 28: 2511–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Findlay MD, Dawson J, Dickie DA. et al. Investigating the relationship between cerebral blood flow and cognitive function in hemodialysis patients. J Am Soc Nephrol 2019; 30: 147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meyring-Wösten A, Zhang H, Ye X. et al. Intradialytic hypoxemia and clinical outcomes in patients on hemodialysis. Clin J Am Soc Nephrol 2016; 11: 616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Sandwijk MS, ten Berge IJM, Caan MWA. et al. Cognitive improvement after kidney transplantation is associated with structural and functional changes on MRI. Transplant Direct 2020; 6: e531. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7056275/ (28 January 2021, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chu NM, Gross AL, Shaffer AA. et al. Frailty and changes in cognitive function after kidney transplantation. J Am Soc Nephrol 2019; 30: 336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liabeuf S, Cheddani L, Massy ZA.. Uremic toxins and clinical outcomes: The impact of kidney transplantation. Toxins 2018; 10: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liabeuf S, Laville SM, Glorieux G. et al. Difference in profiles of the gut-derived tryptophan metabolite indole acetic acid between transplanted and non-transplanted patients with chronic kidney disease. Int J Mol Sci 2020; 21: 2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duranton F, Cohen G, De Smet R. et al. ; European Uremic Toxin Work Group. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 2012; 23: 1258–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oshima N, Onimaru H, Matsubara H. et al. Uric acid, indoxyl sulfate, and methylguanidine activate bulbospinal neurons in the RVLM via their specific transporters and by producing oxidative stress. Neuroscience 2015; 304: 133–145 [DOI] [PubMed] [Google Scholar]
- 56. Lin YT, Wu PH, Tsai YC. et al. Indoxyl sulfate induces apoptosis through oxidative stress and mitogen-activated protein kinase signaling pathway inhibition in human astrocytes. J Clin Med 2019; 8: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Watanabe K, Sato E, Mishima E. et al. Effect of uremic toxins on hippocampal cell damage: Analysis in vitro and in rat model of chronic kidney disease. Heliyon 2021; 7: e06221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ohtsuki S, Asaba H, Takanaga H. et al. Role of blood–brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: Its involvement in neurotransmitter metabolite clearance from the brain. J Neurochem 2002; 83: 57–66 [DOI] [PubMed] [Google Scholar]
- 59. Sato E, Saigusa D, Mishima E. et al. Impact of the oral adsorbent AST-120 on organ-specific accumulation of uremic toxins: LC-MS/MS and MS imaging techniques. Toxins 2017; 10: 19. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5793106/ (15 February 2021, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mair RD, Nguyen H, Huang TT. et al. Accumulation of uremic solutes in the cerebrospinal fluid in experimental acute renal failure. Am J Physiol Renal Physiol 2019; 317: F296–F302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. ΡBobot M, Thomas L, Moyon A. et al. Uremic toxic blood–brain barrier disruption mediated by AhR activation leads to cognitive impairment during experimental renal dysfunction. J Am Soc Nephrol 2020; 31: 1509–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sun CY, Li JR, Wang YY. et al. p-Cresol sulfate caused behavior disorders and neurodegeneration in mice with unilateral nephrectomy involving oxidative stress and neuroinflammation. Int J Mol Sci 2020; 21: 6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bossola M, Ciciarelli C, Di Stasio E. et al. Correlates of symptoms of depression and anxiety in chronic hemodialysis patients. Gen Hosp Psychiatry 2010; 32: 125–131 [DOI] [PubMed] [Google Scholar]
- 64. ΡHsu HJ, Yen CH, Chen CK. et al. Association between uremic toxins and depression in patients with chronic kidney disease undergoing maintenance hemodialysis. Gen Hosp Psychiatry 2013; 35: 23–27 [DOI] [PubMed] [Google Scholar]
- 65. Yeh YC, Huang MF, Liang SS. et al. Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. Neurotoxicology 2016; 53: 148–152 [DOI] [PubMed] [Google Scholar]
- 66. Ye BS, Lee WW, Ham JH. et al. ; Alzheimer’s Disease Neuroimaging Initiative. Does serum uric acid act as a modulator of cerebrospinal fluid Alzheimer’s disease biomarker related cognitive decline? Eur J Neurol 2016; 23: 948–957 [DOI] [PubMed] [Google Scholar]
- 67. Sleeman I, Lawson RA, Yarnall AJ. et al. Urate and homocysteine: Predicting motor and cognitive changes in newly diagnosed Parkinson’s disease. J Parkinsons Dis 2019; 9: 351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Efstathiadou A, Gill D, McGrane F. et al. Genetically determined uric acid and the risk of cardiovascular and neurovascular diseases: A Mendelian randomization study of outcomes investigated in randomized trials. J Am Heart Assoc 2019; 8: e012738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lin YT, Wu PH, Liang SS. et al. Protein-bound uremic toxins are associated with cognitive function among patients undergoing maintenance hemodialysis. Sci Rep 2019; 9: 20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lin YT, Wu PH, Lee HH. et al. Indole-3 acetic acid increased risk of impaired cognitive function in patients receiving hemodialysis. NeuroToxicology 2019; 73: 85–91 [DOI] [PubMed] [Google Scholar]
- 71. Li Y, Pi HC, Yang ZK. et al. Associations between small and middle molecules clearance and the change of cognitive function in peritoneal dialysis. J Nephrol 2020; 33: 839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Linde ET, Roij CJM, van Meijers BKI. et al. Cognitive function and uremic toxins after kidney transplantation: An exploratory study. Kidney360 2020; 1: 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sankowski B, Księżarczyk K, Raćkowska E. et al. Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with Parkinson’s disease. Clin Chim Acta 2020; 501: 165–173 [DOI] [PubMed] [Google Scholar]
- 74. Hénaut L, Grissi M, Brazier F. et al. Cellular and molecular mechanisms associated with ischemic stroke severity in female mice with chronic kidney disease. Sci Rep 2019; 9: 6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ting EYC, Yang AC, Tsai SJ.. Role of interleukin-6 in depressive disorder. Int J Mol Sci 2020; 21: 2194. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7139933/ (26 April 2021, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Slee AD. Exploring metabolic dysfunction in chronic kidney disease. Nutr Metab (Lond) 2012; 9: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. De Giorgi A, Fabbian F, Pala M. et al. Uric acid: Friend or foe? Uric acid and cognitive function “Gout kills more wise men than simple”. Eur Rev Med Pharmacol Sci 2015; 19: 640–646 [PubMed] [Google Scholar]
- 78. Chamorro A, Amaro S, Castellanos M. et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): A randomised, double-blind phase 2b/3 trial. Lancet Neurol 2014; 13: 453–460 [DOI] [PubMed] [Google Scholar]
- 79. Llull L, Laredo C, Renú A. et al. Uric acid therapy improves clinical outcome in women with acute ischemic stroke. Stroke 2015; 46: 2162–2167 [DOI] [PubMed] [Google Scholar]
- 80. Afsar B, Elsurer R, Covic A. et al. Relationship between uric acid and subtle cognitive dysfunction in chronic kidney disease. Am J Nephrol 2011; 34: 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Watanabe H, Miyamoto Y, Otagiri M. et al. Update on the pharmacokinetics and redox properties of protein-bound uremic toxins. J Pharm Sci 2011; 100: 3682–3695 [DOI] [PubMed] [Google Scholar]
- 82. Six I, Gross P, Rémond MC. et al. Deleterious vascular effects of indoxyl sulfate and reversal by oral adsorbent AST-120. Atherosclerosis 2015; 243: 248–256 [DOI] [PubMed] [Google Scholar]
- 83. Six I, Flissi N, Lenglet G. et al. Uremic toxins and vascular dysfunction. Toxins 2020; 12: 404. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7354618/ (19 November 2020, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jono S, McKee MD, Murry CE. et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 2000; 87: E10– E–17. [DOI] [PubMed] [Google Scholar]
- 85. Barreto FC, Barreto DV, Liabeuf S. et al. ; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 2009; 4: 1551–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liabeuf S, Barreto DV, Barreto FC. et al. ; European Uraemic Toxin Work Group (EUTox). Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 2010; 25: 1183–1191 [DOI] [PubMed] [Google Scholar]
- 87. Addi T, Dou L, Burtey S.. Tryptophan-derived uremic toxins and thrombosis in chronic kidney disease. Toxins 2018; 10: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sallée M, Dou L, Cerini C. et al. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 2014; 6: 934–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pawlak K, Mysliwiec M, Pawlak D.. Haemostatic system, biochemical profiles, kynurenines and the prevalence of cardiovascular disease in peritoneally dialyzed patients. Thromb Res 2010; 125: e40–e45 [DOI] [PubMed] [Google Scholar]
- 90. Laville SM, Massy ZA, Kamel S. et al. Intestinal chelators, sorbants, and gut-derived uremic toxins. Toxins 2021; 13: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bell S, Kolobova I, Crapper L. et al. Lesch-Nyhan syndrome: Models, theories, and therapies. Mol Syndromol 2016; 7: 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Singh JA, Cleveland JD.. Comparative effectiveness of allopurinol versus febuxostat for preventing incident dementia in older adults: A propensity-matched analysis. Arthritis Res Ther 2018; 20: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dawson J, Broomfield N, Dani K. et al. Xanthine oxidase inhibition for the improvement of long-term outcomes following ischaemic stroke and transient ischaemic attack (XILO-FIST)—protocol for a randomised double blind placebo-controlled clinical trial. Eur Stroke J 2018; 3: 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Anders H-J, Andersen K, Stecher B.. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 2013; 83: 1010–1016 [DOI] [PubMed] [Google Scholar]
- 95. Perna AF, Glorieux G, Zacchia M. et al. The role of the intestinal microbiota in uremic solute accumulation: A focus on sulfur compounds. J Nephrol 2019; 32: 733–740 [DOI] [PubMed] [Google Scholar]
- 96. Maheshwari V, Thijssen S, Tao X. et al. In silico comparison of protein-bound uremic toxin removal by hemodialysis, hemodiafiltration, membrane adsorption, and binding competition. Sci Rep 2019; 9: 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Florens N, Yi D, Juillard L. et al. Using binding competitors of albumin to promote the removal of protein-bound uremic toxins in hemodialysis: hope or pipe dream? Biochimie 2018; 144: 1–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.