Abstract
Background
Side effects caused by oral contraceptives discourage compliance with, and continuation of, oral contraceptives. A suggested disadvantage of biphasic compared to triphasic oral contraceptive (OC) pills is an increase in breakthrough bleeding. We conducted this systematic review to examine this potential disadvantage.
Objectives
To compare biphasic with triphasic oral contraceptives in terms of efficacy, cycle control, and discontinuation due to side effects.
Search methods
We searched MEDLINE, POPLINE, LILACS and CENTRAL, as well as clinical trials databases (ClinicalTrials.gov and ICTRP). We searched the reference lists of relevant articles and book chapters. We also contacted the authors of relevant studies and pharmaceutical companies.
Selection criteria
We included randomized controlled trials comparing any biphasic with any triphasic OC when used to prevent pregnancy.
Data collection and analysis
We examined the studies found during the searches for inclusion and assessed methodological quality. We contacted the authors of included studies and of possibly randomized studies for information about the methods and outcomes. We entered the data into RevMan. We calculated Peto odds ratios for incidence of discontinuation due to medical reasons, intermenstrual bleeding, and absence of withdrawal bleeding.
Main results
Only two trials of limited quality met our inclusion criteria. One study compared two biphasic pills and one triphasic pill, each containing levonorgestrel and ethinyl estradiol. No important differences emerged, and the frequency of discontinuation due to medical problems was similar with all three pills. The other trial compared a biphasic pill containing norethindrone (Ortho 10/11) with a triphasic pill containing levonorgestrel (Triphasil) and with another triphasic containing norethindrone (Ortho 7/7/7). The biphasic pill had inferior cycle control compared with the levonorgestrel triphasic. The odds ratio of cycles with intermenstrual bleeding was 1.70 (95% confidence interval (CI) 1.29 to 2.24) for the biphasic compared with the triphasic levonorgestrel pill. The odds ratio of cycles without withdrawal bleeding was 6.48 (95% CI 3.13 to 13.39). In contrast, cycle control with the biphasic pill was comparable to that of the triphasic containing the same progestin (norethindrone).
Authors' conclusions
The available evidence is limited and the internal validity of these trials is questionable. Given the high losses to follow up, these reports may even be considered observational. Given that caveat, the biphasic pill containing norethindrone was associated with inferior cycle control compared with the triphasic pill containing levonorgestrel. The choice of progestin may be more important than the phasic regimen in determining bleeding patterns.
Plain language summary
Birth control pills with two phases versus three phases
Side effects of birth control pills may keep women from using them as planned. Attempts to decrease side effects led to the three‐phase pill in the 1980s. Pills with phases provide different amounts of hormones over three weeks. Whether three‐phase pills lead to fewer pregnancies than two‐phase pills is unknown. Nor is it known if the pills give better cycle control or have fewer side effects. This review looked at whether two‐phase pills worked as well as three‐phase pills. We also studied whether women had fewer side effects with these pills.
We did a computer search for studies of birth control pills with two phases versus pills with three phases. We also wrote to researchers and manufacturers to find other trials. We included randomized trials in any language.
We found only two trials that looked at two‐phase versus three‐phase birth control pills. The studies did not have good methods and the authors did not report all their methods. Many women dropped out of the studies, which affects what can be said about the results. One study compared two types of two‐phase pills with a three‐phase pill. The pills did not differ in any major ways, including the numbers of women who stopped using the pills due to health problems. The other trial compared a two‐phase pill with two different three‐phase pills. The two‐phase pill had worse bleeding patterns than the three‐phase pill with a different hormone (levonorgestrel). In contrast, bleeding with the two‐phase pill was like that of the three‐phase pill with the same hormone (norethindrone). The type of hormone may be more important than the phases for cycle control.
These trials did not provide enough evidence to say if three‐phase pills worked any better than two‐phase types for birth control, bleeding patterns, or staying on the pill. More research would be needed to show whether three‐phase pills were better than two‐phase pills. However, two‐phase pills are not used enough to justify further research.
Background
Side effects caused by oral contraceptives discourage compliance with, and continuation of, oral contraceptives (Hillard 1992). Three approaches have been used to decrease these adverse effects: reduction of the steroid dose, development of new steroids, and new formulas and schedules of administration. The third strategy led to the development of both biphasic and triphasic pills.
Biphasic and triphasic oral contraceptives purportedly attempt to 'mimic' the rising and falling pattern of estrogen and progesterone as seen during the normal menstrual cycle (Upton 1983). The biphasic pill soon yielded to the triphasic pill because of a suggested increase in breakthrough bleeding associated with the use of the biphasic oral contraceptive pill (Mishell 1991). We conducted this systematic review to examine this potential disadvantage of biphasic oral contraceptives as compared with triphasic pills.
Objectives
The aim of this review was to compare biphasic with triphasic oral contraceptive pills. Our a priori hypotheses were that biphasic and triphasic pills have similar contraceptive efficacy but that triphasic oral contraceptives cause fewer side effects, give better cycle control, and have higher continuation rates.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials in this review.
Types of participants
Healthy women of reproductive age without contra‐indications for oral contraceptive use who desired to use oral contraceptives for preventing pregnancy.
Types of interventions
We included any biphasic compared with any triphasic oral contraceptive pill when used to prevent pregnancy. Both 21‐pill and 28‐pill packages were included. We excluded studies examining sequential pills (those containing estrogen alone early in the cycle, followed by estrogen plus progestin later in the cycle). We also excluded studies comparing biphasic with triphasic pills when used as a treatment and not as a contraceptive.
Types of outcome measures
Primary outcomes
Principal outcome measures included the incidence of accidental pregnancy, spotting, breakthrough bleeding, amenorrhea, intermenstrual bleeding, and discontinuation due to side effects.
We excluded studies which focused primarily on metabolic outcome measures and follicular growth.
Search methods for identification of studies
Electronic searches
We searched computerized databases of MEDLINE using PubMed, EMBASE, POPLINE, LILACS and Cochrane Central Register of Controlled Trials (CENTRAL) for publications comparing monophasic, biphasic or triphasic oral contraceptives. In addition, we searched for recent clinical trials through ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). The current search strategies are shown below, along with the search dates. For the initial review (2006) and the 2008 update, we also searched EMBASE (Appendix 1).
MEDLINE via PubMed (Jun 2008 to 02 May 2011)
contraceptives, oral[MeSH Terms] AND (monophasic[ALL] OR biphasic[ALL] OR triphasic[ALL] OR multiphasic[ALL]) AND (clinical trials[MeSH Terms] OR clinical trial*[ALL] OR controlled clinical trial*[ALL] OR comparative stud*[ALL] OR compar* OR randomized controlled trial[ALL] OR random allocation[MeSH Terms] OR random allocation[Text Word] OR random[ALL] OR double‐blind method[MeSH Terms] OR double blind method[Text Word] OR single‐blind method[MeSH Terms] OR single blind method[Text Word] OR multicenter stud*[ALL])
POPLINE (past 5 years to 03 May 2011)
(oral & contracept*) & (monophasic/biphasic/triphasic/multiphasic)
LILACS (to 03 May 2011)
(((("contraceptives, oral") or "contraceptive")) or "contraceptives") or "contraception" [Words] and ((("monophasic") or "biphasic") or "triphasic") or "multiphasic" [Words]
CENTRAL (2008 to 02 May 2011)
oral AND contracept* in Title, Abstract or Keywords AND (monophasic OR biphasic OR triphasic OR multiphasic) in Abstract
ClinicalTrials.gov (03 May 2011)
Search terms: biphasic OR triphasic OR multiphasic Condition: NOT diabetes Interventional Studies Studies with Female Participants Received on or after 07/01/2008
ICTRP (03 May 2011)
Intervention: (monophasic OR biphasic OR triphasic OR multiphasic) NOT insulin Date of registration from 01 Jul 2008
Searching other resources
For the initial review, we searched relevant book chapters and review articles identified with the above strategies for relevant trials. We reviewed the reference lists of identified studies for previously unidentified trials. We also searched the holdings of the FHI Library for relevant trials, book chapters and review articles.
We attempted to contact the authors of all included trials. We also wrote letters to pharmaceutical companies in the USA and in Europe that market oral contraceptives. In the contact letters, we provided a list of studies identified and asked if correspondents knew of unpublished or published trials we had not found.
Data collection and analysis
Selection of studies
For the initial review, two authors evaluated the titles and abstracts found during the literature searches, and all potentially relevant articles were photocopied. We had one French article (Castaigne 1985) translated into English. Then two authors independently examined the retrieved studies for possible inclusion. For the updates, one author reviewed the search results and identified reports for inclusion or exclusion. A second author also examined any reports identified for appropriate categorization.
Data extraction and management
After inclusion of a study, two authors abstracted the data. There was no disagreement about the inclusion of studies or abstracted data. We wrote a letter to the authors of the two included studies and asked for additional information about the methodology of the study and the various outcome measures. One author then entered the abstracted data into RevMan 3.1, and later imported the review into RevMan 4.1. Another author verified that the data had been correctly entered.
Assessment of risk of bias in included studies
The methodological quality of the trial was assessed using Cochrane guidelines. We focused especially on the method of randomization, use of allocation concealment, use of blinding, and exclusion of participants after randomization.
Data synthesis
We calculated Peto odds ratios (OR) with 95% confidence intervals (CI) for the outcome measures of breakthrough bleeding, spotting, withdrawal bleeding, intermenstrual bleeding, and discontinuation due to medical reasons.
Results
Description of studies
Included studies
Two studies met the criteria for this review. Larranaga 1978 compared three pills (two biphasic and one triphasic):
a biphasic pill (termed "Alpha") containing 50 μg levonorgestrel and 50 μg ethinyl estradiol for 11 days, followed by 125 μg levonorgestrel and 50 μg ethinyl estradiol for 10 days;
a biphasic pill (termed "Beta") composed of 150 μg levonorgestrel and 30 μg ethinyl estradiol for 7 days, followed by 200 μg ethinyl estradiol and 40 μg ethinyl estradiol for 14 days;
a triphasic pill (termed "Gamma") containing 50 μg levonorgestrel and 20 μg ethinyl estradiol for 7 days, 50 μg levonorgestrel and 50 μg ethinyl estradiol for 7 days, then 125 μg levonorgestrel and 30 μg ethinyl estradiol for 7 days.
Percival‐Smith 1990 compared three marketed products:
a biphasic pill containing 500‐1000 μg norethindrone and 35 μg ethinyl estradiol (Ortho 10/11);
a triphasic pill containing 50‐75‐125 μg levonorgestrel and 30‐40‐30 μg ethinyl estradiol (Triphasil);
a triphasic pill containing 500‐750‐1000 μg norethindrone and 35 μg ethinyl estradiol (Ortho 7/7/7).
Excluded studies
We excluded four studies. Castaigne 1985 did not report that the allocation method was randomized, and we were unable to reach the author. Gaspard 1983 examined a sequential pill. Because of probable fraud (Rossiter 1992) we excluded two studies by Briggs (Briggs 1980; Briggs 1982).
Risk of bias in included studies
Neither study (Larranaga 1978; Percival‐Smith 1990) adequately described the method of randomization and allocation concealment. One study (Percival‐Smith 1990) sponsored by Parke‐Davis reportedly kept investigators blinded as to treatment. Percival‐Smith 1990 provided an a priori hypothesis and a sample size calculation. Larranaga 1978 did not report an a priori hypothesis or a sample size or power calculation. We received supplemental information from Percival‐Smith 1990 but not from Larranaga 1978.
Both trials had high losses after randomization. Of the 458 women who participated in one trial (Larranaga 1978), 252 women discontinued. Losses to follow‐up ranged from 40% to 54% in the three treatment arms. In Percival‐Smith 1990, 169 out of 469 women participating withdrew during the first six months. Seventy‐eight women dropped out before or during the first cycle of pill use. This raises the strong possibility of selection bias in both studies.
Effects of interventions
We could not aggregate the clinical outcomes of the included studies because they examined different kinds of pills (Larranaga 1978; Percival‐Smith 1990). Moreover, even if they had examined the same pills, we would not choose to aggregate two studies of poor methodological quality.
Larranaga 1978 examined 1269 user cycles of biphasic preparation Alpha, 1163 user cycles of biphasic preparation Beta, and 1154 user cycles of triphasic preparation Gamma. No pregnancies occurred during the study period. Life‐table continuation rates after 12 cycles were lower with the triphasic Gamma pill (40%) than with Alpha (50%) or Beta (44%) pills. The discontinuation rates for medical reasons did not differ significantly among the three preparations (Analysis 1.1; Analysis 2.1). Patterns of breakthrough bleeding, spotting, and absence of withdrawal bleeding are impossible to interpret because of insufficient detail.
1.1. Analysis.
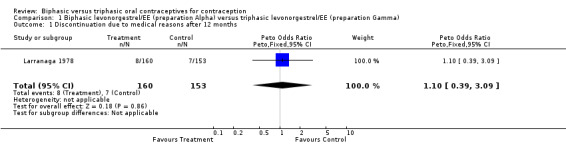
Comparison 1 Biphasic levonorgestrel/EE (preparation Alpha) versus triphasic levonorgestrel/EE (preparation Gamma), Outcome 1 Discontinuation due to medical reasons after 12 months.
2.1. Analysis.
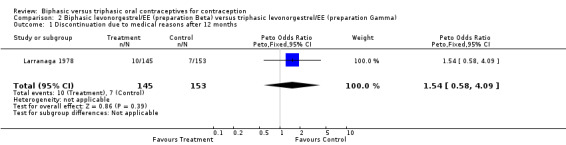
Comparison 2 Biphasic levonorgestrel/EE (preparation Beta) versus triphasic levonorgestrel/EE (preparation Gamma), Outcome 1 Discontinuation due to medical reasons after 12 months.
Percival‐Smith 1990 examined 533 user cycles of a biphasic Ortho 10/11, 506 cycles of Triphasil, and 524 cycles of the triphasic Ortho 7/7/7. In the comparison between Ortho 10/11 and Triphasil, the biphasic pill (Ortho 10/11) was associated with significantly more cycles with intermenstrual bleeding (Analysis 3.1: OR 1.70; 95% CI 1.29 to 2.24). Similarly, cycles without a withdrawal bleed were significantly more common with the biphasic pill (Analysis 3.2: OR 6.48; 95% CI 3.13 to 13.39). However, rates of study discontinuation due to intermenstrual bleeding were similar for both groups (Analysis 3.3).
3.1. Analysis.
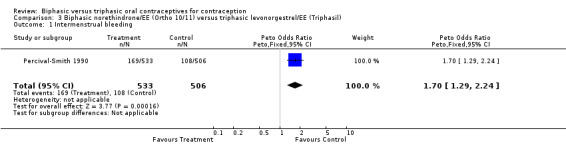
Comparison 3 Biphasic norethindrone/EE (Ortho 10/11) versus triphasic levonorgestrel/EE (Triphasil), Outcome 1 Intermenstrual bleeding.
3.2. Analysis.
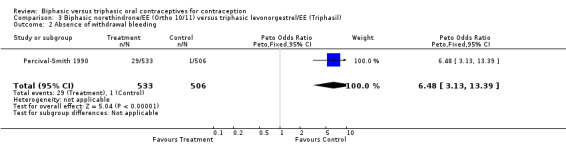
Comparison 3 Biphasic norethindrone/EE (Ortho 10/11) versus triphasic levonorgestrel/EE (Triphasil), Outcome 2 Absence of withdrawal bleeding.
3.3. Analysis.
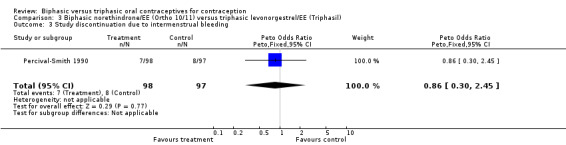
Comparison 3 Biphasic norethindrone/EE (Ortho 10/11) versus triphasic levonorgestrel/EE (Triphasil), Outcome 3 Study discontinuation due to intermenstrual bleeding.
In the comparison between Ortho 10/11 and Ortho 7/7/7, the preparations had similar frequencies for cycles with intermenstrual bleeding (Analysis 4.1). The proportions of cycles without withdrawal bleeding were also comparable (Analysis 4.2). Finally, the proportion of women who discontinued the study because of intermenstrual bleeding was the same in both groups (Analysis 4.3).
4.1. Analysis.
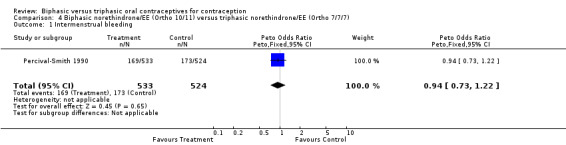
Comparison 4 Biphasic norethindrone/EE (Ortho 10/11) versus triphasic norethindrone/EE (Ortho 7/7/7), Outcome 1 Intermenstrual bleeding.
4.2. Analysis.
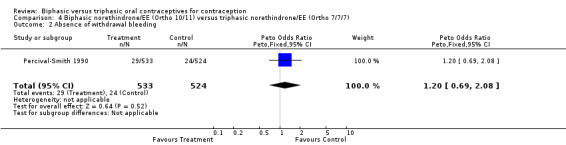
Comparison 4 Biphasic norethindrone/EE (Ortho 10/11) versus triphasic norethindrone/EE (Ortho 7/7/7), Outcome 2 Absence of withdrawal bleeding.
4.3. Analysis.
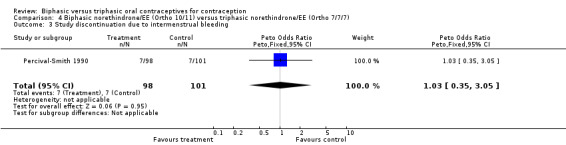
Comparison 4 Biphasic norethindrone/EE (Ortho 10/11) versus triphasic norethindrone/EE (Ortho 7/7/7), Outcome 3 Study discontinuation due to intermenstrual bleeding.
Discussion
We identified only two randomized controlled trials comparing a biphasic with a triphasic regimen (Larranaga 1978; Percival‐Smith 1990). The reports do not describe the method of randomization and the use (if any) of allocation concealment. Both studies examined a modest number of user cycles. More importantly, a large proportion of women in both trials were lost to follow up after randomization. Because of the large losses after randomization, these data may be more appropriately considered observational, and the internal validity of these trials is questionable. Because of small sample sizes, we were unable to address our a priori hypothesis concerning contraceptive efficacy. However, we found some support for our hypothesis of better cycle control with at least one triphasic regimen (Percival‐Smith 1990).
An earlier trial (Larranaga 1978) contributed little information. However, it showed no important differences among the three pills studied. A later trial (Percival‐Smith 1990) found that the biphasic pill (Ortho 10/11) caused significantly more cycles with intermenstrual bleeding or no withdrawal bleeding than did one triphasic pill (Triphasil). This difference was not seen when the biphasic pill was compared with a triphasic pill (Ortho 7/7/7) containing the same progestin (norethindrone).
Other randomized controlled trials have found that triphasic pills containing levonorgestrel provide better cycle control than do those containing norethindrone. One trial comparing two norethindrone‐containing triphasic pills versus a levonorgestrel‐containing triphasic pill (Droegemueller 1989) found that levonorgestrel was associated with the lowest frequency of intermenstrual bleeding. Another trial comparing these three triphasic formulations (Schilling 1989) corroborated this observation. Thus, the poorer cycle control with the biphasic pill (Ortho 10/11) versus Triphasil in this review (Percival‐Smith 1990) may reflect the progestin used (norethindrone) rather than the phasic formulation itself. Levonorgestrel has a greater bioavailability, longer serum half‐life, and greater relative binding affinity in humans than does norethindrone (Wallach 2000). These features may translate into better control of menstrual bleeding.
Authors' conclusions
Implications for practice.
One study found that a biphasic pill containing norethindrone (Ortho 10/11) provided inferior cycle control to a triphasic pill containing levonorgestrel (Triphasil). Aside from this, clinicians have limited information to distinguish between biphasic and triphasic pills.
Implications for research.
Given the infrequent use of biphasic pills in most of the world, further comparative trials do not appear justified.
What's new
| Date | Event | Description |
|---|---|---|
| 4 May 2011 | New search has been performed | Searches updated; no new trials found. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 25 November 2008 | New search has been performed | Searches were updated in Oct and Nov 2008; no new trials were found. Added searches of clinical trials databases. |
| 14 April 2008 | Amended | Converted to new review format. |
| 15 May 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Carol Manion of FHI assisted with the literature searches.
Appendices
Appendix 1. Previous search strategies
Initial review (2006) and 2008 update
EMBASE
1. oral contraceptive agent 2. biphasic 3. triphasic 4. multiphasic 5. 2 OR 3 OR 4 6. 1 AND 5 7. monophasic 8. 6 AND 7
CENTRAL
1. (contraceptives and oral) 2. monophasic 3. biphasic 4. triphasic 5. multiphasic 6. (((#2 or #3) or #4) or #5) 7. (#1 and #6)
POPLINE
(kw) oral contraceptives AND (tw) (monophasic OR biphasic OR triphasic OR multiphasic) AND (tw) (compar* OR clinical trials OR comparative studies OR random OR double blind studies)
ClinicalTrials.gov
Search terms: biphasic OR triphasic OR multiphasic Condition: oral contraceptive
ICTRP
Title: monophasic OR biphasic OR triphasic OR multiphasic Intervention or condition: contraception OR contraceptive
Data and analyses
Comparison 1. Biphasic levonorgestrel/EE (preparation Alpha) versus triphasic levonorgestrel/EE (preparation Gamma).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to medical reasons after 12 months | 1 | 313 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.39, 3.09] |
Comparison 2. Biphasic levonorgestrel/EE (preparation Beta) versus triphasic levonorgestrel/EE (preparation Gamma).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to medical reasons after 12 months | 1 | 298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [0.58, 4.09] |
Comparison 3. Biphasic norethindrone/EE (Ortho 10/11) versus triphasic levonorgestrel/EE (Triphasil).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intermenstrual bleeding | 1 | 1039 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.70 [1.29, 2.24] |
| 2 Absence of withdrawal bleeding | 1 | 1039 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.48 [3.13, 13.39] |
| 3 Study discontinuation due to intermenstrual bleeding | 1 | 195 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.30, 2.45] |
Comparison 4. Biphasic norethindrone/EE (Ortho 10/11) versus triphasic norethindrone/EE (Ortho 7/7/7).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intermenstrual bleeding | 1 | 1057 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.73, 1.22] |
| 2 Absence of withdrawal bleeding | 1 | 1057 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.69, 2.08] |
| 3 Study discontinuation due to intermenstrual bleeding | 1 | 199 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.35, 3.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Larranaga 1978.
| Methods | Randomized controlled trial without blinding. The method of randomization and the use of allocation concealment are not described. | |
| Participants | 458 women of childbearing age in Lima, Peru. Women were of low income and requesting the pill for socioeconomic reasons. More than 50% of the participants had been pregnant more than 4 times. | |
| Interventions | Biphasic levonorgestrel plus EE (LNG 50‐125 μg and EE 50 μg in a 10/11 day regimen) (preparation Alpha) versus biphasic levonorgestrel plus EE (LNG 150‐200 μg and EE 30‐40 μg in a 7/14 day regimen) (preparation Beta) versus triphasic levonorgestrel plus EE (LNG 50‐50‐125 μg and EE 20‐50‐30 μg in a 7/7/7 day regimen) (preparation Gamma). | |
| Outcomes | Primary outcome measures were pregnancy, cycle control, side effects, number and reasons for discontinuation. | |
| Notes | The report does not provide an a priori hypothesis or a sample size or power calculation. Large losses to follow up (40% to 54% in each treatment group). | |
Percival‐Smith 1990.
| Methods | Randomized controlled trial with blinding (of investigator). The method of randomization and the use of allocation concealment are not described. | |
| Participants | 469 women at 4 Canadian sites who were aged 15 to 35 years. However, only 391 women were admitted to the study and used the pills for at least one month. | |
| Interventions | Biphasic norethindrone plus EE (NET 500‐1000 μg and ethinyl estradiol 35 μg in a 10/11 day regimen) (Ortho 10/11) versus triphasic levonorgestrel plus EE (LNG 50‐75‐125 μg and EE 30‐40‐30 μg in a 6/5/10 day regimen) (Triphasil) versus triphasic norethindrone plus EE (NET 500‐750‐1000 μg and EE 35 μg in a 7/7/7 day regimen) (Ortho 7/7/7). | |
| Outcomes | Primary outcomes measures were side effects, cycle control, continuation, discontinuation rates, and reasons for discontinuation. | |
| Notes | The report provides an a priori hypothesis and an adequate sample size calculation. 169 women discontinued, but the reasons for discontinuation are unclear. Intermenstrual bleeding is breakthrough bleeding and spotting. Continuing menstrual flow is not included in the intermenstrual bleeding data. | |
EE = ethinyl estradiol
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Briggs 1980 | Briggs is suspected of scientific fraud (Rossiter 1992). Report focuses on metabolic outcomes. |
| Briggs 1982 | Briggs is suspected of scientific fraud (Rossiter 1992). Report describes 2 studies. One study compares 4 monophasic oral contraceptives in terms of metabolic changes. The other is a duplicate publication (Briggs 1980). |
| Castaigne 1985 | The report does not provide the method used to generate the allocation sequence. We were unable to contact the author. |
| Gaspard 1983 | The study examines a sequential pill (Ovidol, 7 days of EE 50 μg and 14 days of desogestrel 125 μg plus EE 50 μg). The study primarily looks at metabolic outcomes. |
EE = ethinyl estradiol
Contributions of authors
F Helmerhorst came up with the idea of comparing multiphasic with monophasic oral contraceptives. For the initial review, D Grimes and H Van Vliet developed the protocol, conducted the literature searches, assessed the methodological quality of the studies, abstracted the data and entered the data in RevMan. K Schulz verified the correct entry of the data. D Grimes and H Van Vliet wrote the manuscript. K Schulz and F Helmerhorst advised on, commentated and proof‐read the manuscript. For the 2006 update, H Van Vliet reviewed the search results; L Lopez edited the review for Cochrane style issues, and wrote the Plain Language Summary. For the 2008 and 2011 updates, L Lopez conducted the searches, reviewed the search results, and edited the review.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute of Child Health and Human Development, USA.
U.S. Agency for International Development, USA.
Declarations of interest
DA Grimes has consulted with the pharmaceutical companies Bayer Healthcare Pharmaceuticals and Merck & Co, Inc.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Larranaga 1978 {published data only}
- Larranaga A, Sartoretto JN, Winterhalter M, Navas Filho F. Clinical evaluation of two biphasic and one triphasic norgestrel/ethinyl estradiol regimens. International Journal of Fertility 1978;23:193‐9. [PubMed] [Google Scholar]
Percival‐Smith 1990 {published and unpublished data}
- Percival‐Smith RK, Yuzpe AA, Desrosiers JA, Rioux JE, Guilbert E. Cycle control on low‐dose oral contraceptives: a comparative trial. Contraception 1990;42:253‐62. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Briggs 1980 {published data only}
- Briggs M, Briggs M. A randomized study of metabolic effects of four oral contraceptive preparations containing levonorgestrel plus ethinylestradiol in different regimens. The development of a new triphasic oral contraceptive. Proceedings of a Special Symposium held at the 10th World Congress on Fertility and Sterility; 1980 July; Madrid. Lancaster (England): MTP Press, 1980:79‐88.
Briggs 1982 {published data only}
- Briggs MH, Briggs M. Randomized prospective studies on metabolic effects of oral contraceptives. Acta Obstetrica et Gynecologica Scandinavica Supplement 1982;105:25‐32. [DOI] [PubMed] [Google Scholar]
Castaigne 1985 {published data only}
- Castaigne JP. Triella: a French multicenter double blind clinical study [Triella‐Etude clinique multicentrique francaise menee en double aveugle]. Contraception, Fertilité, Sexualité 1985;13 Suppl:445‐53. [PubMed] [Google Scholar]
Gaspard 1983 {published data only}
- Gaspard UJ, Romus MA, Gillain D, Duvivier J, Demey‐Ponsart E, Franchimont P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception 1983;27:577‐90. [DOI] [PubMed] [Google Scholar]
Additional references
Droegemueller 1989
- Droegemueller W, Katta LR, Bright TG, Bowes WA Jr. Triphasic randomized clinical trial: comparative frequency of intermenstrual bleeding. American Journal of Obstetrics and Gynecology 1989;161:1407‐11. [DOI] [PubMed] [Google Scholar]
Hillard 1992
- Hillard PJ. Oral contraception noncompliance: the extent of the problem. Advances in Contraception 1992;8(Suppl 1):13‐20. [DOI] [PubMed] [Google Scholar]
Mishell 1991
- Mishell DR. Oral contraception: past, present, and future perspectives. International Journal of Fertility 1991;36 Suppl:7‐18. [PubMed] [Google Scholar]
Rossiter 1992
- Rossiter EJR. Reflections of a whistle‐blower. Nature 1992;357:434‐6. [DOI] [PubMed] [Google Scholar]
Schilling 1989
- Schilling LH, Bolding OT, Chenault CB, Chong AP, Fleury F, Forrest K, et al. Evaluation of the clinical performance of three triphasic oral contraceptives: a multicenter, randomized comparative trial. American Journal of Obstetrics and Gynecology 1989;160(5 Pt 2):1264‐8. [DOI] [PubMed] [Google Scholar]
Upton 1983
- Upton GV. The phasic approach to oral contraception: the triphasic concept and its clinical application. International Journal of Fertility 1983;28:121‐40. [PubMed] [Google Scholar]
Wallach 2000
- Wallach M, Grimes DA, Chaney EJ, Connell EB, Creinin MD, Emans SJ, et al. Modern Oral Contraception. Totowa (NJ): Emron, Inc., 2000. [Google Scholar]
References to other published versions of this review
Van Vliet 2002
- Vliet H, Grimes D, Helmerhorst F, Schulz K. Biphasic versus triphasic oral contraceptives for contraception. Contraception 2002;65:321‐4. [DOI] [PubMed] [Google Scholar]


