Abstract
Objectives: Over the last decades, there has been a significant increase in antimicrobial prescribing and consumption associated with the development of patients' adverse events and antimicrobial resistance (AMR) to the point of becoming a global priority. This study aims at evaluating antibiotic prescribing during COVID-19 pandemic from November 2019 to December 2020.
Materials and Methods: A systematic review was conducted primarily through the NCBI database, using PRISMA guidelines to identify relevant literature for the period between November 1, 2019 and December 19, 2020, using the keywords: COVID-19 OR SARS-Cov-2 AND antibiotics restricted to the English language excluding nonclinical articles. Five hundred twenty-seven titles were identified; all articles fulfilling the study criteria were included, 133 through the NCBI, and 8 through Google Scholar with a combined total of 141 studies. The patient's spectrum included all ages from neonates to elderly with all associated comorbidities, including immune suppression.
Results: Of 28,093 patients included in the combined studies, 58.7% received antibiotics (16,490/28,093), ranging from 1.3% to 100% coverage. Antibiotics coverage was less in children (57%) than in adults with comorbidities (75%). Broad-spectrum antibiotics were prescribed presumptively without pathogen identifications, which might contribute to adverse outcomes.
Conclusions: During the COVID-19 pandemic, there has been a significant and wide range of antibiotic prescribing in patients affected by the disease, particularly in adults with underlying comorbidities, despite the paucity of evidence of associated bacterial infections. The current practice might increase patients' immediate and long-term risks of adverse events, susceptibility to secondary infections as well as aggravating AMR.
Keywords: COVID-19, antibiotics, antimicrobial stewardship, resistance, AMR
Introduction
The discovery of antibiotics in the middle of the 20th century was a significant breakthrough for humanity saving millions of lives and preventing significant morbidity and mortality associated with infectious diseases.1 A decade after the historical discovery, a noticeable antimicrobial resistance (AMR) was observed escalating to an alarming scale over recent years.2 It has been estimated that about 700,000 annual global mortality is attributed to AMR, which attracted the attention of world leaders and international organizations such as the World Health Organization (WHO) all advocating regional and global initiatives to contain the problem.3 Antimicrobial Stewardship Programs (ASPs) have been implemented in many health care settings worldwide to curtail inappropriate and excessive antibiotic prescribing, particularly for broad-spectrum antibiotics.4 At the end of 2019, the world witnessed a worrying herald of a global pandemic caused by a novel coronavirus coined SAR-CoV-2 leading to the clinical syndrome of COVID-19 disease.5 Although the disease causes a respiratory illness primarily, it was noticed from the beginning it is associated with significant secondary presentations, including multisystem complications in need of critical care, particularly for server disease. Since there was no available effective management, antibiotics were frequently prescribed for various rationales with the potential of contributing to AMR.6 Although COVID-19 principally is a viral infection not usually responding to antibiotics, it is capable of causing an acute respiratory disease indistinguishable from bacterial infections and creating an environment and complications favoring secondary bacterial infections.7 For such reasons, health care professionals were confounded to prescribe antibiotics to treat potential bacterial infections or secondary complications. To comprehend the scale of the problem, a study conducted by the WHO demonstrated that 72% of COVID-19 patients received antibiotics. Nevertheless, only 8% had evidence of documented superimposed bacterial infections.8
To add to the complexity of the situation, unverified research at the start of the pandemic advocated combined management with chloroquine/hydroxychloroquine together with the macrolide antibiotic azithromycin led to hasty inclusion in many COVID-19 management guidelines across the globe before establishing better-evaluated efficacy.9 Even for patients who warrant treatment during the pandemic, Getahun et al.8 indicated that antimicrobials were overprescribed for patients admitted to intensive care units (ICUs) in 88 countries where 70% of patients received antibiotics. However, only 54% of patients had suspected or proven bacterial infections. Because of the gravity of the situation, confusion of the optimal management approaches for the novel disease together with the stretching of physical limits and capabilities of health care ASPs; the COVID-19 pandemic created an environment for inappropriate and excessive antibiotic prescribing, which might worsen future AMR through selective pressures. The presented literature review is conducted to examine and highlight the spectrum of antimicrobial prescribing during the COVID-19 pandemic to raise awareness toward potential consequences.
Materials and Methods
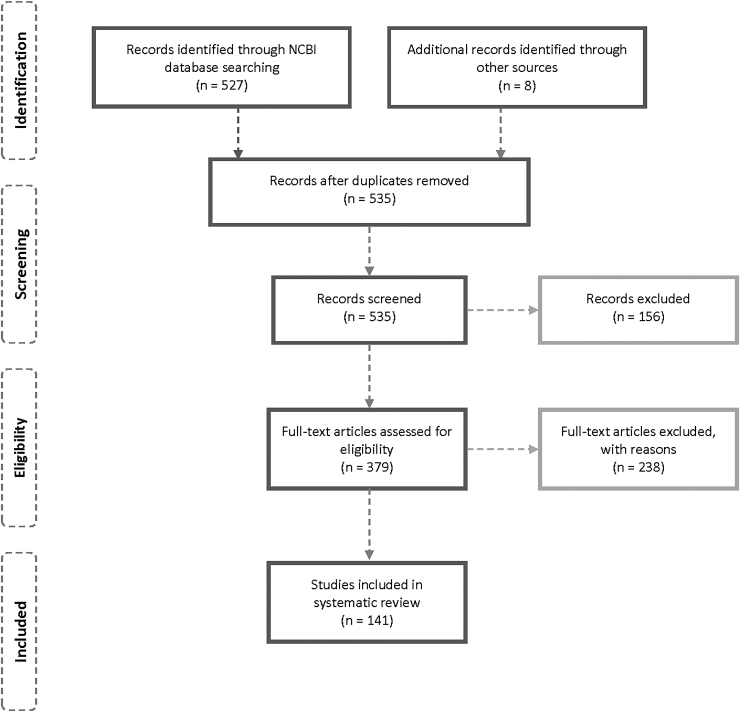
A literature search was conducted using the PRISMA guidelines for systematic reviews.172 The NCBI database was identified as a primary source of related literature because of clinical relevance between November 2019 and December 19, 2020. Adopted search keywords were COVID-19 OR Sars-Cov-2 AND antibiotics restricted to the English language. The search initially resulted in 527 identified titles eventually limited to 133 following applying restrictive criteria. An additional 8 articles were included following searching Google Scholar search engine, bringing the total number to 141 studies. As per the study protocol, only articles covering clinical settings were included, articles limited to basic science, solely microbiological characteristics, experiments, surveys, guidelines, and hypotheses. Those not providing details of antibiotic prescribing were excluded (n = 386) (Fig. 1). The information extracted from the included articles comprises types of antibiotics prescribed for COVID-19 patients and the number of those patients, bacterial coinfection, and relevant patient demographic data (age, gender, and country). In addition, if the COVID-19 patient is suffering from any other complications such as hypertension, cardiac disease, diabetes, pregnancy, cancer, and human immunodeficiency virus (HIV) were reported.
FIG. 1.
Schematic selection process of included studies.
Results
One hundred forty-one articles were included in this review from 28 different countries. The majority of them are from countries worst affected by the pandemic: China (n = 55), followed by the USA (n = 18), Italy (n = 10), UK (n = 5), Spain (n = 5), Brazil (n = 4), Iran (n = 4), and India (n = 3). Two articles were incorporated from Belgium, Germany, Japan, South Korea, Netherlands, and Saudi Arabia and one from Bhutan, Colombia, France, Ireland, Morocco, Niger, Oman, Philippines, Qatar, Singapore, Switzerland, Taiwan, and Uganda. Fourteen articles were included with no identified country (Table 1).
Table 1.
Showing Affected Countries, the Total Number of Patients, Number and Percentage of Patients Prescribed Antibiotics, Gender, Age, Prescribed Antibiotics, and Comorbidities
| Country | Total number of patients | Number of patients prescribed antibiotic therapy, n (%) | Gender | Age, mean ± SD (range) | Prescribed antibiotics and the number of patients prescribed | Comorbidities and the number of patients |
|---|---|---|---|---|---|---|
| South Korea10 | 7,339 | 2,820 (38.1) | 2,970 Male 4,369 Female |
47.1 ± 19.0 | Unspecified antibiotic: 3,174 Penicillin: 646 Cephalosporins: 1,649 Sulfamethoxazole/trimethoprim: 43 Tetracycline: 33 |
HT: 1,373 Tuberculosis: 28 COPD: 81 Pneumonia: 513 Asthma: 387 DM: 857 CKD: 48 CLD: 645 CVDs: 455 Cancer: 162 HIV: 4 |
| USA11 | 5,853 | 4,130 (71) | NA | NA | Doxycycline, azithromycin, levofloxacin, ciprofloxacin, ceftriaxone, and cefepime | Not reported |
| China12 | 3,309 | 2,127 (64.28) | 1,642 Male 1,667 Female |
62 (median) | Unspecified antibiotics | HT: 988 DM: 464 CVD: 242 Cerebrovascular disease: 130 Cancer: 93 CKD: 57 COPD: 42 |
| China13 | 1,123 | 792 (70.5) | 560 Male 563 Female |
61 (median) | Azithromycin: 63 Fluoroquinolones: 666 Levofloxacin: 77 Moxifloxacin: 690 Cephalosporins: 220 Penicillin: 50 Carbapenems: 108 Meropenem: 77 |
HT: 361 Coronary heart disease: 95 Other heart diseases: 46 DM: 147 Cancer: 40 COPD: 40 |
| China14 | 1,099 | 637 (58) | 640 Male 459 Female |
47 (median) | Unspecified antibiotics | Not reported |
| China15 | 970 | 505 (52.1) | 561 Male 409 Female |
45.1 ± 17.3 | Teicoplanin | Not reported |
| Netherlands16 | 925 | 669 (72.3) | 583 Male 324 Female |
70 (median) | Cefuroxime, amoxicillin, ciprofloxacin | Not reported |
| China17 | 476 | 319 (67) | 319 Male 205 Female |
53 (median) | Unspecified antibiotics | Not reported |
| China15 | 468 | 264/330 (80.0) | 282 Male 282 Female |
53.1 ± 27.6 | Teicoplanin | Not reported |
| China18 | 465 | 218 (46.88) | 243 Male 222 Female |
45 (5–88) | Cephalosporins, quinolones, carbapenem, tigecycline, and linezolid | HT: 82 DM: 28 CLD: 19 Cancer: 5 (1.08%) CKD: 5 Heart disease: 3 Pediatric: 3 Pregnancy: 2 |
| China19 | 450 | 225 (50) | 228 Male 222 Female |
46.2 ± 15.1 | Quinolones: 190 Cephalosporins: 22 Carbapenems: 8 Macrolides: 4 Penicillin: 33 Linezolid: 6 Polymyxin: 1 Teicoplanin: 1 |
HT: 75 DM: 45 CVD: 22 CLD: 11 CKD: 1 Cerebrovascular disease: 11 COPD: 10 Cancer: 5 Rheumatic disease: 2 |
| China20 | 350 | 177 (50.6) | 173 Male 177 Female |
43 (median) | Moxifloxacin: 156 Levofloxacin: 25 Piperacillin/tazobactam: 9 Unspecified antibiotics: 11 |
HT: 51 DM: 26 CVD: 15 Chronic pulmonary disease: 7 CKD: 9 CLD: 14 Cancer: 1 |
| China21 | 334 | 167 (50) | 173 female 161 Male |
60 (21–90) | Unspecified antibiotics | Not reported |
| USA22 | 321 | 222 (69) | 155 Male 166 Female |
60 ± 17 | Unspecified antibiotics | Not reported |
| USA23 | 242 | 162 (67) | 123 Male 119 Female |
50–82 | Unspecified antibiotics | COPD: 30 Asthma: 18 Heart failure: 35 Atrial fibrillation: 24 Liver cirrhosis: 8 DM: 118 CKD: 42 Renal disease: 19 Coronary artery disease: 45 HT: 180 |
| China24 | 204 | 141 (69.12) | 107 Male 97 Female |
52.91 ± 15.98 | Antibiotic treatment | Not reported |
| China25 | 200 | 141 (70.5) | 98 Male 102 Female |
55 ± 17.1 | Moxifloxacin, ceftriaxone | Not reported |
| China26 | 195 | 115 (59.0) | 100 Male 95 Female |
64 (median) | Unspecified antibiotics | Not reported |
| Brazil27 | 181 | 148 (81.8) | Male 71 110 Female |
55.3 ± 21.1 | Unspecified antibiotics | Cancer: 181 HT: 77 DM: 31 Chronic renal failure: 10 COPD/asthma: 7 |
| China28 | 169 | 87 (51.5) | 86 Male 83 Female |
45 (median) | Unspecified antibiotics | HT: 19 DM: 13 COPD: 3 Cancer: 2 CVD and cerebrovascular diseases: 10 |
| USA, Italy, Spain29 | 144 | 106 (74) | 94 Male 50 Female |
62 (median) | Unspecified antibiotics | Kidney transplant: 144 |
| Germany23 | 140 | 121 (86.4) | 90 Male 50 Female |
63.5 (17–99) | Ampicillin/sulbactam: 56 Piperacillin/tazobactam: 26 Azithromycin: 38 Meropenem: 6 Moxifloxacin: 4 Cephalosporin: 3 |
HT: 68 (48.6%) DM: 30 (21.4%) Coronary heart disease: 26 (18.6%) Congestive heart failure: 12 (8.6%) COPD: 7 (5.0%) Bronchial asthma: 15 (10.7%) CKD: 16 (11.4%) Cancer: 29 (20.7%) HIV: 5 (3.6%) CLD: 7 (5.0%) |
| China30 | 138 | NA | 75 Male 63 Female |
56 (median) | Moxifloxacin: 89 Ceftriaxone: 34 Azithromycin: 25 |
Not reported |
| China31 | 136 | NA | 66 Male 70 Female |
56 (median) | Moxifloxacin: 51 Cefoperazone-sodium/sulbactam-sodium: 88 Imipenem/cilastatin: 4 |
Not reported |
| China32 | 135 | 131 (97) | 57 Male 78 Female |
53.53 ± 13.22 | Moxifloxacin | Not reported |
| China33 | 135 | 59 (43.7) | 72 Male 63 Female |
47 (median) | Unspecified antibiotics | Not reported |
| China34 | 132 | 92 (69.6) | 74 Male 58 Female |
58.8 ± 12.9 | Unspecified antibiotics | CVD: 52 Cancer: 7 CKD: 1 |
| China35 | 107 | 85 (79.4) | 57 Male 50 Female |
51 (median) | Unspecified antibiotics | Not reported |
| China36 | 101 | 99 (98) | 48 Male 53 Female |
51 (median) | Unspecified antibiotics | Not reported |
| China37 | 99 | 70 (71) | 67 Male 32 Female |
55 · 5 ± 13 · 1 (21–82) | Cephalosporins, quinolones, carbapenems, tigecycline, and linezolid | Not reported |
| South Korea38 | 98 | 98 (100) | 38 Male 60 Female |
55.4 ± 17.1 | Unspecified antibiotics | Not reported |
| China39 | 93 | 84 (90.3) | 54 Male 39 Female |
43 ± 17.34 | Moxifloxacin: 54 Levofloxacin: 5 Azithromycin: 1 Amoxicillin: 1 Cefepime: 1 Cefperazone-sulbactam: 1 Cefixime: 1 Other: 23 |
HT: 6 DM: 6 Heart disease: 3 Stroke: 2 Hypothyroidism: 2 COPD or chronic bronchitis: 2 |
| China40 | 90 | 47 (52) | 48 Male 42 Female |
64 (median) | Unspecified antibiotics | CVD: 11 HT: 38 DM: 17 COPD: 4 CKD: 1 Cerebrovascular disease: 6 Cancer: 10 |
| China41 | 85 | 77 (90.6) | 62 Male 23 Female |
65.8 ± 14.2 | Meropenem: 38 Imipenem/cilastatin: 1 Moxifloxacin: 40 Levofloxacin: 4 Linezolid: 18 Vancomycin: 2 Teicoplanin: 2 Tigecycline: 2 Piperacillin/tazobactam: 9 Ceftriaxone sodium: 3 Cefoperazone/sulbactam: 2 Ceftazidime/tazobactam: 2 |
Not reported |
| China21 | 82 | 68 (82.9) | 44 Male 38 Female |
74 (34–95) | Unspecified broad-spectrum antibiotics | Cardiac disease, injury, and surgery: 82 |
| Brazil42 | 79 | 60 (76) | 43 Male 36 Female |
4 (median) | Unspecified antibiotics | Pediatric: 79 |
| China43 | 74 | 31 (41.89) | 37 Male 37 Female |
46.14 ± 14.19 | Unspecified antibiotics | Not reported |
| Italy44 | 70 | 32 (45.7) | 41 Male 29 Female |
45–74 | Azithromycin | Not reported |
| China45 | 68 | 24 (35.3) | 25 Male 43 Female |
44.3 ± 16.4 | Moxifloxacin: 21 Cephalosporin: 9 Azithromycin:2 Amoxicillin: 2 |
Not reported |
| UK46 | 68 | 9 (1.3) | 32 Male 36 Female |
42.5 (0.5–76) | Doxycycline, moxifloxacin | Not reported |
| France47 | 66 | 34 (51.5) | 15 Male 51 Female |
87.7 ± 9.0 | Azithromycin and rovamycin | Not reported |
| China48 | 64 | 45 (70.3) | 20 Male 44 Female |
61 (median) | Unspecified antibiotics | HT: 32 |
| Oman49 | 63 | NA | 53 Male 10 Female |
48 ± 16 | Ceftriaxone:50 Azithromycin:45 Piperacillin/tazobactam: 49 |
Not reported |
| China26 | 63 | 47 (74.6) | 38 Male 25 Female |
65 (57–71) | Unspecified broad-spectrum antibiotics | Diabetic: 63 |
| Saudi Arabia50 | 61 | 61 (100) | 54 Male 7 Female |
51 (median) | Azithromycin, ceftriaxone, and piperacillin/tazobactam | DM: 24 HT: 13 Hypothyroidism: 1 |
| Spain51 | 60 | 5 (8.3) | 60 Female | NA | Unspecified antibiotics | Pregnant: 60 |
| NA52 | 58 | 29 (50.0) | NA | >20 years | Levofloxacin, moxifloxacin, meropenem, and cefixime | Not reported |
| Europe53 | 57 | 35 (63) | 40 Male 17 Female |
65 (57–70) | 1 or more unspecified antibiotics and azithromycin as COVID-19 treatment | Liver transplant |
| Brazil54 | 56 | 33 (58.9) | 39 Male 17 Female |
6.2 (median) | Unspecified antibiotics | Pediatric: 56 |
| China55 | 55 | 29 (52.7) | 31 Male 24 Female |
44 (median) | Unspecified antibiotics | HT: 8 DM: 5 Respiratory diseases: 4 Thyroid disease: 3 CLD: 3 CKD: 1 CVD: 1 |
| China32 | 52 | 52 (100) | 34 Male 18 Female |
71.40 + 9.43 | Moxifloxacin | Cardiac disease, injury and surgery: 52 |
| China56 | 47 | 25 (53.19) | 21 Male 26 Female |
45 (median) | Unspecified antibiotics | HT: 10 DM: 9 Coronary heart disease: 6 COPD: 10 |
| China57 | 44 | 16 (36.4) | 22 Male 22 Female |
(1–18) years | Unspecified antibiotics | Pediatric: 44 |
| China58 | 41 | 41 (100) | 30 Male 11 Female |
49 (median) | Unspecified antibiotics | Not reported |
| China59 | 34 | 29 (85) | 14 Male 20 Female |
33 (10.00–94.25) months | Azithromycin was given to 9 patients with pneumonia infection | Pediatric: 34 |
| Italy60 | 33 | NA | 30 Male 3 Female |
64 (median) | Carbapenem: 4 Cephalosporin: 7 Macrolide: 18 Penicillin: 23 Unspecified antibiotics: 2 |
Heart disease: 14 Lung disease: 4 DM: 2 Autoimmune disease or immunodeficiency: 1 |
| NA61 | 32 | 18 (56.3) | NA | NA | Initial antibiotic therapy: cefuroxime 7 Amoxicillin-clavulanic acid 1 Piperacillin/tazobactam Subsequent antibiotic therapy: 7 Cases treated with cefuroxime, 1 amoxicillin-clavulanic acid, 1 Ceftazidime, 2 vancomycin 2, flucloxacillin 3 |
Not reported |
| China62 | 31 | 6 (19.4) | NA | 7 years and 1 month (6 months–17 years) | Unspecified antibiotics | Pediatric: 31 |
| Iran63 | 30 | NA | 14 Male 16 Female |
0–18 years | Ceftriaxone: 17 Azithromycin: 2 Meropenem: 6 Clindamycin: 3 Vancomycin: 6 |
Pediatric: 30 |
| China64 | 28 | 23 (82.1) | 17 Male 11 Female |
65 (median) | Unspecified antibiotics | Cancer: 28 |
| Italy65 | 25 | 20 (80) | 20 Male 5 Female |
71.64 ± 10.08 | Ceftriaxone and azithromycin | Cancer: 25 |
| China66 | 25 | 13 (56) | 14 Male 11 Female |
3 (2–9) | For 2 critical cases: Case 1: cefoperazone/sulbactam Case 2: meropenem, linezolid |
Pediatric: 25 |
| China57 | 23 | 6 (26.1) | 10 Male 13 Female |
0 day–1 year | Unspecified antibiotics | Neonate and infant: 23 |
| China67 | 20 | 17 (85.0) | 10 Male 10 Female |
43.2 ± 14.0 | Unspecified antibiotics | Not reported |
| China68 | 17 | 13 (76.5) | 12 Male 5 Female |
88 (median) | Unspecified antibiotics | HT: 9 CVD: 8 CKD: 6 DM: 5 Neurodegenerative diseases 5 COPD: 3 Cancer: 2 |
| China69 | 16 | 8 (50) | 6 Male 10 Female |
44.1 (5–70) | Unspecified antibiotics | Not reported |
| China70 | 15 | 15 (100) | Female | 32 ± 5 | Unspecified antibiotics | Pregnant: 15 |
| China71 | 11 | 11 (100) | 5 Male 6 Female |
36.6 (2–69) | Ceftriaxone and moxifloxacin initially and changed to cefoperazone sulbactam, linezolid, and polymyxin later | Not reported |
| China72 | 10 | 5 (50) | 4 Male 6 Female |
74 (3–131) months | Unspecified antibiotics | Pediatric: 10 |
| Spain73 | 10 | 10 (100) | 3 Male 7 Female |
54 ± 10 | Cephalosporin: 7 Carbapenem: 4 Macrolide: 8 Linezolid: 2 |
HT: 9 DM: 4 Kidney transplant: 10 |
| China74 | 9 | 4 (44.4) | 5 Male 4 Female |
42 (14–56) | Moxifloxacin | Not reported |
| China75 | 9 | 9 (100) | Female | 29.9 (26–40) | Unspecified antibiotics | Pregnant: 9 |
| NA76 | 8 | 4 (50) | 2 Male 6 Female |
5 days–12 month | Amoxicillin, cefotaxime and gentamicin | Neonate and infant: 8 |
| UK76 | 8 | 4 (50) | 2 Male 6 Female |
5.1 months (5 days–12 months) | Unspecified antibiotics | Not reported |
| China77 | 6 | 6 (100) | 2 Male 6 Female |
3 (1–7) | Unspecified antibiotics | Not reported |
| Italy78 | 6 | 6 (100) | 5 Male 1 Female |
66.5 (50–82) | Unspecified antibiotics | Not reported |
| Spain79 | 5 | 5 (100) | 3 Female 2 Male |
62 (38–86) | All patient received azithromycin and ceftriaxone In addition, case 1: ceftaroline Case 2 and 5: oral cefixime Case 3: levofloxacin |
Not reported |
| China80 | 5 | 5 (100) | 4 Male 1 Female |
≥55 years | Unspecified antibiotics | Not reported |
| China82 | 5 | 5 (100) | 2 Male 3 Female |
50.2 (39–66) | Unspecified antibiotics | Not reported |
| Spain83 | 5 | 4 (80) | 3 Male 2 Transgender |
37.8 (29–49) | Case1: — Case 2: meropenem (for 16 days) Case 3: azithromycin (for 5 days) Case 4: azithromycin (for 5 days), cefixime (for 5 days) Case 5: azithromycin (for 5 days), ceftaroline fosamil (for 7 days), co-trimoxazole (for 21 days, followed by secondary prophylaxis) |
HIV: 5 |
| China82 | 5 | 5 (100) | 2 Male 3 Female |
50.2 (39–66) | Unspecified antibiotics | HT: 2 CVD: 1 |
| China81 | 5 | 4 (80) | 1 Male 4 Female |
65.8 (51–79) | Levofloxacin, moxifloxacin, ceftriaxone, piperacillin-tazobactam, and meropenem | Rheumatic diseases: 5 |
| Australia84 | 5 | 5 (100) | 5 Males | 63 (46–74) | Unspecified antibiotics | HT: 2 DM: 2 Aortic valve replacement: 1 Asthma: 1 |
| USA85 | 4 | 2 (50) | 2 Male 2 Female |
54.3 (38–64) | Azithromycin, also ceftriaxone, was given to one patient | Cardiac disease, injury, and surgery: 4 |
| Italy86 | 4 | 4 (100) | 2 Male 2 Female |
61 (48–70) | Case 1: piperacillin/tazobactam and levofloxacin Case 2: meropenem Case 3: iv meropenem Case 4: piperacillin/tazobactam |
Lung transplant: 4 |
| NA87 | 3 | 3 (100) | 3 Male | 56 (38–74) | Azithromycin | Not reported |
| China88 | 3 | 1 (33.3) | 3 Male | 7.6 (6–9) | Ceftriaxone | Pediatric: 3 |
| Belgium89 | 3 | 3 (100) | 1 Male 2 Female |
51.6 (44–64) | Unspecified antibiotics | CVDs: 1 |
| Philippines90 | 2 | 1 (50) | 1 Male 1 Female |
44 years 39 years |
Vancomycin | None reported |
| China91 | 2 | 2 (100) | 1 Male 1 Female |
40 years 79 years |
Unspecified antibiotics | Renal failure: 2 |
| China92 | 2 | 2 (100) | Male | 47–60 | Case 1: moxifloxacin, ceftriaxone, and tazobactam Case 2: moxifloxacin |
HIV: 2 |
| Italy93 | 2 | 1 (50) | Male | 69–73 | Azithromycin | Cancer: 2 |
| NA94 | 2 | 1 (50) | 1 Male 1 Female |
59–75 | Sulfamethoxazole-trimethoprim-ds | Heart transplant: 2 |
| China95 | 2 | 2 (100) | 2 Male | 51–58 | Case 1: moxifloxacin, cephalosporin, linezolid, and meropenem Case 2: moxifloxacin |
Case 1: allogeneic bone marrow transplantation Case 2: kidney transplantation |
| USA94 | 2 | 1 (50) | 1 Male 1 Female |
59–75 | Case1: cefepime Vancomycin Doxycycline sulfamethoxazole-trimethoprim Tobramycin Linezolid |
Case 1 and 2: heart transplant DM, HT, CKD |
| USA96 | 2 | 1 (50) | 2 Male | NA | Case 2: ceftriaxone, piperacillin-tazobactam | Pediatric: 2 |
| USA97 | 2 | 2 (100) | 1 Male 1 Female |
55–57 | Azithromycin: 2 | Case 1: asthma, HT case 2: DM, HT |
| Iran98 | 2 | 1 (50) | 2 Male | 0 months | Unspecified antibiotics | Neonate and infant: 2 |
| USA99 | 2 | 2 (100) | 2 Female | 26–77 | Ceftriaxone, azithromycin | Not reported |
| Switzerland100 | 2 | 2 (100) | Male | 59 | Levofloxacin: 1 Amoxicillin/clavulanate: 1 |
HT: 1 |
| Ireland101 | 1 | 1 (100) | Male | 25 | Unspecified antibiotics | Not reported |
| Japan102 | 1 | 1 (100) | Male | 59 | Unspecified antibiotics | Not reported |
| Taiwan103 | 1 | 1 (100) | Female | 55 | Ceftriaxone replaced by oral amoxicillin/clavulanate | Not reported |
| Bhutan104 | 1 | 1 (100) | Male | 76 | Ceftriaxone and doxycycline switched to meropenem and vancomycin | Not reported |
| Colombia105 | 1 | 1 (100) | Male | 34 | Unspecified broad-spectrum antibiotics | Not reported |
| Japan106 | 1 | 1 (100) | Female | 72 | Cefepime and clindamycin phosphate | Not reported |
| NA107 | 1 | 1 (100) | Male | 33 | Piperacillin–tazobactam | Not reported |
| China108 | 1 | 1 (100) | Male | 23 | Meropenem and linezolid | DM: 1 |
| Italy109 | 1 | 1 (100) | Male | 56 | Piperacillin/tazobactam | Spinal cord injury patient: 1 |
| China110 | 1 | 1 (100) | Male | 50 | Moxifloxacin | Renal failure: 1 |
| NA111 | 1 | 1 (100) | Male | 59 | Cefepime, piperacillin/tazobactam, linezolid, gentamicin and meropenem and amikacin | Not reported |
| Italy112 | 1 | 1 (100) | Female | 54 | Unspecified broad-spectrum antibiotics | Diaphragmatic rupture and gastric perforation: 1 |
| NA113 | 1 | 1 (100) | Male | 64 | Amoxicillin/clavulanic | Cardiac disease, injury, and surgery: 1 |
| NA114 | 1 | 1 (100) | Male | 63 | Piperacillin–tazobactam | Cardiac disease, injury, and surgery: 1 |
| NA115 | 1 | 1 (100) | Male | 37 | Piperacillin sulbactam | Cardiac disease, injury, and surgery: 1 |
| NA116 | 1 | 1 (100) | Male | 75 | Azithromycin with hydroxychloroquine | HIV: 1 |
| NA117 | 1 | 1 (100) | Female | 56 | Zosyn and vancomycin | Liver failure: 1 |
| China118 | 1 | 1 (100) | Female | 62 | Meropenem and teicoplanin, followed by linezolid and tigecycline | Cancer: 1 |
| NA119 | 1 | 1 (100) | Male | 63 | Ceftizoxime sodium+moxifloxacin to ceftizoxime sodium+teicoplanin | Cancer: 1 |
| Iran120 | 1 | 1 (100) | Male | 15 days | Vancomycin and amikacin | Neonate: 1 |
| Morocco121 | 1 | 1 (100) | Female | 17 months | Amoxicilline-acide clavulanique and azithromycin | Infant: 1 |
| China122 | 1 | 1 (100) | NA | NA | Meropenem and linezolid | Pediatric: 1 |
| Uganda123 | 1 | 1 (100) | Female | 34 years | Unspecified antibiotics | HIV: 1 |
| UK124 | 1 | 1 | Female | 22 | Ceftriaxone | None reported |
| Saudi Arabia125 | 1 | 1 (100) | Male | 45 | Meropenem and vancomycin | None reported |
| India126 | 1 | 1 | NA | 1 week | Ampicillin, amoxicillin/clavulanate, meropenem, vancomycin | Neonate and infant: 1 |
| India127 | 1 | 1 | Male | 60 | Unspecified antibiotics | DM, HT, and biclonal gammopathy: 1 |
| USA128 | 1 | 1 (100) | Male | 23 | Unspecified antibiotics | Not reported |
| UK129 | 1 | 1 (100) | Male | 77 | Levofloxacin | HT: 1 |
| US130 | 1 | 1 (100) | Male | 20 | Unspecified antibiotics | None reported |
| USA131 | 1 | 1 (100) | Male | 88 | Unspecified antibiotics | HT: 1 |
| India132 | 1 | 1 (100) | Male | 60 | Meropenem, vancomycin | DM: 1 |
| USA133 | 1 | 1 (100) | Male | 58 | Azithromycin, piperacillin/tazobactam | Not reported |
| China110 | 1 | 2 (100) | Male | 79 | Moxifloxacin | End-stage renal disease: 1 |
| Germany134 | 1 | 1 (100) | Male | 46 | Ampicillin/sulbactam | HT: 1 |
| USA135 | 1 | 1 (100) | Male | 24 | Vancomycin, cefepime, meropenem | DM: 1 |
| Netherlands136 | 1 | 1 (100) | Male | 7 | Amoxicillin | Not reported |
| Singapore137 | 1 | 1 (100) | Male | 77 | Unspecified antibiotics | HT, coronary artery disease, and asthma-COPD overlap syndrome: 1 |
| Niger138 | 1 | 1 (100) | Male | 8 months | Ceftriaxone, gentamycin | Neonate and infant |
| US139 | 1 | 1 (100) | Male | 49 | Ceftriaxone, azithromycin | Not reported |
| Qatar140 | 1 | 1 (100) | Female | 40 | Azithromycin, piperacillin/tazobactam, meropenem | Not reported |
| Belgium113 | 1 | 1 (100) | Male | 64 | Amoxicillin/clavulanate | HT and aortic dissection: 1 |
| Italy141 | 1 | 1 (100) | Female | 78 | Ceftriaxone, piperacillin/tazobactam, levofloxacin | Not reported |
| USA142 | 1 | 1 (100) | Female | 13 | Ceftriaxone, metronidazole | Pediatric: 1 |
| China143 | 1 | 1 (100) | Female | 65 | Moxifloxacin | Not reported |
| Brazil144 | 1 | 1 (100) | Male | 65 | Meropenem, vancomycin | DM, HT, and cancer: 1 |
| China145 | 1 | 1 (100) | Male | 64 | Unspecified antibiotics | Cancer: 1 |
| USA146 | 1 | 1 (100) | Male | 78 | Cefepime | Not reported |
| USA147 | 1 | 1 (100) | Male | 51 | Ceftriaxone, azithromycin | Diabetes: 1 |
CKD, chronic kidney disease; CLD, chronic liver disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; HIV, human immunodeficiency virus; HT, hypertension.
The study population's demographic and clinical characteristics included all ages from neonates, children, and adults, including pregnant women and the elderly. Associated underlying conditions included hypertension, diabetes mellitus, heart, respiratory, renal, liver, thyroid, cerebrovascular, rheumatic diseases, and HIV and organ transplantation (heart, lung, kidney, liver, and bone marrow). Of 28,093 patients included in the combined studies, 58.7% received antibiotics (16,490/28,093). The percentage of patients prescribed antibiotics in each article differs, ranging from 1.3% to 100% coverage, with only 9.9% of the articles reporting less than 50% antibiotic covering (14/141). Most included articles did not present clear data on an antibiotic prescription for patients with other complications versus those without comorbidities. Comparing the articles that include the population who suffered from other diseases to those with no other complications, we found that antibiotic coverage did not differ significantly between patients with and without comorbidities (75.2%, 415/552), and 71% (8,449/11,886), respectively (Fig. 2).
FIG. 2.
Comparison of percentage antibiotic prescription in studied population compared to patients with reported comorbidities, children, and pregnant women.
Antibiotics coverage was less in children, 57% (187/329) compared to adults, and it was least in pregnant women (34.5%, 29/84). Despite the high percentage of antibiotic prescribing, most articles did not report bacterial coinfection (75.36%), indicating that probably a significant amount of antibiotics were empirically and unnecessarily prescribed.
The spectrum of antimicrobial prescreening is broad since more than 40 different antimicrobials were used to manage patients with COVID-19 disease (Table 2).
Table 2.
Showing the Number of Articles Reporting Each Antibiotic
| Antibiotic | No. of articles |
|---|---|
| Unspecified antibiotics | 68 |
| Cephalosporins | 38 |
| Azithromycin | 27 |
| Moxifloxacin | 23 |
| Meropenem | 20 |
| Piperacillin/tazobactam | 18 |
| Levofloxacin | 13 |
| Linezolid | 12 |
| Vancomycin | 9 |
| Amoxicillin/clavulanate | 8 |
| Teicoplanin | 6 |
| Carbapenem | 6 |
| Amoxicillin | 6 |
| Cefepime | 6 |
| Tigecycline | 4 |
| Cefoperazone/sulbactam | 4 |
| Cefixime | 4 |
| Penicillin | 4 |
| Doxycycline | 4 |
| Fluoroquinolones | 3 |
| Imipenem/cilastatin | 2 |
| Clindamycin | 2 |
| Amikacin | 2 |
| Gentamicin | 2 |
| Trimoxazole | 2 |
| Sulfamethoxazole/trimethoprim | 2 |
| Ampicillin/sulbactam | 2 |
| Flucloxacillin | 1 |
| Ceftazidime/tazobactam | 1 |
| Cefotaxime | 1 |
| Ceftaroline fosamil | 1 |
| Ceftizoxime sodium | 1 |
| Meropenem/vancomycin | 1 |
| Piperacillin/sulbactam | 1 |
| Tazobactam | 1 |
| Spiramycin | 1 |
| Tobramycin | 1 |
| Clarithromycin | 1 |
| Ampicillin | 1 |
| Tetracycline | 1 |
| Polymyxin | 1 |
| Metronidazole | 1 |
Inferring from the number of articles reporting the use of specific antibiotics, cephalosporins followed by azithromycin and moxifloxacin were the predominant oral antibiotics while piperacillin/tazobactam was the prevalent parenteral antibiotic. However, when subdividing cephalosporins into distinct classes based on their generation (first vs. second vs. third vs. fourth), azithromycin becomes the predominant antibiotic reported, which reflects its prominent role during the pandemic. Nevertheless, most studies highlighted that the majority of antibiotics were prescribed empirically as prophylaxis to prevent secondary bacterial infection,70 to treat secondary bacterial infection such as pneumonia,59 or as potential COVID-19 treatment agents.53 Other described drugs reported include meropenem, levofloxacin, linezolid, vancomycin, amoxicillin/clavulanate, Teicoplanin, and carbapenem.
Discussion
The excessive and inappropriate prescribing of antibiotics is a significant challenge for health care across the globe. The escalating problem has been directly associated with detrimental patients' safety through the development of direct adverse events, indirect acquisition of secondary health care-associated infections, propagation of AMR, worsening infection control and prevention measures, as well as substantial cost implications.148,149 Of all infectious diseases, respiratory infections are the leading cause of inappropriate antibiotic prescribing and overuse. The majority of upper respiratory tract infections are caused by viruses, and only less than 10% are caused by bacteria150; nevertheless, the WHO reported that in 2016, 71% of patients with UTRIs had been prescribed antibiotics.151
The COVID-19 pandemic caught all health care settings across the globe by surprise; the novel SARS-CoV-2 virus caused an unprecedented universal health scare since there was little preceding knowledge about the disease and its implications, particularly potential secondary infections. Furthermore, the disease presents primarily as a respiratory illness mimicking bacterial infections hence confounding clinical assessment; conversely, critical patients need invasive procedures often associated with secondary health care-associated infections. To add the disease complexity, unverified early clinical reports and trials advocated using antibiotics to hinder disease progression and hasten viral clearance, despite the discouragement of such an approach by international guidelines.8 Consequent to all these factors, antibiotic prescribing was noticeably frequent in patients with COVID-19 disease.
Our search encompassed about 28,000 patients from 28 different countries, to evaluate the problem systematically, the majority of which were severely affected by the pandemic, such as China, Iran, Italy, Spain, UK, and the USA, demonstrated widespread practice of prescribing antibiotics particularly in adults underlying clinical with conditions. The overall percentage of cases prescribed antimicrobial therapy is evident in 58.7% of cases being more common with premorbid or immune-compromised conditions (Fig. 1). Several authors reported treatment strategies for COVID-19 patients incorporating empirical antibiotic treatment.14,30,37,58,152 Such observations are in line with early pandemic epidemiological reports since it was apparent that more severe and critical disease is predominant in the elderly and those with underlying premorbid conditions such as diabetes, heart failure, and the immune-compromised. Conversely, severity markers included acute kidney and liver injuries, explaining antibiotic prescribing prevalence in such populations.
It is worth noticing; prescribed antibiotics are not necessarily to cover documented secondary bacterial infections since, in many studies, the presence of bacterial coinfection or secondary infection is much lower than the number of patients prescribed antimicrobial therapy. In their review, Lai et al.153 reviewed 13 papers for the presence of bacterial coinfection or secondary infection, 5 of which reported 0% bacterial coinfection or secondary infection. In contrast, three reported a low percentage of 1%, 3.4%, and 4.8%, respectively. Similarly, a large-scale study from New York described 5,700 patients with only 3 secondary bacterial infections.154 On the contrary, this in contrast with Italy's study, where 17.2% of patients had bacterial pneumonia and 37% suffered from secondary bacteremia.155 Lansbury et al. covered 30 studies and 3,834 patients, demonstrating only 7% of the hospitalized patients infected with COVID-19 had a bacterial coinfection.156 Understandably, the presence of bacterial coinfection was highest in ICU patients (14%) compared to patients in mixed wards (4%). A third review reported 8% of bacterial or fungal coinfection.7
The reviewed evidence supports the discrepancy between inappropriate and excessive antibiotic prescribing in patients with COVID-19 disease and the presence of bacterial coinfections. Nevertheless, Chien-Yi Chang and Kok-Gan Chan argue that the low rate of coinfection could result from prescribing antibiotics on a large scale to avoid overwhelming health systems during the early pandemic.157 Furthermore, some have argued that the lack of clear antimicrobial stewardship guidance for the frontline clinician at the early stages of the pandemic probably resulted in an inclination toward antimicrobial prescribing, especially in the early stages of the pandemic. In addition, Lansbury et al.'s156 analysis shows that more than 90% of the patients in 10 out of 17 studies, in which patients were prescribed antibiotics, received the antimicrobial therapy empirically. It is also worth mentioning that in patients with moderate and severe symptoms, those who received antibiotics or corticosteroids had more extended hospital stays than those who did not.17
It is worth noting that the high percentage of antibiotic prescribing in patients with no comorbidities (71%) could be confounded by not reporting them in some of the articles, which does not equate to their absence. It is quite possible that an undetermined percentage of patients in such studies suffer from comorbidities. The review also demonstrated lower antibiotic prescribing patterns in the pediatrics population; from 329 neonates, infants, and children included in the review, only 187 (57%) were prescribed antimicrobial therapy. This is a lower rate but might also be appropriate since coinfection is expected in the pediatric population since two studies reported 40% and 51.3% coinfection rates, respectively.158,159 This indicates that the pediatric population might have been better managed during the pandemic from the ASP point of view. Pregnant women were the least to be prescribed antimicrobial therapy, with only 34.5%, which might be due to fears of prescribing antimicrobials during pregnancy rather than its liberal use when compared to a similar cohort, however, we are not sure of the reason for this lower rate in antimicrobial prescription in pregnant women.
The macrolide antibiotic azithromycin was the predominant antimicrobial agents reported in the management of COVID-19 disease (Table 2). Most possible, it was used for its claimed anti-inflammatory effect.160 Before the start of the pandemic, it was used mostly to treat community-acquired pneumonia as well as exacerbations of chronic obstructive pulmonary disease.161 Azithromycin's role has been recognized by previous reports of efficacy against other RNA viruses such as Zika and Ebola virus disease162–164 and has been speared when suggested as an adjunct to hydroxychloroquine leading to rapid viral clearance in COVID-19 patients through unclear mechanisms.9 This probably reflects the highlighted issue with the drug in the foremost pandemic history.160 Although some limited reports support improved outcomes with adjunctive macrolides in the treatment of COVID-19 disease stemming from previous observations of moderate-to-severe acute respiratory distress syndrome, this has not been materialized in COVID-19 clinical trials.165 Furthermore, both hydroxychloroquine/chloroquine and azithromycin have been associated with cardiotoxicity by prolonging the QT intervals (the time it takes for the ventricles of the heart to contract and relax), which might precipitate arrhythmias in susceptible patients, particularly those with cardiac diseases, the impact of which is yet to be thoroughly evaluated.166 The widely used antibiotic azithromycin was gradually recognized as a rare cause of prolonged QT, severe arrhythmia, and increased risk of sudden death.167–170 Beović et al.171 reported that broad-spectrum antibiotic use in patients with COVID-19 is widespread, according to his survey study administered across 82 hospitals in 23 countries. Importantly, different broad-spectrum antibiotics have been frequently prescribed, including piperacillin/tazobactam, meropenem, vancomycin, and teicoplanin, highlighting potential further development of current or future AMR. More than half of the respondents reported combined use of β-lactams and macrolides or fluoroquinolones, and the most commonly prescribed antibiotic in the COVID-19 ICU was piperacillin/tazobactam.171 Worryingly, most broad-spectrum antibiotics have been prescribed empirically as prophylaxis to prevent secondary bacterial infection,70 or to treat bacterial secondary infection and pneumonia,59 or as part of COVID-19 treatment53
Although the systematic search captured a significant number of studies in a short time frame, we acknowledge there are some accompanying limitations. Restricting inclusion to the English language probably omitted other thematic studies. The pandemic's dynamic nature and short time reporting scope probably caused reporting bias, which might be corrected over time. Nevertheless, our report outcomes are in line with other conducted cross-sectional studies such as the WHO studied report.8
In summary, this systematic review demonstrated the widespread practice of antibiotic prescribing for COVID-19 patients during the pandemic with little supporting evidence of secondary bacterial infections. While the practice is more frequent in adult patients with comorbidities than in the younger population, this might reflect more advanced and severe diseases in this population. We encourage the appropriate and judicious use of antimicrobials, particularly broad-spectrum antibiotics, to avoid short- and long-term consequences. We anticipate if no appropriate actions have been taken throughout the pandemic through various elements of ASPs or tailored COVID-19 management guidelines, such practice might become an established culture with all its detrimental consequences.
Authors' Contributions
Conceptualization, N.O.E.; methodology, N.O.E., S.H.A., and H.A.; resources, S.H.A. and H.A.; writing—original draft preparation, S.H.A., H.A., A.J., and H.A.H.; writing—review and editing, N.O.E., H.A.H., H.M.Y., and A.A.A.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Biomedical Research Center, Qatar University.
References
- 1. Aminov, R.I. 2010. A brief history of the antibiotic era: lessons learned and challenges for the future. Front. Microbiol. 1:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frost, I., T.P. Van Boeckel, Pires J., Craig J., and Laxminarayan R.. 2019. Global geographic trends in antimicrobial resistance: the role of international travel. J. Travel Med. 26:taz036. [DOI] [PubMed] [Google Scholar]
- 3. Bloom, D.E., and Cadarette D.. 2019. Infectious disease threats in the twenty-first century: strengthening the global response. Front. Immunol. 10:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacDougall, C., and Polk R.E.. 2005. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 18:638–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi, Y., Wang G., X.P. Cai, et al. 2020. An overview of COVID-19. J. Zhejiang Univ. Sci. B. 21:343–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clancy, C.J., and Nguyen M.H.. 2020. Coronavirus disease 2019, superinfections, and antimicrobial development: what can we expect?. Clin. Infect. Dis. 71:2736–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rawson, T.M., L.S. Moore, Zhu N., et al. 2020. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 71:2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Getahun, H., Smith I., Trivedi K., Paulin S., and Balkhy H.H.. 2020. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 98:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gautret, P., J.C. Lagier, Parola P., et al. 2020. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Lee, S.G., G.U. Park, Y.R. Moon, and Sung K.. 2020. Clinical characteristics and risk factors for fatality and severity in patients with coronavirus disease in Korea: a nationwide population-based retrospective study using the Korean Health Insurance Review and Assessment Service (HIRA) Database. Int. J. Environ. Res. Public Health 17:8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nori, P., Cowman K., Chen V., et al. 2021. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect. Control Hosp. Epidemiol. 42:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen, J., Bai H., Liu J., et al. 2020. Distinct clinical characteristics and risk factors for mortality in female inpatients with coronavirus disease 2019 (COVID-19): a sex-stratified, large-scale cohort study in Wuhan, China. Clin. Infect. Dis. 71:3188–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu, C., Wen Y., Wan W., Lei J., and Jiang X.. 2021. Clinical characteristics and antibiotics treatment in suspected bacterial infection patients with COVID-19. Int. Immunopharmacol. 90:107157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan, W.J., Z.Y. Ni, Hu Y., et al. 2020. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai, C.C., Y.H. Liu, C.Y. Wang, et al. 2020. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J. Microbiol. Immunol. Infect. 53:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karami, Z., B.T. Knoop, A.S. Dofferhoff, et al. 2021. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in The Netherlands. Infect. Dis. 53:102–110. [DOI] [PubMed] [Google Scholar]
- 17. Feng, Y., Ling Y., Bai T., et al. 2020. COVID-19 with different severities: a multicenter study of clinical features. Am. J. Respir. Crit. Care Med. 201:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lian, J., Jin X., Hao S., et al. 2020. Epidemiological, clinical, and virological characteristics of 465 hospitalized cases of coronavirus disease 2019 (COVID-19) from Zhejiang province in China. Influenza Other Respir. Viruses 14:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma, Y., Zeng H., Zhan Z., et al. 2020. Corticosteroid use in the treatment of COVID-19: a multicenter retrospective study in Hunan, China. Front. Pharmacol. 11:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo, T., Shen Q., Zhou Z., et al. 2020. Combined Interventions for Severe Novel Coronavirus Disease (COVID-19): experience from 350 Patients. Infect. Drug Resist. 13:3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi, S., Qin M., Shen B., et al. 2020. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lehmann, C.J., M.T. Pho, Pitrak D., J.P. Ridgway, and Pettit N.N.. 2021. Community acquired co-infection in COVID-19: a retrospective observational experience. Clin. Infect. Dis. 72:1450–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rothe, K., Feihl S., Schneider J., et al. 2021. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur. J. Clin. Microbiol. Infect. Dis. 40:859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan, L., Mu M., Yang P., et al. 2020. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 115:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang, L., Liu J., Zhang R., et al. 2020. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: a descriptive study. J. Clin. Virol. 129:10; 4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang, Y., Cui Y., Shen M., et al. 2020. Association of diabetes mellitus with disease severity and prognosis in COVID-19: a retrospective cohort study. Diabetes Res. Clin. Pract. 165:108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Melo, A.C., L.C. Thuler, J.L. da Silva, et al. 2020. Cancer inpatients with COVID-19: a report from the Brazilian National Cancer Institute. PLoS One 15:e0241261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang, C., Zhou L., Chen J., et al. 2020. The differences of clinical characteristics and outcomes between imported and local patients of COVID-19 in Hunan: a two-center retrospective study. Respir. Res. 21:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cravedi, P., S.S. Mothi, Azzi Y., et al. 2020. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am. J. Transplant. 20:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bai, Y., Yao L., Wei T., et al. 2020. Presumed asymptomatic carrier transmission of COVID-19. JAMA 323:1406–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang, Q., Xie L., Zhang W., et al. 2020. Analysis of the clinical characteristics, drug treatments and prognoses of 136 patients with coronavirus disease 2019. J. Clin. Pharm. Ther. 45:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo, T., Fan Y., Chen M., et al. 2020. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan, S., Y.I. Xiang, Fang W., et al. 2020. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 92:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li, H.Y., J.W. Wang, L.W. Xu, X.L. Zhao, J.X. Feng, and Xu Y.Z.. 2020. Clinical analysis of 132 cases COVID-19 from Wuhan. Medicine (Baltimore) 99:e22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang, D., Yin Y., Hu C., et al. 2020. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit. Care 24:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ji, M., Yuan L., Shen W., et al. 2020. Characteristics of disease progress in patients with coronavirus disease 2019 in Wuhan, China. Epidemiol. Infect. 148:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen, N., Zhou M., Dong X., et al. 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hong, K.S., K.H. Lee, J.H. Chung, et al. 2020. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med. J. 61:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu, H., Gao J., Y.Wang, et al. 2020. Epidemiological and clinical characteristics of 2019 novel coronavirus disease (COVID-19) in Jilin, China: a descriptive study. Medicine (Baltimore) 99:e2; 3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tian, R., Wu W., Wang C., et al. 2020. Clinical characteristics and survival analysis in critical and non-critical patients with COVID-19 in Wuhan, China: a single-center retrospective case control study. Sci. Rep. 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Du, Y., Tu L., Zhu P., et al. 2020. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 201:1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prata-Barbosa, A., Lima-Setta F., G.R. Santos, et al. 2020. Pediatric patients with COVID-19 admitted to intensive care units in Brazil: a prospective multicenter study. J. Pediatr. 96:582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jin, X., J.S. Lian, J.H. Hu, et al. 2020. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 69:1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. d'Ettorre, G., Ceccarelli G., Marazzato M., et al. 2020. Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front. Med. 7:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu, B.M., Q.Q. Yang, L.Y. Zhao, et al. 2020. Epidemiological characteristics of COVID-19 patients in convalescence period. Epidemiol. Infect. 148:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Easom, N., Moss P., Barlow G., et al. 2020. Sixty-eight consecutive patients assessed for COVID-19 infection: experience from a UK regional infectious diseases unit. Influenza Other Respir. Viruses 14:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Annweiler, C., Hanotte B., C.G. de l'Eprevier, J.M. Sabatier, Lafaie L., and Célarier T.. 2020. Vitamin D and survival in COVID-19 patients: a quasi-experimental study. J. Steroid Biochem. Mol. Biol. 204:105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hu, C., Xiao L., Zhu H., et al. 2020. Effect of hypertension on outcomes of patients with COVID-19. J. Southern Med. Univ. 40:1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khamis, F., Al-Zakwani I., Al Naamani H., et al. 2020. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J. Infect. Public Health. 13:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mady, A., Aletreby W., Abdulrahman B., et al. 2020. Tocilizumab in the treatment of rapidly evolving COVID-19 pneumonia and multifaceted critical illness: a retrospective case series. Ann. Med. Surg. 60:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pereira, A., Cruz-Melguizo S., Adrien M., Fuentes L., Marin E., and Perez-Medina T.. 2020. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet. Gynecol. Scand. 99:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bai, P., He W., Zhang X., Liu S., and Jin J.. 2020. Analysis of clinical features of 58 patients with severe or critical 2019 novel coronavirus pneumonia. Chin. J. Emerg. Med. [Epub ahead of print]. [Google Scholar]
- 53. Becchetti, C., M.F. Zambelli, Pasulo L., et al. 2020. COVID-19 in an international European liver transplant recipient cohort. Gut 69:1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lima-Setta, F., M.C. de Magalhães-Barbosa, Rodrigues-Santos G., et al. 2020. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J. Pediatr. (Rio J). [Epub ahead of print]; DOI: 10.1016/j.jped.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun, L., Shen L., Fan J., et al. 2020. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J. Med. Virol. 92:2055–; 2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lu, J., Yin Q., Li Q., et al. 2020. Clinical characteristics and factors affecting the duration of positive nucleic acid test for patients of COVID-19 in XinYu, China. J. Clin. Lab. Anal. 34:e2; 3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yu, Y., and Chen P.. 2020. Coronavirus disease 2019 (COVID-19) in neonates and children from China: a review. Front. Pediatr. 8:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang, C., Wang Y., Li X., et al. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang, C., Gu J., Chen Q., et al. 2020. Clinical and epidemiological characteristics of pediatric SARS-CoV-2 infections in China: a multicenter case series. PLoS Med. 17:e100; 3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Piva, S., Filippini M., Turla F., et al. 2020. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia, Italy. J. Crit. Care 58:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Verroken, A., Scohy A., Gérard L., Wittebole X., Collienne C., and Laterre P.F.. 2020. Co-infections in COVID-19 critically ill and antibiotic management: a prospective cohort analysis. Crit. Care 24:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang, D., X.L. Ju, Xie F., et al. 2020. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China [in Chinese]. Zhonghua Er Ke Za Zhi 58:269–274. [DOI] [PubMed] [Google Scholar]
- 63. Soltani, J., Sedighi I., Shalchi Z., Sami G., Moradveisi B., and Nahidi S.. 2020. Pediatric coronavirus disease 2019 (COVID-19): an insight from west of Iran. North. Clin. Istanb. 7:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang, L., Zhu F., Xie L., et al. 2020. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 31:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stroppa, E.M., Toscani I., Citterio C., et al. 2020. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy). Future Oncol. 16:1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zheng, F., Liao C., Q.H. Fan, et al. 2020. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr. Med. Sci. 24:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lei, Z., Cao H., Jie Y., et al. 2020. A cross-sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China. Travel Med. Infect. Dis. 35:10; 1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dang, J.Z., G.Y. Zhu, Y.J. Yang, and Zheng F.. 2020. Clinical characteristics of coronavirus disease 2019 in patients aged 80 years and older. J. Integr. Med. 18:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hu, W., Chen X., He B., et al. 2020. Clinical characteristics of 16 patients with COVID-19 infection outside of Wuhan, China: a retrospective, single-center study. Ann. Transl. Med. 8:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu, D., Li L., Wu X., et al. 2020. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. Am. J. Roentgenol. 215:127–132. [DOI] [PubMed] [Google Scholar]
- 71. Dong, X., Y.Y. Cao, X.X. Lu, et al. 2020. Eleven faces of coronavirus disease 2019. Allergy 75:1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jiehao, C., Jin X., Daojiong L., et al. 2020. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis. 71:1547–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Trujillo, H., Caravaca-Fontán F., Á. Sevillano, et al. 2020. Tocilizumab use in kidney transplant patients with COVID-19. Clin. Transplant. 34:e14072. [DOI] [PubMed] [Google Scholar]
- 74. Chen, Q., Quan B., Li X., et al. 2020. A report of clinical diagnosis and treatment of nine cases of coronavirus disease 2019. J. Med. Virol. 92:683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen, H., Guo J., Wang C., et al. 2020. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 395:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ng, K.F., Bandi S., P.W. Bird, and Tang J.W.. 2020. COVID-19 in neonates and infants: progression and recovery. Pediatr. Infect. Dis. J. 39:e140–e142. [DOI] [PubMed] [Google Scholar]
- 77. Liu, W., Q.I. Zhang, Chen J., et al. 2020. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N. Engl. J. Med. 382:1370–; 1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Caraffa, R., Bagozzi L., Fiocco A., et al. 2020. Coronavirus disease 2019 (COVID-19) in the heart transplant population: a single-centre experience. Eur. J. CardioThorac. Surg. 58:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cucchiari, D., J.M. Pericàs, Riera J., Gumucio R., E.C. Md, and D. Nicolás; Hospital Clínic 4H Team. 2020. Pneumococcal superinfection in COVID-19 patients: a series of 5 cases. Med. Clin. 155:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fu, Y., Yang Q., Xu M., et al. 2020. Secondary bacterial infections in critical ill patients of COVID-19. Open Forum Infect. Dis. 7:ofaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cheng, C., Li C., Zhao T., et al. 2020. COVID-19 with rheumatic diseases: a report of 5 cases. Clin. Rheumatol. 39:2025–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ding, Q., Lu P., Fan Y., Xia Y., and Liu M.. 2020. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 92:1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Blanco, J.L., Ambrosioni J., Garcia F., et al. 2020. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 7:e314–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. West, T.A., Malik S., Nalpantidis A., et al. 2020. Tocilizumab for severe COVID-19 pneumonia: case series of 5 Australian patients. Int. J. Rheum. Dis. 23:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fried, J.A., Ramasubbu K., Bhatt R., et al. 2020. The variety of cardiovascular presentations of COVID-19. Circulation. 141:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Morlacchi, L.C., Rossetti V., Gigli L., et al. 2020. COVID-19 in lung transplant recipients: a case series from Milan, Italy. Transplant Infect. Dis. 22:e1: 3356. [DOI] [PubMed] [Google Scholar]
- 87. Sattar, Y., Connerney M., Rauf H., et al. 2020. Three cases of COVID-19 disease with colonic manifestations. Am. J. Gastroenterol. 115:948–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang, T., Cui X., Zhao X., et al. 2020. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J. Med. Virol. 92:909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dhont, S., Callens R., Stevens D., et al. 2020. Myotonic dystrophy type 1 as a major risk factor for severe COVID-19?. Acta Neurol. Belg. [Epub ahead of print]; DOI: 10.1007/s13760-020-01514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Edrada, E.M., E.B. Lopez, J.B. Villarama, et al. 2020. First COVID-19 infections in the Philippines: a case report. Trop. Med. Health. 48:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ke, C., Wang Y., Zeng X., Yang C., and Hu Z.. 2020. 2019 Novel coronavirus disease (COVID-19) in hemodialysis patients: a report of two cases. Clin. Biochem. 81:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wu, Q., Chen T., and Zhang H.. 2020. Recovery from the coronavirus disease-2019 (COVID-19) in two patients with coexisted (HIV) infection. J. Med. Virol 92:2325–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Di Lorenzo, G., Buonerba L., Ingenito C., et al. 2020. Clinical characteristics of metastatic prostate cancer patients infected with COVID-19 in South Italy. Oncology 98:743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Holzhauser, L., Lourenco L., Sarswat N., Kim G., Chung B., and Nguyen A.B.. 2020. Early experience of COVID-19 in 2 heart transplant recipients: case reports and review of treatment options. Am. J. Transplant. 20:2916–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Huang, J., Lin H., Wu Y., et al. 2020. COVID-19 in posttransplant patients—report of 2 cases. Am. J. Transplant. 20:1879–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Basalely, A., Brathwaite K., M.D. Duong, et al. 2021. COVID-19 in children with kidney disease: a report of 2 cases. Kidney Med. 3:120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hazariwala, V., Hadid H., Kirsch D., and Big C.. 2020. Spontaneous pneumomediastinum, pneumopericardium, pneumothorax and subcutaneous emphysema in patients with COVID-19 pneumonia, a case report. J. Cardiothorac. Surg. 15:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sagheb, S., Lamsehchi A., Jafary M., Atef-Yekta R., and Sadeghi K.. 2020. Two seriously ill neonates born to mothers with COVID-19 pneumonia-a case report. Ital. J. Pediatr. 46:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gupta, A.K., B.M. Parker, Priyadarshi V., and Parker J.. 2020. Cardiac adverse events with remdesivir in COVID-19 infection. Cureus 12:e11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Muheim, M., F.J. Weber, Muggensturm P., and Seiler E.. 2020. An unusual course of disease in two patients with COVID-19: pulmonary cavitation. BMJ Case Rep. 13:e237967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Faller, E., Lapthorne S., Barry R., et al. 2020. The presentation and diagnosis of the first known community transmitted case of SARS-CoV-2 in the Republic of Ireland. Irish Med. J. 113:1–5. [PubMed] [Google Scholar]
- 102. Yokoo, K., Sugaya F., Matsuzaka S., et al. 2020. The first case of COVID-19 occurring as community-acquired pneumonia in Hokkaido, Japan and our preventive measures against nosocomial infection. Respir. Med. Case Rep. 30:101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cheng, S.C., Y.C. Chang, Y.L. Chiang, et al. 2020. First case of Coronavirus Disease 2019 (COVID-19) pneumonia in Taiwan. J. Formos. Med. Assoc. 119:747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. LeVine, S., G.P. Dhakal, Penjor T., Chuki P., Namgyal K., and Watts M.. 2020. Case report: the first case of COVID-19 in Bhutan. Am. J. Trop. Med. Hyg. 102:1205–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Millán-Oñate, J., Millan W., L.A. Mendoza, et al. 2020. Successful recovery of COVID-19 pneumonia in a patient from Colombia after receiving chloroquine and clarithromycin. Ann. Clin. Microbiol. Antimicrob. 19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Taniguchi, H., Ogawa F., Honzawa H., et al. 2020. Veno-venous extracorporeal membrane oxygenation for severe pneumonia: COVID-19 case in Japan. Acute Med. Surg. 7:e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Walpole, S.C., McHugh R., Samuel J., and Schmid M.L.. 2020. COVID-19 presenting as severe, persistent abdominal pain and causing late respiratory compromise in a 33-year-old man. BMJ Case Rep. 13:e236030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Han, X., Fan Y., Y.L. Wan, and Shi H.. 2020. A diabetic patient with 2019-nCoV (COVID-19) infection who recovered and was discharged from hospital. J. Thorac. Imaging. 35:W94–W95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Righi, G., and Del Popolo G.. 2020. COVID-19 tsunami: the first case of a spinal cord injury patient in Italy. Spinal Cord Ser. Cases 6:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Huang, L., Wang Y., Wang L., Lv Y., and Liu Q.. 2020. Coronavirus disease 2019 (COVID-19) pneumonia in a hemodialysis patient: a case report. Medicine (Baltimore) 99:e20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Caputo, V., Schroeder J., and Rongioletti F.. 2020. A generalized purpuric eruption with histopathologic features of leucocytoclastic vasculitis in a patient severely ill with COVID-19. J. Eur. Acad. Dermatol. Venereol. 34:e579–e581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Poggiali, E., Vercelli A., Demichele E., Ioannilli E., and Magnacavallo A.. 2020. Diaphragmatic rupture and gastric perforation in a patient with COVID-19 pneumonia. Eur. J. Case Rep. Intern. Med. 7:001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Martens, T., Y.V. Weygaerde, Vermassen J., and Malfait T.. 2020. Acute type A aortic dissection complicated by COVID-19 infection. Ann. Thorac. Surg. 110:e421–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zeng, J.H., Y.X. Liu, Yuan J., et al. 2020. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection 48:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hu, H., Ma F., Wei X., and Fang Y.. 2021. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur. Heart J. 42:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Di Giambenedetto, S., Del Giacomo P., Ciccullo A., et al. 2020. SARS-CoV-2 infection in a highly experienced person living with HIV. AIDS 34:1257–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Qiu, H., Wander P., Bernstein D., and Satapathy S.K.. 2020. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Liver Int. 40:1590–1593. [DOI] [PubMed] [Google Scholar]
- 118. Wu, Y., Lin H., Xie Q., et al. 2020. COVID-19 in a patient with pre-existing acute lymphoblastic leukaemia. Br. J. Haematol. 190:e13–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Han, P., Li F., Cao P., et al. 2020. A case report with COVID-19 during perioperative period of lobectomy. Medicine (Baltimore) 99:e20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kamali Aghdam, M., Jafari N., and Eftekhari K.. 2020. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect. Dis. 52:427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lahfaoui, M., Azizi M., Elbakkaoui M., El Amrani R., Kamaoui I., and Benhaddou H.. 2020. Acute respiratory distress syndrome secondary to SARS-CoV-2 infection in an infant [in French]. Rev. Mal. Respir. 37:502–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chen, F., Liu Z., F.R. Zhang, et al. 2020. First case of severe childhood novel coronavirus pneumonia in China [in Chinese]. Zhonghua Er Ke Za Zhi 58:179–182. [DOI] [PubMed] [Google Scholar]
- 123. Baluku, J.B., Mwebaza S., Ingabire G., Nsereko C., and Muwanga M.. 2020. HIV and SARS-CoV-2 coinfection: a case report from Uganda. J. Med. Virol. 92:2351–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gallacher, S.D., and Seaton A.. 2020. Meningococcal meningitis and COVID-19 co-infection. BMJ Case Rep. 13:e237366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Alharthy, A., Balhamar A., Faqihi F., et al. 2020. Rare case of COVID-19 presenting as acute abdomen and sepsis. New Microbes New Infect. 38:100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kulkarni, R., Rajput U., Dawre R., et al. 2021. Early-onset symptomatic neonatal COVID-19 infection with high probability of vertical transmission. Infection. 49:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vashistha, P., A.K. Gupta, Arya M., V.K. Singh, Dubey A., and Koner B.C.. 2020. Biclonal gammopathay in a case of severe COVID-19. Clin. Chim. Acta 511:342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mohan, S., Workman A., Barshak M., D.B. Welling, and Abdul-Aziz D.. 2021. Considerations in Management of Acute Otitis Media in the COVID-19 Era. Ann. Otol. Rhinol. Laryngol. 130:520–527. [DOI] [PubMed] [Google Scholar]
- 129. Butt, I., Sawlani V., and Geberhiwot T.. 2020. Prolonged confusional state as first manifestation of COVID-19. Ann. Clin. Transl. Neurol. 7:1450–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chavis, A., Bakken H., Ellenby M., and Hasan R.. 2020. COVID-19 and telehealth: prevention of exposure in a medically complex patient with a mild presentation. J. Adolesc. Health 67:456–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Elkattawy, S., Alyacoub R., Mowafy A., Younes I., and Remolina C.. 2020. Unfortunate outcomes in patients with SARS-CoV-2 superimposed on pneumococcal pneumonia. Cureus 12:e10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mehta, S., and Pandey A.. 2020. Rhino-orbital mucormycosis associated with COVID-19. Cureus 12:e10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Elnadoury, O., Beattie J., and Lubinsky A.S.. 2020. Uninterrupted continuous and intermittent nebulizer therapy in a COVID-19 patient using sequential vibratory mesh nebulizers: a case report. J. Aerosol Med. Pulm. Drug Deliv. 33:357–360. [DOI] [PubMed] [Google Scholar]
- 134. Hornuss, D., Laubner K., Monasterio C., Thimme R., and Wagner D.. 2020. COVID-19 associated pneumonia despite repeatedly negative PCR-analysis from oropharyngeal swabs [in German]. Dtsch. Med. Wochenschr. 145:844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Singh, S., Foster A., Khan Z., Siddiqui A., Atere M., and Nfonoyim J.M.. 2020. COVID-19-induced diabetic ketoacidosis and acute respiratory distress syndrome in an obese 24-year-old type I diabetic. Am. J. Case Rep. 21:e925586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Slaats, M.A., Versteylen M., K.B. Gast, et al. 2020. Case report of a neonate with high viral SARSCoV-2 loads and long-term virus shedding. J. Infect. Public Health 13:1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lau, J.Y., H.W. Khoo, T.C. Hui, G.J. Kaw, and Tan C.H.. 2020. Atypical chest computed tomography finding of predominant interstitial thickening in a patient with coronavirus disease 2019 (COVID-19) pneumonia. Am. J. Case Rep. 21:e926781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Soumana, A., Samaila A., L.M. Moustapha, et al. 2020. A fatal case of COVID-19 in an infant with severe acute malnutrition admitted to a paediatric ward in Niger. Case Rep. Pediatr. 2020:8847415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Placik, D.A., W.L. Taylor, and Wnuk N.M.. 2020. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol. Case Rep. 15:2378–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Alhassan, S.M., Iqbal P., Fikrey L., et al. 2020. Post COVID 19 acute acalculous cholecystitis raising the possibility of underlying dysregulated immune response, a case report. Ann. Med. Surg. 60:434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Rossi, C.M., F.N. Beretta, Traverso G., Mancarella S., and Zenoni D.. 2020. A case report of toxic epidermal necrolysis (TEN) in a patient with COVID-19 treated with hydroxychloroquine: are these two partners in crime?. Clin. Mol. Allergy. 18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jones, B.A., and Slater B.J.. 2020. Non-operative management of acute appendicitis in a pediatric patient with concomitant COVID-19 infection. J. Pediatr. Surg. Case Rep. 59:101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Liang, B., Chen J., Li T., et al. 2020. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine (Baltimore) 99:e21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Silveira, R.Q., V.T. Carvalho, H.N. Cavalcanti, F.C. Rodrigues, C.B. Braune, and Ramírez E.P.. 2020. Multiple cranial nerve palsies in malignant external otitis: a rare presentation of a rare condition. IDCases 22:e00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Liu, Y., Wang M., Luo G., et al. 2020. Experience of N-acetylcysteine airway management in the successful treatment of one case of critical condition with COVID-19: a case report. Medicine (Baltimore) 99:e22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Haraszti, S., Sendil S., and Jensen N.. 2020. Delayed presentation of acute generalized exanthematous pustulosis following treatment with cefepime in a patient with COVID-19 without the use of hydroxychloroquine. Am. J. Case Rep. 21:e926901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rizvi, S., Danic M., Silver M., and LaBond V.. 2021. Cytosorb filter: an adjunct for survival in the COVID-19 patient in cytokine storm? a case report. Heart Lung. 50:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gandra, S., D.M. Barter, and Laxminarayan R.. 2014. Economic burden of antibiotic resistance: how much do we really know?. Clin. Microbiol. Infect. 20:973–980. [DOI] [PubMed] [Google Scholar]
- 149. Woolhouse, M., Waugh C., M.R. Perry, and Nair H.. 2016. Global disease burden due to antibiotic resistance–state of the evidence. J. Glob. Health 6:010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Li, J., Song X., Yang T., et al. 2016. A systematic review of antibiotic prescription associated with upper respiratory tract infections in China. Medicine (Baltimore) 95:e3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Shankar, P.R. 2009. Medicines use in primary care in developing and transitional countries: fact book summarizing results from studies reported between 1990 and 2006. Bull. World Health Organ. . Available at: https://apps.who.int/iris/handle/10665/70032
- 152. Xu, X.W., X.X. Wu, X.G. Jiang, et al. 2020. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lai, C.C., C.Y. Wang, and Hsueh P.R.. 2020. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents?. J. Microbiol. Immunol. Infect. 53:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Richardson, S., J.S. Hirsch, Narasimhan M., et al. 2020. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zangrillo, A., Beretta L., A.M. Scandroglio, et al. 2020. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit. Care Resusc. 22:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lansbury, L., Lim B., Baskaran V., and Lim W.S.. 2020. Co-infections in people with COVID-19: a systematic review and meta-analysis. J. Infect. 81:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chang, C.Y., and Chan K.G.. 2020. Underestimation of co-infections in COVID-19 due to non-discriminatory use of antibiotics. J Infect. 81:e29–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Xia, W., Shao J., Guo Y., Peng X., Li Z., and Hu D.. 2020. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr. Pulmonol. 55:1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wu, Q., Xing Y., Shi L., et al. 2020. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics 146:e20200961. [DOI] [PubMed] [Google Scholar]
- 160. Simoens, S., Laekeman G., and Decramer M.. 2013. Preventing COPD exacerbations with macrolides: a review and budget impact analysis. Respir. Med. 107:637–648. [DOI] [PubMed] [Google Scholar]
- 161. Zimmermann, P., V.C. Ziesenitz, Curtis N., and Ritz N.. 2018. The immunomodulatory effects of macrolides—a systematic review of the underlying mechanisms. Front. Immunol. 9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Retallack, H., Di Lullo E., Arias C., et al. 2016. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. U.S.A. 113:14408–1; 4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bosseboeuf, E., Aubry M., Nhan T., et al. 2018. Azithromycin Inhibits the Replication of Zika Virus. J. Antivir. Antiretrovir. 10:6–11. [Google Scholar]
- 164. Madrid, P.B., R.G. Panchal, T.K. Warren, et al. 2015. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect. Dis. 1:317–326. [DOI] [PubMed] [Google Scholar]
- 165. Kawamura, K., Ichikado K., Takaki M., Eguchi Y., Anan K., and Suga M.. 2018. Adjunctive therapy with azithromycin for moderate and severe acute respiratory distress syndrome: a retrospective, propensity score-matching analysis of prospectively collected data at a single center. Int. J. Antimicrob. Agents 51:918–924. [DOI] [PubMed] [Google Scholar]
- 166. Pani, A., Lauriola M., Romandini A., and Scaglione F.. 2020. Macrolides and viral infections: focus on azithromycin in COVID-19 pathology. Int. J. Antimicrob. Agents 56:106053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Choi, Y., H.S. Lim, Chung D., J.G. Choi, and Yoon D.. 2018. Risk evaluation of azithromycin-induced QT prolongation in real-world practice. Biomed Res. Int. 2018:1574806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sears, S.P., T.W. Getz, C.O. Austin, W.C. Palmer, E.A. Boyd, and Stancampiano F.F.. 2016. Incidence of sustained ventricular tachycardia in patients with prolonged QTc after the administration of azithromycin: a retrospective study. Drugs Real World Outcomes 3:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kezerashvili, A., Khattak H., Barsky A., Nazari R., and Fisher J.D.. 2007. Azithromycin as a cause of QT-interval prolongation and torsade de pointes in the absence of other known precipitating factors. J. Interv. Card. Electrophysiol. 18:243–246. [DOI] [PubMed] [Google Scholar]
- 170. Chugh, S.S., Reinier K., Singh T., et al. 2009. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation 119:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Beović, B., Doušak M., Ferreira-Coimbra J., et al. 2020. Antibiotic use in patients with COVID-19: a ‘snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J. Antimicrob. Chemother. 75:3386–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Moher, D., Liberati A., Tetzlaff J., and Altman; Prisma Group D.G.. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]