Abstract
Background: Cachexia is a prevalent condition associated with underlying chronic disease. Wasting of skeletal muscle and adipose tissue loss in cachectic patients is associated with higher rates of disability, reduced quality of life (QoL), and worse prognosis. There is a large unmet need to develop strategies to treat cachexia as there are currently no standardized guidelines in the management of cachexia. Activation of endogenous cannabinoid receptors, through exogenous cannabinoids, has demonstrated potential in increasing appetite, reducing catabolism, and has shown anti-inflammatory properties. Since no single pharmacological agent is currently recommended for use in cachexia, the potential of cannabinoids as an appetite stimulant warrants further research and assessment of current evidence.
Objective: This review aims to evaluate the evidence for the efficacy of cannabis-based medicinal products, against placebo and other active treatments, in anorexia–cachexia syndrome in improving appetite, weight, and QoL.
Methods: A literature search of the Medline, EMBASE, CENTRAL, and the Web of Science Core Collection, for articles published up to February 2020, was conducted. All randomized controlled trials comparing the use of cannabis-based medicine versus placebo/active treatments for patients with cachexia were screened. The quality of evidence in included studies was assessed using the GRADE framework and any risk of bias was judged using the Cochrane risk of bias tool.
Results: A total of five studies, encompassing 934 participants, were found to be eligible. The pooled group effect size for change in appetite was −1.79 (95% confidence interval: −3.77 to 0.19) favoring the control group (p=0.08). Additionally, no significant difference for weight change or change in QoL for cannabinoids versus placebo/other treatment was observed. The quality of evidence for all five studies was assessed to be low.
Conclusion: There is a lack of high-quality evidence to recommend the use of cannabinoids in the treatment of cachexia. Given the limited available pharmacological options for cachexia and the potential for cannabinoids to increase appetite and alter the immune system, further research is needed before clinical recommendations on the pharmacological management of cachexia can be made.
Keywords: cachexia, cannabinoids, cannabis, weight loss, appetite
Background
Cachexia is defined as “a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass.”1 It affects ∼9 million patients globally, with incidence rising in line with increasing multimorbidity of chronic disease.2 Cachexia is particularly associated with cancer and AIDS affecting 30% and 35% of patients, respectively.3 It has a prevalence of up to 80% in advanced cancer patients and an approximate mortality rate of 30% and 80% in chronic heart failure and cancer patients, respectively.4,5 Cachectic patients show higher rates of disability, prolonged hospital admission,6 decreased psychological wellbeing,7 reduced quality of life (QoL),8 and an overall poorer prognosis.9,10 There is a wide variety of cachexia–death associations, including increased risk of thrombosis,11 immune deficiency,12,13 reduced cardiac output,14 and increased risk of adverse cardiovascular events.11,12,15–17 There exist no standardized guidelines for the management of cachexia and there is currently no agreed gold-standard pharmacological agent for the treatment of cachexia.
Multiple pathways have been identified in the pathophysiology of cachexia. An upregulation of pathways that catabolize muscle and fat and a downregulation of pathways that stimulate muscle growth lead to an overall catabolic state, clinically manifesting as weight loss, weakness, and wasting.3 Cytokines, such as tumor necrosis factor-α (TNF-α) and Interferon-γ, are upregulated in cachexia and directly inhibit synthesis of heavy chains of myosin.18 Myoblast determination protein 1, a transcription factor essential for repair and differentiation of skeletal muscles, is also inhibited by TNF-α through activation of nuclear factor kappa-light-chain-enhancer of activated B cells leading to dysfunction of skeletal muscle production.19,20 Cytokines activate proteolysis through a ubiquitin-mediated system—targeting specific proteins within skeletal muscle, breaking them down for use in hepatic synthesis of acute phase proteins.3,21 Resistance of appropriate hypothalamic responses to orexigenic and anorexigenic signaling has been observed.22 In cachexia proinflammatory cytokines mimic the negative feedback leptin exerts on orexigenic pathways.23–25 This excess, unopposed negative feedback combined with resistance to orexigenic pathways leads to the loss of appetite and induction of an overall catabolic state resulting in the sustained, uncontrolled weight loss.23
The cannabinoid receptor type 1 (CB1R) is thought to play a significant role in modulating appetite and satiety through presynaptic activity at both orexigenic and anorexigenic neurons. This serves to increase caloric intake and reduce catabolism.26,27 Activation of this system has been observed to increase abdominal adiposity.26,27 Hence, there is increasing interest in targeting the endocannabinoid system for the treatment of conditions, such as anorexia–cachexia syndrome. This has previously been suggested to potentially result in a better prognosis, owing to increased appetite and weight gain.9,10 As such, there is on-going research into the potential benefits to treat anorexia–cachexia syndrome. The (−)-trans-Δ9-tetrahydrocannabinol (THC), a partial agonist of CB1R, and its isomers are currently the most widely researched cannabis-based medicine for cachexia treatment. THC has been shown to stimulate appetite, reduce nausea, and improve the functional status in cachexia patients.28–31
The aim of this systematic review and meta-analysis was to compare the effects of cannabis-based medicinal products against both placebo and active treatment in anorexia–cachexia syndrome for appetite stimulation, change in body mass, and QoL. The review assessed the quality of evidence for whether cannabis-based medicine is effective in the treatment of anorexia–cachexia syndrome and hopefully provides a rationale for any recommendation for use in clinical practice.
Methods
Selection criteria for studies
The PICOS (patient, intervention, control, outcome, study type) acronym was used to define the research question by specific criteria. The population in this case were patients with cachexia, from any underlying illness, as defined by official diagnostic criteria, having had a sustained weight loss >5% (or body mass index <20 kg/m2) in less than 12 months with three of the five of the following characteristics: decreased muscle strength, fatigue, anorexia, low fat-free mass index, and abnormal biochemistry.1 The intervention was the use of cannabis-based medicines or their synthetic analogs, which could be compared with either a placebo or other interventions used in the active treatment of cachexia. The chosen outcomes were objective measurements, such as weight gain and additionally subjective measurements such as patient-reported QoL and their change in appetite. Only randomized controlled trials (RCTs) were included, where sample size was >10 with follow-up ≥4 weeks. The following criteria were to be excluded: volunteers below 18 years of age; healthy volunteers; patients with anorexia nervosa; patients with a normal nutritional status in the presence of chronic illness; and animal studies and non-RCTs.
Search strategies for identification of studies
Extensive keywords and MeSH terms were used to search Medline and EMBASE databases using the OVID platform. Equally, similar keywords were used to search the Cochrane Central Register of Controlled Trials (CENTRAL) and the Web of Science Core Collection—to include gray literature in the search. See Supplementary Appendix SA1 for a full description of search strategy developed in consultation with an experienced medical librarian. In addition, citations and bibliographic references of included studies and relevant reviews were manually searched to identify any further studies or search keywords.
Selection of studies
The search was performed by two authors independently (S.H. and S.E.); studies selected for full-text review were assessed independently and any disagreement would be resolved by a third author (M.H.S.)—however, this was not necessary in this instance.
Data extraction
The following data were extracted for analysis from the included studies for both the cannabis-based medicines and placebo/comparator arms:
□ Sample size
□ Patient demographics (age, sex, ethnicity (where possible), and weight loss history)
□ Appetite change, through a Visual Analog Scale (VAS) score, at baseline and endpoint
□ Weight change (kg)
□ Change in QoL assessment (baseline to endpoint)
□ Nausea, mood, and vomiting VAS score change (baseline to endpoint)
□ Adverse outcomes (type and frequency)
Across studies, follow-up times and study length varied.
Quality of evidence and bias risk assessment
The GRADE framework was used to assess the quality of evidence within each study, where evidence from each study is given a certainty grading from very low to high. Where evidence has a very low certainty, the true effect is likely very different from the estimated effect seen in the study outcome. These levels of certainty are calculated based on a subjective approach. The risk of bias, level of imprecision in 95% confidence intervals (CIs), inconsistency, indirectness, and publication bias are all aspects that need to be analyzed before a GRADE level can be given.32
The Cochrane risk of bias tool for randomized trials was utilized to assess the risk of bias for each study.33 The items assessed within the risk of bias tool include:
□ Bias arising from randomization (selection bias)
□ Bias from deviations from intended interventions (performance bias)
□ Bias due to missing outcome data (attrition bias)
□ Bias due to measurement of the outcome (detection bias)
□ Bias in selection of the reported result (reporting bias)
Meta-analysis
Measures of treatment effect
For continuous outcomes, a pooled mean difference (MD) and 95% CI was calculated. However, in studies using different scales measuring appetite, pain, and nausea, the standardized MD and 95% CI were calculated. For studies that reported baseline and endpoint data, we calculated the standard deviation (SD) of the mean change from the baseline according to reported CI. A decision was made not to pool studies together if considerable clinical heterogeneity exists. All data were calculated using the Review Manager (Cochrane, v5.3).34
Unit of analysis issues
Unit of analysis issues were dealt with depending on the specific study design. The relevance of each intervention group to this systematic review was determined by types of population and types of intervention. The control arm was divided equally by the number of included intervention groups in studies that contain two or more groups as described by Deeks et al.35 If the study already presented separate subgroup analyses, then the control group was considered as a whole.
Dealing with missing data
Where necessary, the authors of selected studies were contacted to obtain any missing data. When this was not be possible, SDs were calculated using the data available.36
Assessment of heterogeneity
Clinical heterogeneity (differences in participant type or characteristics, timing of outcome measurements, and intervention characteristics) was assessed by reviewing the treatments used across studies and the characteristics of included participants to assess for any substantial differences. Statistical heterogeneity was assessed using the χ2 test and I2 statistic. A p-value of 0.05 was considered statistically significant for the χ2 test. The I2 statistic was used to quantify the proportion of variation between studies that is due to heterogeneity rather than to chance. This interpretation was in keeping with the Cochrane Handbook of systematic reviews.37 An I2 value of 0–40% indicates heterogeneity may be not be important; 30–60% indicates moderate heterogeneity; 50–90% indicates substantial heterogeneity, and 75–100% indicates considerable heterogeneity. Forest plots were created and visibly inspected to identify any outliers.
Assessment of reporting biases
Reporting bias was assessed by comparing prespecified outcomes in pretrial registry entries/study protocols to outcomes reported in final articles, where available. If registry entries or protocols were unavailable, reporting bias was assessed by comparing outcomes specified in the methodology compared with those reported in the results section. Publication bias could not be calculated using a funnel plot due to fewer than 10 studies being included.38
Results
Search results
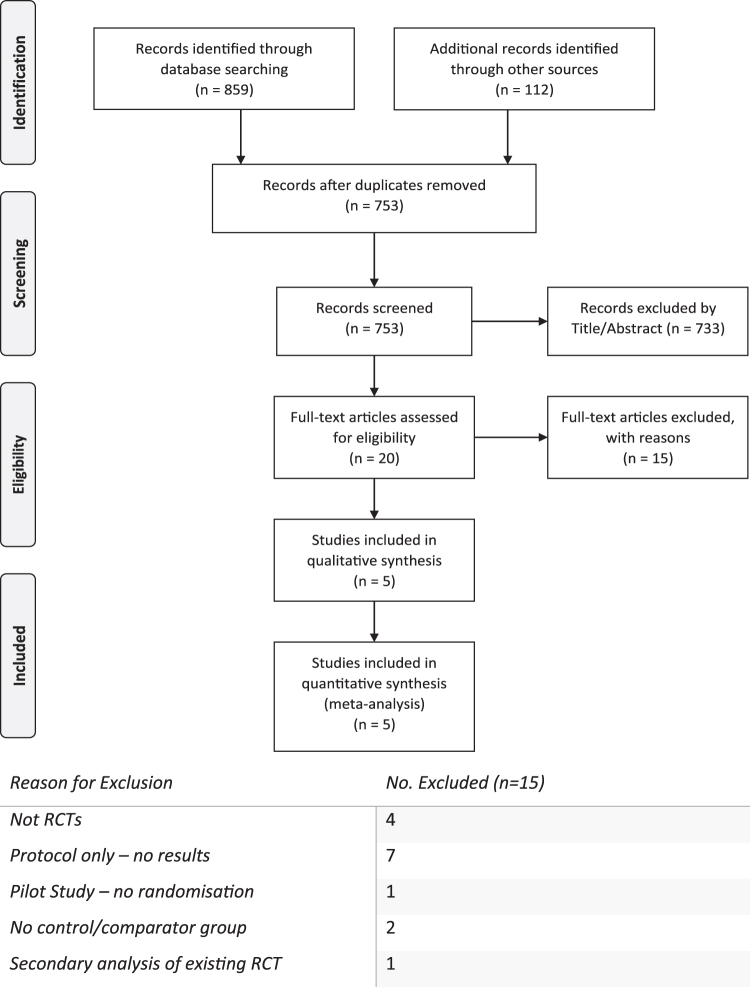
A summary of the study selection process is provided in Figure 1. A total of 859 abstracts were identified through searches of databases, whereas 112 records were identified through hand-searching relevant bibliographies and from gray literature. Once duplicates (n=218) were removed, 753 records were screened by the title and/or abstract, with 733 being excluded. Twenty full-text articles were assessed for eligibility and five articles were included in this review. Figure 1 lists the reasons for exclusion at the full-text level.
FIG. 1.
A flowchart depicting the study selection from identification to inclusion using the PRISMA flowchart, a recommended method of reporting the flow of information during a systematic review,44 alongside the reasons for exclusion at full-text level. RCT, randomized controlled trial.
Characteristics of included studies
The characteristics of each study are presented in Table 1. Relevant information on participant demographics, interventional groups, control groups, any follow-ups, and outcomes were extracted as detailed in the methodology.
Table 1.
Table Displaying the Characteristics of Each Study
| Study | Population | Intervention (n=number of participants) |
|
Outcome |
Duration | |||
|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Comparison | Primary | Secondary | |||
| Beal et al. (1995)39 | AIDS patients with anorexia-associated weight loss | 2.5 mg dronabinol b.d. (n=72) | — | — | Placebo (n=67) | Change in appetite (VAS) Change in weight (kg) |
Mood, nausea, and vomiting (VAS) | 6 weeks |
| Jatoi et al. (2002)40 | Cancer-associated Cachexia | 2.5 mg dronabinol+placebo b.d. (n=152) | Oral megestrol acetate 800 mg/day suspension o.d. +2.5 mg dronabinol b.d. (n=158) | — | oral megestrol acetate 800 mg/day o.d.+placebo (n=159) | Change in appetite (VAS) | Patient and Physician reported weight change, kg QoL assessment (FAACT) |
4 weeks |
| Strasser et al. (2006)43 | Cancer-associated Cachexia | Cannabis Extract (2.5 mg THC +1 mg CBD) b.d. (n=95) | 2.5 mg THC b.d. (n=100) | — | Placebo (n=48) | Change in appetite (VAS) | QoL Assessment (Global Health Status Score and EPRTC QLQ-C30) | 6 weeks |
| Timpone et al. (1997)41 | HIV wasting syndrome | 2.5 mg Dronabinol b.d. (n=12) | 750 mg megestrol acetate o.d. +2.5 mg dronabinol b.d. (n=13) | 250 mg megestrol acetate o.d. +2.5 mg dronabinol b.d. (n=13) | 750 mg megestrol acetate o.d. (n=12) | Pharmacokinetics (through plasma sampling) | Weight Change (kg and BMI) Appetite, mood, and nausea (VAS) |
12 weeks |
| Turcott et al. (2018)42 | Nonsmall-cell lung cancer patients with anorexia | 0.5 mg Nabilone o.d. for 2 wks then 1.0 mg Nabilone o.d. (n=14) |
— | — | Placebo (n=19) | Energy intake (g and kcal) QoL (EORTC-QLQ-C30 and LC13) AC/S score Change in appetite (VAS) |
BMI Weight change Proinflammatory markers (through plasma sampling) |
8 weeks |
o.d.—once daily; b.d.—twice daily; EORTC-QLQ—European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire (C30 and LC13—specific for cancer and lung cancer, respectively).
AC/S, anorexia–cachexia subscale of FAACT; AIDS, acquired immunodeficiency syndrome; BMI, body mass index; CBD, cannabidiol; FAACT, Functional Assessment of Anorexia–Cachexia Therapy instrument; QoL, quality of life; THC, tetrahydrocannabinol; VAS, Visual Analog Scale.
Intervention
Each of the five studies were RCTs that used some form of cannabis-based medicine (dronabinol n=3; cannabis extract and THC n=1; nabilone n=1) in cachectic patients against a control group (placebo, n=3) or alternative pharmacological agent (megestrol acetate n=2). The median length of treatment was 6 weeks (range 4–12 weeks). Three of the studies implemented a twice-daily regimen of 2.5 mg of dronabinol as the intervention arm compared with either placebo or megestrol acetate once daily.39–41 Two of these studies also investigated a combination of megestrol acetate and dronabinol together.40,41 Jatoi et al.40 investigated 2.5 mg dronabinol+placebo versus oral megestrol acetate 800 mg/day+dronabinol 2.5 mg versus oral megestrol acetate 800 mg/day+placebo. Timpone et al.41 had four treatment arms looking at 2.5 mg dronabinol versus a combination of 2.5 mg dronabinol with either 250 mg or 750 mg of once daily megestrol acetate versus just 750 mg megestrol acetate. Turcott et al.42 compared an initial 2-week 0.5 mg dose of nabilone against placebo followed by 6 weeks of 1.0 mg nabilone, whereas Strasser et al.43 looked at the effects of oral cannabis extract, 2.5 mg THC, and 1 mg cannabidiol (CBD), versus both placebo and 2.5 mg THC alone.
Participants
A total of 934 adult patients took part across all five studies. Two studies focused on HIV-positive wasting syndrome with a clinical diagnosis of cachexia through a >10% or >2.3 kg of weight loss in the preceding 2 months.39–41 In three other studies, patients diagnosed with advanced cancer with an estimated life expectancy of at least 3 months and self-reported weight loss of >5% or >2.3 kg in the preceding months, not explained by other disease or recent surgery, were eligible.40,42,43 These cancer patients all had to have a European Cooperative Oncology Group Performance Status (ECOG) of 0–2, which determined they were capable of self-care for example, independent in daily activities. All five studies mandated either no previous use or a greater-than-1-month washout period of any appetite stimulants, including corticosteroids and cannabis products for at least the past month. All patients were required to be able to tolerate oral intake with no parenteral nutrition. For four studies, the majority of patients were male with just the nabilone study on nonsmall-cell lung cancer patients42 having a female majority. The mean age across all five studies was 53 years (SD=12).
Risk of bias assessment
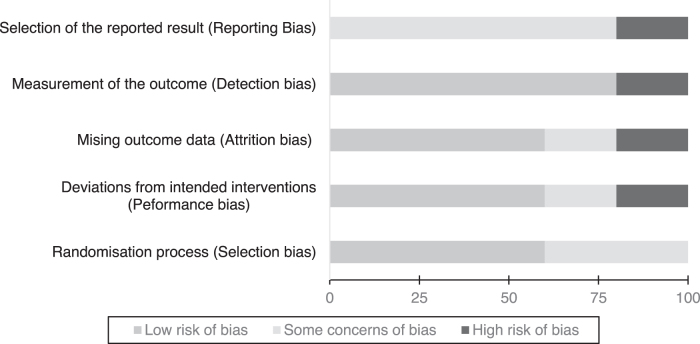
Figure 2 demonstrates the risk of bias across all five studies as assessed by the author using the Cochrane risk of bias tool.33 Supplementary Appendix SA2 provides a table summarizing the risk of bias in each domain for each of the included studies as well as the quality of evidence assessment through the GRADE framework.32
FIG. 2.
Overall risk of bias graph: authors' judgments about each risk of bias item presented as percentages across all included studies, using the Cochrane risk of bias tool (RoB 2)33
Domain 1: Randomization process
All studies stated randomization occurred but provided no exact methodology to how (e.g., random number generator). Two studies42,43 were assessed to have some concerns of bias owing to significant differences in baseline characteristics between intervention and control arms (weight loss, performance status, and age). Allocation was also concealed in four of five studies before assessment.
Domain 2: Deviations from intended interventions
Four of the five studies were stated to be double-blind, where unblinding was only permitted for safety reasons. One study41 explicitly states that participants were instructed on when and how to take their assigned intervention showing it was unblinded and was therefore assessed to have some concerns of bias. Beal et al.39 excluded patients postrandomization, despite eligibility, due to protocol violations, taking less than 75% of planned medication, and were observed less than 4 weeks. This naive “per-protocol” approach, only evaluating those strictly adhering to the assigned intervention, was deemed to be at high risk of bias, according to Cochrane.33
Domain 3: Missing outcome data
All five studies had missing outcome data mainly due to loss to follow-up or death, some studies handled this incomplete data appropriately using “intention-to-treat” (ITT) analyses. Strasser et al. explicitly states that missing values were substituted using the nearest neighbor approach to allow for an ITT analysis.43 Beal et al. eliminated 41 of 139 patients from analysis based on protocol violations or taking less than the planned dosages of medication—since no sensitivity analysis was conducted to indicate that this missing outcome data had no effect on the estimations of the intervention's effects, this was deemed as high risk of bias.39
Domain 4: Measurement of the outcome
Blinding of the outcome measurements was described in four of the five studies. Timpone et al. did not describe any blinding, so it is assumed that both self-reported outcomes for QoL and physician measurements could have been influenced by knowledge of the intervention.41 This study was therefore assessed to have high risk of bias for this domain.
Domain 5: Selection of the reported result
For all five studies, no prespecified trial protocols could be identified. Although attempts to contact the study authors were made, to identify any prespecified analysis plans, this was unsuccessful for all included articles. Raw data for outcome measurements was also missing in all five studies. Although the reported outcome measurements were in-keeping with the methodology described in each study—this was written retrospectively once the study had finished and as such all five studies were judged as having at least some concerns for reporting bias. However, four studies reported comprehensive outcome measurements at multiple time points for all intervention arms. Jatoi et al. only reported outcome measurements as “some point during the study” rather than specific time points and as such was judged as high risk of reporting bias.40
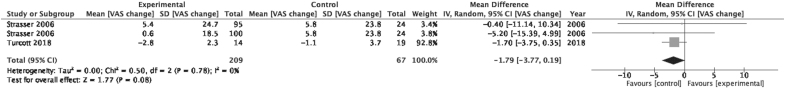
Change in appetite
Figure 3 is a forest plot showing the effect sizes of two included studies looking at the change in appetite for the cannabis-based medicine (experimental) and placebo (control) arms.42,43 An effect size above 0 favors the experimental arm, whereas below 0 favors the control arm. Strasser et al. provided a unit of analysis issue as it contained two intervention arms (Cannabis Extract+THC vs. THC alone)—as such the control arm was divided equally into two groups for data analysis.43 The pooled group effect size for change in appetite was −1.79 (95% CI: −3.77 to 0.19) favoring control but this effect was found to be not significant (p=0.08). Low heterogeneity existed between these studies (I2=0%).
FIG. 3.
Forest plot displaying effect sizes of studies comparing change in appetite, as scored using VAS, for cannabis-based medicines versus control in adults. VAS, Visual Analog Scale.
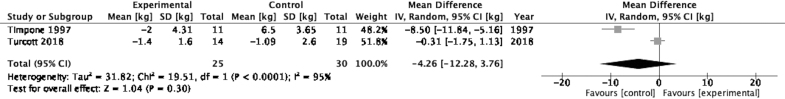
Change in weight
Figure 4 illustrates a forest plot for the effect sizes of change in weight reported in two studies between cannabis-based medicine and the control groups.41,42 The pooled between-group effect size for change in weight was −4.26 (95% CI: −12.28 to 3.76) although this result was not statistically significant (p=0.30). Moreover, the I2 value is 95% suggesting a high likelihood of study heterogeneity.
FIG. 4.
Forest plot displaying effect sizes of studies comparing change in weight for cannabis-based medicines versus control in adults.
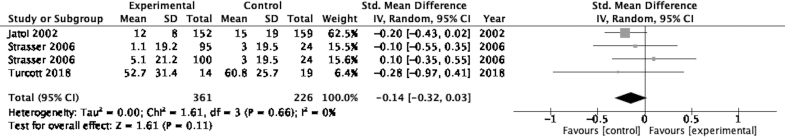
Quality of life
Figure 5 displays a forest plot for the effect sizes for change in QoL reported in three studies.40,42,43 The pooled between-group effect size for change in QoL was −0.14 (95% CI: −0.32 to 0.03) favoring control, although this was not statistically significant (p=0.11). The I2 value of 0% suggests there was low heterogeneity between included studies. The standard MD was used here due to the QoL measurements using different scales/instruments.
FIG. 5.
Forest plot displaying effect sizes of studies comparing change in QoL for cannabis-based medicines versus control in adults. QoL, quality of life.
Acceptability of treatment
All five included studies utilized some form of patient self-reporting through QoL questionnaires allowing additional monitoring of adverse events.
Beal et al. reported that 43% of the experimental arm reported treatment-related adverse events compared with 13% of the control group (p<0.001).39 Additionally, nervous system events (dizziness, euphoria, and drowsiness) were the most common adverse events seen in the experimental group (35%) compared with placebo (9%) with a p-value <0.001.
In the two studies that compared cannabis-based medicine with megestrol acetate, neither found a statistically significant difference with respect to the frequency of adverse events.40,41 Jatoi et al. found that 18% of male patients receiving megestrol acetate reported impotence contrasting with 4% on dronabinol (p=0.002).40
Three studies comparing cannabis-based medicines against placebo found no statistically significant difference with respect to the frequency of adverse events.39,42,43 Turcott et al. observed a significant reduction in both pain and insomnia for the nabilone arm compared with the placebo group.42
Discussion
This systematic review and meta-analysis aimed to assess the effect of cannabis-based medicine on patients with cachexia. The primary objective being to compare the available evidence on how appetite is affected while secondarily looking at change in body mass and QoL.
The literature search identified five RCTs evaluating the efficacy of cannabis-based medicine in the treatment of cachexia in both advanced cancer (three studies) and HIV patients (two studies). GRADE methodology and the risk of bias tool were utilized for the analysis and interpretation of study results.
Although two studies found an overall trend that appetite scores had improved with cannabis-based medicine use, no statistically significant change in appetite was observed across all five studies.39,42 For two pooled studies, an MD of −1.79 (p=0.08) favoring the control group of patients was calculated.42,43 The quality of evidence was assessed to be low. These studies had small sample sizes and wide CIs that crossed 0.
No statistically significant change in weight was observed in the three studies measuring weight change.39,41,42 However, the quality of evidence for this outcome was assessed as very low due to identified risk of bias in outcome measurement and a likelihood of high study heterogeneity. Patients in one study measuring weight change as an outcome were aware of their intervention, which could have influenced their self-reported weight change.45–47 Moreover, study duration varied from 4 to 12 weeks, this relatively short duration combined with an increasing loss to follow-up, observed as trial duration continued, calls in to question the validity of these findings.48 Clinical heterogeneity in the form of different routes of administration, dosage, and plasma levels may confound the outcomes leading to the overall study outcome. Future clinical studies may better benefit from pharmacological evaluation to identify an optimum treatment regimen particularly in view to identifying if a dose–response relationship exists.
QoL data were pooled for three studies but no statistically significant change was observed.40,42,43 The quality of evidence here was again considered low. The risk of bias in reporting outcomes was also high in one included study.
All five studies suffered from missing outcome data due to loss to follow-up. In some cases, evaluable patients postrandomization were also excluded following protocol violations. Only one study explicitly explained how it handled missing outcome data through a nearest neighbor approach.43 The inappropriate handling of missing outcome data in some of the studies calls in to question the interpretation of the results. If the characteristics of patients that dropped out differed across treatment arms or the reasons for their loss to follow-up were not thoroughly explored, the estimate of the effect of cannabis may be incorrect.49 For example, drop-out patients could have been of certain ethnicities, disease status, or age ranges that suffered more severe adverse events or felt insufficient benefit from the treatment to warrant continuation.
Small sample sizes and low quality of evidence were consistent limitations at study level. A limited number of studies were identified as suitable for inclusion and the lack of raw data available for each included study restricted the quality of analysis possible. The small study number also meant that it was not possible to conduct subgroup analyses despite study differences (treatment type, study duration, and methodology). Publication bias could not be assessed using funnel plot asymmetry because fewer than 10 studies were included within the meta-analysis.34,50
The average age of the entire cohort for the included studies is ∼53 years old with the majority being Caucasian males. The applicability of findings in these studies may be less generalizable to the population due to differences in pharmacokinetics among age, gender, and ethnicity.51 Differences in fat distribution and muscle mass across different populations may affect the pharmacodynamics of cannabinoids.52,53 Formal phase I/II studies to elicit pharmacokinetic data on cannabinoids in cachexia are still awaited.54 This study aimed to mitigate this by only specifically including those with diagnosed cachexia who would therefore share a more similar body phenotype, in contrast to previous systematic reviews, which had included patients without meeting a definition for cachexia.29 Patients being entered onto trials concerning nutritional status could also be more likely to be better informed and more knowledgeable about good nutritional regimes versus the general population. This could confound findings in studies that assess pharmacological interventions for appetite and weight change, biasing toward the null.
The findings in this review are in line with a previous systematic review on cannabinoid use in palliative medicine that observed no significant effect of cannabinoids on appetite or weight change.28 A larger systematic review and meta-analyses on multiple pharmacological management options for cachexia found no robust evidence to recommend any single pharmacological agent, including cannabis, for the treatment of cachexia.55 Both reviews similarly found that most studies suffer from at least some risk of bias and low quality of evidence.
Cannabinoids possess sufficient pharmacological potential for use in cachexia. CB1R agonists increase appetite in orexigenic and anorexigenic neurones.26,27 Moreover, THC and in particular CBD have demonstrated immunoregulatory function, particularly through TNF-α and interleukin-6, suggesting a mechanism through which to treat cachexia.56,57 Most patients are accepting of cannabis-based medicines as therapeutics, despite negative connotations associated with its recreational use. Multiple studies conducted in different patient populations (cancer, acute perioperative pain, and chronic pain) confirm this notion.58–61 Over 500 patients surveyed in the United States judged oral administration in capsules or pills as the most acceptable, which is the format of administration used in all five included studies.62
Besides the low quality of evidence in the included studies for cachexia and cannabinoid use, there exist a myriad of barriers and challenges to conducting wider research into cannabinoids in general. Aside from legal, regulatory, and funding barriers, cannabis can exist as a whole plant extract or active pharmaceuticals can be isolated and administered to patients.63–65 Cannabis cultivation yields highly heterogeneous products based on growth techniques (light, temperature, humidity, and nutrient type), and therefore all studies utilizing plant-sourced cannabinoids need assurance that good manufacturing practice was used throughout.66 The Cannabis plant contains over 100 cannabinoids, in addition, hundreds of terpenes, flavonoids, stilbenoids, amino acids, fatty acids, alkaloids, hydrocarbons, carbohydrates, and phenols.67,68 Ben-Shabat et al. first highlighted how varying concentrations of each constituent compound in the plant may alter the end effect on a patient, known as the “entourage effect.”69 While there is much controversy over the strength of this posited theory, with evidence both supporting and refuting its existence, the heterogony of cannabis chemovars does provide challenges in performing RCTs on whole plant extract of unprocessed flower as the effects of each compound need to be considered for their effects on the clinical results.69,70–73 Successful clinical trials require large sample sizes over long durations with tightly controlled methodology that can be replicated and consistent results be reproduced.74
Conclusion
This review found no high-quality evidence to recommend the use of cannabis-based medicine for the treatment of cachexia. It supports previous findings that there is no high-quality evidence to support the use of any pharmacological agents in isolation for cachexia. In view of this, it is recommended that, based on the pharmacological potential of cannabinoids for increasing appetite and modulating immune function combined with the unmet need to develop an effective treatment option for cachectic patients, further trials be conducted. Studies with larger sample sizes and longer trial durations, to produce a higher quality of evidence, are required. In particular, THC/CBD combination regimens may warrant specific further evaluation, as THC induces appetite stimulation through CB1R agonist activity, while CBD is immunomodulatory. Moreover, further studies are needed to identify if whole plant extract does exhibit an “entourage effect” over single agent isolates and how this alters treatment efficacy for patients.
Ethical Considerations
The authors have reviewed the journal guidance on ethical standards and have nothing to declare.75
Supplementary Material
Acknowledgment
The authors thank Ms. Rebecca Jones, medical librarian, who contributed to the development of search strategy.
Abbreviations Used
- AC/S
anorexia–cachexia subscale of FAACT
- AIDS
acquired immunodeficiency syndrome
- BMI
body mass index
- CB1R
cannabinoid receptor type 1
- CBD
cannabidiol
- CI
confidence interval
- EORTC-QLQ
European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire
- FAACT
Functional Assessment of Anorexia–Cachexia Therapy instrument
- ITT
intention-to-treat
- MD
mean difference
- PICOS
patient, intervention, control, outcome, study type
- QoL
quality of life
- SD
standard deviation
- THC
tetrahydrocannabinol
- TNF-α
tumor necrosis factor-α
- VAS
Visual Analog Scale
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received in support of this study.
Supplementary Material
Cite this article as: Hammond S, Erridge S, Mangal N, Pacchetti B, Sodergren MH (2021) The effect of cannabis-based medicine in the treatment of cachexia: a systematic review and meta-analysis, Cannabis and Cannabinoid Research 6:6, 474–487, DOI: 10.1089/can.2021.0048.
References
- 1. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. [DOI] [PubMed] [Google Scholar]
- 2. von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopeni. 2010;1:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morley JE, Thomas DR, Wilson MG. Cachexia: pathophysiology and clinical relevance. AM J Clin Nutr. 2006;83:735–743. [DOI] [PubMed] [Google Scholar]
- 4. Haehling Sv, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers—update 2014. J Cachexia Sarcopeni. 2014;5:261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farkas J, von Haehling S, Kalantar-Zadeh K, et al. Cachexia as a major public health problem: frequent, costly, and deadly. J Cachexia Sarcopenia Muscle. 2013;4:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naito T, Okayama T, Aoyama T, et al. Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non-small-cell lung cancer in Japan: a prospective longitudinal observational study. BMC Cancer. 2017;17:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hopkinson JB. Psychosocial impact of cancer cachexia. J Cachexia Sarcopeni. 2014;5:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takayama K, Atagi S, Imamura F, et al. Quality of life and survival survey of cancer cachexia in advanced non-small cell lung cancer patients—Japan nutrition and QOL survey in patients with advanced non-small cell lung cancer study. Support Care Cancer. 2016;24:3473–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vagnildhaug OM, Blum D, Wilcock A, et al. The applicability of a weight loss grading system in cancer cachexia: a longitudinal analysis. J Cachexia Sarcopeni. 2017;8:789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. [DOI] [PubMed] [Google Scholar]
- 11. Utech AE, Tadros EM, Hayes TG, et al. Predicting survival in cancer patients: the role of cachexia and hormonal, nutritional and inflammatory markers. J Cachexia Sarcopeni. 2012;3:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalantar-Zadeh K, Rhee C, Sim J, et al. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopeni. 2013;4:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lesinski G, Young G, Brasdovich M, et al. Cancer cachexia is associated with decreased responsiveness of immune cells to immunomodulatory cytokines. Cancer Res. 2008;68(9 Supplement):3792. [Google Scholar]
- 14. Webb JG, Kiess MC, Chan-Yan CC. Malnutrition and the heart. Can Med Assoc J. 1986;135:753–758. [PMC free article] [PubMed] [Google Scholar]
- 15. Keung Y, Owen J. Iron deficiency and thrombosis: literature review. Clin Appl ThrombHem. 2004;10:387–391. [DOI] [PubMed] [Google Scholar]
- 16. Tancini G, Barni S, Crispino S, et al. A study of thyroid function in cancer cachexia. Tumori J. 1989;75:185–188. [DOI] [PubMed] [Google Scholar]
- 17. Brown AA, Hu FB. Dietary modulation of endothelial function: implications for cardiovascular disease. Am J Clin Nutr. 2001;73:673–686. [DOI] [PubMed] [Google Scholar]
- 18. Acharyya S, Ladner KJ, Nelsen LL, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tapscott SJ. The circuitry of a master switch: myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. [DOI] [PubMed] [Google Scholar]
- 20. Guttridge DC, Mayo MW, Madrid LV, et al. NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. SCIEAS. 2000;289:2363–2366. [DOI] [PubMed] [Google Scholar]
- 21. Llovera M, Garcia-Martinez C, Agell N, et al. Muscle wasting associated with cancer cachexia is linked to an important activation of the ATP-dependent ubiquitin-mediated proteolysis. Int J Cancer. 1995;61:138–141. [DOI] [PubMed] [Google Scholar]
- 22. Fujitsuka N, Asakawa A, Uezono Y, et al. Potentiation of ghrelin signaling attenuates cancer anorexia–cachexia and prolongs survival. Transl Psychiatry. 2011;1:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suzuki H, Asakawa A, Amitani H, et al. Cancer cachexia—pathophysiology and management. Am J Gastroenterol. 2013;48:574–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inui A, Meguid MM. Cachexia and obesity: two sides of one coin? Curr Opin Clin Nutr. 2003;6:395–399. [DOI] [PubMed] [Google Scholar]
- 25. Perboni S, Inui A. Anorexia in cancer: role of feeding-regulatory peptides. Philos Trans R Soc Lond B Biol Sci. 2006;361:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazier W, Saucisse N, Gatta-Cherifi B, et al. The endocannabinoid system: pivotal orchestrator of obesity and metabolic disease. Trends Endocrinol Metabol. 2015;26:524–537. [DOI] [PubMed] [Google Scholar]
- 27. Woods SC. The endocannabinoid system: mechanisms behind metabolic homeostasis and imbalance. AM J Clin Med. 2007;120(2 Suppl 1):S9–S32. [DOI] [PubMed] [Google Scholar]
- 28. Mücke M, Weier M, Carter C, et al. Systematic review and meta-analysis of cannabinoids in palliative medicine. J Cachexia Sarcopeni. 2018;9:220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J, Wang Y, Tong M, et al. Medical cannabinoids for cancer cachexia: a systematic review and meta-analysis. BioMed Res Int. 2019;2019:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456–2473. [DOI] [PubMed] [Google Scholar]
- 31. Martin BR, Wiley JL. Mechanism of action of cannabinoids: how it may lead to treatment of cachexia, emesis, and pain. J Support Oncol. 2004;2:305–316. [PubMed] [Google Scholar]
- 32. Schünemann H, Brożek J, Guyatt G, et al. The GRADE Handbook. Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group. 2013. Available from: https://gdt.gradepro.org/app/handbook/handbook.html
- 33. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 34. Deeks JJ, Higgins JP, Altman DG. Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley & Sons, 2019; 241–284. [Google Scholar]
- 35. Deeks JJ, Higgins JPT, Altman DG (editors) on behalf ofthe Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook. [Google Scholar]
- 36. Altman DG, Bland JM. Standard deviations and standard errors. BMJ. 2005;331:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley & Sons, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beal EJ, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manag. 1995;10:89–97. [DOI] [PubMed] [Google Scholar]
- 40. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group Study. J Clin Oncol. 2002;20:567–573. [DOI] [PubMed] [Google Scholar]
- 41. Timpone JG, Wright DJ, Li N, et al. The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. The DATRI 004 Study Group. Division of AIDS Treatment Research Initiative. AIDS Res Hum Retroviruses. 1997;13:305–315. [DOI] [PubMed] [Google Scholar]
- 42. Turcott JG, Del Rocio Guillen Núñez M, Flores-Estrada D, et al. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: a randomized, double-blind clinical trial. Support Care Cancer. 2018;26:3029–3038. [DOI] [PubMed] [Google Scholar]
- 43. Strasser F, Luftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol. 2006;24:3394–3400. [DOI] [PubMed] [Google Scholar]
- 44. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deutz NEP, Safar A, Schutzler S, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sánchez-Lara K, Turcott JG, Juárez-Hernández E, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr. 2014;33:1017–1023. [DOI] [PubMed] [Google Scholar]
- 47. Engelen MPKJ, Safar AM, Bartter T, et al. High anabolic potential of essential amino acid mixtures in advanced non-small cell lung cancer. Ann Oncol. 2015;26:1960–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dettori JR. Loss to follow-up. Evidence Spince Care J. 2011;2:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Groenwold RHH, Moons KGM, Vandenbroucke JP. Randomised trials with missing outcome data: how to analyse and what to report. CMAJ. 2014;186:1153–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in a meta-analyses using individual participant data: a database survey. BMJ. 2012;344:d7762. [DOI] [PubMed] [Google Scholar]
- 51. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fattore L, Fratta W. How important are sex differences in cannabinoid action? Br J Pharmacol. 2010;160:544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. J Neuropsychoph. 2018;43:34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reuter SE, Martin JH. Pharmacokinetics of cannabis in cancer cachexia-anorexia syndrome. Clin Pharmacokinet. 2016;55:807–812. [DOI] [PubMed] [Google Scholar]
- 55. Advani SM, Advani PG, VonVille HM, et al. Pharmacological management of cachexia in adult cancer patients: a systematic review of clinical trials. BMC Cancer. 2018;18:1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nichols JM, Kaplan BLF. Immune responses regulated by cannabidoil. Cannabis Cannabinoid Res. 2020;5:12–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nagarkatti P, Pandey R, Rieder SA, et al. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1:1333–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tanco K, Dumlao D, Kreis R, et al. Attitudes and beliefs about medical usefulness and legalisation of marijuana among cancer patients in a legalised and a nonlegalized state. J Palliat Med. 2019;22:1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Erridge S, Miller M, Gall T, et al. A comprehensive patient and public involvement program evaluating perception of cannabis-derived medicinal products in the treatment of acute postoperative pain, nausea, and vomiting using a qualitative thematic framework. Cannabis Cannbinoid Res. 2020;5:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Khelemsky Y, Goldberg AT, Hurd YL, et al. Perioperative patient beliefs regarding potential effectiveness of marijuana (cannabinoids) for treatment of pain: a prospective population survey. Reg Anesth Pain Med. 2017;42:652–659. [DOI] [PubMed] [Google Scholar]
- 61. Rochford C, Edgeworth D, Hashim M, et al. Attitudes of Irish patients with chronic pain towards medical cannabis. Ir J Med Sci. 2019;188:267–272. [DOI] [PubMed] [Google Scholar]
- 62. Rudski JM. Treatment acceptability, stigma, and legal concerns of medical marijuana are affected by method of administration. J Drug Issues. 2013;44:308–320. [Google Scholar]
- 63. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. Challenges and Barriers in Conducting Cannabis Research. In: The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press (US), 2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425757/
- 64. Ware MA. Medical cannabis research: issues and priorities. J Neuropsychoph. 2018;43:214–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychoph. 2012;2:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chandra S, Lata H, El-Sohly MA, et al. Cannabis cultivation: methodological issues for obtaining medical-grade product. Epilepsy Behav. 2017;70:302–312. [DOI] [PubMed] [Google Scholar]
- 67. Russo EB. The case for the entourage effect and conventional breeding of clinical cannabis: no “Strain,” no gain. Front Plant Sci. 2019;9:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Andre CM, Hausman JF, Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 2016;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ben-Shabat S, Fride E, Sheskin T, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353:23–31. [DOI] [PubMed] [Google Scholar]
- 70. Finlay DB, Sircombe KJ, Nimick M, et al. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front Pharmacol. 2020;11:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Santiago M, Sachdev S, Arnold JC, et al. Absence of entourage: terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis Cannabinoid Res. 2019;4:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Piomelli D. Waiting for the entourage. Cannabis Cannabinoid Res. 2019;4:137–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anderson LL, Etchart MG, Bahceci D, et al. Cannabis constituents interact at the drug efflux pump BCRP to markedly increase plasma cannabidiolic acid concentrations. Sci Rep. 2021;11:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Katz S, Dufficy H, John C. Keys to success with clinical trials. J Gastroenterol Hepatol. 2011;7:100–105. [PMC free article] [PubMed] [Google Scholar]
- 75. von Haehling S, Morley JE, Coats AJS, et al. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle. 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.