Abstract
Objective: We assessed the efficacy, safety, and tolerability of cannabidivarin (CBDV) as add-on therapy in adults with inadequately controlled focal seizures.
Materials and Methods: One hundred and sixty-two participants (CBDV n=81; placebo n=81) were enrolled. After a 4-week baseline, participants titrated from 400 to 800 mg CBDV twice daily (b.i.d.) (or placebo) over 2 weeks, followed by 6 weeks stable dosing (at 800 mg b.i.d.) and a 12-day taper period. The primary endpoint was the change from baseline in focal seizure frequency during the 8-week treatment period. Secondary endpoints included additional efficacy measures relating to seizures, physician- and participant-reported outcomes, change in the use of rescue medication, cognitive assessments, and safety.
Results: Median baseline focal seizure frequencies were 17–18 per 28 days in both groups, and similar reductions in frequency were observed in the CBDV (40.5%) and placebo (37.7%) groups during the treatment period (treatment ratio [% reduction] CBDV/placebo: 0.95 [4.6]; confidence interval: 0.78–1.17 [−16.7 to 21.9]; p=0.648). There were no differences between the CBDV and placebo groups for any seizure subtype. There were no significant treatment differences between CBDV and placebo groups for any of the secondary efficacy outcome measures. Overall, 59 (72.8%) of participants in the CBDV group and 39 (48.1%) in the placebo group had ≥1 treatment-emergent adverse event (AE); the 3 most common were diarrhea, nausea, and somnolence. The incidence of serious AEs was low (3.7% in the CBDV group vs. 1.2% in the placebo group). There was little or no effect of CBDV on vital signs, physical examination, or electrocardiogram findings. Elevations in serum transaminases (alanine aminotransferase or aspartate aminotransferase) to levels >3×upper limit of normal occurred in three participants taking CBDV (two discontinued as a result) and one taking placebo; however, none met the criteria for potential Hy's Law cases.
Conclusion: It is likely the 40.5% seizure reduction with CBDV represents an appropriate pharmacological response in this population with focal seizures. The placebo response was, however, high, which may reflect the participants' expectations of CBDV, and a treatment difference from placebo was not observed. CBDV was generally well tolerated.
Clinical Trial Registration number: NCT02365610.
Keywords: antiepileptic drug, cannabinoid, GWP42006, epilepsy
Introduction
Cannabidivarin (CBDV) is a phytocannabinoid derived from the Cannabis sativa L. plant,1 which is currently being developed for the treatment of several conditions, including epilepsy. CBDV lacks appreciable affinity and activity at the human cannabinoid receptor, CB1, responsible for the euphoric effects associated with cannabis use.2 CBDV has been shown to reduce epileptiform activity in vitro,3 and to exert anticonvulsant effects in a broad range of seizure models in vivo, without significantly affecting normal motor function.2,4 CBDV was also found to ameliorate the induction of neuronal degeneration in seizure models in immature rats, suggesting potential therapeutic value.4 A role has been suggested for the transient receptor potential vanilloid receptor 1 in CBDVs anticonvulsant effects.4
To date, there have been limited clinical data on CBDV. Before this trial, the effect of concomitant antiseizure medications (ASMs) on the safety and pharmacokinetics of CBDV (after 2 weeks dosing at 400 mg twice daily [b.i.d.]) was assessed. This was done to assess potential for drug interactions with available ASMs. No potential interactions were found, and CBDV had an acceptable safety profile (NCT02369471, data on file).
The current trial aimed to evaluate the efficacy and safety of CBDV administered concomitantly with other ASMs, compared with placebo, to treat focal seizures.
Materials and Methods
Participants
Eligible participants were adults aged between 18 and 65 years (inclusive) with a documented history of focal epilepsy (documented by compatible electroencephalogram and clinical history). Eligible participants had seizures despite prior treatment with at least two ASMs and were receiving one to three concomitant ASMs. They were required to have experienced ≥1 weekly seizure during the 28 days preceding screening and 1 weekly seizure during the 28-day baseline. Countable seizures included the following: focal motor seizures without impairment of consciousness or awareness, focal seizures with impairment of consciousness or awareness, and focal seizures evolving to bilateral convulsive seizures. All medications or interventions for epilepsy had to be stable for 4 weeks before screening and throughout the trial. More detailed inclusion criteria can be found in Supplementary Data.
Trial design
This phase 2 randomized, double-blind, placebo-controlled trial was conducted between March 2016 and September 2017. It included 34 trial sites (5 in the Czech Republic, 7 in Hungary, 1 in Italy [no participants screened], 12 in Poland, 4 in Spain, and 5 in the United Kingdom).
Participants were randomized 1:1 to receive CBDV or placebo. There was a 4-week baseline period, a 2-week dose escalation period in which participants received 400 mg CBDV b.i.d. for 7 days (or placebo equivalent), then 600 mg CBDV b.i.d. for 7 days (or placebo equivalent). This was followed by a 6-week stable treatment period at the target dose of 800 mg b.i.d. (or placebo equivalent) and a 12-day taper period. Individual dose escalation was subject to the investigator assessment of safety and tolerability. Participants were required to attend eight trial visits (screening [days −28 to 3], randomization [day 1], during treatment [days 8±3, 15±3, 29±3, and 43±3], end of treatment [day 57±3], and end of taper [day 69±3]), with a follow-up call 4 weeks (±3 days) after the last dose.
CBDV and placebo were oral solutions containing 50 mg/mL CBDV and excipients or excipients only (placebo). Participants were instructed to fast for at least 2 h before taking the trial compounds and for 30 min afterward.
Randomization and blinding
Randomization occurred before treatment administration on day 1. Allocation to each treatment group was conducted through an interactive voice/web response system, according to a randomization schedule produced by an independent statistician. The randomization schedule was held centrally and not divulged to any other person involved in the trial until the database was locked and unblinding authorized by the relevant sponsor personnel. Investigators, participants, and the sponsor were blinded to the treatment allocation.
Outcome measures
The primary objective was to evaluate the efficacy of CBDV by measuring the change from 4-week baseline in focal seizure frequency during the 8-week treatment period for those taking CBDV compared with placebo. Secondary objectives were to evaluate the effects of CBDV compared with placebo on other measures of efficacy, including summary of treatment responders, proportion of participants with reductions in focal seizure frequency [>0% and ≤25%, >25% and ≤50%, >50% and ≤75%, and >75% and ≤100%], change in seizure subtype frequency, change in composite seizure score (the weighted sum of seizure subtypes, where weights were the severity of seizure subtypes—Type 1 seizures [least severe] were graded as “1,” Type 2 seizures as “2,” and Type 3 seizures [most severe] as “3”; see trial procedures for seizure type definition), change in number of focal seizure free days, change in rescue medication use, Physician Global Impression of Change (PGIC), and Subject Global Impression of Change (SGIC). Safety was monitored through adverse event (AE) recording, clinical laboratory tests, vital signs, physical examination, urinalysis, and 12-lead electrocardiograms (ECGs). Suicidal behavior and ideation were monitored using the Columbia Suicide Severity Rating Scale,5 and withdrawal symptoms were monitored using the cannabis withdrawal scale at randomization (day 1), the end of treatment, 1 day after the last dose, and 7, 14, 21, and 28 days after the last dose of CBDV.6 Finally, to investigate cognitive function at the start and end of treatment, the Trail Making Test (Halstead-Reitan), Lafayette Grooved Pegboard, and Symbol Digit Modalities Test were employed.
Trial procedures
Safety was monitored throughout the trial, using the parameters above.
Participants recorded seizure data in a diary throughout the trial. Seizures were classified into four categories: Type 1: focal seizures, which did not impair consciousness or awareness and had a motor component; Type 2: focal seizures, which impaired consciousness or awareness; Type 3: focal seizures, which evolved to bilateral convulsive seizures; and Type 4: other (seizures other than those listed above, i.e., focal seizures without impairment of consciousness or awareness and without an observable motor component). Seizure data were used for analysis of the primary (Types 1–3) and several secondary endpoints. An independent committee of experts from the Epilepsy Study Consortium reviewed the individuals' documented seizure history and subtype classification.
At the end of treatment (day 57), the PGIC and SGIC were administered. Both are 7-point Likert scales with markers from “very much improved” to “very much worse.” Physicians were asked to assess changes in the participants' general functional abilities since starting treatment, and participants were asked to rate their overall condition since starting treatment. Before starting treatment (day 1), participants wrote a brief description of their overall condition as a memory aid for completion of the SGIC at treatment end.
A cognitive assessment battery to assess coordination, attention, and visual-spatial integration was performed at the start and end of treatment (days 1 and 57), including Trail Making Test (Halstead-Reitan), Lafayette Grooved Pegboard, and Symbol Digit Modalities Test.
Statistical analysis
The trial intended to enroll 140 adults randomized in a 1:1 ratio to receive CBDV or placebo (70 per group). It was assumed that those in the placebo group would experience a mean reduction in focal seizure frequency of 15% (from baseline), meaning a sample size of 70 per group would allow detection of a difference of 35% between treatments. This was based on a standard deviation (SD) of 63%, using a 2-sided 5% significance level and 90% power.
There were two analysis sets for the reported trial data: intention to treat (ITT) and safety. Both included all participants who received at least one dose of CBDV or placebo. Efficacy endpoints were described and compared by group. All statistical analysis was 2-tailed and carried out at the 5% level of significance. The primary outcome variable for the determination of efficacy was the change from baseline in focal seizure frequency per 28 days during the 8-week treatment period. Data were analyzed using percentage change from baseline, if found to be normally distributed; however, due to non-normality of the seizure data, negative binomial regression analyses were conducted, where treatment ratios <1 indicate a difference in favor of CBDV. Safety data were summarized using appropriate summary statistics.
Compliance with ethical standards
This trial was conducted in accordance with International Council for Harmonisation Good Clinical Practice guidelines and ethical principles that have their origin in the Declaration of Helsinki. The protocol was approved by all relevant local Ethics Committee, and all participants provided written informed consent. The trial is registered on the clinicaltrials.gov website.
Results
Disposition of participants
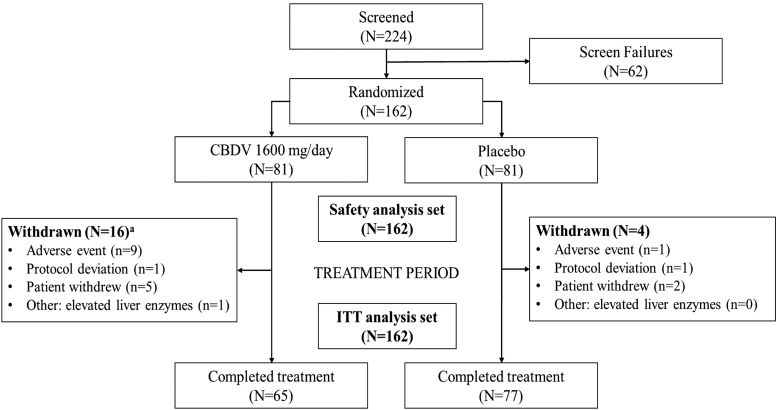
A total of 162 participants were randomized to receive CBDV (n=81) or placebo (n=81), and were included in the ITT and safety analysis sets. Participant disposition is summarized in Figure 1. In the CBDV group, 16 (20%) participants withdrew early from the trial; 9 (11%) due to AEs, 5 (6%) withdrew consent, 1 (1%) due to a protocol deviation, and 1 (1%) experienced elevated liver enzymes so was withdrawn by the investigator. In the placebo group, 4 (5%) withdrew early from the trial; 1 (1%) due to an AE, 2 (2%) withdrew consent, and 1 (1%) due to a protocol deviation.
FIG. 1.
Disposition of participants. aOne participant in the CBDV group discontinued treatment on day 32 due to an adverse event of severe diarrhea and did not restart treatment after adverse event resolution; however, they attended the end-of-treatment visit and was reported as completing the trial. CBDV, cannabidivarin; ITT, intention to treat.
Demographics are summarized in Table 1 and were similar between the two groups. The median number of prior ASMs was similar between treatment groups (4 for CBDV and 5 for placebo), and the median number of concomitant ASMs taken during the trial was 3 in both treatment groups. The most common concomitant ASMs taken were levetiracetam (43% CBDV vs. 48% placebo), lamotrigine (42% vs. 43%), valproic acid (40% vs. 33%), lacosamide (28% vs. 32%), and carbamazepine (25% vs. 31%). Six (7%) participants randomized to CBDV and five (6%) randomized to placebo had an active vagus nerve stimulator in situ throughout the trial.
Table 1.
Demographics and Characteristics (Safety Analysis Set)
| CBDV (N=81) | Placebo (N=81) | |
|---|---|---|
| Sex | ||
| Male, N (%) | 34 (42) | 38 (47) |
| Female, N (%) | 47 (58) | 43 (53) |
| Race | ||
| White/Caucasian, N (%) | 80 (99) | 81 (100) |
| Asian, N (%) | 1 (1) | 0 |
| Age (years), mean (SD) | 36.0 (11) | 36.1 (13) |
| Weight (kg), mean (SD) | 69.7 (17) | 73.8 (19) |
| Country | ||
| Czech Republic, N (%) | 10 (12) | 13 (16) |
| Hungary, N (%) | 13 (16) | 11 (14) |
| Poland, N (%) | 50 (62) | 48 (59) |
| Spain, N (%) | 4 (5) | 4 (5) |
| United Kingdom, N (%) | 4 (5) | 5 (6) |
| Baseline focal seizure frequency, mean (SD) | 38.7 (54) | 41.7 (74) |
| Baseline seizure frequency | ||
| Type 1, mean (SD)a | 33.5 (64) [N=34] | 25.5 (35) [N=42] |
| Type 2, mean (SD)a | 23.8 (30) [N=66] | 30.9 (72) [N=62] |
| Type 3, mean (SD)a | 11.0 (12) [N=38] | 12.9 (25) [N=30] |
| Type 4, mean (SD)a | 16.5 (36) [N=9] | 8.1 (19) [N=10] |
Type 1: focal seizures, which did not impair consciousness or awareness and had a motor component; Type 2: focal seizures, which impaired consciousness or awareness; Type 3: focal seizures, which evolved to bilateral convulsive seizures; and Type 4: other (seizures other than those listed above, that is, focal seizures without impairment of consciousness or awareness and without an observable motor component).
CBDV, cannabidivarin; N, number of participants; SD, standard deviation.
The median number of seizures during baseline per 28 days was 18.1 in the CBDV group versus 17.0 in the placebo group. Overall, the mean time since onset of seizures was 21.5 years (SD: 10.8) in the CBDV group and 20.9 years (SD: 12.1 years) in the placebo group. The number of participants with each type of focal seizure at baseline (Types 1–4) is presented in Table 1, alongside total seizure frequency by type at baseline. Thirteen participants had previously experienced status epilepticus; the mean time since last episode was 12.5 years; 17.1 years (range: 1.1–41.5 years) in the CBDV group; and 9.6 years (range: 1.1–26.5 years) in the placebo group.
Drug exposure
The mean number of dosing days reported during the treatment period was 49.3 days in the CBDV group and 54.5 days in the placebo group. In both treatment groups, the median number of dosing days during the treatment period was 57.0 days. The mean modal daily CBDV dose during the treatment period was 1480 mg/day.
Safety
A summary of treatment-emergent adverse events (TEAEs) is presented in Table 2. Most AEs were mild (26% CBDV; 30% placebo) or moderate (40% CBDV; 12% placebo) in intensity. The most common AE leading to treatment discontinuation was diarrhea, reported by 4 (5%) participants taking CBDV (vs. none taking placebo); all recovered. Three (4%) participants taking CBDV discontinued treatment due to a maculopapular rash; all recovered. All other AEs leading to treatment discontinuation were reported by ≤1 participant per group.
Table 2.
Summary of Treatment-Emergent Adverse Events
| CBDV N=81 |
Placebo N=81 |
|
|---|---|---|
| No. of participants (%) | ||
| TEAE | ||
| All TEAEs | 59 (73) | 39 (48) |
| TEAEs leading to treatment cessation | 11 (14) | 2 (3) |
| Serious TEAEs | 3 (4) | 1 (1) |
| Mild TEAEs | 21 (26) | 24 (30) |
| Moderate TEAEs | 32 (40) | 10 (12) |
| Severe TEAEs | 6 (7) | 5 (6) |
| TEAEs reported by >5% participants in either treatment group | ||
| Diarrhea | 20 (25) | 6 (7) |
| Nausea | 8 (10) | 8 (10) |
| Abdominal pain upper | 5 (6) | 1 (1) |
| Somnolence | 12 (15) | 2 (3) |
| Headache | 7 (9) | 6 (7) |
| Dizziness | 5 (6) | 3 (4) |
| Decreased appetite | 2 (3) | 6 (7) |
TEAE, treatment-emergent adverse event.
AEs leading to treatment discontinuation were serious for 2 (2%) participants taking CBDV. One participant taking CBDV experienced a serious TEAE of intoxication, pharmacologic, symptoms of which included severe ataxia, nystagmus, diplopia, dysarthria, mild confusion, elevated liver transaminases, and arterial hypotension. The TEAE began on day 31 (midway through maintenance dosing), led to treatment discontinuation after 5 days, and resolved 10 days after treatment discontinuation. The other participant experienced serious AEs of diarrhea, azotemia, and acute renal failure concurrently on day 33 (midway through maintenance dosing), which resolved after treatment discontinuation due to diarrhea. One other participant in the CBDV group experienced a serious AE but did not discontinue. The participant had a history of cholelithiasis and experienced an acute episode during a scheduled cholecystostomy during the taper period.
In the placebo group, one participant experienced status epilepticus 5 days into the taper period, which resolved after 1 day.
No participants in either treatment group reported suicidal behavior on the columbia-suicide severity rating scale during or at the end of treatment. In terms of the cannabis withdrawal scale (CWS), a reduction in score indicates an improvement in withdrawal symptoms. Post-treatment changes in overall CWS scores decreased from baseline in both treatment groups; therefore, there were no signs of withdrawal in either treatment group.
There was little or no effect of CBDV on vital signs, physical examination findings, or ECGs. Overall changes in vital signs or ECG that met predefined criteria for clinical significance were generally similar between the treatment groups. Elevations in serum transaminases (alanine aminotransferase or aspartate aminotransferase) to levels >3×upper limit of normal occurred in three participants taking CBDV (two participants discontinued as a result) and one taking placebo; however, none met the criteria for potential Hy's Law cases. All participants with elevated serum transaminases were taking at least one concomitant medication associated with transaminase elevations, such as carbamazepine, lacosamide, lamotrigine, clarithromycin, or valproic acid. In the CBDV treatment group, no participants shifted from having normal aspartate aminotransferase levels at baseline to high levels (>upper limit of normal) at the end of treatment compared with 2 (2%) participants in the placebo group. There were no significant changes, either between baseline and the end of treatment, or between treatment groups in any of the cognitive assessments: Trail Making Test (Halstead-Reitan), Lafayette Grooved Pegboard, or the Symbol Digit Modalities Test.
Efficacy
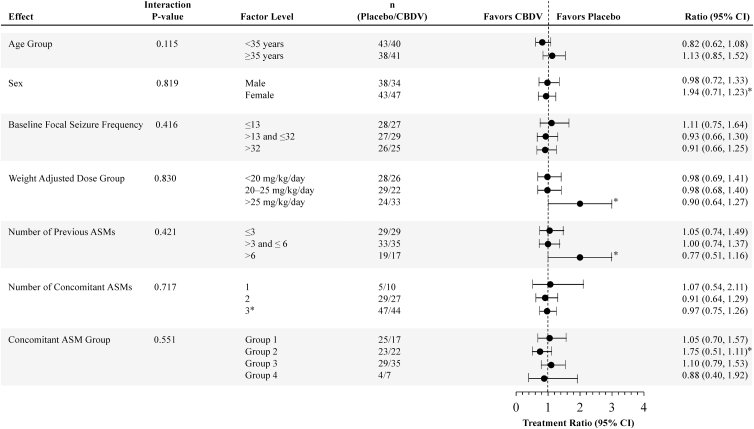
For the primary endpoint, similar reductions in focal seizure frequency were observed in the CBDV (41%) and placebo (38%) groups during the treatment period. The treatment difference was in favor of CBDV but was not statistically significant (Table 3). A summary of effect modifier analyses of the primary efficacy endpoint by subgroup is shown in Figure 2. The treatment ratio was ∼1 for all subgroups, and in all cases the 95% confidence interval (CI) values crossed 1; all interaction p-values were >0.1, suggesting no strong interaction between any of the subgroups analyzed.
Table 3.
Negative Binomial Regression Analysis of Focal Seizure Counts During Baseline and Treatment Periods (Intention-to-Treat Analysis Set)
| CBDV N=81 | Placebo N=81 | |
|---|---|---|
| Focal seizure frequency per 28 days | ||
| Baseline period median (Q1, Q3) | 18.1 (11.0, 35.0) | 17.0 (10.6, 44.0) |
| Treatment period median (Q1, Q3) | 11.3 (6.0, 22.0) | 12.3 (5.9, 26.0) |
| Variable | ||
| Ratio versus baseline (95% CI) | 0.59 (0.52 to 0.69)a | 0.62 (0.54 to 0.72)a |
| % Reduction versus baseline (95% CI) | 40.5 (31.4 to 48.4) | 37.7 (28.2 to 45.9) |
| Treatment ratio [CBDV vs. placebo] (95% CI) | 0.95 (0.78 to 1.17)b | |
| % Reduction (95% CI) | 4.6 (−16.7 to 21.9) | |
| p-value | 0.648 | |
Improvement from baseline.
Result in favor of CBDV.
CI, confidence interval.
FIG. 2.
Effect modifier analysis of primary efficacy: negative binomial regression effect modifier analysis of focal seizure count during baseline (per 28 days) and treatment (ITT analysis set). Concomitant ASM groups are as follows: Group 1: participants on inducer ASMs (and not on inhibitor ASMs); Group 2: participants on inhibitor ASMs (and not on inducer ASMs); Group 3: participants on ASMs that are neither inducer nor inhibitors; Group 4: patients on inducer and inhibitor ASMs. ASMs, antiseizure medications; CI, confidence interval; N/A, not applicable.
*Correction added on April 15, 2021 after first online publication of February 13, 2021: In Fig. 2, (i) the ratio text values under Ratio (95% CI) for Sex factor level Female was incorrect as 1.94 and should be 0.94 and the ratio text values under Ratio (95% CI) for Concomitant ASM Group factor level Group 2 was incorrect as 1.75 and should be 0.75; (ii) the forest plot data points were incorrect for Number of previous ASMs factor level >6 and Weight Adjusted Dose Group factor level >25 mg/kg/day and have been revised; and (iii) in the Factor Level column, the Number of Concomitant ASMs factor level 3 was incorrect and should be ≥3. The figure has been corrected to reflect the correct values.
Reductions in specific seizure types were inconsistent, and no difference between treatments reached statistical significance. Accordingly, no statistical difference was found in the seizure composite score. The proportion of participants with a≥25%, ≥50%, ≥75%, or 100% reduction in seizure frequency was similar between treatment groups.
Participants in the CBDV group achieved a mean gain of 5 days of total seizure freedom over the treatment period versus 4 days in the placebo group; this difference was not statistically significant (treatment difference: 0.9; 95% CI: −0.5 to 2.3; p=0.195). Rescue medication use was relatively infrequent and remained unchanged.
Physicians reported 56% of participants randomized to CBDV and placebo as having improved from baseline (very much, much, or slightly improved) in their general functional abilities at the end of treatment. The respective proportions of participants who reported any improvement in overall condition from baseline were 61% in the CBDV group and 64% in the placebo group. Similar proportions in each treatment group fell into each of the seven change categories for the PGIC and SGIC. There was no statistical difference between the treatment groups for either the PGIC (odds ratio [OR]=0.96; 95% CI: 0.55–1.69; p=0.895) or SGIC (OR=1.05; 95% CI: 0.59–1.86; p=0.866).
Discussion
This trial showed that add-on CBDV treatment in participants with focal seizures had an acceptable safety profile, but there was no difference in seizure reductions between the CBDV and placebo treatment groups after 8 weeks of treatment.
An adult population was targeted given the limited human exposure data available for CBDV. Unlike cannabidiol, plants rich in CBDV are rare in nature. A single Phase 1 study in adult healthy volunteers had been carried out before the start of this study. In addition, at the time this study was run, animal juvenile toxicology data were not available. The dose was selected based on the dose range tested in the Phase 1 study, in which a maximum of 800 mg once daily for 5 days was used. In this study, doses of 800 mg b.i.d. were administered to maximize exposure. The dose selected was supported by the animal toxicology data.
We observed a large placebo effect. The magnitude of the placebo effect in human epilepsy trials has been previously documented in meta-analyses, where estimates of the placebo response were reported to be 13% per data pooled from 1887 placebo-treated individuals7 and ranged from 9% to 17% for 50% responders in a further review of randomized controlled trials.8 In the current trial, 36% of participants taking CBDV were 50% responders versus 33% in those taking placebo. This is a larger placebo effect than has been previously reported.7,8 A large heterogeneity in placebo response rates has also been noted between trials,9 and a correlation within trials between the placebo and drug response has been reported.10 These findings suggest that the magnitude of the placebo effect may be influenced by the trial design, location, and population.
Causes of high placebo response rates in epilepsy trials have been proposed as classical conditioning, the Hawthorne effect, high expectations of the trial drug, regression to the mean, and natural fluctuations of the disease.10,11 In the current trial, baseline seizure frequency was comparable between groups, and effect modifier analyses indicated that there was little interaction between groups in relation to baseline seizure frequency. This suggests that regression to the mean is unlikely to be the main contributor for the observed effects.
In terms of trial design, a factor that may have contributed to the overall response is the relatively short evaluation period. The baseline data consisted of 4 weeks, and the treatment period was 8 weeks. This was a proof-of-concept trial, and the aim was to determine efficacy while minimizing the period of exposure. In a retrospective analysis of pregabalin data, it has been suggested that a 4-week baseline and 3-week treatment could be enough to determine efficacy for a highly efficacious drug.12 Given the unknown efficacy of CBDV and the slow dose escalation adopted, CBDV was generally well tolerated, with low incidence rates of serious TEAEs.
This study has some important limitations. Many factors can potentially affect the results of clinical studies in people with epilepsy, including heterogeneity of the data across geographic regions or study centers, and baseline characteristics of the participants. Unfortunately, the number of participants included in this trial was too small to conclusively evaluate these factors.
Conclusions
CBDV was generally well tolerated, but efficacy of CBDV in the treatment of focal seizures was not demonstrated. A high placebo response was observed. Further studies of ASMs should consider trials designed to control for the large placebo effect often observed in this population. The neuromodulatory potential of CBDV is still under investigation.
Supplementary Material
Acknowledgments
We are indebted to the individuals who took part in the trial, as well as to the staff at the clinical research sites. We thank Dr. Lesley Taylor of Alchemy Medical Writing Ltd for medical writing and editorial support, funded by Greenwich Biosciences, Inc. The study group investigators made significant contributions to the planning and/or execution of the study. Study group members (including authors) confirming approval to list their name are provided below in alphabetical order:
Czech Republic: Tomas Nezadal (INEP medical s.r.o., Křižíkova 264/22, 186 00 Praha 8); Jana Slonkova (JS-GYNAM s.r.o., Studentská 1155/14, 736 01 Havířov). Hungary: Anna Altman (Servus Salvus K ft., Szilagyi Erzsebet fasor 17–21. fel.3., Budapest); Kálmán Füle (Bács-Kiskun Megyei Kórház, SZTE ÁOK Oktató Kórháza, H-6000 Kecskemét, Nyíri út 38); Ágnes Horváth (Markusovszky Egyetemi, EEG Diagnosztikai es Epilepszia Centrum, Markusovszkky L. u. 5. Szombathely); Anna Kelemen (Országos Klinikai Idegtudományi Intézet, H-1145 Budapest, Amerikai út 57); Sámuel Komoly (Pécsi Tudományegyetem, Klinikai Központ Neurológiai Klinika, Ret u. 2., Pecs). Poland: Marta Banach (Specjalistyczny Gabinet Neurologiczny Marta Banach, Plac Lasoty 4, 30–534 Kraków); Iwona Kurkowska-Jastrzebska (Institute of Psychiatry and Neurology, Second Department of Neurology, ul. Sobieskiego 9, Warszawa); Paweł Lisewski (Vitamed Gałaj I Cichomski Spółka Jawna, Ul. Kościuszki 35, Bydgoszcz; Jolanta Zagórska (Centrum Kliniczno-Badawcze J. Brzezicki, B., Gornikiewicz Brzezicka Lekarze Spolka Partnerska, ul. Studzienna 35–36A, Elblag). Spain: Merce Falip (Hospital de Bellvitge, Neurology Department, Barcelona); Rodrigo Rocamora (Hospital del Mar, Neurology Department, Barcelona). United Kingdom: Manny Bagary (Regional Epilepsy Clinic, BSMHFT, Birmingham); Carlo Canepa (James Paget University Hospitals NHS Foundation Trust, Great Yarmouth, Norfolk).
Abbreviations Used
- AE
adverse event
- ASM
antiseizure medication
- CBDV
cannabidivarin
- CI
confidence interval
- CWS
cannabis withdrawal scale
- ECG
electrocardiogram
- ITT
intention to treat
- OR
odds ratio
- PGIC
Physician Global Impression of Change
- SD
standard deviation
- SGIC
Subject Global Impression of Change
- TEAE
treatment-emergent adverse event
Contributor Information
on Behalf of the GWEP1330 Study Group:
Tomas Nezadal, Jana Slonkova, Anna Altman, Kálmán Füle, Ágnes Horváth, Anna Kelemen, Sámuel Komoly, Marta Banach, Iwona Kurkowska-Jastrzebska, Paweł Lisewski, Merce Falip, Rodrigo Rocamora, and Manny Bagary
Collaborators: on Behalf of the GWEP1330 Study Group
Author Disclosure Statement
M.N., F.S., and A.S. are employed by GW Research Ltd and hold share options in GW Research Ltd. L.P. has received consultation fees from GL Pharma, Mylan, Sandoz, and Vipharm. He has also received educational grants and support from Vipharm, GP Pharma, and Mylan. He has accepted honoraria and guest lecture fees for symposia and other events sponsored by Astellas, Lundbeck, Teva, Eisai, and GL Pharma. He has served as principal investigator for >50 sponsor-initiated studies, and has been a national coordinator and key opinion leader for Astellas. He has served as an expert witness and consultant for lawsuits, providing deposition and testimony, including analysis of medical documents. J.W.S. received research grants from UCB and personal fees from UCB and Zogenix outside the submitted work. P.C. has served mainly as principal investigator in 66 industry-sponsored clinical trials in epilepsy and migraine. In seven trials, he was country coordinator. He has co-organized 26 annual conferences, named Psychosocial Aspects of Epilepsy sponsored by Pfizer, Glaxo, and Sanofi in the past and UCB Pharma, Teva, Apotex, Mylan, Glenmark, Accord, and Novartis currently. He has accepted speaking fees for lectures and webinars sponsored by UCB Pharma.
Funding Information
The trial was sponsored by GW Research Ltd.
Supplementary Material
Cite this article as: Brodie MJ, Czapinski P, Pazdera L, Sander JW, Toledo M, Napoles M, Sahebkar F, Schreiber A; on Behalf of the GWEP1330 Study Group (2021) A phase 2 randomized controlled trial of the efficacy and safety of cannabidivarin as add-on therapy in participants with inadequately controlled focal seizures, Cannabis and Cannabinoid Research 6:6, 528–536, DOI: 10.1089/can.2020.0075.
References
- 1. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am J Bot. 2004;91:966–975. [DOI] [PubMed] [Google Scholar]
- 2. Hill TD, Cascio MG, Romano B, et al. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol. 2013;170:679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–1141. [DOI] [PubMed] [Google Scholar]
- 4. Huizenga MN, Sepulveda-Rodriguez A, Forcelli PA. Preclinical safety and efficacy of cannabidivarin for early life seizures. Neuropharmacology. 2019;148:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Posner K, Brent D, Lucas C, et al. Columbia-Suicide Severity Rating Scale (C-SSRS). New York State Psychiatric Institute: New York, NY, 2008. [Google Scholar]
- 6. Allsop DJ, Norberg MM, Copeland J, et al. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–129. [DOI] [PubMed] [Google Scholar]
- 7. Guekht AB, Korczyn AD, Bondareva IB, et al. Placebo responses in randomized trials of antiepileptic drugs. Epilepsy Behav. 2010;17:64–69. [DOI] [PubMed] [Google Scholar]
- 8. Burneo JG, Montori VM, Faught E. Magnitude of the placebo effect in randomized trials of antiepileptic agents. Epilepsy Behav. 2002;3:532–534. [DOI] [PubMed] [Google Scholar]
- 9. Cramer JA, Fisher R, Ben-Menachem E, et al. New antiepileptic drugs: comparison of key clinical trials. Epilepsia. 1999;40:590–600. [DOI] [PubMed] [Google Scholar]
- 10. Zaccara G, Giovannelli F, Cincotta M, et al. Adverse events of placebo-treated, drug-resistant, focal epileptic patients in randomized controlled trials: a systematic review. J Neurol. 2015;262:501–515. [DOI] [PubMed] [Google Scholar]
- 11. Goldenholz DM, Goldenholz SR. Response to placebo in clinical epilepsy trials—Old ideas and new insights. Epilepsy Res. 2016;122:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. French JA, Cabrera J, Emir B, et al. Designing a new proof-of-principle trial for treatment of partial seizures to demonstrate efficacy with minimal sample size and duration-a case study. Epilepsy Res. 2013;106:230–223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.