Abstract
The Bmx gene, a member of the Tec tyrosine kinase gene family, is known to be expressed in subsets of hematopoietic and endothelial cells. In this study, mice were generated in which the first coding exon of the Bmx gene was replaced with the lacZ reporter gene by a knock-in strategy. The homozygous mice lacking Bmx activity were fertile and had a normal life span without an obvious phenotype. Staining of their tissues using β-galactosidase substrate to assess the sites of Bmx expression revealed strong signals in the endothelial cells of large arteries and in the endocardium starting between days 10.5 and 12.5 of embryogenesis and continuing in adult mice, while the venular endothelium showed a weak signal only in the superior and inferior venae cavae. Of the five known endothelial receptor tyrosine kinases tested, activated Tie-2 induced tyrosyl phosphorylation of the Bmx protein and both Tie-2 and vascular endothelial growth factor receptor 1 (VEGFR-1) stimulated Bmx tyrosine kinase activity. Thus, the Bmx tyrosine kinase has a redundant role in arterial endothelial signal transduction downstream of the Tie-2 and VEGFR-1 growth factor receptors.
Endothelial cells perform overlapping but also distinct functions in different parts of the cardiovascular system (28). The pericellular environment of venular and arterial endothelial cells differs primarily in the relative oxygen content of the blood, blood pressure, fluid shear forces, and periendothelial matrix and smooth muscle structures. The arterial endothelium is required to withstand an exceptionally strong stress caused by pulsatile blood flow. It also has a role in the control of smooth muscle cell tone in the vessel wall and thus of blood pressure (22). Dysfunction of the endothelium contributes to pathological conditions of the arterial wall, such as atherosclerosis (23). Recently, molecular cloning has increased our knowledge of gene expression specific for the arterial endothelial cells and of the mechanisms leading to distinct properties of the arteries and veins.
Tyrosine kinases (TKs) play a central role in signal transduction in endothelial cells. They activate growth and differentiation signal cascades through phosphorylation of cellular proteins (8, 27, 31). For example, the EphB4 receptor TK and its ligand ephrin B2 are considered to be responsible for signaling early arteriovenous differentiation (1, 39). Also, the recently reported expression of Dll4, a member of the Delta family of Notch ligands, in arterial endothelial cells indicates the existence of additional arterial endothelium-specific signal transduction cascades (33). In endothelial cells, TKs activate pathways resulting in the expression of proteins and factors which function as regulators of vessel tone (e.g., nitric oxide and endothelin 1), adhesion molecules (e.g., vascular cell adhesion molecule 1), or chemoattractants (e.g., monocyte chemoattractant protein 1) (for reviews, see reference 14). Mechanical stress activates a number of signaling pathways leading to changes in the conformation of arterial endothelial cells and in their interactions with pericellular matrix, for example, in focal adhesions (9, 38).
Bmx is a member of the Tec family of TKs, which also includes the Tec, Btk, Itk/Tsk/Emt, and Rlk/Txk kinases (24). The Tec kinases are expressed mainly in hematopoietic cells, where they transduce growth and differentiation signals from a variety of cytokine and antigen receptors. For example, the Tec TK occurs in most hematopoietic cells, while Btk is expressed selectively at certain stages of lymphocyte development and in the myeloid cell lineage (for reviews, see reference 41). Like the other members of the Tec family, Bmx is expressed in the myeloid hematopoietic cell lineage (15, 40). In myeloid progenitor cells, Bmx was previously shown to regulate cell differentiation and maturation (4). In addition, Bmx mRNA has been detected in the endothelium of large arteries by in situ hybridization of mouse embryos (5).
In this study, we have replaced the first coding exon of the Bmx gene with the lacZ gene encoding β-galactosidase. While the loss of Bmx function gave no obvious phenotype, the model allowed a detailed study of Bmx expression in arterial endothelial cells of fetal and adult tissues. Based on this analysis, we also show that the endothelium-specific TK receptors Tie-2/Tek and vascular endothelial growth factor receptor 1 (VEGFR-1) are upstream regulators of Bmx in the arterial endothelium.
MATERIALS AND METHODS
Construction of the targeting vector.
Mouse 129Sv genomic clones flanking the first coding exon of the Bmx gene were used to generate the targeting vector pPNT-Bmx-LacZ. A 10.5-kb BamHI-KpnI fragment containing the 5′ flanking sequence of the first coding exon was fused to the bacterial lacZ gene and blunt end cloned as the 5′ arm of the pPNT targeting vector described previously (32). A 1.8-kb EcoRI-ClaI fragment 3′ of the first coding exon was blunt end cloned into the pPNT vector and used as the 3′ homology arm. The targeting construct replaces the exon containing the translation initiation codon, thereby inactivating the gene.
Generation and genotyping of the Bmx−/− mice.
Electroporation of embryonic stem (ES) cells and generation of chimeric mice transmitting the mutation in the germ line were followed by mating of heterozygous and homozygous knockout mice (2). To identify Bmx-deficient mice, Southern blot analysis was carried out using genomic DNA purified from tail biopsy specimens of 4-week-old mice. The mouse strain used was DBA/2. All experiments using the mice were approved by the Helsinki University Laboratory Animal Committee. Ten male and ten female mice were used with identical results.
RNA isolation and analysis.
Total RNA from mouse bone marrow was extracted using the RNAeasy kit (Qiagen) according to the manufacturer's instructions. The reverse transcription (RT) reaction was carried out with 0.5 μg of total RNA, using the Omniscript RT Kit (Qiagen) and random hexanucleotide primers in a total volume of 20 μl. Following the RT reaction, the Bmx cDNA was PCR amplified from 4 μl of RT product using 5′-AACATACGCTATATTCCA-3′ and 5′-GCATGCAGATTTTCCTCT-3′ as primers for 25 cycles. The reaction conditions were 94°C for 40 s, 49°C for 30 s, and 72°C for 72 s. The LacZ cDNA was amplified for 15 cycles using 5′-GCTGCATAAACCGACTACAC-3′and 5′-TTCACCCTGCCATAAAGAAAC-3′ as primers with the following conditions: 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s. Both amplifications were carried out using Dynazyme (Finnzymes) polymerase in a total volume of 50 μl. Following the amplification, 10 μl of each PCR mixture was electrophoresed in a 1.5% agarose gel and Southern blotted onto a Hybond N+ (Amersham Pharmacia Biotech) nylon filter under alkaline conditions. The filters were hybridized with Bmx or LacZ cDNA probes using QuickHyb (Stratagene) solution and stringent washing conditions.
β-Galactosidase staining of tissues.
Embryos and whole-mount adult tissues were fixed in 0.2% glutaraldehyde–2 mM MgCl2–5 mM EGTA in 100 mM phosphate buffer at room temperature for 15 to 30 min. The tissues were then washed at room temperature in wash buffer (2 mM MgCl2, 0.01% deoxycholate, 0.02% Nonidet P-40 in 100 mM phosphate buffer) and incubated in 1-mg/ml X-Gal reaction mixture [1 mg of 5-bromo-4-chloro-3-indolyl-β-galactosidase/ml, 5 mmol of K3Fe(CN)6/liter, 5 mmol of K4Fe(CN)6/liter in wash buffer] at 37°C overnight. After staining, the samples were incubated in wash buffer at 4°C, transferred into 4% paraformaldehyde in phosphate-buffered saline (PBS), and fixed at 4°C overnight. The tissues were dehydrated through graded ethanols and embedded in paraffin. Sections were cut at 7 μm, mounted onto glass slides, dewaxed, and stained with nuclear fast red.
Immunostaining.
Tissues were fixed in 4% paraformaldehyde at 4°C overnight, dehydrated, and embedded in paraffin. Five-micrometer-thick paraffin sections were incubated for 20 min in 250 μg of trypsin/ml at 37°C and stained with endothelial-cell-specific platelet endothelial cell adhesion molecule 1 antibodies (Abs) (1:1,000; Pharmingen) using the Tyramide signal amplification (TSA) kit (NEN Life Sciences). For Tie-2 and smooth muscle β-actin (SMA) staining, the sections were heated in a microwave oven in 10 mM sodium citrate, blocked according to the manufacturer's instructions, and incubated with rabbit polyclonal anti-Tie-2 (1:500; Santa Cruz) and monoclonal anti-SMA (1:100, alkaline phosphatase conjugated; Sigma), respectively. The Tie-2-stained sections were processed further using the TSA kit, and the SMA-stained sections were processed using the Alkaline Phosphatase Substrate II kit (Vector Laboratories). The slides were counterstained in hematoxylin.
Lectin staining of blood vessels.
Lectin staining was used to visualize blood vessels in whole mounts of tissue as described previously (36). Biotinylated Lycopersicon esculentum lectin (100 μl of a 1-mg/ml solution; Sigma catalog no. B-1175) was injected intravenously via the femoral vein into anesthetized mice and allowed to circulate for 2 min. The mice were then sacrificed, and the tissues were fixed by intracardial perfusion with 1% paraformaldehyde–0.5% glutaraldehyde in PBS. The ears were dissected and washed with PBS, and the cartilage was removed. Bound lectin was visualized by biotin-peroxidase staining, and the ears were mounted on slides and examined by light microscopy.
Fluorescence in situ hybridization of metaphase chromosomes.
Fetal cell culture was established from a male mouse according to routine protocols (7). Part of the mouse genomic Bmx DNA was identified, isolated, and cloned from a mouse 129Sv genomic DNA library in the lambda FIX II vector (Stratagene), using a PCR fragment containing human Bmx cDNA nucleotides 23 to 162 as a probe. The insert was subcloned as a SalI fragment into pBluescript SK II (Stratagene) and labeled with digoxigenin-11-dUTP (Boehringer Mannheim) according to standard procedures. The single-color metaphase fluorescence in situ hybridization was performed and analyzed essentially as described previously (13).
Cell culture, growth factor stimulation, DNA constructs, and cell transfections.
293T cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, glutamine, and antibiotics. Human dermal microvascular endothelial (HDME) cells, obtained from Promo Cell, were grown in MV low-serum endothelial cell growth medium (Promo Cell) supplemented with the endothelial cell supplement mix provided by Promo Cell. The HDME cells were starved in serum- and growth factor-free media containing 0.2% bovine serum albumin for 45 h prior to 20-min stimulation with either angiopoietin-1 (Ang-1)-conditioned medium produced by transfected 293T cells or 100 ng of hVEGF165 (R&D Systems)/ml. The Ang-1 expression plasmid was produced by PCR amplification of human Ang-1 (21), followed by cloning into the SignalpIG vector (Ingenius; R&D Systems). All other DNA constructs used have been previously described (4, 19). The 293T cells were transiently transfected using a calcium phosphate transfection kit (GIBCO Life Technologies) according to the manufacturer's instructions.
Abs, immunoblotting, and in vitro Bmx kinase assay.
The monoclonal antihemagglutinin (anti-HA) epitope and anti-Bmx Abs were obtained from Berkeley Antibody Company and Transduction Laboratories, respectively, while the phosphotyrosine-specific mouse monoclonal Ab 4G10 was from Upstate Biotechnology. Abs to the endothelial cell receptors studied were the following: for Tie-1, MII (rabbit polyclonal anti-human Tie-1) (25); for Tie-2, goat polyclonal anti-human Tie-2 (R&D Systems); for VEGFR-1, P1-3 rabbit polyclonal anti-human VEGFR-1 (a kind gift from Masabumi Shibuya); for VEGFR-2, RS2 (rabbit polyclonal anti-human-VEGFR-2; a kind gift from Lena Claesson-Welsh); and for VEGFR-3, 9D9 mouse monoclonal anti-human VEGFR-3 (12). For immunoprecipitation, cells were lysed in TKB lysis buffer (1% NP-40, 20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 10% glycerol) supplemented with aprotinin, leupeptin, phenylmethylsulfonyl fluoride, and sodium vanadate. Immunoprecipitation was carried out from equal amounts of cell lysates by adding specific Abs and protein A-Sepharose (Amersham Pharmacia Biotech AB) or protein G-Sepharose (Amersham Pharmacia Biotech AB) and incubating for 1 h. The Bmx in vitro kinase assays were done as described previously (4). Briefly, the immunoprecipitates were washed twice with lysis buffer, once with wash buffer (150 mM NaCl, 20 mM HEPES, pH 7.4), and twice with kinase assay buffer (10 mM HEPES [pH 7.4], 5 mM MnCl2) and resuspended in 25 μl of reaction buffer (kinase assay buffer, 10 μM unlabeled ATP, 5 μCi of γ-ATP). The kinase reactions were carried out at 30°C for 10 min and stopped by adding an equal volume of 2× reducing Laemmli buffer. For Western blotting, the immunoprecipitates were washed four times with the lysis buffer followed by elution with the Laemmli buffer and separation in sodium dodecyl sulfate–7.5% polyacrylamide gels. The proteins were transferred to a nitrocellulose membrane and detected using specific primary Abs, biotinylated anti-mouse or anti-rabbit secondary Abs (Dako A/S), and streptavidin-biotin-horseradish peroxidase conjugate (Amersham Life Science) followed by detection by the ECL method (Amersham Life Science).
RESULTS
Generation of mice expressing the lacZ gene under the mouse Bmx promoter.
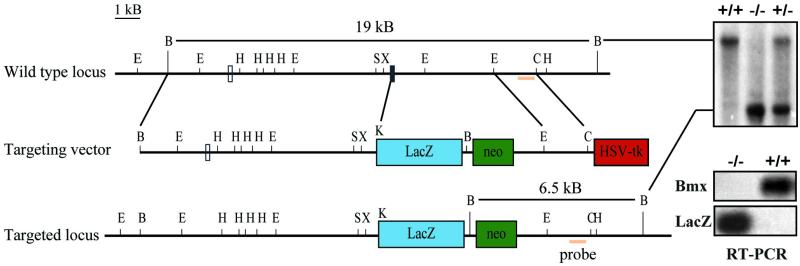
In order to study the expression pattern of the Bmx gene in embryos and adult tissues, it was replaced with the bacterial lacZ gene by gene targeting of ES cells. The exon encoding the translation start codon of Bmx was exchanged with the lacZ gene, placing the latter under the control of the Bmx promoter. R1 ES cells were electroporated with the targeting vector described in Materials and Methods. Several double-resistant drug-selected cell lines were obtained where homologous recombination had occurred, as evidenced by Southern blotting and hybridization of genomic DNA using both internal and external probes (data not shown). Two independent cell clones were used to make aggregation chimeras, which transmitted the BmxlacZ allele into the germ line. For the analysis, mice derived from two different aggregations were used with identical results. The knockout strategy, as well as an example of genotyping of the offspring using Southern blotting, is shown in Fig. 1. The knockout mice were fertile and had a normal life span.
FIG. 1.
Gene targeting of the mouse Bmx locus. The homologous recombination event deletes the exon coding for the translation initiation codon (exon 2, black box) and places the bacterial lacZ gene under the control of the Bmx promoter region. Schematic structures of the wt and recombinant loci and the targeting vector are shown. Open box, exon 1; E, EcoRI; B, BamHI; H, HindIII; S, SalI; X, XhoI; C, ClaI; Neo, neomycin phosphotransferase gene; HSV-TK, herpes simplex virus thymidine kinase gene; kb, kilobase pairs. Genotyping of tail biopsy specimens from 4-week-old mice was carried out by Southern blotting using the indicated fragment as a probe. Shown also are the results of RT-PCR analysis of isolated RNA from bone marrow cells derived from BmxLacZ/BmxLacZ or wt mice. The upper panel shows PCR using Bmx-specific primers, while the lower panel shows PCR using LacZ-specific primers. A faint band seen upon prolonged exposure in samples from the BmxLacZ/BmxLacZ mice corresponded to the signal obtained by a 100-fold dilution of the wt DNA and was considered insignificant (data not shown).
In order to ensure that no Bmx mRNA was produced in the knockout mice, RNA from bone marrow cells from homozygous knockout mice and wild-type (wt) controls was analyzed by RT-PCR. No Bmx mRNA could be detected in the BmxLacZ/BmxLacZ mice, while a clear signal was obtained from the wt mice. Vice versa, only the knockout mice expressed LacZ mRNA. Essentially similar results were also obtained using three other primer pairs located in various regions of Bmx.
Expression of the lacZ gene in the Bmx knock-in locus.
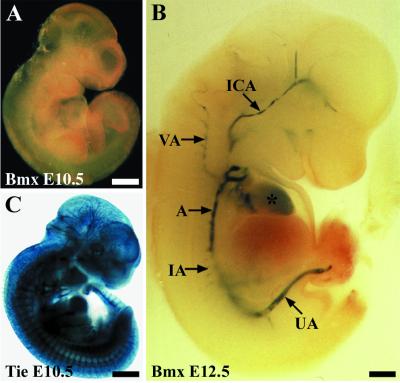
The β-galactosidase activity in the tissues of the Bmx gene-targeted mice was used to assess the sites of BmxlacZ expression by X-Gal staining, as explained in Materials and Methods. Most of the localization studies were carried out using mice homozygous for the Bmx mutation, but heterozygous mice gave essentially similar results, suggesting that deletion of the Bmx gene does not lead to significant alteration of the normal vascular development or architecture. No staining was obtained in 10.5-day-postconception embryos (E10.5; Fig. 2A). At E12.5, strong staining of the endocardium of the heart and the endothelium of large arteries was seen in whole-mount analysis (Fig. 2B). The aortic endothelium was positive both in the thoracic and in the abdominal region, and major arteries branching out from the aortic trunk also showed clear activity in the endothelial cells. For comparison, the Tie promoter-driven β-galactosidase expression was widely observed throughout the vascular endothelium, especially in the capillary network (Fig. 2C) (see also reference 18).
FIG. 2.
β-Galactosidase reporter staining of E10.5 (A) and E12.5 (B) BmxLacZ embryos and an E10.5 TieLacZ/Tie embryo (C). At E12.5, the strongest signals are seen in the aorta (A), internal carotid artery (ICA), vertebral artery (VA), intercostal arteries (IA), and umbilical artery (UA). An asterisk marks the heart. Bar, 500 μm.
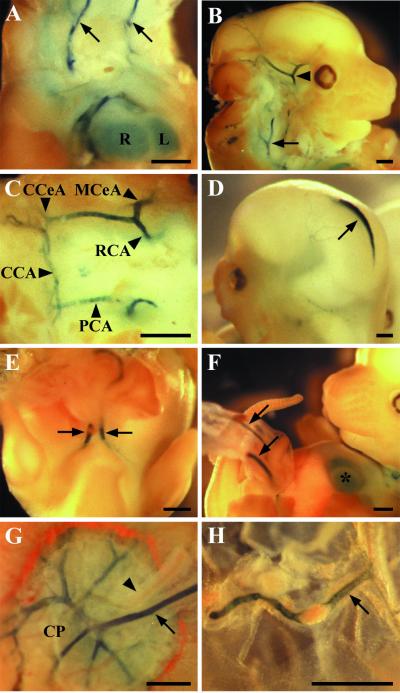
In the E12.5 BmxLacZ embryos, the carotid arteries and their branches in the basal brain were positive for β-galactosidase activity, as were the vertebral arteries (Fig. 3A to C). A conspicuous signal was also obtained from the developing venous sinus in the midline of the roof of the midbrain (Fig. 3D). Intercostal arteries branching from the descending aorta were stained but not as strongly as the larger arteries. In general, the staining became weaker in the more distal branches. A similar pattern of staining was obtained in the pulmonary trunk (data not shown). The umbilical arteries branching from the distal part of the aorta displayed intense β-galactosidase staining in the endothelium, which continued in the placental part, where the umbilical artery divides into smaller vessels (Fig. 3E to G). The amniotic artery was also strongly positive (Fig. 3H).
FIG. 3.
BmxlacZ expression in E12.5 embryos. (A) The carotid arteries (arrows) and the heart (R, right ventricle; L, left ventricle) show β-galactosidase activity in the dissected embryo. (B) Branch point of the internal and external carotid arteries (arrow) and the internal carotid artery at the base of the brain (arrowhead, partially cut out during preparation). (C) The LacZ-positive arteries of the vascular system called the circle of Willis; the anterior part is not visible in the figure. CCeA, caudal cerebral artery; MCeA, medial cerebral artery; CCA, caudal communicating artery; RCA, rostral communicating artery; PCA, posterior communicating artery. (D) Expression in the superior sagittal dural venous sinus (arrow). (E and F) Umbilical arteries (arrows) entering the umbilical cord. The asterisk in panel F indicates the heart. (G) The umbilical artery (arrow) entering the placenta and the chorionic plate (CP). The umbilical vein (arrowhead) is negative. (H) The largest amniotic arteries (arrow) were positive. Bar, 500 μm.
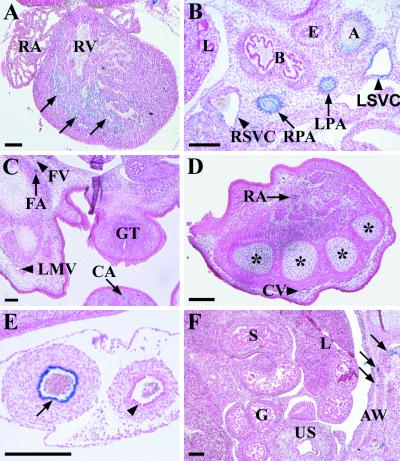
At E15.5, the developing fetal skin is no longer transparent for the visualization of β-galactosidase activity, and thus, only tissue sections were studied. The major sites of expression were the ones identified in the whole-mount analysis of E12.5 embryos, except that the staining was now also apparent in the more peripheral branches of the arteries. The endocardium of the heart was weakly stained, and a weak signal was also present in the endothelium of the superior and inferior venae cavae close to the sites where they enter the heart (Fig. 4A and B). The signals were much stronger in the major arteries around the heart and also in the peripheral arteries of the body wall. Examples of the arterial specificity are further illustrated in Fig. 4C and D, where the femoral artery, but not the vein and the radial artery, is stained. Also, a cross section of the umbilicus is shown in Fig. 4E. However, all blood vessels of the abdominal organs were negative in both E12.5 and E15.5 embryos (Fig. 4F). As in the E12.5 embryos, the endothelial cells lining the superior sagittal dural venous sinus were strongly positive. Remarkably, no other sites of expression could be detected.
FIG. 4.
Staining of sections from E15.5 BmxLacZ embryos. (A) The endocardium of the heart ventricles (arrows). RA, right atrium; RV, the lumen of the right ventricle. (B) The endothelial cells of the aorta (A), the right pulmonary artery (RPA), and the left pulmonary artery (LPA) are also stained. In the right and left superior venae cavae (RSVC and LSVC, respectively), the endothelial lining is weakly positive. L, right lung; B, main bronchi; E, esophagus. (C) In the blood vessels of the hind limbs and tail, BmxlacZ expression occurs in the femoral artery (FA) and caudal artery (CA) but not in the femoral vein (FV) or lateral marginal vein (LMV) of the hind limb. GT, genital tubercle. (D) The endothelium of the radial artery (RA) is positive, whereas the cephalic vein (CV) is negative. Asterisks indicate the primordia of the metacarpal bones. (E) In the umbilical cord, BmxlacZ was expressed specifically in the arterial endothelium (arrow), while the venous endothelial cells (arrowhead) were negative. (F) No positive blood vessels were found in the abdominal organs, while the abdominal wall (AW) contains stained vessels (arrows). US, urogenital sinus; S, stomach; G, lumen of gut; L, left lobe of the liver. Bar, 200 μm.
BmxLacZ activity in adult mice.
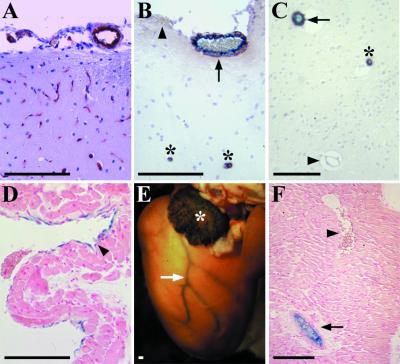
Adult mice were analyzed at 3 to 6 months postpartum. The endothelial cells of the arteries throughout the body showed strong BmxLacZ activity, whereas capillaries were negative. For example, in the ear, the endothelium of the arteries was positive, but such a signal was absent from the capillaries (Fig. 5). In the brain, a signal was obtained from most vessels which were positive for SMA, indicative of their arterial nature. The capillary network and the smallest SMA-positive arterioles were β-galactosidase negative (Fig. 6A to C). Again, the venous endothelium was positive only in the superior and inferior venae cavae (Fig. 6D). In the heart, a strong signal was found in the coronary arteries and in the endothelial cells of the auricles (Fig. 6E and F).
FIG. 5.
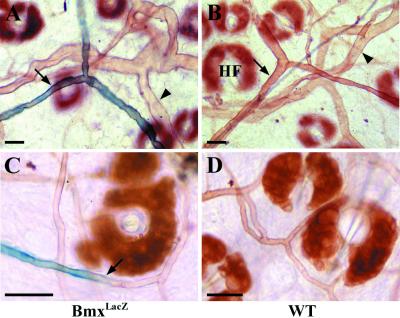
Whole-mount analysis of BmxlacZ expression in lectin-stained blood vessels of the ear. (A). In the β-galactosidase-stained ear of the lectin-perfused BmxLacZ mice, the endothelial cells are positive in the arteries (arrow) but negative in the veins (arrowhead). (B and D) The wt mice were negative for β-galactosidase staining. HF, hair follicle. (C) In the small arteries, the BmxlacZ expression was weaker and the signal was lost (arrow) in the capillary endothelium. Bar, 100 μm.
FIG. 6.
(A to C) BmxlacZ expression in brain microvasculature and major vessels of adult mice. (A) Endothelial cell-specific platelet endothelial cell adhesion molecule 1 staining of the microvasculature of the cerebral cortex. (B) Simultaneous staining for SMA (brown) and β-galactosidase (blue) in cortical brain. The smooth muscle cells decorate the walls of arteries and arterioles, whereas veins (arrowhead) and capillaries are negative. Note that BmxlacZ signal (arrow) is present only in SMA-positive vessels, although not in the smallest ones (asterisks). (C) Overlapping expression patterns in an SMA- and X-Gal-stained section from the brain parenchyma. Symbols are as defined for panel B. (D) Weakly β-galactosidase-positive cells are present in a section from the inferior vena cava (arrowhead). (E) In whole-mount staining of the heart, a strong signal is seen in the coronary arteries (arrow, left coronary artery) and in the endothelial cells of the auricles (asterisk, left auricle). (F) In a section through the heart wall, endothelial expression of BmxlacZ is observed in the coronary artery (arrow) but not in the coronary vein (arrowhead). Bar, 150 μm.
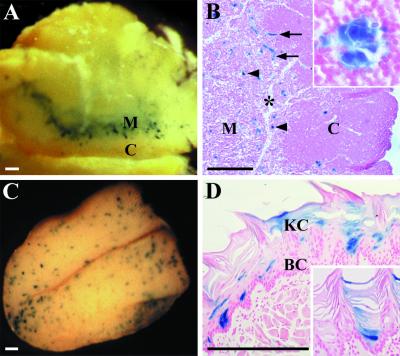
In adult mice, the BmxlacZ gene was also expressed outside the cardiovascular system. A strong signal was present in Hassall's corpuscles in the medulla of the thymus (Fig. 7A). The epithelioreticular cells were stained; these are of oropharyngeal origin and concentrically arranged, with the centers of the corpuscles occasionally displaying keratinization. Additional epithelial expression was found in the mucous membrane of the tongue, where occasional epithelial cells in the suprabasal layer expressed β-galactosidase and some of the staining persisted in the keratinizing layer in a patch-like pattern (Fig. 7B).
FIG. 7.
Whole-mount β-galactosidase staining of the thymus (A and B) and the tongue (C and D). (A) The BmxlacZ gene is expressed in a spot-like pattern in the medulla (M) of the thymus. C, cortex. (B) Histological analysis of the thymus shows positive clusters of cells (arrowheads) and blood vessels (arrows) in the medulla (M). The former are epithelial cells of the Hassall's corpuscles (inset). C, cortex. The asterisk shows the trabecula. (C) BmxlacZ is expressed in a patch-like pattern on the surface of the tongue. (D) Staining is strongest in certain basal cells in the mucosa and becomes weaker when the epithelial cells keratinize and move upward (inset). BC, basal cells; KC, keratinized cells. Bar, 300 μm.
Chimeric expression pattern of the X-chromosomal BmxlacZ locus.
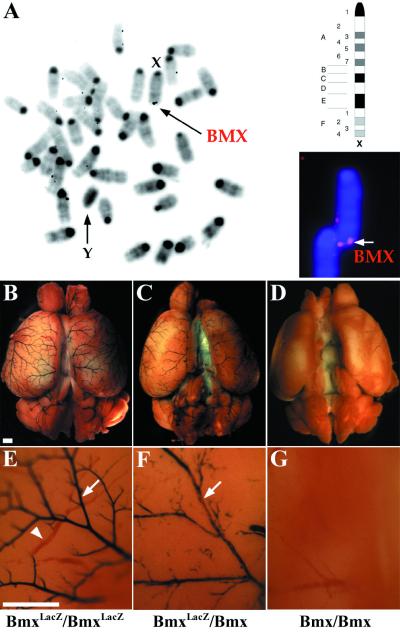
As has been previously shown for the human Bmx locus (35), the mouse Bmx locus is located in the X chromosome as detected by in situ hybridization analysis (Fig. 8A). Because one of the X chromosomes is inactivated in a stochastic manner during embryonic development, it was of interest to compare the staining of homozygous and heterozygous BmxLacZ mice. As expected, a mosaiclike expression pattern was seen in heterozygous female mice, illustrated in Fig. 8B to G.
FIG. 8.
Chromosomal localization and mosaic expression pattern of the BmxlacZ locus. (A) Chromosomal fluorescence in situ hybridization for the mouse Bmx gene. The Bmx gene is located in the telomeric region of the X chromosome, below band F. (B and E) The arteries on the surface of the brain cortex show a strong uniform signal in BmxLacZ/BmxLacZ mice in β-galactosidase staining. In a higher magnification, arteries (arrow, blue) appear darkly stained, whereas the veins (arrowhead, red) are visible only when filled with blood. (C and F) In the BmxLacZ/Bmx mice, the staining of the arterial endothelium is weaker and Bmx is detected in a mosaiclike pattern (arrow). (D and G) Bmx/Bmx control mice were negative in β-galactosidase staining. Bar, 800 μm.
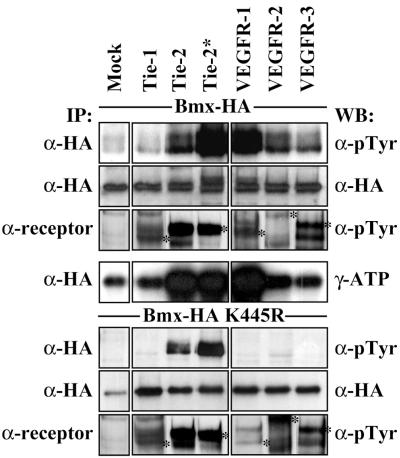
The Bmx kinase activity is regulated by Tie-2 and VEGFR-1 TK receptors.
Bmx expression specifically in arterial endothelial cells suggested that this TK could receive signals from endothelium-specific growth factor receptors. In order to investigate the ability of endothelial cell-specific receptor TKs to phosphorylate and activate Bmx, wt Bmx or “kinase-dead” (kd) Bmx was coexpressed with Tie-1, Tie-2, VEGFR-1, VEGFR-2, or VEGFR-3 in 293T cells. As can be seen from Fig. 9, Tie-2 coexpression increased the tyrosyl phosphorylation of both wt and catalytically inactive kd Bmx while VEGFR-1 increased the phosphorylation of only wt Bmx. This phosphorylation was associated with an increase in wt Bmx kinase activity as measured by the autophosphorylation assay. The other receptors did not affect Bmx phosphorylation. The activated mutant Tie-2*, containing an arginine-to-tryptophan mutation (R849W) in the kinase domain, caused an even more potent phosphorylation of Bmx than did wt Tie-2. Interestingly, this mutation has been linked to venous malformations in humans (37).
FIG. 9.
Bmx is activated by Tie-2 and VEGFR-1. wt Bmx (top four panels) or kd Bmx (bottom three panels) was coexpressed with the indicated endothelial cell-specific growth factor receptors in 293T cells. The cell lysates were immunoprecipitated with either anti-HA, precipitating the Bmx protein, or specific receptor Abs, followed by immunodetection with antiphosphotyrosine (α-pTyr) Ab or anti-HA Ab. Tyrosine-phosphorylated and activated receptors are indicated with asterisks. The immunoprecipitated Bmx was also subjected to in vitro kinase reactions as described in Materials and Methods. Note the slower-migrating (phosphorylated) isoform of Bmx in the anti-HA blot from cells expressing wt Bmx together with Tie-2* or VEGFR-1. IP, immunoprecipitation; WB, Western blotting.
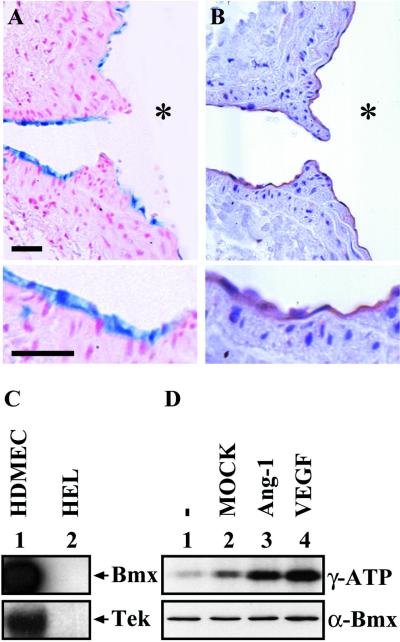
Coexpression of Bmx and Tie-2 in endothelial cells.
The relevance of the observed Bmx regulation by Tie-2 depends on whether these two TKs are expressed in the same cells in vivo. We therefore compared the expression patterns of Bmx and Tie-2 in tissue sections. Significant overlaps were detected. For example, in Fig. 10A, the BmxLacZ staining is shown in a branch of the aorta and the corresponding Bmx-expressing endothelial cells are stained by Abs against Tie-2 in Fig. 10B. We then tested cultured HDME cells for Bmx and Tie-2 mRNA expression. As expected, both mRNAs were expressed at high levels, while neither mRNA was expressed in the HEL leukemia cell line (Fig. 10C). In order to test whether the endogenous Bmx is also activated by the Tie-2 and VEGF receptors, we stimulated the HDME cells with Ang-1-conditioned medium produced by transfected 293T cells (Fig. 10D, lane 3) or with VEGF (lane 4). A clear increase of Bmx kinase activity was detected following both stimulations over that in the unstimulated cells (lane 1) or cells stimulated with 293T mock medium (lane 2), indicating that these receptors activate endogenous Bmx in cultured endothelial cells as well.
FIG. 10.
BmxLacZ and Tie-2 are coexpressed in endothelial cells. (A) β-Galactosidase staining of a section from a branch of the aorta. Note the endothelial staining, shown below in higher magnification. Bars, 40 μm. (B) Immunohistochemical staining of Tie-2 in a similar section. The asterisks in panels A and B indicate the aortic lumen. (C) Northern blotting of RNA isolated from HDME cells (HDMEC) (lane 1) and HEL cells (lane 2). The filter was probed for Bmx (upper panel), followed by reprobing for Tie-2 (lower panel). (D) Serum- and growth factor-starved HDME cells were stimulated for 20 min with the indicated factors. Immunoprecipitated Bmx was subjected to in vitro kinase assay, and the activity was detected as autophosphorylation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, as has been described previously. The lower panel shows control Western blotting using anti-Bmx Abs and 15 μg of total protein per lane.
DISCUSSION
The Bmx TK appears to be the first intracellular TK which is relatively specific for the arterial endothelium in solid tissues. It is also, to our knowledge, the first intracellular TK shown to be regulated by both the Tie-2 and VEGFR-1 receptors. In the present study, we show that the Bmx gene is expressed in endothelial cells of certain blood vessels, in the atria of the heart, and in thymic and lingual epithelial cells. In the venous endothelium, only a weak Bmx signal was seen in the superior and inferior venae cavae near the heart.
Bmx expression was strong at sites where the blood pressure and blood flow are regulated in the more peripheral vessels and more centrally, where atherosclerosis occurs, such as in the coronary arteries, aorta, and carotid and cerebral arteries. According to these data, the Bmx promoter could also be a potential tool for studying the function of the arterial endothelium in transgenic models. By using the Bmx promoter, it could be possible to express a gene of interest specifically in arterial endothelial cells.
Previously, ephrin B2, a transmembrane ligand, has been shown to be expressed in the arterial endothelium, but not in the venous endothelium, in the earliest stages of vasculogenesis. Ephrin B2-knockout mice die in utero before E11.5 because of defective angiogenesis (1, 39). Recently, another membrane-bound signal transduction molecule, Dll4, a member of the Delta family of Notch ligands, was found to be expressed specifically in the arterial endothelium (33), and indeed Notch signaling was shown to be required for vascular remodeling in mice (20).
During mouse development, primitive blood vessels begin to form at E8.0, and remodeling of capillaries into arteries is evident in the yolk sac between E9.0 and E9.5 (39). Unlike ephrin B2 or Dll4, Bmx was activated between E10.5 and 12.5, which suggests that Bmx does not participate in the initial stages of vasculogenesis or arteriogenesis of mouse embryos. The role of Bmx in endothelial cells, as well as the mechanisms that activate the Bmx promoter in arteries but not in veins, is unknown. However, our data show that the Bmx TK activity is increased by VEGFR-1 and Tie-2 in endothelial cells and suggest a role for Bmx in the endothelial signal transduction. Although embryos homozygous for a Tie-2-null allele die at E9.5 (3, 30) and those lacking VEGFR-1 die at E8.5 (6), our present study shows that the Bmx-null mice are viable and without an obvious phenotype, at least in the present DBA/2 background. This suggests that Bmx signaling is a redundant pathway downstream of the Tie-2 receptor. In the case of VEGFR-1, such deductions are less obvious as the TK domain of this receptor also can be deleted without an obvious resulting phenotype (10). The Bmx protein has previously been shown to regulate granulocytic differentiation of cultured myeloid progenitor cells (4). However, in differential counting of peripheral blood leukocytes, no significant differences could be detected between wt and knockout mice. These results further point to the redundancy of the functions of the Bmx TK.
Interestingly, both the Tie-2 and the Bmx TKs are involved in endothelial cell adhesion and migration and the activation of the Stat transcription factors (16, 19, 29, 34; our unpublished data). Thus, it is possible that Bmx provides the Tie-2 receptor a signal amplifier and a link to the cytoskeleton and cell adhesion, which allows the Tie-2 receptor, which is constitutively expressed in the various endothelia, to induce a tightly adherent endothelial cell phenotype in arteriae, where high blood flow and shear stress forces operate. In this way, Bmx function could be involved in cellular responses to the stress caused by blood flow and pressure or perhaps in interactions of endothelial cells with the muscular walls of the arteries.
We have previously shown that Bmx is regulated via phosphatidylinositol 3-kinase (4). This provides a possible route for activation of Bmx by Tie-2 receptor, as it also has been shown previously that p85 becomes highly phosphorylated when coexpressed with activated Tie-2 and following Ang-1 stimulation (11, 17). However, other possibilities also exist. For example, the activation signal could be mediated by some of the docking proteins binding to the Tie-2 receptors (see reference 26). We are currently investigating these proteins in order to exactly determine the route of Bmx activation.
ACKNOWLEDGMENTS
I. Rajantie and N. Ekman contributed equally to this work.
We are grateful to Masabumi Shibuya and Lena Claesson-Welsh for kindly providing the Abs specified in Materials and Methods. We also thank the laboratory technicians Tapio Tainola, Pipsa Ylikantola, Eija Koivunen, and Riikka Kivirikko for excellent technical assistance.
This work was supported by the Finnish Cancer Organizations, the Finnish Academy, the Sigrid Juselius Foundation, the University of Helsinki, and the European Union BioTechnology program (BIO4-CT98-0142). In addition, N.E. was supported by grants from the Helsinki University Biomedical Graduate School, from the Research and Science Foundation of Farmos, and from the Finnish Foundation for Cardiovascular Research.
REFERENCES
- 1.Adams R H, Wilkinson G A, Weiss C, Diella F, Gale N W, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P, Kieckens L, Schoonjans L, Ream B, van Nuffelen A, Prendergast G, Cole M, Bronson R, Collen D, Mulligan R C. Plasminogen activator inhibitor-1 gene-deficient mice. I. Generation by homologous recombination and characterization. J Clin Investig. 1993;92:2746–2755. doi: 10.1172/JCI116892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumont D J, Gradwohl G, Fong G H, Puri M C, Gertsenstein M, Auerbach A, Breitman M L. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 4.Ekman N, Arighi E, Rajantie I, Saharinen P, Ristimäki A, Silvennoinen O, Alitalo K. The Bmx tyrosine kinase is activated by IL-3 and G-CSF in a PI-3K dependent manner. Oncogene. 2000;19:4151–4158. doi: 10.1038/sj.onc.1203763. [DOI] [PubMed] [Google Scholar]
- 5.Ekman N, Lymboussaki A, Västrik I, Sarvas K, Kaipainen A, Alitalo K. The Bmx tyrosine kinase is specifically expressed in the endocardium and the endothelium of large arteries. Circulation. 1997;96:1729–1732. doi: 10.1161/01.cir.96.6.1729. [DOI] [PubMed] [Google Scholar]
- 6.Fong G H, Rossant J, Gertsenstein M, Breitman M L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 7.Freshney R I. Culture of animal cells. A manual of basic techniques. 2nd ed. New York, N.Y: Alan R. Liss; 1998. [Google Scholar]
- 8.Gale N W, Yancopoulos G D. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- 9.Giancotti F G, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 10.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont D J. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem. 1999;274:30896–30905. doi: 10.1074/jbc.274.43.30896. [DOI] [PubMed] [Google Scholar]
- 12.Jussila L, Valtola R, Partanen T A, Salven P, Heikkila P, Matikainen M T, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K. Lymphatic endothelium and Kaposi's sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 1998;58:1599–1604. [PubMed] [Google Scholar]
- 13.Kallioniemi O P, Kallioniemi A, Mascio L, Sudar D, Pinkel D, Deaven L, Gray J. Physical mapping of chromosome 17 cosmids by fluorescence in situ hybridization and digital image analysis. Genomics. 1994;20:125–128. doi: 10.1006/geno.1994.1138. [DOI] [PubMed] [Google Scholar]
- 14.Karkkainen M J, Petrova T V. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598–5605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- 15.Kaukonen J, Lahtinen I, Laine S, Alitalo K, Palotie A. Bmx tyrosine kinase gene is expressed in granulocytes and myeloid leukemias. Br J Haematol. 1996;94:455–460. [PubMed] [Google Scholar]
- 16.Kim I, Kim H G, Moon S O, Chae S W, So J N, Koh K N, Ahn B C, Koh G Y. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86:952–959. doi: 10.1161/01.res.86.9.952. [DOI] [PubMed] [Google Scholar]
- 17.Kontos C D, Stauffer T P, Yang W P, York J D, Huang L, Blanar M A, Meyer T, Peters K G. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol. 1998;18:4131–4140. doi: 10.1128/mcb.18.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korhonen J, Lahtinen I, Halmekyto M, Alhonen L, Janne J, Dumont D, Alitalo K. Endothelial-specific gene expression directed by the tie gene promoter in vivo. Blood. 1995;86:1828–1835. [PubMed] [Google Scholar]
- 19.Korpelainen E I, Karkkainen M, Gunji Y, Vikkula M, Alitalo K. Endothelial receptor tyrosine kinases activate the STAT signaling pathway: mutant Tie-2 causing venous malformations signals a distinct STAT activation response. Oncogene. 1999;18:1–8. doi: 10.1038/sj.onc.1202288. [DOI] [PubMed] [Google Scholar]
- 20.Krebs L T, Xue Y, Norton C R, Shutter J R, Maguire M, Sundberg J P, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith G H, Stark K L, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 21.Kukk E, Wartiovaara U, Gunji Y, Kaukonen J, Buhring H J, Rappold I, Matikainen M T, Vihko P, Partanen J, Palotie A, Alitalo K, Alitalo R. Analysis of Tie receptor tyrosine kinase in haemopoietic progenitor and leukaemia cells. Br J Haematol. 1997;98:195–203. doi: 10.1046/j.1365-2141.1997.1732989.x. [DOI] [PubMed] [Google Scholar]
- 22.Luscher T F, Tanner F C. Endothelial regulation of vascular tone and growth. Am J Hypertens. 1993;6:283S–293S. doi: 10.1093/ajh/6.7.283s. [DOI] [PubMed] [Google Scholar]
- 23.Lusis A J. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mano H. Tec family of protein-tyrosine kinases: an overview of their structure and function. Cytokine Growth Factor Rev. 1999;10:267–280. doi: 10.1016/s1359-6101(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 25.Partanen J, Armstrong E, Makela T P, Korhonen J, Sandberg M, Renkonen R, Knuutila S, Huebner K, Alitalo K. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992;12:1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partanen J, Dumont D J. Functions of Tie1 and Tie2 receptor tyrosine kinases in vascular development. Curr Top Microbiol Immunol. 1999;273:159–172. doi: 10.1007/978-3-642-59953-8_8. [DOI] [PubMed] [Google Scholar]
- 27.Pawson T, Nash P. Protein-protein interactions define specificity in signal transduction. Genes Dev. 2000;14:1027–1047. [PubMed] [Google Scholar]
- 28.Risau W. Differentiation of endothelium. FASEB J. 1995;9:926–933. [PubMed] [Google Scholar]
- 29.Saharinen P, Ekman N, Sarvas K, Parker P, Alitalo K, Silvennoinen O. The Bmx tyrosine kinase induces activation of the Stat signalling pathway, which is specifically inhibited by PKCδ. Blood. 1997;90:4341–4353. [PubMed] [Google Scholar]
- 30.Sato T N, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 31.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 32.Shalaby F, Rossant J, Yamaguchi T P, Gertsenstein M, Wu X F, Breitman M L, Schuh A C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 33.Shutter J R, Scully S, Fan W, Richards W G, Kitajewski J, Deblandre G A, Kintner C R, Stark K L. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 34.Takakura N, Huang X L, Naruse T, Hamaguchi I, Dumont D J, Yancopoulos G D, Suda T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–686. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- 35.Tamagnone L, Lahtinen I, Mustonen T, Virtaneva K, Francis F, Muscatelli F, Alitalo R, Smith C I, Larsson C, Alitalo K. BMX, a novel nonreceptor tyrosine kinase gene of the BTK/ITK/TEC/TXK family located in chromosome Xp22.2. Oncogene. 1994;9:3683–3688. [PubMed] [Google Scholar]
- 36.Thurston G, Murphy T J, Baluk P, Lindsey J R, McDonald D M. Angiogenesis in mice with chronic airway inflammation: strain-dependent differences. Am J Pathol. 1998;153:1099–1112. doi: 10.1016/S0002-9440(10)65654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vikkula M, Boon L M, Carraway K L, 3rd, Calvert J T, Diamonti A J, Goumnerov B, Pasyk K A, Marchuk D A, Warman M L, Cantley L C, Mulliken J B, Olsen B R. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87:1181–1190. doi: 10.1016/s0092-8674(00)81814-0. [DOI] [PubMed] [Google Scholar]
- 38.Vuori K. Integrin signaling: tyrosine phosphorylation events in focal adhesions. J Membr Biol. 1998;165:191–199. doi: 10.1007/s002329900433. [DOI] [PubMed] [Google Scholar]
- 39.Wang H U, Chen Z F, Anderson D J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 40.Weil D, Power M A, Smith S I, Li C H. Predominant expression of murine Bmx tyrosine kinase in the granulo-monocytic lineage. Blood. 1998;90:4332–4340. [PubMed] [Google Scholar]
- 41.Yang W C, Collette Y, Nunes Y, Olive D. Tec kinases: a family with multiple roles in immunity. Immunity. 2000;12:373–382. doi: 10.1016/s1074-7613(00)80189-2. [DOI] [PubMed] [Google Scholar]