Abstract
Background
Helicobacter pylori (H. pylori) is the most common chronic bacterial infection. Its management has to rely on local effectiveness, given the geographical variability of bacterial antibiotic resistance. We evaluated treatment effectiveness in naïve patients in Greece, as part of the European Registry on the management of H. pylori (Hp-EuReg).
Methods
Patients were registered in the AEG-REDCap Electronic Case Report Form from 2013-2020. All cases with a first-line treatment were included. Modified intention-to-treat (mITT) analysis was used.
Results
A total of 547 patients from 5 medical institutions were treated with the following regimens: concomitant with proton pump inhibitors (PPIs), clarithromycin, amoxicillin and metronidazole (concomitant-C+A+M) (38%); hybrid with PPI, clarithromycin, amoxicillin and metronidazole (hybrid-C+A+M) (20%); sequential with PPI, clarithromycin, amoxicillin and tinidazole (sequential-C+A+T) (12%); sequential with PPI, clarithromycin, amoxicillin and metronidazole (sequential-C+A+M) (12%); concomitant with PPI, clarithromycin, amoxicillin and tinidazole (concomitant-C+A+T) (8%); triple with PPI, clarithromycin and amoxicillin (triple-C+A) (7%); and other (3%). Overall compliance was 99%. Triple-C+A, sequential-C+A+T, sequential-C+A+M and concomitant-C+A+T were used from 2013-2015. The respective mITT cure rates (95% confidence interval) were 92% (78-98), 87% (76-94), 67% (54-78) and 91% (79-98). Since 2015, patients were also treated with concomitant-C+A+M and hybrid-C+A+M regimens, with respective mITT cure rates of 90% (85-94) and 88% (80.5-94). Adverse events were reported by 31% of the patients, dysgeusia being the most frequent (15%).
Conclusions
“Optimized” H. pylori therapies should achieve cure rates over 90%. In Greece, at present, only non-bismuth quadruple concomitant regimens achieve this target and can be recommended as first-line treatment.
Keywords: Helicobacter pylori, treatment, Greece, Hp-EuReg
Introduction
Helicobacter pylori (H. pylori) infection is of worldwide concern, as it has been recognized as the main cause of gastritis, peptic ulcer disease, gastric mucosa-associated lymphoid tissue lymphoma and gastric cancer. H. pylori gastritis was formally recognized as an infectious disease in 2015, leading to the recommendation that all patients should receive treatment; since then, the list of indications to test for the presence of the infection has been greatly expanded [1,2]. In 2020 the Taipei Global consensus emphasized the role of H. pylori eradication in accomplishing the goal of reducing or eliminating deaths from gastric cancer [3].
A number of different H. pylori therapies have been introduced, including triple therapies consisting of a proton pump inhibitor (PPI), amoxicillin and clarithromycin, metronidazole, a fluoroquinolone, or rifabutin; bismuth quadruple therapy; dual therapy (PPI and amoxicillin) and a group of 4-drug therapies consisting of a PPI, amoxicillin, clarithromycin and metronidazole, called sequential, concomitant, hybrid and reverse hybrid therapies, depending on the manner of administration. However, the management of H. pylori infection has to be tailored according to the local, geographical variability of bacterial antibiotic resistance.
A multinational and multicenter prospective ongoing registry of the clinical practice of European gastroenterologists concerning H. pylori infection (Hp-EuReg) has been developed, including patients from 2013 to date. Greece has been participating in the Hp-EuReg from the beginning. The aim of the present study was to analyze the data relating to first-line H. pylori eradication treatments in Greece during the period 2013-2020, as part of the Hp-EuReg.
Materials and methods
European Registry on H. pylori management
The “European Registry on H. pylori Management” (Hp-EuReg) is an international multicenter prospective non-interventional registry, recording information about H. pylori infection management (30 countries) since May 2013. It was prospectively registered in ClinicalTrials.gov (NCT02328131) and was promoted by the European Helicobacter and Microbiota Study Group (www.helicobacter.org) [4]. The current study is a sub-analysis focusing on a cohort of Greek H. pylori positive adult patients from centers actively participating in the Hp-EuReg.
National coordinators
Thirty European countries were selected for this registry, with national coordinators for each country. For Greece, the National Coordinator was author TR, who was responsible for supervising data inclusion, monitoring and the drafting of this study.
Recruiting investigators
The recruiting investigators were gastroenterologists attending an adult population with a gastroenterology outpatient clinic treating patients infected with H. pylori. Patients were managed and registered following routine clinical practice. Greek recruiting investigators were authors TR, SG, SM, VN and CL.
Data extraction and analysis
Data were recorded in an Electronic Case Report Form (e-CRF) using a web-based application, REDCap (Research Electronic Data Capture), a platform managed and hosted by the “Asociación Española de Gastroenterología” (AEG; www.aegastro.es), a non-profit Scientific and Medical Society that focuses on gastroenterology research.
The patient’s demographics, the previous eradication attempts, the treatments employed, and the outcomes: cure rates, compliance, adverse events and the follow up were recorded. All patient data were anonymized. The main outcome was confirmed eradication at least 4 weeks after treatment. Data were collected up to February 2021.
Effectiveness analysis
For the purposes of this study, a modified intention to treat (mITT) analysis was used to achieve the closest result to those obtained in clinical practice. The mITT includes all cases that had completed the follow up (i.e., had undertaken a valid confirmatory test after the eradication treatment), regardless of compliance with treatment, and excluding those cases with an incomplete follow up (lost to follow up). All patients who were empirically treated (i.e., not susceptibility-guided) were included in the effectiveness analysis. Patient compliance with treatment was defined as more than 90% drug intake.
Statistical analysis
Continuous variables were presented as arithmetic means and standard deviations. Qualitative variables were presented as proportions and 95% confidence intervals (CIs). The statistical analyses of the results were carried out using the χ2-Pearson test. One- and 2-sided tests were used for the analyses and the P-value cutoff for significance was set to less than 0.05. The analyses were carried out using IBM SPSS Statistics 22.0.0.
Results
Patient characteristics and regimens used
Overall, the Greek medical institutions reported data on 653 H. pylori-positive patients who had been treated for H. pylori infection; of these, 547 had received a first-line eradication treatment. The main characteristics of the cohort are shown in Table 1. Patients treated with first-line eradication treatment received the following regimens: concomitant with a PPI, clarithromycin, amoxicillin and metronidazole (concomitant-C+A+M) was received by 208 patients (38%); hybrid PPI, clarithromycin, amoxicillin and metronidazole (hybrid-C+A+M) was received by 109 patients (20%); sequential PPI, clarithromycin, amoxicillin and tinidazole (sequential-C+A+T) was received by 66 patients (12%); sequential PPI, clarithromycin, amoxicillin and metronidazole (sequential-C+A+M) was received by 66 patients (12%); concomitant PPI, clarithromycin, amoxicillin and tinidazole (concomitant-C+A+T) was received by 44 patients (8%); triple PPI, clarithromycin, amoxicillin (triple-C+A) was received by 208 patients (7%); and other treatment was received by 16 patients (3%) (Fig. 1). Triple-C+A, sequential-C+A+T, sequential-C+A+M and concomitant-C+A+T were mainly used in the period from 2013-2015. Since 2016, patients were treated with concomitant-C+A+M and hybrid-C+A+M regimens. Concerning the H. pylori diagnostic test, in 92% of the patients an invasive test was used, as opposed to the 8% who underwent noninvasive testing (Table 1).
Table 1.
Baseline characteristics of the patients in the Greek cohort of Hp-EuReg
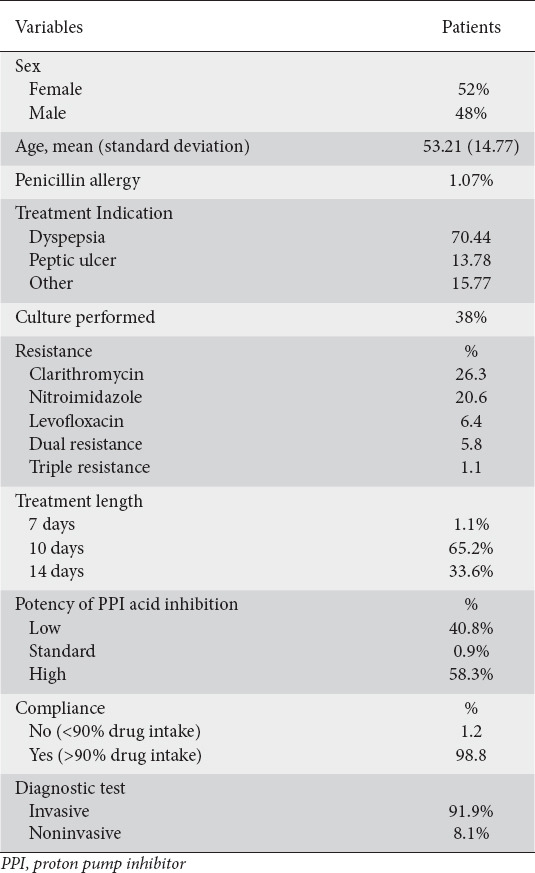
Figure 1.
Percentages of regimens used as empirical first-line treatment in Greece
C, clarithromycin; A, amoxicillin; M, metronidazole; T, tinidazole
First-line empiric treatment cure rates
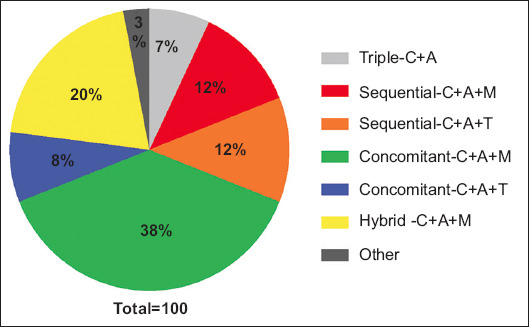
The mITT cure rates for triple-C+A, sequential-C+A+T, sequential-C+A+M, concomitant-C+A+T, concomitant-C+A+M and hybrid-C+A+M regimens were 92% (95%CI 78-98), 87% (95%CI 76-94), 67% (95%CI 54-78), 91% (95%CI 79-98), 90% (95%CI 85-94), and 88% (95%CI 80.5-94), respectively (Fig. 2). Three regimens, i.e., triple-C+A, concomitant-C+A+T and concomitant-C+A+M achieved cure rates of more than the acceptable level of 90%. The remaining 3 regimens, i.e., sequential-C+A+T, sequential-C+A+M and hybrid-C+A+M failed to reach this level of acceptability. The sequential-C+A+M regimen was the least efficacious, and the comparisons between this and the other reported regimens yielded statistically significant results, i.e., P=0.003 vs. triple-C+A, P=0.02 vs. sequential-C+A+T, P=0.003 vs. concomitant-C+A+T, P<0.001 vs. concomitant-C+A+M and P=0.006 vs. hybrid-C+A+M. Accordingly, the best H. pylori therapy in Greece was the concomitant-C+A+imidazole (either metronidazole or tinidazole) regimen.
Figure 2.
Effectiveness of the regimens used in the Greek cohort. Modified intention to treat results are expressed as percentages with 95% confidence intervals (CI)
C, clarithromycin; A, amoxicillin; M, metronidazole; T, tinidazole
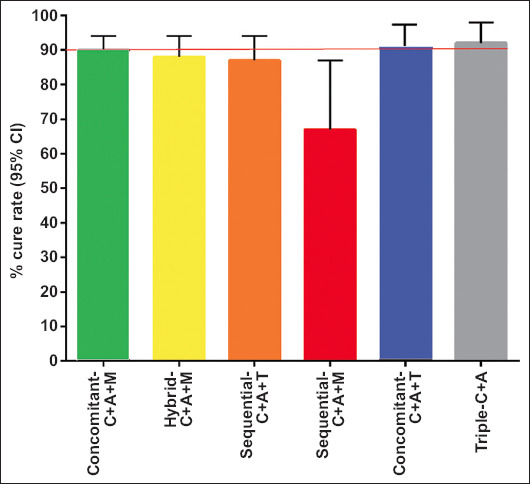
The treatment prescriptions by treatment length, i.e., 7, 10 and 14 days, as well as the potency of PPI acid inhibition, i.e., low, standard and high, of the regimens used in this Greek cohort of the Hp-EuReg, are shown in Table 2. Thus, the percentages of treatment prescriptions for 7, 10 and 14 days were 1.1%, 65.2% and 33.65%, respectively. Concerning the potency of PPI acid inhibition, the relevant percentages were 40.8% for low, 0.9% for standard and 58.3% for high potency. The trend in the prescription of treatments (2013-2020) is shown in Fig. 3. There was a significant trend (P<0.001) towards an increase in the use of non-bismuth quadruple regimens, administered as concomitant-C+A+M and hybrid therapy.
Table 2.
Treatment length and potency of PPI acid inhibition of the regimens used in the Greek cohort of the Hp-EuReg
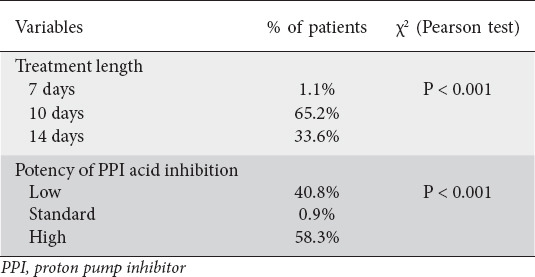
Figure 3.
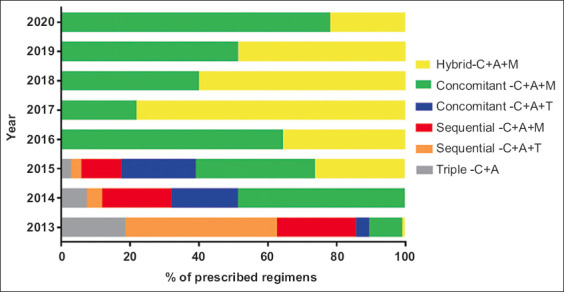
Treatment prescription trends (2013-2020) in Greece
C, clarithromycin; A, amoxicillin; M, metronidazole; T, tinidazole
Compliance and adverse events
The compliance of treated patients, was reported as 99% (95%CI 97.5-99.4). Concerning adverse events, they were reported in 31% of the naïve patients, dysgeusia being the most frequent (15%), followed by diarrhea (12%), nausea (6%), abdominal pain (3%), dyspepsia (3%), anorexia (2%), and vomiting (1.3%). No serious adverse events were reported.
Discussion
The Hp-EuReg was started in 2013. The idea was to collect treatment results, side effects, patients’ compliance and antimicrobial susceptibility from different European countries. Thirty countries and more than 200 recruiting investigators are actively participating in the Hp-EuReg [5].
In this study, data concerning the first-line eradication treatments for the Greek cohort were analyzed and showed that 3 regimens, i.e., triple-C+A, concomitant-C+A+T and concomitant-C+A+M, performed well in that they achieved cure rates of more than the acceptable level of 90%. Furthermore, the evolution of the prescriptions of first-line treatments over the examined period 2013-2020, showed a significant shift towards a higher use of non-bismuth quadruple regimens, mainly concomitant-C+A+M and hybrid regimens. Bismuth is not available in Greece, so regimens including this drug were not used. Triple therapy was prescribed only in the first 3 years (2013-2016), and a similar trend was found for sequential regimens. Since then, the empiric use of these regimens was abandoned and they were replaced by quadruple regimens, i.e., non-bismuth concomitant and hybrid. The reason for this shift could be attributed primarily to the fact that antibiotic resistance in Greece is increasing, which is particularly true for clarithromycin [6,7]. In 2017, the Maastricht V consensus report was published [8], which stated that when clarithromycin resistance rate exceeds 15%, triple therapy should be abandoned. Therefore, it would be possible that, as the aforementioned clinical guidelines became widely adopted in Greece, as expressed by the consensus report of the Hellenic H. pylori and Microbiota Study Group [9], Greek doctors became aware of the fact that when clarithromycin resistance increases, the cure rate drops to unacceptable levels [10-11]. It should be mentioned that 26% of the patients included in the present study had clarithromycin resistance, which is clearly higher than the 15% standard threshold [8]. However, there is a possibility that the cure rate achieved by triple therapy at the beginning of the Greek participation in the Hp-EuReg, might be the result of antibiotic susceptibility testing (either by culture or molecular based test), although these data are not available in this cohort. It is accepted that triple therapy is highly effective after susceptibility testing [10]. This is in agreement with the notion of antibiotic stewardship [11-14], which maintains that treatments, instead of being empirical, should be based on susceptibility testing. However, in different populations, apart from antibiotic resistance, a number of other factors should be taken into account when trying to interpret H. pylori cure rates, such as the relative effects of different degrees of acid suppression in relation to potency and dosage, as these markedly affect the effectiveness of the regimens [5].
Despite the interesting findings, this study has some limitations. The main limitation is the relatively small number of recruiting centers. This is expected to improve with the recruitment of more centers from various parts of Greece. An additional limitation is the fact that not all recruited patients underwent culture testing. It is obvious that the promotion and creation of large databases to record local antibiotic resistance information could improve effectiveness. Nonetheless, the current study was performed to evaluate the routine empirical practice of gastroenterologists in Greece concerning first-line treatment. Therefore, the present study provides a comprehensive overview of current local empirical first-line H. pylori treatment in Greece; consequently, the conclusions drawn are easily applicable in routine clinical practice, i.e., the use of non-bismuth quadruple concomitant regimens (with a PPI, clarithromycin, amoxicillin and a nitroimidazole) can achieve over 90% cure rates and therefore can be recommended as empirical first-line treatment in Greece.
Nonetheless, H. pylori treatment continues to be a challenge, as bacterial antibiotic resistance continues to emerge and impair clinicians’ ability to cure H. pylori infection. Recently, a consensus report recommended that clarithromycin, metronidazole, and levofloxacin should not be used empirically unless proven to be reliably effective locally [2]. Under these circumstances, a new approach concerning H. pylori treatment is needed, and towards this end there are views supporting the notion that it is now time to transition from trial and error to antimicrobial stewardship, as in other infectious diseases [15]. This approach requires therapy to be restricted to optimized therapies that have been proven to be reliably highly effective in the local population (e.g., cure rate of more than 90%), and therefore comparisons should be restricted to regimens that meet these criteria.
In conclusion, in recent years in Greece, only non-bismuth quadruple concomitant regimens (with a PPI, clarithromycin, amoxicillin and a nitroimidazole) can achieve over 90% cure rates and therefore can be recommended as empirical first-line treatment.
Summary Box.
What is already known:
• A multinational and multicenter prospective ongoing registry of the clinical practice of European gastroenterologists concerning Helicobacter pylori (H. pylori) infection (Hp-EuReg) has been developed, including patients from 2013 to date
• Greece has been participating in the Hp-EuReg from the beginning
What the new findings are:
• In this study we analyzed the data of first-line H. pylori eradication treatments in Greece during the 2013-2020 period, as part of the Hp-EuReg
• “Optimized” H. pylori therapies should achieve over 90% cure rates
• In Greece, at present, only non-bismuth quadruple concomitant regimens achieve this target and can be recommended as first-line treatment
Biography
Henry Dunant Hospital, Athens, Greece; Athens Medical Paleo Faliron Hospital, Athens, Greece; Alexandra Hospital, Athens, Greece; Tzaneio General Hospital of Piraeus, Greece; 401 Military Hospital, Athens, Greece; Althaia Xarxa Assistencial Universitària de Manresa and Universitat de Vic-Universitat Central de Catalunya (UVicUCC), Manresa, Spain; Hospital Universitario de La Princesa, Institute de Investigación Sanitaria Princesa (IIS-IP), Universidad Autónoma de Madrid (UAM), and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Madrid, Spain; Laboratoire de Bactériologie, Hôpital Pellegrin, Bordeaux, France; Trinity College Dublin, Dublin, Ireland
Footnotes
Conflicts of interest: Dr. Megraud received research support from Aptalis Pharma, bioMerieux and Mobidiag and is a consultant for Phathom Pharmaceuticals. Dr. Gisbert has served as speaker, consultant, and advisory member for, or has received research funding from Mayoly, Allergan, Diasorin, Phathom and Gebro Pharma. Dr Nyssen has received research funding from Mayoly and Allergan
References
- 1.Sugano K, Tack J, Kuipers EJ, et al. faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Kao JY, Kanwal F, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16:992–1002. doi: 10.1016/j.cgh.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liou JM, Malfertheiner P, Lee YC, et al. Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM) Screening and eradication of Helicobacter pylori for gastric cancer prevention:the Taipei global consensus. Gut. 2020;69:2093–2112. doi: 10.1136/gutjnl-2020-322368. [DOI] [PubMed] [Google Scholar]
- 4.McNicholl AG, O'Morain CA, Megraud F, Gisbert JP As Scientific Committee of the Hp-Eureg on Behalf of the National Coordinators. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg) Helicobacter. 2019;24:e12630. doi: 10.1111/hel.12630. [DOI] [PubMed] [Google Scholar]
- 5.Nyssen OP, Bordin D, Tepes B, et al. Hp-EuReg Investigators. European Registry on Helicobacter pylori management (Hp-EuReg):patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21,533 patients. Gut. 2021;70:40–54. doi: 10.1136/gutjnl-2020-321372. [DOI] [PubMed] [Google Scholar]
- 6.Georgopoulos SD, Papastergiou V, Martinez-Gonzalez B, et al. Hybrid therapy as first-line regimen for Helicobacter pylori eradication in a high clarithromycin resistance area:a prospective open-label trial. Ann Gastroenterol. 2018;31:205–210. doi: 10.20524/aog.2017.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Megraud F, Coenen S, Versporten A, et al. Study Group participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 9.Georgopoulos SD, Michopoulos S, Rokkas T, et al. Hellenic consensus on Helicobacter pylori infection. Ann Gastroenterol. 2020;33:105–124. doi: 10.20524/aog.2020.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Long X, Ji Y, et al. Randomised controlled trial:susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther. 2019;49:1385–1394. doi: 10.1111/apt.15273. [DOI] [PubMed] [Google Scholar]
- 11.Graham DY. Helicobacter pylori eradication therapy research:ethical issues and description of results. Clin Gastroenterol Hepatol. 2010;8:1032–1036. doi: 10.1016/j.cgh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–278. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 13.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy:evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177–186.e3. doi: 10.1016/j.cgh.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rokkas T, Gisbert JP, Malfertheiner P, et al. Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection:a network meta-analysis. Gastroenterology. 2021;161:495–507.e4. doi: 10.1053/j.gastro.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Graham DY. Transitioning of Helicobacter pylori therapy from trial and error to antimicrobial stewardship. Antibiotics (Basel) 2020;9:671. doi: 10.3390/antibiotics9100671. [DOI] [PMC free article] [PubMed] [Google Scholar]


