Abstract
SARS-CoV-2 vaccination is the most powerful and promising tool against the COVID-19 pandemic. Millions of people have been vaccinated worldwide. Recently, few cases of subacute thyroiditis following SARS-CoV-2 vaccination with various types of vaccine have been reported. We describe here a 36-year-old woman who presented with subacute thyroiditis 10 days after she had received her first dose of the SARS-CoV-2 mRNA vaccine Comirnaty (Pfizer/BioNTech); the condition receded but then recurred 10 days after she received her second dose. As vaccination programmes proceed, clinicians' attention and vigilance for such cases will be increased. Physicians need to know that subacute thyroiditis is a mild and self-limiting condition in the majority of cases. Last but not least, the benefits of vaccination against COVID-19 outweigh the side-effects reported so far.
Keywords: COVID-19, SARS-CoV-2, Vaccination, Thyroiditis, mRNA vaccine
Highlights
-
•
A 36-year-old woman presented subacute thyroiditis after the SARS-CoV-2 mRNA vaccine.
-
•
It receded but recurred after the 2nd dose.
-
•
It is usually a mild and self-limiting condition .
-
•
Benefits of vaccination against COVID-19 outweigh side effects.
1. Introduction
Subacute thyroiditis (SAT) is a self-limiting inflammatory thyroid disorder. The presenting symptoms usually appear 2–8 weeks after an acute symptomatic or asymptomatic viral infection. Clinical presentation may include general signs of infection, fatigue, front-neck pain and thyroid dysfunction, usually with transient thyrotoxicosis [1].
Many types of virus have been suggested as possible causative agents for SAT, [2] including SARS-CoV-2 [[3], [4], [5], [6]]. In addition, SAT has been described following influenza or hepatitis B vaccination [[7], [8], [9], [10], [11], [12]]. Recently, cases have been reported of SAT following SARS-CoV-2 vaccination with CoronaVac (Sinovac Life Sciences, China) [[13], [14], [15]], Vaxzevria (AstraZeneca, Sweden) [[16], [17], [18], [19], [20]], Spikevax (Moderna Biotech, Spain) [20], Covaxin (Bharat Biotech, India) [21], and Pfizer-BioNTech SARS-CoV-2 mRNA vaccine (BioNTech, Fosun Pharma, Pfizer, Germany and USA) [[22], [23], [24], [25], [26], [27], [28]].
We report here the case of a 36-year-old woman who presented with SAT 10 days after she had received her first dose of the SARS-CoV-2 mRNA vaccine COMIRNATY (Pfizer/BioNTech); the condition receded but then recurred 10 days after the patient had received her second dose.
2. Case presentation
A 36-year-old Caucasian woman was admitted to the outpatient clinic complaining for anterior neck pain that radiated to the ear and jaw, mostly on the left side. She also had fever of up to 37.8 °C, fatigue and palpitations. Her symptoms first appeared 10 days after she had received her first dose of the SARS-CoV-2 mRNA vaccine COMIRNATY (Pfizer/BioNTech) but the condition remitted a few days later with no medication. Therefore, the patient did not seek medical attention and proceeded with her second dose of the same vaccine. Ten days after that second dose, the symptoms recurred, with greater intensity of the neck pain and fatigue.
She had no medical history of thyroid disease, nor did she report any viral infection, including SARS-CoV-2 infection, during the previous 3 months. She had had recent diagnoses of ulcerative gastritis and intraocular hypertension. She was being treated for endometriosis with progesterone on a daily basis. Otherwise, her personal and family history were not remarkable. She did not report any adverse events after vaccination in the past. Her clinical examination revealed tenderness in the thyroid region and a mild tremor. Her blood pressure was 115/75 mmHg and heart rate 100 beats/min; her body temperature was normal. The patient weighed 56.5 kg and her height was 1.61 m and she reported no weight loss over the preceding months.
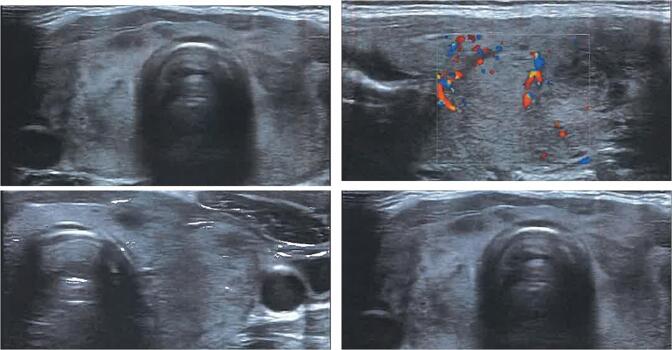
Her laboratory tests 26 days after 2nd dose of vaccine revealed low TSH concentrations, with slightly elevated FT4 and normal T3 levels. ESR and CRP levels were increased, while blood count was normal. Anti-thyroid peroxidase (anti-TPO) and thyroid stimulating hormone receptor antibodies (TRAb or TSI) were negative. There was an elevation of anti-thyroglobulin (anti-TG) antibodies levels (Table 1). Ultrasound of the neck showed slightly elevated dimensions of the thyroid gland with heterogeneous echogenicity and bilateral hypoechoic areas (Fig. 1). Cervical lymph nodes were not obvious. The Tc99 pertechnetate radionuclide thyroid scan showed poor thyroid uptake (Fig. 2). A diagnosis of subacute thyroiditis was made.
Table 1.
Laboratory blood tests.
| TSH | T4 | FT4 | T3 | CRP | ESR | anti-TPO | anti-Tg | TRAb (TSI) | |
|---|---|---|---|---|---|---|---|---|---|
| 26 days after 2nd dose | 0.225 mUI/ml | 149 nmol/l | 22.01 pmol/l | 2.29 nmol/l | 1.96 mg/dl | 59 | 15.72 | 292 | 0.1 |
| 40 days after 2nd dose | 0.028 mUI/ml | 185 nmol/l | 31.67 pmol/l | 2.66 nmol/l | 6.46 mg/dl | – | – | – | – |
| 54 days after 2nd dose | 0.173 mIU/ml | – | 16.42 pmol/l | 0.773 nmol/l | – | – | – | – | – |
| Normal range | 0.4–4 mIU/ml | 58–161 nmol/l | 12–22 nmol/l | 0.84–2.6 nmol/l | < 0.5 mg/dl | 0–20 mm/h | < 34 IU/ml | < 40 IU/ml | < 1.75 U/L |
Fig. 1.
Thyroid ultrasound.
Fig. 2.
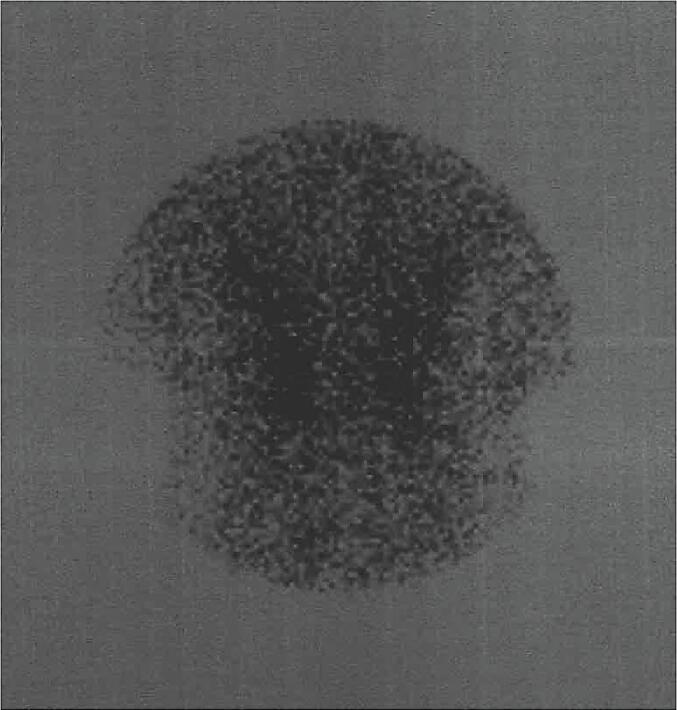
Radionuclide thyroid scan.
Due to the mildness of symptoms, the patient commenced treatment with paracetamol and ibuprofen. Ten days later, she appeared at the endocrine department complaining of worsening of her neck pain, fever, myalgia and fatigue. Her symptoms were no longer relieved by treatment with NSAIDs. She also reported 1 kg weight loss and cough. TSH was suppressed, FT4 and T3 levels were clearly elevated, and CRP was still high. Her thyroid was enlarged and was tender on palpitation. Treatment with methylprednisolone (16 mg twice a day) was initiated.
The pain and tenderness resolved completely within 48 h and 2 weeks later TSH, FT4 and T3 levels were within normal limits (Table 1). The patient was followed-up on tapering treatment with methylprednisolone. Follow-up was performed by clinical examination and measurement of thyroid hormones.
3. Discussion
SAT is a self-limiting thyroid disorder, commonly associated with upper respiratory viral infection [1]. Several viruses have been considered as causes of SAT, including adenovirus, enterovirus, influenza virus, cytomegalovirus, rubella virus, Epstein Barr virus, Coxsackie virus, and measles virus [2]. SAT may be rarely associated with SARS-CoV-2 infection, as a few cases have been reported recently in the literature [[3], [4], [5], [6]]. Moreover, several cases of SAT after viral vaccines have been reported. Most of them are related to influenza, H1N1 or HBV vaccines [[7], [8], [9], [10], [11], [12]].
Recently, following the massive vaccination programme worldwide, there have been a few reports of thyroid dysfunction after SARS-CoV-2 vaccination, including SAT [[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]] and Graves' disease [19,[29], [30], [31]]. All types of vaccines have been involved, namely inactivated virus SARS-CoV-2 vaccines CORONAVAC [[13], [14], [15]] and COVAXIN [21], viral vector-based vaccines VAXZEVRIA [[16], [17], [18], [19], [20],31] and Janssen [19] and mRNA-based vaccines COMIRNATY [[22], [23], [24], [25], [26], [27], [28]] and MODERNA [20].
In this case, the course of disease did not differ from the usual clinical expression of SAT nor did response to treatment. The patient's age and gender were typical: SAT connected to SARS-CoV-2 vaccination concerns predominantly young and middle-aged women (18 out of 21 cases) (ged 26 to 55 years) while males and one female were older (67 to 75 years old). Reports concerning inactivated virus and mRNA-based SARS-CoV-2 vaccines refer to the appearance of symptoms 4–12 days after the first or second dose of vaccine. SAT has been reported 2 weeks after the first dose of the viral vector-based vaccine VAXZEVRIA. In all cases, rapid improvement of clinical symptoms and laboratory findings was achieved either by administration of NSAIDS in most cases or by short-duration corticosteroid treatment. Follow-up in all cases was consistent with mild and uncomplicated course of disease.
Various hypotheses have been raised regarding thyroid autoimmunity after vaccination, such as autoimmune inflammatory syndrome induced by adjuvants (ASIA) or molecular mimicry. Adjuvants are an essential component of vaccines, used mainly to increase the response to vaccination, and may play a role in producing diverse autoimmune and inflammatory responses. Post-vaccination phenomena have been described, including autoimmune endocrine diseases, mostly after receipt of an HPV, influenza or hepatitis B vaccine. The clinical spectrum of reported thyroid disease includes both Hashimoto's thyroiditis and Graves' disease [32]. Regarding molecular mimicry, cross-reactivity between the coronavirus spike protein produced after vaccination and thyroid cell antigens is suggested as underlying mechanism [33].
The fact that SAT may arise a few days after SARS-Cov-2 vaccination requires medical attention for a possible causal relation. Interestingly, in our case, SAT recurrence and deterioration were observed after the second dose of the mRNA vaccine. This manifestation is consistent with the rechallenge criterion for causality assessment according to the WHO-UMC system [34], strongly suggesting a possible cause-and-effect relationship.
SAT after COVID-19 vaccination is a very rare condition taking into consideration the vaccination of millions of people worldwide. On the other hand, because of its mild clinical course, also it may be under-reported as an adverse event. As vaccination programmes proceed, clinicians' attention will be probably increased. However, physicians need to know that SAT is a self-limiting and mild disease with only transient thyroid dysregulation in the vast majority of cases. Last but not least, the benefits of vaccination against COVID-19 outweigh any mild and rare side-effects reported so far.
Acknowledgments
Contributors
Vasiliki Vasileiou wrote the initial draft.
Stavroula A. Paschou wrote the initial draft.
Xakousti Tzamali wrote the initial draft.
Marina Mitropoulou revised the manuscript.
Fotini Kanouta revised the manuscript.
Theodora Psaltopoulou revised the manuscript.
Georgia N. Kassi revised the manuscript.
All authors took care of the patient. All authors approved the final version of the article.
Funding
The authors received no funding from an external source for the publication of this case report.
Patient consent
Obtained.
Provenance and peer review
This article was not commissioned. Peer review was directed by Professor Margaret Rees independently of Stavroula A. Paschou, one of the authors and a member of the Case Reports in Women's Health editorial board, who was blinded to the process.
Acknowledgments
Conflict of interest statement
The authors declare that they have no conflict of interest regarding the publication of this case report.
References
- 1.Tabassom A., Chippa V., Edens M.A. StatPearls Publishing; Treasure Island (FL): 2021. StatPearls [Internet] [Google Scholar]
- 2.Desailloud R., Hober D. Viruses and thyroiditis: an update. Virol. J. 2009;6:5. doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dworakowska D., Morley S., Mulholland N., Grossman A.B. COVID-19-related thyroiditis: a novel disease entity? 2021 Sep;95(3):369–377. doi: 10.1111/cen.14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brancatella A., Ricci D., Cappellani D., et al. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights From a Case Series. J. Clin. Endocrinol. Metab. 2020 Aug 11 doi: 10.1210/clinem/dgaa537. dgaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller I., Cannavaro D., Dazzi D., Covelli D., Mantovani G., Muscatello A., Ferrante E., Orsi E., Resi V., Longari V., Cuzzocrea M., Bandera A., Lazzaroni E., Dolci A., Ceriotti F., Re T.E., Gori A., Arosio M., Salvi M. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020 Sep;8(9):739–741. doi: 10.1016/S2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caron P. Thyroiditis and SARS-CoV-2 pandemic: a review. Endocrine. 2021;72:326–331. doi: 10.1007/s12020-021-02689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girgis C.M., Russo R.R., Benson K. Subacute thyroiditis following the H1N1 vaccine. J. Endocrinol. Investig. 2010;33:506. doi: 10.1007/BF03346633. [DOI] [PubMed] [Google Scholar]
- 8.Hernan Martinez J., Corder E., Uzcategui M., Garcia M., Sostre S., Garcia A. Subacute thyroiditis and dyserythropoesis after influenza vaccination suggesting immune dysregulation. Bol. Asoc. Med. P R. Apr-Jun 2011;103(2):48–52. [PubMed] [Google Scholar]
- 9.Altay F.A., Guz G., Altay M. Subacute thyroiditis following seasonal influenza vaccination. Hum. Vaccin Immunother. 2016;12:1033–1034. doi: 10.1080/21645515.2015.1117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passah A., Arora S., Damle N.A., Reddy K.S., Khandelwal D., Aggarwal S. Occurrence of subacute thyroiditis following influenza vaccination. Indian J. Endocrinol. Metab. 2018;22:713–714. doi: 10.4103/ijem.IJEM_237_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toft J., Larsen S., Toft H. Subacute thyroiditis after hepatitis B vaccination. Endocr. J. 1998;45:135. [PubMed] [Google Scholar]
- 12.Hsiao J.Y., Hsin S.C., Hsieh M.C., Hsia P.J., Shin S.J. Subacute thyroiditis following influenza vaccine (Vaxigrip) in a young female. Kaohsiung J. Med. Sci. 2006;22:297–300. doi: 10.1016/S1607-551X(09)70315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iremli B.G., Sendur S.N., Unluturk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: post-vaccination ASIA syndrome. J. Clin. Endocrinol. Metab. 2021 doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saygılı E.S., Karakilic E. Subacute thyroiditis after inactive SARS-CoV-2 vaccine. BMJ Case Rep. 2021 Oct 1;14(10) doi: 10.1136/bcr-2021-244711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Şahin Tekin M., Şaylısoy S., Yorulmaz G. Subacute thyroiditis following COVID-19 vaccination in a 67-year-old male patient: a case report. Hum. Vaccin Immunother. 2021 Jul 1:1–3. doi: 10.1080/21645515.2021.1947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oyibo S. Subacute thyroiditis after receiving the adenovirus vectored vaccine for coronavirus disease (COVID-19) Cureus. 2021;13 doi: 10.7759/cureus.16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratnayake G.M., Dworakowska D., Grossman A.B. Can COVID-19 immunisation cause subacute thyroiditis? Clin. Endocrinol. 2021:1–2. doi: 10.1111/cen.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das L., Bhadada S.K., Sood A. post-COVID-vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J. Endocrinol. Investig. 2021 Sep 28:1–3. doi: 10.1007/s40618-021-01681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K.A., Kim Y.J., Jin H.Y. Thyrotoxicosis after COVID-19 vaccination: seven case reports and a literature review. Endocrine. 2021;74(3):470–472. doi: 10.1007/s12020-021-02898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bornemann C., Woyk K., Bouter C. Case report: two cases of subacute thyroiditis following SARS-CoV-2 vaccination. Front. Med. (Lausanne). 2021 Aug 24;(8) doi: 10.3389/fmed.2021.737142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soltanpoor P., Norouzi G. Subacute thyroiditis following COVID-19 vaccination. Clin. Case Rep. 2021 Oct 4;9(10) doi: 10.1002/ccr3.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franquemont F., Galvez J. Subacute thyroiditis after mRNA vaccine for Covid-19. J. Endocrine Soc. 2021;5 doi: 10.1210/jendso/bvab048.1954. A956–7. [DOI] [Google Scholar]
- 23.Schimmel J., Alba E.L., Chen A., Russell M., Srinath R. Letter to the editor: thyroiditis and thyrotoxicosis after the SARS-CoV-2 mRNA vaccine. Thyroid. 2021 doi: 10.1089/thy.2021.0184. [DOI] [PubMed] [Google Scholar]
- 24.Kyriacou A., Ioakim S., Syed A.A. COVID-19 vaccination and a severe pain in the neck. Eur. J. Intern. Med. 2021 Oct 18;S0953-6205(21) doi: 10.1016/j.ejim.2021.10.008. 00338–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siolos A., Gartzonika K., Tigas S. Thyroiditis following vaccination against COVID-19: report of two cases and review of the literature. Metabol. Open. 2021 Dec;12 doi: 10.1016/j.metop.2021.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatzi S., Karampela A., Spiliopoulou C., Boutzios G. Subacute thyroiditis after SARS-CoV-2 vaccination: a report of two sisters and summary of the literature. Hormones (Athens). 2021 Oct 22:1–3. doi: 10.1007/s42000-021-00332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeeyavudeen M.S., Patrick A.W., Gibb F.W., Dover A.R. COVID-19 vaccine-associated subacute thyroiditis: an unusual suspect for de Quervain’s thyroiditis. BMJ Case Rep. 2021;14(11) doi: 10.1136/bcr-2021-246425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raven L.M., McCormack A.I., Greenfield J.R. Letter to the editor from Raven: three cases of subacute thyroiditis following SARS-CoV-2 vaccine. J. Clin. Endocrinol. Metab. 2021 Nov 9:dgab822. doi: 10.1210/clinem/dgab822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vera-Lastra O., Ordinola Navarro A., Cruz Domiguez M.P., Medina G., Sánchez Valadez T.I., Jara L.J. Two cases of Graves’ disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021 Sep;31(9):1436–1439. doi: 10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 30.Zettinig G., Krebs M. Two further cases of Graves’ disease following SARS-Cov-2 vaccination. J. Endocrinol. Investig. 2021 Aug 3:1–2. doi: 10.1007/s40618-021-01650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.di Filippo L., Castellino L., Giustina A. Occurrence and response to treatment of Graves’ disease after COVID vaccination in two male patients. Endocrine. 2021 Nov 2:1–3. doi: 10.1007/s12020-021-02919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watad A., David P., Brown S., Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front. Endocrinol. (Lausanne). 2016;7:150. doi: 10.3389/fendo.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The use of the WHO-UMC system for standardised case causality assessment. https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf The Upsala Monitoring Center. [last accessed on 18 December 2021]