Abstract
Background
Our objective was to characterize the frequency, early impact, and risk factors for neurological manifestations in hospitalized children with acute severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or multisystem inflammatory syndrome in children (MIS-C).
Methods
Multicenter, cross-sectional study of neurological manifestations in children aged <18 years hospitalized with positive SARS-CoV-2 test or clinical diagnosis of a SARS-CoV-2-related condition between January 2020 and April 2021. Multivariable logistic regression to identify risk factors for neurological manifestations was performed.
Results
Of 1493 children, 1278 (86%) were diagnosed with acute SARS-CoV-2 and 215 (14%) with MIS-C. Overall, 44% of the cohort (40% acute SARS-CoV-2 and 66% MIS-C) had at least one neurological manifestation. The most common neurological findings in children with acute SARS-CoV-2 and MIS-C diagnosis were headache (16% and 47%) and acute encephalopathy (15% and 22%), both P < 0.05. Children with neurological manifestations were more likely to require intensive care unit (ICU) care (51% vs 22%), P < 0.001. In multivariable logistic regression, children with neurological manifestations were older (odds ratio [OR] 1.1 and 95% confidence interval [CI] 1.07 to 1.13) and more likely to have MIS-C versus acute SARS-CoV-2 (OR 2.16, 95% CI 1.45 to 3.24), pre-existing neurological and metabolic conditions (OR 3.48, 95% CI 2.37 to 5.15; and OR 1.65, 95% CI 1.04 to 2.66, respectively), and pharyngeal (OR 1.74, 95% CI 1.16 to 2.64) or abdominal pain (OR 1.43, 95% CI 1.03 to 2.00); all P < 0.05.
Conclusions
In this multicenter study, 44% of children hospitalized with SARS-CoV-2-related conditions experienced neurological manifestations, which were associated with ICU admission and pre-existing neurological condition. Posthospital assessment for, and support of, functional impairment and neuroprotective strategies are vitally needed.
Keywords: Neurological manifestations, Pediatrics, SARS-CoV-2, Child development
Introduction
Globally, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has led to an estimated 13.5 million cases and 10,600 deaths in children and young adults younger than 20 years as of May 2021 (https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-dashboard/). Among hospitalized adults with coronavirus disease 2019 (COVID-19), the acute disease caused by SARS-CoV-2 infection, 36% to 82% experienced central and peripheral central nervous system manifestations associated with increased risk of mortality.1 , 2 Reports show that neurological signs and symptoms such as headache and altered mental status,3 , 4 and conditions such as Guillain-Barré syndrome5 and encephalitis,6 , 7 occur in children with COVID-19 and the postinfectious multisystem inflammatory syndrome in children (MIS-C) as well. However, coordinated, multinational studies of neurological manifestations in children with SARS-CoV-2-associated conditions are lacking.
The Global Consortium Study of Neurologic Dysfunction in COVID-19 (GCS-NeuroCOVID) is a multinational research collaborative initiated in April 2020 to describe the prevalence and outcomes of neurological manifestations of acute SARS-CoV-2 and MIS-C in adults and children.8 Pediatric outcome data collection is ongoing. Herein we present an interim analysis of the prevalence and characteristics of the neurological manifestations in hospitalized children with acute SARS-CoV-2 or MIS-C with a focus on potential risk factors.
Materials and Methods
Study design and participants
This is a preliminary analysis of a multinational, observational cohort study conducted between January 1, 2020, and April 30, 2021. Screening was performed at each center using locally approved methods including chart review and hospital registries. Local regulatory approval was obtained at each study site. The University of Pittsburgh Institutional Review Board (STUDY20060012) approved the Data Coordinating Center at the University of Pittsburgh to receive and analyze the data.
Inclusion criteria
Children aged <18 years who were admitted to the hospital with SARS-CoV-2-related condition were included. Acute SARS-CoV-2 patient cases were either confirmed (positive SARS-CoV-2 virus or antibody test) or presumed (clinical diagnosis), may or may not have been symptomatic, and did not receive a diagnosis of MIS-C. Presumed acute SARS-CoV-2 infection was defined as a patient who was diagnosed clinically due to clinical suspicion and/or a close contact being positive for the virus; this situation occurred most often early in the pandemic when testing was restricted due to lack of testing availability. MIS-C diagnosis was determined by treating physicians with guidance from the Centers for Disease Control and Prevention (https://www.cdc.gov/mis/hcp/index.html).
Exclusion criteria
Exclusion criterion was previous enrollment.
Study consortium
The GCS-NeuroCOVID Consortium studies hospitalized adult (≥18 years) and pediatric (<18 years of age) patients with SARS-CoV-2-related conditions. The overarching goals of this consortium include to9 (1) characterize neurological manifestations, (2) identify predictors of neurological manifestations, (3) determine the impact of neurological manifestations on posthospital outcomes, and (4) explore mechanisms and predict outcome of neurological injuries.
Participating centers in the pediatrics core
Pediatric centers were recruited from pediatric critical care professional networks with endorsements from the Neurocritical Care Society (NCS) and the Pediatric Neurocritical Care Research Group (PNCRG) and registered on an NCS webpage.10 Thirty centers submitted data for this preliminary analysis (Supplemental Table 1). Twenty-six (n = 1440 patients) centers were in North America. Nearly all centers were university-affiliated (99.8%) and 64% were free-standing children's hospitals.
Data collection
A Case Report Form (CRF)11 with common data elements, data dictionary, and guide to data collection was provided to the centers. The following data types were extracted from the medical record and entered into the CRF: (1) patient characteristics (e.g., pre-existing condition), (2) disease details (e.g., neurological manifestations, initial Glasgow Coma Scale [GCS] score, Pediatric Logistic Organ Dysfunction score if the child was admitted to an intensive care unit [ICU]),12 (3) testing results (e.g., SARS-CoV-2 testing), (4) acute SARS-CoV-2- and MIS-C-related treatments (e.g., steroids), (5) patient outcomes at hospital discharge (e.g., mortality), (5) center characteristics (e.g., number of hospital beds), and (6) rehabilitation consultations (e.g., physical therapy). Testing for SARS-CoV-2-related conditions was determined by individual center clinicians and availability of resources. The definitions of neurological manifestations studied here were previously published by the consortium.11 Conditions such as stroke, which included both ischemic and hemorrhagic stroke, and seizure were diagnosed by local clinicians without specific study criteria. Encephalopathy was defined as new-onset altered mental status, lethargy, or drowsiness not otherwise diagnosed as delirium. Delirium was diagnosed clinically or through a delirium scoring tool used at the center. Body mass index was calculated as follows: weight (kg)/[height (m)]2. Obesity was defined as body mass index ≥ 30.13
Outcomes
The primary outcome was frequency and type of neurological manifestations, overall and by acute SARS-CoV-2 and MIS-C groups. Secondary outcomes included risk factors for neurological manifestations, overall and by acute SARS-CoV-2 and MIS-C groups.
Data management
Each site was assigned a study identification code and entered data into a custom Microsoft Excel (2019) CRF. Data entry was performed by faculty, trainees, and/or research coordinators. Webinars and e-mail served to provide regular study updates and training for study startup and execution.
The Data Coordinating Center (DCC) primary investigator and coordinator team worked with the Clinical Research, Investigation, and Systems Modeling of Acute Illness (CRISMA) Center at the University of Pittsburgh to manage central data collection, quality, security, and analysis. Centers with a data use agreement in place with the University of Pittsburgh submitted patient data to the DCC using encrypted e-mail or via upload to a secure cloud (https://www.globus.org/). Data were stored on a password-protected network at the DCC, with additional periodic secure offsite backups to the database. Data were screened for missing or implausible information, and queries were issued for clarification and adjusted.
Statistical analysis
Most data were nonparametric and presented as median (interquartile range [IQR]). Comparisons were made between children (1) with and without neurological manifestations and (2) acute SARS-CoV-2 versus MIS-C groups. Kruskal-Wallis, Mann-Whitney, Fisher exact, and chi-square tests were used as appropriate. Multivariable logistic regression modeling was performed to identify patient and disease characteristics associated with neurological manifestation in the overall cohort and by acute SARS-CoV-2 and MIS-C groups. Spearman correlations for neurological conditions (e.g., stroke) and symptoms (e.g., headache) were performed to explore common patient presentations. No adjustment was made for multiple comparisons except for correlations; secondary outcomes results should be interpreted as hypothesis-generating. Statistical analysis by region was not performed due to the small number of centers and subjects in some regions. The majority of variables had less than 10% missing data, and missing data were not imputed (thus sample sizes for variables and denominators varied slightly). All P values were two-sided, and P < 0.05 was considered statistically significant. The Statistical Package for the Social Sciences version 20 (Armonk, NY, USA) was used for statistical analyses. This article was written according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) initiative.14
Results
Patient and clinical characteristics: overall and by acute SARS-CoV-2 versus MIS-C groups
The median age of the 1493 children analyzed in the overall cohort was 8 (IQR 1.1 to 14.0) years and 47% were female (Table 1 ). Forty-two per cent and 28% identified as white or black race, respectively, and 37% as Hispanic or Latino. Most patients were admitted during the July to December 2020 epoch (55%) versus January to June 2020 (34%) and January to April 2021 (10%), P < 0.001. Of 863 (58%) children with a pre-existing condition, the most common were respiratory and neurological (20% each). The most common acute, constitutional, nonneurological symptoms reported were fever (64%), cough (36%), and anorexia (29%). Six per cent of children had a GCS score ≤12 on hospital admission. Thirty-five per cent of children required ICU care, with Pediatric Logistic Organ Dysfunction score 7 (1 to 11). Ninety-five per cent of children were discharged to home, 2% were discharged to inpatient rehabilitation, and 1% died.
TABLE 1.
Child Characteristics, SARS-CoV-2 Testing, and Nonneurological Manifestations by Overall, Neurological Manifestation Status, and Acute SARS-CoV-2 or MIS-C Group
| Variables | Overall N = 1493 |
Neurological Manifestations N = 652 (44%) |
No Neurological Manifestations N = 841 (56%) |
P Value | Acute SARS-CoV-2 N = 1278 (86%) |
MIS-C N = 215 (14%) |
P Value |
|---|---|---|---|---|---|---|---|
| Epoch | n = 1331 | n = 581 | n = 750 | n = 1156 | n = 175 | <0.001 | |
| January 2020-June 2020 | 457 (34.3) | 197 (33.9) | 260 (34.7) | 0.869 | 417 (36.1) | 40 (22.9) | |
| July 2020-December 2020 | 736 (55.3) | 321 (55.3) | 415 (55.3) | 632 (54.7) | 104 (59.4) | ||
| January 2021-April 2021 | 138 (10.4) | 63 (10.8) | 75 (10.0) | 107 (9.3) | 31 (17.7) | ||
| Age, y | 8 (1.1-14.0) | 11·5 (6.0-15.0) | 4·6 (0.6-13.0) | 0.006 | 8·0 (1.0-14.8) | 8.3 (5.0-13.0) | <0.001 |
| Female sex | n = 1459 | n = 652 | n = 808 | n = 1244 | n = 215 | ||
| 691 (47.4) | 310 (47.5) | 381 (47.2) | 0.980 | 601 (48.3) | 90 (41.9) | 0.175 | |
| Race | n = 1430 | n = 640 | n = 790 | n = 507 | n = 923 | ||
| White | 599 (41.9) | 284 (44.4) | 315 (39.8) | 522 (42.9) | 77 (36.2) | ||
| Black or African American | 404 (28.3) | 171 (26.7) | 233 (29.5) | 331 (27.2) | 73 (34.3) | ||
| Asian | 51 (3.6) | 24 (3.8) | 27 (3.4) | 0.555 | 41 (3.4) | 10 (4.7) | 0.111 |
| American Indian or Alaskan Native | 7 (0.5) | 4 (0.6) | 3 (0.4) | 6 (0.5) | 1 (0.5) | ||
| Native Hawaiian or other Pacific Islander | 5 (0.4) | 2 (0.3) | 3 (0.4) | 3 (0.3) | 2 (0.9) | ||
| Other | 364 (25.5) | 155 (24.2) | 209 (26.5) | 314 (25.8) | 50 (23.5) | ||
| Hispanic | n = 1399 | n = 624 | n = 775 | 0.828 | n = 1190 | n = 209 | |
| Ethnicity | 518 (37.0) | 233 (37.3) | 285 (36.8) | 456 (38.3) | 62 (29.7) | 0.017 | |
| Acute SARS-CoV-2 versus MIS-C diagnosis and test results | |||||||
| Acute SARS-CoV-2 diagnosis | 1278 (85.6) | 510 (39.9) | 768 (60.1) | ||||
| PCR/Ag+ (n = 1480) | 1217 (82.2) | 470 (72.6) | 747 (89.7) | <0.001 | 1217 (82.2) | <0.001∗ | |
| Ab+ (n = 1092) | 121 (11.1) | 89 (17.4) | 32 (5.5) | <0.001 | 121 (11.1) | <0.001∗ | |
| Suspected/presumed (n = 808) | 35 (4.33%) | 28 (7.7) | 7 (1.6) | <0.001 | 35 (4.3) | 0.003∗ | |
| MIS-C diagnosis | 215 (14.4) | 142 (66.0) | 73 (34.0) | ||||
| PCR+ (n = 1480) | 135 (9.1) | 84 (13.0) | 51 (6.1) | <0.001 | 215 (14·4) | ||
| Ab+ (n = 1092) | 178 (16.3) | 119 (23.3) | 59 (10.2) | <0.001 | 135 (9.1) | ||
| Suspected/presumed (n = 808) | 14 (1.7) | 13 (3.6) | 1 (0.2) | <0.001 | 178 (16.3) | ||
| Pre-existing condition (n = 1493) | 863 (57.8) | 421 (64.6) | 442 (52.6) | <0.001 | 783 (61.3) | 80 (37.2) | <0.001 |
| Respiratory (n = 1452) | 285 (19.6) | 142 (21.8) | 143 (17.8) | 0.055 | 257 (20.8) | 28 (13.0) | 0.008 |
| Neurological (n = 1451) | 287 (19.8) | 176 (27.0) | 111 (13.9) | <0.001 | 268 (21.7) | 19 (8.8) | <0.001 |
| Gastrointestinal (n = 1453) | 200 (13.8) | 84 (12.9) | 116 (14.5) | 0.391 | 190 (15.3) | 10 (4.7) | <0.001 |
| Obesity (n = 1271) | 175 (13.8) | 111 (19.0) | 64 (9.3) | <0.001 | 157 (14.8) | 18 (8.7) | 0.019 |
| Congenital/genetic (n = 1454) | 179 (12.3) | 84 (12.9) | 95 (11.8) | 0.536 | 170 (13.7) | 9 (4.2) | <0.001 |
| Hematologic/immunologic (n = 1452) | 155 (10.7) | 63 (9.7) | 92 (11.5) | 0.267 | 148 (12.0) | 7 (3.3) | 0.001 |
| Metabolic (n = 1454) | 135 (9.3) | 83 (12.7) | 52 (6.5) | <0.001 | 123 (9.9) | 12 (5.6) | 0.043 |
| Cardiovascular (n = 1452) | 133 (9.2) | 51 (7.9) | 82 (10.2) | 0.118 | 123 (9.9) | 10 (4.7) | 0.013 |
| Premature (n = 1397) | 125 (9.0) | 47 (7.7) | 78 (10.0) | <0.001 | 119 (9.9) | 6 (3.1) | <0.001 |
| Technology dependent (n = 1454) | 110 (7.6) | 48 (7.4) | 62 (7.7) | 0.803 | 102 (8.2) | 8 (3.7) | 0.021 |
| Renal/urologic (n = 1453) | 75 (5.2) | 32 (4.9) | 43 (5.4) | 0.702 | 70 (5.7) | 5 (2.3) | 0.042 |
| Malignancy (n = 1453) | 68 (4.7) | 33 (5.1) | 35 (4.4) | 0.519 | 65 (5.3) | 3 (1.4) | 0.014 |
| Transplantation (n = 1453) | 35 (2.4) | 15 (2.3) | 20 (2.5) | 0.818 | 33 (2.7) | 2 (0.9) | 0.126 |
| Other (n = 1453) | 144 (9.9) | 79 (12.1) | 65 (8.1) | 0.011 | 135 (10.9) | 9 (4.2) | 0.003 |
| Nonneurological manifestation | |||||||
| Fever (n = 1493) | 955 (64.0) | 448 (68.7) | 507 (60.3) | 0.001 | 749 (58.6) | 206 (95.8) | <0.001 |
| Cough (n = 1493) | 531 (35.6) | 250 (38.3) | 281 (33.4) | 0.048 | 465 (36.4) | 66 (30.7) | 0.107 |
| Anorexia (n = 1493) | 429 (28.7) | 215 (33.0) | 214 (25.4) | 0.001 | 305 (23·9) | 124 (57.7) | <0.001 |
| Abdominal pain (n = 1493) | 360 (24.1) | 211 (32.4) | 149 (17.7) | <0.001 | 239 (18.7) | 121 (56.3) | <0.001 |
| Diarrhea (n = 1493) | 316 (21.2) | 165 (25.3) | 151 (18.0) | 0.001 | 210 (16.4) | 106 (49.3) | <0.001 |
| Throat pain (n = 1493) | 196 (13.1) | 133 (20.4) | 63 (7.5) | <0.001 | 139 (10.9) | 57 (26.5) | <0.001 |
Abbreviations:
Ab = Antibody
Ag = Antigen
IQR = Interquartile range
MIS-C = Multisystem inflammatory syndrome in children (MIS-C)
PCR = Polymerase chain reaction
SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2
Results are reported as median (IQR) versus n (%).
Acute SARS-CoV-2 versus MIS-C.
Eighty-six percent of children were diagnosed with acute SARS-CoV-2 versus 14% with MIS-C. Children with acute SARS-CoV-2 were more often admitted in earlier epochs, were younger, and of Hispanic ethnicity than children with MIS-C, all P < 0.05. SARS-CoV-2 polymerase chain reaction or antigen tests were positive in 96% and 64% of children with acute SARS-CoV-2 and MIS-C, respectively, whereas antibody tests were positive in 14% and 86% of these populations. Pre-existing conditions were more common in children with acute SARS-CoV-2 (61%) than those with MIS-C (37%), P < 0.001. Initial GCS scores were similar between acute SARS-CoV-2 and MIS-C groups. Children with MIS-C were more frequently admitted to the ICU (69% vs 29%) and had longer lengths of ICU and hospital stay than children with acute SARS-CoV-2, all P < 0.05. Eleven (1%) children with acute SARS-CoV-2 and 4 (2%) children with MIS-C died by hospital discharge, P = 0.174. Children with MIS-C had increased frequency of all nonneurological symptoms compared with children with acute SARS-CoV-2; the most common nonneurological symptoms for both groups was fever (59% vs 96%, acute SARS-CoV-2 versus MIS-C, respectively, P < 0.001).
Neurological manifestations: overall and by acute SARS-CoV-2 versus MIS-C
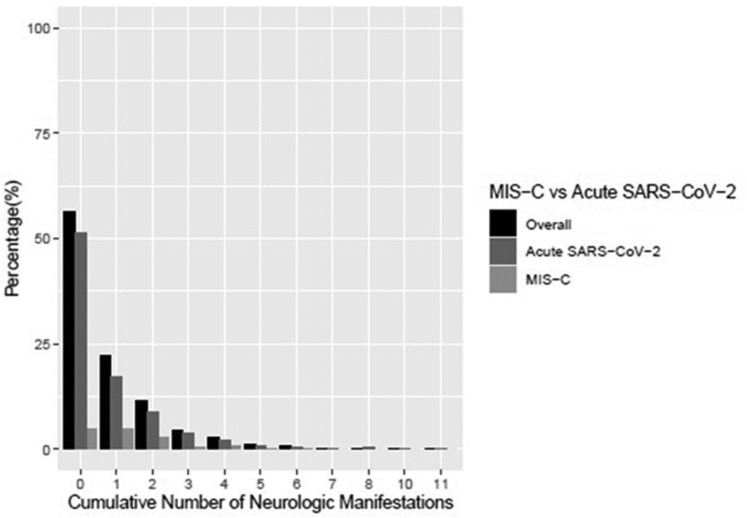
Forty-four percent of children presented with at least one neurological sign or symptom and 12% of children had two or more (Table 2 , Fig 1 ). Headache (20%) and encephalopathy (16%) were the most common neurological manifestations in children overall, followed by seizures (8%). Anosmia (4%), ageusia (3.6%), meningitis/encephalitis (1.3%), and stroke (0.9%) were less common. Nonneurological symptoms were generally more common in children with neurological manifestations (Table 1). More children with neurological manifestations had moderate (GCS 8 to 12, 8% versus 1%) or severe (GCS<8, 5% vs 0.4%) impairment of consciousness on admission compared with children without neurological manifestation, P < 0.05 (Table 3). More children with neurological manifestations required ICU care compared with children without neurological manifestations (51% vs 22%, P < 0.05). Hospital (5 [2 to 9] vs 3 [2 to 6] days) and ICU (5 [3 to 8] vs 3 [2 to 5] days) lengths of stay were longer for children with neurological manifestations compared with children without neurological manifestations, both P < 0.05.
TABLE 2.
Frequency of Neurological and Nonneurological Manifestations by Overall and Acute SARS-CoV-2 and MIS-C Group
| Manifestations | Overall N = 1493 |
Acute SARS-CoV-2 N = 1278 (86%) | MIS-C N = 215 (14%) |
P Value |
|---|---|---|---|---|
| Headache | 309 (20.7) | 209 (16.4) | 100 (46.5) | <0.001 |
| Acute encephalopathy | 241 (16.1) | 193 (15.1) | 48 (22.3) | 0.008 |
| Clinical seizures/status epilepticus | 115 (7.7) | 108 (8.5) | 7 (3.3) | 0.005 |
| Weakness | 109 (7.3) | 89 (7.0) | 20 (9.3) | 0.223 |
| Dizziness | 95 (6.4) | 69 (5.4) | 26 (12.1) | <0.001 |
| Anosmia | 59 (4.0) | 51 (4.0) | 8 (3.7) | 0.851 |
| Ageusia | 54 (3.6) | 43 (3.4) | 11 (5.1) | 0.203 |
| Delirium | 43 (2.9) | 38 (3.0) | 5 (2.3) | 0.599 |
| Vision impairment | 37 (2.5) | 29 (2.3) | 8 (3.7) | 0.205 |
| Ataxia | 31 (2.1) | 28 (2.2) | 3 (1.4) | 0.449 |
| Numbness | 27 (1.8) | 26 (2.0) | 1 (0.5) | 0.110 |
| Syncope | 26 (1.7) | 23 (1.8) | 3 (1.4) | 0.675 |
| Coma | 25 (1.7) | 21 (1.6) | 4 (1.9) | 0.818 |
| Paresthesia | 23 (1.5) | 21 (1.6) | 2 (0.9) | 0.432 |
| Meningitis/encephalitis | 19 (1.3) | 15 (1.2) | 4 (1.9) | 0.406 |
| Sympathetic storming/dysautonomia | 21 (1.4) | 12 (0.9) | 9 (4.2) | <0.001 |
| Cardiac arrest | 16 (1.1) | 12 (0.9) | 4 (1.9) | 0.225 |
| Stroke | 13 (0.9) | 12 (0.9) | 1 (0.5) | 0.489 |
| Neuropathy | 12 (0.8) | 12 (0.9) | 0 (0.0) | 0.154 |
| Myelopathy | 6 (0.4) | 6 (0.5) | 0 (0.0) | 0.314 |
| Other reported neurological manifestations (free text) | ||||
| Coacute neurological condition∗ | 13 | 13 | 0 | |
| Acute psychosis | 7 | 4 | 3 | |
| Photophobia/phonophobia | 7 | 4 | 3 | |
| Abnormal motor movements | 6 | 6 | 0 | |
| Cranial nerve abnormality | 6 | 5 | 1 | |
| Hypotonia | 4 | 4 | 0 | |
| Papilledema | 2 | 1 | 1 | |
| Dysarthria | 2 | 2 | 0 | |
| Meningismus | 1 | 1 | 0 | |
| Arthralgia | 1 | 0 | 1 | |
| Dysphagia | 1 | 1 | 0 | |
| Moyamoya disease | 1 | 1 | 0 | |
| Unspecified | 1 | 1 | 0 |
Abbreviations:
MIS-C = Multisystem inflammatory syndrome in children
SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2
Results are reported as n (%).
For example, traumatic brain injury.
FIGURE 1.
Cumulative number of neurological manifestations by overall, and grouped by acute SARS-CoV-2 versus MIS-C. MIS-C, multisystem inflammatory syndrome in children; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
TABLE 3.
Hospital Outcomes by Neurological Manifestation Status and Acute SARS-CoV-2 or MIS-C Clinical Diagnosis
| Variables | Overall N = 1493 |
Neurological Manifestation N = 652 (44%) |
No Neurological Manifestation N = 841 (56%) |
P Value | Acute SARS-CoV-2 N = 1278 (86%) |
MIS-C N = 215 (14%) |
P Value |
|---|---|---|---|---|---|---|---|
| Highest level of care | |||||||
| Ward | 975 (65.3) | 319 (48.9) | 656 (78.0) | <0.001 | 909 (71.1) | 66 (30.7) | <0.001 |
| ICU | 518 (34.7) | 333 (51.1) | 185 (22.0) | 369 (28.9) | 149 (69.3) | ||
| Initial Glasgow Coma Scale score | n = 990 | n = 421 | n = 569 | n = 785 | n = 205 | ||
| 13-15 | 928 (93.7) | 369 (87.7) | 559 (98.3) | <0.001 | 735 (93.6) | 193 (94.1) | 0.094 |
| 9-12 | 40 (4.0) | 32 (7.6) | 8 (1.4) | 30 (3.8) | 10 (4.9) | ||
| 3-8 | 22 (2.2) | 20 (4.8) | 2 (0.4) | 20 (2.6) | 2 (1.0) | ||
| Initial PELOD (if ICU) | n = 229 | n = 149 | n = 80 | n = 118 | n = 111 | ||
| Median | 5 (1-11) | 6 (2-11) | 2 (1-10) | 0.086 | 3 (1-11) | 5 (2-11) | 0.032 |
| Hospital length of stay, days | 4.00 (2-7) | 5.00 (2-9) | 3.00 (2-6) | <0.001 | 3.00 (2-7) | 7.00 (5-9) | 0.008 |
| ICU length of stay, days | 4.00 (3-7) | 5.00 (3-8) | 3·00 (2-5.3) | <0.001 | 4.00 (2-7) | 4.00 (3-6) | 0.046 |
| Hospital mortality | 15 (1.0) | 14 (2·2) | 1 (0.1) | <0.001 | 11 (0.9) | 4 (1.9) | 0.174 |
| Hospital disposition | n = 1462 | n = 633 | n = 829 | n = 1247 | n = 215 | ||
| Home | 1391 (95.2) | 680 (91·6) | 811 (97.8) | <0.001 | 1186 (95.1) | 205 (95.3) | 0.730 |
| Inpatient rehabilitation | 25 (1.7) | 22 (3·5) | 3 (0.4) | 20 (1.6) | 5 (2.3) | ||
| Long-term care facility | 2 (0.1) | 2 (0·3) | 0 (0.0) | 2 (0.2) | 0 (0.0) | ||
| Other | 44 (3.0) | 29 (4·6) | 15 (1.8) | 39 (3.1) | 5 (2.3) |
Abbreviations:
ICU = Intensive care unit
IQR = Interquartile range
MIS-C = Multisystem inflammatory syndrome in children
PELOD = Pediatric Logistic Organ Dysfunction
SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2
Results are reported as median (IQR) versus n (%).
Forty percent of children with acute SARS-CoV-2 and 66% with MIS-C presented with at least one neurological sign or symptom. The most common neurological manifestations in children with acute SARS-CoV-2 were headache (16%), acute encephalopathy (15%), and seizures (8%), whereas children with MIS-C most commonly had headache (47%), acute encephalopathy (22%), and dizziness (12%). Anosmia and vision impairment had similar prevalence between acute SARS-CoV-2 and MIS-C populations. Stroke was reported in 12 (0.9%) and 1 (0.5%) children with acute SARS-CoV-2 and MIS-C, respectively. Other neurological manifestations reported by participating centers as write-ins included coacute neurological conditions such as traumatic brain injury (n = 13, all in the acute SARS-CoV-2 group) and acute psychosis (n = 7; acute SARS-CoV-2 n = 4 and MIS-C n = 3). More children with MIS-C versus acute SARS-CoV-2 had 2 or more neurological manifestations (66% vs 40%), P < 0.001.
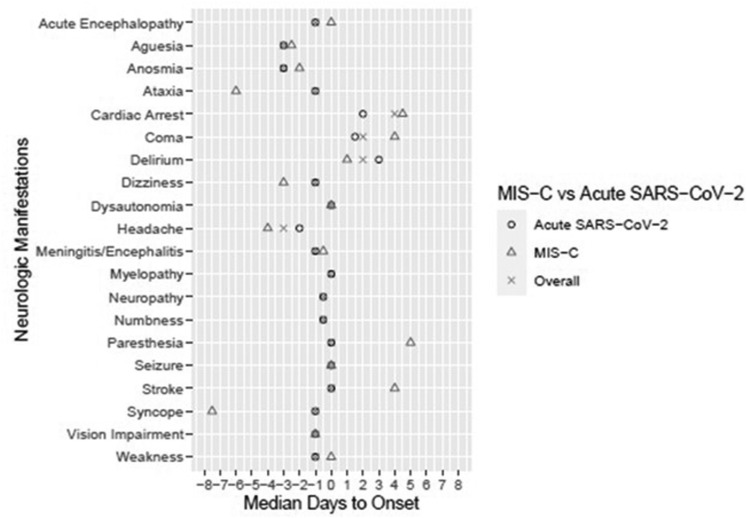
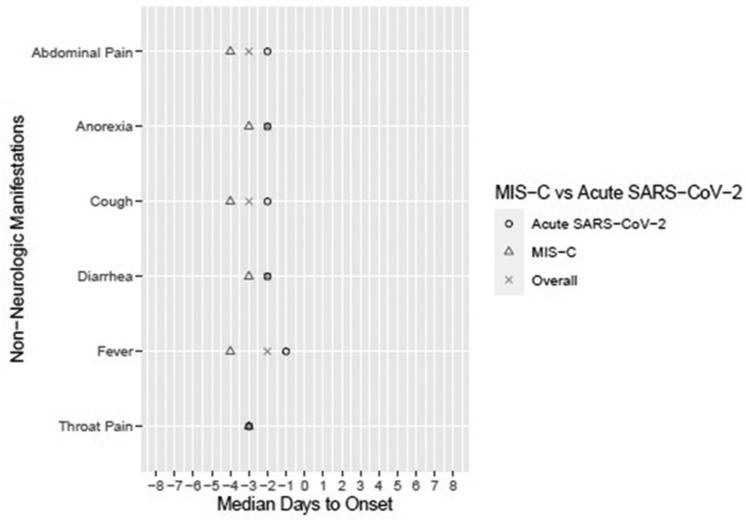
Median days to onset of neurological and nonneurological symptom(s) and neurological condition(s) in the overall cohort and acute SARS-CoV-2 and MIS-C subcohorts are presented in Figs 2 and 3 , with day 0 corresponding to the day of hospitalization. In the overall and acute SARS-CoV-2 groups, the earliest prehospitalization neurological symptoms included headache, ageusia, and anosmia, all occurring at median 3 days before hospitalization, except headache in the acute SARS-CoV-2 group occurring at median 2 days before hospitalization. In the MIS-C group, the earliest prehospital neurological symptoms included syncope (median 7.5 days before hospitalization), ataxia (6 days), headache (4 days), and dizziness (3 days). All nonneurological manifestations occurred before hospitalization (Fig 3). Correlations between symptoms and conditions are in Supplemental Table 4. Weak correlations were found, with unique patterns of neurological symptoms for each neurological condition.
FIGURE 2.
Median days to neurological manifestation by overall and acute SARS-CoV-2 and MIS-C groups. Day 0 is the day of hospitalization; thus, negative days represent days leading up to hospitalization. MIS-C, multisystem inflammatory syndrome in children; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
FIGURE 3.
Median days to nonneurological manifestation by overall and acute SARS-CoV-2 and MIS-C groups. Day 0 is the day of hospitalization; thus, negative days represent days leading up to hospitalization. MIS-C, multisystem inflammatory syndrome in children; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Ward versus ICU
Twenty-eight percent of children received ICU care. Children who were older (10 [4.9 to 15.0] versus 7 [0.8 to 14.0] years), male (58% vs 50%), and those with pre-existing conditions (66% vs 53%) were more likely to be admitted to an ICU, all P < 0.05 (Supplemental Tables 1 and 2). Critically ill children generally had more neurological manifestations reported compared with children admitted to the ward, all P < 0.05.
Multivariable logistic regression analyses for neurological manifestations
In a multivariable logistic regression in the overall cohort, older age (adjusted odds ratio [OR] 1.10, 95% confidence interval [95% CI] 1.07 to 1.13), MIS-C versus acute SARS-CoV-2 diagnosis (OR 2.16, 95% CI 1.45 to 3.24), neurological (OR 3.48, 95% CI 2.37 to 5.15) and metabolic pre-existing condition (OR 1.65, 95% CI 1.04 to 2.66), and throat (OR 1.74, 95% CI 1.16 to 2.64) and abdominal pain (OR 1.43, 95% CI 1.03 to 2.00) were associated with neurological manifestations, all P < 0.05 (Table 4 ).
TABLE 4.
Multivariable Logistic Regression for the Association of Patient Characteristics With Occurrence of Any Neurological Manifestation (Overall Cohort)
| Variable | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age | 1.10 | 1.07, 1.13 | <0.001 |
| Female sex | 1.16 | 0.04, 29.84 | 0.917 |
| Race | |||
| Asian | 0.48 | 0.05, 3.41 | 0.466 |
| Black | 0.30 | 0.04, 1.95 | 0.210 |
| Native American or Pacific Islander | 0.30 | 0.01, 6.85 | 0.465 |
| White | 0.45 | 0.05, 2.87 | 0.397 |
| American Indian or Alaskan Native | 1.00 | - | - |
| Other | 0.39 | 0.05, 2.60 | 0.336 |
| Hispanic ethnicity | 0.88 | 0.61, 1.27 | 0.499 |
| MIS-C versus acute SARS-CoV-2 | 2.16 | 1.45, 3.24 | <0.001 |
| Pre-existing condition | |||
| Neurological | 3.48 | 2.37, 5.15 | <0.001 |
| Cardiovascular | 0.61 | 0.37, 0.99 | 0.051 |
| Respiratory | 0.88 | 0.61, 1.29 | 0.514 |
| Renal or urologic | 0.64 | 0.34, 1.17 | 0.153 |
| Gastrointestinal | 0.68 | 0.44, 1.07 | 0.095 |
| Hematologic or immunologic | 0.74 | 0.47, 1.17 | 0.206 |
| Metabolic | 1.65 | 1.04, 2.66 | 0.036 |
| Congenital or genetic defect | 1.09 | 0.68, 1.76 | 0.709 |
| Malignancy | 1.03 | 0.57, 1.88 | 0.917 |
| Premature or neonatal | 1.27 | 0.78, 2.03 | 0.332 |
| Technology dependence | 0.81 | 0.44, 1.47 | 0.497 |
| Transplantation | 1.18 | 0.51, 2.71 | 0.695 |
| Other, nonneurological | 1.08 | 0.70, 1.66 | 0.729 |
| Constitutional symptoms | |||
| Fever | 1.30 | 0.68, 1.24 | 0.093 |
| Cough | 0.92 | 0.68, 1.24 | 0.607 |
| Anorexia | 1.28 | 0.94, 1.74 | 0.116 |
| Diarrhea | 0.99 | 0.70, 1.39 | 0.949 |
| Throat pain | 1.74 | 1.16, 2.64 | 0.008 |
| Abdominal pain | 1.43 | 1.03, 2.00 | 0.035 |
| Obesity | 1.13 | 0.73, 1.75 | 0.575 |
Abbreviations:
MIS-C = Multisystem inflammatory syndrome in children
SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2
In the acute SARS-CoV-2 group, older age (OR 1.10, 95% CI 1.07 to 1.13) and pre-existing neurological (OR 3.64, 95% CI 2.45 to 5.48) or metabolic (OR 1.78, 95% CI 1.09 to 2.96) condition, anorexia (OR 1.56, 95% CI 1.10 to 2.22), and throat pain (OR 1.85, 95% CI 1.15 to 3.00) were associated with neurological manifestations, all P < 0.05. Black race (OR 0.63, 95% CI 0.42 to 0.94) and pre-existing cardiovascular condition (OR 0.52, 95% CI 0.30 to 0.89) were protective factors, both P < 0.05 (Table 5 ).
TABLE 5.
Multivariable Logistic Regression for the Association of Patient Characteristics With Occurrence of Any Neurological Manifestation (Acute SARS-CoV-2 Subcohort)
| Variable | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age | 1.10 | 1.07, 1.13 | <0.001 |
| Female sex | 1.23 | 0.05, 31.87 | 0.884 |
| Race | |||
| Asian | 1.03 | 0.45, 2.27 | 0.952 |
| Black | 0.63 | 0.42, 0.94 | 0.022 |
| White | Reference | - | - |
| Other | 0.96 | 0.65, 1.41 | 0.825 |
| Hispanic ethnicity | 0.96 | 0.65, 1.42 | 0.845 |
| Pre-existing condition | |||
| Neurological | 3.64 | 2.45, 5.48 | <0.001 |
| Cardiovascular | 0.52 | 0.30, 0.89 | 0.018 |
| Respiratory | 0.84 | 0.57, 1.26 | 0.406 |
| Renal or urologic | 0.63 | 0.33, 1.19 | 0.158 |
| Gastrointestinal | 0.83 | 0.52, 1.32 | 0.438 |
| Hematologic or immunologic | 0.69 | 0.43, 1.11 | 0.127 |
| Metabolic | 1.78 | 1.09, 2.96 | 0.023 |
| Congenital or genetic defect | 1.07 | 0.65, 1.74 | 0.799 |
| Malignancy | 1.14 | 0.62, 2.10 | 0.679 |
| Premature or neonatal | 1.27 | 0.77, 2.08 | 0.334 |
| Technology dependence | 0.71 | 0.38, 1.33 | 0.287 |
| Transplantation | 1.44 | 0.60, 3.41 | 0.402 |
| Other, nonneurological | 1.22 | 0.78, 1.91 | 0.378 |
| Constitutional symptoms | |||
| Fever | 1.25 | 0.92, 1.72 | 0.160 |
| Cough | 0.89 | 0.64, 1.23 | 0.482 |
| Anorexia | 1.56 | 1.10, 2.22 | 0.013 |
| Diarrhea | 0.99 | 0.66, 1.47 | 0.960 |
| Throat pain | 1.85 | 1.15, 3.00 | 0.012 |
| Abdominal pain | 0.94 | 0.64, 1.37 | 0.739 |
| Obesity | 1.15 | 0.72, 1.83 | 0.551 |
Abbreviation:
SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2
In the MIS-C group, older age (OR 1.16, 95% CI 1.06 to 1.27), pre-existing respiratory condition (OR 4.98, 95% CI 1.21 to 27.88), and abdominal pain (OR 5.36, 95% CI 2.39 to 12.63) were associated with neurological manifestations, all P < 0.05 (Table 6). Pre-existing gastrointestinal (OR 0.03, 95% CI 0.001 to 0.27) condition was protective, P < 0.05.
TABLE 6.
Multivariable Logistic Regression for the Association of Patient Characteristics With Occurrence of Any Neurological Manifestation (MIS-C Subcohort)
| Variable | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age | 1.16 | 1.06, 1.27 | 0.001 |
| Female sex | 1.46 | 0.66, 3.27 | 0.353 |
| Race∗ | |||
| Asian | 1.70 | 0.23, 15.83 | 0.612 |
| Black | 0.88 | 0.33, 2.27 | 0.791 |
| White | Reference | - | - |
| Other | 0.31 | 0.16, 1.76 | 0.305 |
| Hispanic ethnicity | 0.45 | 0.14, 1.38 | 0.164 |
| Pre-existing condition | |||
| Neurological | 3.84 | 0.76, 23.91 | 0.121 |
| Cardiovascular | 1.80 | 0.33, 13.31 | 0.525 |
| Respiratory | 4.98 | 1.21, 27.88 | 0.040 |
| Gastrointestinal | 0.03 | 0.001, 0.27 | 0.005 |
| Metabolic | 1.41 | 0.33, 14.78 | 0.678 |
| Other, nonneurological† | 0.63 | 0.20, 2.13 | 0.436 |
| Constitutional symptoms | |||
| Fever | 0.37 | 0.02, 3.31 | 0.416 |
| Cough | 0.98 | 0.43, 2.25 | 0.954 |
| Anorexia | 0.71 | 0.32, 1.55 | 0.391 |
| Diarrhea | 0.86 | 0.38, 1.93 | 0.717 |
| Throat pain | 2.19 | 0.86, 5.97 | 0.110 |
| Abdominal pain | 5.36 | 2.39, 12.63 | <0.001 |
| Obesity | 1.45 | 0.34, 7.94 | 0.641 |
Abbreviations:
MIS-C = Multisystem inflammatory syndrome in children
SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2
American Indian or Alaskan Native and Native American or Pacific Islander collapsed into other race due to small sample size.
Pre-existing Renal, Congenital or Genetic, Defect Malignancy, Premature or Neonatal, Technology dependence, and Transplantation conditions were grouped into the Other, nonneurological group due to small sample size.
Discussion
In this preliminary report of neurological manifestations in children hospitalized with acute SARS-CoV-2 and MIS-C (1) neurological manifestations were common (44%); (2) the frequency of severe neurological conditions including stroke were uncommon, but children with neurological manifestations were more likely to present with abnormal GCS and require ICU care; and (3) older children and those with specific pre-existing conditions and constitutional symptoms were at increased risk of neurological manifestations, although this risk differs by acute SARS-CoV-2 versus MIS-C diagnosis.
The frequency of neurological manifestations in this prospective cohort of hospitalized children is lower than that reported by the GCS-NeuroCOVID Consortium–Adult study (All COVID-19 cohort, 80%).2 Our cohort had higher prevalence of neurological manifestations than reported in a secondary analysis of the Overcoming COVID-19 study (n = 1695 children in US hospitals).6 In the latter cohort, only 22% of children hospitalized with SARS-CoV-2 infection (not reported by acute SARS-CoV-2 or MIS-C separately) had neurological manifestations. In that cohort, fatigue/weakness was most common, followed by altered awareness or confusion, and then headache. One explanation for the difference in neurological manifestation type and frequency is that our study collected more granular data collection on neurological manifestations than the Overcoming COVID-19 study, and our study did not assess fatigue/weakness.11 Finally, a meta-analysis of neurological manifestations in SARS-CoV-2 infection in children found that fatigue/myalgia was most prevalent (14%) followed by acute encephalopathy (13%), with a lower headache and seizure prevalence than we observed at 4% and 3%, respectively.15 Differences in our study population, such as including children with pre-existing neurological conditions and a substantial number of children with MIS-C, may account for some of the differences in reported frequency of neurological manifestations. Many excellent reviews exist regarding the potential mechanisms of neurological manifestations with SARS-CoV-2 in children; detailed discussion of this is outside the scope of this report.16
Headache and acute encephalopathy were the predominant neurological manifestations, especially in children with MIS-C; this differs from adults, in whom acute encephalopathy was the most commonly reported neurological manifestation (50%), which was also associated with mortality.2 An International Pediatric Stroke Study Group multinational study reported that of children with strokes occurring during the pandemic, fewer than half were tested for acute SARS-CoV-2 infection and that most children with stroke had underlying risk factors for stroke.17 In our study, stroke was less prevalent in children than in the adult GCS-NeuroCOVID consortium study (1% vs 3%), but seizure/status epilepticus was more prevalent in children than adults (8% vs 1%). Furthermore, seizure/status epilepticus was more than twice as frequent in children with acute SARS-CoV-2 than MIS-C. In the Overcoming COVID-19 study, seizures were more common in younger versus older children.6 Other more severe conditions occurred rarely, similar to a cohort in the United Kingdom, which prospectively studied children with SARS-CoV-2-related illness who were hospitalized and received a neurology consultation.7 The cohort found more encephalopathy and neuropsychiatric manifestations occurred in the children with MIS-C versus acute SARS-CoV-2 infection.
Children with pre-existing conditions were at increased risk of more severe SARS-CoV-2-related illness and neurological manifestation.6 This finding is similar to newly reported data in children with pre-existing neurological disease with influenza.18 In our study, children with SARS-CoV-2-related illness and pre-existing neurological conditions had 3.48 higher odds for neurological manifestation compared with children without pre-existing neurological condition. It is possible that children with pre-existing neurological conditions have decreased cognitive and functional reserves and hence less tolerance to systemic insults common to hospitalized patients such as oxygen desaturation, hypotension, and fever. Children with MIS-C had more than 2 times higher odds of neurological manifestation compared with the acute SARS-CoV-2 cohort, and this may be due in part to hyperinflammation; however, more research is needed.19 Metabolic disease, which includes type I diabetes mellitus, was also associated with neurological manifestations in children with acute SARS-CoV-2, with SARS-CoV-2 having mechanistic plausibility for “diabetogenic effect,” similar to other viruses.20
Clinical implications and future
Different patterns of neurological and nonneurological symptoms occurred in children with acute SARS-CoV-2 versus MIS-C diagnosis, which may help identify children needing close neurological monitoring. Consequences of critical illness and pediatric sepsis, including neurological manifestations, functional health, and health-related quality of life impairments are increasingly recognized,21, 22, 23, 24 but little is known in children with acute SARS-CoV-2 and MIS-C. Children with life-threatening neurological involvement (n = 43) during admission in Overcoming COVID-19 study were at risk of new neurological deficits at hospital discharge (40%) and death (26%).6 Studies regarding treatment efficacy of interventions in children with neurological manifestations in SARS-CoV-2-related conditions are vitally needed. Finally, important health effects and inequalities are emerging from the SARS-CoV-2 pandemic including poor access to health care and education,25 , 26 exposure to maltreatment,27 developmentally important experiences,28 and parental loss.29 , 30
Limitations
Neurological manifestations were only recorded if present in the medical record; thus, younger age and developmental status as well as inconsistent documentation contribute to lower reporting due to ascertainment bias. Some manifestations, such as encephalopathy, may present differently by age or developmental stage unaccounted for in our data definitions. It is thus possible that encephalopathy was overreported or underreported if a child's baseline developmental status was not known by the documenting clinicians. Hospital presentation GCS scores were recorded but baseline GCS scores were not. Pre-existing conditions were determined by site research investigators using data available in chart review. Despite the goal of diverse geographical inclusion, centers participating in this preliminary report are largely from North America. Some patients of the acute SARS-CoV-2 group were admitted for other primary diagnoses (e.g., trauma) and were found to be positive for SARS-CoV-2 due to center testing policies; we are unable to accurately report the number of these patients. Otherwise asymptomatic children with neurological conditions such as stroke may not have been tested for SARS-CoV-2 due to low clinical suspicion and thus some neurological manifestations may be underreported.17 In children presenting with comorbid acute neurological disease, we were not able to determine whether neurological manifestations were due to SARS-CoV-2-related condition or the comorbid disease. Some patients in our consortium were included in other published US cohort studies. Last, impact of the SARS-CoV-2 delta variant is largely absent from this analysis.
The strengths of our study include prospective data collection using a case report form with defined data elements in a multicenter, multinational consortium. Future consortium reports will focus on relationships between neurological manifestations, physiologic and laboratory data, acute SARS-CoV-2- and MIS-C-specific treatments, and outcomes at hospital discharge. In addition, the consortium has launched a posthospital discharge outcome in a subset of patients. Results of these studies will inform future hypothesis-driven proposals to uncover pathophysiology of neurological manifestations of SARS-CoV-2 conditions in the developing brain and therapeutic opportunities. Finally, long-term goals of this consortium are to create a living platform for the streamlined, global reporting of neurological manifestations in future epidemics and pandemics.
Conclusions
In this multicenter study of children hospitalized with acute SARS-CoV-2 and MIS-C, neurological manifestations were common. Older age, MIS-C diagnosis, pre-existing neurological and metabolic conditions, and nonneurological symptoms were associated with increased risk of neurological manifestations.
Acknowledgments
Research Coordinators: Melissa L. Hutchinson, MD, MA; Josey Hensley RN, BSN, CCRN; Lisa Steele RN, BSN, CCRN (Nationwide Children's Hospital); Tracy Jones, BS (Oklahoma University Health Sciences Center); Geoffrey M. Houtz (University of North Carolina School of Medicine); Jacqueline Lee-Eng, BSc; Mikaela Gatterman, R. EEG T (Seattle Children's Hospital); Jacqueline Harrison, BA (Children's Hospital of Philadelphia); Sarah F Frankl, MD (University of Michigan); Ben Orwoll, MD (Oregon Health & Science University); Travis Kirkpatrick, RN; Aysun Tekin, MD (Mayo Clinic Rochester); Marianne Dufour (Université Laval); Luisa Gil Diaz, BS; Jessica Weibrecht, BA; Amy Ouyang, BA (Washington University in St. Louis); Maureen G Richardson, BSN, RN, CPN (Children's Healthcare of Atlanta); Paige Selenski, Rebecca Rehborg, BA; Rupa Nallamothu, MBBS (Medical College of Wisconsin); Ronke Awojoodu MPH, BSN; Colleen Mennie RN, BSN (John Hopkins University); Dr José Albino da Paz, PhD (University of São Paulo); Min Ye Shen, MD; Mallory Kerner-Rossi, MD; Meghan Gray, MD; Benjamin Hooe, MD; Chelsea Earley, MD; Arsenoi Asfour, MD (Columbia University).
University of Pittsburgh Data Coordinating Center (Department of Critical Care Medicine's Clinical Research, Investigation, and Systems Modeling of Acute Illness (CRISMA) center and UPMC Children's Hospital of Pittsburgh's Division of Pediatric Critical Care Medicine):
Research staff: Pamela Rubin, RN; David Maloney, BS; Nicole Toney, MPH; Ali Smith Scott, BA.
Data coordination: Dan Ricketts, MET; Edvin Music, MSIS, MBA; Jonathan Holton, MSIS.
Statistical analysis: James Yun, MS, Chung-Chou H. Chang, PhD.
GCS-NeuroCOVID Consortium Steering Committee: Sherry H.-Y. Chou, MD, MSc (Northwestern University–Feinberg School of Medicine, USA); Raimund Helbok, MD (Medical University of Innsbruck, Innsbruck, Austria); Paul Vespa, MD (University of California, Los Angeles, USA); Daiwai Olson, RN, PhD (University of Texas Southwestern, USA); Claude Hemphill, MD (University of California, San Francisco, USA); Chethan P Venkatasubba Rao MD (Baylor College of Medicine, USA); Nerissa Ko, MD, MS (University of California, San Francisco, USA); Jose I. Suarez, MD (The Johns Hopkins University School of Medicine, USA); Shraddha Mainali, MD (Virginia Commonwealth University, USA); Molly McNett, PhD (The Ohio State University, USA).
Neurocritical Care Society for hosting the GCS-NeuroCOVID Consortium weblink to register for study participation.
Pediatric research networks. We thank Pediatric Acute Lung Injury and Sepsis Investigators, Pediatric Neurocritical Care Research Group, Canadian Critical Care Trials Group, European Society for Pediatric and Neonatal Intensive Care, Australia and New Zealand Intensive Care Society, World Federation of Pediatric Intensive and Critical Care Societies, Red Colaborativa Pediátrica de Latinoamérica, United Kingdom Paediatric Critical Care Society Study group, Prevalence of Acute critical Neurological disease in children: a Global Epidemiological Assessment Investigators, Brazilian Research in Intensive Care network and the Pediatric Acute & Critical Care Medicine Asian Network for allowing distribution of this study opportunity to their members.
Finally, special thank you to the families and children in our care and to health care providers for their devotion to public health during the pandemic.
Footnotes
Group authorship: Members of “GCS-NeuroCOVID Consortium – Pediatrics”: Marlina E. Lovett, MD, Department of Pediatrics, Division of Critical Care Medicine, Nationwide Children's Hospital, The Ohio State University, Columbus, OH, USA; Casey Stulce, MD, Department of Pediatrics, University of Chicago, Chicago, Il, USA; Mais Yacoub MD, FAAP, Department of Pediatrics, Division of Critical Care, UMC Children's Hospital, Las Vegas, NV, USA; Renee M. Potera, MD, Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA; Elizabeth Zivick, MD, FAAP, Department of Pediatrics, Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston, South Carolina, USA; Adrian Holloway, MD, Department of Pediatrics, Division of Critical Care, University of Maryland Medical Center, Baltimore, Maryland, USA; Ashish Nagpal, MD, Department of Pediatrics, Section of Critical Care Medicine, Oklahoma Children's Hospital at OU health, Oklahoma University College of Medicine, Oklahoma City, OK, USA; Kari Wellnitz, MD, Division of Pediatric Critical Care, Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA; Theresa Czech, MD, Division of Pediatric Neurology, Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA; Katelyn M. Even, MD, Division of Pediatric Critical Care Medicine, Penn State College of Medicine, Hershey, Pennsylvania, USA; Werther Brunow de Carvalho, PhD, FCCS, José Albino da Paz, and Isadora Souza Rodriguez, MD, Department of Pediatrics, University of São Paulo, São Paulo, Brazil; Stephanie P. Schwartz, MD and Tracie C. Walker, MD, Department of Pediatrics, University of North Carolina at Chapel Hill Hospitals, Chapel Hill, North Carolina, USA; Santiago Campos-Miño, MD, MSc, Pediatric Intensive Care Unit, Hospital Metropolitano, Quito, Ecuador; Leslie A. Dervan MD, MS, (University of Washington School of Medicine), Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Washington School of Medicine, Seattle, WA, USA; Andrew S Geneslaw, MD, MS and Taylor B. Sewell, MD, MBA, Department of Pediatrics, Division of Pediatric Critical Care and Hospital Medicine, Columbia University Irving Medical Center, New York, NY, USA; Patrice Pryce, MD, Department of Pediatrics, Division of Pediatric Critical Care and Hospital Medicine, Columbia University Irving Medical Center, Morgan Stanley Children's Hospital New York-Presbyterian Hospital, New York, NY, USA; Wendy G. Silver, MD, MA, Jieru Egeria Lin, MD, PhD, and Wendy S. Vargas, MD, Department of Neurology, Division of Child Neurology, Columbia University Irving Medical Center, New York, NY, USA; Alexis Topjian, MD, MSCE and Alicia M Alcamo MD, MPH, Division of Critical Care Medicine at The Children's Hospital of Philadelphia, Philadelphia, PA; Departments of Anesthesiology and Critical Care Medicine and Pediatrics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; Jennifer L McGuire MD MSCE, Division of Neurology at The Children's Hospital of Philadelphia, Philadelphia, PA; Departments of Neurology and Pediatrics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA; Jesus Angel Domínguez Rojas, MD, and Jaime Tasayco Muñoz, MD, Department of Pediatrics, Division of Pediatric Critical Care, Hospital de Emergencia Villa El Salvador, Lima, Peru; Sue J. Hong, MD; William J. Muller, MD, PhD, and Matthew Doerfler, MD, Department of Pediatrics, Ann & Robert H. Lurie Children's Hospital of Chicago and Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA; Cydni N. Williams, MD, MCR, Department of Pediatrics, Division of Pediatric Critical Care; Pediatric Critical Care and Neurotrauma Recovery Program Portland, Oregon Health & Science University, OR, USA; Kurt Drury, MD, Oregon Health & Science University, Department of Pediatrics, Division of Pediatric Critical Care, Oregon Health & Science University, Portland, OR, USA; Dhristie Bhagat, MD; Aaron Nelson, MD, MBS, and Dana Price, MD, Department of Neurology, NYU Langone Health, New York, NY, USA; Heda Dapul, MD and Laura Santos, MD, MS, Department of Pediatrics, Division of Pediatric Critical Care, Hassenfeld Children's Hospital at NYU Langone Health, New York, NY, USA; Robert Kahoud, MD, Division of Pediatric Critical Care Medicine, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, MN, USA; Conall Francoeur, MDCM, Department of Pediatrics, CHU de Québec – Université Laval Research Center, Quebec City, Quebec, Canada; Brian Appavu, MD, Division of Neurology, Barrow Neurological Institute at Phoenix Children's Hospital, University of Arizona, College of Medicine, Phoenix, AZ, USA; Kristin P. Guilliams, MD, MSCI, and Shannon C. Agner, MD, PhD, Departments of Neurology, Pediatrics, and Radiology, Washington University in St. Louis, St. Louis, Missouri, USA; Karen H Walson, MD, Department of Pediatric Critical Care Medicine, Children's Healthcare of Atlanta, Atlanta, GA, USA; Lindsey Rasmussen, MD, FAAP, and Anna Janas, MD, PhD, Pediatric Critical Care Medicine, Lucile Packard Children's Hospital, Stanford University, Stanford, CA, USA; Peter Ferrazzano, MD, Department of Pediatrics, University of Wisconsin, Madison, WI, USA ferrazzano@pediatrics.wisc.edu; Raquel Farias-Moeller, MD, Department of Neurology, Division Child Neurology, Medical College of Wisconsin, Children's Wisconsin. Milwaukee, WI, USA; Kellie C. Snooks, DO, Department of Pediatrics, Medical College of Wisconsin, Children's Wisconsin. Milwaukee, WI, USA; Chung-Chou H. Chang, PhD, and James Yun, MS, Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA, USA
Authors and contributors: Drs. Fink, Robertson, Schober, Wainwright, and Roa had full access to all of data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Fink, Schober, and Robertson. Acquisition, analysis, and interpretation of data: Fink, Schober, Robertson, Wainwright, and Roa. Drafting of the manuscript: Fink. Critical revision of the manuscript for important intellectual content: Fink, Schober, Robertson, Wainwright, Roa. Lovett, Stulce, Yacoub, Potera, Zivick, Holloway, Nagpal, Wellnitz, Czech, Even, Brunow de Carvalho, Da Paz, Rodriguez, Schwartz, Walker, Campos- Miño, Dervan, Geneslaw, Sewell, Lin, Pryce, Silver, Vargas, Topjian, Alcamo, McGuire, Dominguez, Rojas, Tasayco-Muñoz, Hong, Muller, Doerfler, Williams, Drury, Bhagat, Nelson, Price, Dapul, Santos, Kahoud, Francoeur, Appavu, Guilliams, Agner, Walson, Rasmussen, Ferrazzano. Statistical analysis: Yun, Chang, Fink. Obtained funding: Fink, Schober, Robertson. Administrative, technical, or material support: Fink. Supervision: Chang, Fink, Schober, Robertson.
Declaration of Interest: Related funding: This study was in part funded by the Neurocritical Care Society Investing in Clinical Neurocritical Care Research (INCLINE) grant (EF, MS, CR). Unrelated funding: Fink: National Institutes of Health; payment to the institution. Schober: National Institutes of Health; payment to the institution. Robertson: National Institutes of Health and Army Medical Research and Material Command (AMRMC)/Rubicon Biotechnologies grants; both payments to the institution. National Neurotrauma Society Payment to C. Robertson (travel costs for NNS symposium, Pittsburgh 2019). National Neurotrauma Society President, Council Pediatric Neurocritical Care Research Group Executive Committee, Past-Chair (2016-2018). Wainwright: Sage Therapeutics: Member, Clinical Advisory Board; payment to him. Roa: None.
Role of the funding source: The funder had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pediatrneurol.2021.12.010.
Supplementary data
References
- 1.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou S.H., Beghi E., Helbok R., et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19-a report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Mannan O., Eyre M., Löbel U., et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77:1440–1445. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin J., Lawson E.C., Verma S., Peterson R.B., Sidhu R. Cytotoxic lesion of the corpus callosum in an adolescent with multisystem inflammatory syndrome and SARS-CoV-2 infection. AJNR Am J Neuroradiol. 2020;41:2017–2019. doi: 10.3174/ajnr.A6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank C.H.M., Almeida T.V.R., Marques E.A., et al. Guillain-barré syndrome associated with SARS-CoV-2 infection in a pediatric patient. J Trop Pediatr. 2020;67:fmaa044. doi: 10.1093/tropej/fmaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaRovere K.L., Riggs B.J., Poussaint T.Y., et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray S.T.J., Abdel-Mannan O., Sa M., et al. Neurological manifestations of SARS-CoV-2 infection in hospitalised children and adolescents in the UK: a prospective national cohort study. Lancet Child Adolesc Health. 2021;5:631–641. doi: 10.1016/S2352-4642(21)00193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helbok R., Chou S.H., Beghi E., et al. NeuroCOVID: it's time to join forces globally. Lancet Neurol. 2020;19:805–806. doi: 10.1016/S1474-4422(20)30322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frontera J., Mainali S., Fink E.L., et al. Global consortium study of neurological dysfunction in COVID-19 (GCS-NeuroCOVID): study design and rationale. Neurocrit Care. 2020;33:25–34. doi: 10.1007/s12028-020-00995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Society NC . 2020. COVID-19 Research Opportunities.https://www.neurocriticalcare.org/research/covid-19-research-opportunities Available at: [Google Scholar]
- 11.McNett M., Fink E.L., Schober M., et al. The global consortium study of neurological dysfunction in COVID-19 (GCS-NeuroCOVID): development of case report forms for global use. Neurocrit Care. 2020;33:793–828. doi: 10.1007/s12028-020-01100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leteurtre S., Duhamel A., Salleron J., Grandbastien B., Lacroix J., Leclerc F. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013;41:1761–1773. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 13.Jensen M.D., Ryan D.H., Apovian C.M., et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 15.Panda P.K., Sharawat I.K., Panda P., Natarajan V., Bhakat R., Dawman L. Neurological complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis. J Trop Pediatr. 2020;67:fmaa070. doi: 10.1093/tropej/fmaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schober M.E., Pavia A.T., Bohnsack J.F. Neurologic manifestations of COVID-19 in children: emerging pathophysiologic insights. Pediatr Crit Care Med. 2021;22:655–661. doi: 10.1097/PCC.0000000000002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beslow L.A., Linds A.B., Fox C.K., et al. Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann Neurol. 2021;89:657–665. doi: 10.1002/ana.25991. [DOI] [PubMed] [Google Scholar]
- 18.Frankl S., Coffin S.E., Harrison J.B., Swami S.K., McGuire J.L. Influenza-associated neurologic complications in hospitalized children. J Pediatr. 2021;239:24–31.e1. doi: 10.1016/j.jpeds.2021.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peart Akindele N., Kouo T., Karaba A.H., et al. Distinct cytokine and chemokine dysregulation in hospitalized children with acute coronavirus disease 2019 and multisystem inflammatory syndrome with similar levels of nasopharyngeal severe acute respiratory syndrome coronavirus 2 shedding. J Infect Dis. 2021;224:606–615. doi: 10.1093/infdis/jiab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coppieters K.T., Boettler T., von Herrath M. Virus infections in type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:a007682. doi: 10.1101/cshperspect.a007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman J.J., Banks R., Berg R.A., et al. Critical illness factors associated with long-term mortality and health-related quality of life morbidity following community-acquired pediatric septic shock. Crit Care Med. 2020;48:319–328. doi: 10.1097/CCM.0000000000004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stubbs D.J., Yamamoto A.K., Menon D.K. Imaging in sepsis-associated encephalopathy--insights and opportunities. Nat Rev Neurol. 2013;9:551–561. doi: 10.1038/nrneurol.2013.177. [DOI] [PubMed] [Google Scholar]
- 23.Abend N.S., Arndt D.H., Carpenter J.L., et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013;81:383–391. doi: 10.1212/WNL.0b013e31829c5cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning J.C., Pinto N.P., Rennick J.E., Colville G., Curley M.A.Q. Conceptualizing post intensive care syndrome in children-the PICS-p framework. Pediatr Crit Care Med. 2018;19:298–300. doi: 10.1097/PCC.0000000000001476. [DOI] [PubMed] [Google Scholar]
- 25.Jensen C., McKerrow N.H. Child health services during a COVID-19 outbreak in KwaZulu-Natal Province, South Africa. S Afr Med J. 2020;0:13185. [PubMed] [Google Scholar]
- 26.Chiappini E., Parigi S., Galli L., et al. Impact that the COVID-19 pandemic on routine childhood vaccinations and challenges ahead: a narrative review. Acta Paediatr. 2021;110:2529–2535. doi: 10.1111/apa.15949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawson M., Piel M.H., Simon M. Child maltreatment during the COVID-19 pandemic: consequences of parental job loss on psychological and physical abuse towards children. Child Abuse Negl. 2020;110:104709. doi: 10.1016/j.chiabu.2020.104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christakis D.A., Van Cleve W., Zimmerman F.J. Estimation of US children's educational attainment and years of life lost associated with primary school closures during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.28786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillis S.D., Unwin H.J.T., Chen Y., et al. Global minimum estimates of children affected by COVID-19-associated orphanhood and deaths of caregivers: a modelling study. Lancet. 2021;398:391–402. doi: 10.1016/S0140-6736(21)01253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidman R., Margolis R., Smith-Greenaway E., Verdery A.M. Estimates and projections of COVID-19 and parental death in the US. JAMA Pediatr. 2021;175:745–746. doi: 10.1001/jamapediatrics.2021.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.