Abstract
LGR4-6 (leucine-rich repeating containing, G-protein-coupled receptors 4, 5, and 6) are three related receptors with upregulated expression in gastrointestinal cancers to various extents, and LGR5 is enriched in cancer stem cells. Antibody-drug conjugates (ADCs) targeting LGR5 showed robust antitumor effect in vivo but could not eradicate tumors due to plasticity of LGR5-positive cancer cells. As LGR5-negative cancer cells often express LGR4 or LGR6 or both, we reasoned that simultaneous targeting of all three LGRs may provide a more effective approach. R-spondins (RSPOs) bind to LGR4-6 with high affinity and potentiate Wnt signaling. We identified an RSPO4 furin domain mutant (Q65R) that retains potent LGR binding but no longer potentiates Wnt signaling. Drug conjugates of a peptibody comprising the RSPO4 mutant and IgG1-Fc showed potent cytotoxic effects on cancer cell lines expressing any LGR in vitro and suppressed tumor growth in vivo without inducing intestinal enlargement or other adverse effects.
Graphical Abstract
INTRODUCTION
LGR4-6 (leucine-rich repeat containing, G protein-coupled receptor 4, 5, and 6) are three related membrane receptors with a large extracellular domain (ECD) and a seven transmembrane (7TM) domain typical of GPCRs.1,2 In normal adult tissues, LGR4 is expressed broadly at low to moderate levels whereas LGR5 and LGR6 are mostly restricted to adult stem cells in the gastrointestinal system and skin.3–6 The three receptors are often co-upregulated in various cancer types, particularly in cancers of the gastrointestinal system, with LGR4 co-expressed with LGR5 or LGR6.7–12 Furthermore, LGR5 has been shown to be enriched in cancer stem cells13–16 and LGR5-positive cancer cells were shown to fuel the growth of tumor mass and metastasis.17, 18 LGR6 is a marker of cancer stem cells of squamous carcinoma.16 TCGA’s RNA-Seq data of colon, liver, and stomach cancers confirmed that LGR4 expression at high levels in nearly all cases while LGR5 and LGR6 were co-expressed with LGR4 in the majority of colon cancer and substantial fractions of liver and stomach cancer. LGR5 is also a marker of stem cells in the liver and highly expressed in hepatocellular carcinomas (HCC) with β-catenin mutations.19, 20
R-spondins are a group of four related secreted proteins (RSPO1-4) that play critical roles in normal development and survival of adult stem cells.21 RSPOs function as ligands of LGR4-6 to potentiate Wnt signaling and support stem cell growth.22–25 Mechanistically, RSPO-LGR4 form a complex to inhibit the function of RNF43 and ZNRF3, two E3 ligases that ubiquitinate Wnt receptors for degradation, leading to higher Wnt receptor levels and stronger signaling.26, 27 In contrast, RSPO-LGR5 potentiates Wnt signaling through a different mechanism and although RSPO4 has been shown to bind LGR5 it is unable to activate LGR5-mediated Wnt signaling.28 All four RSPOs comprise a N-terminal furin-like domain with 2 repeats (Fu1 and Fu2) and thrombospondin-like domain (TSP) at the C-terminus.21 The furin-like domain is both necessary and sufficient to bind LGRs to potentiate Wnt signaling whereas the TSP domain enhances the potency of furin-like domain.29–31 Crystal structure analysis revealed that the Fu1 domain binds to the E3 ligases while the Fu2 domain binds to the extracellular domain of LGR.32–35
Antibody–drug conjugates (ADCs) are monoclonal antibodies that are covalently linked to cell-killing cytotoxins (payloads).36 We and others have shown that anti-LGR5 ADCs are highly effective in inhibiting the growth of LGR5-positive colon cancers in xenograft without major adverse effect.12,37 However, tumors eventually came back due to plasticity of colon cancer cells,12,18,37 which have been shown to interconvert between an LGR5-positive and LGR5-negative phenotype.14 Both LGR5-positive and LGR5-negative colon cancer cells have been shown to express LGR4.38 Given that RSPOs bind to all three LGRs with high affinity, we reasoned that drug conjugates of an RSPO mutant protein that maintains binding to LGR without activating Wnt signaling would be able to target cancer cells expressing any LGR and therefore overcome plasticity of cancer cells. Hereby we report that an RSPO4 mutant defective in Wnt signaling was able to inhibit tumor cell growth in vitro and in vivo after being fused to the Fc domain of IgG1 and conjugated with cytotoxin.
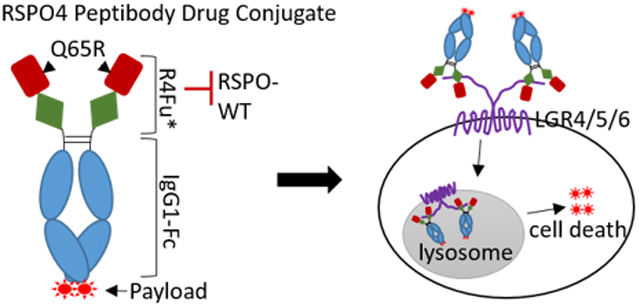
RRESULTS and DISCUSSION
Since RSPO4 has high binding affinity for LGR4-6 yet the lowest functional activity in potentiating Wnt signaling,22,39,40 we reasoned that RSPO4-Fu may be the best starting ligand for development of drug conjugates that can target all three LGRs. Based on co-crystal structures of RSPO1/2 furin domain with RNF43/ZNRF3-ECD (Extracellular Domain) and LGR5-ECD,32,33 Gln65 (Q65) of RSPO4 is predicted to be essential for interacting with RNF43/ZNRF3 (Fig. 1A). Homozygous mutation of RSPO4 Q65R in human causes nail formation defects,41, 42 and introduction of this mutation to the corresponding Gln residues in RSPO1 (Q71) and RSPO2 (Q75) led to near total loss of activity in potentiating Wnt signaling.34, 40 Wild-type (WT) and Q65R of RSPO4 furin domain (R4Fu, AA27-124) was fused to the N-terminus of the Fc domain of human IgG1 antibody with a (G4S)x3 linker in between R4Fu and Fc. The two fusion proteins, considered peptibodies (fusion of peptide and antibody), were designated R4Fu-WT and R4Fu-Q65R, respectively. They were expressed in FreeStyle™ 293-F Cells and purified by protein A-based affinity chromatography and compared in binding affinity to LGR4 and LGR5 using HEK293T cells expressing LGR4 or LGR5 or vector alone. For LGR4, WT and mutant showed Kd of 3.2 and 6.2 nM, respectively, an approximate 2-fold increase (Fig. 1B) while only a 33% increase (0.6 vs 0.8 nM) was found on LGR5 (Fig. 1B). No binding of R4Fu-Q65R was detected in HEK293T cells expressing vector alone (Fig. 1B). We then compared functional activity of the WT and mutant R4Fu in STF cells which express LGR4 but not LGR5 endogenously using the TOPFlash Wnt signaling assays.31, 43 The mutant was completely inactive in potentiating Wnt/β-catenin signaling whereas the WT protein showed robust, dose-dependent activity in STF cells (Fig. 1C). Since RSPO1 and RSPO2 have complete and partial dependence, respectively, on LGR4 for potentiating Wnt signaling,31, 44 R4Fu-Q65R was expected to be able to bind to LGR4 and prevent the binding of RSPO1/2, and therefore antagonize RSPO1/2 activity. Indeed, R4Fu-Q65R antagonized both RSPO1 and RSPO2 activity with better potency and efficacy on RSPO1 (Fig. 1D), consistent with complete and partial dependence of RSPO1 and RSPO4 on LGR4. Overall, these results indicate that the peptibody R4Fu-Q65R was able to bind both LGR4 and LGR5 with high affinity without potentiating Wnt signaling. Furthermore, the mutant could competitively block functions of RSPOs that require LGR4/5 in Wnt signaling and partially blocks RSPO2 and presumably RSPO3 which are not completely dependent on LGR4/5.
Figure 1.
RSPO4-Fu-Q65R retained high affinity binding to LGR4/5 but antagonized RSPO1/2 activity in Wnt signaling. (A) A proposed structure of RSPO4 Fu binding to RNF43/ZNRF3 and LGR4, modeled after LGR5ECD-RSPO2Fu-ZNRF3 (PDB 4UFS). (B) Binding analysis of purified R4Fu-WT and -Q65R to HEK293T cells overexpressing LGR4, LGR5 or vector control (N=2). (C) TOPFlash Wnt/β-catenin signaling activity of R4Fu-WT and -Q65R in HEK293-STF cells (N=4). (D) Antagonist activity of R4Fu-Q65R on RSPO1 and RSPO2 in STF TOPFlash Wnt/β-catenin signaling assay (N=2). Each experiment was repeated at least three times and shown here are representative graphs. Error bars are SD.
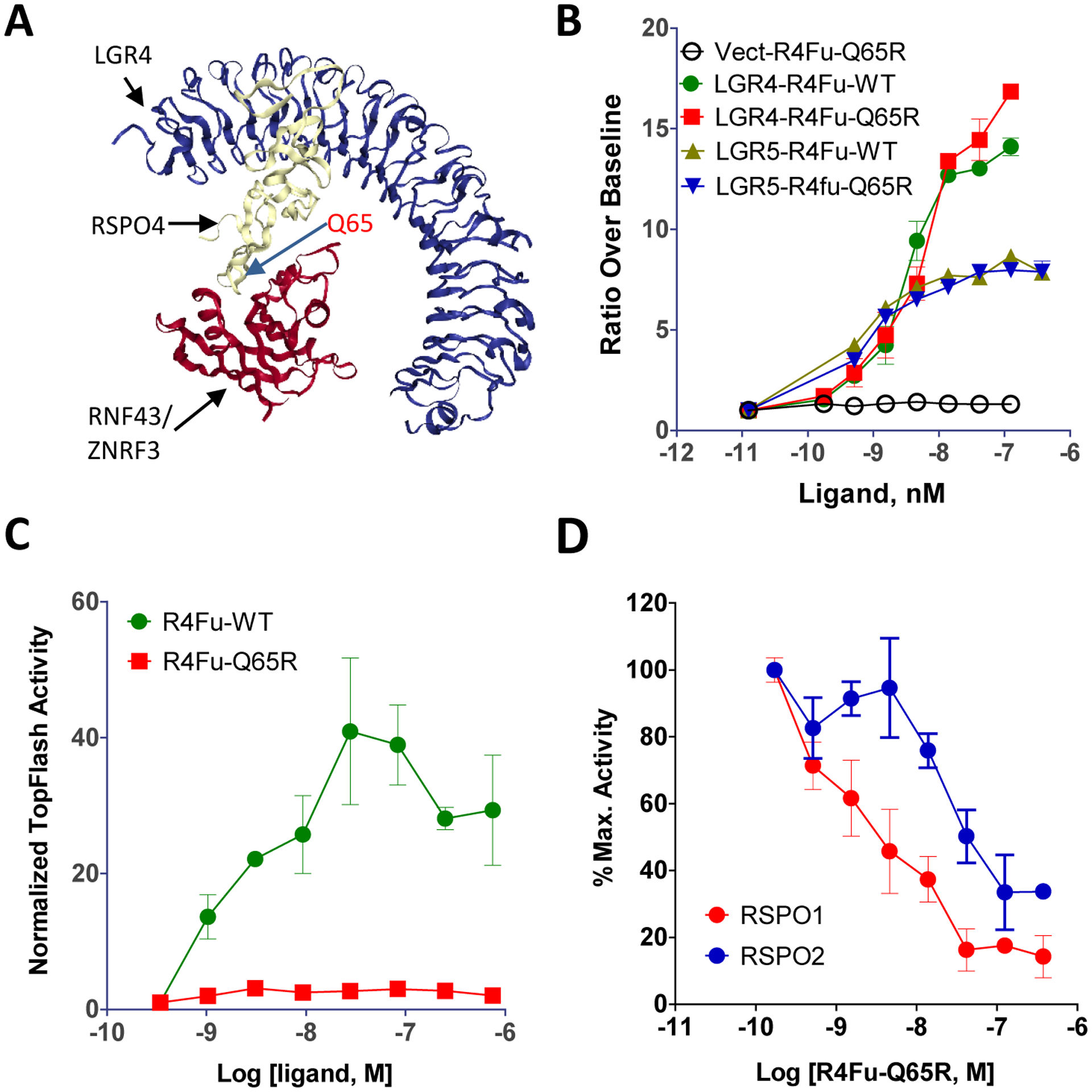
To evaluate the potential of R4Fu-Q65R as a drug carrier, a cleavable cytotoxin was covalently linked to the peptibody by site-specific conjugation as schematically illustrated (Fig. 2A). In brief, R4Fu-Q65R incorporating an LLQGA tag at the C-terminus was expressed and purified with the Q (Gln) residue in the LLQGA tag serving as the recipient group for transgluminase.45 PEG4-VC-PAB-MMAE (ethylene glycol-4x-valine-citruline-p-aminobenzoyloxycarbonyl-MMAE) was incubated with the peptibody in the presence of bacterial transglutaminase as described previously45. The peptibody-drug conjugate (PDC) product, designated R4Fu-Q65R-MMAE, was purified and analyzed for integrity and receptor binding. Gel electrophoresis showed increased molecular weight (MW) in the PDC (Fig. 2B). Receptor binding showed that the PDC retained the binding affinity of the free peptibody for LGR4 (Fig. 2C). Mass spectrometry analysis confirmed the expected MWs of the peptibody and PDC (38,867 and 40220 Da, respectively; Fig. 2D), indicating only one MMAE was conjugated per monomer unit of each peptibody. Peptide mapping validated the conjugation was specific to the LLQGA tag (see Supporting information Fig. S1). A similar PDC using duocarmycin SA (DMSA) as payload was also generated, and the product was designated R4Fu-Q65R-DMSA.
Figure 2.
Site-specific conjugation of MMAE to R4Fu-Q65R. (A) A schematic diagram of the R4Fu-Q65R with an LLQGA tag at the C-terminus. (B) Coomassie blue staining of R4Fu-Q65R before (lane 1) and after conjugation (lane 2). (C) Binding of unconjugated R4Fu-Q65R and R4Fu-Q65R-MMAE to HEK293 cells over-expressing LGR4 (N=2, Error bars are SD). The experiment was repeated more than 3 times and shown here is a representative graph. (D) Q-TOF mass spectrometry analysis of R4Fu-Q65R and R4Fu-Q65R-MMAE.
To further characterize the integrity of R4Fu-Q65R and R4Fu-Q65R-MMAE, the samples were analyzed by size exclusion chromatography. Unconjugated R4Fu-Q65R displayed a single peak with a MW of ~80 kD, consistent with the expected size of R4Fu-Q65R as a dimer enforced by the Fc domain (Fig. 2A, Supporting Information Fig. S2A). The conjugated PDC showed two peaks, one (Peak1 or P1) with a MW of ~500 kD and the other (Peake 2 or P2) with a MW of ~80 kD that was slightly larger than unconjugated peptibody (Supporting Information Fig. S2A). P1 is most likely an oligomer of 6–7 PDC dimers. Gel analysis showed that pre-separation and P1 samples contained high molecular weight whereas P2 was largely monomer/dimer under reducing/non-reducing conditions (Supporting Information Fig. S2B). P1 and P2, along with the pre-separation sample, were examined for binding to LGR4 and the results showed that both P1 and P2 bound to LGR4 with high affinity with P2 showing higher affinity (Supporting Information Fig. S2C). Importantly, all three products showed little binding to control cells without over-expressing LGR4, suggesting that PDC was not simply an aggregate that would have non-specific binding to cells. Unseparated PDC was used for all studies described below.
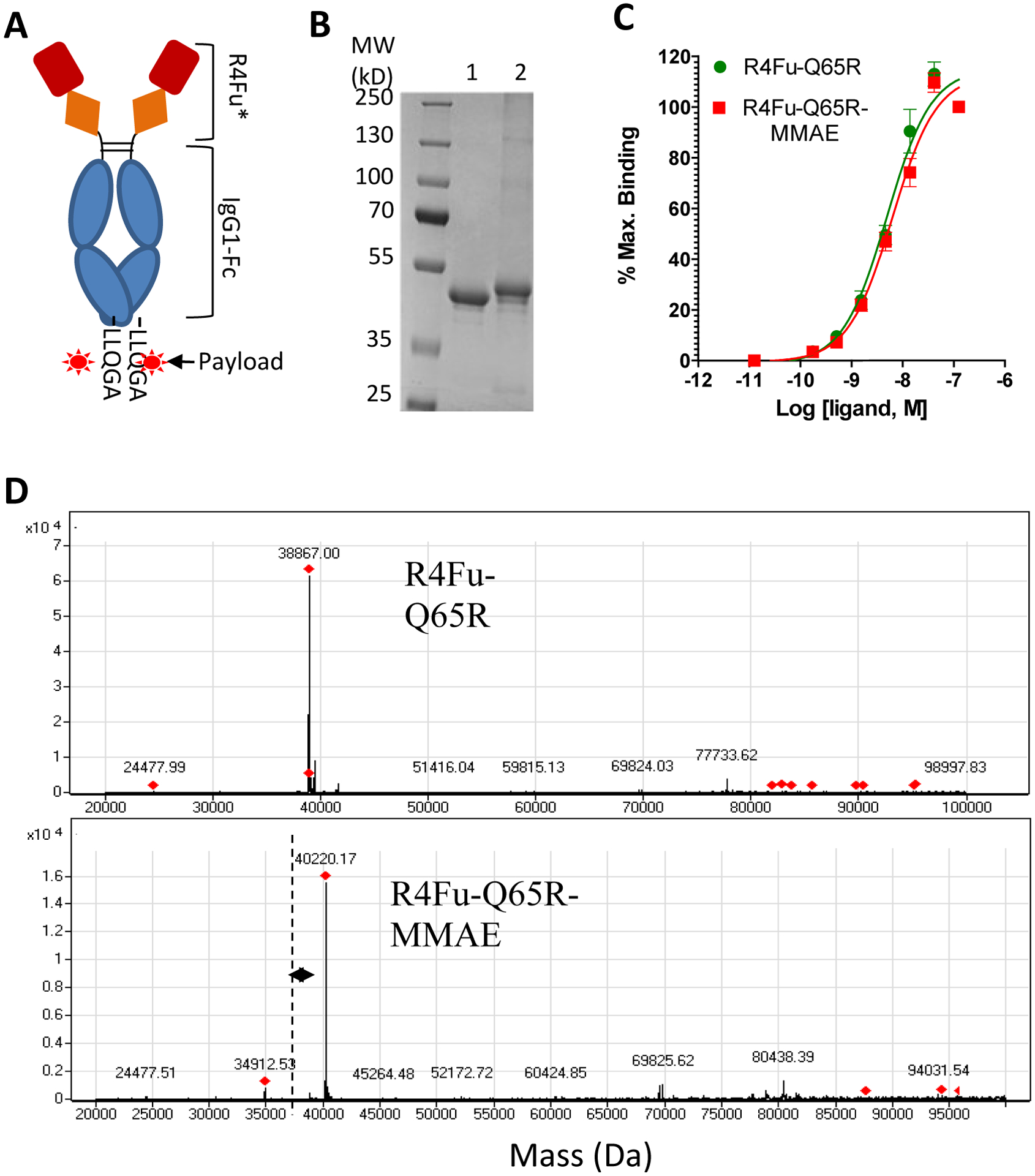
Target expression level is key to efficacy of ADCs and thus we analyzed expression level of LGR4 and LGR5 in over-expressing cells and three GI cancer cell lines (LoVo-colon cancer, AGS-stomach cancer, and PLC/PRF5-liver cancer). These three cancer cell lines were known to have relatively high levels of LGR4 and LGR5 based on RNA-seq data (Supporting Information Table S1) and other published results.37 We also generated a LoVo cell line with knockout of LGR4 (LoVo-4KO) using the CRISPR/Cas9 method.46 Total cell lysate proteins from the four cancer cell lines, parental HEK293T cells, HEK293T-LGR4, and HEK293T-LGR5 (titrated down to the range of cancer cell lines) were detected with LGR4 or LGR5 antibodies by Western blot (WB). AGS and PLC/PRF cells had similar levels of LGR4 that was approximately 2-fold higher than that of LoVo cells (Fig. 3A). LoVo-4KO cells had lost LGR4 as expected (Fig. 3A) while LGR4 in HEK293T cells are comparable to cancer cell lines. Over-expression of LGR4 led to ~60x increase compared to cancer cell lines (Fig. 3A). For LGR5, LoVo and AGS cells showed similar levels of endogenous expression while PLC/PRF5 and HEK293T cells had none detected (Fig. 3B), consistent with RNA-seq data (Table S1). LGR5 level in HEK293T-LGR5 cells is ~150x higher than that of LoVo and AGS cells (Fig. 3B), and surprisingly, LoVo-4KO cells also lost LGR5 expression.
Figure 3.
Analysis of LGR4 and LGR5 expression and R4Fu binding in cancer cell lines. (A). WB of LGR4 in recombinant and cancer cell lines. Actin is loading control. (B) WB of LGR5 in HEK293T and cancer cell lines. GAPDH is loading control. (C) Binding and internalization of peptibody and PDC in LoVo and LoVo-4KO cells by fluorescence confocal microscopy. Cell were incubated with the ligands at 36 nM, fixed, permeabilized, and stained with Alexa-488-labeled anti-human IgG (green). Nuclei were counter-stained with To-PRO3 (blue).
Next we examined binding and internalization of R4Fu-Q65R and the PDC in LoVo and LoVo-4KO cells by immunofluorescence confocal microscopy. After incubating for one hour at 37 °C, both unconjugated and conjugated R4Fu-Q65R were detected in a dose-dependent fashion in endocytic vesicles in parental LoVo cells (Fig. 3C, left panel; Supporting Information Fig. S3) while little signal was detected in LoVo-4KO cells (Fig. 3C, right panel; Supporting Information Figure S3). These results suggest that both peptibody and PDC were rapidly internalized following binding to LGR4/LGR5 with little non-specific binding.
Cytotoxic activities of the PDCs was then analyzed on recombinant and cancer cell lines. The R4Fu-Q65R-MMAE showed very potent cytotoxic effect on HEK293T overexpressing any one of the LGRs (IC50 = 0.5 nM, 1.1 nM, and 0.9 nM for LGR4, LGR5, and LGR6, respectively, Fig. 4A). The PDC showed much lower potency with IC50 >10 nM on parental HEK293T cells (Fig. 4A). When tested on the LoVo colon cancer cell line, which expresses both LGR4 and LGR5, R4Fu-Q65R-MMAE inhibited cell growth with an IC50 of 0.8 nM. LoVo-4KO cells or LoVo cells with knockdown (KD) of LGR5 led to much reduced sensitivity to the PDC (IC50 > 10 nM, Fig. 4B), indicating the cytotoxicity was mediated mostly by LGR5 in LoVo cells. We also compared cytotoxic activities of the two peaks of R4Fu-Q65R-MMAE separated by gel filtration and found that the two peaks had nearly identical activity on LoVo cells with little activity on LoVo-4KO cells (Supporting Information Fig. S4A and Fig. S4B). The results suggest that cytotoxic activity was not due to potential non-specific binding by PDC oligomers.
Figure 4.
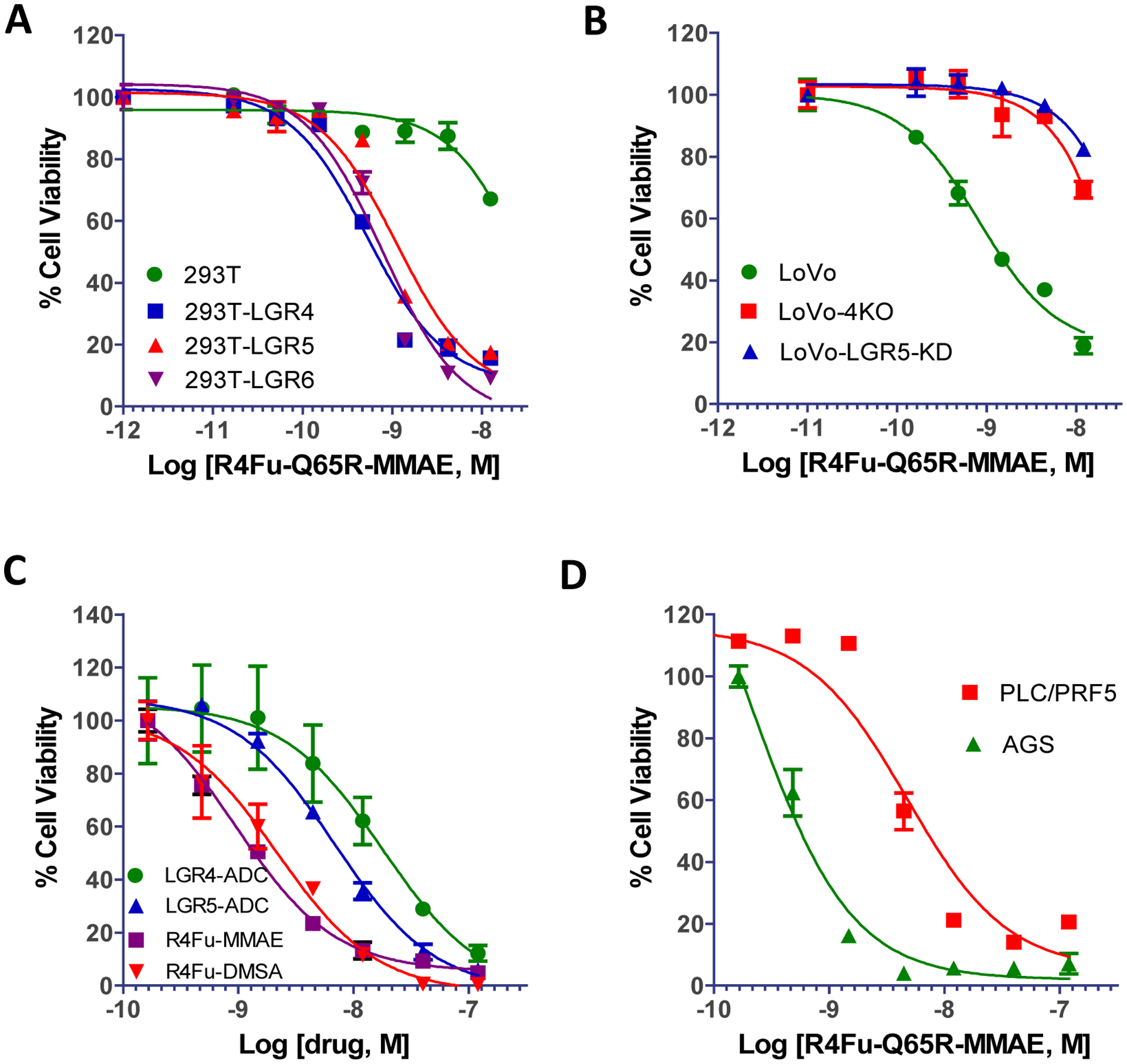
Cytotoxic activity of R4Fu-Q65R-MMAE and –DMSA in various cell lines. (A) Viability of HEK293T cells with or without over-expressing LGR4, LGR5, or LGR6. (B) Viability of LoVo cells with KO of LGR4 or KD of LGR5 treated with R4Fu-Q65R PDC. (C) Viability of LoVo cells treated with ADCs of LGR4, LGR5, or R4Fu-Q65R PDC. (D) Viability of PLC/PRF5 and AGS cells treated with R4Fu-Q65R-MMAE. Each experiment was repeated at least three times and shown here are representative graphs. Error bars are SD (N = 2).
We also compared the R4Fu-Q65R PDCs with anti-LGR5 ADC37 and anti-LGR4 ADC side-by-side on LoVo cells. Both PDCs were ~3x more potent than the anti-LGR5 ADC and ~10x more potent than the anti-LGR4 ADC (Fig. 4C). Of note, the PDC had a drug-protein ratio of 2.0 whereas the ADCs had a drug-antibody ratio of ~4.0. The higher potencies of PDCs, despite similar affinity of PDCs and ADCs in binding to the receptors, may be due to simultaneous targeting of two receptors and/or differential intracellular trafficking induced by ligand versus antibody. In the stomach cancer cell line AGS and liver cancer cell lines PLC/PRF5, both have high LGR expression, R4Fu-Q65R-MMAE showed IC50s of 0.4 nM and 3.9 nM, respectively (Fig. 4D). The lower potency on PLC/PRF5 cells could be due to its lower sensitivity to MMAE (Supporting Information Fig. S5) or lack of LGR5 (Fig. 3B). These results suggest that RSPO4-Fu PDCs were able to inhibit the growth of cancer cell lines expressing LGRs at sufficient levels.
We then asked if R4Fu-Q65R-MMAE and R4Fu-65R-DMSA could inhibit tumor growth in vivo. Stability of R4Fu-Q65R and its MMAE conjugate were analyzed in mice and both had half-life of <24 hours (Fig. 5A). In a xenograft model of LoVo cells in athymic nude mice, the animals were randomized into 4 groups with 5–6 per group when tumors reached the size of ~130 mm3. The animals were administered PBS (vehicle), unconjugated R4Fu-Q65R, R4Fu-Q65R-MMAE, or R4Fu-Q65R-DMSA at 5 mg/kg by intraperitoneal injection every other day for a total of eight injections. Tumor sizes were measured and animals were monitored for general health. Both MMAE and DMSA-conjugated PDC showed significant anti-tumor effect with 70–80% inhibition of tumor growth (Fig. 5B) and significant increase in survival (Fig. 5C). Neither body weight loss nor other gross toxicity was observed with any of the treatment groups. However, tumors eventually came back in both PDC-treated groups after treatment stopped (Supporting Information Fig. S6).
Figure 5.
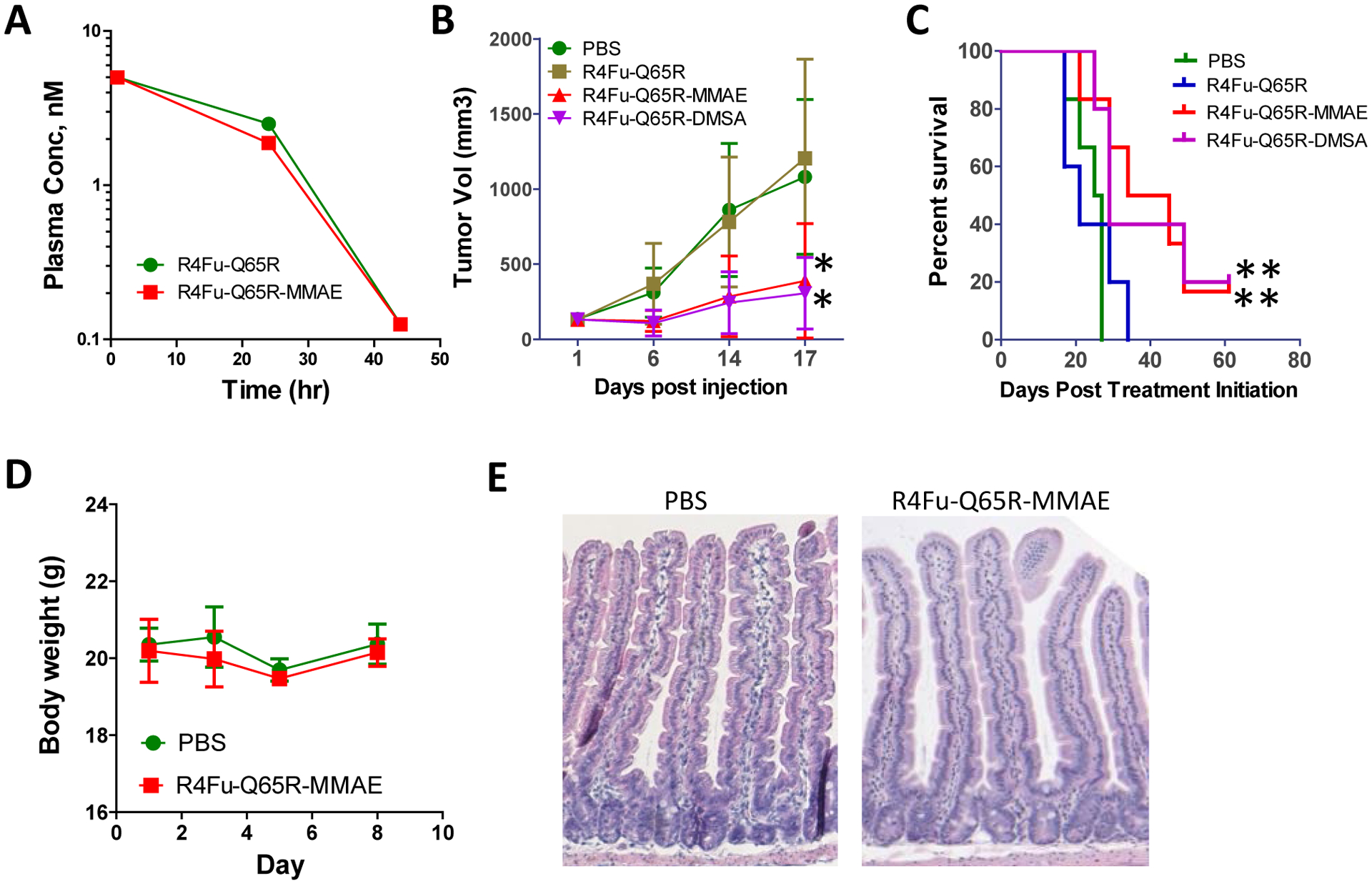
Anti-tumor effect of R4Fu-Q65R PDCs in xenograft models of LoVo cells and in vivo toxicity. (A) Pharmacokinetic analysis (2 mice per group). (B) Tumor growth curve (6 mice per group). *p< 0.05 vs PBS group. (C) Kaplan-Meier survival plot of R4Fu-Q65R-MMAE and –DMSA vs PBS. **p=0.02 vs PBS, Log-rank test. Last treatment was on Day 17 (N = 6 at starting). (D) Body weights of C57/Bl mice dosed with vehicle ((PBS) or PDC at 15 mg/kg on Days 1, 3, and 5 (N=3 per group). (E) H&E staining of small intestine at termination (Day 8) from vehicle or PDC-treated animals. Error bars are SD.
To evaluate tolerability of the PDCs, R4Fu-Q65R-MMAE was given to normal C57/Bl mice at 15 mg/kg on Days 1, 3, and 5, and the animals were monitored body weight and general well-being. The study was terminated on Day 8 and the animals showed no obvious signs of toxicity nor significant loss of body weight (Fig. 5D). H&E staining of intestines revealed no effect on crypt size and epithelium histology (Fig. 5E). Importantly, the lack of effect on crypt size indicated that the PDC at this dose level was not able to antagonize the activity RSPO2 and RSPO3 which are essential for self-renewal and proliferation of crypt stem cells.47 This is most likely because RSPO2/3 could partially function without LGR.31,44 It also suggests that the combined LGR levels in intestinal stem cells are too low to mediate sufficient cytotoxicity delivered by the PDCs.
LGR4-6 are well known to be upregulated in GI and other cancers at different extents, and various approaches, including anti-LGR5 ADC and neutralizing anti-RSPO2/3, had been attempted to target the RSPO-LGR pathway for cancers associated with aberrant RSPO-LGR activity.12,37,47,48. However, these one target-based agents could not eradicate tumors due to plasticity and heterogeneity of tumor cells. Here we found that an RSPO4 mutant may be used to deliver cytotoxins into cancer cells expressing any of the three LGRs while acting as an RSPO antagonist at the same time. This approach has the potential of overcoming plasticity since LGR5-negative cells often still express LGR4 or LGR6 or both.38, 49.
However, in xenograft models, R4Fu-Q65R-MMAE or –DMSA still could not eradicate tumors, most likely due to the poor pharmacokinetics of the PDC when compared to antibodies, insufficient drug load per peptibody or both. We are now evaluating other RSPO-based peptibodies and their drug conjugates to identify one with better in vivo stability, and optimizing the drug-peptibody ratio with various linker-payloads to identify PDCs with better efficacy and potency in vivo.
While this manuscript was in preparation, Yu et al published a paper that described the use of RSPO1 furin domain as a carrier to deliver MMAE into cancer cells with LGR expression.50 The authors showed modest efficacy of the protein-drug conjugate in a number of ovarian cancer models. Notably, they used a wild-type form of RSPO1 furin domain which is expected to strongly potentiate Wnt signaling and induce hyperproliferation of crypt stem cells in vivo,51 making it unsuitable for use as a drug delivery carrier.
CONCLUSION
The RSPO-LGR pathway plays critical roles in cancer development and progression, and various approaches have attempted to target it for cancer treatment. Here we described the use of an RSPO4 mutant-based peptibody that binds to all three LGRs with high affinity but no longer potentiates Wnt signaling. Drug conjugates of the peptibody displayed specific binding and cytotoxicity to cancer cells expressing any LGR in vitro and anti-tumor effect in vivo, yet tumors still came back after treatment stopped. Optimization of pharmacokinetics and payload class as well as conjugation ratio may lead to better candidates that could eradicate tumors by simultaneous targeting of the LGRs.
EXPERIMENTAL SECTION
Cell lines.
All cancer cell lines were obtained from ATCC. Knockout of LGR4 in LoVo cells were carried out using the CRISPR/Cas9 lentiviral vector with the same guide sequence as described.31, 46 HEK293T cells expressing LGR4 or LGR5 or LGR6 were generated as previously reported.22,25 All cell lines were cultured as before22 or using conditions recommended by ATCC.
Protein expression, purification, and characterization.
R4Fu-WT and R4Fu-Q65R fused to the N-terminus of human IgG1-Fc (starting at DKTHT of CH2 domain) with a signal peptide (MKHLWFFLLLVAAPRWVLS) upstream of R4Fu and a linker sequence SGGGGSGGGGSGGGGS between R4Fu and Fc were cloned into the vector pCEP5 using standard cloning methods. Protein production and characterization were carried out as we described previously.31 Protein purity is estimated to be ≥ 95% based on Coomassie blue staining. Receptor binding, Wnt signaling assays (TOPFlash) were also performed as before.31 For receptor binding, HEK293T cells expressing LGR4, LGR5 or vector control were seeded into poly-D-lysine-coated, clear-bottom black 96-well plates and cultured to confluency. On the day of binding assay, the plates were chilled on ice and ligands diluted in culture media were added to the indicated concentrations and incubated on ice for 2 hours. The cells were washed 3 times with cold PBS+1% BSA, fixed with 4% paraformaldehyde, and incubated with Alexa-555-labeled anti-human IgG (Invitrogen Cat # A-21433) at 1:500 dilution. The cells were then washed 3 times with cold PBS+1% BSA, and fluorescence was measured on a Tecan plate reader using excitation at 555 nm, and emission at 580 nm with bandwidth of 5 nm and 10 nm, respectively.
ADC synthesis and characterization.
NH2-PEG4-VC-PAB-MMAE was purchased from Levena Biopharma (San Diego, California). Activa® TI transgluminase was purchased from Modern Pantry (Eliot, Maine). Conjugation of R4Fu-Q65R was carried out using bacterial transgluminase as described by Strop et al.45 In brief, R4Fu-Q65R (5 mg/ml) was mixed with the linker payload at a molar ratio of 1:5 and the transglutaminase at 0.5% in 25 mM Tris-Cl, 150 mM NaCl, pH8.0 and incubated at room temperate for 16 hours with gentle agitation. The sample was diluted 10x with cold PBS, and loaded onto protein A column, washed with 10 column volume of cold PBS, and eluted with 100 mM glycine, pH2.6, and neutralized by 1 M Tris, pH9.0, followed by dialysis in PBS. The product was then concentrated and stored in PBS. Size exclusion chromatography was performed on a Cytiva Superdex™ 200 Increase 10/300 GL Prepacked Tricorn™ Column using an AKTA pure FPLC system.
Mass spectrometry.
Molecular weights of R4Fu-Q65R and R4FuQ65R-MMAE were determined on an Agilent 6538 UHD Accurate-Mass Quadrupole Time-of–Flight (Q-TOF) mass spectrometer coupled with an Agilent 1200 series HPLC. Conjugation site analysis were carried out with tryptic digested samples by LC/MS/MS using an Orbitrap FusionTM TribridTM mass spectrometer (Thermo ScientificTM) interfaced with a Dionex UltiMate 3000 Binary RSLCnano HPLC System. Details are described in Supporting Information.
Western blot and confocal microscopy.
WB was performed using standard procedures with anti-LGR4 antibody 7E7 and anti-LGR5 antibody ab75850 (abcam). Immunofluorescence and confocal microscopy were performed as described before.8, 52
In vitro cell viability assays.
Cells were seeded at various numbers (depending on growth rate) in 96-well plates and serial dilutions of PDC were added. The cells were incubated for 4–5 days and cell viability was measured using CellTiter-Glo® Luminescent Cell Viability Assay (Promega).
Pharmacokinetics analysis in mice:
R4Fu-Q65R and R4Fu-Q65R-MMAE were administered at 5 mg/kg by intraperitoneal injection into 11-week old, female C57BL/6J mice and blood was collected at 1, 24, and 48 fours post injection. Peptibody and PDC level in the plasma were determined by ligand binding analysis. In brief, plasma were diluted by 10x or 20x and were incubated with HEK293T-LGR4 cells. Ligand concentrations were calculated using standard curves established with purified R4Fu-Q65R on the same cell line.
In vivo studies.
Animal studies were carried out in strict accordance with the recommendations of the Institutional Animal Care and Use Committee of the respective institutes. For LoVo xenograft study, female 9-week-old nu/nu mice (Charles River Laboratories) were subcutaneously inoculated with 5 × 106 cells in 1:1 mixture with Matrigel (BD Biosciences). After 3 weeks, when tumor size reached approximately ~100 mm3, mice were randomized into 5 groups of 6 mice per group and given vehicle (PBS), unconjugated R4Fu-Q65R, R4Fu-Q65R-MMAE or –DMSA once every other day at 5 mg/kg by intraperitoneal injection for a total of 8 doses. For tolerability study in normal animals, 11-week old female C57BL/6 mice were given PBS or R4Fu-Q65R-MMAE at 15 mg/kg by IV injection on Days 1, 3, and 5. The animals were monitored for body weight and general behavior, and sacrificed on Day 8. Intestines were harvested and examined following H&E staining.
Statistical analysis.
Data are expressed as mean ± SEM or SD as indicated in the Results section. Multiple comparisons used one-way ANOVA and Dunnett’s post hoc analysis. P ≤ 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by funding from Wntrix Inc (to QJL), Cancer Prevention and Research Institute of Texas (RP190542, to QJL and KSC), the Janice David Gordon for Bowel Cancer Research Endowment (to QJL), and the National Cancer Institute (R01CA226894, to KSC).
ABBREVIATIONS USED
- PDC
peptibody drug conjugate
- ADC
antibody drug conjugate
- RSPO
R-spondin
- R4Fu
RSPO4 furin domain
- LGR4/5/6
leucine-rich repeat containing, G protein-coupled receptor 4, 5, 6
- KO
knockout
- CRISPR
clustered regularly interspaced short palindromic repeats
- MMAE
monomethyl auristatin
- GAPDH
glyceraldehyde-3-phosphate
- PBS
-
phosphate buffered saline
dehydrogenase
Footnotes
The authors declare the following competing financial interest(s): C.J., S.P., K.S.C., Q.J.L., and the Regents of the University of Texas have filed a provisional patent application related to this project. An initial version of the manuscript was deposited the preprint server bioRxiv (https://www.biorxiv.org/content/10.1101/2021.02.05.429956v1).
REFERENCES
- 1.McDonald T; Wang R; Bailey W; Xie G; Chen F; Caskey CT; Liu Q Identification and cloning of an orphan G protein-coupled receptor of the glycoprotein hormone receptor subfamily. Biochem Biophys Res Commun 1998, 247, 266–270. [DOI] [PubMed] [Google Scholar]
- 2.Hsu SY; Kudo M; Chen T; Nakabayashi K; Bhalla A; van der Spek PJ; van Duin M; Hsueh AJ The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol 2000, 14, 1257–1171. [DOI] [PubMed] [Google Scholar]
- 3.Hsu SY; Liang SG; Hsueh AJ Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol Endocrinol 1998, 12, 1830–1845. [DOI] [PubMed] [Google Scholar]
- 4.Van Schoore G; Mendive F; Pochet R; Vassart G Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochem Cell Biol 2005, 124, 35–50. [DOI] [PubMed] [Google Scholar]
- 5.Barker N; Clevers H Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 2010, 138, 1681–1696. [DOI] [PubMed] [Google Scholar]
- 6.Snippert HJ; Haegebarth A; Kasper M; Jaks V; van Es JH; Barker N; van de Wetering M; van den Born M; Begthel H; Vries RG; Stange DE; Toftgard R; Clevers H Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 2010, 327, 1385–1389. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y; Kitagawa K; Hiramatsu Y; Kikuchi H; Isobe T; Shimada M; Uchida C; Hattori T; Oda T; Nakayama K; Nakayama KI; Tanaka T; Konno H; Kitagawa M Up-regulation of GPR48 induced by down-regulation of p27Kip1 enhances carcinoma cell invasiveness and metastasis. Cancer Res 2006, 66, 11623–11631. [DOI] [PubMed] [Google Scholar]
- 8.Yi J; Xiong W; Gong X; Bellister S; Ellis LM; Liu Q Analysis of LGR4 receptor distribution in human and mouse Tissues. PLoS One 2013, 8, e78144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gugger M; White R; Song S; Waser B; Cescato R; Riviere P; Reubi JC GPR87 is an overexpressed G-protein coupled receptor in squamous cell carcinoma of the lung. Dis Markers 2008, 24, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong X; Yi J; Carmon KS; Crumbley CA; Xiong W; Thomas A; Fan X; Guo S; An Z; Chang JT; Liu QJ Aberrant RSPO3-LGR4 signaling in Keap1-deficient lung adenocarcinomas promotes tumor aggressiveness. Oncogene 2015, 34, 4692–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue Z; Yuan Z; Zeng L; Wang Y; Lai L; Li J; Sun P; Xue X; Qi J; Yang Z; Zheng Y; Fang Y; Li D; Siwko S; Li Y; Luo J; Liu M LGR4 modulates breast cancer initiation, metastasis, and cancer stem cells. FASEB J 2018, 32, 2422–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junttila MR; Mao W; Wang X; Wang BE; Pham T; Flygare J; Yu SF; Yee S; Goldenberg D; Fields C; Eastham-Anderson J; Singh M; Vij R; Hongo JA; Firestein R; Schutten M; Flagella K; Polakis P; Polson AG Targeting LGR5+ cells with an antibody-drug conjugate for the treatment of colon cancer. Science translational medicine 2015, 7, 314ra186. [DOI] [PubMed] [Google Scholar]
- 13.Vermeulen L; Todaro M; de Sousa Mello F; Sprick MR; Kemper K; Perez Alea M; Richel DJ; Stassi G; Medema JP Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci U S A 2008, 105, 13427–13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi S; Yamada-Okabe H; Suzuki M; Natori O; Kato A; Matsubara K; Jau Chen Y; Yamazaki M; Funahashi S; Yoshida K; Hashimoto E; Watanabe Y; Mutoh H; Ashihara M; Kato C; Watanabe T; Yoshikubo T; Tamaoki N; Ochiya T; Kuroda M; Levine AJ; Yamazaki T LGR5-positive colon cancer stem cells interconvert with drug-resistant LGR5-negative cells and are capable of tumor reconstitution. Stem Cells 2012, 30, 2631–2644. [DOI] [PubMed] [Google Scholar]
- 15.Kemper K; Prasetyanti PR; De Lau W; Rodermond H; Clevers H; Medema JP Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem Cells 2012, 30, 2378–2386. [DOI] [PubMed] [Google Scholar]
- 16.Huang PY; Kandyba E; Jabouille A; Sjolund J; Kumar A; Halliwill K; McCreery M; DelRosario R; Kang HC; Wong CE; Seibler J; Beuger V; Pellegrino M; Sciambi A; Eastburn DJ; Balmain A Lgr6 is a stem cell marker in mouse skin squamous cell carcinoma. Nat Genet 2017, 49, 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimokawa M; Ohta Y; Nishikori S; Matano M; Takano A; Fujii M; Date S; Sugimoto S; Kanai T; Sato T Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature 2017, 545, 187–192. [DOI] [PubMed] [Google Scholar]
- 18.de Sousa e Melo F; Kurtova AV; Harnoss JM; Kljavin N; Hoeck JD; Hung J; Anderson JE; Storm EE; Modrusan Z; Koeppen H; Dijkgraaf GJ; Piskol R; de Sauvage FJ A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature 2017, 543, 676–680. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y; Sakamoto M; Fujii G; Tsuiji H; Kenetaka K; Asaka M; Hirohashi S Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology 2003, 37, 528–533. [DOI] [PubMed] [Google Scholar]
- 20.Huch M; Dorrell C; Boj SF; van Es JH; Li VS; van de Wetering M; Sato T; Hamer K; Sasaki N; Finegold MJ; Haft A; Vries RG; Grompe M; Clevers H In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lau WB; Snel B; Clevers HC The R-spondin protein family. Genome biology 2012, 13, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmon KS; Gong X; Lin Q; Thomas A; Liu Q R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 2011, 108, 11452–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lau W; Barker N; Low TY; Koo BK; Li VS; Teunissen H; Kujala P; Haegebarth A; Peters PJ; van de Wetering M; Stange DE; van Es JE; Guardavaccaro D; Schasfoort RB; Mohri Y; Nishimori K; Mohammed S; Heck AJ; Clevers H Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011, 476, 293–297. [DOI] [PubMed] [Google Scholar]
- 24.Glinka A; Dolde C; Kirsch N; Huang YL; Kazanskaya O; Ingelfinger D; Boutros M; Cruciat CM; Niehrs C LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep 2011, 12, 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong X; Carmon KS; Lin Q; Thomas A; Yi J; Liu Q LGR6 Is a high affinity receptor of R-Spondins and potentially functions as a tumor suppressor. PLoS One 2012, 7, e37137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao HX; Xie Y; Zhang Y; Charlat O; Oster E; Avello M; Lei H; Mickanin C; Liu D; Ruffner H; Mao X; Ma Q; Zamponi R; Bouwmeester T; Finan PM; Kirschner MW; Porter JA; Serluca FC; Cong F ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–200. [DOI] [PubMed] [Google Scholar]
- 27.Koo BK; Spit M; Jordens I; Low TY; Stange DE; van de Wetering M; van Es JH; Mohammed S; Heck AJ; Maurice MM; Clevers H Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012, 488, 665–669. [DOI] [PubMed] [Google Scholar]
- 28.Park S; Wu L; Tu J; Yu W; Toh Y; Carmon KS; Liu QJ Unlike LGR4, LGR5 potentiates Wnt-beta-catenin signaling without sequestering E3 ligases. Sci Signal 2020, 13, eaaz4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nam JS; Park E; Turcotte TJ; Palencia S; Zhan X; Lee J; Yun K; Funk WD; Yoon JK Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev Biol 2007, 311, 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Q; Yokota C; Semenov MV; Doble B; Woodgett J; He X R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J Biol Chem 2007, 282, 15903–15911. [DOI] [PubMed] [Google Scholar]
- 31.Park S; Cui J; Yu W; Wu L; Carmon KS; Liu QJ Differential activities and mechanisms of the four R-spondins in potentiating Wnt/beta-catenin signaling. J Biol Chem 2018, 293, 9759–9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen PH; Chen X; Lin Z; Fang D; He X The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev 2013, 27, 1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zebisch M; Jones EY Crystal structure of R-spondin 2 in complex with the ectodomains of its receptors LGR5 and ZNRF3. J Struct Biol 2015, 191, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng WC; de Lau W; Forneris F; Granneman JC; Huch M; Clevers H; Gros P Structure of stem cell growth factor R-spondin 1 in complex with the ectodomain of its receptor LGR5. Cell reports 2013, 3, 1885–1892. [DOI] [PubMed] [Google Scholar]
- 35.Xu K; Xu Y; Rajashankar KR; Robev D; Nikolov DB Crystal structures of Lgr4 and its complex with R-spondin1. Structure 2013, 21, 1683–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter PJ; Senter PD Antibody-drug conjugates for cancer therapy. Cancer J 2008, 14, 154–169. [DOI] [PubMed] [Google Scholar]
- 37.Gong X; Azhdarinia A; Ghosh SC; Xiong W; An Z; Liu Q; Carmon KS LGR5-targeted antibody-drug conjugate eradicates gastrointestinal tumors and prevents recurrence. Mol Cancer Ther 2016, 15, 1580–1590. [DOI] [PubMed] [Google Scholar]
- 38.Dame MK; Attili D; McClintock SD; Dedhia PH; Ouillette P; Hardt O; Chin AM; Xue X; Laliberte J; Katz EL; Newsome GM; Hill DR; Miller AJ; Tsai YH; Agorku D; Altheim CH; Bosio A; Simon B; Samuelson LC; Stoerker JA; Appelman HD; Varani J; Wicha MS; Brenner DE; Shah YM; Spence JR; Colacino JA Identification, isolation and characterization of human LGR5-positive colon adenoma cells. Development 2018, 145, dev153029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warner ML; Bell T; Pioszak AA Engineering high-potency R-spondin adult stem cell growth factors. Mol Pharmacol 2015, 87, 410–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zebisch M; Xu Y; Krastev C; MacDonald BT; Chen M; Gilbert RJ; He X; Jones EY Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nature communications 2013, 4, 2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blaydon DC; Ishii Y; O’Toole EA; Unsworth HC; Teh MT; Ruschendorf F; Sinclair C; Hopsu-Havu VK; Tidman N; Moss C; Watson R; de Berker D; Wajid M; Christiano AM; Kelsell DP The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet 2006, 38, 1245–1247. [DOI] [PubMed] [Google Scholar]
- 42.Bergmann C; Senderek J; Anhuf D; Thiel CT; Ekici AB; Poblete-Gutierrez P; van Steensel M; Seelow D; Nurnberg G; Schild HH; Nurnberg P; Reis A; Frank J; Zerres K Mutations in the gene encoding the Wnt-signaling component R-spondin 4 (RSPO4) cause autosomal recessive anonychia. Am J Hum Genet 2006, 79, 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Q; Wang Y; Dabdoub A; Smallwood PM; Williams J; Woods C; Kelley MW; Jiang L; Tasman W; Zhang K; Nathans J Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 2004, 116, 883–895. [DOI] [PubMed] [Google Scholar]
- 44.Lebensohn AM; Rohatgi R R-spondins can potentiate WNT signaling without LGRs. eLife 2018, 7, e33126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strop P; Liu SH; Dorywalska M; Delaria K; Dushin RG; Tran TT; Ho WH; Farias S; Casas MG; Abdiche Y; Zhou D; Chandrasekaran R; Samain C; Loo C; Rossi A; Rickert M; Krimm S; Wong T; Chin SM; Yu J; Dilley J; Chaparro-Riggers J; Filzen GF; O’Donnell CJ; Wang F; Myers JS; Pons J; Shelton DL; Rajpal A Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chemistry & biology 2013, 20, 161–167. [DOI] [PubMed] [Google Scholar]
- 46.Shalem O; Sanjana NE; Hartenian E; Shi X; Scott DA; Mikkelsen TS; Heckl D; Ebert BL; Root DE; Doench JG; Zhang F Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014, 343, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Storm EE; Durinck S; de Sousa e Melo F; Tremayne J; Kljavin N; Tan C; Ye X; Chiu C; Pham T; Hongo JA; Bainbridge T; Firestein R; Blackwood E; Metcalfe C; Stawiski EW; Yauch RL; Wu Y; de Sauvage FJ Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature 2016, 529, 97–100. [DOI] [PubMed] [Google Scholar]
- 48.Chartier C; Raval J; Axelrod F; Bond C; Cain J; Dee-Hoskins C; Ma S; Fischer MM; Shah J; Wei J; Ji M; Lam A; Stroud M; Yen WC; Yeung P; Cancilla B; O’Young G; Wang M; Kapoun AM; Lewicki J; Hoey T; Gurney A Therapeutic targeting of tumor-derived R-Spondin tttenuates beta-Catenin signaling and tumorigenesis in multiple cancer types. Cancer Res 2016, 76, 713–723. [DOI] [PubMed] [Google Scholar]
- 49.Fumagalli A; Oost KC; Kester L; Morgner J; Bornes L; Bruens L; Spaargaren L; Azkanaz M; Schelfhorst T; Beerling E; Heinz MC; Postrach D; Seinstra D; Sieuwerts AM; Martens JWM; van der Elst S; van Baalen M; Bhowmick D; Vrisekoop N; Ellenbroek SIJ; Suijkerbuijk SJE; Snippert HJ; van Rheenen J Plasticity of Lgr5-negative cancer cells drives metastasis in colorectal cancer. Cell Stem Cell 2020, 26, 569–578 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu S; Mulero MC; Chen W; Shang X; Tian S; Watanabe J; Watanabe A; Vorberg T; Wong C; Gately D; Howell SB Therapeutic targeting of tumor cells rich in LGR stem cell receptors. Bioconjug Chem 2021, 32, 376–384. [DOI] [PubMed] [Google Scholar]
- 51.Kim KA; Kakitani M; Zhao J; Oshima T; Tang T; Binnerts M; Liu Y; Boyle B; Park E; Emtage P; Funk WD; Tomizuka K Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 2005, 309, 1256–1259. [DOI] [PubMed] [Google Scholar]
- 52.Carmon KS; Gong X; Yi J; Wu L; Thomas A; Moore CM; Masuho I; Timson DJ; Martemyanov KA; Liu QJ LGR5 receptor promotes cell-cell adhesion in stem cells and colon cancer cells via the IQGAP1-Rac1 pathway. J Biol Chem 2017, 292, 14989–15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


