Abstract
Background:
There is growing recognition for a reciprocal, bidirectional link between anxiety disorders and obesity. Although the mechanisms linking obesity and anxiety remain speculative, this bidirectionality suggests shared pathophysiological processes. Neuroinflammation and oxidative damage are implicated in both pathological anxiety and obesity. This study investigates the relative contribution of comorbid diet-induced obesity and stress-induced anxiety to neuroinflammation and oxidative stress.
Methods:
Thirty-six (36) male Lewis rats were divided into four groups based on diet type and stress exposure: 1) control diet unexposed (CDU) and 2) exposed (CDE), 3) Western-like high-saturated fat diet unexposed (WDU) and 4) exposed (WDE). Neurobehavioral tests were performed to assess anxiety-like behaviors. The catalytic concentrations of glutathione peroxidase and reductase were measured from plasma samples, and neuroinflammatory/oxidative stress biomarkers were measured from brain samples using Western blot. Correlations between behavioral phenotypes and biomarkers were assessed with Pearson’s correlation procedures.
Results:
We found that WDE rats exhibited markedly increased levels of glial fibrillary acidic protein (185%), catalase protein (215%), and glutathione reductase (GSHR) enzymatic activity (418%) relative to CDU rats. Interestingly, the brain protein levels of and behavioral indices of anxiety.
Conclusions:
Together, our results support a role for neuroinflammation and oxidative stress in heightened emotional reactivity to obesogenic environments and psychogenic stress. Uncovering adaptive responses to obesogenic environments characterized by high access to high-saturated fat/high-sugar diets and toxic stress has the potential to strongly impact how we treat psychiatric disorders in at-risk populations.
Keywords: PTSD, Diet-induced Obesity Rat, Reactive Oxygen Species, Neuroinflammation
Introduction
The prevalence of trauma exposure during adolescence is concerning and has severe consequences for the emergence of psychiatric disorders [1]. While the majority of traumatized children are not chronically affected, it is estimated that approximately one-third of trauma-exposed adolescents will develop anxiety symptomatology and posttraumatic stress disorder (PTSD) [2,3]. A better understanding of specific vulnerability factors may facilitate the development of tailored preventive and curative interventions. Obesity and the consumption of imbalanced diets rich in saturated fats and sugars have emerged as risk factors for the development of anxiety and stress-related disorders [4–11]. We have modeled this relationship by measuring stress reactivity to traumatic stress in a rat model of diet-induced obesity (DIO). Consistent with studies in obese adolescents, we reported that adolescent diet-induced obesity (DIO) in rats: 1) reduces hippocampal volume [12], 2) impairs the maturation of the corticolimbic fear circuits [13], 3) enhances behavioral vulnerabilities to psychosocial stress [12–14], and 4) results in profound fear extinction learning deficits [13,14], even in the absence of an obesogenic phenotype [15]. However, the molecular factors underlying these vulnerabilities are poorly understood.
Several lines of evidence indicate that obesity and the consumption of obesogenic diets lead to brain inflammation [16–18]. We demonstrated that the intake of an obesogenic diet upregulates the expression of the glial fibrillary acidic protein (GFAP) in the brain [13]. GFAP expression has been associated with activated astrocytes, which can lead to astrogliosis. Although the implications of this (mal)adaptive astrocytic response to obesogenic diets and brain insults remain controversial [19–23], astrocytes play critical roles in regulating oxidative stress in the brain [24,25]. Unlike neurons, astrocytes use glycolysis for energy production and, as a consequence, have been associated with a higher generation of mitochondrial reactive oxygen species (ROS) [26]. It is known that ROS accumulate following exposure to obesogenic diets and obesity in rodents and humans [27–31]. Due to its high lipid content, the brain is vulnerable to ROS damage, which can have devastating effects on brain function and in the etiology of anxiety and stress-related disorders [32–35]. Together, these findings underscore the importance of oxidative stress as a potential molecular link connecting obesity to increased stress vulnerability.
The functional coupling between astrocytes and neurons contributes to redox metabolism, which is tightly regulated by antioxidant systems [26]. Influential members of this defense system include catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GSHR), and superoxide dismutases (SOD) [36,37]. In general, the antioxidant defense system works as follows: SOD converts O2. into hydrogen peroxide (H2O2), which is transformed into water by CAT and GPx [38,39]. Otherwise, H2O2 can react with iron and produce •OH radicals in a process known as the Fenton reaction. GSHR’s role relies on regenerating the reduced glutathione product of the GPx following GPx activities on lipid and non-lipid hydro-peroxides [40]. Studies demonstrate that lack of activity on this system leads to ROS buildup, inflammation, and may represent an important predisposing factor for anxiety and stress-related neuropsychiatric disorders [32–35].
Given that obesogenic diets induce oxidative stress, it is logical to hypothesize that the antioxidant network will mount a compensatory response to preserve redox homeostasis. Therefore, examining the response of the antioxidant system to obesogenic diets may provide valuable insight into the potential contribution of oxidative stress to anxiety and other stress-related psychopathologies. This study is the first to investigate the combined effects of an obesogenic diet and psychological stress on the expression profile and activities of the ROS defense system in the rat brain.
Methods
Experimental procedures involving rats were performed in compliance with the Loma Linda University School of Medicine regulations and institutional guidelines consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. We made efforts to reduce the number of rats used in the study as well as to minimize animal suffering and discomfort. This study reanalyzes behavioral data from the same animals that were used in our recent work [13]. While previous reports focused on the impact of the obesogenic diet on brain structure and behavioral outcomes, this study was carried out to identify the joint effects of an obesogenic diet and predatory-based stress on fear, anxiety-like behaviors, and oxidative stress markers. Furthermore, we now report additional ethological measures in the elevated plus maze (EPM). All the molecular experiments reported in this study were performed using tissue samples collected from the rats used in our previous work [13].
Animals
Thirty-six (36) adolescent male Lewis rats (postnatal day, PND, 21–23) were acquired from Charles River Laboratories (Portage, MI). The rats were pair-housed and maintained in conventional housing conditions which are, 21 ± 2 °C, relative humidity of 45%, and a 12-hour light/dark cycle with lights turned on at 7:00 am. The animals had ad libitum access to food and water.
Study Design
The rats were allowed to habituate to the animal facility for one week. Afterward, they were divided at random into two groups: one received the control diet (CD; n = 18) while the other received the Western-like high-fat diet (WD; n = 18). The rats consumed the diets for 8 weeks before exposure to the psychogenic stressor and behavioral testing. During the acclimation/handling sessions, the rats were handled for 5 minutes in their room for 3 consecutive days during the morning hours. Subsequently, the rats were brought to the behavior facility and acclimated to the room for 30 min and then handled for 5 min. Twenty-four hours following the acclimation to the testing environment, the rats were habituated to the Acoustic Startle Reflex (ASR) chamber. This habituation protocol consisted of placing the rats inside the testing enclosure and chamber for 5 min and then gently returning them to their home cage. Following forty-eight hours after habituation, ASR baseline measurements were acquired. Three days after acquiring the baseline ASR measurements, the rats were matched and subdivided into four groups based on exposure to psychogenic stress (control diet unexposed, CDU, n = 8; control diet exposed, CDE, n = 10; Western high-fat diet unexposed, WDU, n = 8; Western high-fat diet exposed, WDE, n = 10). The ASR-based group matching resulted in an uneven distribution of rats with similar startle responses in all groups. Given the inherent behavioral variation between stressed animals, we reasoned that an unbalanced design would be more appropriate.
While existing PTSD models have contributed greatly to understanding anxiety symptoms that develop after psychogenic stress, the investigation of fear processing occurring shortly after the traumatic event is lacking. We reasoned that prior stress will facilitate fear learning and result in fear extinction deficits in rats exposed to PS. Here, we tested whether predispositions to abnormalities in fear behaviors could be influenced by diet and mild psychogenic stress (PS). We used cat urine as a mild PS to provide continuity to our published studies [12]. Twenty-four hours after exposure to the mild psychogenic stress, the 6-day fear-potentiated startle (FPS) protocol was commenced. Twenty-four hours after FPS day 6, the rats were tested in the elevated plus-maze (EPM) and ASR. The seven-day delay between PS and EPM/ASR testing was used to reproduce the conditions found in previous studies [12]. All behaviors were recorded between 10:00–15:00 h. The rats were euthanized 24 h after completion of the behavioral assessments, and plasma and brain tissue were collected.
Diets
The rats were given ad libitum access to water and either standard chow control diet (CD; 13% kcal fats and 53% kcal from carbohydrates; total density = 3.1 kcal; Harlan Laboratories Inc., Indianapolis, IN) or a Western-like high-saturated fat diet (WD; catalog #F6724; 41% kcal fats and 43% kcal from carbohydrates; total density = 4.6 kcal; Bio-Serv, Frenchtown, NJ). The composition of the diets is fully detailed in previous reports (Table 1) [13,15].
Table 1.
Diet Composition.
| CD | WD | ||
|---|---|---|---|
|
| |||
| % kcal Carbohydrates | 53 | 43 | |
| % kcal Protein | 34 | 16 | |
| % kcal Fat | 13 | 41 | |
| Total kcal/gram | 3.0 | 4.6 | |
|
| |||
|
| |||
| C4:0 (Butyric Acid) | NR | 4.89 | |
| C6:0 (Caproic Acid) | NR | 3.37 | |
| C8:0 (Caprylic Acid) | NR | 2.11 | |
| C10:0 (Capric Acid) | NR | 5.08 | |
| C12:0 (Lauric Acid) | NR | 5.93 | |
| C14:0 (Myristic Acid) | NR | 18.8 | |
| C15:0 (Pentadecylic Acid) |
NR | 2.01 | |
| C16:0 (Palmitic Acid) | 7.0 | 56.1 | |
| C17:0 (Margaric Acid) | NR | 1.01 | |
| C18:0 (Stearic Acid) | 3.0 | 19.6 | |
| C20:0 (Arachidic Acid) | NR | 0.29 | |
| Total SFA | 11.0 | 113 | |
|
| |||
|
| |||
| C14:1 (Myristoleate Acid) |
NR | 1.46 | |
| C16:1 (Palmitoneic Acid) |
NR | 2.62 | |
| C18:1(Oleic Acid) | 12.0 | 36.7 | |
| Total MUFA | 14.0 | 45.5 | |
|
| |||
|
| |||
| C18:2n6 (Linoleic Acid) | 8.0 | 10.2 | |
| C18:3n3 (Alpha-Linoleic Acid) | 1.0 | 0.82 | |
| C20:3n6 (Dihomogamma-linoleic Acid) | NR | 0.22 | |
| C20:4n6
(Arachidonic Acid) |
NR | 0.29 | |
| C20:5n3
(Eicosapentaenoic Acid) |
NR | <0.14 | |
| C22:6n3
(Docosahexaenoic Acid) |
NR | <0.14 | |
| Total PUFA | 9.0 | 11.1 | |
Predator Odor Stress
Psychogenic stress (PS) was induced by exposing the rats to predator odor [12,13,41–43]. In this model, the rats are exposed to soiled cat litter that was in use by a domestic male cat for 2 days and sifted for stools (Fresh Step Unscented Multi-Cat Litter from Clorox Pet Products Company). To replicate previous studies, 400 total grams of urine were collected in the cat litter. The soiled cat litter was placed in small glass containers before the experiments (∼100 grams/container). Each rat was placed in an empty standard plastic mouse cage with a filtered plastic top for 15 min. Predatory odor exposure took place at normal lighting conditions (269 lux illumination). Subsequently, either a clean, fresh litter container (unexposed group) or a soiled cat litter container (exposed group) was placed inside the cage. Each rat was allowed to interact with the container for 15 min. The total time in the cage was 30 min.
Acoustic startle reflex (ASR)
The ASR experiments were performed using SR-Lab acoustic chambers (San Diego Instruments, San Diego, CA, USA). The ASR magnitudes were measured by placing the rats inside startle enclosures that contained piezoelectric transducers. The acoustic stimuli intensities and response sensitivities were calibrated prior to commencing the experiments. The ASR protocol has been previously described by our group [12,13]. The experimental sessions were 22 min long and started with a 5-min habituation period (background noise = 55 decibels, dB; light conditions during the session were 400 lux). The rats were then presented with a series of 30 tones (10 tones at each intensity: 90 dB, 95 dB, and 105 dB) using a 30-s inter-trial interval (ITI). The acoustic stimuli had a 20 millisecond (ms) duration, and the trials were presented in a quasi-random order. The rats were returned to their home cages after the completion of the session. The enclosures were cleaned with soap and water and thoroughly dried between sessions. The ASR magnitudes were averaged and normalized to body weight to eliminate confounding factors associated with a differential body weight between groups (Weight-corrected ASR = maximum startle magnitude in mV divided by body weight at testing day) [12,13,44–46].
Fear potentiated startle (FPS)
The fear-potentiated startle (FPS) protocol was adapted from Dr. Michael Davis [47] and detailed in previous studies from our laboratory [13–15]. Each FPS session started with a 5-min acclimation period (background noise = 55 dB). During the first session of the paradigm (fear training), the rats were conditioned to associate a light stimulus (conditioned stimulus, CS) with a 0.6 mA foot shock (unconditioned stimulus, US). This conditioning session involved 10 CS + US pairing presentations. During each CS + US presentation, the light (3200 ms duration) was paired with a co-terminating foot shock (500 ms duration). Light stimuli during FPS conditioning was delivered via a bare 5-W incandescent bulb located on the ceiling of the testing chamber (400 lux). Light-shock pairings were presented in a quasi-random manner (ITI = 3–5 min). The average startle amplitude following each foot shock was used as a measure of shock reactivity. The acquisition of fear-related responses was measured 24 h later. During this second session (fear acquisition testing), the rats were first presented with 15 startle-inducing tones (leader trials; 5 trials for each tone: 90 dB, 95 dB, and 105 dB) delivered alone at 30 sec ITI. Subsequently, the rats were presented with 60 test trials. For half of these test trials, a 20 ms tone was presented alone (tone alone trials; 10 trials for each tone: 90 dB, 95 dB, and 105 dB). For the other half, the tone was paired with a 3200 ms light (light + tone trials; 10 trials for each pairing: 90 dB, 95 dB, and 105 dB). To conclude the acquisition testing session, the rats were presented with 15 startle-inducing tones (trailer trials; 5 trials of each tone: 90 dB, 95 dB, and 105 dB) delivered at 30 sec ITI. The trials in this session were presented in a quasi-random order (ITI = 30 sec). The startle-eliciting tones had a 20 ms duration. One day after fear acquisition testing, the rats were exposed to three (3) consecutive daily fear extinction-training sessions. The fear extinction training session consisted of 30 CS alone presentations (light without shock or noise bursts) with a duration of 3700 ms (ITI = 30 sec). Twenty-four hours after the last extinction session, we determined the FPS responses using a testing session identical to that used to measure the acquisition of fear. Thus, the fear extinction testing session measured learned fear extinction responses, not only spontaneous fear extinction responses. The persistence of potentiated startle responses in the presence of the CS during this fear extinction acquisition session indicated a failure to acquire extinction learning. FPS data were reported as the proportional change between US and CS + US [%FPS = ((Light + Tone Startle) − (Tone Alone Startle)) / (Tone Alone Startle) × 100] [48]. Fear extinction was scored as the FPS difference between the fear learning and the fear extinction testing sessions (pre-extinction FPS – post-extinction FPS).
Elevated Plus Maze (EPM)
The near infrared-backlit (NIR) elevated plus maze (EPM) consisted of two opposite open arms (50.8 × 10.2 × 0.5 cm) and two opposite enclosed arms (50.8 × 10.2 × 40.6 cm) (Med Associates Inc., Fairfax, VT). The arms were connected by a central 10 × 10 cm square-shaped area. The maze was elevated 72.4 cm from the floor. Behaviors were recorded in the dark (< 10 Lux). While the main rationale for testing in the light phase under dark lighting was to reproduce the conditions reported in previous studies [12,49], there are two important aspects to consider. 1) Regarding EPM testing in dark conditions: Previous studies indicate that open arm aversion in the EPM is not dependent on light intensity but appears to be an all-or-none phenomenon in rats [50]. 2) Regarding EPM testing during the light phase: A few lines of evidence support that measures of anxiety in the EPM are not significantly influenced by the circadian phase in male rats [51,52].
The rats were placed on the central platform facing an open arm and allowed to explore the EPM for 5 min. The apparatus was cleaned after each trial (70% ethanol, rinsed with water, and then dried with paper towels). The behaviors were monitored via a monochrome video camera equipped with a NIR filter and recorded and tracked using Ethovision XT (Noldus Information Technology, Leesburg, VA). In rats, changes in the percentage of time spent on the open arms (OA) indicate changes in anxiety, and the number of closed arms (CA) entries is the best measure of locomotor activity [53,54]. These data are used to calculate the anxiety index [12,55–57]. Anxiety Index = 1 − [([OA cumulative duration / Total test duration] + [OA entries / Total number of entries to CA + OA]) / 2].
Several ethological-oriented measures were analyzed during the EPM. Risk assessment behaviors included the stretch-attend posture (the trunk extends and then flexes back to original position) and head dipping (the head flexes below the edge of the open arm) were analyzed from Ethovision XT-tracked data. In addition, we manually scored exploratory behaviors: supported rearing (standing on the hind legs with hands on the wall) and unsupported rearing (standing on hind legs). Grooming was also manually scored as a measure of non-exploratory behavior (paw licking and paw used to wash face and ears, and subsequent shoulder and hind leg licking).
Immunoblotting
A group of rats (n = 24; 6 rats per group) were randomly selected and deeply anesthetized with an intraperitoneal overdose injection of Euthasol (150 mg/kg; Virbac, Fort Worth, TX) and perfused transcardially with PBS. Following perfusions, the rats were rapidly decapitated, and the brains isolated. The brain tissue was homogenized and transferred to a 1.5 mL microtube containing 700 μl of cold Cell Lytic MT Lysis Extraction Buffer (catalog #C3228; Sigma-Aldrich, St. Louis, MO), 1% phosphatase inhibitor cocktail 3 (catalog #P0044; Sigma-Aldrich), and a FAST Protease Inhibitor Cocktail Tablet, EDTA free (catalog #S8830; Sigma-Aldrich). Protein extraction was performed as suggested by the manufacturer. Briefly, the samples were centrifuged for 10 min at 4°C (20,000 rpm), and the supernatant was collected and stored at −80°C until needed for further processing. As for protein quantification, the Bio-Rad protein assay was used according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA). Proteins were separated on a 12% polyacrylamide-SDS gel (40 μg of protein/lane) and wet transferred to a nitrocellulose membrane from 1h at 4°C. The membrane was blocked with Odyssey Blocking Buffer (catalog #927–40000; LI-COR Biosciences, Lincoln, NE, USA) for 1 hour at room temperature. The proteins of interest were detected using the following primary antibodies: anti-rabbit Catalase (1:375; catalog #Ab52477; Abcam, Cambridge, MA); anti-rabbit Glutathione Peroxidase (1:500; catalog #Ab22604; Abcam); anti-rabbit Glutathione Reductase (1:2000; catalog #Ab16801; Abcam); anti-rabbit SOD-1 (1:500; catalog #Ab16831; Abcam); anti-rabbit SOD-2 (1:2000; catalog #13141S; Cell Signaling, Danvers, MA); anti-rabbit GFAP (1:5000; cat# 556,327, BD Biosciences, San Jose, CA, USA); and anti-rabbit Iba-1 (1:1000; cat# 016–20,001 Wako Chemicals, Richmond, VA, USA). All the antibodies were incubated in a blocking solution and overnight at 4°C. Anti-mouse ß-actin (1:10000; catalog #A5441; Sigma-Aldrich, St. Louis, MO) was used as a loading control for antioxidant proteins. GFAP and Iba-1 protein analyses were generated from previously collected data and normalized to GAPDH (1:5000; cat# G9545, Sigma-Aldrich) [13]. As for secondary antibodies, we used goat anti-rabbit (1:25000; catalog #926–32211; LI-COR Biosciences) and goat anti-mouse (1:25000; catalog #925–68070; LI-COR Biosciences) for 1h at room temperature. Infrared signals from membranes were detected using the Odyssey CLx Scanner (LI-COR Biosciences, Lincoln, NE). Densitometric analyses were performed using the Image Studio 5.2 Software (LI-COR Biosciences, Lincoln, NE). The background correction method used in the software was the median background option. The parameters for the imaging used in all the cases were as follows: channels: 700 nm (red) and 800 nm (green); resolution: 169 μm; intensities: automatic for both channels.
Glutathione Peroxidase and Reductase Activity
Following Euthasol injections to induce euthanasia (150 mg/kg; Virbac, Fort Worth, TX), blood was collected from cardiac puncture during the perfusion procedure and added to tubes with EDTA. Blood was collected between 9:00 and 15:00 hours. Blood samples were centrifuged (2,000 rpm for 20 min at 4°C) and plasma samples were collected and placed in new tubes and frozen at −80°C until extraction. Rat plasma samples were randomly selected for enzymatic activity assays. The plasma glutathione peroxidase (GPx) and reductase (GSHR) activities were determined using a Glutathione Peroxidase Assay Kit (#703102) and a Glutathione Reductase Assay Kit (#703202; Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer’s instructions. The rate of change of absorbance (optical density at 340 nm) per minute was measured using the SpectraMax® i3x Multi-Mode Microplate Reader (Molecular Devices, Molecular Devices, Sunnyvale, CA, USA) and expressed as nmol/min/ml.
Statistical Analysis
We recorded and tabulated all data using Excel (Microsoft, Redmond, WA, USA). Statistical analyses were performed using Graphpad Prism version 8.0 (Graphpad Software, La Jolla, CA, USA). Shapiro–Wilk statistical analyses were used to determine sample distribution. The Brown–Forsythe test was used to test for the equality of group variances. When appropriate, main (diet, stress) and interaction (diet x stress) effects were determined with two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. The ROUT method was used to investigate outliers. To explore associations between behavioral phenotypes and ROS biomarkers, we used Pearson’s correlation test. When appropriate, multiple testing adjustments of the correlations were determined by the false discovery rate (FDR). The two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli (Q = 5%) was used to analyze FDRs. For all analyses, we considered differences significant if p < 0.05; all data are shown as the mean ± SEM.
Results
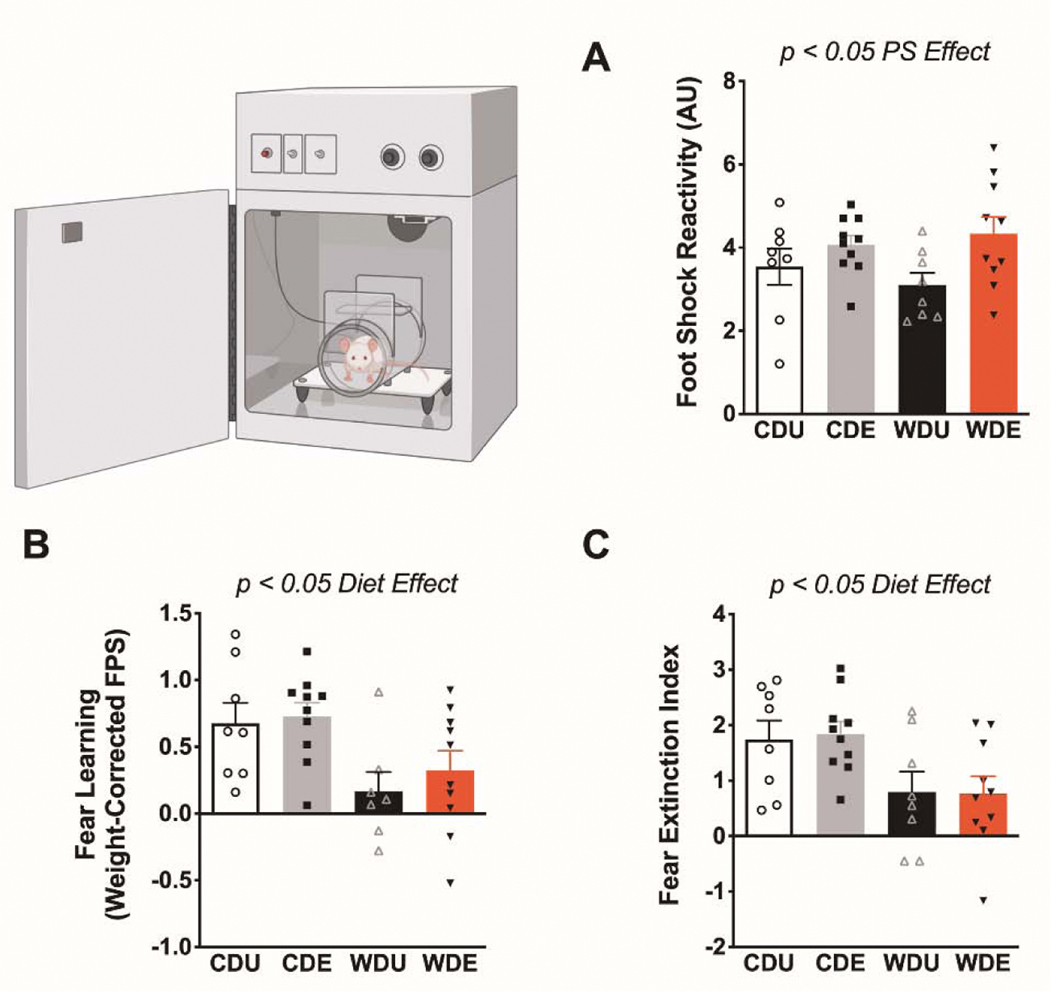
This study investigated the relative and combined effects of the obesogenic Western-like diet (WD) and psychogenic stress (PS) on anxiety-like behaviors and neuroinflammation/oxidative stress biomarkers. Figure 1 illustrates the experimental timeline and procedures (adapted from Vega-Torres et al., 2018) [13]. Body weight, food consumption, and obesogenic biomarker data was previously reported and indicates that the rats that consumed the WD exhibited an obesogenic phenotype [13]. Supplemental Figure 1 shows growth chart and food consumption for study groups and validates previous observations on this diet-induced obesity (DIO) rat model [12]. We found that the exposure to the psychogenic stressor increased the reactivity to the footshocks during the fear conditioning session (FPS day 1) [F(1,32) = 6.27, p = 0.02] (Figure 2A). Analyses revealed no significant effects of the diet type [F(1,32) = 0.06, p = 0.81] or interaction between factors [F(1,32) = 1.02, p = 0.32] on footshock reactivity. We investigated the effect of the obesogenic diet and psychogenic stress in fear-related behaviors (Supplemental Figures 2–4). As previously reported, analysis of the effect of diet revealed that the obesogenic WD significantly reduced fear learning, as evaluated by the fear-potentiated startle [F(1,31) = 10.80, p = 0.003] (Figure 2B). Psychogenic stress exposure [F(1,32) = 0.56, p = 0.46] and interaction between the obesogenic diet and PS [F(1,32) = 0.15, p = 0.70] did not have a significant effect on fear acquisition. Analysis of the effect of the diet on fear extinction learning revealed a significantly attenuated fear extinction index in the rats that consumed the obesogenic diet [F(1,32) = 10.57, p = 0.003] (Figure 2C). The psychogenic stressor [F(1,32) = 0.01, p = 0.91] and interaction [F(1,32) = 0.04, p = 0.81] did not affect fear extinction significantly.
Figure 1. Study design and timeline of experimental procedures, behavioral tests, and outcome measures.
This study used the same timeline and rats from our previous study [13]. While the previous report focused on the impact of diet on brain and behavior, here we subdivided the rats by exposure to predatory odor stress (PS). Furthermore, tissue collected from the previous study was processed and analyzed to determine the impact of diet and stress on oxidative stress and neuroinflammation markers. Abbreviations: PND, postnatal day; CDU, control diet unexposed; CDE, control diet exposed; WDU, Western high-fat diet unexposed; WDE, Western high-fat diet exposed; ASR, acoustic startle reflex; EPM, elevated plus maze; FPS, Fear Potentiated Startle.
Figure 2. Deficits in delay cued fear conditioning and fear extinction in rats that consume an obesogenic Western-like diet.
(A) Psychogenic stress (PS) significantly increase footshock reactivity during the fear-conditioning session [stress: F(1,32) = 6.27, p = 0.018]. (B) Average fear-potentiated startle (FPS) responses 24 h after conditioning. The rats that consumed the obesogenic Western-like diet (WD) exhibited reduced fear learning relative to controls [diet: F(1,31) = 10.80, p = 0.003]. (C) Average extinction index following three consecutive daily fear extinction-training sessions. WD rats displayed deficits in fear extinction [diet: F(1,32) = 10.57, p = 0.003]. *, p < .05; n = 8–10 rats/group. Error bars are S.E.M.
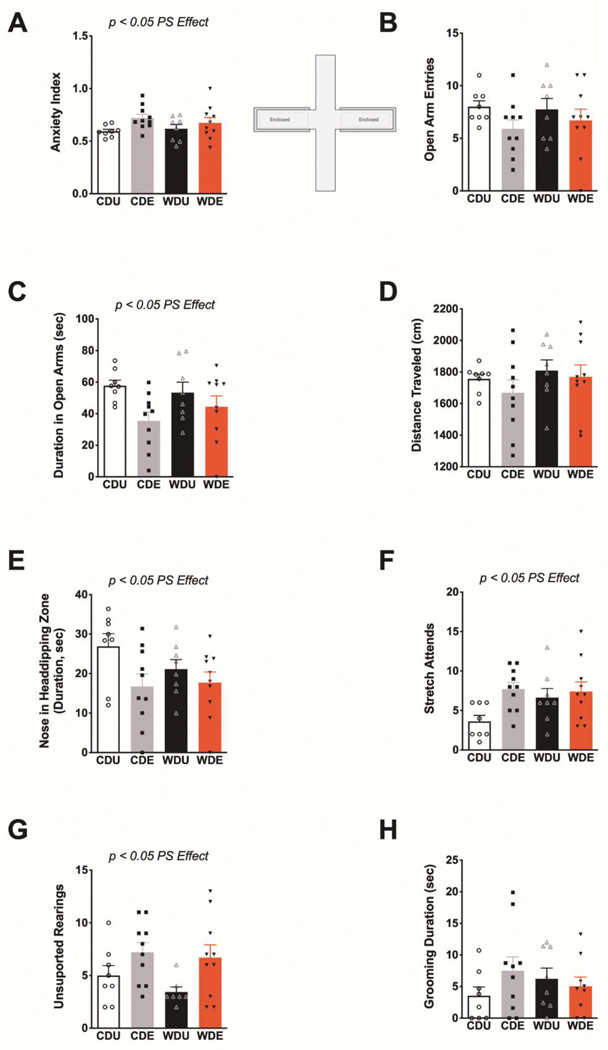
Next, we tested whether the obesogenic WD, PS, or interactions between these factors had a significant effect on anxiety-like behaviors in the elevated plus maze (EPM). Exposure to the PS increased general measures of anxiety in the EPM [F(1,32) = 5.17, p = 0.03] (Figure 3A). The diet type [F(1,32) = 0.08, p = 0.78] and interactions between factors [F(1,32) = 0.78, p = 0.38] did not have significant effects on the anxiety index. We found no significant main effects or interactions on the number of open arm entries (diet: F(1,32) = 0.89, p = 0.77; PS: F(1,32) = 2.91, p = 0.10; interaction: F(1,32) = 32.33, p = 0.57) (Figure 3B). Analyses revealed that rats exposed to the PS exhibited reduced duration in the open arms of the EPM [F(1,32) = 6.73, p = 0.01] (Figure 3C). We found no significant diet [F(1,32) = 0.14, p = 0.71] and interaction [F(1,32) = 1.23, p = 0.28] effects on the duration spent in the open arms. Analyses showed no significant main effects or interactions on the total distance traveled in the EPM (diet: F(1,32) = 1.18, p = 0.29; PS: F(1,32) = 0.82, p = 0.37; interaction: F(1,32) = 0.12, p = 0.73) (Figure 3D). PS exposure reduced nose duration in head-dipping zones of the EPM [F(1,32) = 5.43, p = 0.03], supporting the anxiogenic effect of the stressor (Figure 3E). Post hoc analyses revealed that the CD rats exposed to PS exhibited a significantly reduced duration in head-dipping zones relative to unexposed CD rats (p = 0.04). The diet type [F(1,32) = 1.68, p = 0.20] and interactions [F(1,32) = 2.51, p = 0.12] did not have a significant effect on the duration of the rats in head-dipping zones. PS increased the frequency of ethological measures of anxiety in the EPM. We found that both the frequency of stretch attend postures (PS: F(1,32) = 5.26, p = 0.03; diet: F(1,32) = 0.66, p = 0.42; interaction: F(1,32) = 1.29, p = 0.26) (Figure 3F) and unsupported rearing (PS: F(1,31) = 7.42, p = 0.01; diet: F(1,31) = 1.06, p = 0.31; interaction: F(1,31) = 0.28, p = 0.60) (Figure 3G) were significantly increased in PS-exposed rats. The time spent performing spontaneous self-grooming behaviors in the EPM was not significantly affected by the diet, PS, or the interaction between these factors (diet: F(1,31) = 0.003, p = 0.96; PS: F(1,31) = 0.60, p = 0.44; interaction: F(1,31) = 2.09, p = 0.16) (Figure 3H).
Figure 3. Selective anxiogenic effects of predatory odor stress in the elevated plus maze.
(A) Average anxiety index scores calculated from behavioral outcomes in the elevated plus maze (EPM). PS heightened anxiety-like behaviors in the EPM [stress: F(1,32) = 5.17, p = 0.030]. (B) Average open arm entries were similar between groups (p > 0.05). (C) Average duration in the open arms. PS rats exhibited reduced duration in the open arms of the EPM [stress: F(1,32) = 6.73, p = 0.014]. (D) Average distance traveled was similar between groups (p > 0.05). (E) Average duration of nose in head-dipping zones of the EPM, expressed in seconds. PS significantly decreased the extent of nose in head-dipping zones [stress: F(1,32) = 5.43, p = 0.026]. (F) Average frequency of stretch attend postures (SAP). PS increased SAP frequency [stress: F(1,32) = 5.26, p = 0.030]. (G) Average frequency of unsupported rearing behaviors during EPM testing. PS increased the frequency of unsupported rearings [F(1,31) = 7.42, p = 0.011]. (H) Average grooming duration (in seconds) was similar between groups (p > 0.05). n = 8–10 rats/group. Error bars are S.E.M.
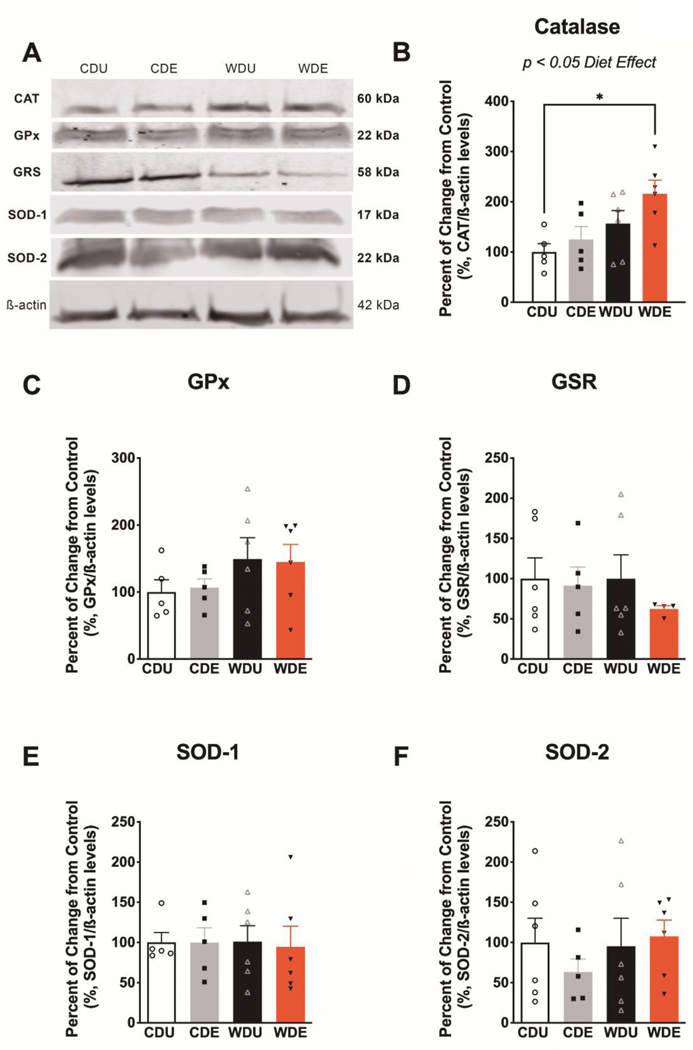
To examine the adaptive responses of the brain antioxidant network to the obesogenic WD and PS, we focused on measuring the expression levels of critical proteins implicated with oxidative stress (Figure 4A). We found that the WD had a robust effect on the expression of catalase in the brain (diet: F(1,18) = 8.60, p = 0.01; PS: F(1,18) = 2.84, p = 0.11; interaction: F(1,18) = 0.47 p = 0.50) (Figure 4B). Post hoc revealed significantly increased catalase protein expression levels in the brain of WDE rats relative to CDU (p = 0.03), indicating that the obesogenic WD exacerbates the effect of PS on catalase protein expression. We found no significant main or interaction effects on the protein expression levels of glutathione peroxidase (diet: F(1,18) = 3.08, p = 0.10; PS: F(1,18) = 0.002, p = 0.10; interaction: F(1,18) = 0.050, p = 0.83) (Figure 4C), glutathione reductase (diet: F(1,17) = 0.32, p = 0.58], PS: F(1,17) = 0.81, p = 0.38; interaction: F(1,17) = 0.31, p = 0.58) (Figure 4D), superoxide dismutase-1 (diet: F(1,18) = 0.01, p = 0.91; PS: F(1,18) = 0.03, p = 0.87; interaction: F(1,18) = 0.02, p = 0.88) (Figure 4E), and superoxide dismutase-2 (diet: F(1,19) = 0.53, p = 0.48; PS: F(1,19) = 0.20, p = 0.66; interaction: F(1,19) = 0.81, p = 0.38) (Figure 4F).
Figure 4. Increased catalase protein levels in the brain of rats exposed to an obesogenic diet.
(A) Representative Western blot bands from whole-brain homogenates illustrating the relative levels of critical proteins involved in the brain antioxidant network. Protein levels are expressed in percent from control and normalized in relation to 𝝱-actin. (B) The obesogenic WD increased catalase protein levels in the brain [diet: F(1,18) = 8.60, p = 0.0090]. This effect was particularly evident in WDE rats relative to CDU (post-hoc p = 0.03), indicating that PS exacerbates the effect of an obesogenic diet on catalase protein levels. The protein levels of glutathione peroxidase (C), glutathione reductase (D), superoxide dismutase 1 (E), and superoxide dismutase 2 (F) were not significantly affected by the dietary and stress manipulations (for main and interaction effects: p > 0.05). n = 5–6 rats/group. Error bars are S.E.M.
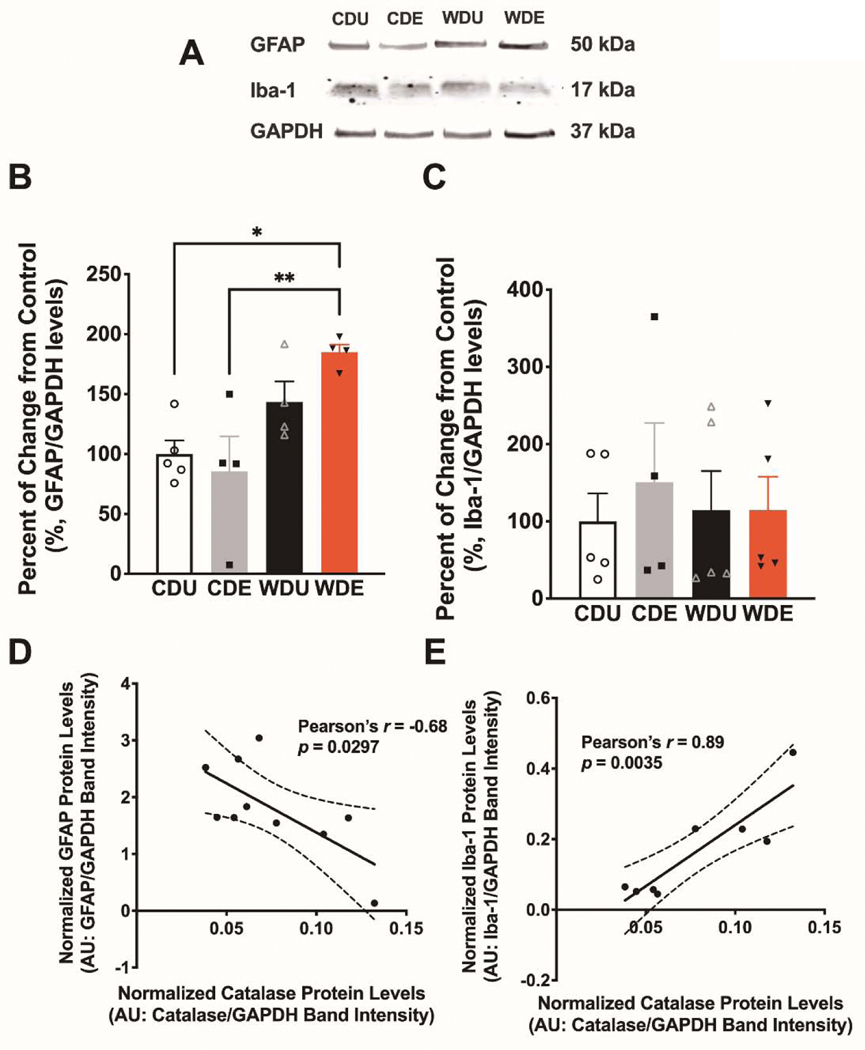
Astrocytes and microglia are resident immune-competent cells with high expression of catalase and important roles regulating oxidative stress [58–61]. With the ultimate aim of identifying whether the obesogenic WD and PS impacted these relevant cellular phenotypes, we next determined the expression levels of GFAP and Iba-1 via Western blot (Figure 5A). In agreement with previous reports from our group, we found that the obesogenic diet increased GFAP protein levels in the brain (diet: F(1,13) = 16.50, p = 0.001; PS: F(1,13) = 0.59, p = 0.46; interaction: F(1,13) = 2.54, p = 0.14) (Figure 5B). Post hoc analyses demonstrated that WDE rats had increased GFAP levels relative to CDU (p = 0.02) and CDE (p < 0.01) rats. Interestingly, we found no significant main or interaction effects on the expression of Iba-1 in the brain (diet: F(1,15) = 0.045, p = 0.83; PS: F(1,15) = 0.25, p = 0.63; interaction: F(1,15) = 0.24, p = 0.63 (Figure 5C), revealing a selective effect of the experimental manipulations on glial markers. We found strong associations between the glial markers and catalase levels in the brain. Notably, while GFAP levels were negatively associated with catalase protein expression levels (Pearson’s r = −0.68, p = 0.03) (Figure 5D), Iba-1 protein levels were positively associated (Pearson’s r = 0.89, p = 0.004) (Figure 5E).
Figure 5. Psychogenic stress synergizes with an obesogenic WD to increase GFAP protein levels.
(A) Western blotdata from Vega-Torres et al. 2018 was reanalyzed to include the effects of PS on neuroinflammation (CDE and WDE groups were included here) [13]. (B) The obesogenic WD increased GFAP (glial fibrillary acidic protein) levels in whole-brain homogenates [F(1,13) = 16.50, p = 0.0013]. Interestingly, analyses demonstrate that WDE rats had increased GFAP levels relative to CDU (p = 0.02) and CDE (p < 0.01) rats. (C) WD and PS exposure did not alter Iba-1 (Ionized calcium-binding adaptor molecule 1) protein levels (p > 0.05). n = 5–6 rats/group. Error bars are S.E.M. Protein levels are expressed in percent from control and normalized to GAPDH. (D) A scatter plot shows a significant inverse relationship between GFAP and catalase protein levels in the brain (Pearson’s r = −0.68; p = 0.030). (E) A scatter plot illustrates a robust positive association between Iba-1 and catalase levels in the brain (Pearson’s r = 0.89; p = 0.0035).
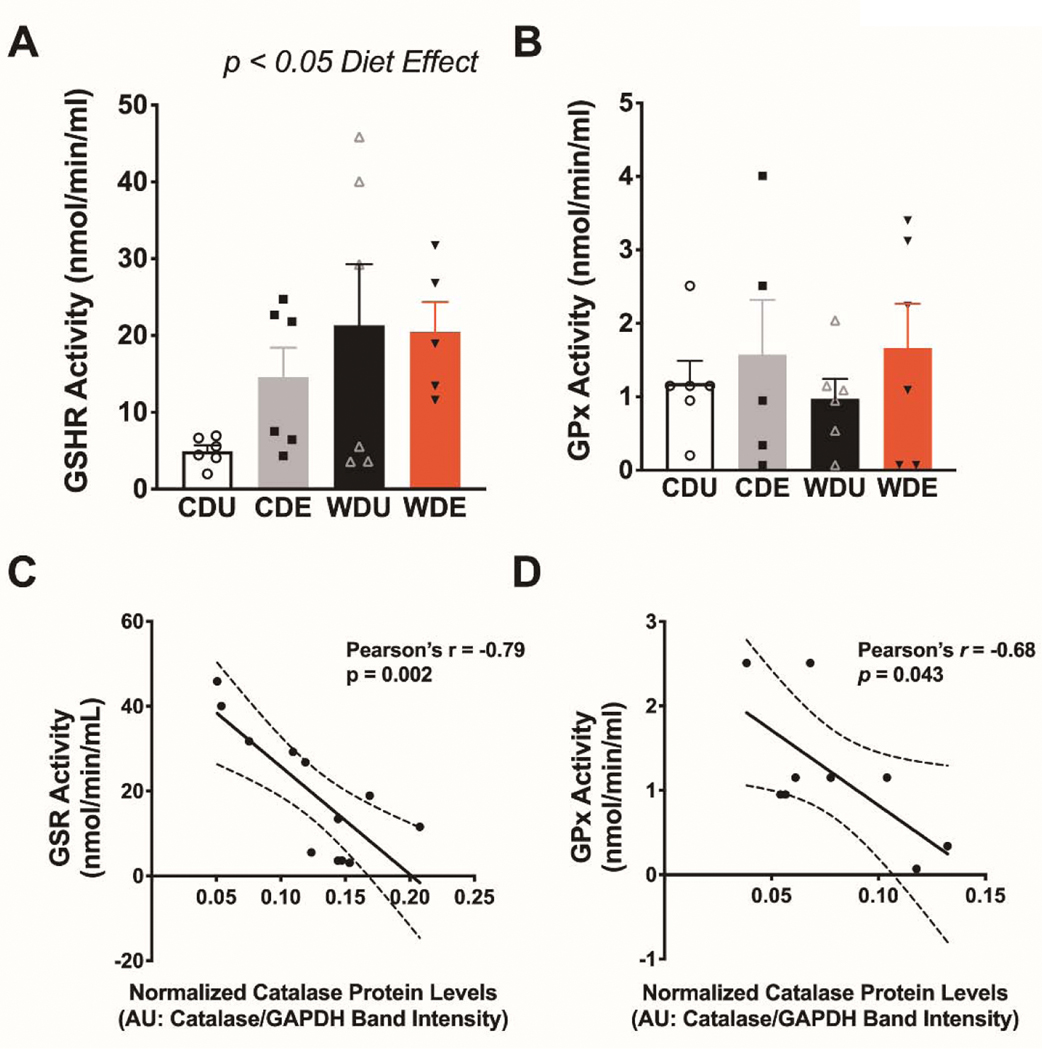
We then determined the enzymatic activity of glutathione peroxidase and reductase in plasma to more clearly define the contributions of the obesogenic WD and PS on peripheral biomarkers of oxidative stress status. A growing body of evidence indicates that the glutathione antioxidant system may contribute to the pathophysiology of PTSD [62]. Because obesity and obesogenic diets alter this antioxidant system [63–65], we reasoned that the obesogenic diet may predispose to PTSD-like behaviors by interfering with glutathione metabolism. Therefore, we focused our initial experiments on investigating the enzymatic activities of glutathione peroxidase (GPx) and reductase (GSHR) in rat plasma. Our analyses demonstrate that the obesogenic WD increased the activities of glutathione reductase in plasma (diet: F(1,19) = 5.08, p = 0.04; PS: F(1,19) = 0.79, p = 0.38; interaction: F(1,19) = 1.12, p = 0.30) (Figure 6A). On the other hand, we found no significant main or interaction effects for the enzymatic activity of glutathione peroxidase in the plasma (diet: F(1,19) = 0.02, p = 0.90; PS: F(1,19) = 1.17, p = 0.29; interaction: F(1,19) = 1.09, p = 0.77). Given that peripheral ROS can influence brain oxidative status [66–69], we investigated associations between the enzymatic activities of glutathione peroxidase and reductase and catalase protein levels in the brain. Pearson’s correlation analyses revealed a robust negative association between glutathione reductase activity in plasma and catalase protein expression in the brain (Pearson’s r = −0.79, p = 0.002) (Figure 6C). We also found a significant relationship between glutathione peroxidase activity in plasma and catalase protein expression in the brain (Pearson’s r = −0.68, p = 0.04) (Figure 6D).
Figure 6. Increased plasma glutathione reductase activities in rats exposed to an obesogenic diet.
(A) The obesogenic WD increased the averaged glutathione reductase enzymatic activity in plasma [F(1,19) = 5.08, p = 0.036]. (B) Average plasma glutathione peroxidase activities were similar between diet groups (p > 0.05). For A and B: n = 5–6 rats/group, error bars are S.E.M. (C) Scatter plot shows a significant inverse relationship between GSHR plasma activity and catalase protein levels in the brain (Pearson’s r = −0.79; p = 0.0020). (D) A scatter plot illustrates a significant inverse association between GPx plasma activity and catalase levels in the brain (Pearson’s r = 0.68; p = 0.0043).
Finally, to investigate correlations between behavioral outcomes and neuroinflammation/oxidative stress biomarkers, we performed Pearson’s correlation analyses. Emerging evidence suggests that the molecular consequences of PTSD include elevated levels of oxidative stress and inflammation [62,70]. Thus, we investigated associations between neuroinflammation and oxidative stress biomarkers and stress-related behavioral outcomes. Previous fecal corticosterone (CORT) data from these animals was used to determine the potential associations between HPA axis reactivity and neuroinflammation/oxidative stress. Results revealed significant associations between behavioral outcomes implicated with fear and anxiety and inflammation/ROS biomarkers (Table 2). In summary, we found a positive relationship between GFAP, CAT, GPx, and body weight. Interestingly, GFAP, GPx and SOD-1 brain protein levels were negatively associated with fear learning deficits and anxiety-like behaviors in the EPM. Plasma GSHR activity was positively associated with increased CORT levels after experimental manipulations.
Table 2.
Associations between neuroinflammatory and oxidative stress biomarkers and behavioral outcomes.
| Pearson’s r | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| GFAP | Iba-1 | Catalase | GSHR | GPx | SOD-1 | SOD-2 | GSHR Activity | GPx Activity | |
| Body Weight | 0.525 | 0.008 | 0.600 | 0.302 | 0.486 | −0.150 | 0.190 | 0.330 | −0.069 |
| CORT | 0.489 | 0.263 | −0.003 | −0.060 | 0.082 | −0.253 | 0.020 | 0.447 | 0.054 |
| Startle Change | −0.111 | 0.217 | 0.077 | −0.155 | 0.067 | −0.068 | 0.134 | 0.044 | −0.187 |
| Fear learning | −0.219 | 0.268 | −0.316 | −0.400 | −0.429 | 0.230 | −0.403 | 0.311 | −0.025 |
| Fear extinction | −0.252 | 0.297 | −0.305 | −0.149 | −0.371 | 0.080 | −0.260 | 0.190 | −0.093 |
| Anxiety Index | 0.388 | 0.184 | 0.207 | 0.045 | 0.332 | 0.250 | −0.026 | −0.028 | 0.104 |
| Stretch attends | 0.416 | 0.156 | 0.383 | 0.361 | 0.453 | 0.161 | −0.198 | −0.031 | −0.029 |
| Head dips | −0.692 | −0.110 | −0.048 | 0.035 | −0.223 | −0.473 | 0.025 | −0.141 | −0.364 |
| p Value | |||||||||
| GFAP | Iba-1 | Catalase | GSHR | GPx | SOD-1 | SOD-2 | GSHR Activity | GPx Activity | |
| Body Weight | 0.030 | 0.973 | 0.003 | 0.162 | 0.022 | 0.505 | 0.386 | 0.134 | 0.761 |
| CORT | 0.055 | 0.277 | 0.991 | 0.790 | 0.724 | 0.269 | 0.929 | 0.042 | 0.816 |
| Startle Change | 0.670 | 0.373 | 0.732 | 0.480 | 0.767 | 0.763 | 0.542 | 0.846 | 0.404 |
| Fear learning | 0.399 | 0.268 | 0.152 | 0.058 | 0.046 | 0.303 | 0.057 | 0.158 | 0.910 |
| Fear extinction | 0.330 | 0.217 | 0.168 | 0.498 | 0.090 | 0.722 | 0.232 | 0.398 | 0.679 |
| Anxiety Index | 0.124 | 0.450 | 0.356 | 0.838 | 0.131 | 0.263 | 0.906 | 0.902 | 0.645 |
| Stretch attends | 0.097 | 0.524 | 0.079 | 0.090 | 0.034 | 0.475 | 0.366 | 0.892 | 0.898 |
| Head dips | 0.004 | 0.676 | 0.840 | 0.879 | 0.344 | 0.035 | 0.916 | 0.554 | 0.105 |
| q Value | |||||||||
| GFAP | Iba-1 | Catalase | GSHR | GPx | SOD-1 | SOD-2 | GSHR Activity | GPx Activity | |
| Body Weight | 0.255 | 1.000 | 0.023 | 0.319 | 0.194 | 0.770 | 0.721 | 0.542 | 0.956 |
| CORT | 0.283 | 0.756 | 0.915 | 0.825 | 0.920 | 0.770 | 0.975 | 0.470 | 0.956 |
| Startle Change | 0.804 | 0.756 | 0.828 | 0.654 | 0.920 | 0.915 | 0.840 | 0.974 | 0.956 |
| Fear learning | 0.671 | 0.756 | 0.353 | 0.283 | 0.194 | 0.770 | 0.318 | 0.542 | 0.956 |
| Fear extinction | 0.615 | 0.756 | 0.353 | 0.654 | 0.251 | 0.915 | 0.649 | 0.836 | 0.956 |
| Anxiety Index | 0.347 | 0.756 | 0.582 | 0.825 | 0.315 | 0.770 | 0.975 | 0.974 | 0.956 |
| Stretch attends | 0.326 | 0.800 | 0.290 | 0.283 | 0.194 | 0.770 | 0.721 | 0.974 | 0.956 |
| Head dips | 0.107 | 0.597 | 0.980 | 0.844 | 0.597 | 0.313 | 0.597 | 0.915 | 0.554 |
| Sample Size | |||||||||
| GFAP | Iba-1 | Catalase | GSHR | GPx | SOD-1 | SOD-2 | GSHR Activity | GPx Activity | |
| Body Weight | 17 | 19 | 22 | 23 | 22 | 22 | 23 | 22 | 22 |
| CORT | 16 | 19 | 21 | 22 | 21 | 21 | 22 | 21 | 21 |
| Startle Change | 17 | 19 | 22 | 23 | 22 | 22 | 23 | 22 | 22 |
| Fear learning | 17 | 19 | 22 | 23 | 22 | 22 | 23 | 22 | 22 |
| Fear extinction | 17 | 19 | 22 | 23 | 22 | 22 | 23 | 22 | 22 |
| Anxiety Index | 17 | 19 | 22 | 23 | 22 | 22 | 23 | 22 | 22 |
| Stretch attends | 17 | 19 | 22 | 23 | 22 | 22 | 23 | 22 | 22 |
| Head dips | 15 | 17 | 20 | 21 | 20 | 20 | 21 | 20 | 21 |
Discussion
This study investigated the individual and combined effects of a Western-like high-saturated fat obesogenic diet (WD) and acute exposure to predator odor stress (PS) on critical endogenous antioxidants and redox regulators. The main finding of this study is that WD intake increased the brain protein levels of specific redox/neuroinflammatory biomarkers in the rats that were exposed to the PS. In particular, the protein levels of catalase (CAT) and the glial fibrillary acidic protein (GFAP). We demonstrate that the levels and activities of some of these biomarkers are closely associated with behavioral proxies related to fear and anxiety in rats.
Obesogenic diets trigger redox dysregulation and neuroinflammation: Implications for stress-related disorders.
Obesity is highly comorbid with anxiety and stress-related disorders [71–74]. Our studies indicate that consumption of an obesogenic WD during adolescence heightens stress reactivity, promotes anxiety-like behaviors, and impairs the structural integrity of neural substrates implicated in stress-induced psychopathology in rats [12–15]. Furthermore, there is support to the notion that impairments in brain and behavior can occur even in the absence of an obesogenic phenotype [13,75–81]. Yet, the molecular players underlying these abnormalities remain unclear. There is mounting evidence demonstrating that diet-induced obesity (DIO) triggers oxidative brain damage and mitochondrial dysfunction in critical corticolimbic regions implicated with stress-related disorders [27,63,82]. Paralleling studies in obese humans [64,83], a large body of evidence indicates that dietary obesity leads to increased production of ROS while altering the brain antioxidant capacity in rats [82]. Under normal circumstances, this ROS buildup will lead to increased expression and activities of several proteins with ROS scavenging capabilities, particularly the glutathione system [84]. Glutathione (GSH) plays an important role in cellular defense against oxidative stress, since it eliminates in a non-enzymatic way, hydroxyl radicals and singlet oxygen. Glutathione reductase (GSHR) reduces GSSG into GSH to continue the antioxidant cycle during detoxification of oxidants [40]. In this study, we found increased levels of GSHR activity in the plasma of rats that consumed the WD without changes in protein levels in the brain. This suggests a compensatory response to the oxidative stress generated due to metabolic imbalances [85]. Furthermore, this suggests that GSHR adaptive changes to the obesogenic diet have somewhat different control mechanisms. Because it has been reported that subjects with metabolic imbalances exhibit low levels of GSH, it is likely that this adaptive response to increase GSHR is ineffective in obesity [64,86]. Our results are consistent with the results reported in Gawlik et al., demonstrating increased GSHR plasma activity in subjects with metabolic disorders [85]. Notably, DIO-resistant rats exhibit normal ROS levels and no oxidative brain damage, even after the consumption of a high-fat obesogenic diet [27]. This finding highlights the impact of DIO on brain structure and function while suggesting that the genetic background strongly influences dietary obesity and oxidative brain damage.
Emerging evidence suggests that the molecular consequences of PTSD include elevated systemic levels of oxidative stress and inflammation [62,70]. Clinical studies have reported significant differences in blood antioxidant enzyme concentrations and ROS-related gene expression between PTSD patients and controls [62,87]. Animal studies have also found evidence consistent with an association between stress reactivity and ROS markers [70]. For example, studies demonstrate the potential involvement of ROS in rat models of PTSD [70,88]. Brain nitric oxide plays a critical role in facilitating fear conditioning [89]. In support of these observations, a study published by Mejia-Carmona et al. showed that predatory stress induces changes in oxidative stress biomarkers in the amygdala and prefrontal cortex in rats [90,91]. Furthermore, ROS heightens stress responsivity and alters fear and anxiety responses in rats [32,92–94]. Interestingly, the PTSD-like rats that were treated with a vitamin E-based antioxidant therapy exhibited resilience to the effects of stress on cognitive function [88]. While we did not detect changes in redox status biomarkers following PS exposure, we identified significant associations between several behaviors implicated in fear and stress reactivity and the protein levels of several ROS status biomarkers. The discrepancies between studies may be related to the mild psychogenic stressor used in this study, a potential masking effect of footshock stress, and the rat strain used in this study. For instance, Lewis rats display higher ROS levels relative to the Brown Norway rat strain, which is protective against Toxoplasma infection resistance [95]. Notably, Lewis rats are highly susceptible to inflammatory challenges and stress [96–98]. Overall, these studies indicate that obesity and the consumption of obesogenic diets rich in saturated fats could affect the mitochondrial redox balance and contribute to brain structural abnormalities and stress-related psychopathology. It is plausible that the consumption of obesogenic diets may “prime” the brain for heightened stress reactivity through increased ROS levels. Thus, ROS levels may represent a risk factor for anxiety and stress-related disorders.
An interesting finding of this study is the identification of selective neuroinflammatory and oxidative stress-related responses to an obesogenic WD and PS. In particular, catalase (CAT) protein levels were upregulated in the brain of the rats that consumed the obesogenic diet and were exposed to the psychogenic stressor. CAT is one of the major endogenous antioxidant enzymes that detoxify the reactive oxygen species (ROS) hydrogen peroxide (H2O2) to water and oxygen. While brain CAT activity is low compared to other tissues and organs [25], this enzyme regulates macrophage polarization in adipose tissue [99]. Overexpression of catalase is beneficial in the context of obesity. For instance, overexpression of a mitochondria-specific catalase prevents insulin impairments and inflammation while attenuating mitochondrial ROS production in mice on a brief high-fat diet [100].
On the other hand, catalase deletion leads to metabolic impairments and an obesogenic phenotype in mice [101]. Interestingly, it has been demonstrated that the reaction of CAT to dietary fat can occur before the manifestation of obesity-related phenotypes in mice[102]. It was reported that CAT protein levels and activity are elevated in the heart of mice that consumed a high-fat diet for only one day [102]. Thus, it appears that a robust switch to fatty acid oxidation is an adaptive cellular detoxification response to high-fat intake. It was proposed that since fatty acid oxidation enhances mitochondrial H2O2 production, high levels of CAT are needed to restore redox balance [102]. The findings of this study support the idea that obesogenic diets trigger an adaptive metabolic process in which CAT expression is upregulated to prevent cellular damage [103]. Furthermore, it indicates that catalase may represent a promising candidate for modulating redox dysregulation in obesity.
Glial cells have a high-energy demand, which is achieved by a strictly regulated cellular metabolism and high expression of redox proteins, including CAT [24,25,61]. The association between CAT and the ionized calcium-binding adaptor molecule 1 (Iba-1) and the glial fibrillary acidic protein (GFAP) levels validate a role for CAT in glial cells [99,104,105]. Furthermore, the upregulation of GFAP in the rats exposed to the obesogenic WD and PS provides support to the role of glial cells in ROS [106]. Several reports demonstrate that glial cells and CAT activities are particularly susceptible to the effects of high-fat diets and stress [20,22,107–109]. The brain redox state is sensitive to predator odor stress exposure in rats [90,91]. While these investigations show reduced CAT activities in the prefrontal cortex and amygdala of rats exposed to predator odor stress, the protein expression levels were not reported. A likely interpretation of this observation is that a reduction in CAT activity leads to increased CAT expression. It is widely accepted that peripheral circulation is a significant source of ROS, which can impact brain homeostasis [110]. Given this cooperativity, it is plausible that the high-fat diet-induced peripheral ROS and deficient antioxidant activities are reflected by changes in catalase expression in the brain [102]. Our data suggest that CAT upregulation may contribute to fear and anxiety-like phenotypes in rats exposed to an obesogenic diet and stress.
Decomposition of superoxide into hydrogen peroxide (H2O2) is catalyzed by superoxide dismutases (SOD). Two SOD proteins, cytoplasmic Cu/Zn-superoxide dismutase (SOD1) and mitochondrial Mn-superoxide dismutase (SOD2) are the among the most abundant antioxidant proteins in the brain and play critical roles in protecting neurons from oxidative stress. Several studies have reported increased activity of SODs in response to increased ROS production in psychiatric patients [111], including PTSD [112]. While we did not detect increased SOD protein levels in our experimental conditions, we identified a significant association between SOD-1 brain protein levels and increased anxiety-like behavior (reduced head dipping in the EPM). Our results provide support to previous studies in patients diagnosed with PTSD [112] and anxiety [113]. This study supports the potential role of diet-induced redox dysregulation in behavioral correlates implicated in anxiety and stress-related disorders [114]. Oxidative stress could be a consequence of perturbations within several systems known to be disrupted in obesity and may be involved in predisposition and pathogenesis of stress-related disease.
Limitations
The overarching hypothesis that abnormal fear network maturation and function in obesity implicates redox dysregulation/oxidative stress mechanisms should be further tested. Studies should be done to elucidate more specifically the molecular events that occur in WD-induced redox dysregulation, and more importantly, to establish causal relationships. Next investigations should also consider 1) using purified ingredient-matched control diets, 2) including female animals, and 3) testing the effects of DIO and PS on different rat strains. While this study did not provide redox status data from specific brain regions implicated in fear, whole-brain analyses provide fast and quantitative information on the general diet- and stress-induced redox status. Future studies are needed to validate the specific impact of obesogenic diets and stress on specific brain regions implicated with anxiety and stress-related disorders (e.g., hippocampus, prefrontal cortex, and amygdala).
Conclusion
More comprehensive knowledge about the effects of obesity and poor dietary practices on stress reactivity can help to build up a more compelling panorama of the benefits of an early healthy antioxidant diet and, more importantly, help to identify biomarkers of psychiatric risk in affected obese individuals.
Supplementary Material
Highlights:
Predatory odor stress heightens footshock reactivity and anxiety-like behaviors in Lewis rats.
WD intake increases glutathione reductase activity in plasma.
WD intake increases the brain protein levels of catalase and the glial fibrillary acidic protein.
The protein levels and activities of some redox/neuroinflammatory biomarkers are closely associated with behavioral proxies related to fear and anxiety in rats.
Acknowledgements
This study was partly supported by the NIH (P20MD006988 and 2R25 GM060507), Cooperative Title V (P031S130068), the University of Puerto Rico, Carolina Campus, Seed Funds Granted to JMS, and the Loma Linda University School of Medicine Seed Grant Funds to JDF.
We would like to thank the staff at the animal care facility.
Acknowledgements Statement: All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing assistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgements and have given us their written permission to be named. If we have not included an Acknowledgements, then that indicates that we have not received substantial contributions from non-authors.
Footnotes
Authorship Statement: All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, although this manuscript appears as unreviewed material in BioRxiv, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in Behavioural Brain Research.
Financial Disclosures
All authors report no financial interests or potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Friedman MJ, Keane TM, Resick PA, Handbook of PTSD, Second Edition, Guilford Publications, 2014. [Google Scholar]
- [2].Pine DS, Cohen JA, Trauma in children and adolescents: risk and treatment of psychiatric sequelae., Biol Psychiat. 51 (2002) 519 531. 10.1016/s0006-3223(01)01352-x. [DOI] [PubMed] [Google Scholar]
- [3].Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J, Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A), J Am Acad Child Adolesc Psychiatry. 49 (2010) 980 989. 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Smith BN, Tyzik AL, Neylan TC, Cohen BE, PTSD and obesity in younger and older veterans: Results from the mind your heart study., Psychiat Res. 229 (2015) 895 900. 10.1016/j.psychres.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bartoli F, Crocamo C, Alamia A, Amidani F, Paggi E, Pini E, Clerici M, Carrà G, Posttraumatic Stress Disorder and Risk of Obesity, J Clin Psychiatry. 76 (2015) e1253 61. 10.4088/jcp.14r09199. [DOI] [PubMed] [Google Scholar]
- [6].Kubzansky LD, Bordelois P, Jun HJ, Roberts AL, Cerda M, Bluestone N, Koenen KC, The Weight of Traumatic Stress: A Prospective Study of Posttraumatic Stress Disorder Symptoms and Weight Status in Women, Jama Psychiat. 71 (2014) 44–51. 10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mitchell KS, Aiello AE, Galea S, Uddin M, Wildman D, Koenen KC, PTSD and obesity in the Detroit neighborhood health study., Gen Hosp Psychiat. 35 (2013) 671 673. 10.1016/j.genhosppsych.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maguen S, Madden E, Cohen B, Bertenthal D, Neylan T, Talbot L, Grunfeld C, Seal K, The relationship between body mass index and mental health among Iraq and Afghanistan veterans., J Gen Intern Med. 28 Suppl 2 (2013) S563 70. 10.1007/s11606-013-2374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Johannessen KB, Berntsen D, Losing the symptoms: weight loss and decrease in posttraumatic stress disorder symptoms., J Clin Psychol. 69 (2013) 655 660. 10.1002/jclp.21962. [DOI] [PubMed] [Google Scholar]
- [10].Perkonigg A, Owashi T, Stein MB, Kirschbaum C, Wittchen H-U, Posttraumatic stress disorder and obesity: evidence for a risk association., Am J Prev Med. 36 (2009) 1 8. 10.1016/j.amepre.2008.09.026. [DOI] [PubMed] [Google Scholar]
- [11].Scott KM, McGee MA, Wells JE, Browne MAO, Obesity and mental disorders in the adult general population., J Psychosom Res. 64 (2008) 97 105. 10.1016/j.jpsychores.2007.09.006. [DOI] [PubMed] [Google Scholar]
- [12].Kalyan-Masih P, Vega-Torres JD, Miles C, Haddad E, Rainsbury S, Baghchechi M, Obenaus A, Figueroa JD, Western High-fat Diet Consumption During Adolescence Increases Susceptibility to Traumatic Stress while Selectively Disrupting Hippocampal and Ventricular Volumes, Eneuro. 3 (2016) ENEURO.0125–16.2016. 10.1523/eneuro.0125-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vega-Torres JD, Haddad E, Lee JB, Kalyan-Masih P, George WIM, Pérez LL, Vázquez DMP, Torres YA, Santana JMS, Obenaus A, Figueroa JD, Exposure to an obesogenic diet during adolescence leads to abnormal maturation of neural and behavioral substrates underpinning fear and anxiety., Brain Behav Immun. (2018). 10.1016/j.bbi.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vega-Torres JD, Reyes-Rivera AL, Figueroa JD, Developmental regulation of fear memories by an obesogenic high-saturated fat/high-sugar diet, Biorxiv. (2019) 748079. 10.1101/748079. [DOI] [Google Scholar]
- [15].Vega-Torres JD, Azadian M, Rios-Orsini R, Reyes-Rivera AL, Ontiveros-Angel P, Figueroa JD, Early maturational emergence of adult-like emotional reactivity and anxiety after brief exposure to an obesogenic diet, Biorxiv. (2020) 2020.03.03.975789. 10.1101/2020.03.03.975789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Layé S, Ferreira G, Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats., Brain Behav Immun. 40 (2014) 9 17. 10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- [17].Byrne ML, O’Brien-Simpson NM, Mitchell SA, Allen NB, Adolescent-Onset Depression: Are Obesity and Inflammation Developmental Mechanisms or Outcomes?, Child Psychiat Hum D. 46 (2015) 839 850. 10.1007/s10578-014-0524-9. [DOI] [PubMed] [Google Scholar]
- [18].Bolton JL, Bilbo SD, Developmental programming of brain and behavior by perinatal diet: focus on inflammatory mechanisms., Dialogues Clin Neurosci. 16 (2014) 307 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sofroniew MV, Vinters HV, Astrocytes: biology and pathology, Acta Neuropathol. 119 (2010) 7 35. 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jayaram B, Pan W, Wang Y, Hsuchou H, Mace A, Cornelissen-Guillaume GG, Mishra PK, Koza RA, Kastin AJ, Astrocytic leptin-receptor knockout mice show partial rescue of leptin resistance in diet-induced obesity, J Appl Physiol. 114 (2013) 734–741. 10.1152/japplphysiol.01499.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Buckman LB, Thompson MM, Moreno HN, Ellacott KLJ, Regional astrogliosis in the mouse hypothalamus in response to obesity., J Comp Neurol. 521 (2013) 1322 1333. 10.1002/cne.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Calvo-Ochoa E, Hernández-Ortega K, Ferrera P, Morimoto S, Arias C, Short-term high-fat-and-fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus., J Cereb Blood Flow Metabolism. 34 (2014) 1001 1008. 10.1038/jcbfm.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rottkamp DM, Rudenko IA, Maier MT, Roshanbin S, Yulyaningsih E, Perez L, Valdearcos M, Chua S, Koliwad SK, Xu AW, Leptin potentiates astrogenesis in the developing hypothalamus., Mol Metab. 4 (2015) 881 889. 10.1016/j.molmet.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baxter PS, Hardingham GE, Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes, Free Radical Bio Med. 100 (2016) 147–152. 10.1016/j.freeradbiomed.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wilson JX, Antioxidant defense of the brain: a role for astrocytes, Can J Physiol Pharm. 75 (1997) 1149–1163. 10.1139/y97-146. [DOI] [PubMed] [Google Scholar]
- [26].Gonçalves C-A, Rodrigues L, Bobermin LD, Zanotto C, Vizuete A, Quincozes-Santos A, Souza DO, Leite MC, Glycolysis-Derived Compounds From Astrocytes That Modulate Synaptic Communication, Front Neurosci-Switz. 12 (2019) 1035. 10.3389/fnins.2018.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ma W, Yuan L, Yu H, Xi Y, Xiao R, Mitochondrial dysfunction and oxidative damage in the brain of diet-induced obese rats but not in diet-resistant rats, Life Sci. 110 (2014) 53–60. 10.1016/j.lfs.2014.07.018. [DOI] [PubMed] [Google Scholar]
- [28].Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I, Increased oxidative stress in obesity and its impact on metabolic syndrome, J Clin Invest. 114 (2004) 1752–1761. 10.1172/jci21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maslov LN, Naryzhnaya NV, Boshchenko AA, Popov SV, Ivanov VV, Oeltgen PR, Is oxidative stress of adipocytes a cause or a consequence of the metabolic syndrome?, J Clin Transl Endocrinol. 15 (2018) 1–5. 10.1016/j.jcte.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sun M, Huang X, Yan Y, Chen J, Wang Z, Xie M, Li J, Rac1 is a possible link between obesity and oxidative stress in Chinese overweight adolescents., Obes Silver Spring Md. 20 (2012) 2233–40. 10.1038/oby.2012.63. [DOI] [PubMed] [Google Scholar]
- [31].Karbownik-Lewinska M, Szosland J, Kokoszko-Bilska A, Stępniak J, Zasada K, Gesing A, Lewinski A, Direct contribution of obesity to oxidative damage to macromolecules., Neuro Endocrinol Lett. 33 (2012) 453–61. [PubMed] [Google Scholar]
- [32].Hassan W, Silva CEB, Mohammadzai IU, da Rocha JBT, J L-F, Association of oxidative stress to the genesis of anxiety: implications for possible therapeutic interventions., Curr Neuropharmacol. 12 (2014) 120–39. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang XY, Yao JK, Oxidative stress and therapeutic implications in psychiatric disorders., Prog Neuro-Psychoph. 46 (2013) 197–9. 10.1016/j.pnpbp.2013.03.003. [DOI] [PubMed] [Google Scholar]
- [34].Zalachoras I, Hollis F, Ramos-Fernández E, Trovo L, Sonnay S, Geiser E, Preitner N, Steiner P, Sandi C, Morató L, Therapeutic potential of glutathione-enhancers in stress-related psychopathologies, Neurosci Biobehav Rev. 114 (2020) 134–155. 10.1016/j.neubiorev.2020.03.015. [DOI] [PubMed] [Google Scholar]
- [35].Pandya CD, Howell KR, Pillai A, Antioxidants as potential therapeutics for neuropsychiatric disorders., Prog Neuro-Psychopharmacology Biological Psychiatry. 46 (2013) 214 223. 10.1016/j.pnpbp.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gandhi S, Abramov AY, Mechanism of oxidative stress in neurodegeneration., Oxid Med Cell Longev. 2012 (2012) 428010. 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kumar A, Yegla B, Foster TC, Redox Signaling in Neurotransmission and Cognition During Aging, Antioxid Redox Sign. 28 (2018) 1724–1745. 10.1089/ars.2017.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chelikani P, Fita I, Loewen PC, Diversity of structures and properties among catalases, Cell Mol Life Sci Cmls. 61 (2004) 192–208. 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McCord JM, Fridovich I, Superoxide dismutase: The first twenty years (1968–1988), Free Radical Bio Med. 5 (1988) 363–369. 10.1016/0891-5849(88)90109-8. [DOI] [PubMed] [Google Scholar]
- [40].Barker JE, Heales SJR, Cassidy A, Bolaños JP, Land JM, Clark JB, Depletion of brain glutathione results in a decrease of glutathione reductase activity; an enzyme susceptible to oxidative damage, Brain Res. 716 (1996) 118–122. 10.1016/0006-8993(96)00003-0. [DOI] [PubMed] [Google Scholar]
- [41].Goswami S, Samuel S, Sierra OR, Cascardi M, Paré D, A rat model of post-traumatic stress disorder reproduces the hippocampal deficits seen in the human syndrome., Front Behav Neurosci. 6 (2012) 26. 10.3389/fnbeh.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cohen H, Matar MA, Richter-Levin G, Zohar J, The contribution of an animal model toward uncovering biological risk factors for PTSD., Ann Ny Acad Sci. 1071 (2006) 335 350. 10.1196/annals.1364.026. [DOI] [PubMed] [Google Scholar]
- [43].Cohen H, Zohar J, An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria., Ann Ny Acad Sci. 1032 (2004) 167 178. 10.1196/annals.1314.014. [DOI] [PubMed] [Google Scholar]
- [44].Elkin TD, Wollan MO, Anderson SL, Gaston R, Meyer W, Fuemmeler BF, Holloway FA, Martin RE, Dietary essential fatty acids and gender-specific behavioral responses in cranially irradiated rats., Neuropsychiatric Disease and Treatment. 2 (2006) 365 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gogos JA, Santha M, Takacs Z, Beck KD, Luine V, Lucas LR, Nadler JV, Karayiorgou M, The gene encoding proline dehydrogenase modulates sensorimotor gating in mice., Nat Genet. 21 (1999) 434 439. 10.1038/7777. [DOI] [PubMed] [Google Scholar]
- [46].Grimsley CA, Longenecker RJ, Rosen MJ, Young JW, Grimsley JM, Galazyuk AV, An improved approach to separating startle data from noise., J Neurosci Meth. 253 (2015) 206 217. 10.1016/j.jneumeth.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Davis M, Fear-Potentiated Startle in Rats, John Wiley & Sons, Inc., 2001. 10.1002/0471142301.ns0811as14. [DOI] [PubMed] [Google Scholar]
- [48].Walker DL, Davis M, Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: implications for the neurocircuitry of fear and anxiety., Psychopharmacology. 164 (2002) 318 328. 10.1007/s00213-002-1213-0. [DOI] [PubMed] [Google Scholar]
- [49].Goswami S, Cascardi M, Rodríguez-Sierra OE, Duvarci S, Paré D, Impact of predatory threat on fear extinction in Lewis rats, Learn Memory. 17 (2010) 494–501. 10.1101/lm.1948910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Garcia AMB, Cardenas FP, Morato S, Effect of different illumination levels on rat behavior in the elevated plus-maze, Physiol Behav. 85 (2005) 265–270. 10.1016/j.physbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [51].Jones N, King SM, Influence of circadian phase and test illumination on pre-clinical models of anxiety, Physiol Behav. 72 (2001) 99–106. 10.1016/s0031-9384(00)00388-7. [DOI] [PubMed] [Google Scholar]
- [52].Verma P, Hellemans KGC, Choi FY, Yu W, Weinberg J, Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress, Physiol Behav. 99 (2010) 276–285. 10.1016/j.physbeh.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pellow S, Chopin P, File SE, Briley M, Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat., Journal of Neuroscience Methods. 14 (1985) 149 167. [DOI] [PubMed] [Google Scholar]
- [54].File SE, The interplay of learning and anxiety in the elevated plus-maze, Behav Brain Res. 58 (1993) 199–202. 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- [55].Contreras CM, Rodríguez-Landa JF, García-Ríos RI, Cueto-Escobedo J, Guillen-Ruiz G, Bernal-Morales B, Myristic Acid Produces Anxiolytic-Like Effects in Wistar Rats in the Elevated Plus Maze, Biomed Res Int. 2014 (2014) 1–8. 10.1155/2014/492141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z, Animal model for PTSD: from clinical concept to translational research., Neuropharmacology. 62 (2012) 715 724. 10.1016/j.neuropharm.2011.04.023. [DOI] [PubMed] [Google Scholar]
- [57].Vega-Torres JD, Kalyan-Masih P, Argueta DA, DiPatrizio NV, Figueroa JD, Endocrine, metabolic, and endocannabinoid correlates of obesity in rats exhibiting high anxiety-related behaviors, Matters Sel. (2019). 10.19185/matters.201906000003. [DOI] [Google Scholar]
- [58].Rojo AI, McBean G, Cindric M, Egea J, López MG, Rada P, Zarkovic N, Cuadrado A, Redox control of microglial function: molecular mechanisms and functional significance., Antioxid Redox Sign. 21 (2014) 1766–801. 10.1089/ars.2013.5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gray E, Kemp K, Hares K, Redondo J, Rice C, Scolding N, Wilkins A, Increased microglial catalase activity in multiple sclerosis grey matter, Brain Res. 1559 (2014) 55–64. 10.1016/j.brainres.2014.02.042. [DOI] [PubMed] [Google Scholar]
- [60].Liddell JR, Robinson SR, Dringen R, Endogenous glutathione and catalase protect cultured rat astrocytes from the iron-mediated toxicity of hydrogen peroxide, Neurosci Lett. 364 (2004) 164–167. 10.1016/j.neulet.2004.04.042. [DOI] [PubMed] [Google Scholar]
- [61].Desagher S, Glowinski J, Premont J, Astrocytes protect neurons from hydrogen peroxide toxicity, J Neurosci. 16 (1996) 2553–2562. 10.1523/jneurosci.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Miller MW, Lin AP, Wolf EJ, Miller DR, Oxidative Stress, Inflammation, and Neuroprogression in Chronic PTSD., Harvard Rev Psychiat. 26 (2017) 1. 10.1097/hrp.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Freeman LR, Zhang L, Nair A, Dasuri K, Francis J, Fernandez-Kim S-O, Bruce-Keller AJ, Keller JN, Obesity increases cerebrocortical reactive oxygen species and impairs brain function., Free Radic Biology Medicine. 56 (2012) 226–33. 10.1016/j.freeradbiomed.2012.08.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Vincent HK, Innes KE, Vincent KR, Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity, Diabetes Obes Metabolism. 9 (2007) 813–839. 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- [65].Lee YS, Kim AY, Choi JW, Kim M, Yasue S, Son HJ, Masuzaki H, Park KS, Kim JB, Dysregulation of Adipose Glutathione Peroxidase 3 in Obesity Contributes to Local and Systemic Oxidative Stress, Mol Endocrinol. 22 (2008) 2176–2189. 10.1210/me.2008-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Forman HJ, Bernardo A, Davies KJA, What is the concentration of hydrogen peroxide in blood and plasma?, Arch Biochem Biophys. 603 (2016) 48–53. 10.1016/j.abb.2016.05.005. [DOI] [PubMed] [Google Scholar]
- [67].Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, Zahoor H, Saeed D, Natteru PA, Iyer S, Zaheer A, Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration, Front Cell Neurosci. 11 (2017) 216. 10.3389/fncel.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shaji CA, Robinson BD, Yeager A, Beeram MR, Davis ML, Isbell CL, Huang JH, Tharakan B, The Tri-phasic Role of Hydrogen Peroxide in Blood-Brain Barrier Endothelial cells, Sci Rep-Uk. 9 (2019) 133. 10.1038/s41598-018-36769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Morris G, Fernandes BS, Puri BK, Walker AJ, Carvalho AF, Berk M, Leaky brain in neurological and psychiatric disorders: Drivers and consequences, Australian New Zealand J Psychiatry. 52 (2018) 924–948. 10.1177/0004867418796955. [DOI] [PubMed] [Google Scholar]
- [70].Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J, Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model., Plos One. 8 (2013) e76146. 10.1371/journal.pone.0076146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gariepy G, Nitka D, Schmitz N, The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis, Int J Obesity. 34 (2010) 407–419. 10.1038/ijo.2009.252. [DOI] [PubMed] [Google Scholar]
- [72].Stefanovics EA, Potenza MN, Pietrzak RH, PTSD and Obesity in U.S. Military Veterans: Prevalence, Health Burden, and Suicidality., Psychiat Res. 291 (2020) 113242. 10.1016/j.psychres.2020.113242. [DOI] [PubMed] [Google Scholar]
- [73].Hryhorczuk C, Sharma S, Fulton SE, Metabolic disturbances connecting obesity and depression, Front Neurosci-Switz. 7 (2013) 177. 10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Masodkar K, Johnson J, Peterson MJ, A Review of Posttraumatic Stress Disorder and Obesity: Exploring the Link., Prim Care Companion Cns Disord. 18 (2016). 10.4088/pcc.15r01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Beilharz JE, Maniam J, Morris MJ, Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats., Brain Behav Immun. 37 (2014) 134 141. 10.1016/j.bbi.2013.11.016. [DOI] [PubMed] [Google Scholar]
- [76].Beilharz JE, Maniam J, Morris MJ, Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation., Behav Brain Res. 306 (2016) 1 7. 10.1016/j.bbr.2016.03.018. [DOI] [PubMed] [Google Scholar]
- [77].Edwards LM, Murray AJ, Holloway CJ, Carter EE, Kemp GJ, Codreanu I, Brooker H, Tyler DJ, Robbins PA, Clarke K, Short-term consumption of a high-fat diet impairs whole-body efficiency and cognitive function in sedentary men, Faseb J. 25 (2011) 1088 1096. 10.1096/fj.10-171983. [DOI] [PubMed] [Google Scholar]
- [78].Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK, Lambert B, Thompson C, Neubauer S, Clarke K, A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects., Am J Clin Nutrition. 93 (2011) 748 755. 10.3945/ajcn.110.002758. [DOI] [PubMed] [Google Scholar]
- [79].Kanoski SE, Davidson TL, Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet., J Exp Psychology Animal Behav Process. 36 (2010) 313 319. 10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- [80].Sobesky JL, D’Angelo HM, Weber MD, Anderson ND, Frank MG, Watkins LR, Maier SF, Barrientos RM, Glucocorticoids Mediate Short-Term High-Fat Diet Induction of Neuroinflammatory Priming, the NLRP3 Inflammasome, and the Danger Signal HMGB1., Eneuro. 3 (2016) ENEURO.0113–16.2016. 10.1523/eneuro.0113-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Khazen T, Hatoum OA, Ferreira G, Maroun M, Acute exposure to a high-fat diet in juvenile male rats disrupts hippocampal-dependent memory and plasticity through glucocorticoids., Sci Rep-Uk. 9 (2019) 12270. 10.1038/s41598-01948800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].de Aquino CC, Leitão RA, Alves LAO, Coelho-Santos V, Guerrant RL, Ribeiro CF, Malva JO, Silva AP, Oriá RB, Effect of Hypoproteic and High-Fat Diets on Hippocampal Blood-Brain Barrier Permeability and Oxidative Stress., Frontiers Nutrition. 5 (2018) 131. 10.3389/fnut.2018.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Roh H-T, Cho S-Y, So W-Y, Obesity promotes oxidative stress and exacerbates blood-brain barrier disruption after high-intensity exercise., J Sport Health Sci. 6 (2016) 225–230. 10.1016/j.jshs.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rani V, Deep G, Singh RK, Palle K, Yadav UCS, Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies, Life Sci. 148 (2016) 183–193. 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- [85].Gawlik K, Naskalski JW, Fedak D, Pawlica-Gosiewska D, Grudzień U, Dumnicka P, Małecki MT, Solnica B, Markers of Antioxidant Defense in Patients with Type 2 Diabetes, Oxid Med Cell Longev. 2016 (2016) 1–6. 10.1155/2016/2352361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Olusi S, Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans, Int J Obesity. 26 (2002) 1159–1164. 10.1038/sj.ijo.0802066. [DOI] [PubMed] [Google Scholar]
- [87].Şimsek Ş, Yüksel T, Kaplan İ, Uysal C, Aktaş H, The Levels of Cortisol and Oxidative Stress and DNA Damage in Child and Adolescent Victims of Sexual Abuse with or without Post-Traumatic Stress Disorder, Psychiatry Investig. 13 (2016) 616. 10.4306/pi.2016.13.6.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ahmed M, Alzoubi KH, Khabour OF, Vitamin E prevents the cognitive impairments in post-traumatic stress disorder rat model: behavioral and molecular study., Psychopharmacology. 237 (2019) 599–607. 10.1007/s00213-019-05395-w. [DOI] [PubMed] [Google Scholar]
- [89].Kelley JB, Balda MA, Anderson KL, Itzhak Y, Impairments in fear conditioning in mice lacking the nNOS gene., Learn Mem Cold Spring Harb N Y. 16 (2009) 371–8. 10.1101/lm.1329209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mejia-Carmona GE, Gosselink KL, de la Rosa LA, Pérez-Ishiwara G, Martínez-Martínez A, Evaluation of antioxidant enzymes in response to predator odor stress in prefrontal cortex and amygdala, Neurochem J. 8 (2014) 125–128. 10.1134/s181971241402007x. [DOI] [Google Scholar]
- [91].Mejia-Carmona GE, Gosselink KL, Pérez-Ishiwara G, Martínez-Martínez A, Oxidant/antioxidant effects of chronic exposure to predator odor in prefrontal cortex, amygdala, and hypothalamus., Mol Cell Biochem. 406 (2015) 121–9. 10.1007/s11010-015-2430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hassan W, de C V. Gomes, S. Pinton, J.B.T. da Rocha, J. Landeira-Fernandez, Association between oxidative stress and contextual fear conditioning in Carioca high- and low-conditioned freezing rats., Brain Res. 1512 (2013) 60–7. 10.1016/j.brainres.2013.03.039. [DOI] [PubMed] [Google Scholar]
- [93].DEOLIVEIRA M, SILVESTRIN R, MELLOESOUZA T, MOREIRA J, Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: Effects of sub acute vitamin A supplementation at therapeutic doses, Neurotoxicology. 28 (2007) 1191–1199. 10.1016/j.neuro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- [94].Brocardo PS, Boehme F, Patten A, Cox A, Gil-Mohapel J, Christie BR, Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise., Neuropharmacology. 62 (2011) 1607–18. 10.1016/j.neuropharm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- [95].Witola WH, Kim CY, Zhang X, Inherent Oxidative Stress in the Lewis Rat Is Associated with Resistance to Toxoplasmosis., Infect Immun. 85 (2017) 323. 10.1128/iai.00289-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z, Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats., Biol Psychiat. 59 (2006) 1208 1218. 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- [97].Khanna A, Guo M, Mehra M, Royal W, Inflammation and oxidative stress induced by cigarette smoke in Lewis rat brains., J Neuroimmunol. 254 (2013) 69 75. 10.1016/j.jneuroim.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chover-Gonzalez AJ, Jessop DS, Tejedor-Real P, Gibert-Rahola J, Harbuz MS, Onset and severity of inflammation in rats exposed to the learned helplessness paradigm., Rheumatology. 39 (2000) 764 771. 10.1093/rheumatology/39.7.764. [DOI] [PubMed] [Google Scholar]
- [99].Park YS, Uddin MJ, Piao L, Hwang I, Lee JH, Ha H, Novel Role of Endogenous Catalase in Macrophage Polarization in Adipose Tissue., Mediat Inflamm. 2016 (2016) 8675905. 10.1155/2016/8675905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Paglialunga S, Ludzki A, Root-McCaig J, Holloway GP, In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice., Diabetologia. 58 (2015) 1071–80. 10.1007/s00125-015-3531-x. [DOI] [PubMed] [Google Scholar]
- [101].Heit C, Marshall S, Singh S, Yu X, Charkoftaki G, Zhao H, Orlicky DJ, Fritz KS, Thompson DC, Vasiliou V, Catalase deletion promotes prediabetic phenotype in mice., Free Radic Biology Medicine. 103 (2016) 48–56. 10.1016/j.freeradbiomed.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Rindler PM, Plafker SM, Szweda LI, Kinter M, High dietary fat selectively increases catalase expression within cardiac mitochondria., J Biological Chem. 288 (2012) 1979–90. 10.1074/jbc.m112.412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin C-T, Price JW, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD, Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans., J Clin Investigation. 119 (2009) 573–81. 10.1172/jci37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pathipati P, Müller S, Jiang X, Ferriero D, Phenotype and Secretory Responses to Oxidative Stress in Microglia, Dev Neurosci-Basel. 35 (2013) 241–254. 10.1159/000346159. [DOI] [PubMed] [Google Scholar]
- [105].Dringen R, Oxidative and Antioxidative Potential of Brain Microglial Cells, Antioxid Redox Sign. 7 (2005) 1223–1233. 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- [106].Chen Y, Qin C, Huang J, Tang X, Liu C, Huang K, Xu J, Guo G, Tong A, Zhou L, The role of astrocytes in oxidative stress of central nervous system: A mixed blessing, Cell Proliferat. 53 (2020) e12781. 10.1111/cpr.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP, Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation., Mol Metab. 6 (2017) 366 373. 10.1016/j.molmet.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ, Cognitive impairment following high fat diet consumption is associated with brain inflammation., J Neuroimmunol. 219 (2010) 25 32. 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Camargo N, Brouwers JF, Loos M, Gutmann DH, Smit AB, Verheijen MHG, High-fat diet ameliorates neurological deficits caused by defective astrocyte lipid metabolism., Faseb J. 26 (2012) 4302 4315. 10.1096/fj.12-205807. [DOI] [PubMed] [Google Scholar]
- [110].Dringen R, Pawlowski PG, Hirrlinger J, Peroxide detoxification by brain cells, J Neurosci Res. 79 (2005) 157–165. 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- [111].Ng F, Berk M, Dean O, Bush AI, Oxidative stress in psychiatric disorders: evidence base and therapeutic implications, Int J Neuropsychoph. 11 (2008) 851–876. 10.1017/s1461145707008401. [DOI] [PubMed] [Google Scholar]
- [112].Tezcan E, Atmaca M, Kuloglu M, Ustundag B, Free radicals in patients with post-traumatic stress disorder, Eur Arch Psy Clin N. 253 (2003) 89–91. 10.1007/s00406-003-0413-x. [DOI] [PubMed] [Google Scholar]
- [113].Russo AJ, Increased Serum Cu/Zn Superoxide Dismutase in Individuals with Anxiety, Proteom Insights. 3 (2010) PRI.S5180. 10.4137/pri.s5180. [DOI] [Google Scholar]
- [114].Agrimi J, Spalletti C, Baroni C, Keceli G, Zhu G, Caragnano A, Matteucci M, Chelko S, Ramirez-Correa GA, Bedja D, Casieri V, Lascio ND, Scalco A, Beltrami AP, Paolocci N, Caleo M, Lionetti V, Obese mice exposed to psychosocial stress display cardiac and hippocampal dysfunction associated with local brain-derived neurotrophic factor depletion., Ebiomedicine. 47 (2019) 384–401. 10.1016/j.ebiom.2019.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.