Abstract
Purpose of the review
We report the current state of HIV-to-HIV kidney transplantation in the United States and remaining challenges in implementing this practice nationally.
Recent findings
The HIV Organ Policy Equity (HOPE) Act, which was the first step in unlocking the potential of HIV+ organ donors, mandates clinical research on HIV+ to HIV+ transplantation. As of March 2019, there have been 57 HOPE donors, including both true and false positive HOPE donors resulting in more than 120 transplants.
Summary
The HOPE Act, signed in 2013, reversed the federal ban on the transplantation of organs from HIV+ donors into HIV+ recipients. Ongoing national studies are exploring the safety, feasibility and efficacy of both kidney and liver transplantation in this population. If successfully and fully implemented, HIV+ to HIV+ transplantation could attenuate the organ shortage for everyone waiting, resulting in a far-reaching public health impact.
Keywords: HIV infection, solid organ transplantation, end stage renal disease, kidney transplant, HOPE Act
INTRODUCTION
The HOPE (HIV Organ Policy Equity) Act was passed in 2013 to initiate research on the use of organs from HIV-infected (HIV+) donors (D+) for transplantation into HIV+ recipients (HIV D+/R+). This review serves to outline key issues in kidney transplantation in the HIV R+, major milestones in the implementation of the HOPE Act, the aims of ongoing HOPE in Action studies, and discuss ongoing clinical and logistical challenges.
KIDNEY TRANSPLANTATION IN THE HIV+ PATIENT
Effective antiretroviral therapy (ART) is responsible for the dramatic increase in life expectancy for HIV+ individuals. Though many HIV+ individuals with access to high quality health care can have normal life expectancies, chronic kidney and liver diseases are growing causes of morbidity and now surpass opportunistic complications of HIV as leading causes of death in some cohorts [1]. Approximately 10–30% of HIV+ people develop chronic kidney disease (CKD) [2] secondary to HIV associated nephropathy (HIVAN), nephrotoxic ART, renal disease associated with common co-infections such as hepatitis B (HBV) and hepatitis C (HCV), as well as concomitant atherosclerotic cardiovascular disease, hypertension, and diabetes [3]. Approximately 0.5–1.4% of the nearly 500,000 patients on dialysis in the United States are HIV+; which translates to approximately 900 incident cases per year [4].
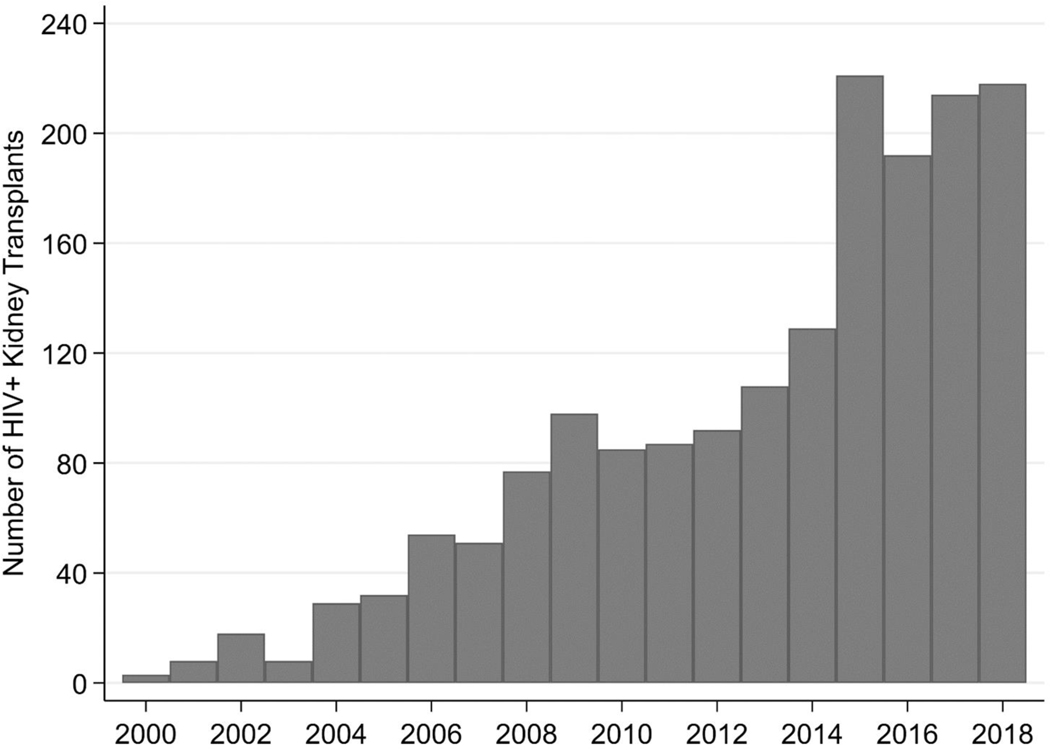
Once on dialysis, survival of HIV+ patients is poor: a study in the modern ART era reported a 63% 5-year survival compared to 94% in matched HIV- counterparts [5]. Consequently, over the past 20 years, kidney transplantation (with organs from HIV- donors) has emerged as the ideal treatment for carefully selected HIV+ transplant candidates [6, 7], offering an 80% lower mortality than dialysis [8]. We conducted a study of the Scientific Registry of Transplant Recipients (SRTR) and found 1,698 HIV+ patients on the kidney wait list nationally [9]. These patients were disproportionately young, black and male. Since 2003, there has been a more than a ten-fold increase in the number of HIV+ kidney transplants performed (Figure 1).
FIGURE 1. Transplants of HIV+ Recipients in the United States 2000–2018.
This figure represents the number of HIV+ transplant recipients (of both HIV+ and HIV- organs) between 2000 and 2018 based on SRTR data.
We conducted a national study examining long-term outcomes among 510 HIV+ kidney transplant recipients in which we found similar 5-year (75.0% versus 75.8%, P=0.58) and 10-year graft survival (55.9% versus 56.0%, P=0.49) compared with HIV- controls (HR, 1.06; 95% CI, 0.85 to 1.33; P=0.61). HIV+ recipients had similar overall survival compared with HIV- controls at 5 years (88.7% versus 89.1%, P=0.50) and 10 years post-transplant (63.5% versus 77.6%, P=0.10) (HR, 1.26; 95% CI, 0.98 to 1.69; P=0.13) [10]. In the NIH Multi-Site Study (HIV-TR), patient and graft survival were excellent at 5 years: 88% and 70% respectively [11]. The successful implementation of transplantation for appropriate HIV+ transplant candidates, however, is limited by the ever-present organ shortage [12].
In South Africa, where access to dialysis is limited and HIV prevalence is staggeringly high, there have been encouraging outcomes of 30 HIV D+/R+ transplants [13, 14]. The first four were reported in NEJM 2010, with 100% patient survival, excellent graft function, no episodes of rejection, and maintenance of HIV control; subsequent transplants have proven similarly successful. This practice was not possible initially in the US since recovering organs from HIV D+ was federally banned until the HOPE Act.
To provide evidence for HIV D+/R+ transplantation in the US, we analyzed data from two national registries and conservatively estimated a potential of 300–500 HIV D+ per year [15, 16]. After engaging national HIV/AIDS advocacy groups and transplantation organizations, we lobbied Congress to draft a bill which was unanimously passed; and in November 2013, the HOPE Act was signed into law [17]. The worsening and catastrophic opioid epidemic is likely to contribute more HIV+ deceased donors to the pool: the number of donors nationally with drug intoxication reported as the mechanism of death increased from 342 (4.3%) in 2010 to 1,382 (13.4%) in 2017 (p<0.001) [18, 19].
MILESTONES IN HOPE ACT IMPLEMENTATION
The HOPE Act had a three-part mandate. First, the Department of Health and Human Service (DHHS) revised the federal ban on recovery of organs from HIV D+ to allow transplantation within research protocols; this was accomplished on June 8, 2015 [20]. Second, the Organ Procurement Transplant Network (OPTN) wrote policies for the use of HIV D+, including documentation of local Institutional Review Board (IRB) approval and a requirement to submit regular safety reports. These changes were implemented on November 21, 2015 [21]. Third, the National Institutes of Health (NIH) developed the Final Human HOPE Act Safeguards and Research Criteria on November 25, 2015 [22]. Our institution opened the first clinical trial under these criteria and received approval from OPTN on January 8, 2016. The objective of this pilot study was to evaluate the safety of HIV D+/R+ kidney and liver transplantation [23]. In March 2016, as part of this protocol, the first HIV D+/R+ kidney and liver transplants were performed at Johns Hopkins Hospital.
HOPE IN ACTION TRIALS
Encouraging results from the pilot study provided the foundation for two NIH-funded U01 multicenter trials of kidney and liver transplantation which launched in April 2018 and January 2019, respectively [24]. These studies are ongoing and designed to generate evidence and guidance necessary to inform HIV D+/R+ policies by studying the feasibility, safety and effectiveness of HIV-to-HIV transplantation.
HIV+ DECEASED ORGAN DONORS
Federal deceased donor criteria state that eligible HIV D+ must be free of active invasive opportunistic complications of HIV. There is no viral load or CD4 requirement. A donor organ biopsy and description of effective post-transplant ART is required. Other criteria are left to investigator teams including allowing consideration of donors with untreated HIV, co-infection with HCV or HBV, prior AIDS diagnosis, and donation after cardiac death (DCD). The optimal evaluation for a potential HIV D+ would include comprehensive data on the donor’s HIV history, ART treatment history and resistance, however the time constraints of the deceased donor process is such that it is not always feasible to obtain comprehensive information prior to organ procurement as some of these tests take weeks to complete.
There are several reasons why the experience and characteristics of HIV+ kidney donors in South Africa is not generalizable to the US. There are key differences between the HIV+ population with respect to race, gender, age, and socio-economic status. In addition, there are differences in HIV subtypes, modes of HIV acquisition, prevalence of HCV coinfection, average viral load and CD4 count, access to ART, type and number of available ART regimens, and access to HIV-specific health care. Other epidemiologic differences include HIV prevalence (17.8% in South Africa vs. 0.6% in US), annual HIV deaths (310,000 in South Africa vs. 17,000 in US), and transmitted drug resistance (< 5% in South Africa vs. 10–18% in US) [25].
HIV+ LIVING KIDNEY DONORS
HIV+ kidney transplant candidates on the wait list have a 28% lower likelihood of receiving a transplant and a 47% lower rate of living donor transplantation compared to their HIV- counterparts [9]. Wait list candidates are most likely to identify a potential living donor from within their own social network, and for HIV+ individuals, this may translate into identification of donors who are also HIV+. There have been concerns about whether donating a kidney would be safe for an HIV+ individual given the increased risk of CKD in this population. In order to quantify this risk, our group compared the cumulative incidence of ERSD among 41,698 HIV+ individuals in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) with 16,025 HIV- matched peers in the National Health and Nutrition Examination (NHANES) III for various low-risk clinical scenarios based on age, race, renal function, HIV viral load, and CD4+ count [26]. We found that in subgroups with no co-morbidities and well-controlled HIV, the risk increase associated with HIV for a 40-year old was 1 per 10,000 among white females, 2 per 10,000 among white males, 10 per 10,000 among black females, and 11 per 10,000 among black males, risks comparable to that of cigarette smoking, which is not a contraindication to kidney donation. National criteria state that living donors should have well controlled HIV (CD4+ T-cell count > 500 for 6 months before donation; undetectable viral load, no evidence of opportunistic infections).
FALSE POSITIVE DONORS
An unexpected benefit of the HOPE Act is that it has facilitated the allocation of kidneys from donors with suspected false-positive HIV tests, that is, potential donors who had no known history of HIV but had unanticipated, discordant HIV screening tests. Prior to the HOPE Act, kidneys from these donors were generally discarded because of the potential risk of unintentionally transmitting HIV. All deceased donors are screened with an HIV antibody (Ab) test and increased risk donors must also have an HIV nucleic acid test (NAT) [27, 28]. Most (68%) OPOs test all donors with HIV NAT, regardless of infectious risk donor (IRD) status [29]. After acquisition, HIV Ab can take 3 to 12 weeks to develop. During this window period, molecular assays that detect viral particles, i.e., a NAT, or that detect HIV antigen (Ag) can diagnose infection within days [30]. The combination of tests is designed to capture acute HIV infection in window period, in other words, Ab-/ NAT+; however, these assays have false positive rates of 0.1–0.5%. In current practice in deceased donor evaluation, there may be insufficient time for confirmation of suspected false-positive HIV screening tests and current OPTN policy does not provide guidance on what to do in these cases [31]. Since the HOPE Act, 16 kidney transplants from the first 10 donors with suspected false-positive HIV tests have been reported with excellent outcomes. These young donors had minimal medical co-morbidities. Given the number of deceased donors tested each with HIV Ab and NAT and the known false positive rates of these assays, we estimated that there will be 50–100 false-positive HOPE donors annually [30].
CLINICAL CHALLENGES
There are biologic issues specific to HIV that are important in the implementation of HIV D+/R+ transplantation [32–34]. One of the main theoretical risks is donor-to-recipient HIV superinfection, defined as recipient acquisition of a distinct HIV strain from the donor. HIV superinfection has been reported with ongoing intravenous drug use (IVDU) and sexual transmission [35]. Potential HIV+ transplant recipients must be on effective ART, and superinfection is thought to occur rarely on ART, however the viral inoculum that occurs with an organ is much higher than that associated with IVDU or sexual transmission. If the donor’s virus has significant antiretroviral resistance, superinfection could lead to HIV breakthrough and progression of HIV. Ideally, one would have complete information on any donor HIV resistance at the time of transplant however HIV resistance tests take several weeks to result. While this testing is possible with living kidney donors, in deceased donor transplantation, physicians must rely on medical record review and clinical judgement. Future studies of rates of antiretroviral resistance among HIV+ deceased donors in the US are anticipated.
Increased rates of acute rejection in HIV+ kidney recipients have been reported [36]. Debate continues on the ideal immunosuppression regimen in this population due to concerns about increased rates of infection with lymphocyte depleting regimens. We studied 830 HIV+ recipients between 2000 and 2014 captured by the SRTR and found that those who received induction with anti-thymocyte globulin (ATG) had lower rates of AR (wRR 0.59, 95% CI 0.35–0.99). Induction was not associated with increased infections.
Another theoretical risk of HIV+ donor kidney transplantation is the risk of HIV-associated kidney disease in the allograft. One study of 19 HIV+ kidney transplant recipients (of HIV- organs) demonstrated that although plasma HIV RNA was undetectable, in some recipients, HIV infection of podocytes was observed, resulting in nephrotic-range proteinuria, progressive focal segmental glomerulosclerosis (FSGS) and subsequent renal dysfunction. This complication was not reported in the NIH HIV-TR study [37] or in the South African studies of HIV D+/R+ transplantation [14]. In this study, HIV was also seen to asymptomatically infect tubular cells with little clinical manifestation [38].
IMPACT OF HOPE IN ACTION IN THE FIRST THREE YEARS
Successful implementation of HIV D+/R+ transplantation requires that the HIV+ community, organ procurement organizations (OPOs) and transplant centers are appropriately informed and prepared. As part of HOPE in Action, we have explored knowledge and attitudes towards the HOPE Act among these key stakeholders.
We conduced 114 one-on-one surveys with HIV+ individuals at a clinic in Baltimore, MD between August and October 2016. Among participants (median age 55; 48% female; 91% Black), 90% of respondents believed that HIV+ people should be permitted to donate organs, and 73% thought that using HIV+ donor organs would reduce discrimination [39]. Eighty percent of respondents were willing to be deceased donors. Although nearly all respondents (93.0%) had discussed their HIV status with their next-of-kin, few had discussed their willingness to donate with them (17.8%) or were registered organ donors (21.1%).
We performed a survey of all 58 OPOs regarding knowledge and attitudes towards the HOPE Act. Fifty-five (95%) OPOS responded and reported support for the HOPE Act and research related to HIV D+/R+ transplantation. The number of referrals of HIV+ donors per OPO was highly variable, ranging from 0 to 276 with 3 OPOs reporting more than 100 referrals. We estimated a potential of 2,164 HIV D+ referrals nationally per year [40], but recognize that many of these referrals might not be eligible donors due to medical reasons or systemic barriers such as late referrals by donor hospitals, lack of brain death testing, low donor registration or authorization rates. With education and training, OPO engagement in HOPE is increasing. In 2016, 16/58 OPOs had evaluated a potential HIV D+; in 2018, the number had increased to 37, and as of March 2019, 46 OPOs (79.3%) were evaluating HOPE donor referrals.
In 2018, we conducted a national survey of transplant centers and found that 50/209 transplant centers reported plans to perform HIV D+/R+ transplants. While ≥1 center in every UNOS region reported planning HIV D+/R+ protocols, the vast majority were clustered in the eastern US [41]. These centers had 2.9-fold higher median annual transplant volume (159 vs 54, P < 0.01), higher proportion of transplants using infectious risk donor organs (15% vs 12%, P < 0.01), and were located in areas with a higher prevalence of HIV compared to centers not planning to implement HIV D+/R+ transplantation. As of March 2019 according to the OPTN, 31 transplant centers have an active HOPE variance [42].
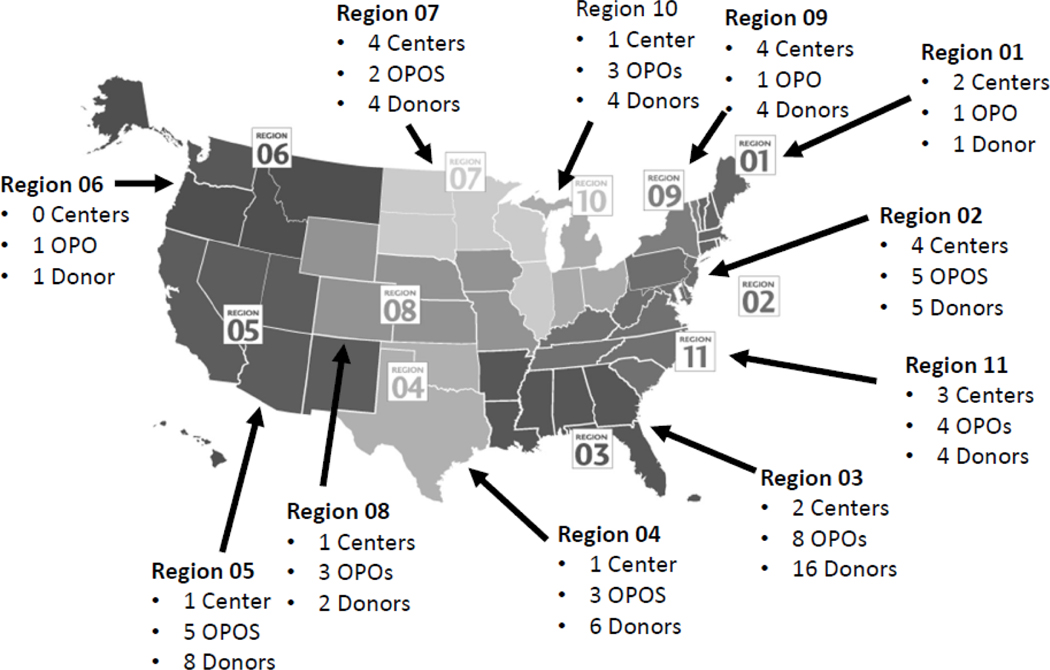
Since the first HOPE deceased donor kidney transplant in March 2016, there have been 57 HOPE donors as of March 2019, including both true and false positive HOPE donors resulting in more than 120 transplants. Kidney transplant outcomes have not yet been published but reports are anticipated in the near future. The HIV+ first HIV living donor kidney transplant took place at Johns Hopkins in March 2019; and several centers have active protocols listed with OPTN with more HIV+ living kidney donations expected (Figure 2).
FIGURE 2. National Transplant Center, OPO, HOPE Donors January 2016 - March 2019.
This is a map of the US divided by United Network for Organ Sharing (UNOS) regions. Each region has listed the number of transplant centers with an ongoing HOPE protocol, OPOs actively evaluating HOPE donors, and the number of HOPE donors who have had organs recovered for transplant as of March 2019.
CONCLUSION
Organs from HIV D+ represent a unique resource for HIV+ individuals on the transplant waitlist. In addition, every HIV R+ that receives an organ from an HIV D+ decreases the wait time and mortality for HIV- individuals. Therefore, if successfully and fully implemented, HIV D+/R+ transplantation could attenuate the organ shortage for everyone waiting, resulting in a far-reaching public health impact. Future directions include exploring short-term and long-term graft and patient survival.
KEY POINTS.
The HIV Organ Policy Equity (HOPE) Act was the first step in unlocking the potential of HIV+ organ donors; ongoing national studies are exploring the safety, feasibility, and efficacy of kidney transplantation in this population.
As of March 2019, there have been 57 HOPE donors, including both true and false positive HOPE donors resulting in more than 120 transplants.
So as to maximize the benefit of utilizing HIV+ organ donors, successful implementation of the HOPE Act requires that the HIV+ community, organ procurement organizations, and transplant centers are appropriately informed and prepared.
ACKNOWLEDGEMENTS
This study used data from the Scientific Registry of Transplant Recipients (SRTR) September 2018 public release. The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
We would like to thank Brianna Doby, Oyinkansola Kusemiju, and Shanti Seaman for their careful review of the data on active HOPE transplant centers, OPOs, and HOPE donors to date.
FINANCIAL SUPPORT
This work was supported by the National Institutes of Health grants K23CA177321-01A1 (Durand), R34AI123023 (Durand), U01AI134591 (Durand, Segev), K24DK101828 (Segev), F30DK11665 (Shaffer) and Johns Hopkins University Center for AIDS Research 1P30AI094189 (Durand).
Footnotes
CONFLICTS OF INTEREST
None
REFERENCES
- [1].Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies, Clin Infect Dis 50(10) (2010) 1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, Atta MG, Wools-Kaloustian KK, Pham PA, Bruggeman LA, Lennox JL, Ray PE, Kalayjian RC, Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America, Clin Infect Dis 59(9) (2014) e96–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maciel RA, Kluck HM, Durand M, Sprinz E, Comorbidity is more common and occurs earlier in persons living with HIV than in HIV-uninfected matched controls, aged 50 years and older: A cross-sectional study, Int J Infect Dis 70 (2018) 30–35. [DOI] [PubMed] [Google Scholar]
- [4].Chaudhary S. Razzak, Workeneh BT, Montez-Rath ME, Zolopa AR, Klotman PE, Winkelmayer WC, Trends in the outcomes of end-stage renal disease secondary to human immunodeficiency virus-associated nephropathy, Nephrol Dial Transplant 30(10) (2015) 1734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Trullas JC, Cofan F, Barril G, Martinez-Castelao A, Jofre R, Rivera M, Martinez-Ara J, Ros S, Perez I, Moreno A, Miro JM, H.I.V.I.i.D.S.G. Spanish, Outcome and prognostic factors in HIV-1-infected patients on dialysis in the cART era: a GESIDA/SEN cohort study, J Acquir Immune Defic Syndr 57(4) (2011) 276–83. [DOI] [PubMed] [Google Scholar]
- [6]. Locke JE, Reed RD, Mehta SG, Durand C, Mannon RB, MacLennan P, Shelton B, Martin MY, Qu H, Shewchuk R, Segev DL, Center-Level Experience and Kidney Transplant Outcomes in HIV-Infected Recipients, Am J Transplant 15(8) (2015) 2096–104. ** Study reports improved outcomes after HIV+ kidney transplantation over time
- [7].Shaffer AA, Durand CM, Solid Organ Transplantation for HIV-Infected Individuals, Curr Treat Options Infect Dis 10(1) (2018) 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Locke JE, Gustafson S, Mehta S, Reed RD, Shelton B, MacLennan PA, Durand C, Snyder J, Salkowski N, Massie A, Sawinski D, Segev DL, Survival Benefit of Kidney Transplantation in HIV-infected Patients, Ann Surg 265(3) (2017) 604–608. ** Evidence suggests that for HIV+ ESRD patients, KT is associated with a significant survival benefit compared with remaining on dialysis.
- [9]. Locke JE, Mehta S, Sawinski D, Gustafson S, Shelton BA, Reed RD, MacLennan P, Bolch C, Durand C, Massie A, Mannon RB, Gaston R, Saag M, Overton T, Segev DL, Access to Kidney Transplantation among HIV-Infected Waitlist Candidates, Clin J Am Soc Nephrol 12(3) (2017) 467–475. ** Describes disparities in access to transplantation among HIV+ patients with ESRD.
- [10]. Locke JE, Mehta S, Reed RD, MacLennan P, Massie A, Nellore A, Durand C, Segev DL, A National Study of Outcomes among HIV-Infected Kidney Transplant Recipients, J Am Soc Nephrol 26(9) (2015) 2222–9. ** HIV-negative and HIV+ kidney transplant recipients had similar graft and patient survival
- [11].Roland ME, Barin B, Huprikar S, Murphy B, Hanto DW, Blumberg E, Olthoff K, Simon D, Hardy WD, Beatty G, Stock PG, H.S. Team, Survival in HIV-positive transplant recipients compared with transplant candidates and with HIV-negative controls, AIDS 30(3) (2016) 435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ruck JM, Segev DL, Expanding deceased donor kidney transplantation: medical risk, infectious risk, hepatitis C virus, and HIV, Curr Opin Nephrol Hypertens 27(6) (2018) 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Muller E, Kahn D, Mendelson M, Renal transplantation between HIV-positive donors and recipients, N Engl J Med, United States, 2010, pp. 2336–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Muller E, Barday Z, Mendelson M, Kahn D, HIV-positive-to-HIV-positive kidney transplantation--results at 3 to 5 years, N Engl J Med 372(7) (2015) 613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Boyarsky BJ, Hall EC, Singer AL, Montgomery RA, Gebo KA, Segev DL, Estimating the potential pool of HIV-infected deceased organ donors in the United States, Am J Transplant 11(6) (2011) 1209–17. ** Estimates 300–500 potential HIV+ deceased donors per year in the United States.
- [16].Richterman A, Sawinski D, Reese PP, Lee DH, Clauss H, Hasz RD, Thomasson A, Goldberg DS, Abt PL, Forde KA, Bloom RD, Doll SL, Brady KA, Blumberg EA, An Assessment of HIV-Infected Patients Dying in Care for Deceased Organ Donation in a United States Urban Center, Am J Transplant 15(8) (2015) 2105–16. [DOI] [PubMed] [Google Scholar]
- [17]. Boyarsky BJ, Segev DL, From Bench to Bill: How a Transplant Nuance Became 1 of Only 57 Laws Passed in 2013, Ann Surg 263(3) (2016) 430–3. * Describes the process of writing the HIV Organ Policy Equity (HOPE) Act.
- [18].Abara WE, Collier MG, Moorman A, Bixler D, Jones J, Annambhotla P, Bowman J, Levi ME, Brooks JT, Basavaraju SV, Characteristics of Deceased Solid Organ Donors and Screening Results for Hepatitis B, C, and Human Immunodeficiency Viruses - United States, 2010–2017, MMWR Morb Mortal Wkly Rep 68(3) (2019) 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Durand CM, Bowring MG, Thomas AG, Kucirka LM, Massie AB, Cameron A, Desai NM, Sulkowski M, Segev DL, The Drug Overdose Epidemic and Deceased-Donor Transplantation in the United States: A National Registry Study, Ann Intern Med 168(10) (2018) 702–711. * Describes that transplantation with overdose deceased donor organs has increased dramatically
- [20].H.R.S.A.H.D.o.H.a.H.S. (HHS). Organ procurement and transplantation: Implementation of the HIV Organ Policy Equity Act. Final Rule, Federal Register., 2015, pp. 26464–7. [PubMed] [Google Scholar]
- [21].Changes to HOPE Act open variance, 2015.
- [22].H.R.S. Administration, Final Human Immunodeficiency Virus Organ Policy Equity (HOPE) Act safeguards and research criteria for transplantation of organs infected with HIV., Federal Register, 2015, pp. 34912–21. [Google Scholar]
- [23].Durand CM, Segev D, Sugarman J, Realizing HOPE: The Ethics of Organ Transplantation From HIV-Positive Donors, Ann Intern Med 165(2) (2016) 138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Doby BL, Tobian AAR, Segev DL, Durand CM, Moving from the HIV Organ Policy Equity Act to HIV Organ Policy Equity in action: changing practice and challenging stigma, Curr Opin Organ Transplant 23(2) (2018) 271–278. ** Describes the logistal challenges of implementing the HOPE Act.
- [25].Boyarsky BJ, Durand CM, Palella FJ Jr., Segev DL, Challenges and Clinical Decision-Making in HIV-to-HIV Transplantation: Insights From the HIV Literature, Am J Transplant 15(8) (2015) 2023–30. [DOI] [PubMed] [Google Scholar]
- [26]. Muzaale AD, Althoff KN, Sperati CJ, Abraham AG, Kucirka LM, Massie AB, Kitahata MM, Horberg MA, Justice AC, Fischer MJ, Silverberg MJ, Butt AA, Boswell SL, Rachlis AR, Mayor AM, Gill MJ, Eron JJ, Napravnik S, Drozd DR, Martin JN, Bosch RJ, Durand CM, Locke JE, Moore RD, Lucas GM, Segev DL, Risk of End-Stage Renal Disease in HIV-Positive Potential Live Kidney Donors, Am J Transplant 17(7) (2017) 1823–1832. ** HIV-positive individuals with no comorbidities and well-controlled disease may be considered low-risk kidney donor candidates.
- [27].Seem DL, Lee I, Umscheid CA, Kuehnert MJ, PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation, Public Health Rep 128(4) (2013) 247–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. OPTN Policies, pp. 20–32.
- [29].Theodoropoulos N, Jaramillo A, Ladner DP, Ison MG, Deceased organ donor screening for HIV, hepatitis B, and hepatitis C viruses: a survey of organ procurement organization practices, Am J Transplant 13(8) (2013) 2186–90. [DOI] [PubMed] [Google Scholar]
- [30]. Durand CM, Halpern SE, Bowring MG, Bismut GA, Kusemiju OT, Doby B, Fernandez RE, Kirby CS, Ostrander D, Stock PG, Mehta S, Turgeon NA, Wojciechowski D, Huprikar S, Florman S, Ottmann S, Desai NM, Cameron A, Massie AB, Tobian AAR, Redd AD, Segev DL, Organs from deceased donors with false-positive HIV screening tests: An unexpected benefit of the HOPE act, Am J Transplant 18(10) (2018) 2579–2586. ** Organ transplantation from suspected HIV false-positive donors is an unexpected benefit of the HOPE Act that provides another novel organ source
- [31].Fischer SA, Avery RK, Screening of donor and recipient prior to solid organ transplantation, Am J Transplant 9 Suppl 4 (2009) S7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Sawinski D, Shelton BA, Mehta S, Reed RD, MacLennan PA, Gustafson S, Segev DL, Locke JE, Impact of Protease Inhibitor-Based Anti-Retroviral Therapy on Outcomes for HIV+ Kidney Transplant Recipients, Am J Transplant 17(12) (2017) 3114–3122. ** Suggests that HIV+ kidney recipients should be converted to a non-protease inhibtor regimen prior to kidney transplantation
- [33].Locke JE, Shelton BA, Reed RD, MacLennan PA, Mehta S, Sawinski D, Segev DL, Identification of Optimal Donor-Recipient Combinations Among Human Immunodeficiency Virus (HIV)-Positive Kidney Transplant Recipients, Am J Transplant 16(8) (2016) 2377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miro JM, Grossi PA, Durand CM, Challenges in solid organ transplantation in people living with HIV, Intensive Care Med, United States, 2019, pp. 398–400. [DOI] [PubMed] [Google Scholar]
- [35].Vesa J, Chaillon A, Wagner GA, Anderson CM, Richman DD, Smith DM, Little SJ, Increased HIV-1 superinfection risk in carriers of specific human leukocyte antigen alleles, Aids 31(8) (2017) 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Kucirka LM, Durand CM, Bae S, Avery RK, Locke JE, Orandi BJ, McAdams-DeMarco M, Grams ME, Segev DL, Induction Immunosuppression and Clinical Outcomes in Kidney Transplant Recipients Infected With Human Immunodeficiency Virus, Am J Transplant 16(8) (2016) 2368–76. ** HIV+ kidney recipients who received induction with anti-thymocyte globulin (ATG) had lower rates of AR
- [37].Roland ME, Barin B, Huprikar S, Murphy B, Hanto DW, Blumberg E, Olthoff K, Simon D, Hardy WD, Beatty G, Stock PG, Survival in HIV-positive transplant recipients compared with transplant candidates and with HIV-negative controls, Aids 30(3) (2016) 435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Canaud G, Dejucq-Rainsford N, Avettand-Fenoel V, Viard JP, Anglicheau D, Bienaime F, Muorah M, Galmiche L, Gribouval O, Noel LH, Satie AP, Martinez F, Sberro-Soussan R, Scemla A, Gubler MC, Friedlander G, Antignac C, Timsit MO, Muda A. Onetti, Terzi F, Rouzioux C, Legendre C, The kidney as a reservoir for HIV-1 after renal transplantation, J Am Soc Nephrol 25(2) (2014) 407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Nguyen AQ, Anjum SK, Halpern SE, Kumar K, Van Pilsum Rasmussen SE, Doby B, Shaffer AA, Massie AB, Tobian AAR, Segev DL, Sugarman J, Durand CM, Willingness to Donate Organs Among People Living With HIV, J Acquir Immune Defic Syndr 79(1) (2018) e30–e36. ** Many of HIV+ patients surveyed expressed willingness to be organ donors.
- [40]. Cash A, Luo X, Chow EKH, Bowring MG, Shaffer AA, Doby B, Wickliffe CE, Alexander C, McRann D, Tobian AAR, Segev DL, Durand CM, HIV+ deceased donor referrals: A national survey of organ procurement organizations, Clin Transplant 32(2) (2018). ** OPOs reported a high volume of HIV+ referrals annually, of which a subset will be medically eligible for donation.
- [41]. Van Pilsum Rasmussen SE, Bowring MG, Shaffer AA, Henderson ML, Massie A, Tobian AAR, Segev DL, Durand CM, Knowledge, attitudes, and planned practice of HIV-positive to HIV-positive transplantation in US transplant centers, Clin Transplant 32(10) (2018) e13365. ** Though many programs plan HIV D+/R+ transplantation, center-level barriers remain including geographic clustering of programs.
- [42].HOPE Act, 2018. https://optn.transplant.hrsa.gov/learn/professional-education/hope-act/. 2019).